Abstract
Background
Nosocomial spread of coronavirus disease 2019 (COVID-19) causes clusters of infection among high-risk individuals. Controlling this spread is critical to reducing COVID-19 morbidity and mortality. We describe an outbreak of COVID-19 in Keio University Hospital, Japan, and its control and propose effective control measures.
Methods
When an outbreak was suspected, immediate isolation and thorough polymerase chain reaction (PCR) testing of patients and health care workers (HCWs) using an in-house system, together with extensive contact tracing and social distancing measures, were conducted. Nosocomial infections (NIs) were defined as having an onset or positive test after the fifth day of admission for patients and having high-risk contacts in our hospital for HCWs. We performed descriptive analyses for this outbreak.
Results
Between March 24 and April 24, 2020, 27 of 562 tested patients were confirmed positive, of whom 5 (18.5%) were suspected as NIs. For HCWs, 52 of 697 tested positive, and 40 (76.9%) were considered NIs. Among transmissions, 95.5% were suspected of having occurred during the asymptomatic period. Large-scale isolation and testing at the first sign of outbreak terminated NIs. The number of secondary cases directly generated by a single primary case found before March 31 was 1.74, compared with 0 after April 1. Only 4 of 28 primary cases generated definite secondary infection; these were all asymptomatic.
Conclusions
Viral shedding from asymptomatic cases played a major role in NIs. PCR screening of asymptomatic individuals helped clarify the pattern of spread. Immediate large-scale isolation, contact tracing, and social distancing measures were essential to containing outbreaks.
Keywords: COVID-19, infection control strategy, nosocomial outbreak, nosocomial infection
Asymptomatic transmissions played a major role in nosocomial COVID-19 infections among patients and healthcare workers. Large-scale isolation at the first sign of outbreak, rigorous contact tracing, extensive PCR testing, and strict distancing measures contributed to its containment.
The coronavirus disease 2019 (COVID-19) pandemic has put hospitals at risk of nosocomial infection (NI) worldwide. Health care workers (HCWs) account for 4%–6% of cases in several countries [1–3]. In Japan, more than 9.4% of all COVID-19 cases were reported as NIs [4]. With its markedly higher rate of morbidity and mortality in people with underlying diseases [5], the first nosocomial outbreak in a hospital in Tokyo was in 109 patients, with 38 deaths resulting within 43 days [6]. This underscores the urgent need for preventive measures, not only to reduce severe complications and deaths, but also to preserve hospital function to provide adequate care to all patients.
Located in Tokyo, Japan, Keio University Hospital confirmed its first NI case on March 24, when an outbreak occurred in the hospital. The cumulative numbers of confirmed cases and deaths from COVID-19 in the Tokyo metropolitan area on that day were reported to be 172 and 5, respectively [7]. Within the next month, those increased to 3742 (0.27 per 1000 of the population in Tokyo) and 93 (0.007 per 1000), respectively (as of April 24). Given that governmentally sanctioned testing was limited to patients with severe symptoms or who were symptomatic for >4 days [8] and that most infected individuals remain mildly symptomatic or asymptomatic [9–11], the number of confirmed cases was in danger of being underestimated. Approximately 40% of transmissions might occur from presymptomatic cases [12, 13]; thus, growing community-wide infections could increase presymptomatic infections among HCWs and pre-admission patients and lead to outbreaks of NIs.
Identification of NI is particularly challenging for COVID-19. As SARS-CoV-2 can be transmitted during the incubation period [12], it is possible to come into contact with an infected person without being aware of it. A literature review and case series from China reported that nosocomial transmission occurred in >40% of diagnosed cases [14, 15], whereas a multicenter international cohort study in the United Kingdom and Italy estimated the proportion of NI cases at 12.5% [16]. The numbers could vary depending on the definition of NI or the number of hospitalizations for community-acquired infection (CAI), which makes it difficult to argue whether a facility has more or fewer NIs per patient population. Although there is no dispute that NIs need to be reduced, discussing actions to reduce them requires careful visualization of patient numbers, infection dynamics, and preventive measures.
Controlling nosocomial and institutional infections of COVID-19 presents a marked challenge [11, 15]; however, measures to mitigate outbreaks in large acute care hospitals have scarcely been reported. Thus, we describe our strict and immediate isolation measures, with the establishment of an in-house polymerase chain reaction (PCR) testing system with contact tracing, to control the outbreak.
