Abstract
There is growing interest in understanding the behavioral and neural mechanisms of catatonia. Here, we examine cognition and brain structure in schizophrenia spectrum disorder (SSD) patients with a history of catatonia. A total of 172 subjects were selected from a data repository; these included SSD patients with (n = 43) and without (n = 43) a history of catatonia and healthy control subjects (n = 86). Cognitive functioning was assessed using the Screen for Cognitive Impairment in Psychiatry (SCIP) and brain structure was assessed using voxel-based morphometry (VBM) in the CAT12 toolbox.
SSD patients with a history of catatonia showed worse performance on tests of verbal fluency and processing speed compared to SSD patients without such a history, even after controlling for current antipsychotic and benzodiazepine use. No differences were found between patients with and without a history of catatonia in terms of brain structure. Both patient groups combined showed significantly smaller grey matter volumes compared to healthy control subjects in brain regions consistent with prior studies, including the anterior cingulate, insular, temporal, and medial frontal cortices.
The results highlight a cognitive-motor impairment in SSD patients with a history of catatonia. Challenges and limitations of examining brain structure in patients with a history of catatonia are discussed.
Keywords: Catatonia, Psychosis, Schizophrenia, Cognition, Brain structure, Verbal fluency, Processing speed
1. Introduction
Catatonia is a psychomotor syndrome seen in approximately 10–15% of psychiatric inpatients (Stuivenga and Morrens, 2014). The symptoms of catatonia range from immobility to excessive and bizarre movement as well as affective and neurological components, making it a heterogeneous and difficult syndrome to understand (Walther et al., 2019). Despite effective treatments with benzodiazepine and electroconvulsive therapy, which help to differentiate catatonia from other psychoses, there remain open questions about the behavioral and neural mechanisms underlying the unique pathophysiology of catatonia within schizophrenia (Cuesta et al., 2015; Shorter and Fink, 2018; Walther and Strik, 2016).
In line with an effort to understand behavioral mechanisms underlying catatonia in schizophrenia, cognition remains a little understood domain. Research by Northoff and colleagues investigated general intelligence, attention, executive function, and visuo-spatial abilities between schizophrenia patients with and without catatonia and healthy controls, finding specific differences in visuo-spatial ability in catatonia compared to patient and healthy control groups (Northoff, 2002; Northoff et al., 2000). A case report of a 46 year-old man with major depression and catatonia was notable for significant long term impairment in memory and executive functioning abilities (Baker et al., 2005). In a study examining cognitive correlates of catatonia, Docx et al. did not find any relationship between scores on a catatonia rating scale and cognitive motor subtests including tests for processing speed, executive functioning, attention, or verbal working memory in a large sample of medically stable patients with schizophrenia (Docx et al., 2012). An update to the cognitive functioning of psychosis patients with catatonia is sorely needed to reconcile and improve our understanding of cognitive functioning in patients with catatonia.
Neuroimaging in catatonia has largely been characterized by case reports and cross sectional studies from the late 1990s to mid 2000s using positron emission tomography, spectroscopy, and functional magnetic resonance imaging (for a review see Hirjak et al., 2019b) with few studies examining brain structure (Wilcox, 1993). Several recent papers have explored brain structure in schizophrenia patients exhibiting signs of catatonia (Hirjak et al., 2019a, 2019c; Walther et al., 2017). Unfortunately, these studies showed little agreement in terms of which brain structures were affected in catatonia. For example, Walther et al., found a cluster in cerebellum lobule VII that differentiated patients with and without catatonia (Walther et al., 2017). Hirjak and colleagues, using a joint independent components analysis, found associations with catatonia in the striatum, thalamus, cerebellum, and several cortical areas (anterior cingulate, somatosensory, primary motor, inferior parietal, temporal, and medial frontal) (Hirjak et al., 2019c). Previous functional imaging has highlighted a diffuse network of brain regions involved in catatonia such as orbitofrontal, prefrontal, premotor, supplementary motor, and primary motor, as well as cerebellum, and parietal cortex (Northoff et al., 2004; Payoux et al., 2004; Richter et al., 2010). Guided by recent work using resting state functional connectivity in schizophrenia patients with catatonia (Walther, 2015; Walther et al., 2017; Walther and Strik, 2016), a conceptual model posits a prominent role of cortical-striatal brain circuits in the pathophysiology of psychomotor symptoms of catatonia (Walther et al., 2019). Relevant brain regions within this network include caudate, putamen, thalamus, cerebellum, anterior cingulate, supplementary motor area, and primary and secondary motor cortex (Obeso et al., 2014). Following this conceptual model using cortico-striatal regions of interest may provide a stepping stone to reconcile the discrepant findings of different brain structure between psychosis patients with and without a history of catatonia.
In this study, we sought to test cognition and brain structure in a matched sample of 86 patients with a schizophrenia spectrum disorder (SSD) who did (n = 43) or did not (n = 43) have a documented history of catatonia. A matched sample of healthy control subjects (n = 86) was also included in comparisons to gauge the relative differences between patient groups. Cognitive functioning was examined across multiple domains including immediate memory, delayed memory, working memory, verbal fluency, and processing speed. We hypothesized that cognitive domains that rely more on motor performance, i.e., verbal fluency and processing speed, would be most impacted in SSD patients with a history of catatonia (SSD with catatonia) compared to those without such a history (SSD without catatonia). Following this analysis, we used VBM to examine differences in brain structure between patient groups and healthy controls. We hypothesized that schizophrenia patients with catatonia would demonstrate structural brain differences that map onto the cortico-striatal network compared to schizophrenia patients without catatonia.
