Abstract
Background
People experiencing homelessness are at increased risk of coronavirus disease 2019 (COVID-19), but little is known about specific risk factors for infection within homeless shelters.
Methods
We performed widespread severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction testing and collected risk factor information at all homeless shelters in Chicago with at least 1 reported case of COVID-19 (n = 21). Multivariable, mixed-effects log-binomial models were built to estimate adjusted prevalence ratios (aPRs) for SARS-CoV-2 infection for both individual- and facility-level risk factors.
Results
During March 1 to May 1, 2020, 1717 shelter residents and staff were tested for SARS-CoV-2; 472 (27%) persons tested positive. Prevalence of infection was higher for residents (431 of 1435, 30%) than for staff (41 of 282, 15%) (prevalence ratio = 2.52; 95% confidence interval [CI], 1.78–3.58). The majority of residents with SARS-CoV-2 infection (293 of 406 with available information about symptoms, 72%) reported no symptoms at the time of specimen collection or within the following 2 weeks. Among residents, sharing a room with a large number of people was associated with increased likelihood of infection (aPR for sharing with >20 people compared with single rooms = 1.76; 95% CI, 1.11–2.80), and current smoking was associated with reduced likelihood of infection (aPR = 0.71; 95% CI, 0.60–0.85). At the facility level, a higher proportion of residents leaving and returning each day was associated with increased prevalence (aPR = 1.08; 95% CI, 1.01–1.16), whereas an increase in the number of private bathrooms was associated with reduced prevalence (aPR for 1 additional private bathroom per 100 people = 0.92; 95% CI, 0.87–0.98).
Conclusions
We identified a high prevalence of SARS-CoV-2 infections in homeless shelters. Reducing the number of residents sharing dormitories might reduce the likelihood of SARS-CoV-2 infection. When community transmission is high, limiting movement of persons experiencing homelessness into and out of shelters might also be beneficial.
Keywords: congregate settings, COVID-19, homeless, SARS-CoV-2, transmission
Risk factors for SARS-CoV-2 infection included being a homeless shelter resident compared to a staff member, sleeping in a room with large numbers of other residents, and residing in a shelter where many residents leave and return each day.
People staying in homeless shelters are at increased risk of acquiring respiratory pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1–3]. In addition, people experiencing homelessness have a high prevalence of chronic conditions that place them at higher risk for severe coronavirus disease 2019 (COVID-19) [4–6]. Furthermore, non-Hispanic black persons—who are overrepresented among homeless shelter residents [7]—seem to have an increased risk of severe disease compared with non-Hispanic white persons [8, 9].
In March 2020, the Chicago Department of Public Health (CDPH) detected the first of several cases of COVID-19 in residents of homeless shelters. Given the large outbreaks in homeless shelters in other US cities [1], and documentation of asymptomatic spread of SARS-CoV-2 in other congregate settings [10], the CDPH collaborated with healthcare partners to offer testing to residents and staff of shelters from which cases were reported to rapidly identify and isolate people with SARS-CoV-2 infection. This article describes the findings of point-prevalence surveys at 21 homeless shelters across Chicago. We gathered associated clinical and epidemiological information to describe the spectrum of illness and identify individual-level and facility-level risk factors for SARS-CoV-2 infection in homeless shelters.
METHODS
Study Population
In January 2019, an estimated 5290 people were experiencing homelessness in Chicago, 4030 of which were accommodated in homeless shelters [11]. Chicago shelters include dormitory-style accommodation with shared bathrooms; rooms accommodating family units but with shared bathrooms and dining facilities between families; and shelters with single rooms for each resident. Several shelters employ their residents—for this analysis, we defined anyone living in the shelter as a resident, including those who also worked at the shelter. Shelter staff comprised a range of full-time and part-time staff, paid staff and volunteers, and people with lived experience of homelessness.
