Abstract
Background
Assessment of the impact of cerebrospinal fluid (CSF) analysis including investigation for the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is essential for the optimization of patient care.
Methods
In this case series, we review patients diagnosed with SARS-CoV-2 undergoing lumbar puncture (LP) admitted to Columbia University Irving Medical Center (New York, NY, USA) from March 1 to May 26, 2020. In a subset of patients, CSF SARS-CoV-2 quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) testing is performed.
Results
The average age of 27 patients who underwent LP with definitive SARS-CoV-2 (SD) was 37.5 (28.7) years. CSF profiles showed elevated white blood cell counts and protein in 44% and 52% of patients, respectively. LP results impacted treatment decisions in 10 (37%) patients, either by change of antibiotics, influence in disposition decision, or by providing an alternative diagnosis. CSF SARS-CoV-2 qRT-PCR was performed on 8 (30%) patients, with negative results in all samples.
Conclusions
Among patients diagnosed with SARS-CoV-2, CSF results changed treatment decisions or disposition in over one-third of our patient cohort. CSF was frequently abnormal, though CSF SARS-CoV-2 qRT-PCR was negative in all samples. Further studies are required to define whether CSF SARS-CoV-2 testing is warranted in certain clinical contexts.
Keywords: cerebrospinal fluid (CSF), lumbar puncture, neuroinvasion, SARS-CoV-2
The global pandemic of the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in >34 million cases worldwide, with >1 million deaths as of October 2, 2020 [1]. There are growing reports of neurological symptoms and conditions seen in the context of coronavirus disease 2019 (COVID-19), including headaches, encephalopathy, seizures, cerebrovascular accidents, acute hemorrhagic necrotizing encephalopathy (AHNE), and Guillain-Barre syndrome (GBS) [2–15]. A major knowledge gap exists with regards to the frequency and mechanisms of neurological injury in COVID-19 patients. Whether these conditions are due to systemic effects of COVID-19 including multiorgan damage, coagulopathy, and systemic inflammation or whether direct viral neuroinvasion contributes remains unknown [16].
Little is known regarding the role of lumbar puncture (LP) in COVID-19 patients with neurological symptoms. Few case reports have identified SARS-CoV-2 in the cerebrospinal fluid (CSF) by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) or sequencing techniques [14, 17, 18]. There are several recent case studies of presumed meningoencephalitis, GBS, AHNE, and strokes in COVID-19 patients who had negative CSF SARS-CoV-2 qRT-PCR results [6, 10, 12, 19]. This may represent a lack of viral presence in CSF or may be impacted by factors such as timing of testing within the natural course of COVID-19 and CSF sampling techniques.
The clinical indications for performing LPs in patients with SARS-CoV-2 infection remain unclear, and decisions are complicated by limitations in personal protective equipment (PPE) during hospital surges in cases and the potential exposure of health care workers. Additionally, how clinicians can use information gained from LPs, such as cell counts and infectious workup, in the management of COVID-19 and neurological symptoms has not been established. Here, we describe how results of LP influence clinical decision-making in a case series of patients with COVID-19 and assess whether SARS-CoV-2 is detected in the CSF by qRT-PCR in a subset of patients undergoing LP for clinical purposes.
METHODS
Patient Consent Statement
Before initiation of data collection, the study was approved by the CUIMC Institutional Review Board. The study was approved as medical chart review and CSF collection and CSF testing for SARS CoV-2 rtPCR on CSF specimens obtained for clinical purposes, and the requirement for obtaining written informed consent was waived.
We performed a retrospective analysis of patients admitted to New York Presbyterian–Columbia Irving Medical Center (CUIMC; New York, NY, USA) between March 1, 2020, and May 26, 2020. All patients meeting Centers for Disease Control and Prevention (CDC) criteria for confirmed COVID-19 infection (ie, patients with a positive SARS-CoV-2 RNA amplification test via nasopharyngeal swab) who underwent lumbar puncture during the same admission were included [20]. Patients were excluded if LP was therapeutic (ie, intrathecal chemotherapy) or if LP was carried out during a different admission from nasopharyngeal (NP) SARS-CoV-2 qRT-PCR-positive testing result. Demographic, clinical, and laboratory data including indication for LP and CSF results, treatment, and patient outcome (alive, deceased, or remains admitted) were collected through electronic medical record (EMR) review. The patient cohort included adults (>18 years of age) as well as pediatric patients. The effect of LP on clinical management was determined by clinician review of change in antimicrobials (addition or removal), ordering of additional tests, and change in disposition in direct response to LP results by review of an infectious diseases physician (E.H.M.) and a neurologist (K.T.T.).