2. Material and methods
2.1. Sample selection
Patients and healthy control subjects were selected from the Psychiatric Genotype/Phenotype Project repository (https://clinicaltrials.gov/ct2/show/NCT00762866). Psychosis patients met criteria for a schizophrenia spectrum disorder as assessed by a trained rater using the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 1995). The schizophrenia group included N = 27 schizophrenia, N = 6 schizoaffective disorder, and N = 53 schizophreniform disorder patients. Here we will refer to the schizophrenia spectrum disorder patients simply as SSD. Psychiatric diagnoses were confirmed via consensus meetings with an expert psychiatrist (SH). General exclusion criteria for all participants were < 16 years of age or > 65 years of age, a history of significant head injury, major medical (i.e., human immunodeficiency virus or cancer) or neurological illness, any contraindication for MRI scanning, current alcohol or substance abuse within the past month, and intelligence quotient <75. For healthy control subjects, exclusion criteria included any history of Axis I disorders or a first degree relative with a psychotic illness. Importantly, patients were asked to participate in the research study after their treating provider determined that they could independently consent verbally and in writing their willingness to participate in the study procedures.
Schizophrenia patients with a lifetime history of catatonia (SSD with catatonia) were selected using the SCID. SSD with catatonia subjects endorsed at least 1/5 symptom domains of catatonia that included motoric immobility (72% of SSD with catatonia), excessive motor activity (33%), extreme negativism (56%), posturing or stereotyped movements (70%), and echolalia or echopraxia (25.5%). Further evidence of catatonia was verified by a careful case-by-case review of all available medical records from Vanderbilt Psychiatric Hospital (VPH). Medical records were reviewed for a Bush Francis Catatonia Rating Scale (BFCRS) assessment or detailed record of catatonia symptoms noted by trained psychiatrists during psychiatric hospitalization. Patients with a history of catatonia had at least 2 catatonia symptoms based on the BFCRS or medical record. In addition, patients with catatonia needed to show a treatment response to benzodiazepine and/or ECT treatment based on a description of improvement in psychomotor behavior from the treating physician.
A comparison group of schizophrenia patients without a history of catatonia (SSD without catatonia) were selected based on the absence of catatonia symptoms defined on the SCID. Further supporting evidence for the absence of catatonia symptoms was gathered using all available clinical and medical record information.
A propensity scoring matching algorithm (MatchIt package v.3.0.2) was used to match SSD with catatonia based on age, sex, and MRI protocol to the SSD with catatonia sample and healthy control subjects. See Fig. 1 for a flow chart describing steps to arrive at final sample and Table 1 for sample demographics.
Fig. 1.
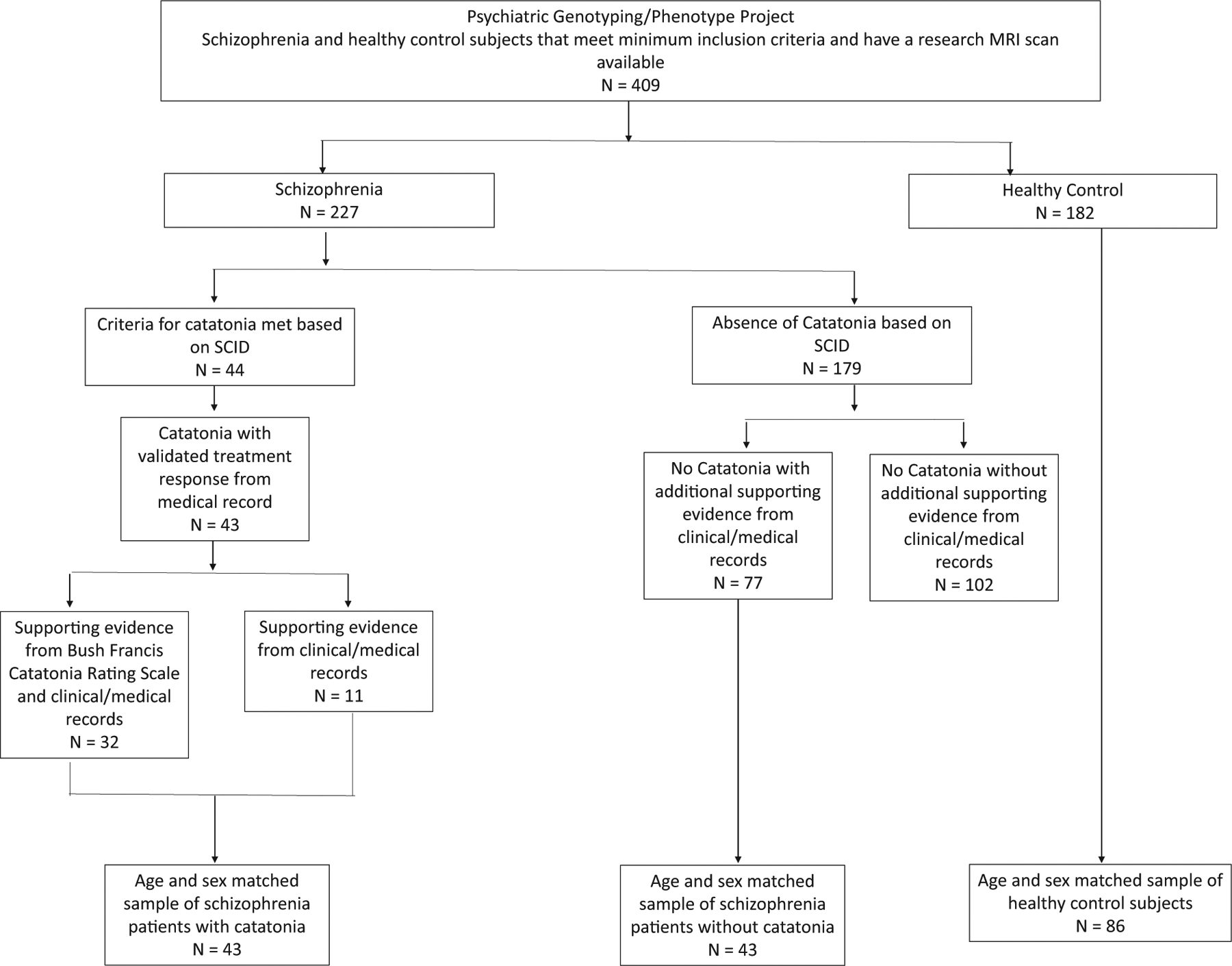
Flowchart detailing criteria for selection of study subjects from the Psychiatric Genotype/Phenotype Project repository.