METHODS
Setting
A patient without symptoms of COVID-19 was transferred on March 19 from another hospital (hospital A) for the treatment of lower limb ischemia to Keio University Hospital, a tertiary care hospital located in the central area of Tokyo, with 960 beds, 26 inpatient wards, and ~2700 HCWs. On March 23, members of the Division of Infectious Diseases and Infection Control were informed that an outbreak of COVID-19 had occurred in hospital A. The transferred patient tested positive for COVID-19 and was assumed to be the first case of COVID-19 in the presymptomatic state on March 24. Infection control measurements and contact tracing revealed multiple clusters of NIs in our hospital. The infection control team, which included infectious diseases physicians, skilled infection control specialists, and epidemiologists, acted rigorously to lead the assessment and control of the status of infection.
Case Definition and Contact Tracing
Persons under investigation (PUI) were defined as patients hospitalized between March 1 and April 24, 2020, and HCWs who worked at Keio University Hospital during the same period. We defined a confirmed case as a PUI with a positive PCR test for SARS-CoV-2 regardless of symptomatic status. In-house PCR testing was performed on all patients and HCWs clinically suspected of infection based on fever or respiratory symptoms. PCR testing was also performed on all high-risk contacts based on thorough contact tracing regardless of symptomatic status. Patients were defined as high-risk contacts if they had been examined or nursed by an infected HCW or had been admitted in the same room as an infected patient or to a ward where a nosocomial outbreak occurred. HCWs were defined as high-risk contacts if they had had exposure to an infected patient (exposure grading A–C, Supplementary Table 1), had been at a conversational distance from an infected person without either person wearing a mask for more than a few minutes, such as during breaks and meals, or belonged to a division or ward where multiple confirmed cases among HCWs were noted. From April 6, universal PCR testing on patients before hospital admission was implemented. Each case was monitored for a minimum of 2 weeks after the test was found positive until May 8, 2020.
Cases were classified according to the maximum observed severity: asymptomatic, mild, severe, and critical [17]. Mild cases presented with fever and respiratory symptoms (cough, sputum, or sore throat). Severe cases were those with oxygen saturation of ≤93% in ambient air and requiring oxygen administration. Critical cases were defined as having respiratory failure requiring mechanical ventilation.
Confirmed cases were assigned to 1 of 4 groups according to their status (patients/HCWs) and place of virus transmission (community-acquired/nosocomial). Patients were defined as having NI if they developed symptoms, and their specimens were collected after the fifth day of admission, which is the median incubation period of COVID-19 [18, 19]. All remaining patient cases were defined as having community-acquired infections (CAIs), except those with a history of hospitalization within 2 weeks (unclassifiable cases). HCWs were defined as having NI if they were high-risk contacts, as defined above, and CAI if they had close contacts with positive cases outside of our hospital. If the links were unknown, or both NI and CAI were probable for HCWs, we defined them as unclassifiable. Identification of infection sources was based on the information gathered from thorough contact tracing using structured questionnaires (Supplementary Table 2) or telephone interviews, if needed, dating back to 1 month before the positive date. When we suspected that a case had initiated a chain of nosocomial transmission, we considered the case to be the primary case, and the accumulation of those NI cases was determined to be a cluster.
Presumptive sources of infection were classified as symptomatic, presymptomatic, or never-symptomatic based on the symptoms at the time of transmission. Asymptomatic sources were classified as presymptomatic if they developed symptoms within 2 weeks of contact. If they did not develop symptoms within 2 weeks of contact, they were classified as never-symptomatic. If multiple sources are assumed possible in NIs, including sources of symptomatic transmission, we defined it as a symptomatic transmission. Information on symptoms in positive patients was collected from medical records and telephone interviews with their physicians, and that of positive HCWs was based on daily health monitoring and contact tracing.
Response Measures
Universal masking had been instituted for all HCWs since the beginning of January for seasonal influenza infection control and for all patients and visitors since the end of March. After February 17, all the HCWs were required to report fever (≥37.5°C) or respiratory symptoms to their department head as well as to Keio University Health Center, after which they were asked to self-isolate at home. Permission from the Health Center was required to return to work. From April 8, negative PCR tests were also required. From April 6, universal PCR testing on patients before hospital admission was implemented. In addition, all emergently admitted patients were routinely isolated for 2 to 7 days and relaxed according to their clinical course or condition.
After March 24, when clusters of COVID-19 had been identified, immediate isolation of the index case and thorough contact tracing were performed. Specifically, all HCWs who had had contact with a confirmed patient were assessed on their exposure level according to our exposure grading (Supplementary Table 1). A 14-day work restriction was instituted regardless of PCR test results for those without a face shield during aerosol-generating procedures. When transmission of infection was suspected within a ward, the ward was immediately closed and disinfected, and comprehensive PCR testing of all patients and HCWs assigned to the ward was conducted, followed by 14-day quarantine for HCWs irrespective of the result of PCR testing. From March 30, stringent social distancing measures were implemented (eg, HCWs were required to have meals alone).
