Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a critical concern among healthcare workers (HCWs). Other studies have assessed SARS-CoV-2 virus and antibodies in HCWs, with disparate findings regarding risk based on role and demographics.
Methods
We screened 3904 employees and clinicians for SARS-CoV-2 virus positivity and serum immunoglobulin (Ig)G at a major New Jersey hospital from April 28 to June 30, 2020. We assessed positive tests in relation to demographic and occupational characteristics and prior coronavirus disease 2019 symptoms using multivariable logistic regression models.
Results
Thirteen participants (0.3%) tested positive for virus and 374 (9.6%) tested positive for IgG (total positive: 381 [9.8%]). Compared with participants with no patient care duties, the odds of positive testing (virus or antibodies) were higher for those with direct patient contact: below-median patient contact, adjusted odds ratio (aOR) = 1.71 and 95% confidence interval [CI] = 1.18–2.48; above-median patient contact, aOR = 1.98 and 95% CI = 1.35–2.91. The proportion of participants testing positive was highest for phlebotomists (23.9%), maintenance/housekeeping (17.3%), dining/food services (16.9%), and interpersonal/support roles (13.7%) despite lower levels of direct patient care duties. Positivity rates were lower among doctors (7.2%) and nurses (9.1%), roles with fewer underrepresented minorities. After adjusting for job role and patient care responsibilities and other factors, Black and Latinx workers had 2-fold increased odds of a positive test compared with white workers. Loss of smell, taste, and fever were associated with positive testing.
Conclusions
The HCW categories at highest risk for SARS-CoV-2 infection include support staff and underrepresented minorities with and without patient care responsibilities. Future work is needed to examine potential sources of community and nosocomial exposure among these understudied HCWs.
Keywords: COVID-19, disparities, healthcare workers, hospital epidemiology, SARS-CoV-2
In a screening of 3904 New Jersey hospital employees and clinicians, 9.8% tested SARS-CoV-2 virus or IgG antibody positive. In multivariable models, direct patient contact, job role, and black and Latinx race/ethnicity were all associated with positive test results.
As the global community continues to confront the coronavirus disease 2019 (COVID-19) pandemic, the potential for occupational exposure among healthcare workers (HCWs) remains a critical concern. Multiple studies comparing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among HCWs and non-HCWs have found higher rates of infection among HCWs, a disparity persisting after adjustment for the likelihood of being tested [1–3]. Nevertheless, uncertainty remains as to what extent SARS-CoV-2 transmission is heightened within hospitals and what factors put hospital employees at greater risk for infection. For example, several studies have observed that HCWs with greater exposure to patients with COVID-19 were more likely to be SARS-CoV-2-infected [2–5], and inadequate personal protective equipment (PPE) was identified as a risk factor for infection [2]. However, other studies have observed no associations [6, 7]. For example, at a large Belgian hospital with strong, early infection control measures, no occupational risk factors were associated with SARS-CoV-2 antibody positivity in HCWs; positivity was only associated with having household contacts with suspected or confirmed COVID-19 [7]. This finding suggests that little occupational transmission occurred, but whether household contacts were infected before or after HCWs was not defined.
More importantly, despite the higher number of confirmed US COVID-19 cases (>6 million of >26 million worldwide) and deaths (>180 000 of >860 000 worldwide) as of September 4, 2020 [8], relatively few studies have directly measured infections among US HCWs [6, 9–12]. Healthcare workers in highly impacted areas may be at particular risk, due both to community transmission and the surge in COVID-19 patients; this may have been particularly true especially during the early phase of the pandemic when PPE and other protections were limited [9–12].
With proximity, strong economic and social ties to New York City (NYC), a large commuter population, and the densest population of any US state, New Jersey (NJ) was particularly hard hit by COVID-19 in March–April 2020 [13]. As of August 2020, the NJ per capita death rate was the second highest in the United States [14]. To assess occupational safety, we conducted a voluntary SARS-CoV-2 virus and antibody screening of all personnel of a university-affiliated NJ teaching hospital. We aimed to (1) characterize SARS-CoV-2 virus and antibody status, (2) examine demographic and occupational risk factors for infection, and (3) assess positivity in relation to self-reported symptoms.
