Abstract
Background
Infection in acute-on-chronic liver failure (ACLF) patients is known to cause higher mortality. The current approach is to culture all patient samples. There are no published data evaluating fungal infections in acutely decompensated patients. In this study, we aim to identify clinical factors predictive of infections within ACLF patients and assess workup compliance within 24 h of hospital admission.
Methods
We retrospectively analyzed the charts of 457 ACLF patients seen at the University of Arizona between January 1, 2014 and December 31, 2014. We used logistic regression to identify potential risk indicators for bacterial, fungal, and any infections. In order to proceed to a systemic infection workup, the following parameters were assessed: complete blood count, urinalysis, urine culture, bacterial blood culture, chest X-ray, and ascitic fluid analysis in patients with ascites. Additionally, serological markers were also assessed in patient samples. Systemic inflammatory response syndrome (SIRS) was defined as the presence of two or more of the following criteria: temperature > 38 °C or < 36 °C, heart rate > 90 beats/min, respiratory rate > 20 breaths/min, white blood cell count > 12,000 or < 4,000 cells/mm or > 10% bands.
Results
An established infection was observed in 60.61% of ACLF patients. SIRS criteria predicted infections with concordance statistic (C-statistic) of 0.71 (odds ratio (OR) 6.85, 95% confidence interval (CI): 4.33, 10.85) for any infection, 0.63 (OR 2.88, 95% CI: 1.96, 4.23) for bacterial infection, and 0.53 (OR 1.32, 95% CI: 0.59, 2.96) for fungal infection. After including other significant variables (over 10 additional variables), predictive ability improved, C-statistic 0.83 (95% CI: 0.77, 0.90) for any infection and 0.71 (95% CI: 0.65, 0.77) for bacterial infections. The combination of model for end-stage liver disease (MELD) and hemoglobin (Hb) predicted fungal infections with C-statistic 0.74 (95% CI: 0.63, 0.84). Workup within 24 h of admission was obtained in 12% of patients.
Conclusions
Fungal infections in ACLF patients results in an increased mortality rate. Elevated MELD and low Hb in combination predict fungal infections. Compliance is very poor to obtain diagnostic workup efficiently, better tools are needed to predict infection upon admission.
Keywords: Liver disease, Acute-on-chronic liver failure, Bacterial infection, Fungal infection
Introduction
Identification and adequate treatment of infection within cirrhotic patients presenting with acute deterioration is the first step of management. In a review of bacterial infections within cirrhotic patients, Bruns et al detail how common indicators for infection must be used with caution within cirrhotic patients, and that the medical community has recommended research on novel biomarkers for diagnosis [1, 2]. The significance of diagnosis and prediction of infection stems from findings that cirrhotic patients are at an increased risk of infection and mortality from infection when compared to the general population. Overall, approximately 25% of cirrhotic patients have an infection [3], increasing mortality risk four-fold [4]. The most common types of infection among cirrhotic patients are spontaneous bacterial peritonitis (31.1%), urinary tract infections (22.6%), pneumonia and cellulitis (11.3%) [3]. Although bacteria are the most common microorganisms (in a good proportion of patients), microorganisms are not identifiable. Despite adequate treatment with antibiotics, mortality in such patients remains high. Factors that could be responsible for high mortality may include multi-resistant bacterial infections, fungal infections, viral infections, or atypical infections. Currently, there are no published data on true incidence and prevalence of fungal and viral infections that lead to acute deterioration in previously compensated or decompensated cirrhotic patients.
Cirrhotic and acute-on-chronic liver failure (ACLF) patients suffer when infections are treated inappropriately. Adverse events can occur from hepatotoxic or nephrotoxic antibiotics, such as amoxicillin with clavulanic acid, the beta lactam group of antibiotics, vancomycin and aminoglycosides [5-7]. To avoid or minimize the necessity of these antimicrobial agents, it is prudent that diagnosis is timely and accurate. In clinical practice, obtaining timely results to infection tests upon hospital admission varies among centers. There are no published data on what and when diagnostic tests should be obtained.
Infection identification is the most challenging aspect of care in ACLF patients. The current approach is to culture patient samples to define the infection, which is time-consuming and prone to cross-contamination. Therefore, it is desirable to detect infection based on other clinical markers while awaiting the results of an infectious workup. The most common infection indicators currently used in clinical practice for the general patient population include systemic inflammatory response syndrome (SIRS), procalcitonin, and serum lactate, among others. Although research supports a high association between SIRS and infection in patient with sepsis without liver disease, other studies question the sensitivity of SIRS to identify infection and its accuracy among critically ill and cirrhotic patients [2, 8]. Serum lactate on the other hand has been identified as a significant clinical marker for bacteremia and sepsis in select studies [9, 10]. In clinical practice, we apply these tools to critically ill cirrhotic patients, but the predictive ability of surrogate markers (such as SIRS and serum lactate) has not been studied. Secondly, we suspect that in real world clinical practice, compliance to obtain appropriate diagnostic tests within 24 h of hospital admission, to identify and diagnose infections is poor. Specific diagnostic tests are required to identify fungal infections such as fungal cultures, serologies, or fungal tissue staining. Such tests are not ordered in routine upon initial presentation in acutely decompensated patients. In our study, we aim to assess prevalence and identify surrogate markers of fungal infections in addition to evaluating compliance in obtaining the adequate tests necessary for diagnosis at initial presentation. We designed this study to evaluate prevalence of infection, evaluate compliance to obtain diagnostic tests within 24 h of admission in a tertiary center teaching hospital, determine predictive value of SIRS to identify bacterial and fungal infections, and identify other variables as predictors of infections in patients who present to the hospital with cirrhosis, as well as acute decompensation of the liver.
