Abstract
Background
Complications in cancer patients with coronavirus disease 2019 (COVID-19) have not been examined. This analysis aimed to compare characteristics of COVID-19 patients with and without cancer and assess whether cancer is associated with COVID-19 morbidity or mortality.
Methods
COVID-19–positive patients with an inpatient or emergency encounter at the Mount Sinai Health System between March 1, 2020, and May 27, 2020, were included and compared across cancer status on demographics and clinical characteristics. Multivariable logistic regressions were used to model the associations of cancer with sepsis, venous thromboembolism, acute kidney injury, intensive care unit admission, and all-cause mortality.
Results
There were 5556 COVID-19–positive patients included, 421 (7.6%) with cancer (325 solid, 96 nonsolid). Those with cancer were statistically significantly older, more likely to be non-Hispanic Black and to be admitted to the hospital during their encounter, and had more comorbidities than noncancer COVID-19 patients. Cancer patients were statistically significantly more likely to develop sepsis (adjusted odds ratio [ORadj] = 1.31, 95% confidence interval [CI] = 1.06 to 1.61) and venous thromboembolism (ORadj = 1.77, 95% CI = 1.01 to 3.09); there was no statistically significant difference in acute kidney injury (ORadj = 1.10, 95% CI = 0.87 to 1.39), intensive care unit admissions (ORadj = 1.04, 95% CI = 0.80 to 1.34), or mortality (ORadj = 1.02, 95% CI = 0.81 to 1.29).
Conclusions
COVID-19 patients with cancer may have a higher risk for adverse outcomes. Although there was no statistically significant difference in mortality, COVID-19 patients with cancer have statistically significantly higher risk of thromboembolism and sepsis. Further research is warranted into the potential effects of cancer treatments on inflammatory and immune responses to COVID-19 and on the efficacy of anticoagulant therapy in these patients.
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged at the end of 2019 (1), resulting in millions of people infected and more than 770 000 deaths worldwide as of August 18, 2020 (2). The SARS-CoV-2 infection is associated with a wide array of pulmonary, cardiovascular, and neurological complications (3,4) and has been shown to stimulate a “cytokine storm,” which results in an uncontrolled systemic inflammatory response that can affect many organs (5).
There is an urgent need to understand the risk factors associated with COVID-19 morbidity and mortality to accurately predict which patients will need the most aggressive care. Initial studies identified patients with chronic pulmonary and cardiovascular diseases and diabetes at particularly high risk of severe COVID-19 disease (6-9). It is currently unclear, however, whether active cancer confers an increased risk of morbidity and mortality in COVID-19 patients. Cancer patients are particularly vulnerable, as they may be immunosuppressed because of the cancer itself or as a consequence of cancer treatment (10). Additionally, cancer is generally considered a hypercoaguable state (11,12), and the finding that SARS-CoV-2 infection is associated with thrombotic complications (13) has raised concerns that cancer patients with COVID-19 could be particularly at risk of thrombotic events.
To date, research on COVID-19 in cancer patients has largely focused on how this infection affects cancer care. The pandemic has clearly led to interruptions in cancer patients’ care, including altered chemotherapy schedules, delays in scheduled curative surgery, and inaccessibility to clinical trials (14-16). The few studies that have assessed risks those cancer patients with COVID-19 face involve a relatively small subset of patients, are largely focused on hematologic malignancies (17), and have provided conflicting data. Although there is some evidence that COVID-19 patients with cancer, particularly those with hematological malignancies, have higher mortality risk (17,18), other studies have found no increased risk of intubation or mortality in cancer patients (19). Additionally, it is unclear what role cancer therapies play in altering the response to the COVID-19 infection.
We analyzed a large registry-based dataset to compare the demographic and clinical characteristics of COVID-19 patients with and without an active cancer to determine whether an active cancer diagnosis was an independent risk factor for adverse outcomes.
Methods
Patients
We used anonymous data from the Mount Sinai Health System (MSHS) COVID-19 registry, which includes all patients with an encounter at a MSHS facility who were diagnosed with, under investigation, or screened for COVID-19. This research on a deidentified dataset was deemed exempt by the Mount Sinai Institutional Review Board (institutional review board# 20–03334, FWA #00005656).