METHODS
Setting and Data Collection
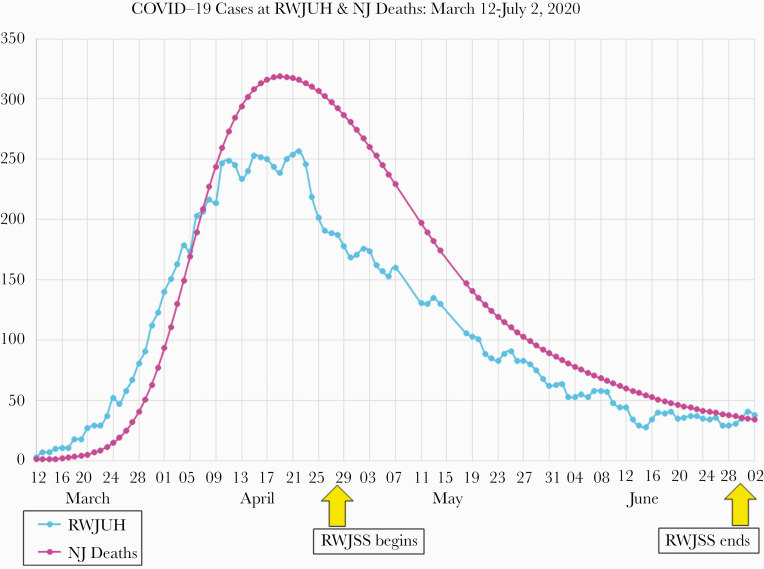
Robert Wood Johnson University Hospital (RWJUH) is an academic medical center located in central NJ. Affiliated with Rutgers University and the RWJBarnabas Health network, RWJUH has 614 licensed beds and approximately 5200 employees and 2300 affiliated attending and trainee physicians. The first patients with COVID-19 were admitted to RWJUH in early March 2020. To date, the hospital has treated >1270 inpatients and 520 outpatients with test-positive COVID-19, with cases peaking in mid-April (Figure 1).
Figure 1.
Coronavirus disease 2019 (COVID-19) cases at Robert Wood Johnson University Hospital (RWJUH) and New Jersey (NJ) COVID-19 deaths in Spring 2020. RWJSS, Robert Wood Johnson Screening Study.
Robert Wood Johnson University Hospital employees and affiliated Rutgers Robert Wood Johnson Medical School (RWJMS) clinicians were invited via email and posters to participate in onsite, voluntary, no-cost screening for SARS-CoV-2 virus and antibodies. Additional in-person recruitment and testing occurred from April 28 to June 30, 2020, at a central hospital location. Employees ill with suspected COVID-19 were directed to employee health and not permitted to participate in screening. Thus, the study population represented personnel at work with no major symptoms of active infection. After consent, interested employees ≥18 years old completed a questionnaire providing information about demographics, job role and duties, medical history and comorbidities, symptoms since the pandemic began locally (fever, cough, shortness of breath, vomiting, diarrhea, loss of smell or taste, muscle aches, or headache), and potential recent sources of exposure. Obesity was classified as body mass index (BMI) ≥30 kg/m2, calculated based on self-reported height and weight.
After completing the questionnaire, each employee provided a blood sample and self-collected nasal swab for antibody and viral testing, respectively. Study data were collected and managed using REDCap electronic data capture tools hosted at RWJMS [15].
Patient Consent Statement
All study activities were approved by the Rutgers University Institutional Review Board (Pro2020000902), and all participants provided electronic informed consent before engaging in study activities.
Laboratory Assays
Detection of SARS-CoV-2 viral ribonucleic acid was performed using real-time, reverse-transcription polymerase chain reaction (PCR) on the cobas 6800 System (Roche Diagnostics, Indianapolis, IN) within the RWJUH Clinical Virology laboratory. The assay, which received Emergency Use Authorization from the US Food and Drug Administration for clinical detection of SARS-CoV-2 RNA in upper respiratory samples including nasal swab [16], targets 2 viral genes: (1) ORF1a/b, a nonstructural region unique to SARS-CoV-2 (limit of detection 25 copies/mL) and (2) E-gene encoding a structural protein envelope gene specific for pan-Sarbecovirus, including SARS-CoV-2 (limit of detection 32 copies/mL).
The SARS-CoV-2 antibody screening of human serum was performed using the Abbott Architect SARS-CoV-2 assay (Abbott Laboratories, Chicago, IL) [16]. The assay detects immunoglobulin class G (IgG) antibodies to the nucleocapsid protein of the SARS-CoV-2 virus. Prior testing showed sensitivity of 96.9% in serum from SARS-CoV-2 PCR-positive patients 14 days after COVID-19 symptom onset and specificity of ≥99.6% in serum from patients collected prepandemic and in patients without COVID-19 but who exhibited respiratory symptoms [16, 17].
Statistical Analysis
We first calculated descriptive statistics for all variables of interest. Given the low prevalence of viral PCR positivity, we aggregated positive tests results by PCR and antibody screening. We examined the relationship between job roles, proportion reporting direct patient care duties, and positive testing prevalence for SARS-CoV-2.
We evaluated the association between variables of interest and SARS-CoV-2 positivity using multivariable logistic regression. We first fit minimally adjusted models examining demographic and lifestyle factors only (gender, age, race/ethnicity, BMI, tobacco use, and comorbidities) in relation to virus/antibody positivity, calculating odds ratios (ORs), and 95% confidence intervals (CIs). We initially considered comorbidities individually and then aggregated them to a binary “any comorbidity” variable, given the similar point estimates observed across individual comorbidities. Fully adjusted models were then fit including those demographic and lifestyle covariates, as well as level of direct patient care and hospital job roles. For this analysis, we consolidated job titles as follows: physicians (including trainees); nurses (including nurse practitioners and trainees); technicians, technologists, transport staff, and phlebotomists (roles with generally brief but numerous patient interactions); interpersonal and support roles (including clerks, counselors, security, monitors, social workers, dieticians, and chaplains); other patient care roles; other nonpatient care roles; and unclassified workers. Physicians were selected as the reference group because they represented one of the larger groups of participants and were considered lower risk than others. We imputed missing data (<5% for most variables) with multiple imputation using chained equations and conducted multivariable models based on 20 imputed datasets [18].
