Abstract
Objectives
The Janus kinase (JAK) inhibitor baricitinib may block viral entry into pneumocytes and prevent cytokine storm in patients with SARS-CoV-2 pneumonia. We aimed to assess whether baricitinib improved pulmonary function in patients treated with high-dose corticosteroids for moderate to severe SARS-CoV-2 pneumonia.
Methods
This observational study enrolled patients with moderate to severe SARS-CoV-2 pneumonia [arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) <200 mmHg] who received lopinavir/ritonavir and HCQ plus either corticosteroids (CS group, n = 50) or corticosteroids and baricitinib (BCT-CS group, n = 62). The primary end point was the change in oxygen saturation as measured by pulse oximetry (SpO2)/FiO2 from hospitalization to discharge. Secondary end points included the proportion of patients requiring supplemental oxygen at discharge and 1 month later. Statistics were adjusted by the inverse propensity score weighting (IPSW).
Results
A greater improvement in SpO2/FiO2 from hospitalization to discharge was observed in the BCT-CS vs CS group (mean differences adjusted for IPSW, 49; 95% CI: 22, 77; P < 0.001). A higher proportion of patients required supplemental oxygen both at discharge (62.0% vs 25.8%; reduction of the risk by 82%, OR adjusted for IPSW, 0.18; 95% CI: 0.08, 0.43; P < 0.001) and 1 month later (28.0% vs 12.9%, reduction of the risk by 69%, OR adjusted for IPSW, 0.31; 95% CI: 0.11, 0.86; P = 0.024) in the CS vs BCT-CS group.
Conclusions
. In patients with moderate to severe SARS-CoV-2 pneumonia a combination of baricitinib with corticosteroids was associated with greater improvement in pulmonary function when compared with corticosteroids alone.
Trial registration
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, ENCEPP (EUPAS34966, http://www.encepp.eu/encepp/viewResource.htm? id = 34967)
Keywords: SARS-CoV-2, COVID-19, baricitinib, corticosteroids
Rheumatology key messages
We present the first evidence for a possible synergistic effect of baricitinib and corticosteroids in SARS-CoV-2 pneumonia.
A decrease of D-dimer might reflect a protective effect of baricitinib on lung endothelium.
A dose of 4 mg baricitinib seems more effective than 2 mg in treating SARS-CoV-2 pneumonia.
Introduction
The clinical spectrum of SARS-CoV-2 ranges from asymptomatic infection to viral pneumonia. Some patients develop acute respiratory failure with severe hypoxaemia that requires mechanical ventilation. However, in a crisis scenario with limited resources, a patient’s care often involves management in the ward by means of non-invasive ventilation or high-flow nasal oxygen. Management also includes a combination of antiviral and/or anti-inflammatory drugs, based on clinical judgement, for critically ill patients in order to delay or avoid mechanical ventilation in the intensive care unit (ICU) [1].
Corticosteroids suppress lung inflammation but also inhibit immune responses and pathogen clearance [2]. Thus, their use in the treatment SARS-CoV-2 pneumonia is controversial. However, early use of a short course of methylprednisolone in patients with moderate to severe SARS-CoV-2 has prevented disease progression and improved a primary composite end point of escalation of care from hospital ward to ICU and mortality [3].
In addition, Janus kinase (JAK) inhibitors, particularly baricitinib, a drug that is currently used to treat RA, may be beneficial in treating SARS-CoV-2 infection. Binding of S1 proteins at the surface of SARS-CoV-2 to the ACE2 molecules on lung cells facilitates viral entry to pneumocytes by endocytosis. The ACE2 receptor has several regulators among which AP2-associated protein kinase-1 (AAK1) and cyclin G-associated kinase mediate clathrin-dependent endocytosis. Baricitinib not only interrupts the passage and intracellular assembly of SARS-CoV-2 into the target cells via disruption of AAK1 signalling but also reduces inflammation in patients with acute respiratory distress syndrome (ARDS) [4, 5]. Furthermore, there is the potential for combining baricitinib with direct-acting antivirals (lopinavir or ritonavir and remdesivir) currently being used in the SARS-CoV-2 outbreak, since baricitinib has a minimal interaction with the relevant cytochrome drug-metabolizing enzymes. Combinations of baricitinib with these direct-acting antivirals could reduce viral infectivity, viral replication and the aberrant host inflammatory response [6].
While mild to moderate forms of SARS-CoV-2 pneumonia require in-hospital surveillance plus antiviral therapy, anti-inflammatory drugs and immunomodulators could be therapeutic options for patients with severe and life-threating pneumonia. Research is needed to define both the role of different medications and customized strategies of management in patients with SARS-CoV-2 at risk of progression to critical condition. As hypoxaemia is a key marker of severity in pneumonia, the purpose of our study was to determine whether the JAK inhibitor baricitinib could offer a beneficial or additive effect to corticosteroids on respiratory function in patients with moderate to severe ARDS due to SARS-CoV-2 pneumonia.
Methods
We carried out a prospective observational study from 15 March to 26 April 2020 at a single centre (General University Hospital of Albacete, Spain) in patients older than 18 years admitted to the hospital because of SARS-CoV-2 pneumonia who had an arterial oxygen partial pressure (PaO2)/fractional inspired oxygen (FiO2) ratio <200 mmHg on the hospital ward (moderate to severe SARS-CoV-2 pneumonia).