PCR Testing for SARS-CoV-2
Real-time 1-step RT-PCR assays were performed using a BD MAX system with BD MAX TNA MMK SPC and BD MAX ExK TNA reagents (Becton Dickinson, Franklin Lakes, NJ, USA). We used 2 primer and probe sets to detect 2 regions in the SARS-CoV-2 nucleocapsid (N) gene (N1 and N2) [20]. Assays in which either N1 or N2 or both were positive before 45 cycles were judged as positive. In cases of single N1 gene positivity or unclear amplification curves, testing was repeated to avoid false-positive results [21].
Data Collection and Analysis
Data were collected ambidirectionally from March 24 as part of the nosocomial infection control program, including contact tracing. Demographic and clinical information was collected from medical records. We performed descriptive analyses for this outbreak. The number of secondary cases directly generated by a single primary case was calculated both before and after March 31, when the more rigid safety measures had been put in place. Resident physicians were assumed to represent an independent population, and the basic reproduction number (R0) as the expected number of cases directly generated by 1 case in this population was estimated using the susceptible‐exposed‐infectious‐recovered (SEIR) model [22]. Details are shown in the Appendix (Supplementary Material). Statistical analyses were performed using R.3.6.2 (R Core Team 2019, R Foundation for Statistical Computing, Vienna, Austria), except for SEIR modeling, which was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
This study was approved by the Institutional Review Board of Keio University School of Medicine (approval number 20200063).
RESULTS
Results of PCR Testing of Patients and Health Care Workers
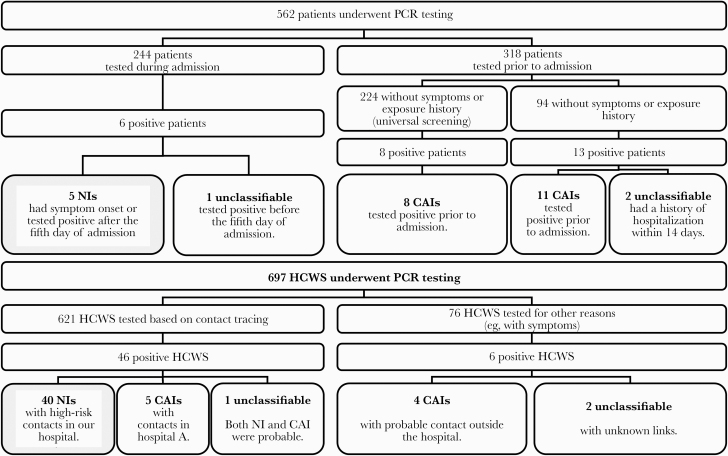
Of 562 patients tested between March 24 and April 24, 2020, 27 (4.8%) were confirmed positive (Figure 1). Five (18.5%) had NIs, 19 (70.4%) had CAIs, and the other 3 (11.1%) positive patients were unclassifiable. NI patients were confirmed positive between 9 and 86 days after admission and developed symptoms between 8 and 82 days after admission. Of 697 health care workers (HCWs) tested, 52 (7.5%) were confirmed positive. Forty (76.9%) had NIs, 9 (17.3%) had CAIs, and the other 3 (5.8%) positive HCWs were unclassifiable.
Figure 1.
Flowchart of polymerase chain reaction testing for coronavirus disease 2019 of patients and health care workers between March 24 and April 24 in our hospital. Abbreviations: CAI, community-acquired infection; HCW, health care worker; NI, nosocomial infection; PCR, polymerase chain reaction.
Presumptive sources of infection are shown in Table 1. Of the 44 transmissions, including both patients and HCWs whose presumptive sources were identified, 42 (95.5%) were transmissions from asymptomatic sources, including 19 transmissions among the resident physicians’ cluster, and 2 (4.5%) were transmissions from sources who were symptomatic but not confirmed positive at the time. Most of the asymptomatic transmissions were presymptomatic, although the resident physicians’ transmissions could not be clearly distinguished because of the group infection. Although all HCWs wore surgical masks during patient care, 9 were likely to have contracted the virus from infected patients who had not yet been confirmed positive. A total of 31 HCWs were suspected to have contracted the disease through staff-to-staff transmission, such as working in a shared medical office and taking meals in a staff lounge.
Table 1.