We additionally fit logistic regression models to examine odds of SARS-CoV-2 virus or antibody positivity in relation to self-report symptoms since the pandemic began. The first model included all participants; the second model included only participants who reported ≥1 symptom. A final linear regression model limited to participants with positive testing examined antibody concentrations in relation to self-reported symptoms, history of hospital-based care, and time since first symptom. All statistical analyses were performed using SAS 9.4.
RESULTS
Of an estimated 7500 employees, 4482 (59.8%) consented to participate in the screening. Of these, 3907 (87.2%) ultimately provided blood and nasal swab samples for testing. In total, 13 (0.3%) participants were positive for the virus and 374 (9.6%) were positive for IgG antibodies, corresponding to an overall positive result (virus or IgG antibodies) in 381 (9.8%) of those screened.
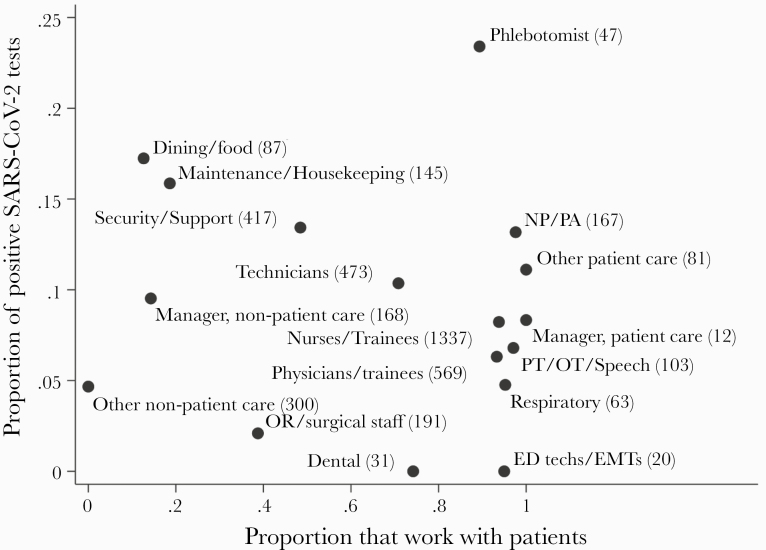
The majority of participants (74.0%) were female, and the sample was relatively diverse, including 22.5% non-Hispanic Asian, 13.8% non-Hispanic black, 14.5% Hispanic/Latinx, and 7.9% identifying as belonging to another racial/ethnic group (Table 1). Approximately one fifth (19.2%) reported at least 1 symptom consistent with COVID-19 in the prior 2 weeks, and approximately one quarter (24.1%) reported at least 1 symptom with a prior illness consistent with COVID-19. Over two thirds of participants (68.8%) reported having direct patient care responsibilities, with a wide variety of roles included. The proportion of participants testing positive was highest for phlebotomists (23.9%), followed by maintenance/housekeeping (17.3%), dining/food services (16.9%), and interpersonal/support (13.7%). By comparison, positivity rates were lower among doctors (7.2%) and nurses (9.1%) (Figure 2). Of note, participants in higher risk roles had greater proportions of underrepresented minorities than doctors and nurses (Supplementary Table 1).
Table 1.
Characteristics of Robert Wood Johnson Screening Study Participants
| All Participants (n = 3904)a | Negative on Both SARS-CoV-2 Virus and Antibody Test (n = 3523; 90.2%) | Positive on SARS-CoV-2 Virus or Antibody Test (n = 381; 9.8%) | ||
|---|---|---|---|---|
| Characteristics | N (Column %) | N (Row %) | N (Row %) | P Valueb |
| Demographics | ||||
| Gender | ||||
| Female | 2874 (74.0) | 2593 (90.2) | 281 (9.8) | .93 |
| Male | 1030 (26.0) | 930 (90.3) | 100 (9.7) | |
| Age (Years) | <.001 | |||
| 20–39 | 1703 (44.1) | 1502 (88.2) | 201 (11.8) | |
| 40–59 | 1690 (43.7) | 1548 (91.6) | 142 (8.4) | |
| ≥60 | 472 (12.2) | 439 (93.0) | 33 (7.0) | |
| Race Ethnicity | <.001 | |||
| Non-Hispanic white | 1612 (41.3) | 1492 (92.6) | 120 (7.4) | |
| Non-Hispanic black | 540 (13.8) | 460 (85.2) | 80 (14.8) | |
| Non-Hispanic Asian | 878 (22.5) | 819 (93.3) | 59 (6.7) | |
| Hispanic | 565 (14.5) | 478 (84.6) | 87 (15.4) | |
| Other/missing | 309 (7.9) | 1492 (92.6) | 120 (7.4) | |
| Clinical Variables | ||||
| BMI | ||||
| <18.5 | 94 (2.5) | 85 (90.4) | 9 (9.6) | .19 |
| 18.5 ≤ BMI < 25 | 1438 (38.2) | 1309 (91.0) | 129 (9.0) | |
| 25 ≤ BMI < 30 | 1271 (33.7) | 1154 (90.8) | 117 (9.2) | |
| 30 ≤ BMI < 40 | 827 (21.9) | 729 (88.1) | 98 (11.9) | |
