Abstract
Background
There is growing concern about the potential harmful effects of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) in patients with coronavirus disease 2019 (COVID-19) and cardiovascular diseases (CVDs). The aim of this study was to evaluate the association between recent exposure to ACEIs/ARBs and in-hospital mortality in patients with COVID-19.
Methods
We used data from a nationwide cohort of patients with COVID-19 from the health insurance claims data of South Korea, which were released for research purposes for public health by the Ministry of Health and Welfare of South Korea. Patients with COVID-19 were identified using the relevant diagnostic code. Propensity score matching (1:1) was carried out among patients with CVD according to the type of medication (ACEIs/ARBs vs other), and the risk of death was assessed.
Results
A total of 4936 patients with COVID-19 were analyzed, of whom 1048 (21.2%) had CVD. Of the 1048 patients with CVD, 864 (82.4%) received at least 1 antihypertensive medication before the diagnosis of COVID-19, including 359 (41.6%) who received ACEIs/ARBs and 505 (58.4%) who received drugs other than ACEIs/ARBs. Using the propensity scores for ACEI/ARB use, we matched 305 pairs of patients receiving ACEIs/ARBs and patients receiving other drugs. Recent use of ACEIs/ARBs was not significantly associated with in-hospital mortality in unadjusted analysis (odds ratio [OR], 0.62; 95% CI, 0.33–1.14) or propensity score matching analysis (OR, 1.00; 95% CI, 0.46–2.16).
Conclusions
In patients with COVID-19 and underlying CVDs, the recent use of ACEIs/ARBs was not significantly associated with in-hospital mortality. These findings do not support stopping or modifying ACEIs/ARBs in patients during the current COVID-19 pandemic.
Keywords: angiotensin-converting enzyme 2, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2
The morbidity and mortality associated with the ongoing coronavirus disease 2019 (COVID-19) pandemic are particularly severe in older adults with underlying cardiovascular diseases (CVDs), who are commonly treated with renin-angiotensin system (RAS) inhibitors such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) [1, 2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters host cells by interacting with angiotensin-converting enzyme 2 (ACE2) as the receptor [3]. Notably, increased ACE2 expression has been shown to be associated with RAS inhibitors in an animal model [4], and there is a growing concern that continuing RAS inhibitor treatment in patients with COVID-19 may negatively affect clinical outcomes. On the other hand, ACE2 has been shown to have a significant protective role in severe acute lung injuries [5, 6], suggesting that RAS inhibitors may confer clinical benefits rather than harm in COVID-19 by upregulating the expression of ACE2. We thus evaluated the effects of recent exposure to ACEIs/ARBs on in-hospital mortality in patients with COVID-19 and underlying CVDs.
METHODS
Study Design and Database
This study was conducted using the National Health Insurance (NHI) claims data of the COVID-19 patient cohort released by the Ministry of Health and Welfare of South Korea. This claims database includes comprehensive information on health care services such as procedures, diagnostic tests, and treatment, including prescriptions as well as the demographic characteristics of patients [7, 8]. Adult patients (aged 18 years or older) who were hospitalized for COVID-19 between January 20, 2020, and March 31, 2020, were identified. In this population with confirmed COVID-19 patients, individuals with CVDs, including hypertension, ischemic heart disease, or heart failure, were identified. Among these CVD patients, recent use of ACEIs/ARBs or other medicines for CVD was assessed by checking prescription records. The in-hospital mortality of patients with CVD receiving ACEIs/ARBs was comparted with that of patients with CVD receiving cardiovascular medication other than ACEIs/ARBs.
Patient Consent Statement
The protocol of this study was approved by the Institutional Review Board of Asan Medical Center (S2020-0489), which waived the requirement for written or verbal consent from the patients based on the observational nature of the study and the fact that the patient identifiers were fully encrypted before analysis.
