Abstract
Passive transfer of antibodies from COVID-19 convalescent patients is being used as an experimental treatment for eligible patients with SARS-CoV-2 infections. The United States Food and Drug Administration’s (FDA) guidelines for convalescent plasma initially recommended target antibody titers of 160. We evaluated SARS-CoV-2 neutralizing antibodies in sera from recovered COVID-19 patients using plaque reduction neutralization tests (PRNT) at moderate (PRNT50) and high (PRNT90) stringency thresholds. We found that neutralizing activity significantly increased with time post symptom onset (PSO), reaching a peak at 31–35 days PSO. At this point, the number of sera having neutralizing titers of at least 160 was approximately 93% (PRNT50) and approximately 54% (PRNT90). Sera with high SARS-CoV-2 antibody levels (>960 enzyme-linked immunosorbent assay titers) showed maximal activity, but not all high-titer sera contained neutralizing antibody at FDA recommended levels, particularly at high stringency. These results underscore the value of serum characterization for neutralization activity.
Keywords: COVID-19, SARS-CoV-2, convalescent plasma, neutralizing antibodies
There is a wide range of neutralizing antibodies in convalescent plasma with many sera having lower neutralizing titers than recommended by the FDA. It is essential to characterize the potential of donor sera prior to use as therapy.
The United States has been profoundly impacted by coronavirus disease 2019 (COVID-19) since the movement of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral infections out of China and across the globe in early 2020. As of 9 October 2020, the United States has had 7 611 616 confirmed COVID-19 cases and more than 212 840 COVID-19–related deaths [1, 2]. The first documented COVID-19 case in New York was reported on 1 March 2020. Through the spring of 2020, New York State accounted for the largest portion of cases in the United States (currently the fourth most cases) [1, 3].
Infection with SARS-CoV-2 can be severe, especially among at-risk populations (eg, the elderly, especially with preexisting comorbidities) [4]. Nonetheless, up to 80% of infections are thought to be mild or asymptomatic [5]. Antibody (Ab)-mediated, immunity is thought to protect an individual from viral infection by interfering with virus-host cell interactions required for viral entry and replication. Vaccinated or previously infected individuals with virus-specific Abs can also help prevent new infections through herd immunity [6]. However, the duration and degree to which asymptomatic infection or recovery from COVID-19 disease confers prolonged immunity from reinfection with SARS-CoV-2 is unclear, even among individuals who produce virus-specific antibodies [7, 8]. Consequently, there is great interest in gaining a better understanding of Ab responses to SARS-CoV-2 and the serological tests that measure them [9–11].
Due to the lack of effective treatments, current COVID-19 outbreak management emphasizes social distancing, expanded diagnostic testing, and isolation of known cases with quarantine of close contacts. The resulting economic and societal disruption exacerbates the public health impact of COVID-19, and there is an urgent need to define correlates of immunological status to identify individuals who may have protective immunity. Passive transfer of Ab from convalescent COVID-19 patients is being used as treatment of seriously ill COVID-19 patients, although controlled studies are still needed to rigorously evaluate efficacy [12–15]. Recovered COVID-19 patients who have generated robust Ab responses to SARS-CoV-2 are sought to fill a growing need for plasma donors to support this therapy. A lack of standardization or information about the relative neutralizing capacity of Abs from convalescent donors complicates implementation and evaluation of plasma therapy protocols for COVID-19. The US Food and Drug Administration (FDA) has issued a recommendation that the titer of neutralizing Abs in convalescent plasma should be at least 160, but allows that an 80 titer is acceptable in the absence of a better match [16]. However, this guidance does not specify the level of virus neutralization that should be achieved at these titers or how to measure it.
Several approaches to measuring relative levels of neutralizing Abs exist, including plaque reduction neutralization tests (PRNT) and microneutralization (MN) [17–19]. Here we chose the PRNT, which measures the ability of Ab in serially diluted sera or plasma to decrease virus infection of cultured cells at a minimum threshold (eg, 50% or 90%). PRNT is a relatively low throughput assay because it takes several days for development of virus plaques, which are the units of measurement. MN assays can provide a higher throughput alternative, but the need for BSL3 containment during SARS-CoV-2 culture remains a barrier. As a result of these limitations, determination of Ab neutralization activity has not been routinely adopted by many COVID-19 plasma donor screening programs; instead donors are chosen based on Ab levels or time since clinical recovery from polymerase chain reaction (PCR) confirmed disease.
The purpose of this study was to measure SARS-CoV-2–specific Abs in the sera of COVID-19 convalescent individuals to characterize the humoral immune response in these individuals and correlate this response to Ab neutralizing activity, and evaluate neutralizing activity in the context of FDA-recommended guidelines for selection of convalescent plasma donors.
METHODS
Study Participants
Specimens were collected with informed consent obtained from individuals in accordance with guidelines of Mount Sinai Hospital (MSH) and the Westchester County Department of Health (WCDH). Testing at the Wadsworth Center was done under a declared Public Health Emergency with a waiver from the New York State Department of Health Institutional Review Board.