City-Wide Interventions
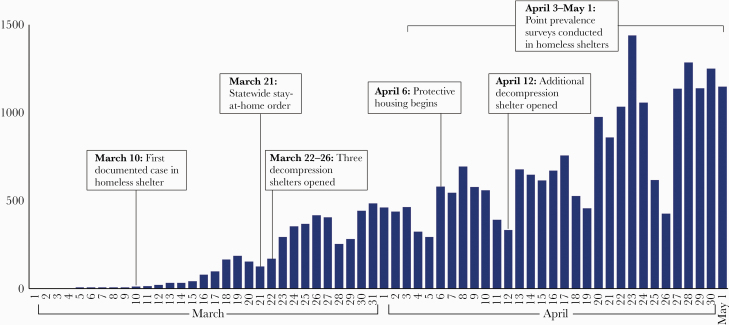
In March, 2020, the Chicago Department of Family and Support Services opened 3 auxiliary shelters with capacity to accommodate 344 residents to reduce crowding within existing shelters (decompression). Two additional shelters with capacity to accommodate 392 residents were opened in April and May. After a statewide stay-at-home order on March 21, 2020, shelter residents were permitted to leave and return to a facility only for essential activities [12]. Beginning April 6, 2020, individuals living in dormitory-style accommodation and identified to be at risk of severe COVID-19 illness as a result of age (over 60 years) or comorbidities (aged over 55 with a condition defined as increasing the risk of severe disease [as defined by the CDC, https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-increased-risk.html]) were offered protective housing in individual hotel rooms off-site—these individuals were tested on entry to the hotel and are included in these data with their shelter of origin and accommodation characteristics before entering the hotel (Figure 1).
Figure 1.
Number of daily cases of coronavirus disease 2019 across Chicago, with a timeline of interventions relevant to homeless shelters—Chicago, Illinois, March–May 2020.
Enhanced Reporting of Coronavirus Disease 2019 Cases in Shelters
All cases of COVID-19 in Chicago are reported through the Illinois’ National Electronic Disease Surveillance System (I-NEDSS). Since March 19, 2020, all congregate living facilities have been required to report to CDPH if ≥2 cases have been identified among residents or staff at their facility [13]—homeless shelters were additionally encouraged to report individual cases via a widely publicized online reporting form (https://redcap.dph.illinois.gov/surveys/?s=FR7MAJAY84) and targeted telephone outreach to shelter managers. In addition, cases in residents were detected through matching addresses reported into I-NEDSS to those of homeless shelters.
Point Prevalence Surveys
During April 1–May 1, 2020, point-prevalence surveys were conducted at all shelters with a reported case of COVID-19 in either a resident or staff member. Testing was scheduled, whenever possible, within 7–10 days of a reported index case and at a convenient time to maximize participation. Testing was offered to all residents and staff. Nasopharyngeal swab specimens were tested for SARS-CoV-2 by real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) [14]. Shelters with a point prevalence of ≥5% were offered further testing approximately 1–2 weeks later; at subsequent visits, testing was offered to any individual not previously tested and to those who previously tested negative.
Patient Consent Statement
Verbal consent was obtained from all participants. This study was reviewed by the US Centers for Disease Control and Prevention (CDC) and the CDPH institutional review boards or equivalent and received a nonresearch determination as part of public health response.
Clinical and Epidemiologic Data
A standardized questionnaire collected information about demographics, role (resident or staff) accommodation details, whether they leave the shelter, symptoms, and past medical history. To ascertain subsequent development of symptoms and other clinical outcomes, residents who tested positive for SARS-CoV-2 were reinterviewed by telephone 14 days after their test date. To identify hospitalizations and intensive care unit (ICU) admissions, names and dates of birth of residents testing positive were matched to data from locally available surveillance from acute care hospital emergency departments. To identify deaths, these individuals were also matched to a list of COVID-19-related deaths that occurred under the jurisdiction of the Cook County Medical Examiner’s Office and to state vital records data.
Shelter Assessments
During April 2020, the CDPH deployed medical staff to all shelters to provide one-time on-site training in infection prevention and control to shelter staff. While on-site, CDPH staff collected facility information, including the number of residents and staff, sleeping arrangements, and availability of hand hygiene materials. Follow-up telephone calls with shelter managers collected additional information according to a standard assessment form designed in collaboration with the CDC (the tool used was similar to that now published by the CDC: https://www.cdc.gov/coronavirus/2019-ncov/community/homeless-shelters/infection-control-inventory-planning-tool.pdf). Variables included the size of the facility in square feet, symptom screening practices, the level of decompression compared with pre-COVID-19 maximum occupancy, and the number of bathrooms per facility, including whether these were for the exclusive use of 1 person or family (private bathroom) or used by many individuals (shared bathrooms). Facility factors were reviewed by a team of clinicians and epidemiologists, and factors deemed most relevant to transmission of SARS-CoV-2 were included in statistical analysis.