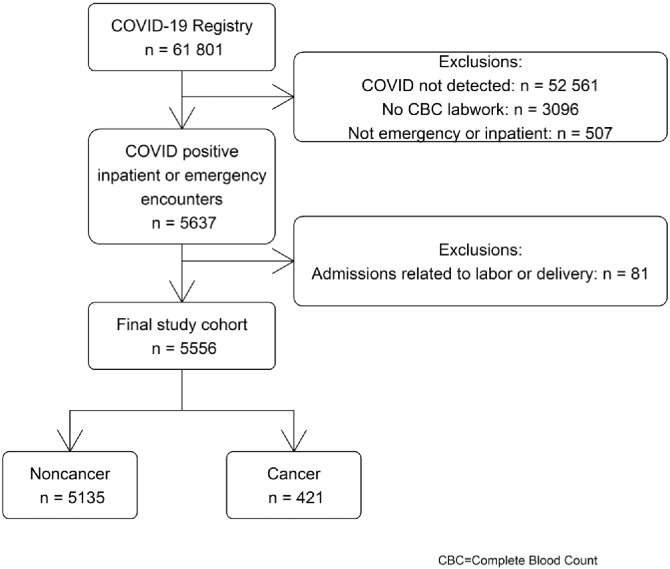
Patient encounters from March 1, 2020, to May 27, 2020, were considered (n = 61 081). COVID-19–positive patients with blood work performed were selected (n = 6144). To exclude asymptomatic cancer patients who had COVID-19 tests during encounters unrelated to COVID-19 and to identify patients who were likely to have data on clinical outcomes, only those with an inpatient or emergency encounter (n = 5637) were included in this analysis. The first such record for each patient was selected. Newborns and women who were at the hospital for labor and delivery were excluded (n = 81), resulting in a final sample of 5556 patients (Figure 1).
Figure 1.
Selection criteria.
Patients were identified as having cancer if they had any International Classification of Diseases (ICD)-10-CM code starting with “C” that was listed as “active” in their medical record. This included patients with a newly diagnosed cancer or those on an active course of treatment, but not those with a history of cancer. Leukemia, lymphoma, and myeloma were classified as nonsolid tumors. Additional comorbidities, including asthma, chronic obstructive pulmonary disease, hypertension, obesity, diabetes, chronic kidney disease, heart failure, atrial fibrillation, liver disease, and coronary artery disease, were also defined if they were active in the patient’s medical record.
Labs of interest included blood cell counts, serum creatinine, C-reactive protein, fibrinogen, D-dimer, ferritin, partial thromboplastin time, prothrombin time, tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, IL-8, and IL-1β.
Outcomes of interest included acute kidney injury, venous thromboembolism (VTE), sepsis (as defined by Bone et al.) (20), all-cause mortality, and, among those with an inpatient encounter, admissions to the intensive care unit (ICU).
Statistical Analysis
Cancer and noncancer patients were compared on baseline demographics, comorbidities, and outcomes using χ2 and t tests for categorical and continuous variables. Laboratory values were compared using linear regression for continuous values and logistic regression for categorical variables, adjusted for age, sex, and number of comorbidities. Lab value observations outside 3 SDs of the mean were excluded (range nexcluded = 2 for IL-8 [0.2% of available observations] to 148 for serum creatinine [2.7%]). Univariate and multivariable logistic regressions were conducted to assess the associations of cancer with outcomes. Multivariable analyses were adjusted for age, sex, and number of additional comorbidities and stratified into solid and nonsolid cancers. Outcomes were also assessed using a 1:2 optimal propensity score (21) matched analysis (maximum difference = 0.001), matching on age, sex, and number of comorbidities. All tests of statistical significance were 2-sided at α = .05. A Bonferroni-Holm correction for multiple comparisons was applied to P values of outcomes analyses. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographics and Comorbidities
There were 5556 patients who met the selection criteria, of whom 421 (7.6%) had cancer (325 solid tumors, 96 nonsolid tumors) (Table 1). Those with cancer were statistically significantly older, more likely to be non-Hispanic Black, and more likely to be admitted to the hospital than noncancer patients. Those with cancer were statistically significantly more likely to have additional comorbidities and were more frequently obese. Those without cancer had statistically significantly worse O2 saturation than cancer patients (Table 1).
Table 1.