Study Set 1
Sera were obtained from individuals who had recovered from COVID-19 during a recruitment effort to identify plasma donors for passive Ab transfer therapy. Seropositivity assessment on these specimens was done by enzyme-linked immunosorbent assay (ELISA) at MSH Laboratory [20, 21]. A sliding recruitment was done to increase the selection of recovered COVID-19 patients at later stages of convalescence. Of the 3277 people who entered the study by 12 April 2020, 227 had a positive SARS-CoV-2 PCR test at MSH, Labcorp, or the New York City Department of Health and Mental Hygiene, and 621 had a self-reported positive PCR result. An additional 1423 had antibodies reactive in the ELISA at 1:50 dilution and were considered “true” COVID-19 recovered individuals.
A progressive approach was used to define eligibility for the donor candidate screening in this cohort. For the first 3 days of the study, the Ab screening criteria included patients who were at least 10 days beyond the onset of symptoms or diagnostic PCR test and asymptomatic for 3 days. With each subsequent week of the study, selected patients were deeper into the convalescent phase, where increased Ab responses would be expected based on other viral infections [22, 23]. By the third week, all patients had to be 21 or more days from the start of infection, and 14 or more days from full recovery.
Study Set 2
To specifically determine Ab levels and neutralizing antibodies in individuals well-removed from the onset of symptoms, sera from 149 healthy, recovered COVID-19 individuals from Westchester County were screened as potential donors of passive Ab therapy. The WCDH recruited individuals as potential plasma donors if they had confirmed positive SARS-CoV-2 PCR results at least 21 days before the date of serum collection and were symptom free for at least 14 days. Ascertainment of symptom-free status was done by interviewing the individual at the time the arrangements were made for phlebotomy. Ab testing for the Westchester cohort was done at the Wadsworth Center using a microsphere immunoassay (MIA).
Immunoassays
Enzyme-Linked Immunosorbent Assay
The initial description of this FDA Emergency Use Authorization (EUA) test and methodological details are reported elsewhere [20, 21]. This ELISA detects Abs that are reactive with the SARS-CoV-2 receptor binding domain (RBD) and the entire spike protein ectodomain. For most of the sera described in this report, specimens were screened initially at a 1:50 dilution using the SARS-CoV-2 RBD as the target antigen and specific immunoglobulin G (IgG) was detected. The endpoint titers of the presumptive screen-positive sera were then determined by ELISA using the whole recombinant spike ectodomain as the target antigen.
Microsphere Immunoassay
The NY SARS-CoV-MIA is a suspension-phase assay using the Luminex platform. This is an FDA EUA test and the details and performance characteristics are described elsewhere [24]. For all specimens included in this study, the MIA measured reactivity to the SARS-CoV-1 nucleoprotein (N) antigen that had been coupled to polystyrene microspheres. Total antigen-specific immunoglobulin (IgM, IgA, and IgG) was measured. Anti-SARS-CoV-2 N Abs are strongly identified due to extensive amino acid identity between SARS-CoV-1 and SARS-CoV-2 (approximately 90%), and ongoing studies show that the substitution of the SARS-CoV-2 N protein provides essentially identical results (W. T. L., unpublished observations). Many of the sera assessed in this study were also evaluated using the SARS-CoV-2 RBD antigen in the MIA, multiplexed in conjunction with the N protein. With some exceptions, both antigens were reactive with the same sera. For the MIA assay positivity is defined as 6 standard deviations (SD) above the mean median fluorescence intensity (MFI) of the signal from 90 pre-2019 blood donor sera from presumed healthy individuals. The high cutoff maximizes specificity (99%) to identify sera with moderate to high amounts of SARS-CoV-2–reactive antibodies and excludes potential cross-reactivity with other respiratory viruses. Results for which the MFI signal falls between 3 SD and 6 SD above the mean MFI of normal sera are considered indeterminate.
Plaque Reduction Neutralization Test
This assay has been previously described and is considered the classical standard for detection of virus-specific Abs based on their ability to neutralize their cognate viral infections [18, 25, 26]. For the detection of SARS-CoV-2 neutralizing Abs, 100 μL of 2-fold serially diluted test sera were mixed with 100 μL of 200 plaque-forming units of SARS-CoV-2, isolate USA-WA1/2020 (NR-52281; BEI Resources) and incubated at 37°C in an incubator with 5% CO2 for 1 hour. Virus:serum mixture, 100 μL, was added to Vero E6 cells (C1008, ATCC CRL-1586) and adsorption proceeded at 37°C in an incubator with 5% CO2 for 1 hour, after which a 0.6% agar overlay prepared in cell culture maintenance medium (Eagle’s Minimal Essential Medium, 2% heat-inactivated fetal bovine serum, 100 µg/mL penicillin G, 100 U/mL streptomycin) was applied. At 2 days post infection, a second agar overlay containing 0.2% Neutral Red was applied, and the number of plaques in each sample were recorded after an additional 2 days of incubation. The inverse of the highest dilutions of sera providing 50% (PRNT50) or 90% (PRNT90) viral plaque reduction relative to virus-only infection was reported as the titer.