Statistical Analysis
For individual, self-reported characteristics, we report descriptive statistics according to infection status. Individuals who tested positive at any point were counted as infected with SARS-CoV-2. Prevalence was compared between residents and staff. To understand more about SARS-CoV-2 infections within homeless shelters, subsequent analyses were restricted to residents.
First, we described symptoms on the date of specimen collection and in the following 2 weeks, and we compared their sensitivity and specificity to rRT-PCR. We also describe severe COVID-19 outcomes (hospitalization, admission to ICU, and death). Next, we built individual-level and multilevel models (including facility factors) to identify risk factors for infection. Prevalence ratios (PRs)—with 95% confidence intervals (CIs)—were calculated using log binomial regression models [15]. In the individual, univariable model, factors were analyzed as categorical variables. Multivariable models included all variables with a P value for association of ≤.2 in the univariable analysis and a random effect to control for clustering at the shelter-level and calculated adjusted PRs (aPRs). Facility-level factors were assigned to all residents at each facility. Variables were selected for inclusion by a stepwise selection using the SAS procedure GLMSELECT; factors with a P > .2 were removed from the model. The multilevel model included both facility-level factors and individual-level factors. All analyses were conducted using SAS (version 9.4; SAS Institute). This study was reviewed by CDC and CDPH and deemed to be a public health response.
RESULTS
During March 1–May 1, 2020, 21 homeless shelters with COVID-19 cases in residents or staff were reported to CDPH. Point-prevalence surveys were conducted at all 21 shelters. Although the refusal rate was not formally collected, few residents or staff (estimated <5%) refused specimen collection. The point prevalence found on the first survey in different shelters ranged from 0% to 59%. Eight shelters were tested more than once, 1 site was tested 3 times. Overall, 1717 residents and staff were tested at least once (of which 586 residents and 77 staff were tested more than once) and 472 (27%) were positive for SARS-CoV-2 infection.
The majority of people tested (1435 of 1717, 84%) were residents. Prevalence of SARS-CoV-2 infection was higher for residents (431 of 1435, 30%) than for staff (41 of 282, 15%) (PR = 2.52; 95% CI, 1.78–3.58). Subsequent results refer to the 1435 residents who were tested.
Information on age, gender, and race and ethnicity were available for most residents (age = 99%, gender = 98%, race and ethnicity = 94%). Most were male (1023 of 1412, 72%), and non-Hispanic black (879 of 1353, 65%), with a median age of 52 (interquartile range, 39–60 years). More than 85% of resident respondents completed the questionnaire and provided self-reported information on symptoms, medical history, smoking status, and accommodation details. Many (600 of 1265, 47%) slept in shared rooms of more than 20 people (Table 1).
Table 1.