Definitions
Patients with COVID-19 were identified by the relevant diagnostic code (U07.1) according to the International Classification of Diseases, Tenth Revision (ICD-10). An emergency ICD-10 code for COVID-19 was announced by the World Health Organization, and “U07.1” was assigned to the diagnosis of COVID-19 confirmed by laboratory testing. In South Korea, all COVID-19 cases were diagnosed by reverse transcription PCR (RT-PCR) in lower respiratory tract samples or nasopharyngeal swab samples. The date of hospitalization with diagnosis of COVID-19 was defined as the index date. Underlying comorbidities were identified using ICD-10 codes when 2 or more hospital visits with the relevant diagnostic codes within a year before the index date were recorded. Patients with CVDs included those with hypertension, ischemic heart disease, or heart failure, as described elsewhere [9]. Prescription history was determined when records of reimbursements for ACEIs/ARBs or other drugs were confirmed for at least 30 days until the date of admission for COVID-19. Death was determined by identifying all inpatient claim records that indicated death.
Statistical Analysis
Categorical variables are presented as frequency (percentage), and continuous variables are presented as mean (SD); statistical comparisons were made using the Mann-Whitney U test, Student t test, χ 2 test, or Fisher exact test, as appropriate. For patients with CVD, a propensity score–matched cohort comprising ACEI/ARB users and non–ACEI/ARB users (active comparator group) was created and adjusted for potential confounders including age, sex, types of insurance coverage, diabetes, dyslipidemia, chronic lung disease, chronic renal disease, chronic liver disease, rheumatic disease, inflammatory bowel disease, malignancy including hematologic malignancy and solid tumor, solid organ transplant, HIV infection, depression, and duration of CVD. The drugs used in the comparator group included beta-blockers, calcium-channel blockers, diuretics, and alpha-blockers (Supplementary Table 1).
Each individual in the ACEIs/ARBs group was matched with an individual in the active comparator group at a 1:1 ratio using propensity scores. The propensity score–matched pairs were created using calipers of width equal to 0.1 SDs of the logit of the propensity score. We employed the standardized difference of means (SDM) to check for differences in baseline characteristics, and SDMs <0.1 indicated a negligible difference. Model discrimination was assessed with c-statistics (0.671), and model calibration was assessed with the Hosmer-Lemeshow test (χ 2 = 7.8094; df = 8; P = .4523). After calculating the predicted probabilities, we matched each ACEI/ARB user to the other drug users using the Greedy algorithm. Odds ratios and corresponding confidence intervals for in-hospital death were calculated by conditional logistic regression in the matched samples. In addition, several sensitivity analyses were performed to test the robustness of our findings. In these analyses, we excluded i) patients with heart failure or ii) patients with heart failure or ischemic heart disease.
All reported P values are 2-sided, and those <.05 were considered statistically significant. Data manipulation and statistical analyses were conducted using SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
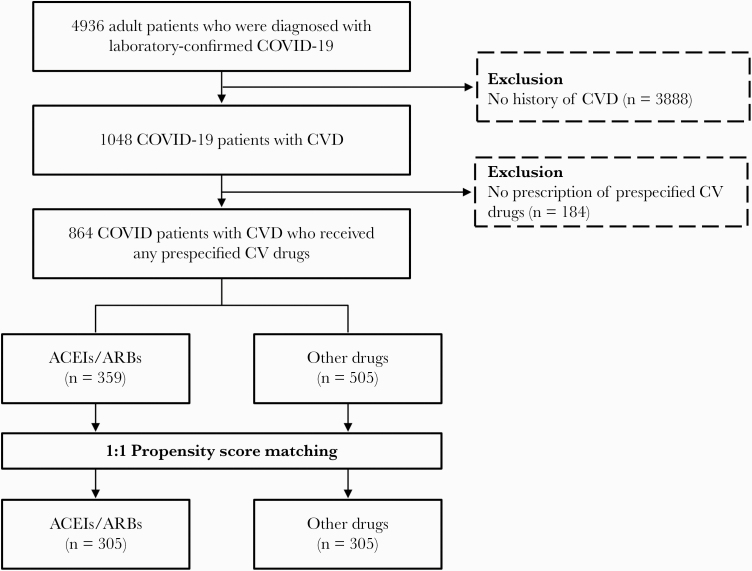
A total of 4936 adult patients (aged 18 years or older) with COVID-19 were hospitalized between January 20, 2020, and March 31, 2020. The mean age was 44.6 years, and 2746 (55.6%) were female. The overall mortality rate was 1.7% (84/4936). Among COVID-19 patients, 1048 (21.2%) had CVD and the remaining 3888 (78.8%) did not. The baseline clinical characteristics of patients with CVD and those without are shown in Supplementary Table 2. Patients with CVD (5.2% [55/1048]) had higher mortality than those without CVD (0.7% [29/3888]; P < .001). Of the 1048 patients with CVD, 184 (17.5%) who did not receive any antihypertensive drug until the diagnosis of COVID-19 were excluded from the final analysis because they could have had different clinical characteristics (ie, mild hypertension, white coat hypertension) compared with those who received antihypertensive drugs. Finally, the remaining 864 patients who had a prescription history of 1 or more predefined drugs before the diagnosis of COVID-19 were analyzed. Of these 864 patients, 359 (41.6%) were ACEI/ARB users, as shown in Figure 1.
