Abstract
This is a retrospective cohort study of hospitalized adults with coronavirus disease 2019 (COVID-19). Fifty-seven patients received treatment alone, and 35 patients received treatment with adjunctive prednisolone. A combination of corticosteroids and antivirals was associated with lower risk of clinical progression and invasive mechanical ventilation or death in early COVID-19 pneumonia.
Keywords: corticosteroids, COVID-19, hydroxychloroquine
Use of corticosteroids and antiviral agent could prevent clinical progression of early COVID-19 pneumonia.
Coronavirus disease 2019 (COVID-19) ranges from asymptomatic to severe pneumonia with respiratory failure [1–3]. Severe COVID-19 is typically marked by rapid clinical deterioration with features of hyperinflammation [4, 5, 6]. Recent studies on use of tocilizumab and dexamethasone have shown promising results in reducing mortality in severe COVID-19 [7, 8]. However, treatment approach for early infection remains unclear.
At our center, treatment of COVID-19 has included interferon beta, lopinavir/ritonavir, and hydroxycholoroquine (HCQ). Prednisolone and tocilizumab were added as adjunctive therapy for COVID-19 in mid-April 2020 when there were reports suggesting potential benefit with its use [9–12]. We aim to evaluate the effect of antivirals with adjunctive corticosteroids in the management of COVID-19.
METHODS
Study Design and Participants
This is a retrospective cohort study of hospitalized adults (≥18 years) with COVID-19, confirmed by polymerase chain reaction on nasopharyngeal swab, who received treatment from February 9, 2020 to May 31, 2020. Those who were on supportive care only, on invasive mechanical ventilation (MV) before treatment, or those admissions not related to respiratory illness were excluded.
Patient Consent Statement
This study was approved by Domain Specific Review Board, National Health Group, Singapore (2020/00850) with waiver of consent.
Treatment
The decision to initiate treatment was at the discretion of the treating physicians in consultation with an Infectious Diseases physician. Treatment regimens included lopinavir/ritonavir 400/100 mg twice a day or HCQ 400 mg twice on day 1, followed by 200 mg 3 times a day on days 2–5 and prednisolone. The dose of prednisolone varied from 30 mg once a day to 40 mg twice a day depending on severity of illness and the patient’s body weight. Corticosteroids use of 3 days or more for any medical indications was considered as adjunctive corticosteroids.
Patients were classified into 2 groups: Group A received treatment without corticosteroids, and Group B received treatment with adjunctive corticosteroids. Clinical conditions of patients with COVID-19 at initiation of treatment were categorized into 3 stages: Stage 1, COVID-19 without pneumonia; Stage 2, COVID-19-related pneumonia not requiring supplemental oxygen; and Stage 3, COVID-19 related pneumonia requiring supplemental oxygen. Chest radiograph was the primary modality used in the assessment of pneumonia in our cohort.
Outcomes
Primary outcome was a composite of clinical progression or death. Clinical progression is defined as progression to requiring supplemental oxygen in Stage 1 and 2 or progression to invasive MV in any stage. Secondary outcome was a composite of invasive MV or death. Subgroup analysis was performed in those with pneumonia (Stage 2 and 3).
Statistical Analysis
Comparison of continuous and categorical data between groups was done using Mann-Whitney U test and χ 2 test or Fisher’s exact test when appropriate. Changes in C-reactive protein (CRP) and daily maximal body temperature were compared between treatment groups.
Data were preprocessed with entropy balancing to achieve covariate balance between the 2 treatment groups and reduce model dependence for estimation of treatment effects [13]. Weight derived from entropy reweighting scheme was used to generate stabilized weight for subjects in the cohort. Balanced covariate is defined by standardized mean difference of less than 0.1 and variance ratio between 0.5 and 2.0. Procedure of covariate balance was performed separately in subgroup with pneumonia. Covariates used in reweighting were age, gender, body mass index, comorbidity, clinical stage, antibiotic use, CRP, procalcitonin, lymphocyte count, neutrophils/lymphocyte ratio, and creatinine (Supplementary Figure S1 and Supplementary Table S1). Multiple imputation was applied for missing data of covariates, which were limited to CRP and procalcitonin (1.1%).
Kaplan-Meier curves and weighted Cox regression models with adjustment for imbalanced covariates were used to estimate effect of adjunctive corticosteroids on composite outcomes. Two-sided P test of <.05 was considered statistically significant. Analysis was performed using Stata version 15 (StataCorp, College Station, TX) and R version 4.0.0 (Foundation for Statistical Computing, Vienna, Austria).