Demographics and clinical characteristics of the sample according to cancer status
| Variable | Noncancer (n = 5135) | Cancer (n = 421) | P a |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Mean O2 saturation (SE), % | 93.7 (0.1) | 94.5 (0.3) | .01 |
| Mean age (SE), y | 63.8 (0.2) | 69.2 (0.7) | <.001 |
| Sex | |||
| Male | 2907 (56.6) | 250 (59.4) | .27 |
| Female | 2228 (43.4) | 171 (40.6) | |
| Race ethnicity | .003 | ||
| Non-Hispanic White | 987 (19.2) | 105 (24.9) | |
| Non-Hispanic Black | 1226 (23.9) | 108 (25.7) | |
| Non-Hispanic Asian/Pacific Islander | 210 (4.1) | 20 (4.8) | |
| Non-Hispanic other | 541 (10.5) | 25 (5.9) | |
| Hispanic | 1438 (28.0) | 115 (27.3) | |
| Missing | 733 (14.3) | 48 (11.4) | |
| Type of encounter | .02 | ||
| Emergency | 892 (17.4) | 54 (12.8) | |
| Inpatient | 4243 (82.6) | 367 (87.2) | |
| Comorbidities | |||
| COPD | 189 (3.7) | 31 (7.4) | <.001 |
| Hypertension | 1677 (32.7) | 223 (53.0) | <.001 |
| Diabetes | 1138 (22.2) | 139 (33.0) | <.001 |
| Chronic kidney disease | 566 (11.0) | 70 (16.6) | <.001 |
| Heart failure | 357 (7.0) | 41 (9.7) | .03 |
| Obesity | 388 (7.6) | 43 (10.2) | .05 |
| Asthma | 238 (4.6) | 33 (7.8) | .003 |
| Atrial fibrillation | 325 (6.3) | 54 (12.8) | <.001 |
| Liver disease | 107 (2.1) | 26 (6.2) | <.001 |
| Coronary artery disease | 618 (12.0) | 82 (19.5) | <.001 |
| No. of comorbidities | <.001 | ||
| 0 | 2686 (52.3) | 108 (25.7) | |
| 1 | 887 (17.3) | 110 (26.1) | |
| ≥2 | 1562 (30.4) | 203 (48.2) | |
| Outcomes | |||
| ICU admission (among inpatient) | 913 (21.5) | 82 (22.3) | .71 |
| Acute kidney injury | 1012 (19.7) | 110 (26.1) | .002 |
| Acute VTE | 113 (2.2) | 15 (3.6) | .07 |
| Sepsisb | 2821 (54.9) | 253 (60.1) | .045 |
| Mortality | 1272 (24.8) | 129 (30.6) | .008 |
| Cancer sitec | |||
| Prostate | — | 69 (16.4) | — |
| Breast | — | 46 (10.9) | — |
| Leukemia | — | 34 (8.1) | — |
| Myeloma | — | 34 (8.1) | — |
| Colon/rectum/anus | — | 32 (7.6) | — |
| Liver | — | 32 (7.6) | — |
| Lymphoma | — | 28 (6.7) | — |
| Uterus/ovary/endometrium | — | 20 (4.8) | — |
| Lung/bronchus | — | 19 (4.5) | — |
| Skin | — | 15 (3.6) | — |
| Kidney | — | 13 (3.1) | — |
| Bladder | — | 12 (2.9) | — |
| Head and neck (including thyroid) | — | 10 (2.4) | — |
| Stomach | — | 5 (1.2) | — |
| Other | — | 26 (6.2) | — |
| Multiple sites | — | 26 (6.2) | — |
P values based on χ2 tests for categorical variables and t tests for continuous variables. COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; VTE = venous thromboembolism.
Defined as more than 1 of the following: temperature greater than 100.4°F; heart rate greater than 90 beats per minute; respiratory rate greater than 20 breaths per minute; white blood cell count less than 4000 or greater than 12 000 cells/μL.
Information available only for cancer patients.
Patients with solid cancers were statistically significantly older (mean = 70.9 vs 63.2 years, P < .001), more likely to be non-Hispanic Black (26.5% vs 22.9%, P = .005), and more likely to be admitted to the hospital (88.3% vs 83.3%, P = .03) than nonsolid cancer patients.