Figure 1.
Flowchart of study population and propensity score–matched cohort. Abbreviations: ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; COVID-19, coronavirus disease 2019; CV, cardiovascular; CVD, cardiovascular disease.
The demographic characteristics of the ACEI/ARB user group and the comparator group that received drugs other than ACEIs/ARBs are summarized in Table 1. Compared with non–ACEI/ARB users, those receiving ACEIs/ARBs had a higher prevalence of diabetes (35.6% vs 46.2%; P = .002) and chronic kidney disease (11.9% vs 30.1%; P < .001). Fourteen (3.9%) patients in the ACEI/ARB group and 32 (6.3%) patients in the non-ACEI/ARB group died during hospitalization; crude analysis revealed that recent use of ACEIs/ARBs was not significantly associated with mortality (odds ratio [OR], 0.62; 95% CI, 0.33–1.14) (Table 2). After propensity score matching, all variables were adjusted, and the SDM for each variable was <10% (Table 1). In the matched cohort, both groups consisted of 305 patients. Propensity score matching analysis also revealed that the recent use of ACEIs/ARBs was not significantly associated with mortality (4.6% [14/305] vs 4.6% [14/305]; OR, 1.00; 95% CI, 0.46–2.16) (Table 2). A difference in COVID-19 mortality according to ACEI/ARB use was not observed in the sensitivity analyses with a modified definition of CVD (Supplementary Table 3). In addition, ACEI/ARB use was not significantly associated with severe COVID-19, including admission to intensive care unit and the need for mechanical ventilation (Supplementary Table 4).
Table 1.
Baseline Clinical Characteristics of Patients With COVID-19 and CVD According to ACEI/ARB Use Before and After Propensity Score Matching
| Before Matching, No. (%) | After Matching, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| ACEIs/ARBs(n = 359) | Others(n = 505) | P | SDM | ACEIs/ARBs(n = 305) | Others(n = 305) | P | SDM | |
| Age, mean (SD), y | 63.3 (14.5) | 65.3 (14.1) | 63.8 (14.5) | 63.5 (14.2) | ||||
| <50 | 61 (17) | 77 (15.2) | .49 | –0.0696 | 49 (16.1) | 51 (16.7) | .83 | 0.0202 |
| ≥50 | 298 (83) | 428 (84.8) | 256 (83.9) | 254 (83.3) | ||||
| Sex | ||||||||
| Male | 199 (55.4) | 245 (48.5) | .045 | 0.1388 | 165 (54.1) | 165 (54.1) | >.99 | 0 |
| Female | 160 (44.6) | 260 (51.5) | 140 (45.9) | 140 (45.9) | ||||
| Type of insurance coverage | ||||||||
| National health insurance | 299 (83.3) | 435 (86.1) | .25 | –0.0793 | 257 (84.3) | 261 (85.6) | .65 | –0.0365 |
| Medicaid | 60 (16.7) | 70 (13.9) | 48 (15.7) | 44 (14.4) | ||||
| Underlying diseases | ||||||||
