Abstract
Background
Limited systematic surveillance for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the early months of the US epidemic curtailed accurate appraisal of transmission intensity. Our objective was to perform case detection of an entire rural community to quantify SARS-CoV-2 transmission using polymerase chain reaction (PCR) and antibody testing.
Methods
We conducted a cross-sectional survey of SARS-CoV-2 infection in the rural town of Bolinas, California (population 1620), 4 weeks after shelter-in-place orders. Participants were tested between April 20 and 24, 2020. Prevalence by PCR and seroprevalence from 2 forms of antibody testing were performed in parallel (Abbott ARCHITECT immunoglobulin [Ig]G and in-house IgG enzyme-linked immunosorbent assay).
Results
Of 1891 participants, 1312 were confirmed Bolinas residents (>80% community ascertainment). Zero participants were PCR positive. Assuming 80% sensitivity, it would have been unlikely to observe these results (P < .05) if there were >3 active infections in the community. Based on antibody results, estimated prevalence of prior infection was 0.16% (95% credible interval [CrI], 0.02%–0.46%). The positive predictive value (PPV) of a positive result on both tests was 99.11% (95% CrI, 95.75%–99.94%), compared with PPV 44.19%–63.32% (95% CrI, 3.25%–98.64%) if 1 test was utilized.
Conclusions
Four weeks after shelter-in-place, SARS-CoV-2 infection in a rural Northern California community was extremely rare. In this low-prevalence setting, use of 2 antibody tests increased seroprevalence estimate precision. This was one of the first community-wide studies to successfully implement synchronous PCR and antibody testing, particularly in a rural setting. Widespread testing remains an underpinning of effective disease control in conjunction with consistent uptake of public health measures.
Keywords: COVID-19, rural population, seroepidemiologic studies, severe acute respiratory syndrome coronavirus 2
During the early months of 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus proliferated in various metropolitan areas across the United States leading to devastating numbers of coronavirus disease 2019 (COVID-19) cases; unfortunately, testing availability lagged behind disease spread [1]. Months into the nation’s epidemic, with numerous reports of asymptomatic and presymptomatic transmission published [2–5], systematic surveillance for SARS-CoV-2 was still absent, eliminating the possibility of containment and limiting mitigation efforts [6]. Widespread testing regardless of symptoms has been proposed as a way to better understand the epidemiology of disease and curtail transmission. For example, in Vo, Italy, repeated testing of an entire town identified a high prevalence of asymptomatic infections and, in conjunction with a community lockdown implemented early after first case detections, demonstrated a reduction in diagnosis of new cases by approximately 50% [7].
In the United States, testing primarily symptomatic patients has revealed stark demographic, clinical, and regional differences in the number and severity of COVID-19 cases. Older age, male sex, and cardiovascular comorbidities are risk factors associated with COVID-19 requiring hospitalization, and black and Latinx individuals have faced disproportionately higher rates of infection and death [8, 9]. There are also clear regional differences in the proportion of positive polymerase chain reaction (PCR) tests, from 33% in New York state to 5% in Los Angeles, although varied testing criteria and limited testing availability, particularly in nonurban areas, make these data difficult to interpret [10, 11]. Measuring antibodies to SARS-CoV-2 can capture prior infections that may be missed by PCR, but many seroprevalence studies published to date have been constrained by accuracy concerns [12, 13]. Furthermore, the penetrance of COVID-19 into rural communities in the United States, which may be particularly vulnerable given a high proportion of elderly residents, was unknown at the time of data collection, and, as others have subsequently described [14–16], it remains an area of relatively limited study.
In the first effort of its kind to offer testing for active and prior COVID-19 infection to an entire town, we sought to (1) perform active case detection in the general population to identify and isolate potential reservoirs of infection and (2) estimate SARS-CoV-2 prevalence and seroprevalence in a Northern California rural community, using PCR and laboratory-based antibody testing to capture those with active and past infection. We hypothesized that prevalence would be relatively low, given the relatively fewer reported cases in the Bay Area compared with other regions, and thus we used 2 orthogonal antibody tests to ensure adequate specificity for assessing prior infection.
METHODS
Four weeks after shelter-in-place health orders, we conducted active population-based surveillance for SARS-CoV-2 infection in Bolinas, California among residents over 4 years of age and in Marin County first responders and essential workers. In close partnership with the community and the Marin Department of Public Health, oropharyngeal and mid-turbinate swabs for reverse-transcription PCR (RT-PCR) testing and blood for antibody testing were collected over 5 days (April 20–April 24, 2020) as previously described in detail and summarized below [17].
Study Setting and Community Partnership
Bolinas is a rural town, 5.8 square miles in size, and located less than 30 miles from the San Francisco metro area. Bolinas is bounded by the Pacific Ocean, a wilderness area, and a lagoon. The 2010 Census estimated that the population was 1620 persons with a population density of 278 persons per square mile [18], whereas in 2018 the American Community Survey (ACS) estimated the population size to have declined to 1077 persons, 46% percent of whom were aged 65 years and older [19]. The majority of the community is White (88%), including 2% Latinx, with 3% Asian/Pacific Islander, and 9% reporting multiple races. The median annual household income was $57 708, and 17% of the community lived in poverty [19].