| ≥40 | 139 (3.7) | 128 (92.1) | 11 (7.9) | |
| Tobacco Use | .25 | |||
| Never | 3175 (82.0) | 2855 (89.9) | 320 (10.1) | |
| Former | 459 (11.9) | 419 (91.3) | 40 (8.7) | |
| Current | 238 (6.1) | 221 (92.9) | 17 (7.1) | |
| Comorbidities | ||||
| No comorbidities | 1810 (48.2) | 1646 (90.9) | 164 (9.1) | .33 |
| Diabetes | 257 (6.7) | 234 (91.1) | 23 (8.9) | .73 |
| High blood pressure | 729 (18.9) | 666 (91.4) | 63 (8.6) | .28 |
| Asthma | 548 (14.3) | 495 (90.3) | 53 (9.7) | .99 |
| Obesity | 966 (25.6) | 857 (88.7) | 109 (11.3) | .055 |
| Otherc | 173 (4.5) | 158 (91.3) | 15 (8.7) | .74 |
| Recent COVID-19 symptoms (any) | 738 (19.2) | 640 (86.7) | 98 (13.3) | <.001 |
| Prior or recent COVID-19 symptoms (any) | 1404 (36.6) | 1175 (83.7) | 229 (16.3) | <.001 |
| Health Exposures | ||||
| Direct Patient Care | .02 | |||
| No | 1171 (31.2) | 1076 (91.9) | 95 (8.1) | |
| Yes, close contact (≤40% of total worktime) | 1358 (36.2) | 1227 (90.4) | 131 (9.6) | |
| Yes, close contact (>40% of total worktime) | 1226 (32.6) | 1085 (88.5) | 141 (11.5) | |
| Household member with COVID-19 diagnosis or symptoms | 208 (5.3) | 161 (77.4) | 47 (22.6) | <.001 |
| Coworker with COVID-19 diagnosis or symptoms | 1108 (28.5) | 988 (89.2) | 120 (10.8) | .15 |
| Other contact with COVID-19 diagnosis or symptoms | 296 (7.6) | 258 (87.2) | 38 (12.8) | .08 |
| Hospital Role | <.001 | |||
| Dentist/Dental | 22 (0.6) | 22 (100.0) | 0 (0.0) | |
| Dining/Food | 89 (2.3) | 74 (83.1) | 15 (16.9) | |
| Interpersonal/Support | 351 (9.0) | 303 (86.3) | 48 (13.7) | |
| Maintenance/Housekeeping | 150 (3.8) | 124 (82.7) | 26 (17.3) | |
| Nonphysician Emergency Workers | 13 (0.3) | 12 (92.3) | 1 (7.7) | |
| NP/PA | 159 (4.1) | 137 (86.2) | 22 (13.8) | |
| Nurse/Trainee | 1239 (31.7) | 1122 (90.6) | 117 (9.4) | |
| OR/Surgical Staff | 54 (1.4) | 52 (96.3) | 2 (3.7) | |
| Other Nonpatient Care | 433 (11.1) | 411 (94.9) | 22 (5.1) | |
| Other Patient Care | 136 (3.5) | 117 (86.0) | 19 (14.0) | |
| Pharmacist | 115 (2.9) | 113 (98.3) | 2 (1.7) | |
| Phlebotomist | 46 (1.2) | 35 (76.1) | 11 (23.9) | |
| Physician/Trainee | 502 (12.9) | 466 (92.8) | 36 (7.2) | |
| PT/OT/ST | 98 (2.5) | 91 (92.9) | 7 (7.1) | |
| Respiratory | 56 (1.4) | 53 (94.6) | 3 (5.4) | |
| Technicians/Technologists | 399 (10.2) | 353 (88.5) | 46 (11.5) | |
| Unclassified | 42 (1.1) | 38 (90.5) | 4 (9.5) |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; NP, nurse practitioner; OR, operating room; OT, occupational therapist; PA, physician’s assistant; PT, physical therapist; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ST, speech therapist.
an for individual variables vary with some missing data from up to 4% of respondents.
bBold font indicates P < .05.
cOther comorbidities include the following: cancer, cardiovascular or cerebrovascular disease, chronic autoimmune disease, chronic kidney disease, chronic obstructive pulmonary disease, or other chronic lung disease.
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and antibody positivity by job role and in relation to patient contact in the RWJSS. ED, emergency department; EMT, emergency medical technician; NP, nurse practitioner; OR, operating room; OT, occupational therapist; PA, physician’s assistant; PT, physical therapist; RWJSS, Robert Wood Johnson Screening Study.