Materials and Methods
We retrospectively reviewed the charts of patients admitted to the University of Arizona, all campuses, between January 1, 2014 and December 13, 2014. The University of Arizona’s Institutional Review Board approved the study protocol and waived the patient consent requirement. We followed the ethical guidelines set forth by the 1975 Declaration of Helsinki.
Patients were included in the study if: they were at least 18 years of age, minimum of 6 months of follow-up following index admission, data available to calculate SIRS, evidence of cirrhosis, and acute decompensation of the liver.
The following definitions were used. SIRS was defined as the presence of two or more of the following criteria: temperature > 38°C or < 36°C, heart rate > 90 beats/min, respiratory rate > 20 breaths/min, white blood cell count > 12,000 or < 4,000 cells/mm or > 10% bands. Acute decompensation was defined as having one or more of the following: gastrointestinal bleeding related to portal hypertension, acute renal failure or hepato-renal syndrome, hepatic encephalopathy or altered mental status, ascites, coagulopathy of liver disease, or jaundice. We also confirmed cirrhosis-related diagnosis codes with available imaging, such as hepatic histology and biochemical tests.
We collected data on all eligible patients, including all historic characteristics and clinical features, upon admission and during clinical course. We characterized disease severity by aspartate aminotransferase (AST) to platelet ratio index, fibrosis-4, Child-Turcotte-Pugh, and model for end-stage liver disease (MELD) score. The primary outcome of the study was presence of infection.
We categorized patients twice, first by presence/absence of infection and again by presence/absence of SIRS. We also created bacterial and fungal infection subgroups to study clinical factors indicating each. We categorized the infected cohort into four microbiological diagnoses: gram-positive bacterial infection, gram-negative bacterial infection, fungal infections, and infections with unidentified organisms (UIOs) to study differences based on microbiological type. Viral infections were also taken into consideration but being that they are less common we did not put a strong emphasis on them.
To identify and diagnose infection, a set of six basic infectious tests were defined as minimum criteria for a basic infectious workup. These tests were: complete blood count, urinalysis, urine culture, bacterial blood culture, chest X-ray, and ascitic fluid analysis in patients with ascites. However, other tests were also looked for, including serological markers. To analyze the compliance to obtain diagnostic workup, patients were categorized into three groups: completed infectious workup within 24 h of admission, completed infectious workup after 24 h of admission, and infectious workup never accomplished during the entire hospital stay.
Statistical analysis
We calculated descriptive statistics to understand the study cohort and its subgroups. To test differences between subgroups, Kruskal-Wallis test was used for continuous variables and Fisher’s exact tests for categorical variables.
We used univariate logistic regression to identify factors associated with any infection, bacterial infections, and fungal infections. Subsequently, we included all statistically significant variables in a multivariable logistic regression. In addition, logistic regression was also performed to derive odds ratio (OR), (95% confidence interval (CI)) and P values.
To explore the effectiveness of the basic infectious workup to identify infection, we derived a ratio of actual to expected number of tests (A/E ratio). Logistic regression was performed relating the diagnosis workup A/E ratio to infectious status. A receiver operating curve was also fit to determine the sensitivity and specificity of A/E ratio. We also performed an adjusted survival analysis to determine if the completeness and timing of basic infectious workup had any association with survival.
The significance level was set at 0.05 for all statistical tests. The statistical analyses were done using SAS 9.4.
Results
A total of 521 patients met the study inclusion criteria. A total of 64 patients had missing data, leaving 457 patients for analysis. Of the 457 patients, 277 patients (60.61%) were identified with established infection. Of the 277 patients with an infection, 148 (53%) cases were detected based on a positive culture, and the remaining 129 (47%) were clinically defined based on serological tests (cocci based on immunoglobulin M (IgM)), clinical examination (cellulitis), radiologist’s imaging report (pneumonia), or neutrophil granulocytes > 250 cells/µL (spontaneous bacterial peritonitis). Distribution of infected patients based on microorganisms were as follows; 43/277 (16%) had a gram-positive bacterial infection (GP), 89/277 (32%) had a gram-negative bacterial infection (GN), 26/277 (9%) had a fungal infection, and the remaining 119/277 (43%) were infected with an UIO. Of those patients with a documented type of infection, 74/228 (33%) had a urinary tract infection, 61/228 (27%) pneumonia, 51/228 (22%) bacteremia, and 78/228 (34%) had multiple types of infections.
Age, sex, alcohol use, MELD, SIRS as well as reason for antibiotics were significantly different between patients who did and did not have an infection. Infection status, reason for antibiotics, and MELD were further significantly different between the more specific groups. We also analyzed etiology of liver disease and found that it was not predictive of infection (data not shown).