Laboratory Values
After adjusting for age, sex, and number of comorbidities, patients with cancer had statistically significantly lower levels of platelets, hemoglobin, red blood cells, and white blood cells than those without cancer. Mean counts of white blood cell subpopulations, including lymphocytes, monocytes, and neutrophils, were statistically significantly lower in cancer patients. Although not significant, cancer patients tended to have lower basophil count and percent (Table 2).
Table 2.
Laboratory measures according to cancer status
| Laboratory measure | nused/navailable | Noncancer mean (SE)a | Cancer mean (SE)a | P b |
|---|---|---|---|---|
| Hemoglobin, g/dL | 5514/5553 | 13.02 (0.03) | 11.90 (0.10) | <.001 |
| Red blood cell, ×106/μL | 5503/5553 | 4.42 (0.01) | 4.06 (0.04) | <.001 |
| Platelet, ×103/μL | 5446/5532 | 224.63 (1.4) | 213.71 (4.49) | .02 |
| White blood cell, ×103/μL | 5495/5551 | 8.39 (0.06) | 7.68 (0.20) | <.001 |
| Lymphocyte, ×103/μL | 5356/5388 | 1.08 (0.01) | 0.99 (0.03) | .005 |
| Lymphocyte, % | 5438/5518 | 14.45 (0.13) | 14.23 (0.42) | .61 |
| Eosinophil, ×103/μL | 5321/5388 | 0.03 (0.001) | 0.03 (0.002) | .89 |
| Eosinophil, % | 5406/5493 | 0.43 (0.01) | 0.43 (0.03) | .88 |
| Basophil, ×103/μL | 5332/5388 | 0.012 (0.001) | 0.009 (0.002) | .08 |
| Basophil, % | 5405/5492 | 0.29 (0.01) | 0.26 (0.02) | .10 |
| Monocyte, ×103/μL | 5334/5388 | 0.55 (0.004) | 0.51 (0.02) | .01 |
| Monocyte, % | 5458/5517 | 7.17 (0.06 ) | 7.41 (0.18) | .20 |
| Neutrophil, ×103/μL | 5482/5544 | 6.70 (0.06) | 6.07 (0.19) | .002 |
| Neutrophil, % | 5441/5518 | 76.64 (0.17) | 76.17 (0.56) | .42 |
| Serum creatinine, mg/dL | 5376/5519 | 1.52 (0.02) | 1.37 (0.07) | .03 |
| Ferritin, ng/mL | 4142/4222 | 1160.61 (23.84) | 1162.62 (73.85) | .97 |
| Percent D-dimer, μg/mL FEU | 4126/4126 | — | — | .13 |
| <0.50 | — | 9.8 | 9.3 | — |
| ≥0.50 | — | 90.2 | 90.7 | — |
| Partial thromboplastin time, s | 3390/3456 | 33.00 (0.12) | 33.01 (0.37) | 1.0 |
| Prothrombin time, s | 3474/3527 | 14.84 (0.05) | 14.97 (0.14) | .39 |
| C-reactive protein, mg/L | 4254/4279 | 128.52 (1.63) | 120.66 (5.03) | .14 |
| Fibrinogen, mg/dL | 2540/2551 | 626.85 (4.31) | 588.85 (12.47) | .004 |
| TNF-alpha, pg/mL | 1316/1226 | 25.94 (0.55) | 25.78 (1.41) | .92 |
| IL-6, pg/mL | 2506/2508 | 178.51 (13.46) | 138.35 (37.72) | .31 |
| IL-8, pg/mL | 1331/1333 | 74.56 (4.84) | 70.56 (12.30) | .76 |
| Percent IL-1β, pg/mL | 1330/1330 | — | — | .99 |
| <0.4 | — | 42.4 | 41.2 | — |
| 0.4-0.5 | — | 21.4 | 21.2 | — |
| 0.6-0.9 | — | 16.7 | 17.7 | — |
| ≥0.9 | — | 19.3 | 19.9 | — |
Values adjusted for age, sex, and number of comorbidities. IL = interleukin; TNF = tumor necrosis factor.
P values based on logistic regression for categorical variables and linear regression for continuous variables, adjusted for age, sex, and number of comorbidities.
Serum creatinine and fibrinogen were statistically significantly higher in noncancer patients than in cancer patients. Although not significant, cancer patients tended to have lower C-reactive protein than noncancer patients (Table 2).