This study was community-initiated and co-led by Bolinas community leaders who contributed throughout the planning and operational process. In addition, key community stakeholders, including the main community-based health organization (Coastal Health Alliance) and the Bolinas Fire Department, provided endorsement and operational support. These community leaders and stakeholders, together with the study leadership, participated in a virtual Town Hall the week before testing and week after results provision to introduce the study to the community, provide education, answer questions, and address concerns [17].
Testing Procedures
We conducted 4 days of drive-through and walk-up testing. On the fifth day of testing, we conducted limited in-home testing for homebound participants. Participants preregistered by completing an online consent and survey (available in both English and Spanish), which included questions related to demographics, movement information, and past and current symptoms. Alternatively, participants were able to register by phone or onsite, although survey data collection was limited for the latter category. The testing was performed at a centrally located outdoor location in town, and participants were encouraged to drive if able. Participants remained in their vehicle (or physically distanced from other participants if on foot) while medical staff first collected blood (300–500 µL) for subsequent antibody testing using fingerstick collection, then performed oropharyngeal and mid-turbinate specimen collection for RT-PCR with spun polyester swabs, following recommended procedures from the UCSF clinical laboratory.
Laboratory Assays
At a CLIA-certified laboratory operated by UCSF and the Chan Zuckerberg Biohub, RT-PCR of SARS-CoV-2 N and E genes as well as human RNAse P gene was completed using a laboratory developed test with a limit of detection of log10 4.5 viral genome copies/mL. The RT-PCR specimens were stored in DNA/RNA Shield (Zymo Research) to inactivate virus and preserve RNA stability. Serum was obtained from fingerprick samples via centrifugation and stored at −20°C until testing. Samples were tested using 2 independent assays: (1) the ARCHITECT SARS-CoV-2 immunoglobulin (Ig)G immunoassay, for antibodies to the SARS-CoV-2 nucleoprotein (Abbott Laboratories, Abbott Park, IL) [20], and (2) an in-house enzyme-linked immunosorbent assay (ELISA) assay detecting IgG to the receptor binding domain of spike protein, based on published protocols [21, 22]. For the ELISA, all samples that had an optical density (OD) above the cutoff (plate-specific OD for the CR3022 monoclonal antibody at 8 ng/µL) were repeated with titering to obtain an estimate of antibody concentration.
Outcomes and Statistical Analyses
The primary outcomes were prevalence of SARS-CoV-2 infection by PCR and seroprevalence by laboratory-based antibody testing. Because we sampled the majority of the population of Bolinas (ie, we obtained a sample without replacement from a finite population), we modeled the number of positive PCR tests as hypergeometric, which will yield increased precision in our prevalence estimate over the binomial, which assumes sampling with replacement from an infinite population.
To further interpret the PCR results, we calculated the probability of observing × PCR-positive cases, conditional on there being K true cases in the population of size N (of which we tested n) [23–25]. Models also accounted for the sensitivity and specificity of the PCR test; we used values of 80% and 100%, respectively [26, 27].
We used a Bayesian modeling approach to jointly estimate population seroprevalence based on the results of the 2 antibody testing platforms, along with their test performance characteristics (ie, sensitivities and specificities) [28]. For validation data, we used the package insert data for the Abbott test [20] (1066 of 1070 negative controls tested negative [99.6% specificity]; 88 of 88 PCR+ positive controls tested positive [100% sensitivity]) and in-house validation of the ELISA test (95 of 95 negative controls tested negative [100% specificity]; 42 of 44 positive controls tested positive [95% sensitivity]). We first estimated seroprevalences independently by assay, and then we estimated a single seroprevalence in a multinomial model that assumed the 2 assays to be conditionally independent [29]. For both scenarios, we calculated positive predictive values (PPVs) based on estimated seroprevalence and test performance. All analyses were conducted using the R statistical software (http://cran.r-project.org) and the Stan programming language (http://mc-stan.org/). See Appendix 1 for a more detailed explanation of the statistical methods; in addition, the codes to reproduce all analyses are available at: https://github.com/EPPIcenter/bolinas-analysis.
Patient Consent Statement
Each participant’s written consent was obtained, and all participant data were anonymized as completely as possible. The study protocol was submitted to UCSF’s Institutional Review Board (IRB), and the study was deemed public health surveillance and did not require IRB oversight (IRB number 20-30636).