Presumptive Sources of Infection and Symptomatic Status of PCR-Confirmed Cases
| Patients | Health Care Workers | ||||
|---|---|---|---|---|---|
| Community-Acquired Infection | Nosocomial Infection | Community-Acquired Infection | Nosocomial Infection | ||
| (n = 19) | (n = 5) | (n = 9) | (n = 40) | ||
| Sex, No. (%) | Male | 12 (63.1) | 5 (100) | 3 (33.3) | 21 (52.5) |
| Female | 7 (36.8) | 0 (0) | 6 (66.7) | 19 (47.5) | |
| Age, mean ± SD, y | 56.9 ± 21.0 | 74.0 ± 9.2 | 31.4 ± 4.0 | 31.7 ± 10.1 | |
| 0–19 y, No. (%) | 1 (5.3) | 0 (0) | 0 (0.0) | 0 (0) | |
| 20–39 y, No. (%) | 2 (10.5) | 0 (0) | 9 (100) | 35 (87.5) | |
| 40–59 y, No. (%) | 5 (26.3) | 0 (0) | 0 (0.0) | 3 (7.5) | |
| ≥60 y, No. (%) | 11 (57.9) | 5 (100) | 0 (0.0) | 2 (5.0) | |
| Presumptive sources of infection in the hospital and their symptomatic status, No. (%) | |||||
| From a patient | - | 4 (80.0) | - | 9 (22.5) | |
| Asymptomatic | - | 4 (100) | - | 7 (77.8) | |
| Presymptomatic | 4 (100) | 7 (100) | |||
| Never-symptomatic | 0 (0) | 0 (0) | |||
| Symptomatic | - | 0 (0) | - | 2 (22.2) | |
| From a worker | - | 0 (0) | - | 31 (77.5) | |
| Asymptomatic | - | 0 (0) | - | 31 (100) | |
| Presymptomatic | - | 10 (32.2) | |||
| Never-symptomatic | - | 2 (6.5) | |||
| Unable to distinguisha | - | 19 (61.3) | |||
| Symptomatic | - | 0 (0) | - | 0 (0) | |
| Unknown | - | 1 (20.0) | - | 0 (0) | |
| Symptom status at PCR testing, No. (%) | |||||
| Symptomatic | 11 (57.9) | 4 (87.5) | 4 (44.4) | 16 (40.0) | |
| Presymptomatic | 0 (0.0) | 1 (12.5) | 0 (0.0) | 4 (10.0) | |
| Asymptomatic | 8 (42.1) | 0 (0) | 5 (55.6) | 20 (50.0) | |
Abbreviation: PCR, polymerase chain reaction.
aResident physicians were not able to distinguish the source of the infection because of the group infection.
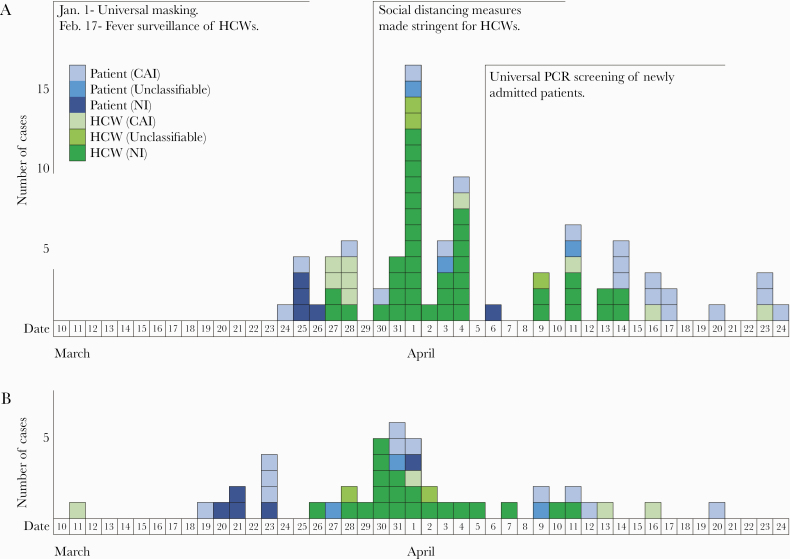
Figure 2 shows the epicurve of confirmed cases by PCR confirmation date (2a) and by illness onset for symptomatic cases (2b). Looking at the dates of PCR confirmation in chronological order, the number of cases reached a peak on April 1, then declined. No NIs were observed from April 15 to May 8.
Figure 2.
A, Epicurve of confirmed cases by date of polymerase chain reaction (PCR) confirmation and (B) symptomatic cases by date of onset for coronavirus disease 2019 outbreak at Keio University Hospital. A, Dates of PCR confirmation in chronological order show that the number of cases peaked on April 1, then decreased. B, Number of onsets in NI cases peaked on March 30, then decreased. Abbreviations: CAI, community-acquired infection; HCW, health care worker; NI, nosocomial infection.
All patients with NIs possessed multiple comorbidities. Three died, 2 from an acute exacerbation of the underlying disease after repeated PCR proved them negative, and 1 from severe gastrointestinal bleeding despite a trend of improvement observed in respiratory status. One critical patient was still ventilated but tested negative on repeated PCR tests. For staff with NIs, neither severe nor critical cases existed. Clinical characteristics of confirmed cases are summarized in Supplementary Table 3.