When cancer patients were stratified into solid and nonsolid cancers, those with nonsolid cancers had lower mean platelet count (184.1 vs 222.6 × 103/μL, P < .001), hemoglobin (11.2 vs 12.1 g/dL, P < .001), red blood cell count (3.7 vs 4.2 × 106/μL, P < .001), and white blood cell count (6.8 vs 7.9 × 103/μL, P < .001) than those with solid cancer. Mean lymphocyte count was statistically significantly lower in the solid cancer group (mean = 0.98, vs 1.04 × 103/μL in nonsolid cancer, P = .01), and mean monocyte counts (nonsolid = 0.47 vs solid = 0.53 × 103/μL, P = .02) and neutrophil counts (nonsolid = 5.3 vs solid = 6.3 × 103/μL, P < .001) were statistically significantly lower in the nonsolid cancer group. Those with nonsolid cancer had a higher percentage of lymphocytes (mean = 16.2% vs 13.7%, P = .05) and a lower percentage of neutrophils (mean = 71.7% vs 77.3%, P < .001) compared with solid-cancer patients. Though not statistically significant, those with nonsolid cancers also tended to have lower basophil counts.
Ferritin was statistically significantly higher in nonsolid cancer patients (mean = 1613.2 vs 1040.9 ng/mL, P = .006) than in solid-cancer patients, as was fibrinogen (mean nonsolid = 599.9 vs solid = 584.6 mg/dL, P = .01).
Outcomes
Patients with cancer were more likely to develop VTE and were statistically significantly more likely to have acute kidney injury and sepsis and to be deceased than those without cancer. Among those with an inpatient encounter, there was no statistically significant difference in ICU admissions between cancer and noncancer patients (Table 1).
After adjusting for age, sex, and the number of additional comorbid conditions, those with cancer were statistically significantly more likely to develop sepsis (adjusted odds ratio [ORadj] = 1.31, 95% confidence interval [CI] = 1.06 to 1.61) and VTE (ORadj = 1.77, 95% CI = 1.01 to 3.09); however, there was no significant difference in acute kidney injury (ORadj = 1.10, 95% CI = 0.87 to 1.39) or all-cause mortality (ORadj = 1.02, 95% CI = 0.81 to 1.29). Among those hospitalized, there was no statistically significant difference in ICU admissions (ORadj = 1.04, 95% CI = 0.80 to 1.34) (Table 3). When the cancer group was stratified into solid and nonsolid cancer types, outcomes did not significantly differ between the 2 cancer groups and differences remained similar compared with noncancer patients. Compared with those without cancer, both patients with nonsolid and solid cancers had similar risk of all-cause mortality, acute kidney injury, or admission to the ICU. Those with nonsolid cancers had statistically significantly higher odds of sepsis (ORadj = 1.83, 95% CI = 1.18 to 2.83); those with solid tumors experienced a slightly higher risk of sepsis (ORadj = 1.19, 95% CI = 0.95 to 1.50) than those without cancer. Those with solid tumors (ORadj = 1.70, 95% CI = 0.89 to 3.23) and nonsolid tumors (ORadj = 1.98, 95% CI = 0.72 to 5.50) had a non-statistically significant higher VTE risk than those without cancer.
Table 3.
Odds of outcomes in cancer vs noncancer patients in the multivariable and propensity matched analyses
| Cancer vs no cancer | Sepsisa (yes vs no) |
VTE (yes vs no) |
Acute kidney injury (yes vs no) |
Mortality (yes vs no) |
ICU admissionc (yes vs no) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORadj (95% CI) | P d | ORadj (95% CI) | P d | ORadj (95% CI) | P d | ORadj (95% CI) | P d | ORadj (95% CI) | P d | |
| Multivariable (n = 5556)b | 1.31 (1.06 to 1.61) | .05 | 1.77 (1.01 to 3.09) | .18 | 1.10 (0.87 to 1.39) | 1.0 | 1.02 (0.81 to 1.29) | .86 | 1.04 (0.80 to 1.34) | 1.0 |
| Propensity matched (n = 1260)b | 1.25 (0.98 to 1.59) | .31 | 2.73 (1.25 to 5.94) | .06 | 1.08 (0.82 to 1.41) | 1.0 | 1.12 (0.86 to 1.45) | 1.0 | 1.07 (0.78 to 1.47) | .68 |
Defined as greater than 1 of the following: temperature greater than 100.4°F; heart rate greater than 90 beats per minute; respiratory rate greater than 20 breaths per minute; white blood cell count less than 4000 or greater than 12 000 cells/μL. CI = confidence interval; ICU = intensive care unit; ORadj = adjusted odds ratio; VTE = venous thromboembolism.