CSF samples were collected by clinical teams performing LPs for clinical purposes. For qRT-pCR testing, DNA/RNA Shield (Zymo) was added in a 1:1 ratio to CSF after collection to inactivate any virus that could have been present. RNA from samples was extracted using the RNeasy Mini Kit (Qiagen). Quantitative RT-PCR was performed on extracted RNA using Taqman 4X master mix and SARS-CoV-2 primer/probe sets (IDT) to detect presence of virus [21, 22]. Each assay included a standard curve to determine the viral load (log10 copies/mL).
Patient characteristics were summarized using frequency and percentage for categorical variables and mean/standard deviation or median/range for continuous variables when evaluating the characteristics of the entire population.
RESULTS
During the study period, 27/3598 (1%) patients admitted with COVID-19 underwent LP at our institution. The average age of patients (SD) was 37.5 (28.7) years, with 10 pediatric patients (70% <3 months of age) (Table 1). Twenty-one (78%) were male, and 11 (41%) were Hispanic. Five (19%) had neurological comorbidities including prior stroke (1, 4%), epilepsy (3, 11%), and cognitive impairment (1, 4%). On admission, fever was the most common presenting symptom, occurring in 13 patients (48%), followed by altered mental status (22%), headache (15%), dyspnea (7%), anosmia (6%), and psychosis, occurring in 1 patient (4%). Eleven (41%) patients, including 1 pediatric patient, presented with predominately neurological symptoms, including altered mental status, focal motor or sensory deficits, and seizures. Six (22%) patients presented with symptoms consistent with COVID-19 including fever, cough, and/or shortness of breath. Within the pediatric patient cohort, the most common presenting symptom was fever (8, 80%). COVID-19 testing was done on all patients being admitted to the hospital at this time, regardless of presenting symptoms.
Table 1.
Patient Characteristics
| Pt. a | Sex | Agea | Race/Ethnicity | Symptoms on Presentation | Medical Comorbidities | Inflammatory Markers on Admissionc | Underlying Neurological Conditions | Indication for LP | Outcomeb |
|---|---|---|---|---|---|---|---|---|---|
| Adult patients (age >18 y) | |||||||||
| 1 | M | 61 | Hispanic | Fatigue, headache, SOB | Asthma, HTN, DM type II | IL6 79, ferritin 199, ESR 118, CRP 153, D-dimer 0.9 | None | Anisocoria, infarct seen on HCT | Still admitted |
| 2 | M | 64 | Hispanic | Left facial droop, left arm weakness, AMS | CKD, DM type II, schizophrenia | IL6 27, ferritin 450, ESR 102, CRP 300, D-dimer 20 | None | Concern for CNS infection | Discharged |
| 3 | M | 39 | Hispanic | SOB, fever worsening for 1 wk | Coarctation of the aorta, endocarditis | IL6 501, ferritin 2304, ESR 92, CRP 263, D-dimer 5.5 | None | Flaccid quadriplegia | Deceased |
| 4 | M | 63 | Caucasian | Aphasia, AMS | HTN, CAD, CKD | IL6 1.7, ferritin 1643, ESR 31, CRP 1.4, D-dimer 2.4 | None | Concern for CNS infection | Discharged |
| 5 | M | 65 | Hispanic | Abd. pain, fever, cough | HTN, DM | IL6 22, ferritin 1815, ESR 71, CRP 249, D-dimer 0.4 | None | Concern for CNS infection | Deceased |
| 6 | M | 80 | African American | Aphasia, AMS | HTN | IL6 19, ferritin 1462, ESR 75, CRP 138, D-dimer 20 | None | AMS | Discharged |
| 7 | M | 44 | Hispanic | Urinary retention, lower limb weakness and numbness, slurring of speech | None | IL6 1.6, ferritin 1507, ESR 34, CRP 1.6, D-dimer 2.8 | None | Transverse myelitis | Discharged |