The number of patients in the hospital, the mean length of hospital stay, and bed occupancy rate in March, April, and May were 25 523, 11.4 days, 88.3% in March; 13 471, 18.3 days, 48.2% in April; and 12 085, 15.9 days, 41.8% in May, respectively.
Description of Infection for Each Cluster
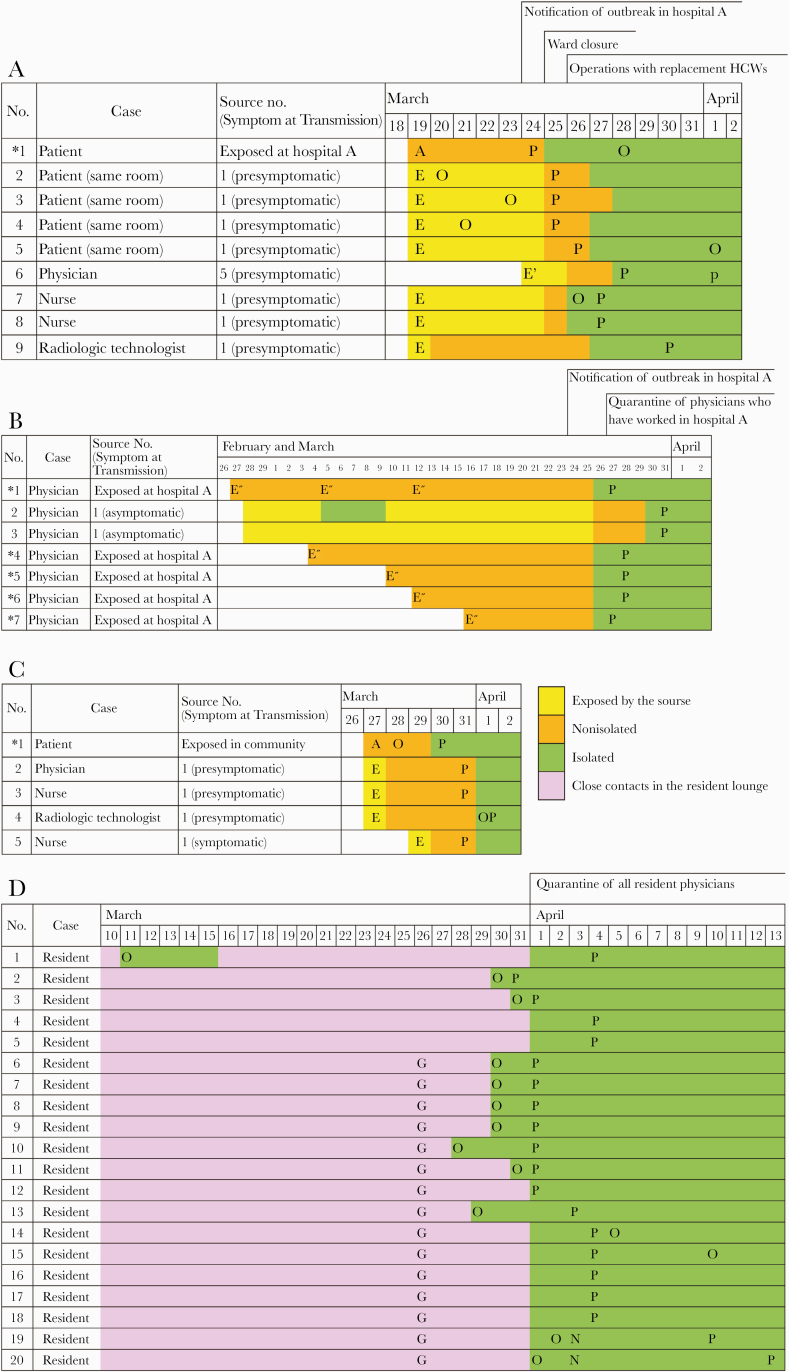
We found 4 major clusters, A–D (Figure 3). The first cluster visualized (cluster A) originated from the index case, the patient transferred to our hospital for the treatment of lower limb ischemia from hospital A. PCR testing of the 3 patients in the same room confirmed transmission of COVID-19. Then, PCR testing was conducted on all 44 inpatients and 100 HCWs assigned to the ward, and 1 patient and 3 HCWs were additionally identified. Although the incubation period was longer in the primary case and shorter in the secondary infected patients, the secondary infected patients in the same room were admitted to our hospital more than 44 days before onset; and considering the spread of infection, the patient from hospital A, where the outbreak of COVID-19 occurred, was most likely to be the primary case. Suspecting that NIs were spreading within the ward, we decided to close it. All HCWs were replaced by employees from other wards after thorough disinfection of the entire floor. The patients still in the hospital a week later were retested for PCR. This cluster numbered 12 cases.