Table 1.
Demographic characteristics of the sample.
| SSD with Catatonia | SSD without Catatonia | Statistic | p = | Healthy Controls | Statistic | p = | |
|---|---|---|---|---|---|---|---|
| Sex, M/F | 32/11 | 35/8 | χ2 = 0.27 | 0.6 | 64/22 | χ2 = 0.86 | 0.65 |
| Age, Years | 27.4 (8.15) | 27.9 (6.63) | t(84) = 0.35 | 0.73 | 29.9 (9.24) | F(2, 169) = 1.59 | 0.21 |
| Education | 12.9 (1.90) | 13.3 (1.88) | t(84) = 0.55 | 0.57 | 13.8 (1.70) | F(2, 173) = 3.39 | 0.04 |
| Parent Education | 14.6 (2.43) | 15.4(3.16) | t(84) = 1.62 | 0.12 | 14.6 (2.46) | F(2,173) = 2.01 | 0.14 |
| Schizophrenia Spectrum Diagnosis | |||||||
| Schizophrenia | 17 | 10 | χ2 = 2.95 | 0.23 | – | – | – |
| Schizoaffective disorder | 2 | 4 | |||||
| Schizophreniform | 24 | 29 | |||||
| CPZ | 351.0 (186.0) | 386 (199.0) | t(69) = 0.61 | 0.54 | – | – | – |
| Benzodiazepine, Y/N | 24/19 | 8/35 | t(84) = 2.04 | 0.04 | – | – | – |
| PANSS | |||||||
| Positive Mean (SD) | 17.2 (8.04) | 19.3 (6.68) | t(84) = 1.40 | 0.16 | – | – | – |
| Negative Mean (SD) | 18.5 (8.35) | 15.4 (6.36) | t(84) = 1.92 | 0.06 | – | – | – |
| General Mean (SD) | 32.4 (8.17) | 31.8 (7.42) | t(84) = 0.28 | 0.78 | – | – | – |
| Total Mean (SD) | 68.0 (19.2) | 66.5 (15.1) | t(84) = 0.34 | 0.73 | – | – | – |
| Scan Procedure | |||||||
| NCT00762866 | 7 | 9 | – | – | 25 | χ2 = 3.44 | 0.49 |
| R01MH070560 | 10 | 10 | – | – | 19 | – | – |
| R01MH102266 | 26 | 24 | – | – | 42 | – | – |
2.2. Cognition
The Screen for Cognitive Impairment In Psychiatry (SCIP; Purdon, 2005) was administered to provide measures of cognitive functioning. The SCIP has good psychometric properties including good decision validity in discriminating cognitive impairment in psychosis clinical samples from healthy controls (sensitivity 87.9 and specificity 80.6), internal consistency (α = 0.73) and test-retest reliability (α > 0.80) (Gómez-Benito et al., 2013; Pino et al., 2008; Rojo et al., 2010). Briefly, the SCIP consists of five subtests: 1) verbal list learning-immediate which entails three repetitions of a 10-item word list; 2) a working memory test similar to the Auditory Consonant Trigrams test (Stuss et al., 1987); 3) phonemic verbal fluency; 4) a coding test of psychomotor processing speed; and 5) a delayed recall trial of the verbal list learning test. SCIP subtest raw scores were converted to Z-scores using the healthy control subjects as the normative sample.
2.3. Neuroimaging
Imaging data included in this investigation were obtained from three studies: NCT00762866, 1R01MH070560, and 1R01MH102266. All imaging data were collected on two identical 3 T Philips Intera Achieva MRI Scanners located at the Vanderbilt University Institute of Imaging Science. Imaging parameters for the T1-weighted scans, which differed slightly across studies, are presented below. The field of view and resolution were identical. NCT00762866 and R01MH070560 T1-weighted anatomical scan: fast field echo (FFE) with 170 sagittal slices, FOV = 256 × 256 matrix (1.0 mm isovoxel resolution), TR/TE = 8.0/3.7 ms. R01MH102266 T1-weighted anatomical scan: turbo field echo (TFE) with 170 sagittal slices, FOV = 256 × 256 matrix (1.0 mm isovoxel resolution), TR/TE = 8.9/4.6 ms.
Voxel-based morphometry (VBM) was performed using the Computational Anatomical Toolbox 12 (CAT12 Version 12.6; C. Gaser, Structural Brain Mapping Group, Jena University Hospital, Jena, Germany; http://dbm.neuro.uni-jena.de/cat/) in Statistical Parametric Mapping 12 (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Following visual inspection, T1 images were corrected for bias-field inhomogeneities, registered using linear (12-parameter affine) and nonlinear transformations, spatially normalized using the DARTEL algorithm (Ashburner, 2007), and segmented into grey matter, white matter, and cerebrospinal fluid (Gaser and Dahnke, 2012). Total grey, white and CSF volumes were calculated as was total intracranial volume (TIV = grey + white + CSF). Following segmentation, each tissue class was warped to a template image comprised of 555 subjects included with the CAT12 toolbox using the high dimension DARTEL normalization method (Ashburner, 2007). The tissue class images were modulated during DARTEL normalization to preserve the original volumes of the tissue classes (Good et al., 2001). Grey matter segmentations were smoothed at 6 mm FWHM gaussian kernel prior to group level analysis.
2.4. Statistical analysis
Demographic and cognitive analyses were conducted in R (v. 3.6.1). One-way analysis of variance (ANOVA), independent samples t-test, and chi-square tests were used to examine group differences for continuous and categorical demographic variables. Antipsychotic medication at the time of the study was converted to chlorpromazine equivalent dosage (CPZ; Gardner et al., 2010). Current benzodiazepine medication prescription was defined using a categorical variable (yes/no) and based on patient report as well as review of the medical record at the time of the cognitive assessment and MRI scan.