Statistical Analyses
One-way ANOVA using the Kruskal-Wallis test with Dunn correction for multiple comparisons was used to measure the differences in means of 3 or more unmatched groups. Spearman r was calculated and significance was assessed using a 2-tailed t test when assessing correlations between PRNT titers and MIA values.
RESULTS
Characterization of Tested Specimens
For this study period, 3277 specimens from convalescent COVID-19 individuals were screened for SARS-CoV-2 RBD Ab reactivity using the ELISA assay at MSH. Of these specimens, 2398 were also tested for Abs at the Wadsworth Center using the MIA. An additional 171 serum specimens, submitted directly to the Wadsworth Center from the WCDH, were tested for the presence of Abs to the SARS-CoV N protein. For the specimens tested at both MSH and the Wadsworth Center, the 2 assays (ELISA and MIA, respectively) showed 79.3% agreement (Supplementary Table 1). If the MIA indeterminates (3 SD) were also counted as reactive, the agreement increased to 87.6%. Inclusion of RBD with the N protein in the MIA resulted in agreement levels of 89.7% (6 SD) and 90.6% (3 SD). A direct comparison of the values provided by the 2 tests showed the strongest correlation between the MIA RBD protein and the ELISA spike protein (Supplementary Figure 1).
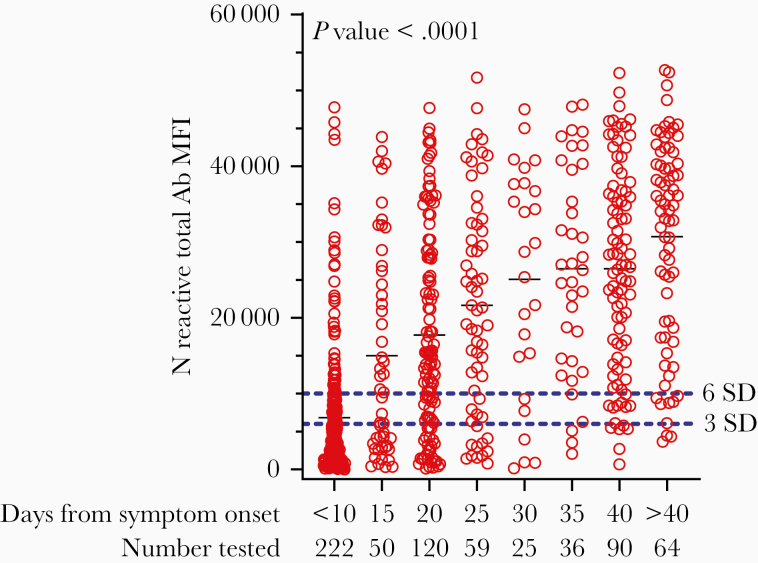
In the MSH cohort, the titers of SARS-CoV-2 Abs were determined for those specimens that were positive for RBD binding at a greater than 1:50 dilution in the initial screen. Over the 3-week course of the study, a higher proportion of sera showed increasingly higher titers, presumably reflecting maturation of the immune response. Supplementary Table 2 shows the distribution of serum titers of samples received during the final 9 days of the study, when most individuals would be expected to be > 1 month post symptom onset (PSO). As indicated, 89.9% of the screen-positive sera had SARS-CoV-2 titers that exceeded a 320 threshold. We noted that 10% of the Ab-positive sera failed to meet a 320-titer threshold for strong Ab responses even 3 weeks from initial symptom onset. In sera that were collected from the WCDH cohort of convalescent individuals who were at least 21 days PSO, the MIA showed reactivity in 83.2% (6 SD) or 90.6% (3 SD) of the specimens. A composite analysis of Ab levels, as indicated by differences in MIA signal intensities, is shown in Figure 1. A 1-way ANOVA using the Kruskal-Wallis test with Dunn correction for multiple comparisons was used to assess the significance of the correlation between days PSO and MIA signal, and the correlation was found to be significant (P value < .0001). All groups ≥11 days post onset had MFI means significantly greater than <10 days post onset.
Figure 1.
SARS-CoV-2 Ab levels in convalescent sera. Serum specimens from COVID-19 convalescent patients (Mount Sinai and Westchester County) were tested at a 1:100 dilution in the MIA. MFI values, based on N antigen reactivity, from individual specimens are presented here grouped into 5-day blocks after symptom onset (except for <10 and >40). The cutoff at 6 SD (upper dotted line) and 3 SD (lower dotted line) are shown. A 1-way ANOVA using the Kruskal-Wallis test with Dunn correction for multiple comparisons was used to assess the significance of correlations between MFI values and days post symptom onset. P value is approximate and is <.0001. All groups ≥11 days post onset have means significantly greater than <10 days post onset. Groups ≥31 days post onset have means significantly greater than 11–15 days post onset. Groups ≥36 days post onset have means significantly greater than 16–20 days post onset. Abbreviations: Ab, antibody; ANOVA, analysis of variance; MFI, mean fluorescence intensity; MIA, microsphere immunoassay; N, nucleocapsid protein.