Treatment guidelines
Standard treatment for all patients included 7–10 days of lopinavir/ritonavir 200/50 mg, two tablets/12 h; and HCQ 200 mg, a loading dose of two tablets/12 h for the first day followed by one tablet/12 h. For patients with respiratory failure, hospital guidelines allowed administration of intravenous immunoglobulin (IVIG, 5 mg/kg daily, 5 days) or interferon β-1b (INF) 8 million international units (0.25 mg) subcutaneously on alternate days (seven doses maximum). In cases of failure of IVIG or INF, or as an alternative option, patients could initiate methylprednisolone (corticosteroids) with or without one or more of the following immunomodulators: baricitinib (see below), anakinra 100 mg subcutaneously every 12 h (5–10 days) and/or tocilizumab as a single patient’s weight-based dose by intravenous drip infusion: 400 mg (<75 kg) or 600 mg (≥75 kg).
Management of coagulopathy was based on low molecular weight heparin following international recommendations and laboratory marker measurements (D-dimer, prothrombin time, platelet count and fibrinogen). In the absence of contraindications, patients received enoxaparin 1 mg/day, and the dose could be higher (1 mg/12 h) if they had a disseminated intravascular coagulation (DIC) score of 5 or more. Low molecular weight heparin was extended 4 weeks after discharge [7, 8].
Inclusion and exclusion criteria
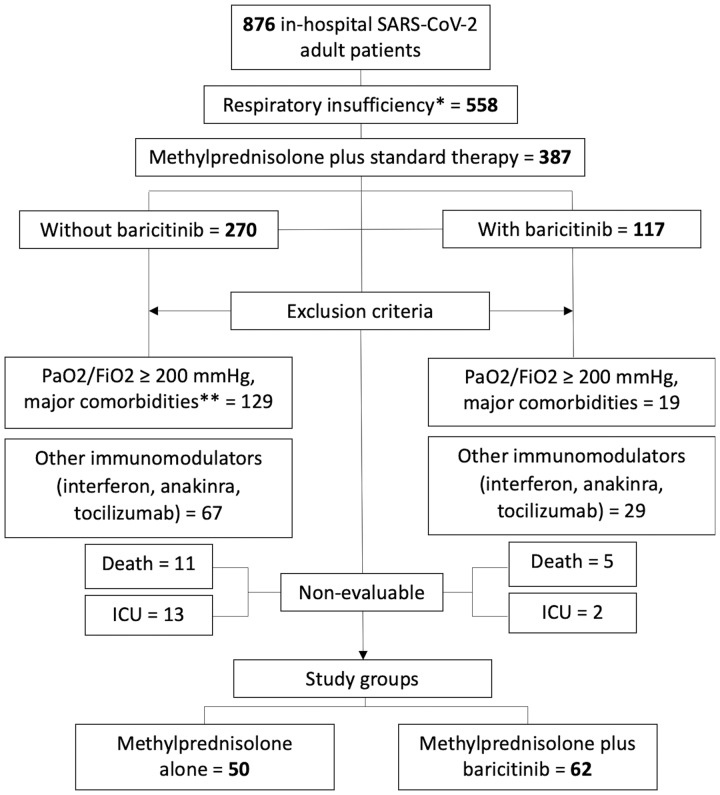
All patients admitted during observation period with SARS-CoV-2 pneumonia and respiratory insufficiency [oxygen saturation as measured by pulse oximetry (SpO2) ≤92% breathing room air] were considered for the study. Patients older than 80 years or discarded for ICU were transferred to a geriatric hospital admission whenever there were beds available. Patients were excluded if they had major comorbidities (chronic heart failure, chronic obstructive pulmonary disease on oxygen therapy, obstructive sleep apnoea syndrome with continuous positive airway pressure, advanced chronic kidney disease, active malignancies). Patients were also excluded if they had previously been treated with other immunomodulators (IVIG, INF, anakinra, tocilizumab). Patients admitted to ICU or who died were considered non-evaluable and thus excluded. Figure 1 shows the flow chart of patients included in the study.
Fig. 1.
Flow chart of patients included in the study
*SpO2 ≤ 92% breathing room air. **Chronic heart failure, chronic obstructive pulmonary disease (COPD) on oxygen therapy, obstructive sleep apnoea syndrome with continuous positive airway pressure, advanced chronic kidney disease, active malignancies. Standard therapy: lopinavir/ritonavir and HCQ. FiO2: fraction of inspired oxygen; PaO2: arterial oxygen partial pressure; SpO2: oxygen saturation as measured by pulse oximetry.
Treatment
In the corticosteroids group (CS, n = 50) patients received three consecutive days of pulse corticosteroid therapy (corticosteroids pulses) followed by prednisone at a starting dose of 30 mg/day. Therapy was discontinued by tapering after 7–10 days of treatment.
In the baricitinib plus corticosteroids group (BCT-CS, n = 62) patients received corticosteroids for 3 days and then prednisone, combined with baricitinib for 5–10 days. Doses of corticosteroids included 6-methylprednisolone 80, 125 or 250 mg/day. Baricitinib was administered under two schemes: a loading dose of 4 mg the first day and then 2 mg daily (low-dose baricitinib, n = 40) or 4 mg daily each day (high-dose baricitinib, n = 22). Patients older than 75 years received low-dose baricitinib.