A linear mixed model using the lme4 package was run to test differences between patient groups and healthy controls on the 5 domains of the SCIP, with subject as a random effect. P-values were obtained using the car package. A separate linear mixed model was run to also test the effect of antipsychotic and benzodiazepine medication on cognitive performance in comparisons involving the patient groups only. Follow-up analyses examining group differences within each domain involved one-way analysis of covariance (ANCOVA). Pairwise post hoc tests involved a Holm adjusted Tukey HSD correction for multiple comparisons.
Smoothed normalized grey matter images were analyzed using a voxel-wise ANCOVA with group (SSD with catatonia, SSD without catatonia, and healthy control subjects) included as a between subjects variable and age, sex, TIV, and scan protocol included as covariates of no interest. To identify grey matter volume abnormalities related to catatonia, a pairwise contrast comparing SSD with catatonia to SSD without catatonia was performed. This contrast was initially masked to a-priori defined brain regions to test our hypothesis that differences between SSD with catatonia and SSD without catatonia would map onto the motor brain network. Motor network regions were based on the model described by Walther et al. (2019) and defined anatomical masks of bilateral caudate, putamen, thalamus, cerebellum, anterior cingulate, supplementary motor area, primary and secondary motor cortex from the Harvard-Oxford Cortical and Subcortical atlases, and Montreal Neurological Institute (MNI) structural atlas in FSL (Desikan et al., 2006; Makris et al., 2006). Regions were combined into a single anatomical mask which was used to mask the voxel-wise analysis. This analysis was followed up with a whole-brain, unmasked analysis to determine if there were any differences between patient groups outside the a-priori defined motor network. Next, to determine how grey matter abnormalities related to normal brain structure, average grey matter was extracted from the clusters identified in the pairwise comparison between patient groups for all subjects, including healthy controls, and analyzed using an ANCOVA in R, comparing each patient group to healthy controls. Finally, a planned contrast comparing SSD (collapsed across catatonia status) to healthy control subjects was performed to identify grey matter abnormalities associated with SSD. All voxel-wise results were thresholded at cluster-level corrected p(FWE) = .05 for voxel-wise p(uncorrected) = .05.
3. Results
3.1. Demographic and clinical characteristics
SSD with catatonia, SSD without catatonia, and healthy control subjects did not differ on demographic variables including age, sex, or parental education (Table 1). SSD patients combined had lower educational attainment than healthy control subjects. There were no differences between patient groups in terms of stage of illness or antipsychotic CPZ equivalent dosage. Significantly more SSD with catatonia patients were currently prescribed benzodiazepines compared to SSD without catatonia. For SSD patients with catatonia, the median time between last recorded catatonia episode and MRI scan was 94 days (range: 1–2676 days).
3.2. Cognition
A linear mixed model comparing all three groups (SSD with catatonia, SSD without catatonia, and healthy control subjects) across the 5 domains of cognitive functioning showed a significant group by cognitive domain interaction (F(8, 675.23) = 2.37, p = .02) (see Fig. 2). As expected, both patient groups (i.e., SSD with and without catatonia) were impaired on all cognitive domains compared to healthy control subjects (see Supplemental Material). Post hoc pairwise contrasts comparing SSD patient groups revealed significantly worse cognitive performance on tests of immediate memory (t = 2.07, p = .04), verbal fluency (t = 2.77, p = .01), and processing speed (t = 2.44, p = .02), but not working memory (t = 0.64, p = .52) or delayed memory (t = 1.38, p = .17) in SSD patients with catatonia compared to SSD patients without catatonia. A linear mixed model comparison of cognitive domains between SSD patient groups, controlling for medication, retained a significant group by cognitive domain interaction (F(4, 271.11) = 2.75, p = .03). SSD with catatonia showed lower scores on verbal fluency (t = 2.15, p = .03) and processing speed (t = 2.53, p = .01) but not immediate memory (t = 1.48, p = .14), working memory (t = .19, p = .85), or delayed memory (t = 1.38, p = .17) compared to SSD without catatonia.
Fig. 2.
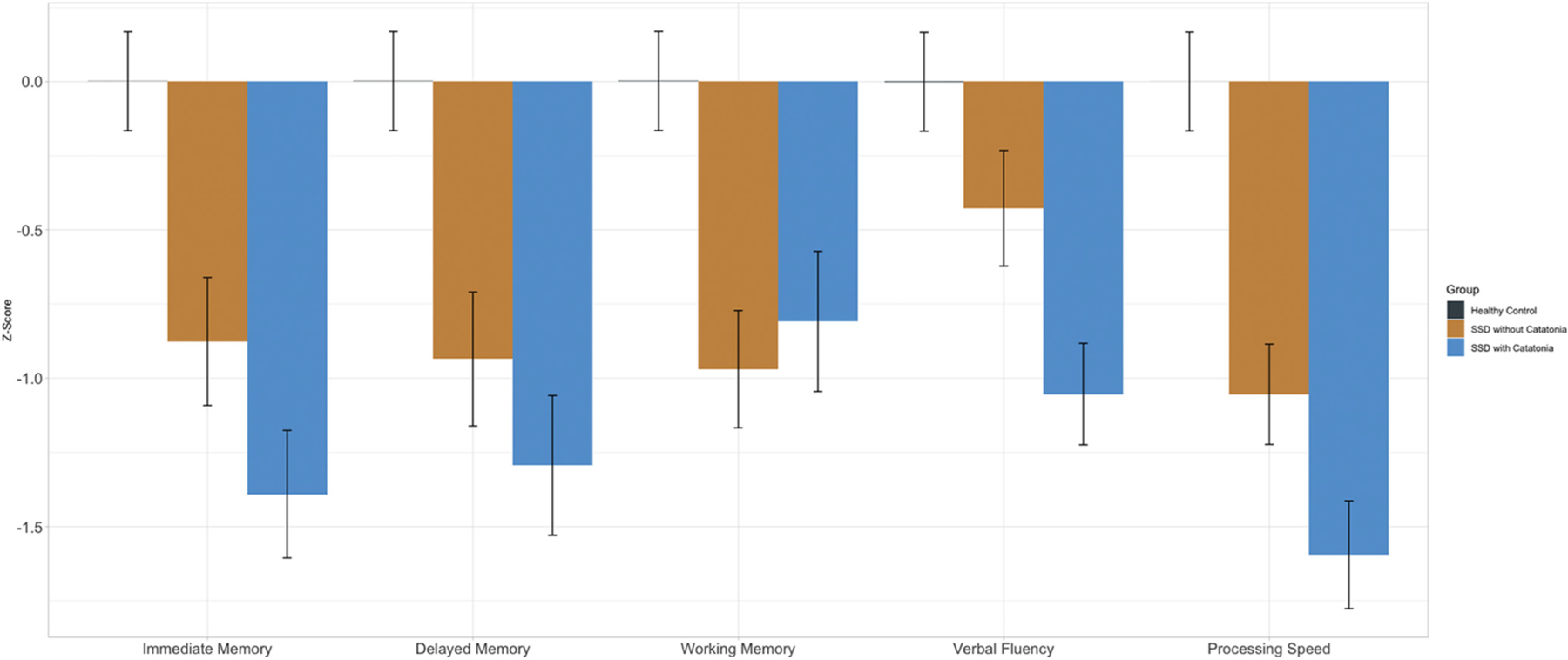
Cognitive differences between patients with and without a history of catatonia and healthy controls using the Screen for Cognitive Impairment in Psychiatry (SCIP). SSD with catatonia showed significantly lower performance on measures of verbal fluency and processing speed compared to SSD without catatonia. Both patient groups showed significantly lower performance across all cognitive variables compared to healthy control subjects.