In minimally adjusted models, compared with non-Hispanic white participants, the odds of positive testing were over 2-fold higher among non-Hispanic black (OR, 2.17; 95% CI, 1.60–2.94) and Hispanic/Latinx participants (OR, 2.13; 95% CI, 1.58–2.87) and 56% higher among participants self-identifying as “other” races (OR, 1.56; 95% CI, 1.05–2.33) (Table 2). The odds of positive testing were also lower among older participants compared with those under 40. Neither gender nor comorbidities (considered individually or collectively) were associated with positive testing, but current smokers were less likely to be positive.
Table 2.
Logistic Regression Models Examining Risk Factors for Positive SARS-CoV-2 Test (Virus and/or Antibody; n = 3904)a
| Characteristic | Minimally Adjusted Model | Fully Adjusted Model |
|---|---|---|
| Risk Factor | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Age | ||
| 18–39 | Ref | Ref |
| 40–59 | 0.71 (0.56–0.90) | 0.74 (0.59–0.95) |
| 60 and above | 0.62 (0.42–0.91) | 0.72 (0.49–1.07) |
| Maleb | 1.03 (0.80–1.32) | 1.12 (0.86–1.45) |
| Race/Ethnicity | ||
| Non-Hispanic white | Ref | Ref |
| Non-Hispanic black | 2.13 (1.58–2.87) | 2.06 (1.51–2.80) |
| Non-Hispanic Asian | 0.89 (0.64–1.24) | 0.90 (0.65–1.24) |
| Hispanic/Latinx | 2.17 (1.60–2.94) | 2.10 (1.53–2.89) |
| Other | 1.56 (1.05–2.33) | 1.48 (0.99–2.23) |
| Tobacco Use | ||
| Never | Ref | Ref |
| Current | 0.61 (0.37–1.02) | 0.59 (0.35–1.00) |
| Former | 0.95 (0.67–1.35) | 0.92 (0.64–1.32) |
| Any comorbidityc | 1.11 (0.89–1.40) | 1.09 (0.87–1.38) |
| Direct Patient Care | ||
| None | Ref | |
| Below median | 1.71 (1.18–2.48) | |
| Above median | 1.98 (1.35–2.91) | |
| Hospital Role | ||
| Physician (including trainee) | Ref | |
| Nurse (including trainee) | 1.33 (0.88–2.01) | |
| Technician/technologist | 1.71 (1.08–2.71) | |
| Interpersonal/support | 2.07 (1.25–3.44) | |
| Other patient care | 0.72 (0.42–1.23) | |
| Other nonpatient care | 1.75 (1.01–3.03) | |
| Unclassified | 1.08 (0.35–3.32) |
Abbreviations: CI, confidence interval; Ref, reference group; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aResults significant at P < .05 are indicated in bold font.
bReference group: female.
cAny comorbidity includes the following: diabetes, hypertension, asthma, obesity, cancer, cardiovascular or cerebrovascular disease, chronic autoimmune disease, chronic kidney disease, chronic obstructive pulmonary disease, or other chronic lung disease.
In fully adjusted models, we examined odds of positive testing in relation to demographic factors (age, sex, race/ethnicity), tobacco use, comorbidities, patient care duties, and job role (Table 2). Adjusting for those covariates, compared with participants with no patient care duties, the odds of testing positive were higher for those with direct patient contact, either below the median (OR, 1.71; 95% CI, 1.18–2.48) or above the median (OR, 1.98; 95% CI, 1.35–2.91). In addition, relative to physicians, workers in several groups had higher odds of positive testing, including interpersonal/support roles (OR, 2.07; 95% CI, 1.25–3.44), technicians/technologists (OR, 1.71; 95% CI, 1.08–2.71), and other nonpatient care (OR, 1.75; 95% CI, 1.01–3.03). Associations between positive testing and age, race, and smoking were consistent with those observed in minimally adjusted models.
In models examining self-reported symptoms, the strongest predictors of positive testing were loss of smell (OR, 5.13; 95% CI, 2.85–9.24) and loss of taste (OR, 4.01; 95% CI, 2.30–7.01) (Table 3). Odds of a positive test were also higher among those reporting recorded fever >100°F (OR, 2.58; 95% CI, 1.75–3.81). In models limited to participants who reported at least 1 symptom consistent with COVID-19 (36.8%), results were largely unchanged. In analyses examining self-reported symptoms in relation to IgG antibody levels, body aches were associated with higher IgG levels (β = 0.75; 95% CI, −0.01 to 1.51), with no strong associations observed for other symptoms (Table 4). Antibody concentrations were nonsignificantly higher among participants reporting symptoms ≤84 days earlier compared with those who tested positive but reported no symptoms, with the highest antibody levels observed among individuals whose symptoms began 14–28 days earlier (Table 4).
Table 3.