| Cardiovascular diseasea | ||||||||
| Hypertension | 355 (98.9) | 491 (97.2) | .09 | 302 (99) | 298 (97.7) | .20 | ||
| Ischemic heart disease | 77 (21.4) | 71 (14.1) | .005 | 67 (22) | 46 (15.1) | .03 | ||
| Heart failure | 73 (20.3) | 70 (13.9) | .01 | 59 (19.3) | 44 (14.4) | .11 | ||
| Diabetes | 166 (46.2) | 180 (35.6) | .002 | –0.2168 | 139 (45.6) | 133 (43.6) | .63 | –0.0402 |
| Dyslipidemia | 232 (64.6) | 316 (62.6) | .54 | –0.0426 | 206 (67.5) | 193 (63.3) | .27 | –0.0886 |
| Chronic lung diseaseb | 95 (26.5) | 160 (31.7) | .10 | 0.1152 | 87 (28.5) | 86 (28.2) | .93 | –0.0072 |
| Chronic kidney disease | 108 (30.1) | 60 (11.9) | <.001 | –0.4586 | 67 (22) | 58 (19) | .37 | –0.0744 |
| Chronic liver disease | 6 (1.7) | 7 (1.4) | .73 | –0.0232 | 5 (1.6) | 4 (1.3) | >.99 | –0.0267 |
| Rheumatic disease | 10 (2.8) | 23 (4.6) | .18 | 0.0942 | 10 (3.3) | 8 (2.6) | .63 | –0.0349 |
| Inflammatory bowel disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Solid tumor | 42 (11.7) | 57 (11.3) | .85 | –0.0129 | 34 (11.1) | 35 (11.5) | .90 | 0.0103 |
| Hematologic malignancy | 1 (0.3) | 2 (0.4) | >.99 | 0.0203 | 0 (0) | 0 (0) | ||
| Solid organ transplantation | 6 (1.7) | 4 (0.8) | –0.0798 | 3 (1) | 3 (1) | >.99 | ||
| HIV | 1 (0.3) | 0 (0) | . | 0 (0) | 0 (0) | . | ||
| Depression | 10 (2.8) | 7 (1.4) | .14 | –0.0980 | 9 (3) | 7 (2.3) | .61 | –0.0459 |
| Treatment | ||||||||
| Lopinavir/ritonavir | 33 (14.4) | 69 (21.8) | .03 | 48 (15.7) | 67 (22.0) | .049 | ||
| Hydroxychloroquine sulfate | 11 (4.8) | 35 (11.1) | .01 | 21 (6.9) | 35 (11.5) | .049 | ||
| Camostat mesilate | 1 (0.4) | 0 (0) | .42 | 1 (0.3) | 0 (0) | >.99 | ||
| Nafamostat mesilate | 0 (0) | 2 (0.6) | .51 | 0 (0) | 1 (0.3) | >.99 | ||
| Methylprednisolone | 1 (0.4) | 11 (3.5) | .02 | 3 (1) | 9 (3.0) | .14 | ||
Abbreviations: ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; SDM, standardized difference of means.
aCardiovascular disease includes hypertension, heart failure, and ischemic heart disease.
bChronic lung disease includes chronic obstructive pulmonary disease and asthma.
Table 2. .
Risk of Mortality in COVID-19 Patients Receiving ACEIs/ARBs Compared With Those Receiving Other Drugs
| ACEIs/ARBs Users, No | Event, No (%) | Other Drug Users, No | Event, No (%) | OR (95% CI) | |
|---|---|---|---|---|---|
| Before PS matching | 359 | 14 (3.9) | 505 | 32 (6.3) | 0.62 (0.33–1.14) |
| After PS matching | 305 | 14 (4.6) | 305 | 14 (4.6) | 1.00 (0.46–2.16) |
Abbreviations: ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; COVID-19, coronavirus disease 2019; OR, odds ratio; PS, propensity score.