3.3. Brain structure
The pairwise contrast comparing SSD patients with catatonia to schizophrenia patients without catatonia did not reveal any significant differences for both the motor network masked and whole-brain analyses (see Fig. 3A). The planned contrast comparing all patients combined to healthy controls revealed smaller grey matter volume in several regions often implicated in schizophrenia including insula, anterior cingulate, medial frontal cortex, middle temporal gyrus and temporal pole, angular gyrus, and lingual gyrus (see Fig. 3B and supplemental material).
Fig. 3.
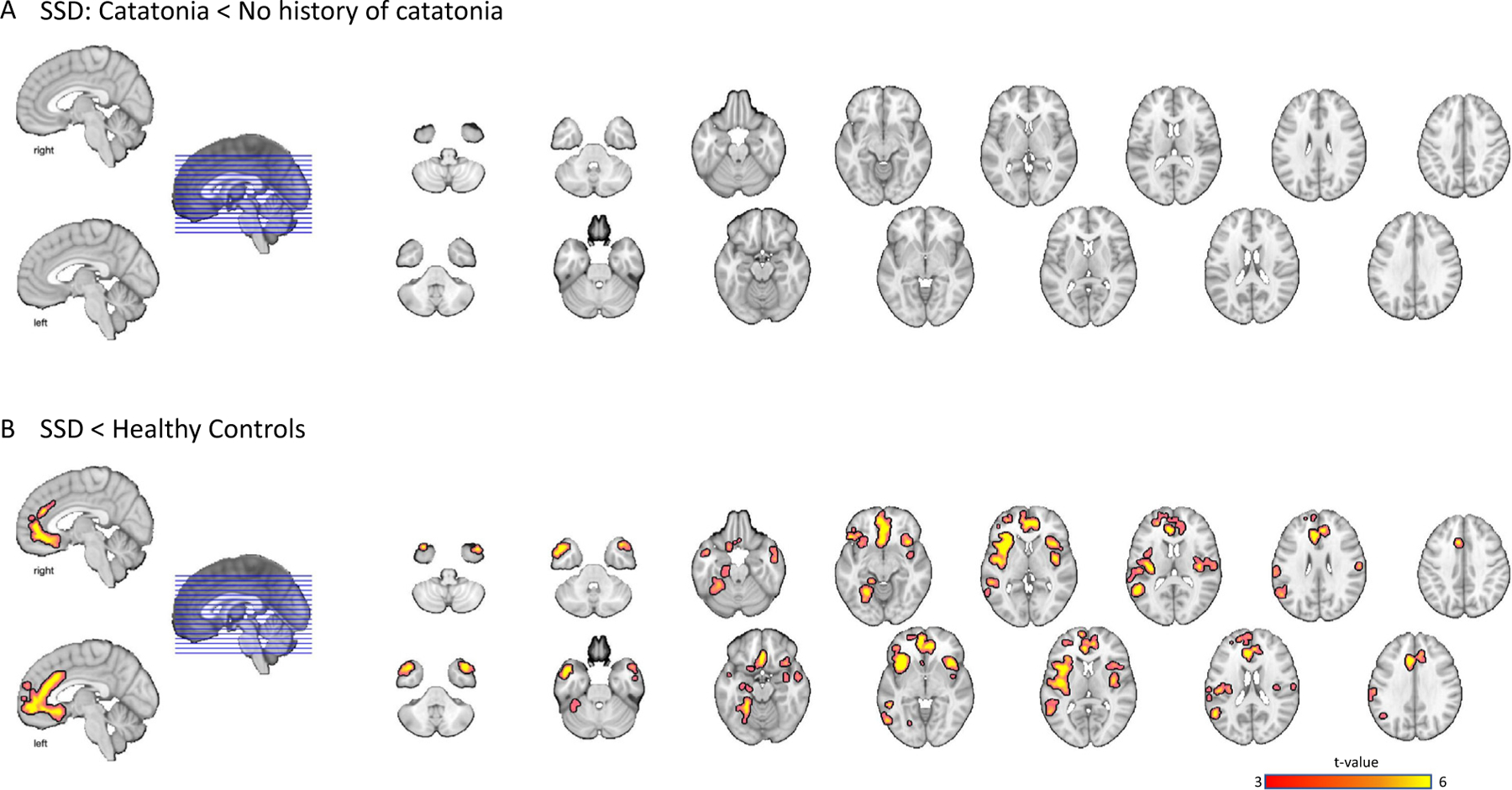
SSD with catatonia did not show grey matter differences compared to SSD without catatonia for both ROI and whole brain analysis (Panel A). A voxel-wise whole brain comparison showed that both patient groups combined had less grey matter volume in comparison to healthy control subjects in regions including anterior cingulate, medial frontal cortex, middle temporal gyrus and temporal pole, angular gyrus, and lingual gyrus (Panel B). Results were thresholded at cluster-level corrected p(FWE) = 0.05 for voxel-wise p(uncorrected) = 0.05. Images are presented in a neurological orientation.