SARS-CoV-2 Test Positivity (Virus and/or Antibody) in Relation to Self-Reported Recent or Prior Symptoms of COVID-19 (n = 3904)a
| Characteristic | All Participants (n = 3904) | Participants With at Least One Symptom (n = 1435) |
|---|---|---|
| Reported symptom | Odds Ratio (95% CI) | |
| Feverb | ||
| Felt feverish but did not take temperature | 0.97 (0.64–1.46) | 0.95 (0.64–1.42) |
| Recorded temperature over 100°F | 2.58 (1.75–3.81) | 2.47 (1.68–3.64) |
| Cough | 1.03 (0.70–1.50) | 0.96 (0.65–1.41) |
| Shortness of breath or difficulty breathing | 0.86 (0.55–1.33) | 0.86 (0.56–1.31) |
| Vomiting | 0.82 (0.40–1.68) | 0.81 (0.40–1.66) |
| Diarrhea/multiple watery stools | 1.23 (0.81–1.85) | 1.19 (0.79–1.78) |
| Loss of smell | 5.13 (2.85–9.24) | 4.97 (2.78–8.90) |
| Loss of taste | 4.01 (2.30–7.01) | 4.00 (2.30–6.94) |
| Body ache | 1.39 (0.95–2.04) | 1.30 (0.88–1.90) |
| Headaches | 1.19 (0.86–1.66) | 1.06 (0.74–1.52) |
| Other symptomsc | 0.52 (0.35–0.78) | 0.52 (0.35–0.76) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Results significant at P < .05 are indicated in bold font.
b Reference group: no fever
c Includes the following: fatigue, nausea, loss of appetite, conjunctivitis, nasal and sinus congestion, runny nose, sneezing, chest tightness, abdominal pain, and rash.
Table 4.
Antibody Levels in Relation to Clinical Features and Timing of Illness Among Antibody-Positive Participants (n = 374)
| Variables | Point Estimates (95% CI) |
|---|---|
| Fevera | |
| Felt feverish but did not take temperature | 0.15 (−0.58 to 0.88) |
| Recorded temperature over 100°F | 0.26 (−0.44 to 0.96) |
| Cough | −0.09 (−0.80 to 0.61) |
| Shortness of breath or difficulty breathing | 0.01 (−0.75 to 0.76) |
| Vomiting | 0.49 (−0.77 to 1.75) |
| Diarrhea/multiple watery stools | 0.32 (−0.47 to 1.11) |
| Loss of smell | −0.21 (−1.43 to 1.02) |
| Loss of taste | −0.64 (−1.87 to 0.60) |
| Body aches | 0.75 (−0.01 to 1.51) |
| Headaches | −0.03 (−0.70 to 0.64) |
| Other Symptomsb | 0.10 (−0.63 to 0.83) |
| Any treatment in hospital for COVID-19 | 0.58 (−0.27 to 1.43) |
| Time Since Beginning of Illnessc | |
| 0–14 days | 0.40 (−2.92 to 3.73) |
| 15–28 days | 1.37 (−0.46 to 3.20) |
| 29–56 days | 0.77 (−0.25 to 1.79) |
| 57–84 days | 0.50 (−0.48 to 1.48) |
| >84 days | −0.41 (−1.52 to 0.69) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
aReference group: no fever.
bIncludes the following: fatigue, nausea, loss of appetite, conjunctivitis, nasal and sinus congestion, runny nose, sneezing, chest tightness, abdominal pain, and rash.
cReference group: no suspected COVID-19 illness.
DISCUSSION
In this screening of approximately 4 thousand hospital employees in NJ, an early epicenter of the US COVID-19 pandemic, approximately 10% of staff tested positive for SARS-CoV-2 virus and/or antibodies. Both demographic and occupational factors were associated with odds of testing positive. In multivariable models, nonwhite hospital staff (particularly those identifying as black, Hispanic/Latinx, or other) were most likely to test positive, consistent with findings reported in the general population [19–21]. After accounting for demographic differences, the odds of infection were higher among employees with patient care duties as well as several groups besides physicians and nurses, including interpersonal/support staff (eg, security staff, clerks) and technicians/technologists (including phlebotomists).
Prevalence of antibody positivity in HCW has been highly variable across studies, hospitals, and geographic settings. Our results are similar to the 12% IgG positivity reported in a small (n = 91) study of NYC anesthesiologists and intensive care providers as well as the 13.7% seropositivity reported in a large (n = 46 117) NYC hospital system [10, 12]. However, another NYC study reported much higher (36%) IgG positivity among high-risk HCWs with direct patient contact (eg, emergency medicine, critical care, and anesthesiology staff) [9]. Even within individual medical systems, HCW seroprevalence can vary considerably across hospitals or geographic areas. In another large NYC study, seroprevalence in HCWs varied more than 2-fold (10.9%–22.7%) by county [12] while in 7 affiliated Houston, Texas hospitals, antibody positivity ranged from 0% to 12.5% [11]. Seroprevalence studies of European HCWs have mostly shown lower rates of antibody positivity, ranging from 1.6% to 6.5% [7, 22, 23]. Overall, the considerable geographic variability likely reflects differences in local trends in community transmission but may also reflect variation in test characteristics and hospital infection control practices, particularly during the early stage of the pandemic when best practices for protecting HCWs were emerging and resources for testing and PPE were limited. In our study, IgG antibody positivity (9.6%) was far more common than SARS-CoV-2 virus positivity (0.03%), suggesting greater prior SARS-CoV-2 exposure among hospital staff. These results are consistent with the timeline of the screening study, which began as the local NJ pandemic was waning after April 2020 (Figure 1).