Variables
We obtained demographics, clinical data, comorbidities, time of disease onset and length of stay. The National Early Warning Score (NEWS) obtained from the emergency department (ED) [9] was used to evaluate baseline illness severity based on vital signs (systolic blood pressure, oxygen saturation, respiratory rate, heart rate, temperature and level of consciousness).
Blood samples were drawn upon admission, at the peak of respiratory impairment whenever it occurred and at discharge. Parameters included blood cell counts, CRP, serum ferritin, lactate dehydrogenase and D-dimer.
To evaluate respiratory function, we used the SpO2/FiO2 ratio, which is known as an adequate non-invasive surrogate marker for PaO2/FiO2 and an indicator of early acute respiratory distress syndrome development among patients at risk. In an analysis of patients from the ARDS Network PaO2/FiO2 ratios of 300, 200 and 100 (severe, moderate and mild ARDS, respectively) correlated with cutoffs of SpO2/FiO2 ratios of 315, 235 and 144 for mild, moderate and severe ARDS, respectively [10].
We evaluated SpO2/FiO2 at the ED, requirement for respiratory support at the ED, decline or worsening of SpO2/FiO2 during hospitalization (difference of SpO2/FiO2 from emergency to hospitalization), and its management (nasal duct or mask, high-flow oxygen, non-invasive ventilation). For SpO2/FiO2 during hospitalization we calculated the mean value of the lowest SpO2/FiO2 on three consecutive days on the ward, which was compared with SpO2/FiO2 at discharge.
After discharge, patients continued with close follow-up by their family physician who decided to discontinue or continue supplemental oxygen at home. Therefore, nurses at the outpatient clinics contacted the patients 1 month after discharge to determine whether they were under supplemental oxygen yet.
Outcomes
The primary end point was defined as the change in SpO2/FiO2 from hospitalization to discharge. Secondary end points included the proportion of patients requiring supplemental oxygen at discharge and 1 month later.
Ethical considerations
All patients provided informed consent before starting treatment. The study was approved by our hospital’s Research Ethics Committee (2020-18 EPA-OD) and the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP; EUPAS34966). We followed the STROBE checklist of items for reports of observational studies [11].
Statistical analyses
The original total sample size was set at 100, since it would provide the trial with 80% power to detect a difference, at a two-sided significance level of α = 0.05, of 40 in the difference of mean change in SpO2/FiO2 from hospitalization to discharge between the two treatment groups (corticosteroids alone or combined with baricitinib) assuming a common standard deviation of 70. Values were reported as absolute and relative (percentages) frequencies or as median and interquartile range (IQR). Boxplots were used for graphical representations of quantitative variables.
Differences of proportions were evaluated by the chi-square test or by Fisher’s exact test as appropriate. Differences of two means between groups were assessed with Student’s t-test for independent samples or by the nonparametric Mann–Whitney U-test as appropriate.
We adjusted the analysis of outcome variables using the inverse propensity score weighting (IPSW), which was based on propensity score, to construct a weighted cohort of patients who differed with respect to treatment received but were similar regarding other measured characteristics. To calculate the IPSW we estimated each patient’s propensity to receive corticosteroids and baricitinib using a logistic-regression model. This model included predictor variables that had been selected on the basis of their a priori possibility of confounding the relationship between treatment received and outcome (age, sex, diabetes, NEWS and SpO2/FiO2 on hospitalization and high-flow oxygen or non-invasive ventilation) for corticosteroids alone vs corticosteroids and baricitinib comparison. It also included age, sex, NEWS, methylprednisolone total dose and high-flow oxygen or non-invasive ventilation for the comparison of low-dose vs high-dose baricitinib. We assigned patients who received baricitinib a weight of 1/(propensity score) and those who received only corticosteroids a weight of 1/(1 − propensity score) [12]. To evaluate comparability between groups we display the histogram of the IPSWs in both groups, (see Supplementary Figs S1 and S2, available at Rheumatology online) and we excluded for adjusted analysis those patients with extreme values of IPSW (>5). Finally, we adjusted the analysis using weighted linear regression and binary logistic regression by the IPSW. All tests were two-tailed. P-values below 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 25.00 (IBM Corp., Armonk, NY, USA) software.
Results
Patients
A total of 876 patients were admitted during the period of time of the study in our hospital. Out of 876 patients, 558 had respiratory insufficiency (SpO2 ≤92% breathing room air). Of these, 387 patients were enrolled. All patients received background lopinavir/ritonavir and HCQ; 270 patients also received corticosteroids and 117 patients received corticosteroids together with baricitinib. After excluding patients with major comorbidities or treated with other immunomodulators as well as non-evaluable cases, a total of 112 patients were analysed: corticosteroids group (CS, n = 50) and baricitinib and corticosteroids group (BCT-CS, n = 62).
There were no significant differences in baseline demographic characteristics, signs and symptoms, distribution of NEWS score at enrolment, or laboratory test results between CS and BCT-CS groups (Table 1). There was a higher proportion of diabetes (P = 0.002) in BCT-CS subjects. Both groups received similar total doses of methylprednisolone and proportion of intensive respiratory support (high-flow oxygen or non-invasive ventilation). There were no significant baseline differences between BCT-CS and CS groups in SpO2/FiO2 at the ED, SpO2/FiO2 on hospitalization, or SpO2/FiO2 change from ED to hospital ward.