| 8 | M | 39 | Hispanic | Fever, headache, stiff neck, fatigue | None | IL6 91, ferritin 512, ESR 39, CRP 77, D-dimer 0.3 | None | Concern for meningitis | Discharged |
| 9 | M | 47 | Hispanic | Nausea, vomiting headache, and fever | None | IL6 48, ferritin 733, ESR 130, CRP 85, D-dimer 20 | None | EVD placed for hydrocephalus | Deceased |
| 10 | F | 62 | Hispanic | AMS, headache, urinary incontinence | HTN | IL6 8.9, ferritin 257, ESR 40, CRP 4.8, D-dimer 0.3 | None | Concern for ventriculitis in setting of EVD | Still admitted |
| 11 | M | 65 | Caucasian | Fever, fatigue, generalized body weakness | HTN, HLD, VF arrest | IL6 8.0, ferritin 739, ESR 58, CRP 281, D-dimer 2.8 | None | Concern for meningitis | Discharged |
| 17 | F | 70 | Hispanic | AMS | None | IL6 44, ferritin 1524, ESR 130, CRP 273, D-dimer 2.3 | Dementia | AMS, agitation | Discharged |
| 21 | F | 61 | African American | AMS, fever, SOB | DM, hypothyroidism, CKD, NASH, s/p liver transplant | IL6 158, ferritin 1206, ESR 83, CRP 300, D-dimer 2.8 | Epilepsy | Concern for CNS infection | Discharged |
| 23 | M | 61 | NR | Fever, AMS, hallucinations, paranoia | HIV, HTN | IL6 8.0, ferritin 732, ESR 119, CRP 50, D-dimer 6.2 | Stroke | AMS | Discharged |
| 24 | F | 57 | NR | AMS, loss of appetite | DM, obesity | IL6 16, ferritin 2470, ESR 30, CRP 141, D-dimer 20 | None | AMS | Discharged |
| 25 | M | 52 | Hispanic | Left-sided weakness | None | IL6 22, ferritin 154, ESR 11, CRP 3.4, D-dimer 7.1 | None | Concern for ventriculitis | Still admitted |
| 27 | M | 56 | NR | Fever, cough, myalgia | None | IL6 15, ferritin 6478, ESR 39, CRP 94, D-dimer 0.5 | None | Concern for CNS infection | Discharged |
| Pediatric patients (birth to 18 y) | |||||||||
| 12 | M | 11 | African American | Fever | None | IL6 158, ferritin 574, ESR 56, CRP 290, D-dimer 2.4 | Febrile seizures | Fever | Discharged |
| 13 | M | 6 wk | Caucasian | Fever, seizure | None | Not sent | None | Seizures/fever in neonate | Discharged |
| 14 | M | 8 | Hispanic | Status epilepticus | None | Ferritin 33, CRP 6.6, D-dimer 0.4 | Epilepsy | Seizures | Discharged |
| 15 | M | 16 d | Hispanic | Fever, lethargy | None | CRP 2.6 | None | Fever in neonate | Discharged |
| 16 | M | 6 d | Other | Jaundice, lethargy | None | Not sent | None | Concern for meningitis | Discharged |
| 18 | M | 17 d | Caucasian | Fever | None | CRP 0.8 | None | Fever in neonate | Discharged |
| 19 | M | 11 d | Caucasian | Cough, fever | None | CRP 1.1 | None | Fever in neonate | Discharged |
| 20 | F | 3 mo | Caucasian | Lethargy, fever | None | CRP 0.4 | None | Fever | Discharged |
| 22 | F | 15 d | Other | Fever, nasal congestion | None | Not sent | None | Fever in neonate | Discharged |
| 26 | M | 6 | Caucasian | Fever, rash, swollen lips | None | Ferritin 379, CRP 0.7, D-dimer 0.8 | None | Concern for elevated intracranial pressure in setting of MIS-C | Discharged |
Abbreviations: AMS, altered mental status; CAD, coronary artery disease; CKD, chronic kidney disease; CNS, central nervous system; CRP, C-reactive protein; DM, diabetes mellitus; ESR, estimated sedimentation rate; HLD, hyperlipidemia; HOCM, hypertrophic obstructive cardiomyopathy; HTN, hypertension; IL6, interleukin-6; MIS-C, multsystem inflammatory syndrome in children; NASH, nonalcoholic steatohepatitis; NR, not reported.
aIn years unless otherwise indicated.
bAs of June 15, 2020.
cIL6: pg/mL; ferritin: ng/mL; ESR: mm/h; CRP: mg/L; D-dimer: μg/mL.