RESULTS
Of 1891 participants tested in Bolinas between April 20 and April 24, 2020, 1312 were confirmed Bolinas residents, 76 were non-Bolinas resident first responders, essential workers, and their families, 47 were nonresident volunteers, and 456 were registered onsite (a mix of Bolinas residents and non-Bolinas first responders/essential workers). Based on the aforementioned 2010 Census and 2018 ACS Bolinas population estimates, we calculated community ascertainment of greater than 80%. Most participants were adults aged 18 and over (90%), with more than one third aged 60 and older (35%). The majority of participants identified as white/Caucasian, with almost one third (31%) reporting annual household income less than $50 000. Demographic, epidemiologic, and symptom-related characteristics of confirmed Bolinas residents are listed in Table 1, with characteristics of all participants shown in Appendix 2. Of note, the 2018 ACS characterization of the population in Bolinas found residents to be older (53% age 60 and older compared with 37% age 60 and older in our sample) and more likely to be white (86% by ACS vs 80% of our sample), suggesting the possibility that our sampling of elderly, white residents of the community was incomplete. The vast majority of survey respondents reported wearing a mask (93%), and most estimated that they left their homes only 0–1 times weekly for work, food, or other reasons (see Table 1). Finally, although only 2% of participants had symptoms consistent with COVID-19 (eg, fever, cough, muscle aches, severe fatigue, difficulty breathing, diarrhea, loss of smell and/or taste) on the day of testing, 31% reported having had at least 1 of the aforementioned symptoms in the month before testing.
Table 1.
Demographic, Epidemiologic, and Symptom-Related Characteristics of Participants
| Characteristics | Confirmed Bolinas Residentsa N = 1312 |
|---|---|
| Demographic Characteristics | |
| Age | |
| <18 | 139 (11%) |
| 18–44 | 363 (28%) |
| 45–60 | 319 (24%) |
| 60 and older | 490 (37%) |
| Sex | |
| Male | 521 (46%) |
| Female | 599 (52%) |
| Declined to state | 22 (2%) |
| Race/Ethnicity | |
| White/Caucasian | 964 (80%) |
| Black/African American | 8 (1%) |
| Hispanic/Latinx | 60 (5%) |
| Asian/Pacific Islander | 23 (2%) |
| Other or Declined | 153 (13%) |
| Income | |
| <50 000 | 295 (33%) |
| 50K–100K | 275 (30%) |
| >100K | 337 (37%) |
| Essential workersb | 182 (14%) |
| Epidemiologic and Behavioral Characteristics | |
| Estimated Number of times Participantsc Left the House per Week for | |
| Work (median, interquartile range) | 0 (0–1) |
| Food | 1 (1–2) |
| Other | 1 (0–2) |
| Endorsed mask-wearing | 984 (92%) |
| Reported travel outside Bolinas in the 2 weeks before testing | 244/346 (73%) |
| Symptom Reporting | |
| Symptoms on Day of Testing | |
| Any symptom below | 32 (2%) |
| Fever | 1 (0%) |
| Cough | 19 (1%) |
| Muscle aches | 1 (0%) |
| Severe fatigue | 4 (0%) |
| Trouble breathing | 6 (6%) |
| Loss of smell or taste | 0 (0%) |
| Symptoms in Month Before Testing | |
| Any symptom below | 350 (31%) |
| Fever | 77 (7%) |
| Cough | 214 (19%) |
| Muscle aches | 143 (13%) |
| Severe fatigue | 134 (12%) |
| Trouble breathing | 72 (6%) |
| Loss of smell or taste | 18 (2%) |
aDoes not include the following: non-Bolinas first-responders, essential workers, and family members; non-Bolinas resident volunteers; or those who registered onsite (likely Bolinas residents, essential workers, or county first responders).
bEssential workers defined as those who self-reported incoming as one of the following: food/beverage, healthcare, tradesperson, and cleaning/personal services.
cn = 1047 confirmed Bolinas residents provided responses.
Reverse-Transcription Polymerase Chain Reaction Results
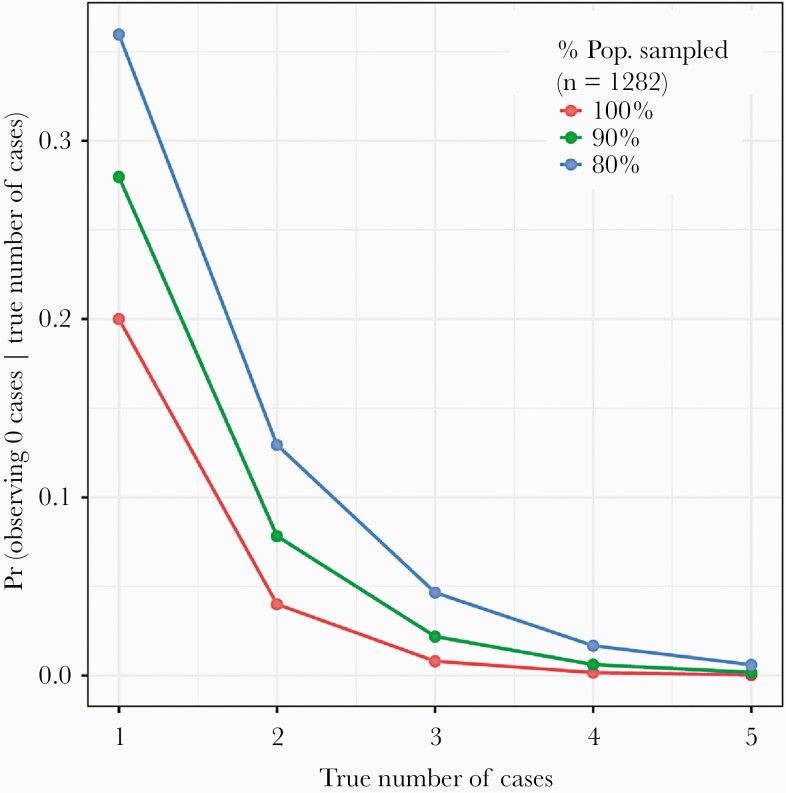
Of 1847 RT-PCR tests performed for active SARS-CoV-2 infection, 0 were positive. Using an estimated test sensitivity of 80%, conservatively assuming 80% of the community was sampled, and only including confirmed Bolinas residents, this corresponds to a population prevalence of 0.00048 (95% credible interval [CrI], 0.00001–0.00176). The calculated probability of observing 0 infections if there were truly 3 active infections in the community was <5%, with probabilities dropping further for higher numbers of infections (Figure 1). Thus, it is likely that few if any active infections were present in the community at the time of sampling.