4. Discussion
This study examined cognitive functioning and brain structure in SSD patients with a history of catatonia compared to a well-matched sample of SSD patients without a history of catatonia. The results showed that patients with a history of catatonia have greater cognitive difficulties with verbal fluency and processing speed compared to patients without a history of catatonia. In contrast to our hypotheses, SSD patients with catatonia did not show differences from SSD patients without catatonia in terms of grey matter volume within the motor brain network. Both patient groups combined had decreased grey matter volume compared to healthy control subjects in several regions including anterior cingulate, medial frontal cortex, middle temporal gyrus and temporal pole, angular gyrus, and lingual gyrus, replicating previous neuroimaging studies in psychosis (Gupta et al., 2015). These results suggest that psychomotor cognitive functioning may differentiate psychosis patients with a history of catatonia from those without such a history.
Motor symptoms distinguish catatonia from other forms of psychosis. They include severe motor retardation (i.e., immobility, stupor, mutism) as well as severe motor excitation (e.g., agitation, verbigeration, stereotypy) and abnormal motor behavior (e.g., waxy flexibility, automatic obedience, ambitendency) (Wilson et al., 2015). These symptoms respond fairly quickly to benzodiazepines and/or electroconvulsive therapy which helps to differentiate catatonia from other disorders that share similar psychomotor features such as anxiety, depression and bipolar disorder (Fink et al., 2004; Rasmussen et al., 2016). However, establishing caseness of catatonia remains difficult and researchers continue to use different methods of categorizing and quantifying the severity of catatonia. For example, recent work by Hirjak used a categorical approach with schizophrenia patients who showed at least 3 symptoms across motor, behavioral and affective categories on the Northoff Catatonia Rating Scale (at least 1 symptom on the motor category was required) and then used a dimensional approach to examine patients with at least 2 symptoms based on DSM-5 criteria for catatonia (Hirjak et al., 2019a). In this study we took a multistep approach to determine a case of catatonia, including clinical interviews, medical record review, the Bush Francis Catatonia Rating Scale, and treatment response to benzodiazepine and/or electroconvulsive therapy.
Distinguishing specific neurocognitive domains that differentiate SSD with catatonia from SSD without catatonia is important for managing the psychological and medical complications that are unique to catatonia (Funayama et al., 2018). Our study revealed that patients with a history of catatonia are especially impaired in cognitive domains that rely on psychomotor functioning, i.e., verbal fluency and processing speed. These differences remained after controlling for both current neuroleptic and benzodiazepine medication, suggesting that these cognitive domains might be unique to catatonia. Importantly, patients with a history of catatonia showed no differences to patients without such a history in other memory domains (i.e., immediate, delayed and working memory). The differences in cognition between patient groups were over and above the impairments that were seen in schizophrenia in general, suggesting some specificity to cognitive impairment in verbal fluency and processing speed with regard to a history of catatonia. The SCIP provides a brief test of multiple cognitive domains including working memory, verbal learning, delayed recall, verbal fluency and processing speed; however, the SCIP does not cover other cognitive domains found to distinguish catatonia patients from psychiatric and healthy control samples such as visuo-spatial ability (Northoff et al., 2000). Future research using a broader array of tests across a larger number of cognitive domains are needed.
The results of the VBM analysis replicated decreased grey matter volumes in schizophrenia, but did not find any evidence that grey matter volume abnormalities are more pronounced in catatonia. This is in contrast to our hypothesis of structural brain differences in the motor network in catatonia. We were unable to replicate the VBM findings from Walther and colleagues (Walther et al., 2017), who found differences between patients with and without catatonia in the cerebellum. One explanation for this lack of group difference in brain structure between patient groups is that our sample is younger (mean age 23.32 ± 7.24 years) and at an earlier stage of the illness in comparison to that of Walther (mean age 35.9 ± 12.7 years). Another explanation is the sampling method: previous studies recruited patients within days or weeks after treatment of catatonia symptoms, assessing brain morphology as close as possible to the episode. Importantly, whole brain group differences between psychosis patients and healthy control subjects map onto established findings of VBM analyses and schizophrenia, with significant decreased grey matter volume in patients in the anterior cingulate, insular, temporal, and medial frontal cortices (Baiano et al., 2007; Honea et al., 2005; Job et al., 2002).
The lack of grey matter volume differences between patients with and without a history of catatonia in motor network regions does not rule out a cortico-striatal impairment. It is possible that the time between a catatonic episode and MRI scanner matters and that plasticity changes may have contributed to these findings (Kays et al., 2012; May, 2011). For example, Viher and colleagues noted increased structural connectivity in catatonia patients within the left corticospinal tract (Viher et al., 2020). Hirjak et al. (2019b) recently noted that studies utilizing the Northoff Catatonia rating scale and fMRI, PET, and SPECT methods propose that in addition to the cortico-striatal network, a frontoparietal network may contribute to motor, cognitive, and affective symptoms in catatonia. While we did not specifically test this network in an ROI analysis, whole brain analysis did not reveal significant differences between SSD with and without catatonia. This might suggest that we did not have the power to detect group differences, or that our ROI and whole brain analyses were not specific enough to detect group differences with voxel and cluster wise correction. More detailed methods of investigating brain structure such as cortical thickness and structural connectivity would be helpful in finding smaller brain ROIs involved in catatonia.