LPs were performed due to clinical concern for central nervous system (CNS) infection given clinical symptoms of altered mental status (5, 19%), clinical symptoms or signs of meningitis or encephalitis (6, 22%), and seizures/status epilepticus (3, 11%). The remaining 13 patients had LPs performed for a variety of indications including EVD placement/concern for ventriculitis (3, 11%), other neurological symptoms such as transverse myelitis and flaccid quadriplegia (3, 11%), fevers in neonates or young children (6, 22%), and multisystem inflammatory syndrome in children (1, 4%) (Table 1). In adult patients, the average duration of symptoms (neurological and/or systemic) before LP (range) was 11.5 (2–>30) days. In the adult cohort, duration of symptoms before presentation could not be obtained for 3 patients due to altered mental status at the time of presentation with no documentation of collateral history. Five adult patients had >30 days between reported onset of symptoms and LP, as 4 (22%) patients initially presented with predominately COVID-19-related respiratory symptoms. For all patients included in the study, the average time from last positive NP swab for SARS-CoV-2 to LP (range) was 8 (0–38) days. In the majority of pediatric patients (8, 80%) who presented with fevers, LP was performed on the same day as the positive SARS-CoV-2 test and on the same day as symptom onset.
Neuroimaging was performed in 18 cases (67%), with 5 cases (27%) notable for acute or subacute ischemic strokes (1 with hemorrhagic conversion), 6 (33%) with intracranial hemorrhages, 2 (11%) with microhemorrhages, 1 (6%) with evidence of kinking of the orbital nerves, 2 (11%) with evidence of focal parenchymal FLAIR/T2 hyperintensities, 2 (11%) with dural enhancement, and 1 (6%) with leptomeningeal enhancement (Figure 1; Supplementary Table 1). Continuous electroencephalogram (EEG) was performed in 14 (52%) patients, with diffuse slowing recorded in 11 (79%) and focal slowing in 1 (7%), and 2 (14%) had evidence of electrographic seizures.
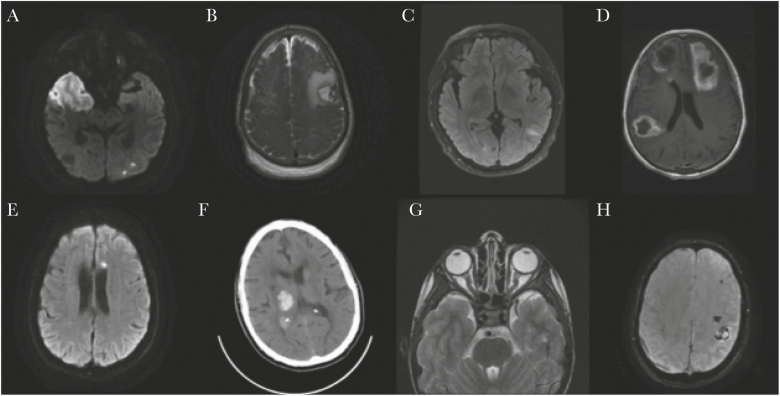
Figure 1.
Select neuroimaging findings. A, Patient 2: MRI of the brain without contrast, axial DWI image showing diffusion restriction in the right middle cerebral arteries and left posterior cerebral artery territories. B, Patient 5: MRI of the brain with and without contrast, axial T2 weighted image showing left frontal parenchymal hemorrhage with surrounding mass effect and nonspecific mild diffuse dural thickening. C, Patient 7: MRI of the brain with and without contrast, axial fluid attenuated recovery image showing left parietal juxtacortical white matter hyperintensity. D, Patient 17: MRI of the brain with and without contrast, axial T1 postcontrast image showing multifocal hypointensities with surrounding enhancement in the left frontal lobe, right temporoparietal lobe, and inferior right frontal lobe with surrounding vasogenic edema. E, Patient 23: MRI of the brain with and without contrast, axial DWI image showing subacute infarct in the left frontal centrum semiovale. F, Patient 25: CT of the head without contrast, axial image showing right thalamic intraparenchymal hemorrhage with associated mass effect on the right lateral ventricle. G, Patient 26: Axial MRI orbits with and without contrast: T2 weighted image showing mild kinking of the intraorbital optic nerves and mildly distended optic nerve sheaths bilaterally. H, Patient 27: Axial MRI of the brain without contrast: susceptibility weighted imaging showing hemorrhagic infarct of the left perirolandic region. Subacute subdural hematoma at the right frontoparietal lobe. Abbreviations: CT, computed tomography; DWI, diffusion weighted imaging; MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging.