Figure 3.
Time course for coronavirus disease 2019 (COVID-19) outbreak in each cluster. *Primary case: A, admission; E, exposure to a primary case; E’, exposure to a secondary case; E’’, exposed at hospital A; O, onset; P, positive polymerase chain reaction test; G, gathering for meals. A, The transmission cluster at a ward. A presymptomatic primary case (case 1) transmitted COVID-19 to 7 individuals in a short period. A presymptomatic patient (case 5) generated 1 definite secondary infection (case 6). B, The transmission cluster of physicians who worked at both Keio University Hospital and hospital A. Case 1 transmitted COVID-19 to 2 health care workers (HCWs; cases 2, 3) having meals together despite never having had any symptoms. No secondary case was discovered for the other 4 primary cases (cases 4–7). C, The transmission cluster at the pediatric outpatient clinic. The primary case transmitted COVID-19 to 4 HCWs in a short period. No secondary infection was discovered. D, The transmission cluster of resident physicians. Five resident physicians were discovered to be febrile on March 30 and confirmed to be COVID-19-positive. Immediate quarantine of all resident physicians was instituted on March 31. With careful contact tracing, no apparent contact with other positive individuals was found. Although the primary case was not clear, we assumed case 1 was the primary case when calculating R0 in this cluster D. Fifteen of 20 in this cluster had gathered for meals on March 26.
Next, a cluster arising among physicians working at both Keio University Hospital and hospital A was identified (cluster B). Quarantine of all 99 physicians was imposed from March 27. Infection was confirmed in 5, and 1 never-symptomatic source had generated 2 secondary cases.
Another cluster appeared from a presymptomatic pediatric outpatient (cluster C). Four HCWs were infected from the primary case; however, no further spread of infection was detected. At the same time, a cluster of resident physicians were discovered to be febrile (cluster D). Quarantine of all 99 resident physicians was imposed; in addition, those whose initial PCR test was negative were retested a week later, and 20 were confirmed to be positive. Detailed contact tracing revealed that none of the resident physicians had contact with confirmed or suspected COVID-19 patients in our hospital for the past month. Transmission was considered due to exposure in the resident lounge. Also, 15, excluding the potential primary case, had gathered for meals on March 26. The number of secondary cases directly generated by the primary case was considered to be 4.4, as the R0 in cluster D was estimated as 4.4 by the SEIR model.
Number of Secondary Cases Generated by a Single Primary Case
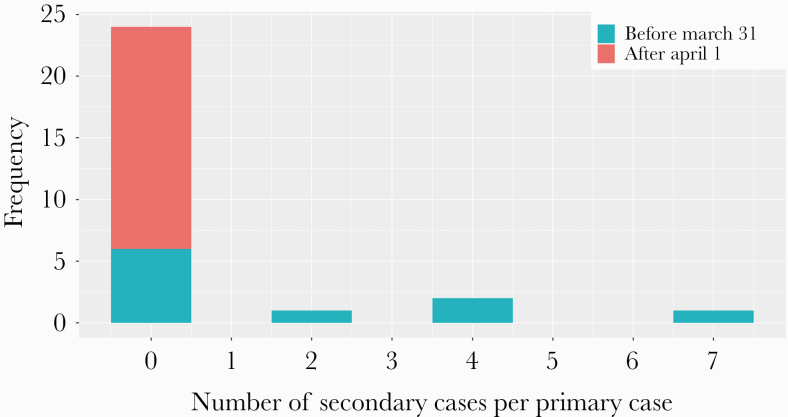
The mean number of secondary cases directly generated by a single primary case in the hospital was 17.4/28 = 0.62, as summarized in Figure 4. It was calculated both before and after March 31, when large-scale isolation and stringent social distancing measures were imposed. This number per primary case testing positive or being symptomatic before March 31 was 17.4/10 = 1.74, and after April 1 it was 0. For these 13 secondary cases except cluster D, the median time from the estimated date of contact to a positive PCR test was 6 days.
Figure 4.
Distribution of the number of secondary cases generated by a single primary case with coronavirus disease 2019 in the hospital. The number of secondary cases per each primary case is shown. Primary cases before March 31 represent primary cases who had onset or were confirmed positive by March 31. Primary cases after April 1 represent primary cases who experienced neither disease onset nor confirmation as positive by March 31. The primary cases, except for those of clusters A–D, did not generate any definite secondary cases.