Relationship Between Antibody Production and Virus Neutralizing Antibody
One protective function of antiviral antibodies is to prevent infection by interfering with essential virus-host cell interactions such as receptor binding, virus entry, and membrane fusion [7, 27]. Neutralization can be measured by PRNT, which we used to examine 2 cohorts of recovered COVID-19 patients. All sera used were reactive in the NYS SARS-CoV MIA, and most specimens were tested using a multiplexed MIA that included the SARS-CoV-2 RBD along with the N protein. Our objective in this portion of the study was to identify those sera that met the recommended (160) or minimal (80) titers for convalescent plasma proposed by the FDA at both the 50% (PRNT50) and 90% (PRNT90) neutralization levels.
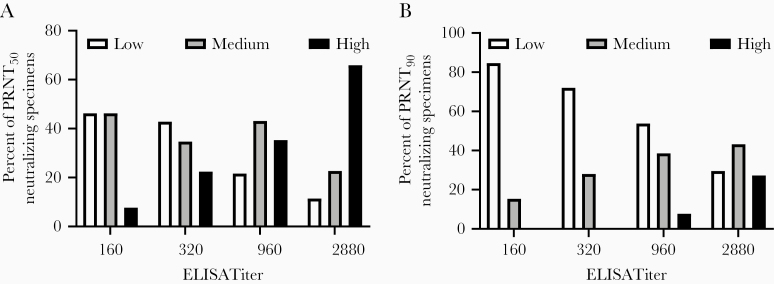
We examined the relationship between ELISA and PRNT titers using sera collected by MSH to determine whether a direct correlation could be made between Ab reactivity in the ELISA and neutralizing activity. Analysis of 159 sera showed a significant, positive correlation between ELISA titers and neutralizing titer at the PRNT50 level (Spearman r = 0.4315, P value < .001) and at the PRNT90 level (Spearman r = 0.4057, P value < .001) (Table 1 and Figure 2). Approximately half (52.9%) of the samples had a ≥160 neutralizing titer PRNT50 level, including 84.1% of sera with an ELISA titer of 2880. However, only 50% of the sera at the highest ELISA Ab titer (2880) had neutralizing titers of 160 or greater when a more stringent (PRNT90) determination for neutralization was used. Figure 2 shows a graphical representation of these data, with results grouped to show the frequencies of specimens that meet the FDA minimal (80) or recommended (160) titers for convalescent plasma use, in addition to frequencies of specimens that either failed to meet or exceeded the recommended titers.
Table 1.
Relationship Between SARS-CoV Neutralizing Antibodies and ELISA Titer
| ELISA Titer | PRNT Tested | Titer | % Neutralizers at FDA Minimal Level | % Neutralizers at FDA Recommended Level | |||
|---|---|---|---|---|---|---|---|
| <80 | 80 | 160 | >320 | ||||
| SARS-CoV-2 PRNT50 Titers | |||||||
| 160 | 13 | 6 | 4 | 2 | 1 | 53.8 | 23.1 |
| 320 | 49 | 21 | 10 | 7 | 11 | 57.1 | 36.7 |
| 960 | 51 | 11 | 15 | 7 | 18 | 78.4 | 49.0 |
| 2880 | 44 | 5 | 2 | 8 | 29 | 88.6 | 84.1 |
| Total | 157 | 43 | 31 | 24 | 59 | 72.6 | 52.9 |
| SARS-CoV-2 PRNT90 Titers | |||||||
| 160 | 13 | 11 | 2 | 0 | 0 | 15.4 | 0 |
| 320 | 50 | 36 | 9 | 5 | 0 | 28.0 | 10.0 |
| 960 | 52 | 28 | 10 | 10 | 4 | 46.2 | 26.9 |
| 2880 | 44 | 13 | 9 | 10 | 12 | 70.4 | 50.0 |
| Total | 159 | 88 | 30 | 25 | 16 | 44.6 | 25.8 |
Serum specimens from COVID-19 convalescent patients that screened Ab positive to SARS-CoV-2 RBD, were titered by ELISA with respect to reactivity to SARS-CoV-2 whole spike protein. Sera with ELISA titers ≥160 were tested for their ability to neutralize SARS-CoV-2 infection of Vero E6 cells. The neutralization titer is the inverse endpoint dilution of sera that could neutralize 50% (top) or 90% (bottom) of viral plaque formation. % Neutralizers at FDA recommended level have a neutralizing titer of 160 or greater. % Neutralizers at FDA minimal level have a neutralizing titer of 80 or greater.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; FDA, Food and Drug Administration; PRNT, plaque reduction neutralization tests; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
Relationship between SARS-CoV neutralizing Abs and ELISA titer. Serum specimens from COVID-19 convalescent patients that screened Ab positive to SARS-CoV-2 RBD were titered by ELISA with respect to reactivity to SARS-CoV-2 whole spike protein. Sera with ELISA titers ≥160 were tested for their ability to neutralize SARS-CoV-2 infection of Vero E6 cells and the PRNT50 (A) and PRNT90 (B) titers were determined. Neutralizing activity is grouped according to titer: <80, white bars; 80/160, gray bars; ≥320, black bars. Spearman r was computed for both groups. PRNT50 r = 0.4057 (95% CI, .2616–.5321), 2-tailed P value < .0001. PRNT90 r = 0.4315 (95% CI, .288–.556), 2-tailed P value < .0001. Abbreviations: Ab, antibody; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; PRNT, plaque reduction neutralization test.