Self-Reported Characteristics of Residents in Homeless Shelters, by SARS-CoV-2 PCR Test Result—Chicago, March–May 2020
| Positive | Negative | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | (%) | n | (%) | n | (%) | Crude Prevalence Ratio (95% CI) | P Value |
| Total | 431 | (30.0) | 1004 | (70.0) | 1435 | (100) | ||
| Age Group (Years) | ||||||||
| <40 | 80 | (18.8) | 307 | (30.6) | 387 | (27.1) | 1.0 | |
| 40–55 | 132 | (31.0) | 330 | (32.9) | 462 | (32.3) | 1.43 (1.13–1.80) | <.01 |
| >55 | 214 | (50.2) | 366 | (36.5) | 580 | (40.6) | 1.74 (1.40–2.16) | <.01 |
| Gender | ||||||||
| Female | 128 | (30.5) | 261 | (26.3) | 389 | (27.6) | 1.0 | |
| Male | 292 | (69.5) | 731 | (73.7) | 1023 | (72.5) | 0.98 (0.83–1.16) | .80 |
| Race or Ethnic Group | ||||||||
| Non-Hispanic Black | 257 | (63.8) | 622 | (65.5) | 879 | (65.0) | 1.0 | |
| Non-Hispanic White | 83 | (20.6) | 145 | (15.3) | 228 | (16.9) | 1.27 (1.04–1.54) | .02 |
| Hispanic | 45 | (11.2) | 139 | (14.6) | 184 | (13.6) | 0.84 (0.64–1.10) | .21 |
| Non-Hispanic Other | 18 | (4.5) | 44 | (4.6) | 62 | (4.6) | 0.96 (0.64–1.43) | .85 |
| Medical Historya | ||||||||
| Respiratory conditionb | 49 | (14.4) | 124 | (13.4) | 173 | (13.7) | 1.00 (0.78–1.29) | .99 |
| Cardiovascular conditionb | 83 | (24.3) | 182 | (19.7) | 265 | (21.0) | 1.16 (0.94–1.42) | .16 |
| Diabetes mellitusb | 43 | (12.6) | 87 | (9.4) | 130 | (10.3) | 1.16 (0.90–1.51) | .26 |
| Smoking Statusa | ||||||||
| Never | 135 | (39.2) | 341 | (37.6) | 476 | (38.0) | 1.0 | |
| Current | 113 | (32.9) | 412 | (45.4) | 525 | (41.9) | 0.80 (0.65–0.99) | .04 |
| Former | 96 | (27.9) | 155 | (17.1) | 251 | (20.1) | 1.29 (1.04–1.59) | .02 |
| Sleeping Arrangementsa | ||||||||
| Single room | 26 | (6.7) | 207 | (23.6) | 233 | (18.4) | 1.0 | |
| Shared room (2–4 people) | 40 | (10.4) | 114 | (13.0) | 154 | (12.2) | 2.33 (1.49–3.64) | <.01 |
| Shared room (5–8 people) | 50 | (13.0) | 79 | (9.0) | 129 | (10.2) | 3.47 (2.28–5.29) | <.01 |
| Shared room (9–20 people) | 47 | (12.2) | 102 | (11.6) | 149 | (11.8) | 2.83 (1.84–4.35) | <.01 |
| Shared room (>20 people) | 223 | (57.8) | 377 | (42.9) | 600 | (47.4) | 3.33 (2.29–4.85) | <.01 |
| Shelter Exposurea | ||||||||
| Leave and return to shelter during dayb | 144 | (49.5) | 405 | (48.8) | 549 | (49.0) | 1.06 (0.87–1.29) | .57 |
Abbreviations: CI, confidence interval; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aMore than 10% missing data in selected variables.
bReference group for each is “not,” eg, not reporting a respiratory condition, or not leaving the shelter during the day.
On the date of specimen collection, 331 (77%) of 431 residents with SARS-CoV-2 infection provided information on their symptoms compared with 898 (89%) of 1004 without SARS-CoV-2 infection. Of the 331 with SARS-CoV-2 infection, 89 (27%) reported symptoms compared with 140 (16%) of 898 without SARS-CoV-2 infection. Among those with symptomatic SARS-CoV-2 infection, the most commonly reported symptoms were cough (n = 56, 63%), congestion or runny nose (n = 38, 43%), and subjective or measured fever (n = 35, 39%). When compared with rRT-PCR, the specificity of reporting any symptoms on the date of specimen collection was 84%. When restricting symptoms to those in the Council of State and Territorial Epidemiologists (CSTE) surveillance case definition [16], specificity improved to 93%, although sensitivity declined to 14%. (The CSTE case definition includes at least 1 of cough, shortness of breath or difficulty breathing, or at least 2 of fever (measured or subjective), chills, rigors, myalgia, headache, sore throat, or new loss of taste or smell. Although the CSTE case definition includes rigors as one of the minor symptoms, rigors were not captured in our questionnaires and were thus not included in our symptom analysis.) In this population, the positive predictive value of reporting any new symptom on the date of specimen collection was 39%.