Table 1.
Baseline demographic, clinical and laboratory data and treatment
| Characteristic | Corticosteroids treatment (n = 50) |
Baricitinib and corticosteroids treatment (n = 62) |
P-value |
|---|---|---|---|
| Baseline patients characteristics | |||
| Age, median (IQR), years | 64 (57–69) | 63 (52–72) | 0.881 |
| Sex, n (%) | 0.734 | ||
| Men | 34 (68) | 44 (71) | |
| Women | 16 (32) | 18 (29) | |
| Time from illness onset, median (IQR), days | 7 (5–10) | 7 (5–10) | 0.464 |
| Length of hospital stay, median (IQR), days | 13 (10–16) | 14 (11–19) | 0.093 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 25 (50.0) | 32 (51.6) | 0.865 |
| Diabetes | 3 (6.0) | 18 (29.0) | 0.002 |
| Hypercholesterolaemia | 16 (32.0) | 23 (37.1) | 0.574 |
| Signs and symptoms, respiratory function and NEWS | |||
| Axillary temperature, median (IQR), °C | 37.6 (37.1–38.2) | 37.4 (36.8–37.9) | 0.233 |
| Altered mental status, n (%) | 2 (4.0) | 2 (3.2) | 1.000 |
| Systolic blood pressure, median (IQR), mmHg | 125 (113–135) | 125 (119–135) | 0.548 |
| Diastolic blood pressure, median (IQR), mmHg | 80 (71–85) | 80 (71–85) | 0.550 |
| Heart rate, median (IQR), beats/min | 90 (81–96) | 90 (80–101) | 0.743 |
| Respiratory rate, median (IQR), breaths/min | 18 (16–24) | 22 (18–26) | 0.012 |
| Oxygen saturation at ED, median (IQR), % | 87 (85–89) | 86 (83–88) | 0.522 |
| Inhaled oxygen at ED | 42 (84.4) | 56 (90.3) | 0.315 |
| High-flow oxygen, non-invasive ventilation (ward), n (%) | 23 (46.0) | 31 (50.0) | 0.674 |
| NEWS score, median (IQR), 0–20 | 6 (5–8) | 7 (6–9) | 0.149 |
| Laboratory parameters | |||
| CRP, median (IQR), mg/l (normal range <6) | 128 (90–194) | 170 (84–232) | 0.205 |
| Ferritin, median (IQR), ng/ml (normal range 30–400) | 1794 (1054–2416) | 1489 (905–2753) | 0.501 |
| Lactate dehydrogenase, median (IQR), U/l (normal range 125–220) | 412 (359–508) | 419 (336–517) | 0.847 |
| D-dimer, median (IQR), ng/ml (≤500) | 897 (658–1859) | 1187 (747–2325) | 0.158 |
| Lymphocyte count, median (IQR), ×103 cells/μl (levels ≥1000) | 590 (410–720) | 610 (533–813) | 0.102 |
| Treatment | |||
| Methylprednisolone, total dose, median (IQR), mg | 500 (375–750) | 500 (375–750) | 0.585 |
| Baricitinib scheme | |||
| Low-dose baricitinib, n (%) | 40 (64.5) | ||
| High-dose baricitinib, n (%) | 22 (35.5) | ||
| Baricitinib days of intake, median (IQR) | 5 (5–6) | ||
| Baricitinib total dose, median (IQR), mg | 15 (12–20) | ||
Laboratory parameters were considered at the peak of the patient’s respiratory deterioration. ED: emergency department; IQR: interquartile range; NEWS: National Early Warning Score.
Primary and secondary end points
A greater improvement in SpO2/FiO2 from hospitalization to discharge was observed in the BCT-CS group vs CS (mean differences adjusted for IPSW, 49; 95% CI: 22, 77; P < 0.001) (Table 2, Fig. 2). Supplementary Fig. S1, available at Rheumatology online, shows a histogram of IPSW scores to evaluate comparability between groups.
Table 2.
Corticosteroids vs baricitinib plus corticosteroids on respiratory function and need of ambulatory supplemental oxygen
| Outcome | Corticosteroids treatment (n = 50) |
Baricitinib and corticosteroids treatment (n = 62) |
P-value |
|---|---|---|---|
| Respiratory function | |||
| SpO2/FiO2 at ED, median (IQR) | 345 (270–410) | 319 (245–405) | 0.372 |
| SpO2/FiO2 on hospitalization, median (IQR) | 155 (122–214) | 154 (128–161) | 0.693 |
| SpO2/FiO2 at discharge, median (IQR) | 410 (332–438) | 442 (428–452) | <0.001 |
| Outcomes | |||
| Change in SpO2/FiO2 from hospitalization to discharge, median (IQR) | 220 (159–284) | 289 (206–308) | 0.002 |
| Patients discharged from hospital requiring supplemental oxygen, n (%) | 31 (62.0) | 16 (25.8) | <0.001 |
| Patients on supplemental oxygen 1 month after discharge, n (%) | 14 (28.0) | 8 (12.9) | 0.046 |
| Differences adjusted for IPSWa | |||
| Change in SpO2/FiO2 from hospitalization to discharge, mean differences (95% CI) | |||
| Unadjusted | 49 (23, 76) | <0.001 | |
| Adjusted for IPSW | 49 (22, 77) | <0.001 | |
| Patients discharged from hospital requiring supplemental oxygen, odds ratio (95% CI) | |||
| Unadjusted | 0.21 (0.10, 0.48) | <0.001 | |
| Adjusted for IPSW | 0.18 (0.08, 0.43) | <0.001 | |
| Patients on supplemental oxygen one month after discharge, odds ratio (95% CI) | |||
| Unadjusted | 0.38 (0.15, 1.00) | 0.050 | |
| Adjusted for IPSW | 0.31 (0.11, 0.86) | 0.024 | |
SpO2/FiO2 on hospitalization: mean of lowest values on three consecutive days.