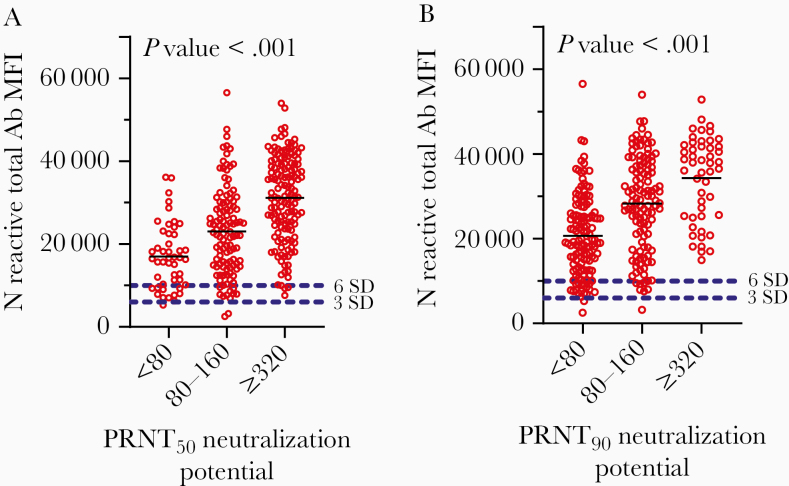
Samples with high Ab titers by ELISA also had high MIA MFIs, consistent with overall agreement between the 2 Ab detection assays. Likewise, we found significant, positive associations between N protein reactive Ab using the MIA and levels of neutralizing Ab activity (Figure 3). Although more Ab led to more neutralizing Ab, no specific MFI value was found to be an absolute predictor of neutralizing activity. Specimens with similar Ab (MFI) levels were found at each level of neutralization, although their frequencies varied. In a smaller sampling, we substituted the RBD antigen for the N antigen in the MIA and, again, found no MFI value that predicted whether the specimen would be a strong neutralizer (data not shown).
Figure 3.
Relationship between SARS-CoV neutralizing Abs and MIA MFI. Serum specimens from COVID-19 convalescent patients were assessed for N protein Ab levels by MIA and then their neutralizing capacity was measured at PRNT50 (A) and PRNT90 (B). Neutralizing activity is grouped according to titer: low < 80, medium 80/160, high ≥ 320. A 1-way ANOVA using the Kruskal-Wallis test with Dunn correction for multiple comparisons was used to assess the significance of correlations between MFI values and neutralizing titers. PRNT50 P value is approximate and is <.0001. The means of the <80 and 80–160 groups are significantly different, with an adjusted P value of .0051. The means of the <80 and ≥320 groups are significantly different, with an adjusted P value of < .0001. The means of the 80–160 and ≥320 groups are significantly different with an adjusted P value of < .0001. PRNT90 P value is approximate and is <.0001. The means of the <80 and 80–160 groups are significantly different, with an adjusted P value of < .0001. The means of the <80 and ≥320 groups are significantly different, with an adjusted P value of < .0001. The means of the 80–160 and ≥320 groups are significantly different, with an adjusted P value of .0093. Abbreviations: Ab, antibody; ANOVA, analysis of variance; MFI, mean fluorescence intensity; MIA, microsphere immunoassay; N, nucleocapsid protein; PRNT, plaque reduction neutralization test.
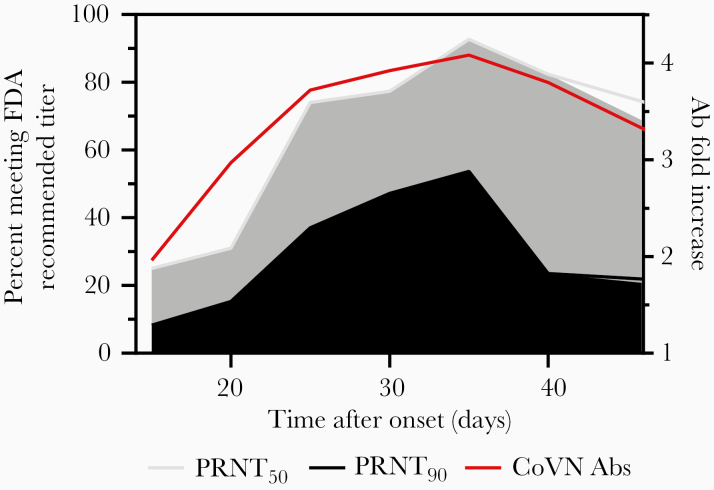
More individuals made higher levels of Ab as time from symptom onset increased, so we examined neutralization titers over different time periods PSO to explore whether more neutralizing Abs were produced after longer recovery periods. PRNT analysis of approximately 300 sera from the combined cohorts demonstrated that, consistent with overall Ab production, neutralization activity initially increased for several weeks after symptom onset. The peak of neutralization in this study was at 31–35 days PSO (Table 2), where approximately 93% of the sera had ≥ 160 PRNT50 titers, while approximately 54% of the sera had ≥ 160 titers using the more stringent PRNT90 evaluation. Beyond 35 days, the number of sera that had high PRNT90 titers decreased significantly, with only approximately 24% of the sera having ≥ 160 neutralizing titers (Table 2 and Figure 4).