This study has several strengths and limitations. First, this is the largest sample of SSD patients with a history of catatonia to be examined with structural brain imaging. Past imaging studies in catatonia have involved samples with n < 33, and many range from single case reports to 10 catatonia patients (De Tiége et al., 2003; Ebert et al., 1992; Galynker et al., 1997; Hirjak et al., 2019a, 2019c; Iseki et al., 2019; Northoff, 2002; Northoff et al., 1999; Satoh et al., 1993; Walther et al., 2017). We used a rigorous approach to determine a history of catatonia through clinical records and response to treatment. However, determining a sample of SSD patients without a history of catatonia proved challenging. After patient interviews and careful medical record review, we were confident that the SSD patients without catatonia did not have a history of catatonia, but recollection and documentation of catatonia signs are often of low quality. It is also possible that the patients will develop catatonia in the future. Along similar lines, we attempted to reduce variability between patient groups by closely matching on important diagnostic and demographic variables. However, patients with affective disorders also experience catatonia (Grover et al., 2015), calling for a wider transdiagnostic approach in studying the neurobiology of catatonia in the future. Finally, the patient sample was selected from a repository, making it difficult to select a large sample of patients who have undergone cognitive assessment and neuroimaging close to an episode of catatonia. Prospective and longitudinal study designs are needed to capture the cognitive, affective and neural basis of catatonia.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jo Ellen Wilson for her thoughtful discussions on catatonia.
Role of the funding source
Supported by the Charlotte and Donald Test Fund; NIMH grants R01 MH102266 (Dr. Woodward), R01 MH70560 (Dr. Heckers), R01 MH118741 (Dr. Walther); Swiss National Science Foundation grants #184717 and #182469 (Dr. Walther); the Vanderbilt Psychiatric Genotype/Phenotype Project NCT00762866, Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH), and the Advanced Computing Center for Research and Education at Vanderbilt University.
Footnotes
Declaration of competing interest
Dr. Walther has received honoraria outside the submitted work from Eli Lilly, Janssen-Cilag, Otsuka, Sunovion, and Lundbeck. The other authors report no financial relationships with commercial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2020.05.012.
References
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P, 2007. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr. Res 93, 1–12. 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Baker IWS, Jackson M, Bass C, 2005. Catatonia causing permanent cognitive impairment: a case study. Cogn. Behav. Neurol 18, 141–143. 10.1097/01.wnn.0000178230.46691.c7. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Moreno-Izco L, Peralta V, 2015. Clinical & diagnostic issues in catatonia. Future Neurol. 10, 405–415. 10.2217/fnl.15.37. [DOI] [Google Scholar]
- De Tiége X, Bier JC, Massat I, Laureys S, Lotstra F, Berré J, Mendlewicz J, Goldman S, Berré J, 2003. Regional cerebral glucose metabolism in akinetic catatonia and after remission. J. Neurol. Neurosurg. Psychiatry 74, 1003–1004. 10.1136/jnnp.74.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, Baeken C, Audenaert K, Sabbe B, 2012. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr. Scand 126, 256–265. 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Kaschka W, 1992. Left temporal hypoperfusion in catatonic syndromes: a SPECT study. Psychiatry Res. Neuroimaging 10.1016/0925-4927(92)90019-Z. [DOI] [PubMed] [Google Scholar]
- Fink M, Taylor M, Caroff SN, Mann SC, Campbell ECSK, 2004. Catatonia: A Clinicians Guide to Diagnosis and Treatment. American Psychiatric Publishing 10.4088/JCP.v67n0421b. [DOI] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL SCID-I/P Version 2.0 Biometrics Research Department, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Funayama M, Takata T, Koreki A, Ogino S, Mimura M, 2018. Catatonic Stupor in Schizophrenic Disorders and Subsequent Medical Complications and Mortality. 10.1097/PSY.0000000000000574. [DOI] [PMC free article] [PubMed]
- Galynker II, Weiss J, Ongseng F, Finestone H, 1997. ECT treatment and cerebral perfusion in catatonia. J. Nucl. Med 38, 251–254. [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ, 2010. International consensus study of antipsychotic dosing. Am. J. Psychiatry 167, 686–693. 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gaser C, Dahnke R, 2012. Computational anatomy toolbox for the analysis of structural MRI. HBM 32. [Google Scholar]
- Gómez-Benito J, Guilera G, Pino Ó, Rojo E, Tabarés-Seisdedos R, Safont G, Martínez-Arán A, Franco M, Cuesta MJ, Crespo-Facorro B, Bernardo M, Vieta E, Purdon SE, Mesa F, Rejas J, 2013. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry 13, 127 10.1186/1471-244X-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ, 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grover S, Chakrabarti S, Ghormode D, Agarwal M, Sharma A, Avasthi A, 2015. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 229, 919–925. 10.1016/j.psychres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, Segall J, Franke B, Zwiers MP, Arias-Vasquez A, Buitelaar J, Fisher SE, Fernandez G, van Erp TGM, Potkin S, Ford J, Mathalon D, McEwen S, Lee HJ, Mueller BA, Greve DN, Andreassen O, Agartz I, Gollub RL, Sponheim SR, Ehrlich S, Wang L, Pearlson G, Glahn DC, Sprooten E, Mayer AR, Stephen J, Jung RE, Canive J, Bustillo J, Turner JA, 2015. Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr. Bull 41, 1133–1142. 10.1093/schbul/sbu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak D, Kubera KM, Northoff G, Fritze S, Bertolino AL, Topor CE, Schmitgen MM, Wolf RC, 2019a. Cortical contributions to distinct symptom dimensions of catatonia. Schizophr. Bull 45, 1184–1194. 