Multivariable analysis adjusted for age, sex, and number of comorbidities. Propensity matched analysis matched on age, sex, and number of comorbidities.
Among patients with an inpatient encounter.
Two-sided, Bonferroni-Holm corrected P values.
After propensity matching, the cancer and noncancer groups were well balanced on age, sex, and number of comorbidities (ncancer = 420, nnoncancer = 840). Results were consistent with the multivariable analysis with increased odds of VTE and sepsis and no statistically significant difference in acute kidney injury, all-cause mortality, or ICU admission (Table 3).
Among cancer patients, those with neutropenia (neutrophil count <1000/μL) had statistically significantly higher rates of sepsis than those without neutropenia (92.3% vs 59.2%, P = .02) and slightly, though not statistically significant, higher rates of mortality (38.5% vs 30.5%, P = .55). There was a non–statistically significant inverse association between platelet number and VTE in cancer patients (mean platelet = 184.8 vs 223.3 × 103/μL in those with and without VTE, P = .23). Among patients with sepsis, cancer patients had non–statistically significantly higher mortality rates (34.0% vs 29.9%, P = .18).
Discussion
This analysis of more than 5000 patients hospitalized for COVID-19 in New York City (NYC) shows that cancer patients are significantly older and are more affected by comorbidities than COVID-19 patients without cancer. Although cancer patients were more likely to have acute kidney injury, VTE, and sepsis, only VTE and sepsis had statistically significantly increased risks after adjustment for covariates. ICU admissions and all-cause mortality were not statistically different between cancer and noncancer COVID-19 patients. Thus, although fatality rates for COVID-19 patients requiring emergency or hospital attention is high, there does not appear to be a significant additional risk to cancer patients. Previous studies have noted high fatality rates for patients with cancer (22,23); however, these analyses did not include a comparison group composed of COVID-19–positive noncancer patients. One study in a geographically similar population on a small sample of 61 cancer patients found a significantly higher case fatality rate for patients with cancer (24) compared with other hospitalized patients as well as the general population of residents in NYC. However, the analysis did not account for differences in other clinical characteristics, including comorbidities, when compared with noncancer patients, a strength of this study.
Cancer patients are more susceptible to infections because of effects of their underlying disease and oncologic treatment regimen, including neutropenia, breakdown of innate mucosal immune barriers, cell-mediated or humoral immune dysfunction, or local tumor effects (25). Infections in cancer patients are complicated by an increased risk for developing sepsis; 1 study indicated that cancer patients are nearly 10 times more susceptible to developing sepsis than noncancer patients (26). This is particularly true for patients with hematological cancers, given their more immunocompromised status (27). Severe sepsis is major cause of mortality among cancer patients (28), and our analysis shows that cancer patients with COVID-19 were more likely to develop sepsis than noncancer patients and, among those with sepsis, had slightly worse mortality. These findings are reported here for the first time, to our knowledge, and open the discussion on appropriate, early treatment to prevent such serious complications.
Cancer patients are characterized by hypercoagulability because of local factors related to the cancer itself, including increased production of inflammatory and cytokinergic factors that result in procoagulant agents (29), aberrant activity of immune cells and hyper activation of platelets (30,31), and the expression of unique oncogenes (32). There are also pro-coagulation events related to the cancer treatment, such as potential long-term immobilization associated with hospitalization, surgical effects, and chemotherapy (33). A prior epidemiological study reported that 20% of all newly diagnosed VTEs were associated with an underlying malignancy (34); another study found patients with active malignancy were 7 times more likely to develop a VTE than noncancer patients (35). VTEs have also been established as a secondary outcome in COVID-19 patients (13). Mechanisms for the development of thromboses in COVID-19 require further investigation, but early evidence suggests the causes are multifactorial (13). Our study shows that COVID-19 patients with cancer were more likely to develop VTEs than those without cancer. Careful precautions should be taken to protect COVID-19 patients with cancer from coagulopathies. More research is needed to further define the role of VTEs in COVID-19 patients.