One patient who presented with occipital headache and fever was positive on the Biofire meningitis-encephalitis (ME) PCR panel for Streptococcus pneumoniae (Table 2) as well as for COVID-19 on NP swab [23]. Ten patients (37%) had bloody CSF samples with >1000 red blood cells (RBC)/mm3, including 4 patients found to have intracranial hemorrhages using CSF from 3 of the patients drawn from external ventricular devices (EVDs). An elevated CSF white blood cell (WBC) count >5/mm3 after correction for CSF RBCs was seen in 12 (44%) patients, including the 3 patients for whom CSF was sampled from an EVD. Of the remaining 9 patients with elevated CSF WBC count on LP, 4 are pediatric patients (3 presented with fever, 1 presented with seizures). The remaining 4 patients are adults, 3 of whom presented with primarily neurological symptoms including weakness, slurred speech, and altered mental status. The final patient presented with symptoms consistent with COVID-19, including abdominal pain, cough, and fever. Protein was also elevated above normal range (>45 mg/dL) in 14 (52%) patients. CSF glucose (corrected for serum glucose) was low (<50 mg/dL) in 2 patients including the patient with S. pneumoniae meningitis who had a CSF glucose of 8 mg/dL. CSF was available for SARS-CoV-2 qRT-PCR in 8 (30%) COVID-19 patients who underwent LP, with negative results in all samples. CSF SARS-CoV-2 testing was performed on 3 additional patients meeting possible or probable criteria for COVID-19, with negative results (Supplementary Table 2). LP analysis resulted in a change in treatment or disposition in 10 (37%) of the patients. This included 4 pediatric cases for fever in an infant where negative cultures and ME panel from LP allowed antibiotics to be stopped and the infants to be discharged shortly after LP results were obtained. In the remaining 6 cases, the LP resulted in change or removal of antibiotic therapy or, in the case of the patient with S. pneumoniae meningitis, a definitive diagnosis for the presenting symptoms being made.
Table 2.
Cerebrospinal Fluid Findings
| Pt No. | Days From Last + COVID NP PCR | Days From Symptom Onset to LP | CSF WBC/mm3,c Differential (%) | CSF RBC, /mm3 | Protein, mg/dL | CSF Glucose, mg/dLb | CSF COVID-19 PCR | CSF Culture/ME Panel and Other Etiological Investigationsd |
|---|---|---|---|---|---|---|---|---|
| Adult patients (age >18 y) | ||||||||
| 1 | 3 | 31 | 1 | 55 | 37 | 98 | N/A | Negative/negative |
| 94L 6M | ||||||||
| 2 | 4 | Unknown | 30 | 27 | 69 | 118 | Negative | Negative/negative |
| 91N 3L 6M | ||||||||
| 3 | 34 | 36 | Hemorrhagic | >2000 | >2000 | 133 | N/A | Negative/none |
| 4 | 8 | 15 | 2 | 49 | 35 | 91 | N/A | Negative/negative |
| 8N, 90 L | ||||||||
| 5 | 29 | 37 | 22 | 13 000 | 58 | 78 | N/A | Negative/negative |
| 83N, 6L, 2M, 3E | ||||||||
| 6 | 0 | 11 | 1 | 0 | 95 | 104 | N/A | Negative/negative |
| 100 L | ||||||||
| 7 | 2 | 4 | 6 | 25 | 36 | 85 | Negative | Negative/negative |
| 8 | 1 | 4 | 11 768 | 1000 | 544 | 8 | N/A | Negative/ME panel positive for S. pneumoniae |
| 9a | 2 | 12 | 112 | 235 000 | >2000 | 48 | Negative | Negative/none |
| 14N, 63L, 22M | ||||||||
| 10a | 1 | 1 | 10 | 2000 | 126 | 66 | Negative | Negative/none |
| 8N, 82L, 8M, 2E | ||||||||
| 11 | 34 | 45 | 2 | 38 | 24 | 61 | N/A | Negative/negative |
| 30N, 60L, 10N | ||||||||
| 17 | 38 | 42 | 435 | 2000 | 110 | 85 | – | Negative/negative |
| 70N, 16M, 14L | ||||||||
| 21 | 30 | 46 | 3 | 0 | 38 | 103 | N/A | Negative/negative |
| 100M | ||||||||
| 23 | 3 | 10 | 2 | 0 | 42 | 62 | Negative | Negative/negative |
| 4N, 88L, 8M | ||||||||