Table 2.
Relationship Between SARS-CoV Neutralizing Antibodies and Onset of Symptoms
| Onset, d | PRNT Tested | Titer | % Neutralizers at FDA Minimal Level | % Neutralizers at FDA Recommended Level | |||
|---|---|---|---|---|---|---|---|
| <80 | 80 | 160 | >320 | ||||
| SARS-CoV-2 PRNT50 Titers | |||||||
| 11–15 | 12 | 5 | 4 | 2 | 1 | 58.3 | 25.0 |
| 16–20 | 58 | 28 | 12 | 7 | 11 | 51.7 | 31.0 |
| 21–25 | 54 | 6 | 8 | 17 | 23 | 88.9 | 74.1 |
| 26–30 | 53 | 2 | 10 | 7 | 34 | 96.2 | 77.4 |
| 31–35 | 41 | 0 | 3 | 8 | 30 | 100 | 92.7 |
| 36–40 | 34 | 1 | 5 | 7 | 21 | 97.1 | 82.4 |
| >40 | 48 | 3 | 12 | 10 | 23 | 93.8 | 68.8 |
| SARS-CoV-2 PRNT90 Titers | |||||||
| 11–15 | 12 | 11 | 0 | 0 | 1 | 8.3 | 8.3 |
| 16–20 | 59 | 42 | 8 | 2 | 7 | 28.8 | 15.3 |
| 21–25 | 54 | 19 | 15 | 13 | 7 | 64.8 | 37.0 |
| 26–30 | 53 | 12 | 16 | 13 | 12 | 77.4 | 47.2 |
| 31–35 | 41 | 7 | 12 | 10 | 12 | 82.9 | 53.7 |
| 36–40 | 34 | 13 | 13 | 3 | 5 | 61.8 | 23.5 |
| >40 | 48 | 28 | 10 | 5 | 5 | 41.7 | 20.8 |
Antibody-positive serum specimens from COVID-19 convalescent patients with onset of symptom and blood collection information were tested. All of the specimens were assessed for either SARS-CoV RBD (Mount Sinai) and SARS-CoV N or SARS-CoV N plus RBD (Mount Sinai, Westchester) reactivity using a microsphere immunoassay. The neutralization titer is the inverse endpoint dilution of sera that could neutralize 50% (top) or 90% (bottom) of viral plaque formation. % Neutralizers at FDA recommended level have a neutralizing titer of 160 or greater. % Neutralizers at FDA minimal level have a neutralizing titer of 80 or greater.
Abbreviations: FDA, Food and Drug Administration; PRNT, plaque reduction neutralization tests; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 4.
Relationship between SARS-CoV neutralizing Abs and onset of symptoms. Graphical representation of the data shown in Figure 1 and Table 2. Shown on the left y-axis are the percentages of specimens that can neutralize 90% (black) or 50% (gray) of viral plaque formation with a titer of 160 or greater. Note that, because the last specimens collected were grouped as >40 days, 45 days was used as an arbitrary end point for graphing purposes. On the right axis is shown the fold increase of the mean levels of Ab production (red line), based on MFIs from Figure 1. Abbreviations: Ab, antibody; CoV N Abs, severe acute respiratory syndrome coronavirus neutralizing antibodies; FDA, Food and Drug Administration; MFI, mean fluorescence intensity; PRNT, plaque reduction neutralization test.
Discussion
At present, plasma from recovered COVID-19 patients for passive Ab transfer is being used as an experimental treatment for patients severely ill with COVID-19. Passive transfer is an old method that has seen recent use during infectious disease outbreaks to combat infections by pathogens such H1N1 influenza virus, Ebola virus, and, notably, MERS CoV and SARS CoV [28]. For COVID-19, there have been reports of compassionate use and reports of administration of “high titer” sera, although a precise standardization of “high titer” is only just beginning to be determined. [14, 15, 29–31]. Controlled trials are urgently needed to establish the parameters for effective therapy, but the success of this approach relies upon the presence of protective antibodies in the sera of recovered, presumably immune, individuals. The FDA had provided limited nonbinding recommendations for investigative COVID-19 convalescent plasma use [16], and has only recently issued more precise guidance [32], although it is not yet clear how the “high titer” standard relates to PRNT.