DISCUSSION
Due to a lack of available clinical data, concerns regarding the possible harmful effects of RAS inhibitors on patients with COVID-19 have been growing. Two recent observational studies from China have provided some evidence supporting the continuous use of RAS inhibitors for patients with hypertension who are hospitalized with COVID-19 [10, 11]. Li et al. found no significant associations between baseline treatment with ACEIs/ARBs and severity of clinical outcomes in a cohort of 362 patients with COVID-19 who were admitted to a single center in Wuhan [10]. Using propensity score–matched analysis in 1128 patients from 9 hospitals in China, Zhang et al. reported similar observations that the inpatient use of ACEIs/ARBs was associated with a lower risk of all-cause mortality [11]. However, these studies focused on the association of the in-hospital use of ACEIs/ARBs with mortality in patients who required hospitalization due to moderate to severe COVID-19. Therefore, such patient selection is likely to have led to bias that a certain proportion of patients with severe COVID-19 showing hypotension might have recently discontinued the use of antihypertensive drugs. Therefore, data on patients with prior or recent exposure to ACEIs/ARBs before the diagnosis of COVID-19 are limited, especially in those with varying degrees of COVID-19 including mild diseases in the general population.
As of May 1, 2020, 2 additional observational studies have been published [12, 13]. Propensity score matching analysis from New York Langone Health comprising 5894 patients with SARS-CoV-2 and 1002 patients with severe COVID-19 showed no significant associations between prior use of ACEIs/ARBs and diagnosis of COVID-19 or severe COVID-19 [12]. A population-based case–control study from Italy also revealed that recent use of ACEIs/ARBs was not significantly associated with likelihood of SARS-CoV-2 infection or severe COVID-19 [13]. Therefore, the results of our analysis using the propensity-matched cohort in patients with CVD according to the recent use of ACEIs/ARBs from nationwide data are in line with the latter studies [12, 13]. Taken together, these observational studies in different populations with different study designs do not consistently provide evidence that recent or in-hospital use of ACEI/ARB is associated with the risk of SARS-CoV-2 infection, severe illness, or death. Therefore, these data support the recommendations of multiple international scientific communities that RAS inhibitors should be continued for indications known to be beneficial in otherwise stable patients with COVID-19 [14, 15].
This study has several limitations inherent to its observational nature and to the use of health insurance claims data, such as possible misclassification. However, the structure of the population in our study in terms of age and sex was consistent with that of the data announced by the Korean government in the same period [16]. Second, there is a possibility of misclassification because the diagnosis of diseases was made only by ICD-10 codes. In addition, we were not able to assess compliance of patients to the prescribed medicine. Third, there were only 46 deaths in the included patients receiving medication for CVD, which may limit the power of the analysis in this study. In addition, information on the specific exposure status to ACEI/ARB during hospitalization and other clinical data (eg, vital signs, clinical presentations, and laboratory values) were not included in our analysis. Therefore, further studies are needed on whether discontinuation or continuation of ACEIs/ARBs in patients before the diagnosis of COVID-19 affects COVID-19 outcomes. Fourth, other indications of ACEI/ARB use, such as diabetes with proteinuria, were not considered in our study. Therefore, the effect of ACEI/ARB use on COVID-19 in patients with diabetes cannot be extrapolated through this study.
In conclusion, recent use of ACEIs/ARBs before diagnosis of COVID-19 was not significantly associated with mortality in patients with CVD. These findings support the recent recommendations of multiple societies on maintaining the prescription of ACEIs/ARBs during the COVID-19 pandemic.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgment
The authors appreciate the health care professionals dedicated to treating patients with COVID-19 in South Korea and the Ministry of Health and Welfare and the Health Insurance Review & Assessment Service of Korea for promptly sharing the invaluable national health insurance claims data.
Financial support. This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI20C0073).
Potential conflicts of interest. There are no potential conflicts of interest for any authors. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–10. [DOI] [PubMed] [Google Scholar]
- 5. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11:875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Lee JS, Park SH, et al. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017; 46:e15. [DOI] [PubMed] [Google Scholar]
- 8. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017; 32:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Wang X, Chen J, et al. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020; 5:825–30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126:1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020; 382:2441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med 2020; 382:2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of Cardiology. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19 Available at: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Accessed 3 May 2020.
- 15. European Society of Cardiology. Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. Available at: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. Accessed 3 May 2020.
- 16. Korea Centers for Disease Control & Prevention (KCDC). The updates on COVID-19 in Korea as of 1 April [press release]. 1 Apr 2020 Available at: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=366702&tag=&nPage=1. Accessed 3 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.