Adjusted for age, sex, diabetes, NEWS, SpO2/FiO2 on hospitalization and high-flow oxygen or non-invasive ventilation (ward), excluding extreme values of IPSW. ED: emergency department; FiO2: fraction of inspired oxygen; IPSW: inverse propensity score weighting; IQR: interquartile range; NEWS: National Early Warning Score; SpO2: oxygen saturation as measured by pulse oximetry.
Fig. 2.
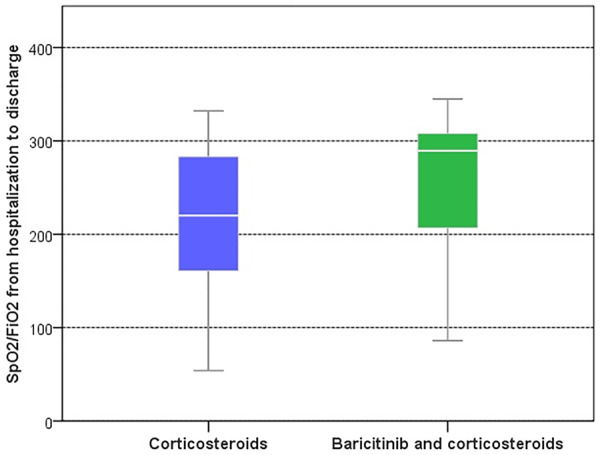
Boxplot of SpO2/FiO2 from hospitalization to discharge by treatment group
FiO2: fraction of inspired oxygen; SpO2: oxygen saturation as measured by pulse oximetry.
A higher proportion of patients required supplemental oxygen both at discharge (62.0% vs 25.8%; risk reduction of 82%, odds ratio (OR) adjusted for IPSW, 0.18; 95% CI: 0.08, 0.43; P < 0.001) and 1 month later (28.0% vs 12.9%, reduction of the risk by 69%, OR adjusted for IPSW, 0.31; 95% CI: 0.11, 0.86; P = 0.024) in CS vs BCT-CS group.
Laboratory parameters
There were no significant differences at baseline between CS vs BCT-CS groups in key laboratory parameters (Table 1). Changes in laboratory parameters from peak to discharge values (CRP, ferritin, lactate dehydrogenase and lymphocyte count), between BCT-CS and CS groups were similar except for D-dimer, as a result of treatment (Supplementary Table S1, available at Rheumatology online). In the BCT-CS subjects, median change of D-dimer from peak to discharge was −497 ng/ml (IQR: −1192 to −253 ng/ml) whereas in patients in the CS group it was −269 ng/ml (IQR: −919 to −3), P = 0.019 (Fig. 3).
Fig. 3.
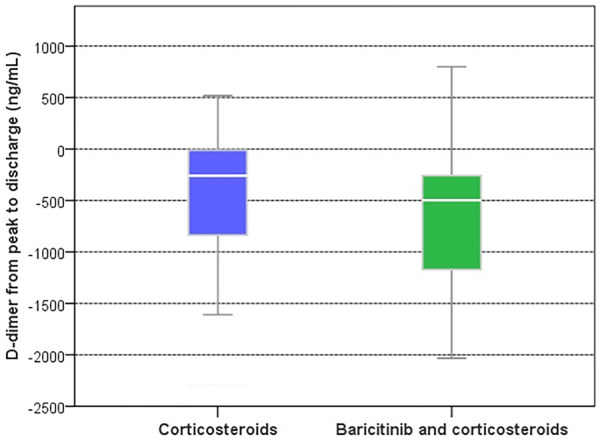
Decrease in D-dimer was more pronounced with baricitinib and corticosteroids vs corticosteroids alone
Comparison of low-dose and high-dose of baricitinib
From 62 patients treated with BCT-CS, low-dose (n = 40) and high-dose (n = 22) groups, median time on baricitinib was 5 days (IQR: 5–6 days) in both subgroups, with a total dose of 12 mg (IQR: 12–14 mg) and 20 mg (IQR: 20–24 mg), P < 0.001, respectively. At baseline, patients receiving BCT-CS high dose differed from patients receiving BCT-CS low dose with respect to diastolic blood pressure, ferritin, lymphocyte count, high-flow oxygen and non-invasive ventilation, and methylprednisolone total dose (Supplementary Table S2, available at Rheumatology online). Patients on the high dose had also lower SpO2/FiO2 on ward, 135 (119–150) vs 156 (148–238), P < 0.003.