Figure 1.
Probability of observing 0 cases given the true number of cases (y-axis), across a range of true numbers of cases (x-axis) and the proportion of the total population that was sampled (red, green, and blue lines). For example, assuming that we had sampled the entire population (red line), the probability of observing 0 cases if there truly had been 1 case is 0.2 or 20%; the probability of observing 0 cases if there truly had been 3 cases is 0.008.
Antibody Results
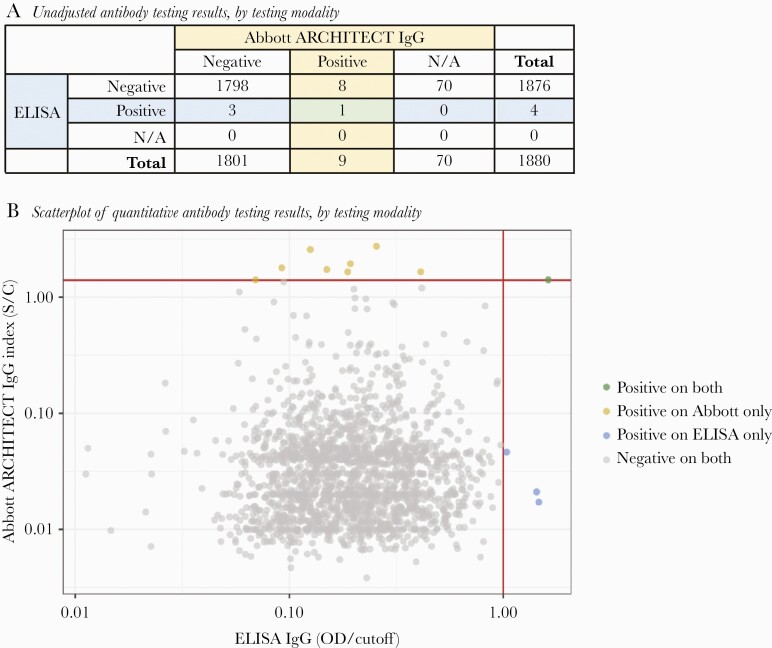
Of 1880 participants with antibody tests performed for prior SARS-CoV-2 infection, 12 participants had a positive result, and 8 of 12 with positive antibody results were confirmed Bolinas residents. Only 1 participant, a Bolinas resident, had a positive result on both antibody assays, whereas 8 others had a positive result on the ARCHITECT Abbott IgG test alone, and 3 other participants had a positive result on the ELISA IgG test alone (see Figure 2). The ELISA titer was the highest for the sample also testing positive via the Abbott test, at >1:100.
Figure 2.
(A) illustrates the number of specimens that were positive and negative, by testing modality. N/A indicates samples not run on Abbott assay due to insufficient plasma volume. (B) illustrates the quantitative results of antibody tests that were run on both assays (n = 1810) with colors denoting positive results. Abbott ARCHITECT immunoglobulin (Ig)G signal to cutoff values are shown on the y-axis with enzyme-linked immunosorbent assay (ELISA) IgG optical density to cutoff values on the x-axis.
Considering each test independently, correcting for the estimated sensitivity and specificities of the assays, and including only confirmed Bolinas residents, we estimated seroprevalence to be 0.29% (95% CrI, 0.01%–0.78%) by the Abbott assay and 0.23% (95% CrI, 0.01%–0.62%) by the ELISA (Table 2). The wide range of the 95% CrI for these prevalence estimates reflects the high uncertainty in interpreting a positive test result in the setting of very low prevalence. Using the data from both tests, we estimated seroprevalence to be 0.16% (95% CrI, 0.02%–0.46%). More importantly, the availability of 2 antibody results per sample sharply increased the PPV: the probability of an individual testing positive on both Abbott and ELISA being truly infected was >99%, whereas the PPV for just 1 of the 2 tests was low (<2% for both configurations). If only 1 of the antibody tests were used, the PPV of a positive test would have been much lower and much more imprecise: between 44% and 63% with CrIs that ranged from ~3% to ~98% (see Table 2).
Table 2.