Most residents with SARS-CoV-2 infection (314 of 431, 73%) were reached for follow up, including 75 who did not provide information on symptoms initially. Twenty-four residents, who initially did not provide information on symptoms or were asymptomatic, reported developing symptoms within 2 weeks of testing positive, such that, overall, 113 (28%) of 406 residents with SARS-CoV-2 infection, who provided some information about symptoms, reported any symptom at any point, whereas 293 (72%) reported no symptoms at the time of specimen collection or within the following 2 weeks.
Fifty-seven (13%) of 431 residents with SARS-CoV-2 were hospitalized due to COVID-19, with 19 requiring ICU admission. Two residents with SARS-CoV-2 infection died (2 of 431, 0.5%).
Risk Factors for Severe Acute Respiratory Syndrome Coronavirus 2 Infection
Individual Model
In the unadjusted analysis, SARS-CoV-2 prevalence was higher for older individuals (PR for those aged >55 years compared with those aged <40 years =1.74; 95% CI, 1.40–2.16; P < .01) and for non-Hispanic white persons compared with non-Hispanic black persons (PR = 1.27; 95% CI, 1.04–1.54; P = .02). Prevalence was higher for individuals sleeping in shared rooms (PR for sharing a room with >20 people compared with a single room = 3.33; 95% CI, 2.29–4.85; P < .01). Prevalence was lower for current smokers compared with people who had never smoked (PR = 0.80; 95% CI, 0.65–0.99; P = .04). Prevalence did not differ by self-reported medical history or gender (Table 1).
After adjusting for individual-level factors (age, race or ethnicity, smoking status, accommodation details) and for clustering at the shelter level, PRs no longer differed by age or race and ethnicity. Sharing a room with >20 people remained associated with a higher prevalence (aPR = 1.70; 95% CI, 1.07–2.69; P = .03), and current smoking remained associated with a lower prevalence of infection compared with never smoking (aPR = 0.73; 95% CI, 0.61–0.87; P < .01) (Table 2).
Table 2.
Adjusted Prevalence Ratios of SARS-CoV-2 Infection by Self-Reported Characteristics of Residents of Homeless Shelters—Chicago, March–May 2020
| Characteristic | Residents Only n = 1148 | ||
|---|---|---|---|
| Adjusted Prevalence Ratioa | (95% CI) | P Value | |
| Age Group (Years) | |||
| <40 | 1.0 | ||
| 40–55 | 0.92 | (0.73–1.17) | .51 |
| >55 | 1.00 | (0.80–1.24) | .98 |
| Race or Ethnic Group | |||
| Non-Hispanic Black | 1.0 | ||
| Non-Hispanic White | 1.03 | (0.84–1.25) | .8 |
| Hispanic | 1.16 | (0.91–1.48) | .22 |
| Non-Hispanic Other | 1.09 | (0.75–1.60) | .65 |
| Cardiovascular Conditionb | |||
| Yes | 1.13 | (0.95–1.34) | .18 |
| Current Smokerc | |||
| Yes | 0.73 | (0.61–0.87) | <.01 |
| Sleeping Arrangements | |||
| Single room | 1.0 | ||
| Shared room (2–4 people) | 1.32 | (0.86–2.02) | .2 |
| Shared room (5–8 people) | 1.50 | (0.95–2.36) | .09 |
| Shared room (9–20 people) | 1.55 | (0.95–2.53) | .08 |
| Shared room (>20 people) | 1.70 | (1.07–2.69) | .03 |
Abbreviations: CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aAdjusted for those variables in the unadjusted model with an association with SARS-CoV-2 infection with a P < .2, and treating shelter as a random effect.
bCompared to not reporting a cardiovascular condition.
cCompared to never and former smokers combined.
Multilevel Model
Consensus review identified 12 facility-level factors suspected to influence transmission (Table 3). Some of these—regular environmental cleaning, use of face coverings, availability of hand hygiene materials, symptom screening, and isolation of symptomatic residents—were near-universally reported and thus excluded from the combined model.
Table 3.