Despite differences in sepsis and VTE risks between cancer and noncancer patients, there was no difference in mortality between the 2 groups. Our findings are contrasted by other studies that found that cancer was predictive of mortality in COVID-19 patients, including a meta-analysis that included data from 8 nations (36). However, this meta-analysis reported crude estimates and did not adjust the analysis for age, which is known to be a major predictor of COVID-19 outcomes and is associated with cancer. Additionally, many of the included studies had few cancer patients, limiting generalizability. The study by Kuderer et al. (22) also indicated that patients with active cancer had higher mortality risk from COVID-19. However, this study included all patients with COVID-19 from Vanderbilt University Medical Center’s data registry and compared them with patients in the recovery phase. Our study was limited to emergency room or inpatient encounters, thus including patients with a clinical symptomatology that required hospital attention. It is possible that cancer patients in the general population are more likely to develop a serious infection from SARS-CoV-2, as suggested by others (22,24,36). However, our research suggests that once an infection has progressed to a stage that requires hospitalization, cancer’s role in increased short-term mortality risk is more limited.
The present analysis also indicates that there are no statistically significant differences in all-cause mortality between solid and nonsolid tumors. This is important given that nonsolid cancers place patients at higher risk for developing sepsis and VTEs. Our results agree with those of Kuderer (22), which also found no significant difference in mortality by tumor type.
This dataset also allowed us to look at inflammatory response parameters; we found no statistically significant difference between cancer and noncancer patients in terms of cytokine response, highlighting that cytokine storm does not appear to be more of a concern for cancer patients than noncancer patients. This is clinically relevant because it can set expectations for future cancer patients admitted with SARS-CoV-2. To our knowledge, this is the first observation of this kind in cancer patients.
Additionally, laboratory tests indicate statistically significant differences in white blood cells response in favor of noncancer patients, even after adjusting for clinical and demographic differences; this could be due to a variety of factors, including the disease itself, cancer therapies, or a dampened immune response (37). The role that prior therapy plays in outcomes is not sufficiently clear, because 1 study of cancer patients indicated no significant difference in mortality by receipt of chemotherapy (38). Although we were unable to explore this more in detail, because the dataset contains no information on treatments before the COVID-19–related encounter, this is important information to consider moving forward.
Results should be interpreted within the possibility of chance finding and in the context of the data limitations. We used a retrospective registry, and therefore we were unable to acquire information on previous treatments or the timing of a patient’s cancer diagnosis, which could have aided in better understanding the complications observed. Because all patients included were required to have either emergency or inpatient care, this analysis does not address whether cancer patients are more likely to contract COVID-19 or to need hospital care if they do. Because cancer patients typically have more contact with health-care systems (39), it is possible that some cancer patients were diagnosed with COVID-19 during an encounter related to cancer care, but not to a COVID-19 clinical manifestation. However, we minimized this effect by excluding patients with routine encounters for laboratory work or outpatient or nurse’s office visits. It is possible that not all patients completed their clinical course before data collection and remained at risk of developing adverse outcomes. Because this dataset is hospital based, it is also difficult to assess outcomes that occurred after discharge. Although this means that we may undercount the number of adverse outcomes, we believe this is unlikely to have biased the results, because we do not expect the distributions of those who had not completed the clinical course or developed outcomes after discharge to differ by cancer status. We do not know how representative the COVID-19 population served by MSHS is of all NYC residents, but previous health assessments show that MSHS patients are very diverse in race, socio-economic status, and insurance. During the COVID pandemic, New York State required all hospitals to enter into an agreement to share patients to provide care to all because hospitals were being overwhelmed (40). Because of these guidelines, we think it is likely that the MSHS may have served an even more representative NYC population. This study adds to the knowledge of risks to patients with a dual COVID-19 and cancer diagnosis. To our knowledge, this is the largest study on COVID-19 cancer patients that includes comprehensive information on comorbidities, laboratory values, and outcomes, including VTE and sepsis. Because follow-up of COVID-19 patients is limited, it is too soon to tell whether there are increased long-term risks of morbidity and mortality for patients with a concurrent cancer. Future research would benefit from a registry coding structure for COVID-19 cancer patients (41) and should include treatment information for these patients and longer follow-up to address and respond to chronic effects of COVID-19 infection on cancer patients.