Surprisingly, we found that while most individuals made moderate to high levels of SARS-CoV-2–specific Ab, a smaller than expected number met the FDA recommended titers of neutralizing Abs [16], as determined by a PRNT. These results suggest that measurement of Ab neutralizing activity has significant potential to enhance the effectiveness of plasma therapy for COVID-19 through improved donor selection criteria. In this study, most recovered COVID-19 individuals made detectable neutralizing Abs when measured using a PRNT50, but only half the sera with a 960 ELISA titer against the spike protein had a neutralizing titer of the FDA recommended level of at least 160. While a 2880 ELISA titer was predictive of neutralizing activity at 160 with PRNT50, only 50% of these individuals reached the 160-titer recommendation at the PRNT90 level. The FDA recently required the use of the Ortho VITROS SARS-CoV-2 test, with plasma that meets a signal-to-cutoff ratio of >12 to qualifying as High Titer COVID-10 Convalescent Plasma. However, this serological test has not been assessed for correlation with neutralizing capacity using genuine SARS-CoV-2 virus and the actual neutralizing capacity of plasma assessed in this manner is uncertain [16, 32, 33].
Our results parallel the findings of Wu et al who showed that approximately 30% of recovered COVID-19 patients examined 2 weeks after discharge from hospitals generated low titers of SARS-CoV-2–specific neutralizing antibodies [34]. However, others have reported higher levels of correlation between Ab levels and neutralizing activity, using a variety of both Ab assays and neutralization tests [35], with the latter including pseudotyped virus neutralization assays [19, 34]. A recent study using the MSH ELISA and an optimized and sensitive MN assay also found high half maximum inhibitory concentration (IC50) neutralization titers and excellent correlation between ELISA and MN assay outcomes [36]. Our results indicate that high-level Ab quantitation is a useful, but imperfect, guide to donor suitability, depending on the required level of neutralization. Additional factors, including the specific target antigen used to measure Ab levels and Ab classes produced, should also be considered.
As expected, we found that timing post infection had a major impact on the degree of neutralizing activity present. Our finding that SARS-CoV-2 neutralizing Ab responses are most abundant at 31–35 days PSO is similar to the >21-days PSO peak in neutralizing Ab titer observed for SARS-CoV-1 [37] but later than the 2 to 3 weeks PSO reported by others [31, 34, 35, 38]. The decrease in neutralizing activity we observed beyond 35 days PSO raises the possibility that there is a relatively narrow window for choosing the “best” sera for treatment, particularly at the more stringent PRNT90 level of neutralization. It is critical to determine the level of neutralization required for effective plasma therapy as this experimental treatment is more heavily used. In particular, the level of stringency required for neutralization must be defined. We note that the initial neutralization recommendation from FDA was 1:160 and the European Commission suggests using a minimum titer of 320 [39].
At present, the extent to which neutralizing Abs contribute to protection from reinfection by SARS-CoV-2 is unknown. Significant protection against influenza virus infection is observed at 40 titers [40] and protection from measles virus infection correlates with 120 PRNT50 titers [41]. However, the optimal level of neutralizing Abs for COVID-19 plasma therapy is likely to differ from that required for an individual’s own immune status, as donor plasma will be diluted more than 10-fold upon transfer into a recipient and protection from viruses after infection or vaccination is not just limited to neutralizing antibodies [42]. There are limited reports about the protective cellular immune response to SARS-CoV-2 [43], but also numerous reports about cellular immunopathology and negative consequences for the disease [44]. Strong cellular immunity could more than compensate for low virus neutralizing capacity in vaccinated or previously infected individuals or due to prior infection from non-SARS-CoV-2 coronaviruses [43, 45, 46]. A recent study testing an inactivated whole-virion SARS-CoV-2 vaccine (PiCoVacc) in nonhuman primates reported low neutralizing titers post vaccination but found evidence of protection from disease in animals challenged with SARS-CoV-2 3 weeks after vaccination [47]. Thus, high neutralizing Ab titers may be desirable for plasma therapy but not be required for protection from reinfection with SARS-CoV-2. We also note that the role or importance of nonneutralizing antibodies for either protection or therapy has not yet been determined but should not be discounted.
While establishing the relationship between PRNT titers and efficacy of plasma therapy for treatment of COVID-19 will require the results of controlled trials, we think the data presented here show that less convalescent sera than expected meet the FDA guidelines for high-titer sera and therefore highlight the need to thoroughly characterize the neutralizing activity in individual plasma before use for passive Ab therapy. At a minimum, better definition of the optimal time period between symptom onset and serum collection and baseline PRNT titers will improve the quality of sera and render convalescent plasma therapy a more effective tool in the COVID-19 treatment arsenal.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge and thank members of the Wadsworth Center Diagnostic Immunology Laboratory for their expert technical assistance: S. Brunt, S. Bush, K. Carson, J. Chan, A. Cukrovany, V. Demarest, A. Furuya, K. Howard, D. Hunt, N. Jones, J. Kenneally, C. Koetzner, M. Marchewka, R. Stone, C. and Walsh, J. Yates; and the Wadsworth Center Tissue Culture and Media core facility. We acknowledge the following members of the Westchester County Department of Health for their many contributions to the success of this project: L. Smittle, R. Recchia, J. Li, T. Peer, J. Falasca, E. Cestone, and L. Goldsmith. We thank Dr R. Amler for helpful discussions and for critical reading of this manuscript
Financial support. This work was supported by the New York State Department of Health.