Characteristics of 21 Homeless Shelters in Chicago With Reported Cases of COVID-19, March–May 2020
| Characteristic | Values | |
|---|---|---|
| Crowding | ||
| Average occupancy (median, range) | 65 | (8–450) |
| Residents per 1000 square feet (median, range) | 4 | (1–17) |
| Percent decompressed (current/maximum occupancy (%), range) | 43 | (13–87) |
| Communal bathrooms per 100 residents (median, range) | 3 | (0–25) |
| Private bathrooms per 100 residents (median, range) | 0.5 | (0–80) |
| Sleeping Arrangements | ||
| Number of dormitory style rooms (median, range) | 2 | (0–38) |
| Distance between beds ≥3feet (n, %) | 19 | (90.5) |
| Barriers between beds (n, %) | 4 | (21.1) |
| Staff | ||
| Staff rotate to other sites (n, %) | 11 | (42.3) |
| Staff: resident ratio (ratio) | 1:4 | (1:1–1:20) |
| Temperature checks for staff before each shift (n, %) | 11 | (50.0) |
| Residents | ||
| Proportion of residents leaving and returning each day (%, range) | 44 | (7–88) |
Abbreviations: COVID-19, coronavirus disease 2019.
In the multilevel model, after adjusting for facility-level factors with a P value for association with SARS-CoV-2 infection of <0.2 (number of residents per 1000 square feet, number of bathrooms per resident, and the proportion of residents leaving and returning each day) and those variables in the individual model, sharing a room with >20 people remained associated with increased prevalence of SARS-CoV-2 infection, and currently smoking remained associated with reduced prevalence of SARS-CoV-2 infection with similar effect sizes. The proportion of residents leaving and returning each day was associated with increased prevalence of infection in the combined model (aPR for an additional 1% of residents reporting leaving the facility and returning each day = 1.08; 95% CI, 1.01–1.16; P = .03), and an increase in the number of private bathrooms was associated with a reduced prevalence of infection (aPR for 1 additional private bathroom per 100 people = 0.92; 95% CI, 0.87–0.98; P = .02) (Table 4).
Table 4.
Prevalence Ratios of SARS-CoV-2 Infection in Residents of Homeless Shelters, Adjusted for Individual- and Facility-Level Factors in a Multilevel Log Binomial Model—Chicago, March–May 2020
| Characteristic | Adjusted Prevalence Ratioa n = 1268 | (95% CI) | P Value |
|---|---|---|---|
| Individual-Level Factors | |||
| Current smoker | 0.71 | (0.60–0.85) | <.01 |
| Sleeping Arrangements | |||
| Single room | 1.0 | ||
| Shared room (2–4 people) | 1.35 | (0.87–2.11) | .19 |
| Shared room (5–8 people) | 1.59 | (1.00–2.53) | .05 |
| Shared room (9–20 people) | 1.64 | (1.00–2.70) | .05 |
| Shared room (>20 people) | 1.76 | (1.11–2.80) | .02 |
| Facility-Level Factors | |||
| Residents per 1000 square feet | 0.86 | (0.68–1.08) | .10 |
| Communal bathrooms per 100 residents | 0.89 | (0.74–1.07) | .22 |
| Private bathrooms per 100 residents | 0.92 | (0.87–0.98) | .02 |
| Proportion of residents leaving and returning each day | 1.08 | (1.01–1.16) | .03 |
DISCUSSION
Our findings demonstrate that SARS-CoV-2 can infect large numbers of people in homeless shelters. Residents were particularly vulnerable to infection, although 15% of staff who live offsite were also found to be infected. Outbreaks involving a similarly high proportion of shelter residents and staff have been reported from Boston [17], Seattle [18], and San Francisco [1]. Our analyses also suggest the shelter residents most likely to acquire SARS-CoV-2 infection were those sleeping in a room with large numbers of other people and those residing in shelters where a higher proportion of residents leave and return to the facility each day, possibly reflecting increased opportunities for exposure and then viral introduction.
Our analysis also found that current smokers appeared less likely to be infected with SARS-CoV-2 than nonsmokers. Current smokers might spend additional time outside due to no smoking policies within shelter, and thus they might avoid some exposures within the congregate setting. In cohorts in China and France, the prevalence of smoking in patients with COVID-19 has also been noted to be lower than that in the general population [19–21]. However, it is well documented that a smoking history is associated with both worse outcomes from COVID-19 [22, 23] and higher rates of other respiratory infections [24, 25]. The significance of this finding in our cohort is therefore not well understood.