Studies focusing on infection among “high-risk” HCW groups reflect the implicit premise that within hospitals, risks of workplace SARS-CoV-2 acquisition likely vary by work unit and role [9, 10, 24]. After adjustment for covariates including race/ethnicity and job role, we observed that participants with patient care responsibilities were more likely to have been infected than those who provided no patient care, which is consistent with some (eg, [3, 25]), but not all (eg, [6, 7]), prior studies. However, we also observed that those with “interpersonal/support” roles (including clerks, counselors, security staff, monitors, social workers, dieticians, and chaplains) and high-throughput patient care roles (technicians, technologists, phlebotomists, and transport staff) had significantly increased likelihood of infection compared with physicians. More granular analyses of infection rates by specific job titles showed highest rates of infections among phlebotomists, dining and food services, maintenance, housekeeping, security, and other support workers (Figure 2). By contrast, physicians, nurses, and emergency medical technicians, who have attracted more attention as “healthcare heroes,” showed much lower infection rates. A recent large study of NYC-area HCWs also observed increased risks of virus positivity among service/maintenance workers vis-a-vis physicians, although differences across roles were less marked than in our study and largely explained by other factors [12]. A smaller study reported that approximately 35% of English housekeeping staff tested positive for antibodies [26]. These results contrast with those of an Italian hospital during the early phase of the pandemic, in which physicians were most likely to be infected and clerical and administrative workers were least likely to be infected [27]. Variation in infection rates among frontline workers with direct patient care responsibilities may reflect differences in hospitals’ abilities to provide adequate PPE and enact precautionary policies during COVID-19 surges. With the safety of frontline HCWs prioritized in many hospital settings, hospital employees without direct patient care roles likely had less access to PPE and enforcement of social distancing and safety protocols.
The higher infection rates we observed may reflect community infection because workers in these support roles were disproportionately black and Hispanic/Latinx, whose communities have been most impacted by the pandemic. After adjusting for job role and other risk factors, the odds of infection were twice as great among black and Latinx workers compared with white workers, consistent with other evidence of social determinants of COVID-19 vulnerability [19, 20]. Similar patterns were observed in a screening of nearly 10 000 United Kingdom university hospital workers, in which black and Asian race as well as hospital porter and cleaning job roles were strong predictors of infection [25]. Because we lack additional information on potential sources of community exposure or additional measures of socioeconomic status in these vulnerable HCWs, we are unable to test hypotheses regarding community versus hospital infection, but we suggest that this is an important future direction. Ultimately, differences in infectivity rates by race/ethnicity and job role warrant additional consideration of individual hospital practices as well as the larger community context. Regardless of whether infections occurred through nosocomial or community transmission, these results suggest a need to enact safety protocols (including marking and social distancing) for all hospital employees to preserve and protect the healthcare workforce through future waves of infection. More importantly, workers in support roles in healthcare settings (with little or no patient contact) have attracted relatively little attention to date; nevertheless, our results suggest potentially high infection rates in this group. Indeed, the 40% of infected HCWs who reported no prior symptoms of infection in our study could indicate an important contribution to nosocomial SARS-CoV-2 spread, even if their infections were initially acquired in the community.
Consistent with prior research in other populations (reviewed in [28]), loss of smell and taste were the symptoms most strongly associated with positive testing. We also observed approximately 2.5-fold increased odds of positive testing among participants reporting a measured fever >100°F during the pandemic. Our results suggest that other symptoms may be less specific and informative for diagnosing SARS-CoV-2 infection. It is interesting to note that fever and symptoms other than body aches were not strongly associated with IgG levels among test-positive participants. Associations between SARS-CoV-2 disease severity and IgG response in patient populations have been reported elsewhere [29–31]. Our data only captured symptom presence, not severity; hospital-based medical care was positively but not significantly associated with antibody level. In a small study of German HCWs, most participants with SARS-CoV-2 infections had detectable IgG antibodies over 12-week follow-up; as in our study, no strong associations with symptoms were observed [32].
Strengths of our study include recruitment from a large hospital with diverse staff and use of a unified testing protocol. Few studies to date have reported on infections among diverse HCW populations or simultaneously tested for both virus and antibodies, signifying current and prior infection, respectively. At the time of recruitment, the hospital and surrounding communities had already experienced the “surge,” making us well positioned to assess infections through antibody testing. Our antibody tests targeted IgG, and unlike most previous studies of HCWs, we analyzed quantitative antibody results among those testing positive (Table 4).