DISCUSSION
We have described a nosocomial outbreak of COVID-19 in a large, acute care hospital in Tokyo, Japan. During this outbreak, 27 of 562 tested patients were confirmed positive, of whom 5 (18.5%) were suspected as NIs. For HCWs, despite universal masking and employee health screening, 52 of 697 tested positive, of whom 40 (76.9%) were considered NIs. The proportion of NIs was much higher than in previous reports [14–16]. This high proportion could be due to 2 factors: One was the limited acceptance of new COVID-19 patients due to the temporary decline in hospital functions, and the other was that the rate of community-acquired infection in Japan was considerably lower than that in other reports. In addition, although the HCWs adhered to universal masks, hand hygiene, and standard precautions during medical practice, there may have been many transmissions outside of practice, such as during use of the staff lounge and eating with other staff members. The implementation of self-quarantine of the hospital staff with close contact for 14 days, universal masking, visitor restriction, and social distancing have been previously reported [23, 24]; however, in the event of a larger-scale nosocomial infection, as presented here, a more consequential decision, such as ward closure, may be necessary. Prompt and rigorous implementation of containment measures led to successful control of viral transmission. One common feature of our primary cases producing widespread transmission was that they were presymptomatic or never-symptomatic, and all transmissions had occurred before their diagnoses. In the hospital, symptomatic individuals were immediately isolated and carefully controlled, and as a result, infection from symptomatic individuals was unlikely to occur, with the majority of infections coming from asymptomatic individuals. In addition, 19 of 42 asymptomatic transmissions were observed from a single cluster, which also contributed to the rise in numbers for asymptomatic transmission. Reports on COVID-19 have demonstrated a large percentage of presymptomatic cases [9–11] and the finding of presymptomatic carriers infecting others [11, 12, 25]. When clusters were discovered, aggressive contact tracing, thorough isolation regardless of PCR results or the presence of symptoms, and testing of highly exposed persons were conducted, which allowed us to further suppress infection. In particular, for cluster A, all employees in the ward were quarantined and temporarily replaced with a different set of workers. For clusters B and D, isolating ~200 physicians at once may have had an effect. Our measures were new in that we closed wards and performed extensive staff self-quarantining rapidly when nosocomial infections were recognized; and because COVID-19 transmission occurs in the asymptomatic phase, follow-up measures and contact tracing only may be inadequate if the number of cases increases. In such cases, large-scale measures such as those we took might have been as effective as the lock down that took place in the city. Quarantine of HCWs can temporarily result in staffing shortages and a reduction in patient care activities. However, implementing active work restrictions, regardless of PCR results or the presence of symptoms, on a large-scale basis at the first sign of an outbreak is essential, especially for COVID-19, where major outbreaks can occur from transmission by presymptomatic individuals.
The mean number of secondary NI cases directly generated by a single primary case was calculated to be 0.62. This number before March 31 was 1.74, whereas that after April 1 was 0, after more aggressive measures had been taken. This value of 1.74 is considered to reflect the reproductive number when the hospital was taking the traditional standard precautions with universal mask and isolation measures for symptomatic workers. This number may contain a margin of error because it was challenging to distinguish between nosocomial and community-acquired infections, and misclassification is likely to have occurred. While this number requires careful interpretation, it is still clear that the measures taken by our hospital reduced the reproductive number and brought the NIs under control. In addition, no new chain of infection had been identified by May 8, even after the conduct of comprehensive surveillance of patients and staff with fevers and symptoms.
NIs of COVID-19 in our hospital did not occur from every case in the same manner: Most cases did not infect anyone, and only 4 of 28 primary cases resulted in clusters. This suggests that even if the reproductive number is large, the spread of infection may be impeded by isolating the most contagious individuals or circumstances that give rise to many infections. The large variance of the number of secondary cases generated by a single primary within the hospital, which is a confined space, is consistent with the findings observed in CAIs in Japan [26].Our findings suggest that screening of symptomatic individuals is not sufficient to prevent nosocomial outbreaks of COVID-19. Detecting and isolating asymptomatic individuals, paying attention to asymptomatic shedding of the virus from patients, and avoiding contact between health care personnel are key to preventing the virus from spreading in a hospital or similar facility.
On April 6, our hospital implemented in-house PCR screening for every patient before admission. This led to a rise in the number of confirmed COVID-19-positive inpatients, who were mostly asymptomatic. No NIs occurred from these confirmed patients who were diagnosed before admission. This suggests that if health care providers are aware of asymptomatic COVID-19 patients and take adequate care to protect against them, the probability of cluster outbreaks from any patient can be reduced. However, PCR testing can have insufficient sensitivity to rule out COVID-19 [27–29], indicating an urgent need to increase the testing capacity and establish sufficiently sensitive systems to rule out COVID-19 within health care facilities in asymptomatic individuals.