Our findings support efforts to reduce the total shelter population and number of individuals sharing rooms at shelters. Based on these findings, protectively housing individuals at increased risk of severe illness in single rooms, away from dormitory-style settings, appears beneficial. In addition, measures to reduce movement into and out of shelters when community transmission is high, such as shelter-in-place orders, might reduce infection risk.
In this population, the majority of people with SARS-CoV-2 infection reported no symptoms. This finding is similar to reports from other cities where widespread testing of people experiencing homelessness has been performed [17, 18]. The true proportion of asymptomatic SARS-CoV-2 infections is not yet known, although most estimates are lower than that reported here [26]. The low proportion of symptomatic individuals identified in our study may reflect the difficulty in detecting new symptoms in this population with a high prevalence of underlying symptoms, including pain, fatigue, gastrointestinal and respiratory complaints [27], and the presence of background symptoms was not elicited in this study. Alternatively, residents may have underreported symptoms due to mistrust of the healthcare system, a belief (mentioned to the testing team) that they may lose their accommodation if openly symptomatic, or substance misuse or mental illness that may reduce ability to articulate symptoms. However, the rates of hospitalization and death were also lower than previously published estimates [28, 29], which was unexpected given the high prevalence of underlying conditions in people experiencing homelessness. Taken together, these findings might represent the true prevalence of asymptomatic and mild infection when widespread asymptomatic testing is conducted in this population or may be due to the policy—implemented before the majority of these point-prevalence surveys—of providing individual hotel rooms to protectively house people most likely to develop severe disease.
The strengths of this study lie in (1) the size of the population and variety of the shelters studied as well as (2) the detailed information collected to facilitate adjustment for individual-level and facility-level confounders. However, these findings are subject to several limitations. First, PCR testing only detects current infections—the data presented here were collected several weeks into the epidemic in Chicago, and so they may underestimate factors that increased risk of infection early in the epidemic. Second, most clinical and epidemiologic data were self-reported and might be subject to reporting biases. Third, some facility-level factors were ascertained during follow-up telephone calls several weeks after testing and, in some instances, data were estimated (eg, square footage of a facility without floor plans). In addition, some important variables likely to impact an individual’s risk of infection were not included in the analysis. For example, the time since detection of the first case in a shelter was excluded given varied access to testing, the use of cloth face coverings was universally reported but regularly observed to be inaccurate by the testing team, and information on the orientation of beds (eg, sleeping head to toe) was not collected. Finally, although the shelters included in our study represent a range of settings, results may not be generalizable to other cities or people living in different types of congregate settings.
CONCLUSIONS
The findings in this report support several recommendations issued by the CDC, including establishing overflow sites to reduce crowding (shelter decompression) and offering protective housing in individual rooms for people who are at highest risk of severe COVID-19 [30]. The nationwide outbreak of COVID-19 continues to put those living in congregate settings, including homeless shelters, at increased risk of infection. Coronavirus disease 2019 has the capacity to exacerbate poor health outcomes; given the potential for large outbreaks in homeless shelters, connecting people experiencing homelessness to primary care to manage modifiable risk factors is likely beneficial. In addition, action beyond the healthcare system—including providing pathways to stable housing—is important to mitigate the impact of COVID-19 in people experiencing homelessness.
Acknowledgments
Disclaimer. The opinions expressed by authors contributing to this article do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Financial support. This work was funded by the Chicago Department of Public Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Mosites E, Parker EM, Clarke KEN, et al. Assessment of SARS-CoV-2 infection prevalence in homeless shelters — four U.S. Cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boonyaratanakornkit J, Ekici S, Magaret A, et al. Respiratory syncytial virus infection in homeless populations, Washington, USA. Emerg Infect Dis 2019; 25:1408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamrah S, Yelk Woodruff RS, Powell K, et al. Tuberculosis among the homeless, United States, 1994–2010. Int J Tuberc Lung Dis 2013; 17:1414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Alliance to End Homelessness. Population at-risk: homelessness and the COVID-19 crisis. Available at: https://endhomelessness.org/wp-content/uploads/2020/03/Covid-Fact-Sheet-3.25.2020-2.pdf. Accessed 11 November 2020.