Modeled Prevalence, Sensitivity, Specificity, and Positive Predictive Value Based on Independent and Conjoined Antibody Testing Results
| Test Characteristics | Estimate and 95% CrI |
|---|---|
| Prevalence | |
| Using Abbott only | 0.29% (0.01%–0.78%) |
| Using ELISA only | 0.23% (0.01%–0.62%) |
| Using Abbott and ELISA | 0.16% (0.02%–0.46%) |
| Sensitivity | |
| Abbott assay (independent) | 99.61% (97.98%–99.99%) |
| ELISA assay (independent) | 96.25% (89.36%–99.51%) |
| Abbott assay, using both | 99.60% (98.00%–99.99%) |
| ELISA assay, using both | 96.23% (89.45%–99.52%) |
| Specificity | |
| Abbott assay (independent) | 99.68% (99.37%–99.88%) |
| ELISA assay (independent) | 99.88% (99.58%–100%) |
| Abbott assay, using both | 99.61% (99.32%–99.82%) |
| ELISA assay, using both | 99.83% (99.56%–99.97%) |
| Positive Predictive Value | |
| Abbott + (independent) | 44.19% (3.25%–83.06%) |
| ELISA + (independent) | 63.32% (5.46%–98.64%) |
| Abbott + and ELISA + | 99.11% (95.75%–99.94%) |
| Abbott + and ELISA – | 1.67% (0.07%–7.47%) |
| Abbott – and ELISA + | 0.56% (0%–3.48%) |
Abbreviations: CrI, credible interval; ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
In the early months of the domestic COVID-19 epidemic, community-wide testing of a rural town comprising mostly older adults with varied socioeconomic status revealed that active and prior SARS-CoV-2 infections were extremely rare 4 weeks after shelter-in-place orders, despite relative geographic proximity to urban areas with higher transmission. In this low-transmission environment, use of 2 highly specific, independent antibody tests allowed for precise estimation of seroprevalence.
As the COVID-19 pandemic has continued, 2 things have become increasingly clear: (1) SARS-CoV-2 testing regardless of symptoms has proved very important and (2) the penetration of SARS-CoV-2 has been alarmingly uneven in different populations. Universal testing in homeless shelters [30], prisons [31], nursing facilities [32], and hospitals [33] has demonstrated high rates of infection without concurrent symptoms. Likewise, in a mass testing campaign conducted in the neighboring Mission District of San Francisco just days after our study, active and prior SARS-CoV-2 infection was more common than passive case detection would have suggested, with 6% cumulative incidence of infection compared with <0.3% estimated seroprevalence in Bolinas [34]. Of note, the vast majority (95%) of active infections in the San Francisco study were among those who identified as Latinx, and additional risk factors for recent infection included frontline service work and inability to shelter-in-place and maintain income [34]. In contrast, we found that SARS-CoV-2 infection was extremely uncommon and potentially nonexistent in our epidemiologically distinct study setting. In retrospect, it is difficult to know whether the relative sparing of the rural town of Bolinas was due to few viral introductions into the community, successful public health measures, or both factors. However, taking into consideration well established evidence supporting the effectiveness of mask wearing and physical distancing [35–37], our findings of approximately zero infections in a community with high uptake of mask wearing and adherence to shelter-in-place health orders could support the effectiveness of these common public health measures. Other possible contributing factors include the Bay Area’s relatively few number of cases; at the time of testing on April 20, 2020, there were 1327 cumulative cases in San Francisco identified by passive case detection [38], compared with over 4000 new cases diagnosed on that day in New York City alone [39]. Another factor may have been the low population density in Bolinas, but this seems to be an insufficient explanation in isolation; although less publicized than urban epidemics, the devastating experience of Navajo Nation [40, 41], and many other rural, native communities [42], stands in stark contrast to our data, illustrating that some rural communities may be particularly vulnerable to severe consequences of SARS-CoV-2 spread. Similarly, in New York City, it was not the most densely population borough (Manhattan) that saw the highest number of cases in the spring of 2020, but rather the borough with the highest proportion of people of color who bore a disproportionate burden of poverty and other consequences of systemic racism that had the highest number of cases [9]. In summary, these data from Bolinas suggest that although rural communities may be vulnerable to severe consequences of SARS-CoV-2 given older age, high rates of poverty, and limited access to testing and medical care, this particular community had almost zero infection 4 weeks into sheltering-in-place. Despite a rate of poverty higher than the national average, this community that sought to test itself with the self-reported goal of protecting its elders, whose constituents were also mostly able to shelter-in-place in a rural coastal setting and reported high uptake of mask wearing, was able to remain close to free from infection early in the epidemic. Of note, even despite a second, larger peak of infections that subsequently occurred throughout the Bay Area in June–August 2020 [38, 43], cumulative incidence of cases in Bolinas has remained between 0 and 9 based on ongoing passive surveillance [43].