After enrolment, patients on high-dose BCT-CS needed more intensive ventilatory support (high-flow oxygen, non-invasive ventilation) (17, 77.3% vs 14, 35.0%, P = 0.001) and received a higher dose of methylprednisolone [750 (375–750) mg vs 375 (375–688) mg, P = 0.026] compared with patients on low-dose BCT-CS.
Although changes in SpO2/FiO2 from hospitalization to discharge were significantly greater in the high-dose BCT-CS compared with the low-dose BCT-CS group, the proportion of patients requiring supplemental oxygen was similar at discharge: 11 (27.5%) in low dose vs 5 (22.7%) in high dose (P = 0.681) and 1 month later [6 (15.0%) vs 2 (9.1%), P = 0.700; Supplementary Table S3, available at Rheumatology online].
When adjusted for IPSW patients in the high-dose BCT-CS group showed a greatest change in SpO2/FiO2 from hospitalization to discharge (mean difference 38; 95% CI: 12, 65; P = 0.004) vs those on baricitinib 2 mg, with no differences in requirement for supplemental oxygen at discharge (OR 1.28; 95% CI: 0.32, 5.03; P = 0.729) or 1 month later (OR 0.97; 95% CI: 0.17, 5.55; P = 0.968) (Supplementary Table S4, Fig S2 and S3, available at Rheumatology online). There were no differences in laboratory parameter changes between the high-dose BCT-CS compared with the low-dose BCT-CS.
Adverse events during hospitalization
In the CS group, treatment-emergent adverse events included: intertriginous candidiasis in the inguinal region treated with topical ketoconazole (one patient), intravascular catheter-related methicillin-resistant Staphylococcus epidermidis bacteraemia treated with vancomycin (two patients), co-infection with Streptococcus pneumoniae diagnosed by antigen excretion in urine and treated with ceftriaxone (one patient), hyperglycaemic decompensation in non-diabetic patients that needed insulin therapy (five patients), delirium treated with neuroleptics (two patients), acute renal failure requiring haemodialysis (one patient), anaemia due to a large arm haematoma treated by red blood cell transfusion (one patient), transient bradycardia associated with prolonged QT interval (one patient) and paroxymal atrial fibrillation that reverted to sinus rhythm after amiodarone therapy (two patients). And in the BCT-CS group: oral candidiasis treated with nystatin (two patients), hyperglycaemic decompensation in non-diabetic subjects (five patients), ketoacidotic decompensation in a diabetic patient (one patient), delirium treated with neuroleptics (two patients) and third degree atrioventricular block that required a permanent pacemaker (one patient). One patient was diagnosed at the ED as having a deep vein thrombosis of the leg associated with subsegmental pulmonary embolism; this patient was later discharged from hospital with no need of oxygen therapy.
Death and ICU admission
From 270 patients who received methylprednisolone, the following were considered non-evaluable: death in 11 cases (4.1%) and ICU admission for mechanical ventilation in 13 (4.8%). The age of patients who died ranged from 47 to 77 years. Five out of 11 subjects had an active neoplasia [leukaemia, thyroid carcinoma with lung metastasis in two patients (medullary and differentiated neoplasm), colonic carcinoma metastatic to lung and liver, and gastric carcinoma] and four patients had cardiac or respiratory disease: heart failure with low left ventricular ejection fraction (two patients), bronchial asthma (one patient), and obstructive sleep apnoea syndrome with continuous positive airway pressure (one patient). From 117 patients who received baricitinib and methylprednisolone, the non-evaluable patients accounted for five deaths (4.3%), while ICU admission for mechanical ventilation corresponded to two (1.7%) patients. The age of patients who died ranged from 49 to 83 years. Four out of five had major comorbidities: renal transplant on immunosuppressants, non-Hodgkin lymphoma on chemotherapy, lung adenocarcinoma with CNS metastases and monoclonal gammopathy of undetermined significance; the fifth was an 80-year-old male with no major comorbidity.
There were no significant differences between CS and BCT-CS groups related to death (P = 1.000), ICU admission (P = 0.249) or combined death and ICU admission (P = 0.333).
Discussion
In our series of patients with moderate to severe ARDS associated with SARS-CoV-2 pneumonia, a short course of in-hospital treatment with baricitinib showed a synergistic effect with corticosteroid treatment on respiratory function improvement. These results might suggest that the immunomodulator could ameliorate both the host systemic inflammatory response to the virus and viral entry into the lung cells by inhibiting the JAK–signal transducer and activator of transcription (STAT) pathway [4, 13, 14].
Regardless of the initial triggering factors, fibrin deposition in the lumen of the lung alveoli from activation of coagulation and inhibition of fibrinolysis is crucial in ARDS pathophysiology [15]. Elevation of D-dimer in patients with SARS-CoV-2 infection indicates a hypercoagulable state. Furthermore, increased D-dimer levels seem to be a predictor of the development of ARDS, the need for admission to an ICU, or death in these patients [16, 17]. An unexpected finding was the significant decrease in D-dimer levels that we observed in patients on baricitinib plus corticosteroids when compared with those on corticosteroids alone. This might reflect a protective effect of baricitinib on lung endothelium and could play a role in the improved respiratory status of baricitinib-treated patients. Since a dysfunction in endothelial cells and cytokine (i.e. IL-6) release may lead to excess thrombin generation, this effect of baricitinib could be mediated by modulation of pro-inflammatory cytokines. In this regard, in vitro assays using human peripheral blood mononuclear cells showed that baricitinib inhibited the signalling of several JAK1/JAK2-dependent cytokines, including IL-6 signalling [18, 19] and also reduced the mean change from baseline of plasma IL‐6 in adult patients with active RA [20].