| 24 | 1 | Unknown | 7 | 2000 | 56 | 138 | N/A | Negative/negative |
| 31N, 46L, 23M | ||||||||
| 25a | 1 | 1 | 22 | 16 000 | 225 | 79 | N/A | Negative/none |
| 14N, 54L, 28M, 4B | ||||||||
| 27 | 2 | 29 | 1 | 60 | 54 | 60 | Negative | Negative/negative |
| 10N, 70L, 20M | ||||||||
| Pediatric patients (birth to 18 y) | ||||||||
| 12 | 0 | 4 | 3 | 0 | 20 | 83 | N/A | Negative/negative |
| 38N, 39L, 15M, 1E | ||||||||
| 13 | 0 | 0 | 2 | 1 | 40 | 50 | N/A | Negative/negative |
| 80M, 20L | ||||||||
| 14 | 1 | 1 | 6 | 32 | 14 | 65 | N/A | Negative/negative |
| 8N, 48L, 44M | ||||||||
| 15 | 10 | 1 | 7 | 4000 | 86 | 43 | N/A | Negative/negative |
| 19N, 23L, 55M, 3E | ||||||||
| 16 | 0 | 0 | 4 | 1 | 64 | 55 | N/A | Negative/negative |
| 6N, 94M | ||||||||
| 18 | 0 | 0 | 1 | 61 | 51 | 48 | N/A | Negative/negative |
| 4N, 52L, 44M | ||||||||
| 19 | 0 | 0 | 8 | 6000 | 129 | 50 | N/A | Negative/negative |
| 19N, 38L, 41M, 2E | ||||||||
| 20 | 0 | 1 | 4 | 1 | 44 | 71 | N/A | Negative/negative |
| 100M | ||||||||
| 22 | 0 | 0 | 6 | 28 | 41 | 50 | N/A | Negative/negative |
| 2N, 23L, 75M | ||||||||
| 26 | 1 | 13 | 2 | 56 | 16 | 68 | Negative | Negative/negative |
| 12N, 60L, 28M |
Abbreviations: B, basophils; CSF, cerebrospinal fluid; COVID-19, coronavirus disease 2019; E, eosinophils; L, lymphocytes; M, monocytes; ME panel, BioFire Meningitis/Encephalitis PCR Panel; N, neutrophils; NP, nasopharyngeal; PCR, polymerase chain reaction; RBC, red blood cells; WBC, white blood cells.
aCSF sample taken from external ventricular drain.
bCSF glucose corrected for serum glucose.
cCSF WBC corrected for CSF RBC (1 WBC:700 RBC).
dOther CSF studies negative unless otherwise indicated.
COVID-19 directed experimental and off-label therapies were used in a number of adult patients (n = 18). Four (22%) received hydroxychloroquine, 2 (11%) received remdesivir (either via compassionate use or through clinical trial), 4 (22%) received off-label tocilizumab, 4 (22%) received 5-day courses of methylprednisolone, and 2 (11%) patients received intravenous immunoglobulin (IVIG). Within this group, 5 (27%) were treated with multiple COVID-19 directed therapies during their hospitalization. Of all patients included in the study, 3 (11%) died, 3 (11%) are still admitted in critical condition due to complications of COVID-19 (1) or complications from intracranial hemorrhage (2), and 21 (78%) were discharged. Of the 3 patients who died, 2 died from complications of intracranial hemorrhages, and 1 died after a prolonged course complicated by hypoxemic respiratory failure due to COVID-19.
DISCUSSION
Here, we describe a case series of COVID-19 patients who underwent LP as part of diagnostic workup at a single academic medical center. A subset of patients had CSF tested for SARS-CoV-2 by qRT-PCR; all of these tests were negative. Most LPs were performed for altered mental status and concern for CNS infection—both viral and bacterial. Many patients included in this case series had complicated and prolonged hospital courses. Most pediatric patients were under 3 months of age and had an LP performed early in their course in the context of fever to rule out bacterial or viral meningoencephalitis. LP results changed management in 10/27 patients. In 1 case, LP provided an alternative diagnosis of S. pneumoniae meningitis and thus need for continued and targeted antibiotic therapy. In the other cases where LP changed treatment, antibiotics and antivirals were either able to be stopped completely or narrowed to no longer include CNS coverage. In pediatric patients, LP data often helped facilitate a rapid discharge by ruling out bacterial or viral meningoencephalitis.