RESULTS
There were 1046 patients with COVID-19 admitted to our institution during the study period. Of these, 94 (9.0%) patients were initiated on treatment. Two patients were excluded because treatment was initiated after invasive MV. In total, 57 patients received treatment without corticosteroids (Group A) and 35 patients received adjunctive prednisolone to treatment (Group B) (Supplementary Figure S2).
Median day of illness at treatment initiation was 4 days and 5 days in Group A and B, respectively. Duration of treatment was 7 days (interquartile range [IQR], 5–7) in Group A and 5 days (IQR, 5–7) in Group B. Median prednisolone use was 5 days (IQR, 3–7).
There were 44 patients in the cohort who had pneumonia, and 68.9% of them did not require supplemental oxygen at treatment initiation. Between the 2 treatment groups, there were higher proportions of patients in Group B who had pneumonia, required supplemental oxygen, had antibiotics, and had a higher CRP level at initiation of treatment (Supplementary Table S2). Eight subjects received tocilizumab in our cohort. All were given post-MV except 2 cases (1 in each treatment group).
After treatment initiation, body temperature of patients trended down in similar fashion between 2 treatment groups. C-reactive protein in Group B trended down; however, Group A trended up at approximately 5–8 days after treatment initiation before declining (Supplementary Figure S3).
Overall, 17 (18.5%) of 92 patients had clinical progression including 13 (22.8%) patients in Group A versus 4 (11.4%) in Group B (P = .172). Eleven patients required invasive MV: 7 (12.2%) patients in Group A and 4 (11.4%) patients in Group B (Supplementary Table S3). One death occurred in Group A after invasive MV, and none occurred in Group B. Analysis of composite outcome was the same as clinical progression.
In the overall cohort, weighted Cox regression analysis showed a statistically significant lower risk of clinical progression in Group B compared with Group A; however, there was no statistically significant difference in risk of invasive MV or death between the 2 groups (Table 1).
Table 1.
Unadjusted and Adjusted Relative Hazards of Adjunctive Corticosteroids on Clinical Progression and Invasive Mechanical Ventilationa
| Outcome | N | Unadjusted Analysis HR (95% CI) | P Value | Adjusted Analysis HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Clinical Progression | |||||
| Overall | 92 | 0.46 (0.15–1.40) | .169 | 0.08 (0.01–0.99) | .049 |
| Pneumonia | 44 | 0.13 (0.04–0.48) | .002 | 0.15 (0.06–0.39) | <.001 |
| Invasive Mechanical Ventilation | |||||
| Overall | 92 | 0.88 (0.26–3.02) | .844 | 0.22 (0.02–2.54) | .22 |
| Pneumonia | 44 | 0.22 (0.06–0.85) | .028 | 0.30 (0.10–0.87) | .029 |
Abbreviations: CI, confidence interval; HR, hazard ratio; N, number.
aCovariates in adjusted analysis were age, gender, body mass index, comorbidity, clinical stage, antibiotic use, C-reactive protein, procalcitonin, lymphocyte count, neutrophils-lymphocyte ratio and creatinine. Clinical progression, progression to requiring supplemental oxygen in Stage 1 and 2, or progression to invasive mechanical ventilation in any stage.
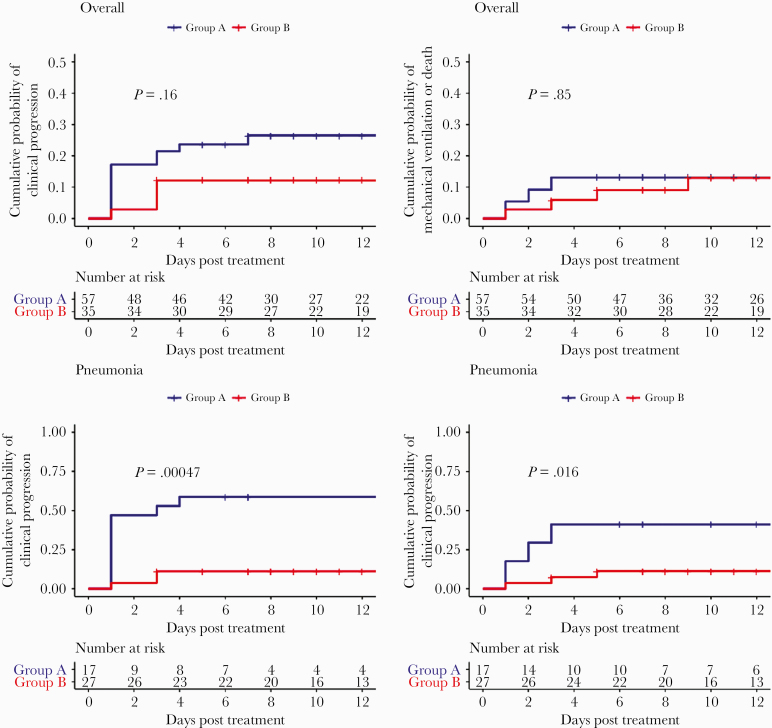
In the subgroup with pneumonia, unweighted Kaplan-Meier estimates showed that 58.8% (95% confidence interval [CI], 27.3–76.7) in Group A versus 11.1% (95% CI, 0.0–22.2) in Group B had clinical progression (log rank P < .001); 41.2% (95% CI, 12.4.–60.5) of patients in Group A required invasive MV versus 11.3% (95% CI, 0.0–22.5) of patients in Group B (log rank P = .016) (Figure 1). In weighted Cox regression, patients in Group B were less likely to have clinical progression (adjusted hazard ratio [aHR], 0.15; 95% CI, 0.06–0.39; P < .001) and require invasive MV compared with Group A (aHR, 0.30; 95% CI, 0.10–0.87; P = .029).