- 5. Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014; 384:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baggett TP, Hwang SW, O’Connell JJ, et al. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern Med 2013; 173:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moses J. Demographic Data Project: Race, Ethnicity, and Homelessness Homeless Research Institute, National Alliance to End Homelessness; Available at: https://endhomelessness.org/wp-content/uploads/2019/07/3rd-Demo-Brief-Race.pdf. Accessed 11 November 2020. [Google Scholar]
- 8. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020; 382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. COVID-19 in Racial and Ethnic Minority Groups Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html. Accessed 11 November 2020.
- 10. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med 2020; 382:2158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. City of Chicago 2019 Homeless Point-in-Time Count & Survey Report Available at: https://www.chicago.gov/content/dam/city/depts/fss/supp_info/Homeless/2019PITReportFinal110819.pdf. Accessed 11 November 2020.
- 12. Pritzker JB. Executive Order 2020–10, issued March 20, 2020: executive order to expand telehealth services and protect health care providers in response to COVID-19 (COVID-19 Executive Order No. 8) Available at: https://www2.illinois.gov/Pages/Executive-Orders/ExecutiveOrder2020-10.aspx. Accessed 11 November 2020.
- 13. Arwady MA. Order of the Commissioner of Health of the City of Chicago No. 2020–2: duties of Hospitals and Other Congregate Facilities Available at: https://www.chicago.gov/content/dam/city/depts/cdph/HealthProtectionandResponse/CDPHOrderDutiesofHospitalsFinal3.19.20.pdf. Accessed 11 November 2020.
- 14. Abbott Molecular, Inc. Abbott RealTime SARS-CoV-2 Assay: Fact Sheet for Healthcare Providers Available at: https://www.molecular.abbott/sal/3-EUA200023-HCP-FS-03182020.pdf. Accessed 11 November 2020.
- 15. Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med 1998; 55:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Council of State and Territorial Epidemiologists (CSTE). Standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Interim-20-ID-01 Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf. Accessed 11 November 2020.
- 17. Baggett TP, Keyes H, Sporn N, Gaeta JM. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 2020; 323:2191–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobolowsky FA, Gonzales E, Self J, et al. COVID -19 outbreak among three affiliated homeless service sites — King County, Washington, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M, Liu S, Yang L, et al. Prevalence of smoking and knowledge about the smoking hazards among 170 000 Chinese adults: a nationally representative survey in 2013–2014. Nicotine Tob Res 2019; 21:1644–51. [DOI] [PubMed] [Google Scholar]
- 21. Miyara M, Tubach F, Pourcher V, Morelot-Panzini C, Pernet J, Lebbah S, et al. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2020. Available at: 10.32388/WPP19W.3. Accessed 11 November 2020. [DOI]
- 22. Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis 2020; 18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res 2020; 22:1653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawrence H, Hunter A, Murray R, et al. Cigarette smoking and the occurrence of influenza - systematic review. J Infect 2019; 79:401–6. [DOI] [PubMed] [Google Scholar]
- 25. Baskaran V, Murray RL, Hunter A, et al. Effect of tobacco smoking on the risk of developing community acquired pneumonia: a systematic review and meta-analysis. PLoS One 2019; 14:e0220204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. CDC. COVID-19 Pandemic Planning Scenarios Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios-h.pdf. Accessed 11 November 2020.
- 27. Patanwala M, Tieu L, Ponath C, et al. Physical, psychological, social, and existential symptoms in older homeless-experienced adults: an observational study of the Hope Home Cohort. J Gen Intern Med 2018; 33:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) – United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69;343–6. [DOI] [PMC free article] [PubMed]
- 29. Rajgor DD, Lee MH, Archuleta S, et al. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis 2020; 20:776–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CDC. Interim Guidance for Homeless Service Providers to Plan and Respond to Coronavirus Disease 2019 (COVID-19) Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/homeless-shelters/plan-prepare-respond.html. Accessed 11 November 2020.