10.1093/schbul/sby192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak D, Kubera KM, Wolf RC, Northoff G, 2019b. Going back to Kahlbaum’s psychomotor (and GABAergic) origins: is catatonia more than just a motor and dopaminergic syndrome? Schizophr. Bull 10.1093/schbul/sbz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak D, Rashidi M, Kubera KM, Northoff G, Fritze S, Schmitgen MM, Sambataro F, Calhoun VD, Wolf RC, 2019c. Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophr. Bull 10.1093/schbul/sbz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE, 2005. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Iseki K, Ikeda A, Kihara T, Kawamoto Y, Mezaki T, Hanakawa T, Hashikawa K, Fukuyama H, Shibasaki H, 2019. Impairment of the Cortical GABAergic Inhibitory System in Catatonic Stupor: A Case Report with Neuroimaging. 10.1684/epd.2009.0257. [DOI] [PubMed]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM, 2002. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage 17, 880–889. 10.1006/nimg.2002.1180. [DOI] [PubMed] [Google Scholar]
- Kays JL, Hurley RA, Taber KH, 2012. The dynamic brain: neuroplasticity and mental health. J. Neuropsychiatry Clin. Neurosci 24, 118–124. 10.1176/appi.neuropsych.12050109. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ, 2006. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res 83, 155–171. 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- May A, 2011. Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, 2002. What catatonia can tell us about “top-down”modulation: a neuropsychiatric hypothesis. Behav. Brain Sci 25, 555–577. [DOI] [PubMed] [Google Scholar]
- Northoff G, Braus DF, Sartorius A, Khoram-Sefat D, Russ M, Eckert J, Herrig M, Leschinger A, Bogerts B, Henn FA, 1999. Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol. Med 29, 997–1002. 10.1017/S0033291798007739. [DOI] [PubMed] [Google Scholar]
- Northoff G, Steinke R, Nagel D, Czerwenka C, Grosser O, Danos P, Genz A, Krause R, Böker H, Otto HJ, Bogerts B, 2000. Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol. Med 30, 583–596. 10.1017/S0033291799002007. [DOI] [PubMed] [Google Scholar]
- Northoff G, Kötter R, Baumgart F, Danos P, Boeker H, Kaulisch T, Schlagenhauf F, Walter H, Heinzel A, Witzel T, Bogerts B, 2004. Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr. Bull 30, 405–427. 10.1093/oxfordjournals.schbul.a007088. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ, 2014. The expanding universe of disorders of the basal ganglia. Lancet 384, 523–531. 10.1016/S0140-6736(13)62418-6. [DOI] [PubMed] [Google Scholar]
- Payoux P, Boulanouar K, Sarramon C, Fabre N, Descombes S, Galitsky M, Thalamas C, Brefel-Courbon C, Sabatini U, Manelfe C, Chollet F, Schmitt L, Rascol O, 2004. Cortical motor activation in akinetic schizoophrenic patients: a pilot functional MRI study. Mov. Disord 19, 83–90. 10.1002/mds.10598. [DOI] [PubMed] [Google Scholar]
- Pino O, Guilera G, Rojo JE, Gómez-Benito J, Bernardo M, Crespo-Facorro B, Cuesta MJ, Franco M, Martinez-Aran A, Segarra N, Tabarés-Seisdedos R, Vieta E, Purdon SE, Díez T, Rejas J, 2008. Spanish version of the Screen for Cognitive Impairment in Psychiatry (SCIP-S): psychometric properties of a brief scale for cognitive evaluation in schizophrenia. Schizophr. Res 99, 139–148. 10.1016/j.schres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Purdon SE, 2005. The Screen for Cognitive Impairment in Psychiatry (SCIP): Administration Manual and Normative Data. Purdon Neuropsychological Labs Inc, Alberta, Canada. [Google Scholar]
- Rasmussen SA, Mazurek MF, Rosebush PI, 2016. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J. Psychiatry 6, 391 10.5498/wjp.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Grimm S, Northoff G, 2010. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum. Psychopharmacol 25, 55–62. 10.1002/hup.1084. [DOI] [PubMed] [Google Scholar]
- Rojo E, Pino O, Guilera G, Gómez-Benito J, Purdon SE, Crespo-Facorro B, Cuesta MJ, Franco M, Martínez-Arán A, Segarra N, Tabarés-Seisdedos R, Vieta E, Bernardo M, Mesa F, Rejas J, 2010. Neurocognitive diagnosis and cut-off scores of the Screen for Cognitive Impairment in Psychiatry (SCIP-S). Schizophr. Res 116, 243–251. 10.1016/j.schres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Satoh K, Narita M, Someya T, Fukuyama H, Yonekura Y, 1993. Functional Brain Imaging of a Catatonic Type of Schizophrenia: PET and SPECT Studies. Psychiatry Clin. Neurosci 47, 881–885. 10.1111/j.1440-1819.1993.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Shorter E, Fink M, 2018. The Madness of Fear. Oxford University Press. [Google Scholar]
- Stuivenga M, Morrens M, 2014. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry 5, 174 10.3389/fpsyt.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Poirier CA, 1987. Comparison of three tests of attention and rapid information processing across six age groups. Clin. Neuropsychol 1, 139–152. 10.1080/13854048708520046. [DOI] [Google Scholar]
- Viher PV, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Walther S, 2020. Altered diffusion in motor white matter tracts in psychosis patients with catatonia. Schizophr. Res 10.1016/j.schres.2020.03.017. [DOI] [PubMed] [Google Scholar]
- Walther S, 2015. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 233, 293–298. 10.1016/j.pscychresns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Walther S, Strik W, 2016. Catatonia. CNS Spectr 21, 341–348. 10.1017/S1092852916000274. [DOI] [PubMed] [Google Scholar]
- Walther S, Schäppi L, Federspiel A, Bohlhalter S, Wiest R, Strik W, Stegmayer K, 2017. Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr. Bull 43, 972–981. 10.1093/schbul/sbw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Wilson JE, Heckers S, 2019. Structure and neural mechanisms of catatonia. Lancet Psychiatry 10.1016/S2215-0366(18)30474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox JA, 1993. Structural brain abnormalities in catatonia. Neuropsychobiology 27, 61–64. 10.1159/000118954. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S, 2015. The diagnostic criteria and structure of catatonia. Schizophr. Res 164, 256–262. 10.1016/j.schres.2014.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