Figure 1.
Kaplan-Meier estimates of the cumulative probability of clinical progression and mechanical ventilation or death by treatment group. Overall, overall cohort; Pneumonia, subgroup with pneumonia, Group A, treatment group without corticosteroids; Group B, treatment group with adjunctive corticosteroids. Clinical progression, progression to requiring supplemental oxygen in Stage 1 and 2, or progression to invasive MV in any stage.
DISCUSSION
Our cohort of patients either received antiviral alone (mostly HCQ) or in combination with prednisolone during the early phase of illness. Early use of prednisolone in our patients with COVID-19 pneumonia had a significant lower risk of clinical progression, invasive MV, or death compared with antiviral alone, despite that 68.9% of patients in this group were not on supplemental oxygen at treatment initiation. The duration of prednisolone use in our cohort was short, with a median of 5 days. The dose of prednisolone varied between 0.5 and 1.0 mg/kg per day.
Emerging data have shown benefits of anti-inflammatory agents in the management of severe COVID-19 pneumonia [7, 8, 14–16]. The RECOVERY trial involving the use of dexamethasone in hospitalized patients with COVID-19 showed a 20% to 40% reduction in mortality in those with pneumonia requiring oxygen and invasive MV. However, there was a trend towards increased mortality when corticosteroids were used in patients not receiving respiratory support at randomization [8]. This appears to correspond to use of corticosteroids in early pneumonia. Earlier observations of corticosteroids use in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS-coronavirus [CoV]) infections may explain the undesired consequences of corticosteroids in early phase of COVID-19 pneumonia at which virus replication is active [17].
Hydroxycholoroquine has been shown to effectively inhibit SARS-CoV-2 infection in vitro [18]. However, its clinical efficacy has largely been reported as ineffective. The RECOVERY trial reported an increased risk of invasive MV or death with use of HCQ compared with supportive care [19]. We found that the group received antivirals without corticosteroids in our cohort had a more protracted course with a higher inflammatory response. Their CRP trended to higher levels at days 5–8 posttreatment despite a similar fever response in both groups. Perhaps this is a paradoxical inflammatory reaction due to treatment.
The use of prednisolone during early pneumonia in our study had a significantly better outcome, which may be due to the concurrent administration of antiviral agents such as HCQ or lopinavir/ritonavir. The prominent protective effect of adjunctive prednisolone observed in our cohort with early pneumonia and the varying effect of dexamethasone on different days of illness of COVID-19 in the RECOVERY trial call for different treatment strategies at different clinical stages of COVID-19. Our results support the hypothesis that control of both viral replication and inflammatory response is important in treatment of early COVID-19 pneumonia [5].
Our study has several limitations. Nonrandomized treatment selection and small sample size could have biased estimates and limit our subgroup analysis. The estimates in weighted regression are dependent on balancing of covariates and model specification. Risk of secondary infection and other adverse outcomes associated with corticosteroids were not evaluated. Finally, younger population in our cohort and single-center design may limit generalizability of these results.
CONCLUSIONS
In summary, our study shows that a combination of antivirals and corticosteroids was associated with lower risk of clinical progression, especially in those with pneumonia. Further prospective studies should be considered to evaluate the clinical efficacy of combination treatment in early COVID-19 pneumonia.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest 2020; 130:2202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020; 39:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020; doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2020; doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020; 92:814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal 2012; 20:25–46. [Google Scholar]
- 14. The Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 2020; doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.