Corticosteroids are not routinely recommended in patients with SARS-CoV-2 without an alternative indication or presence of ARDS. Corticosteroid use in previous viral respiratory illnesses has demonstrated delayed viral clearance and increased mortality [21]. In contrast, other reports showed that a short course of corticosteroids was beneficial and safe in critically ill patients with SARS-CoV-2 and was not found to be an independent risk factor of prolonged viral RNA shedding [22]. As a result, the clinical benefits of corticosteroids might be related to the indication (severity of illness), timing of the intervention, and dose and duration of corticosteroid therapy [23].
Half of the patients in our series required high-flow oxygen or non-invasive ventilation. We used methylprednisolone at a high dose under the hypothesis that severe SARS-CoV-2 shares features with the macrophage activation syndrome leading to cytokine release. Moreover, the ICUs were overwhelmed with severe SARS-CoV-2 pneumonia patients for an extraordinary period of time. In addition, similarly to the management approach used in our patients, in a recent report an early course of i.v. methylprednisolone for 3 days into hospitalization and 8 days from symptom onset improved a primary composite end point of escalation of care from ward to ICU, new requirement for mechanical ventilation and mortality [3].
In summary, our results suggest that baricitinib could have synergistic biological effects when combined with corticosteroids in moderate to severe SARS-CoV-2 pneumonia. The principal anti-inflammatory effect of glucocorticoids is to inhibit many pro-inflammatory genes that encode cytokines, chemokines, cell adhesion molecules, inflammatory enzymes and receptors to address the inflammatory process and restore homeostasis. Furthermore, corticosteroid pulse therapy has induced a significant decrease in serum IL-6 levels in RA [18, 24].
Patients on high-dose (4 mg) baricitinib showed a significantly greater improvement in respiratory function during hospitalization than patients on low-dose (2 mg) baricitinib, although the proportion of patients requiring supplemental oxygen at discharge and after a month was similar and remained low in both groups. Plasma concentration of baricitinib, either as a dose of 2 mg or 4 mg once daily, is sufficient to inhibit AAK1 [13]. However, in the clinical setting, in patients with RA and an inadequate response to conventional and/or biologic DMARDs, baricitinib at a daily dose of 4 mg was associated with a greater, statistically significant clinical improvement at 12 weeks, compared with the 2 mg dose [25]. Some concerns about use of baricitinib in SARS-CoV-2 patients are interference with endogenous interferon and immune response to virus and the risk of increasing thromboembolic events (uncommon adverse event in RA patients) [26, 27].
Interferon release is one of the most potent innate immune responses to prevent viral replication during the early phases of infection, and JAK/STAT blockade with baricitinib may produce an impairment of IFN-mediated antiviral response, potentially facilitating progression of SARS-CoV-2 infection [28]. However, acute clinical deterioration in patients requiring hospital care would be the result of hyperinflammation due to elevated lung cytokine levels and impaired virus-specific T cell responses. The protective antiviral effect of interferon might have less importance in this phase [4].
Baricitinib must be used with caution in patients with risk factors for deep vein thrombosis and/or pulmonary embolism. Hence, its use could be considered of risk in SARS-CoV-2 infection, where the high incidence of thromboembolic events suggests an important role of SARS-CoV-2-induced coagulopathy. In fact, necropsy studies have regularly found histologically microthrombi within small lung arteries [29]. The USA approved only 2 mg because of safety concerns related to opportunistic infections, malignancies and thromboembolic events [30], and the European and Japanese labels for baricitinib 4 and 2 mg were updated to include a precaution related to potential thromboembolic events in patients at risk [31]. As there was no previous experience using baricitinib in SARS-CoV-2 pneumonia, we decided to be cautious and allow two schemes for baricitinib, low and high dose. In our study adverse effects in patients on baricitinib (n = 62) were low and similar to those described in a recent multicentre study of patients with moderate SARS-CoV-2 pneumonia treated with baricitinib 4 mg/day over 2 weeks [32]. In our series there was one case of deep venous thrombosis and pulmonary embolism diagnosed at the time of hospital admission and the patient was later discharged with no need of oxygen therapy.
Several limitations of our study must be addressed. Although this observational study was focused on pulmonary function in patients with no major comorbidities, we also analysed mortality and ICU admission and found no differences between patients who initially received methylprednisolone without baricitinib (n = 270) or with baricitinib (n = 117). Death rate in these patients was 4.0% and 4.2%, respectively, lower than the figure we observed in the whole series of 876 patients, with a median age of 65 years and an overall mortality rate of 8.9%. These results are similar to those reported in a large series of patients hospitalized with SARS-CoV-2 pneumonia in Spain, with 9.1% of deaths in patients aged 60–69 years [33]. Taking into account these findings, it can be hypothesized that corticosteroids (with or without baricitinib) could exert a beneficial effect on mortality in patients from our series and this would be in accordance with a recent report that found a beneficial effect of corticosteroids (dexamethasone) on reduction of deaths in patients with SARS-CoV-2 pneumonia receiving invasive mechanical ventilation or oxygen [34].