In this case series, a subset of patients had CSF tested for the presence of SARS-CoV-2 by qRT-PCR, and all samples tested were negative for the virus. This is consistent with recent case series, which included CSF testing for SARS-CoV-2 by qPCR, in which samples also largely tested negative [18, 24, 25]. Combined, these results suggest that SARS-CoV-2 is unlikely to be present in CSF in the majority of patients. However, several factors may have precluded detection of the virus in CSF. First, the duration of viral illness before LP may be a factor. Prior studies have shown that in the respiratory tract viral levels are highest in the first week of infection [26]. If virus is present in the CSF, it may be present very early on in the disease when viral loads are highest, and therefore timing of the LP would be essential to detect virus in CSF. In the case series from Neumann et al., the average time from positive nasopharyngeal SARS-CoV-2 test to LP (SD) was 5.9 (9.8) days [18]. In this case series, 3 patients had an LP performed within 5 days of their reported onset of symptoms, but the remaining patients tested had a longer time period between reported symptom onset and LP. CSF samples may have been falsely negative for SARS-CoV-2 due to extended periods of time between onset of symptoms and performance of LP, as the viral kinetics in CSF are currently unknown and require further study. Presenting symptoms in many cases are not classic COVID-19-related symptoms (ie, fever, cough, dyspnea); many adult patients present with neurological symptoms such as weakness. Some patients in this study had symptoms that could overlap with both COVID-19 and a primary neurological condition such as headache or fever. Additionally, several patients presented with altered mental status, making it difficult to determine how long symptoms had been present before presentation. Many of these patients had predominately neurological rather than respiratory symptoms at the time of LP, raising the concern for possible COVID-19-related neurological diseases or that neurological symptoms may mask more typical symptoms of COVID-19. In these patients, CSF profiles were abnormal in a majority; however, the role of LP in the diagnosis of potential COVID-19-related CNS disease remains unclear.
Other factors that could have contributed to the negative CSF findings may be related to the test itself. Degradation of viral particles and RNA before qRT-PCR testing could be a factor. CSF for this study was placed in DNA/RNA stabilizing buffer, to preserve any viral RNA present, but time from sample collection to addition of stabilizing buffer varied from case to case. While qRT-PCR is a validated and widely used test for detection of virus in respiratory samples, its use in CSF samples is less well defined. It is possible that an alternative test, such as SARS-CoV-2 antibody testing in CSF, may be a better test of prior viral presence in the CSF, though this remains to be defined. At the time of the study, we did not have the ability to perform CSF antibody testing. Absence of virus in the CSF does not rule out neurotropism of the virus. Recent neuropathological studies of COVID-19 patients have identified viral RNA by qRT-PCR in the brain, though inconsistently, and typically with low viral loads of uncertain clinical significance when reported [27–29].
While SARS-CoV-2 was not detected in CSF samples by qRT-PCR in our small cohort, many patients included in this study had abnormal CSF profiles. In the majority of adult cases, the infectious workup done on the CSF (bacterial gram stain and culture, Biofire ME panel, and other CSF studies) was negative. In these cases, the inflammatory nature of the CSF profile is of particular interest. A significant number of patients had ischemic strokes or intracerebral hemorrhages (ICHs) with history or radiographic pattern concerning for possible CNS infection. Patients presenting with ischemic or hemorrhagic stroke typically have LPs if there is radiographic or clinical concern for vasculitis/vasculopathy or the patient is immunocompromised [30]. Though data are limited, in patients with ischemic strokes, the CSF WBC count can be mildly elevated in the first week with elevated protein, though rarely above 100 mg/dL, in about half of cases [30]. In patients with ICH, CSF profiles can vary significantly and can show a neutrophilic pleocytosis and highly elevated protein, up to 2000 mg/dL, based on limited data [30]. Three patients in this case series had EVDs placed for ICH. Collection of CSF from EVDs may lead to an elevated WBC in CSF and may have accounted for some of the observed increases.
A subset of patients with COVID-19 go on to develop a systemic hyperinflammation syndrome [31]. It is possible that the inflammatory profile of the CSF seen in some of these patients indicates COVID-19-related inflammation. This continues to leave open the possibility that COVID-19 infection may have contributed to the neurological symptoms seen in these patients. As virus was not detected in the CSF, other tests, such as presence of other inflammatory markers in the CSF, may help elucidate the role SARS-CoV-2 may play in these neurological findings.
The strengths of this study include the ability to collect detailed clinical, imaging, laboratory, and EEG data for all patients, presenting a more comprehensive picture of COVID-19 patients undergoing LP. We also report results of 8 SARS-CoV-2 CSF qRT-PCRs from patients who had LPs for a variety of reasons. Additional qRT-PCRs were unable to be performed due to the challenging nature of collecting and preserving samples for research at the height of the COVID-19 surge in New York City and the retrospective case series nature of this type of study. Limitations include that this was a single-institution retrospective case series with a relatively small samples size of LPs compared with the total number of COVID-19 patients admitted to the institution over this time. During this time, the ability to perform LPs was limited due to the clinical volume during the COVID-19 surge in New York City. Additionally, neurological manifestations of COVID-19 may be difficult to detect in patients who are intubated and sedated, leading to fewer LPs being done. A larger evaluation for the extent of possible neurological manifestations of COVID-19 patients in the full patient cohort is needed. Elevated WBC counts were seen in the CSF of 12 patients; however, 3 of these (25%) were taken from external ventrticular drains, the presence of which may have resulted in CSF leukocytosis. Additionally, SARS-CoV-2 qRT-PCR was unable to be performed on CSF samples from all patients included in this case series.