Potential conflicts of interest. M. S. H. is in the process of licensing assays to commercial entities based on the assays described here and has filed for patent protection. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins University. COVID-19 United States cases by county. https://coronavirus.jhu.edu/us-map. Accessed 24 October 2020.
- 3. New York State. COVID alert NY. https://coronavirus.health.ny.gov/home. Accessed 24 October 2020.
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heneghan CB, Brassey J, Jefferson T; Centre for Evidence-Based Medicine COVID-19: What proportion are asymptomatic? https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/. Accessed 24 October 2020.
- 6. Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature 1985; 318:323–9. [DOI] [PubMed] [Google Scholar]
- 7. Liu W, Fontanet A, Zhang PH, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis 2006; 193:792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. Nat. Comm 2020; 11:4704–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020; 58:e00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krammer F, Simon V. Serology assays to manage COVID-19. Science 2020; 68:1060–1. [DOI] [PubMed] [Google Scholar]
- 11. Brigger D, Horn MP, Pennington LF, et al. Accuracy of serological testing for SARS-CoV-2 antibodies: first results of a large mixed-method evaluation study [published online ahead of print 30 September 2020]. Allergy doi: 10.1111/all.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luchsinger LL, Ransegnola B, Jin D, et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID19 patients [published online ahead of print 11 September 2020]. J Clin Microbiol doi: 10.1128/JCM.02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020; 130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv, doi: 10.1101/2020.08.12.20169359, 12. August 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 15. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for COVID-19-potentially hopeful signals. JAMA 2020; 324:455–7. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services Food and Drug Administration. Investigational COVID-19 convalescent plasma: guidance for industry Rockville, MD: FDA, 2020. [Google Scholar]
- 17. Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99:285–90. [PubMed] [Google Scholar]
- 18. Lindsey HS, Calisher CH, Mathews JH. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol 1976; 4:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer EG, Simmons G, Grebe E, et al. Selecting COVID-19 convalescent plasma for neutralizing antibody potency using a high-capacity SARS-CoV-2 antibody assay. medRxiv, doi: 10.1101/2020.08.31.20184895, 31. August 2020, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 2020; 57:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliphant T, Nybakken GE, Austin SK, et al. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol 2007; 81:11828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koraka P, Suharti C, Setiati TE, et al. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 2001; 39:4332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang HS, Racine-Brzostek SE, Lee WT, et al. SARS-CoV-2 antibody characterization in emergency department, hospitalized and convalescent patients by two semi-quantitative immunoassays. Clin Chim Acta 2020; 509:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calisher CH, Karabatsos N, Dalrymple JM, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 1989; 70(Pt 1):37–43. [DOI] [PubMed] [Google Scholar]
- 26. Shambaugh C, Azshirvani S, Yu L, et al. Development of a high-throughput respiratory syncytial virus fluorescent focus-based microneutralization assay. Clin Vaccine Immunol 2017; 24:e00225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burton DR, Saphire EO, Parren P. A model for neutralization of viruses based on antibody coating of the virion surface. In: Burton DR, ed. Antibodies in viral infection. Vol. 260 Berlin: Springer-Verlag, 2001:109–43. [DOI] [PubMed] [Google Scholar]
- 28. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20 000 hospitalized patients. Mayo Clin Proc 2020; 95:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020; 117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed 24 October 2020.
- 33. Luchsinger LL, Ransegnola B, Jin D, et al. Serological analysis of New York City COVID19 convalescent plasma donors. medRxiv, doi: 10.1101/2020.06.08.20124792, 9. June 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 34. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv , doi: 10.1101/2020.03.30.20047365, 30. March 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 35. Okba NMA, Muller MA, Li W, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv, doi: 10.1101/2020.03.18.20038059, 20. March 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 36. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Temperton NJ, Chan PK, Simmons G, et al. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis 2005; 11:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 39. European Commission. Guidance on the collection and transfusion of convalescent COVID-19 plasma. https://ec.europa.eu/health/blood_tissues_organs/covid-19_en. Accessed 24 October 2020.
- 40. Krammer F, Weir JP, Engelhardt O, Katz JM, Cox RJ. Meeting report and review: Immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir Viruses 2020; 14:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis 1990; 162:1036–42. [DOI] [PubMed] [Google Scholar]
- 42. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doshi P. Covid-19: do many people have pre-existing immunity? BMJ 2020; 370:m3563. [DOI] [PubMed] [Google Scholar]
- 46. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity [published online ahead of print 16 September 2020]. Cell doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao Q, Bao L, Mao H, et al. Rapid development of an inactivated vaccine for SARS-CoV-2. bioRxiv, doi: 10.1101/2020.04.17.046375, 17. April 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.