This study should be interpreted in light of several limitations. First, this is single-center descriptive analysis; thus, it is necessary to collect more cases to establish effective measures. Second, the number of nosocomial infections might be overestimated because people defined as NI could have been infected from various sources outside the hospital, including asymptomatic sources. Contact tracing is difficult during epidemic spread in the community, making it challenging to identify the source of infection, if any, in the community. Third, the government declared a state of emergency on April 7 in Tokyo, and the number of confirmed cases peaked on April 16. The spontaneous decrease in CAIs might have contributed to the convergence of nosocomial infections by reducing the number of patients and HCWs with CAIs.
CONCLUSIONS
In conclusion, viral shedding from certain asymptomatic cases played a major role in nosocomial infections with COVID-19 in our institution. Immediate large-scale isolation at the first sign of outbreak, contact tracing, restriction of contact among HCWs, and PCR testing of individuals including asymptomatic ones were effective in terminating the spread of COVID-19 nosocomial infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to offer our sincere condolences to deceased patients and compassion toward the patients affected by the nosocomial outbreak of COVID-19. We thank the physicians, the intensivists, the nursing and medical staff, and the laboratory technicians on the front line of this pandemic. We also thank all the hospital and university staff for their cooperation with the infection control of COVID-19 at Keio University Hospital.
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study was approved by the Institutional Review Board of Keio University School of Medicine (approval number 20200063).
Author contributions. S.H., S.U., and T.A. designed the study, collected and analyzed the data, and drafted the manuscript; M.I., Y.T., Y.I., and Y.U. designed the study and participated in editing the manuscript; T.N., A.Taked, S.U., A.H., M.S., M.M., and A.Takeu participated in data collection and editing the manuscript; H.O., H.Y., K.F., M.A., Y.K., T.T., and N.H. conceptualized the study, participated in editing the manuscript, and revised the article for intellectual content. All authors read and critically revised the first as well as the subsequent and final drafts of this manuscript.
References
- 1. Robert Koch Institute. Coronavirus disease 2019 (COVID-19) daily situation report of the Robert Koch Institute Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-05-03-en.pdf?__blob=publicationFile. Accessed 13 May 2020.
- 2. International Councils of Nurses. High proportion of healthcare workers with COVID-19 in Italy is a stark warning to the world: protecting nurses and their colleagues must be the number one priority Available at: https://www.icn.ch/sites/default/files/inline-files/PR_09_COVID-19%20-%20Italy.pdf. Accessed May 13 May 2020.
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 4. Japan Broadcasting Corporation. Nearly 10% of Japan infections linked to hospitals Available at: https://www3.nhk.or.jp/news/html/20200424/k10012404011000.html. Accessed 13 May 2020.
- 5. Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eiju General Hospital. Confirmed COVID-19 cases in the hospital from March 20 to May 1, 2020 Available at: http://www.eijuhp.com/user/media/20200501koronashuukei.pdf. Accessed 13 May 2020.
- 7. Tokyo Metropolitan Government. Tokyo COVID-19 information Available at: https://stopcovid19.metro.tokyo.lg.jp/. Accessed 13 May 2020.
- 8. Infections Disease Surveillance Center, National Institute of Infectious Diseases. Outline of the active epidemiological investigation for COVID-19 Available at: https://www.niid.go.jp/niid/images/epi/corona/2019nCoV-02-200420.pdf. Accessed 13 May 2020.
- 9. Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ 2020; 368:m1165. [DOI] [PubMed] [Google Scholar]
- 10. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020; 25:2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 13. Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020; 368:eabb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Q, Gao Y, Wang X, et al. ; COVID-19 Evidence and Recommendations Working Group Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med 2020; 8:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter B, Collins JT, Barlow-Pay F, et al. ; COPE Study Collaborators Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J Hosp Infect 2020; 106:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-46. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf. Accessed 17 May 2020.
- 18. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med 2020; 9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shirato K, Nao N, Katano H, et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 21. Japanese Society of Laboratory Medicine. Institutional standards for SARS-Cov-2 testing and policies for specimen transport and accuracy control Available at: http://www.jscm.org/m-info/271.pdf. Accessed 13 May 2020.
- 22. Anderson RM, May RM.. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford University Press; 1991. [Google Scholar]
- 23. Wong SCY, Kwong RT, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 2020; 105:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wee LE, Conceicao EP, Sim XYJ, et al. Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect 2020; 105:113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishiura H, Oshitani H, Kobayashi T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv 2020.02.28.20029272 [Preprint]. 16 April 2020. Available at: 10.1101/2020.02.28.20029272. Accessed 13 May 2020. [DOI] [Google Scholar]
- 27. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 0:200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Long C, Xu H, Shen Q, et al. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 2020; 126:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.