Given the broad spectrum of neurological findings described in this case series, the question of when to obtain an LP in COVID-19 patients remains important. Prior recommendations suggest that LPs be performed in COVID-19 patients where there is concern for encephalitis or neurological findings such as new deficits, CVAs, seizures, or delirium that cannot be explained by other causes, but indications for LP in COVID-19 patients remain challenging [32]. Our data do not support the routine testing of CSF for the presence of SARS-CoV-2; however, this case series shows that LPs were helpful in ruling out other possible causes of neurological findings in patients, specifically bacterial and viral infections. In our clinical experience, LPs may be considered if (1) there is a lack of a direct trigger for a neurological finding (ie, ICH in a patient on anticoagulation), (2) neurological findings cannot be explained by systemic infection, (3) physical examination findings are suggestive of CNS infection (ie, AMS in conjunction with meningismus), (4) specific neuroradiographic patterns are concerning for infectious or postinfectious immune-mediated process (ie, new focal flair signal in brain parenchyma and/or spinal cord or findings suggestive of vasculopathy on neuroimaging). We recommend consultation with neurology and infectious diseases services when considering LP in these patients for aid with decision-making regarding the necessity of testing and for assistance with management changes based on LP results. More studies need to be done to better characterize neurological conditions in the context of COVID-19 and to better understand which CSF profiles and studies may help in the diagnosis of COVID-19, including CSF antibody testing and inflammatory biomarkers.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. Kiran Thakur, MD, funding support from National Institute of Neurological Disorders and Stroke/National Institute of Health K23NS105935-01. Emily Happy Miller, MD, PhD, recieves funding support from National Institute of Allergy and Infectious Diseases T32AI100852-08. Eliza Miller, MD, MS, received funding support from National Institute of Neurological Disorders and Stroke K23NS107645.
Potential conflicts of interest. The authors report no conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. John Hopkins University & Medicine. Coronavirus resource center. Available at: https://coronavirus.jhu.edu/map.html. Accessed 7 July 2020.
- 2. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277:2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382:2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 2020; 91:889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 2020; 382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tunç A, Ünlübaş Y, Alemdar M, Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci 2020; 77:227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020; 19:383–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 2020; 76:233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm 2020; 7:e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol 2020; 267:1877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. In press. [DOI] [PubMed] [Google Scholar]
- 13. Dugue R, Cay-Martínez KC, Thakur KT, et al. Neurologic manifestations in an infant with COVID-19. Neurology 2020; 94:1100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020; 94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020; 296:201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koralnik IJ, Tyler KL. COVID-19: a global threat to the nervous system. Ann Neurol 2020; 88:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis 2020; 36:101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neumann B, Schmidbauer ML, Dimitriadis K, et al. ; PANDEMIC and the IGNITE study groups Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci 2020; 418:117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilotto A, Odolini S, Masciocchi S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Diseaes Control and Prevention. Coronavirus disease 2019 (COVID-19) 2020 interim case definition. Available at: https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/. Accessed 28 June 2020.
- 21. Lu X, Wang L, Sakthivel SK, Whitaker B, et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 2020; 26:1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. CDC 2019-novel coronavirus (2019-nCoV) real-time RT PCR diagnostic panel 2020. Available at: https://www.fda.gov/media/134922/download. Accessed 1 July 2020.
- 23. BioFire Diagnostics. FilmArray meningitis/encephalitis (ME) CE-IVD instruction for use. Available at: https://www.biofiredx.com/products/the-filmarray-panels/filmarrayme/. Accessed 26 June 2020.
- 24. Destras G, Bal A, Escuret V, et al. ; COVID-Diagnosis HCL Study Group Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe 2020; 1:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perchetti GA, Nalla AK, Huang ML, et al. Validation of SARS-CoV-2 detection across multiple specimen types. J Clin Virol 2020; 128:104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 27. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383:989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. 2 ed. Philadelphia: W.B. Saunders Company; 1992. [Google Scholar]
- 31. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transpl 2020; 39:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romoli M, Jelcic I, Bernard-Valnet R, et al. A systematic review of neurological manifestations of SARS-CoV-2 infection: the devil is hidden in the details. Eur J Neurol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.