Given the very low hypothesized prevalence of SARS-CoV-2 infection in this setting, and to minimize the number of false-positive results, we chose to test each sample using 2 specific, laboratory-based tests evaluating responses to different viral proteins of the virus. The implementation and interpretation of SARS-CoV-2 antibody testing has been complicated for several reasons including the following: different indications for antibody testing (eg, diagnosing active infection, confirming convalescent infection for plasma donation, and seroprevalence) that prioritize different optimal test characteristics, use of laboratory and nonlaboratory-based methods with wide variation in accuracy, and sparse and poorly characterized validation data, among other concerns [12, 44]. High specificity of an assay is particularly important when performing serosurveillance in areas with lower transmission, because false positives will be a higher proportion of the total positives. The estimated specificity of our 2 independent tests were both >99.5%, but given the extremely low prevalence of infection in this community, the PPV of either test alone remained low. By jointly analyzing the results of these 2 tests, we were able to more precisely estimate seroprevalence and obtain a high PPV when both tests were positive. In this study, given that only 1 individual was likely to have been truly exposed, we were not able to use these data to systematically risk factors for infection. However, having a high PPV may allow better estimation of risk factors in other studies and may allow for more meaningful communication of results to individuals. Given the aforementioned issues regarding interpretation of antibody data in low prevalence areas [45], it is essential to consider the test characteristics in light of the population studied when designing serosurveillance studies, and using multiple antigenic targets could be a strategy to improve the overall performance of serosurveillance in certain contexts moving forward.
Our study was subject to important limitations. For almost all SARS-CoV-2 antibody tests, the true sensitivity when applied to a community-based sample where the majority of infected individuals will have experienced mild or asymptomatic SARS-CoV-2 infections is unknown, because test performance characteristics have generally been calculated based on severe infections only [46–48]. However, in our study, sensitivity for the ELISA was largely evaluated on mild (although not asymptomatic) infections, although it should be noted that test sensitivities were based on different patient populations (Abbott test based on hospitalized patients, ELISA based predominantly on ambulatory patients) and therefore were not directly comparable. In this particular population with low prevalence of active or prior infection, false-negative results were less of a concern and would have minimally changed seroprevalence estimates. Next, it is possible that we sampled a biased subset of the community, eg, with certain demographic groups or those experiencing illness less likely to leave their homes for testing. However, we estimated relatively high community ascertainment (>80%) and also tested homebound participants, mitigating this factor. Finally, not all participants completed the epidemiologic survey, which may have introduced selection bias in that those who elected to share information about mask wearing and movement during shelter-in-place were more likely to report socially desirable values. However, community members reported anecdotal observations consistent with our overall study findings.
CONCLUSIONS
In conclusion, active and prior SARS-CoV-2 infections were rare in this rural town with a high uptake of mask wearing and compliance with shelter-in-place directives, despite relative proximity to urban areas with significantly higher transmission. Use of 2 independent, highly accurate antibody tests methods allowed for a more precise estimate of seroprevalence and higher PPV than either test alone.
Acknowledgments
We acknowledge the significant contribution to this work made by the following persons: Dr. Matt Willis of the Marin County Public Health Department for his support of this project within his jurisdiction; Jacqueline Martinez and Andrew Kobylinski for their considerable technical expertise in operationalizing multiple aspects of this project; and Ana Vallari, Barb Harris, Ana Olivo, and Chris Lark at Abbott Laboratories for their contribution to generating serology data.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was primarily funded by the Bolinas Community Land Trust (to D. H. and B. G.). Additional sources of support included funding from the Chan Zuckerberg Biohub Investigator program (to B. G.), the National Institutes of Health (Grant 5T32AI007641-17; to A. A.), and the Schmidt Science Fellows, in partnership with the Rhodes Trust (to S. T.).
Potential conflicts of interest. M. R. and J. H. are employees and shareholders of Abbott Laboratories. C. C. is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center and receives research support funding from Abbott Laboratories. T. J. H. reports unrelated research funding obtained from Merck, BMS, and Gilead Biosciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX 1
SUPPLEMENTARY METHODS
1A. Testing the Upper Bound on the True Number of Infections
To test the upper bound on the true number of infections (via PCR), we calculated the probability of observing x = 0 cases, conditional on there being exactly K cases in our population of size N (of which we tested n). We assume that the sensitivity (Se) and specificity (Sp) of the diagnostic test are known and fixed at 80% and 100%, respectively. We assume that the n individuals were sampled at random and so are representative of the entire population N. We use the hypergeometric distribution since we have a finite population size so trials are not independent, and binomial distributions to account for Se and Sp.
Code available at:
https://github.com/EPPIcenter/bolinas-analysis/blob/master/1A_Figure_1.R.
1B. Estimation of PCR Prevalence
We estimated PCR prevalence in 2 ways: first, by using the binomial distribution to model prevalence (p) [24] and second, by using the hypergeometric distribution to model prevalence. The latter is more appropriate in this scenario since testing was performed on a large proportion of the total population (upwards of 80%), so the hypergeometric distribution will yield narrower estimates of uncertainty. We again assume that the Se and Sp of the diagnostic test are known and fixed at 80% and 100%, respectively. As the binomial distribution is more frequently used for prevalence estimation, we provide Stan code to implement both models.