Funding
This work was supported in part by the National Cancer Institute (P30CA196521).
Notes
Role of the funder: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures: The authors declare no potential conflicts of interest.
Author contributions: Conceptualization of the research: BM, RF, ET; Data curation: NA, JLR; Formal analysis: NA; Supervision of analysis: ET; Writing, review, and editing of the manuscript: NA, JLR, BM, WLC, RF, ET.
Acknowledgments: This work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Prior presentations: This work was presented as a short talk at the AACR Virtual Meeting: COVID-19 and Cancer on July 22, 2020.
Data Availability
No new data were generated by the authors of this study.
References
- 1. Guan W-J, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2020. https://covid19.who.int/. Accessed August 18, 2020.
- 3. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahdavinia M, Foster KJ, Jauregui E, et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020;8(7):2388–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14(4):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020;309:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10(6):589–597. [DOI] [PubMed] [Google Scholar]
- 11. Caine GJ, Stonelake PS, Lip GYH, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(S1)(suppl 1):S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tal S, Spectre G, Kornowski R, Perl L. Venous thromboembolism complicated with COVID-19: what do we know so far? Acta Haematol. 2020;143(5):417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Omarini C, Maur M, Luppi G, et al. Cancer treatment during the coronavirus disease 2019 pandemic: do not postpone, do it! Eur J Cancer. 2020;133:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saini KS, de Las Heras B, de Castro J, et al. Effect of the COVID-19 pandemic on cancer treatment and research. Lancet Haematol. 2020;7(6):e432–e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan AC, Ashley DM, Khasraw M. Adapting to a pandemic - conducting oncology trials during the SARS-CoV-2 pandemic. Clin Cancer Res. 2020;26(13):3100–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah V, Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol. 190(5):e279–e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. [DOI] [PubMed] [Google Scholar]
- 21. Murphy B, Fraeman K. A general SAS macro to implement optimal N : 1 propensity score matching within a maximum radius. 2020. https://support.sas.com/resources/papers/proceedings17/0812-2017.pdf. Accessed July 16, 2020.
- 22. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Safdar A, Armstrong D. Infectious morbidity in critically ill patients with cancer. Crit Care Clin. 2001;17(3):531–570. [DOI] [PubMed] [Google Scholar]
- 26. Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129(6):1432–1440. [DOI] [PubMed] [Google Scholar]
- 27. Williams MD, Braun LA, Cooper LM, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5):R291–R298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosolem MM, Rabello LSCF, Lisboa T, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27(3):301–307. [DOI] [PubMed] [Google Scholar]
- 29. Rao LV. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992;11(3-4):249–266. [DOI] [PubMed] [Google Scholar]
- 30. Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol. 2014;8:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karimi M, Cohan N. Cancer-associated thrombosis. Open Cardiovasc Med J. 2010;4(1):78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434(7031):396–400. [DOI] [PubMed] [Google Scholar]
- 33. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6(6):401–410. [DOI] [PubMed] [Google Scholar]
- 34. Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. [DOI] [PubMed] [Google Scholar]
- 36. Ofori-Asenso R, Ogundipe O, Agyeman AA, et al. Cancer is associated with severe disease in COVID-19 patients: a systematic review and meta-analysis. Ecancermedicalscience. 2020;14:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit Rev Oncol Hematol. 2010;74(1):40–60. [DOI] [PubMed] [Google Scholar]
- 38. Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasmussen LA, Jensen H, Virgilsen LF, Falborg AZ, Møller H, Vedsted P. Healthcare utilisation in general practice and hospitals in the year preceding a diagnosis of cancer recurrence or second primary cancer: a population-based register study. BMC Health Serv Res. 2019;19(1):941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. New York State. Amid ongoing COVID-19 pandemic, governor Cuomo announces New Hospital Network Central Coordinating Team. 2020. https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-new-hospital-network-central. Accessed July 16, 2020.
- 41. Greene FL. COVID-19 data and the cancer patient: a need for registry inclusion. Ann Surg Oncol. 2020;27(8):2571–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated by the authors of this study.