Limitations of our study included participation of only half of all employees and affiliated clinicians. Those who suspected current or prior infection may have been more likely to enroll, whereas ill employees with active infection could not attend work and were excluded; thus, the true prevalence of SARS-CoV-2 infections among all employees and clinicians may have been lower or higher than observed. Despite the higher infection rates in those providing patient care, we cannot determine whether infections were contracted in the hospital or community setting. Our measures of socioeconomic status were limited to race/ethnicity and job role; the possibility of residual confounding by additional socioeconomic factors remains. Moreover, because most of the positive cases were identified through antibody testing, which reflects a broader past exposure window than viral testing, our assessment of recent exposures and behaviors (eg, work locations, recent PPE use, and outside infectious contacts in the prior 2 weeks) was not considered informative and thus excluded from analyses. Analyses of factors associated with antibody levels may have been limited by small sample sizes. Other limitations were the accuracy of self-reported data and self-collected nasal swabs.
CONCLUSIONS
In conclusion, in this large hospital-based screening in a US SARS-CoV-2 early US epicenter, we observed that ~10% of HCWs had evidence of active or prior SARS-CoV-2 infection. As expected, those with patient care roles had greater likelihood of infection, but absolute infection rates were highest in black and Latinx HCWs and in those with lower-income support roles. These findings highlight potential opportunities for educational interventions to better protect workers across all job roles as well as foster safe practices outside the hospital.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the many individuals at Rutgers University and Robert Wood Johnson Medical School who contributed to this screening study including Angelo Chaia, Erin McDonnell, Byron Avihai, Carolayn Munoz, Amy Jablonski, Jonathan Bodnar, Mary Kelso, Pat Ondar, Karen Shepherd, Alejandro Morales, Helaine Novek, Judith Argon, Matthew Leonardelli, Angel Chen, and Michael Hoven. Finally, we thank the Robert Wood Johnson University Hospital workers serving on the frontlines of the pandemic.
Financial support. This work was funded by the National Institutes of Health (3U01AI122285-05S1, U01HL133817, UL1TR003017 NCATS, 3UL1TR003017-02S1, UH3AI122309, P30ES005022, R01HL149450, R01HL149450-02S1, K23AR070286).
Potential conflicts of interest. R. A. P. reports the following disclosures: AstraZeneca (consultant/advisory board, research grant, speaker), Medimmune (consultant/advisory board, research grant), RIFM (consultant/advisory board, research grant), Equilium (consultant/advisory board), Theravance (consultant/advisory board), Avilion (consultant/advisory board), Sonfi/Regeneron (speaker), Genentech (speaker, research grant), Novartis (research grant), Optikira (research grant), Metera (research grant). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2-infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. BMC Infectious Diseases 2020; 20: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020; 1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020; 9:e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ran L, Chen X, Wang Y, et al. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020; 2218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mani NS, Budak JZ, Lan KF, et al. Prevalence of COVID-19 infection and outcomes among symptomatic healthcare workers in Seattle, Washington. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020; 324:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus map: tracking the global outbreak. The New York Times, Published 27 August 2020. Available at: https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html. Accessed 27 August 2020.
- 9. Mansour M, Leven E, Muellers K, et al. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med 2020; 35:2485–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morcuende M, Guglielminotti J, Landau R. Anesthesiologists’ and intensive care providers’ exposure to COVID-19 infection in a New York City academic center: a prospective cohort study assessing symptoms and COVID-19 antibody testing. Anesth Analg 2020; 131:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vahidy FS, Bernard DW, Boom ML, et al. Prevalence of SARS-CoV-2 infection among asymptomatic health care workers in the greater Houston, Texas, area. JAMA Netw Open 2020; 3:e2016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA 2020; 324:893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. New Jersey coronavirus map and case count. The New York Times, Published 27 August 2020. Available at: https://www.nytimes.com/interactive/2020/us/new-jersey-coronavirus-cases.html. Accessed 27 August 2020.
- 14. KFF. Confirmed COVID-19 cases and deaths. Available at: https://www.kff.org/other/state-indicator/confirmed-covid-19-cases-and-deaths. Accessed 27 August 2020.
- 15. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. Emergency use authorization: Emergency Use Authorization (EUA) information and list of all current EUAs. Available at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#LDTs. Accessed 27 August 2020.
- 17. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott ARCHITECT SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020; 58:e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30:377–99. [DOI] [PubMed] [Google Scholar]
- 19. Poulson M, Geary A, Annesi C, et al. National disparities in COVID-19 outcomes between black and white Americans. J Natl Med Assoc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol 2020; 48:23–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vahidy FS, Nicolas JC, Meeks JR, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 2020; 10:e039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol 2020; 128:104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt SB, Grüter L, Boltzmann M, Rollnik JD. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS One 2020; 15:e0235417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia 2020; 75:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020; 9:e60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shields AM, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax 2020; 75:1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lombardi A, Consonni D, Carugno M, et al. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect 2020; 26:1413.e9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc 2020; 95:1621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reifer J, Hayum N, Heszkel B, et al. SARS-CoV-2 IgG antibody responses in New York City. Diagn Microbiol Infect Dis 2020; 98:115128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lynch KL, Whitman JD, Lacanienta NP, et al. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fill Malfertheiner S, Brandstetter S, Roth S, et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: a prospective longitudinal study. J Clin Virol 2020; 130:104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.