Code available at:
https://github.com/EPPIcenter/bolinas-analysis/blob/master/1B.1_PCR_prevalence_HGM.R
https://github.com/EPPIcenter/bolinas-analysis/blob/master/1B.2_PCR_prevalence_binomial.R
2A. Estimation of Seroprevalence (One Test)
We used the binomial distribution to estimate seroprevalence (p) separately for each assay j. The binomial distribution will yield conservative intervals compared with the hypergeometric distribution used as above. The difference from the estimation of PCR prevalence is that we also estimate Se and Sp of the assays using validation data. The positive predictive values (PPV) are calculated directly from the estimates.
Code available at:
https://github.com/EPPIcenter/bolinas-analysis/blob/master/2A_Ab_prevalence_separate_binomial.R
2B. Estimation of Seroprevalence (Two Tests)
We extended the approach in 2A above to jointly model the results of both assays , using the multinomial distribution as the generalization of the binomial distribution and now estimating a single seroprevalence p. We necessarily assumed conditional independence between the 2 assays j and k. For samples that were only tested on one platform, we allowed them to contribute to the binomial likelihood for the platform that they were tested on. The positive predictive values, which are now based on the results of both assays, are calculated directly from the estimates.
Code available at:
https://github.com/EPPIcenter/bolinas-analysis/blob/master/2B_Ab_prevalence_joint_multinomial.R
APPENDIX 2
Table 1.
Demographic, Epidemiologic, and Symptom-Related Characteristics of Participants
| Confirmed Bolinas Residentsa N = 1312 | |
|---|---|
| Demographic Characteristics | |
| Age | |
| <18 | 139 (11%) |
| 18–44 | 363 (28%) |
| 45–60 | 319 (24%) |
| 60 and older | 490 (37%) |
| Sex | |
| Male | 521 (46%) |
| Female | 599 (52%) |
| Declined to state | 22 (2%) |
| Race/Ethnicity | |
| White/Caucasian | 964 (80%) |
| Black/African American | 8 (1%) |
| Hispanic/Latinx | 60 (5%) |
| Asian/Pacific Islander | 23 (2%) |
| Other or Declined | 153 (13%) |
| Income | |
| <50 000 | 295 (33%) |
| 50K–100K | 275 (30%) |
| >100K | 337 (37%) |
| Essential workersb | 182 (14%) |
| Epidemiologic and Behavioral Characteristics | |
| Estimated Number of times Participantsc Left the House per Week for | |
| Work (median, interquartile range) | 0 (0–1) |
| Food | 1 (1–2) |
| Other | 1 (0–2) |
| Endorsed mask-wearing | 984 (92%) |
| Reported travel outside Bolinas in the 2 weeks before testing | 244/346 (73%) |
| Symptom Reporting | |
| Symptoms on Day of Testing | |
| Any symptom below | 32 (2%) |
| Fever | 1 (0%) |
| Cough | 19 (1%) |
| Muscle aches | 1 (0%) |
| Severe fatigue | 4 (0%) |
| Trouble breathing | 6 (6%) |
| Loss of smell or taste | 0 (0%) |
| Symptoms in Month Before Testing | |
| Any symptom below | 350 (31%) |
| Fever | 77 (7%) |
| Cough | 214 (19%) |
| Muscle aches | 143 (13%) |
| Severe fatigue | 134 (12%) |
| Trouble breathing | 72 (6%) |
| Loss of smell or taste | 18 (2%) |
aDoes not include the following: non-Bolinas first-responders, essential workers, and family members; non-Bolinas resident volunteers; or those who registered onsite (likely Bolinas residents, essential workers, or county first responders).
bEssential workers defined as those who self-reported incoming as one of the following: food/beverage, healthcare, tradesperson, and cleaning/personal services.
cn = 1047 confirmed Bolinas residents provided responses.
References
- 1. Sharfstein JM, Becker SJ, Mello MM. Diagnostic testing for the novel coronavirus. JAMA 2020; 323:1437–8. [DOI] [PubMed] [Google Scholar]
- 2. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020; 25:2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Volume 26, Number 7–July 2020. Emerging Infectious Diseases Journal. Centers for Disease Control and Prevention. Available at: https://wwwnc.cdc.gov/eid/article/26/7/20-1595_article. Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 6. Walensky RP, del Rio C. From mitigation to containment of the COVID-19 pandemic: putting the SARS-CoV-2 genie back in the bottle. JAMA 2020; 323:1889–90. [DOI] [PubMed] [Google Scholar]
- 7. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. Nature 2020; 584:425–9. [DOI] [PubMed] [Google Scholar]
- 8. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA 2020; 323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spellberg B, Haddix M, Lee R, et al. Community prevalence of SARS-CoV-2 among patients with influenzalike illnesses presenting to a Los Angeles medical center in March 2020. JAMA 2020; 323:1966–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenberg ES, Dufort EM, Blog DS, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State-March 2020. Clin Infect Dis 2020; 71:1953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krammer F, Simon V. Serology assays to manage COVID-19. Science 2020; 368:1060–1. [DOI] [PubMed] [Google Scholar]
- 13. Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS-CoV-2 serological assays. Nat Biotechnol 2020; 38:1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul R, Arif AA, Adeyemi O, et al. Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J Rural Health 2020; 36:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarap M, Conyers J, Cunningham C, et al. US Rural Surgeon Responses to the COVID-19 Pandemic: Leadership in a Time of Crisis: The American Surgeon. Available at: https://journals.sagepub.com/doi/10.1177/0003134820924395. Accessed 15 October 2020. [DOI] [PubMed]
- 16. Pro G, Hubach R, Wheeler D, et al. Differences in US COVID-19 case rates and case fatality rates across the urban–rural continuum. Available at: https://www.rrh.org.au/journal/article/6074/. Accessed 15 October 2020. [DOI] [PMC free article] [PubMed]
- 17. Appa A, Chamie G, Sawyer A, et al. SARS-CoV-2 PCR and antibody testing for an entire rural community: methods and feasibility of high-throughput testing procedures. medRxiv 2020; doi: 10.1101/2020.05.29.20116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CensusViewer. Bolinas, CA Population–Census 2010 and 2000 Interactive Map, Demographics, Statistics, Quick Facts–CensusViewer. Available at: http://censusviewer.com/city/CA/Bolinas. Accessed 19 July 2020.