Beside the need for oxygen supplements, we used a surrogate variable, SpO2/FiO2, to study the effects of baricitinib and corticosteroids on the progression and severity of respiratory function. We believe this variable is easily measurable in all patients, biologically plausible and predictive of disease progression. For this reason, to evaluate the effect of treatments we excluded patients with history of respiratory or cardiac failure and those exposed to other immunomodulators or potential antiviral drugs; therefore, evaluation of hypoxaemia could not be affected by confounding variables.
The absence of randomization should be taken into account when interpreting these findings. However, until more robust evidence is available, we suggest that in the case of patients with moderate to severe SARS-CoV-2 pneumonia, a combination of baricitinib and corticosteroids seems to be a reasonably efficacious and safe option for patients at risk of ICU admission/mechanical ventilation.
Results from randomized control clinical trials are indeed needed to confirm these preliminary results and to further inform guidelines and clinical decisions (COV-BARRIER, TACTIC-R, ACTT-II: https://clinicaltrials.gov/). Furthermore, other studies are required to determine the optimal timing for starting baricitinib relative to onset of symptoms (whether baricitinib could be used earlier in patients with less severe respiratory failure), combination with or without corticosteroids, and dose and duration of treatment.
J.L.R-G. designed this study, was involved in data collection, and drafted the paper. G.S-N. critically revised the paper for important intellectual content. J.A-S. conducted the statistical analysis. C.G-G. supervised protocol guidelines and doses of medications described in the study. J.M.J-V. read and approved the final version of the article. E.M-A. supervised protocol guidelines and approved the final version of the article.
Supplementary Material
Acknowledgements
We would like to express our sincere gratitude to nurses Maria Jose Andicoberry Martinez and Maria Angeles Rubio Andreu.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Rello J, Storti E, Belliato M. et al. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J 2020;55:2001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fadel R, Morrison AR, Vahia A. et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020. (in press), doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seif F, Aazami H, Khoshmirsafa M. et al. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol 2020;181:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lucchino B, Di Franco M, Conti F. COVID-19: an unexpected indication for anti-rheumatic therapies? Rheumatology 2020;59:1200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stebbing J, Phelan A, Griffin I. et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020;20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thachil J, Tang N, Gando S. et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toh CH, Hoots WK; SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost 2007;5:604–6. [DOI] [PubMed] [Google Scholar]
- 9. Doyle DJ. Clinical early warning scores: new clinical tools in evolution. Open Anesthesia J 2018;12:26–33. [Google Scholar]
- 10. Rice TW, Wheeler AP, Bernard GR. et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410–7. [DOI] [PubMed] [Google Scholar]
- 11. Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 12. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson P, Griffin I, Tucker C. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020;395:e30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stebbing J, Krishnan V, Bono S. et al. Mechanism of baricitinib supports artificial intelligence‐predicted testing in COVID‐19 patients. EMBO Mol Med 2020;12:e12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ozolina A, Sarkele M, Sabelnikovs O. et al. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front Med (Lausanne) 2016;3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Yan X, Fan Q. et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marietta M, Ageno W, Artoni A. et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus 2020;18:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choy EHS, Miceli-Richard C, MA G-G. et al. The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib. Clin Exp Rheumatol 2019;37:694–704. [PubMed] [Google Scholar]
- 19. van den BH, van Wik MJ, Geertzen RG. et al. Influence of corticosteroid pulse therapy on the serum levels of soluble interleukin 2 receptor, interleukin 6 and interleukin 8 in patients with rheumatoid arthritis. J Rheumatol 1994;21:430–4. [PubMed] [Google Scholar]
- 20. Tanaka Y, Emoto K, Cai Z, Aoki T. et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12‐week, double‐blind, randomized placebo‐controlled study. J Rheumatol 2016;43:504–11. [DOI] [PubMed] [Google Scholar]
- 21. Arabi YM, Mandourah Y, Al-Hameed F. et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 2018;197:757–67. [DOI] [PubMed] [Google Scholar]
- 22. Xu K, Chen Y, Yuan J. et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020;71:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shang L, Zhao J, Hu Y. et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395:683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Z, Liu J, Zhou Y. et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 2020;81:e13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genovese MC, Kremer J, Zamani O. et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 26. Favalli EG, Ingegnoli F, De Lucia O. et al. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev 2020;19:102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayence A, Van den Eynde JJ. Baricitinib: a 2018 novel FDA-approved small. Molecule inhibiting janus kinases. Clin Exp Rheumatol 2019;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Channappanavar R, Fehr AR, Zheng J. et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest 2019;129:3625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wichmann D, Sperhake JP, Lütgehetmann M. et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020;173:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mogul A, Corsi K, McAuliffe L. Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 2019;53:947–53. [DOI] [PubMed] [Google Scholar]
- 31. Verden A, Dimbil M, Kyle R. et al. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf 2018;41:357–61. [DOI] [PubMed] [Google Scholar]
- 32. Cantini F, Niccoli L, Nannini C. et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect 2020. (in press), doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casas JM, Anton JM, Millan K, SEMICOVID-19 Network. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMICOVID-19 Network. MedRxiv 2020, doi: 10.1101/2020.05.24.20111971.
- 34. Horby P, Lim WS, Emberson JR. et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report. N Engl J Med. 2020; doi:10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.