- 19. United States Census Bureau. Census–Geography Profile. Available at: https://data.census.gov/cedsci/profile?g=1600000US0607316&hidePreview=true&tid=ACSDP5Y2018.DP05. Accessed 19 July 2020.
- 20. Abbott Laboratories. ARCHITECT SARS-CoV-2 IgG Emergency Use Authorization. Available at: https://www.fda.gov/media/137383/download. Accessed 19 July 2020.
- 21. Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 2020; 57:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cameron AR, Baldock FC. A new probability formula for surveys to substantiate freedom from disease. Prev Vet Med 1998; 34:1–17. [DOI] [PubMed] [Google Scholar]
- 24. Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol 1978; 107:71–6. [DOI] [PubMed] [Google Scholar]
- 25. Michael E, Smith ME, Katabarwa MN, et al. Substantiating freedom from parasitic infection by combining transmission model predictions with disease surveys. Nat Commun 2018; 9:4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu X, Wang L, Sakthivel SK, et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome Coronavirus 2–Volume 26, Number 8—August 2020–Emerging Infectious Diseases Journal–Centers for Disease Control and Prevention. Available at: https://wwwnc.cdc.gov/eid/article/26/8/20-1246_article. Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 27. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gelman A, Carpenter B. Bayesian analysis of tests with unknown specificity and sensitivity. J R Stat Soc A Stat Ser C 2020; 69:1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics 1980; 36:167–71. [PubMed] [Google Scholar]
- 30. Baggett TP, Keyes H, Sporn N, Gaeta JM. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 2020; 323:2191–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawks L, Woolhandler S, McCormick D. COVID-19 in prisons and jails in the United States. JAMA Intern Med 2020; 180:1041–2. [DOI] [PubMed] [Google Scholar]
- 32. Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamie G, Marquez C, Crawford E, et al. SARS-CoV-2 community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu DK, Akl EA, Duda S, et al. ; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395: 1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chou R, Dana T, Jungbauer R, et al. Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings. Ann Intern Med 2020; 173:542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff (Millwood) 2020; 39:1419–25. [DOI] [PubMed] [Google Scholar]
- 38. DataSF. COVID-19 Cases and Deaths. Available at: https://data.sfgov.org/stories/s/COVID-19-Cases-and-Deaths/dak2-gvuj/. Accessed 15 October 2020.
- 39. New York Covid Map and Case Count. The New York Times. Available at: https://www.nytimes.com/interactive/2020/us/new-york-coronavirus-cases.html. Accessed 16 October 2020.
- 40. Kristof N. Opinion | The Top U.S. Coronavirus Hot Spots Are All Indian Lands. The New York Times. 2020; Available at: https://www.nytimes.com/2020/05/30/opinion/sunday/coronavirus-native-americans.html. Accessed 19 July 2020.
- 41. Kovich H. Rural matters—Coronavirus and the Navajo Nation. N Eng J Med 2020; 383:105–7. [DOI] [PubMed] [Google Scholar]
- 42. Cheng KJG, Sun Y, Monnat SM. COVID-19 death rates are higher in rural counties with larger shares of blacks and Hispanics. J Rural Health 2020; 36:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marin Health & Human Services. Novel Coronavirus (COVID-19) | Marin County Coronavirus Information. Available at: https://coronavirus.marinhhs.org/surveillance#today. Accessed 16 October 2020.
- 44. Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA 2020; 323:1881–3. [DOI] [PubMed] [Google Scholar]
- 45. Vogel G. First antibody surveys draw fire for quality, bias. Science 2020; 368:350–1. [DOI] [PubMed] [Google Scholar]
- 46. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020; 71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect 2020; 9:833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takahashi S, Greenhouse B, Rodríguez-Barraquer I. Are seroprevalence estimates for severe acute respiratory syndrome coronavirus 2 biased? J Infect Dis 2020; 222:1772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]