Surrogate markers as predictors of infections
We analyzed 34 clinical factors using univariable logistic models with infection as the dependent variable. Procalcitonin was not included in this analysis because there were not enough patients with available procalcitonin values. The results of this analysis for all infection types can be found in Table 1. The following factors were significantly associated with any infection, as determined by their ORs: SIRS criteria, sex, age, diabetes mellitus, hemodialysis, alcohol drinking, antibiotic use, white blood cell count, serum lactate, MELD score, prothrombin time, and creatinine. Among all the variables analyzed, SIRS was the most predictive of any infection (C-statistic = 0.71, 95% CI: 0.67, 0.75). The multivariable logistic regression model including SIRS criteria and all other significant variables yielded a C-statistic of 0.83 (95% CI: 0.77, 0.90). The improvement in prediction of infection based on the increase in C-statistic from 0.71 to 0.83 was statistically significant (P < 0.0001).
Table 1. Identification of Factors Associated With Any Infection (N = 457).
| Variable | Infection frequency (%)/mean ± SD | OR (95% CI) | C-statistics | P value |
|---|---|---|---|---|
| SIRS criteria, Y/N (n = 455) | 159 (84.13)/116 (43.61) | 6.85 (4.33, 10.85) | 0.71 | < 0.0001 |
| Sex, male/female | 164 (55.78)/113 (69.33) | 0.56 (0.37, 0.84) | 0.57 | < 0.01 |
| DM, Y/N (n = 455) | 103 (68.67)/173 (56.72) | 1.67 (1.11, 2.53) | 0.56 | 0.01 |
| CKD, Y/N (n = 456) | 36 (66.67)/240 (59.70) | 1.35 (0.74, 2.46) | 0.52 | 0.33 |
| HD, Y/N | 21 (87.50)/256 (59.12) | 4.84 (1.42, 16.45) | 0.53 | 0.01 |
| Chemotherapy, Y/N (n = 449) | 2 (28.57)/269 (60.86) | 0.26 (0.05, 1.34) | 0.51 | 0.11 |
| Immunotherapy, Y/N (n = 454) | 20 (55.56)/256 (61.24) | 0.79 (0.40, 1.57) | 0.51 | 0.50 |
| History of Clostridium difficile, Y/N | 13 (76.47)/264 (60.00) | 2.17 (0.70, 6.75) | 0.51 | 0.18 |
| History of cocci, Y/N | 7 (50.00)/270 (60.95) | 0.64 (0.22, 1.86) | 0.51 | 0.41 |
| TIPS, Y/N | 28 (65.12)/249 (60.14) | 1.24 (0.64, 2.39) | 0.51 | 0.53 |
| Ever smoker, Y/N (n = 453) | 137 (58.05)/137 (63.13) | 0.81 (0.55, 1.18) | 0.53 | 0.27 |
| Ever drinker, Y/N (n = 442) | 164 (56.55)/103 (67.76) | 0.62 (0.41, 0.94) | 0.55 | 0.02 |
| Antibiotic, Y/N (n = 441) | 38 (76.00)/229 (58.57) | 2.24 (1.14, 4.42) | 0.54 | 0.02 |
| Supplements, Y/N (n = 456) | 36 (61.02)/240 (60.45) | 1.02 (0.59, 1.79) | 0.50 | 0.93 |
| Lactate (n = 314) | 3.29 ± 2.77 | 1.19 (1.05, 1.34) | 0.58 | < 0.01 |
| Platelet | 117.28 ± 78.21 | 0.999 (0.997, 1.002) | 0.53 | 0.58 |
| Fib-4 | 12.09 ± 26.13 | 1.003 (0.995, 1.011) | 0.51 | 0.48 |
| APRI | 5.11 ± 17.57 | 1.01 (0.99, 1.02) | 0.48 | 0.38 |
| CTP | 11.48 ± 1.46 | 1.10 (0.96, 1.25) | 0.54 | 0.16 |
| MELD (n = 428) | 18.73 ± 8.52 | 1.04 (1.02, 1.07) | 0.60 | < 0.01 |
| Age (n = 456) | 55.50 ± 10.87 | 1.03 (1.02, 1.05) | 0.60 | < 0.001 |
| WBC (n = 456) | 9.08 ± 6.03 | 1.08 (1.04, 1.12) | 0.58 | < 0.0001 |
| Hb | 11.16 ± 2.78 | 0.99 (0.92, 1.06) | 0.51 | 0.74 |
| PT (n = 433) | 21.42 ± 17.95 | 1.00 (0.99, 1.01) | 0.56 | 0.67 |
| INR (n= 433) | 1.69 ± 0.96 | 1.25 (0.98, 1.61) | 0.57 | 0.08 |
| PTT (n = 231) | 38.57 ± 16.80 | 1.03 (1.00, 1.06) | 0.58 | < 0.05 |
| TB (n = 453) | 5.00 ± 6.32 | 0.99 (0.97, 1.02) | 0.49 | 0.69 |
| DB (n = 106) | 5.70 ± 5.51 | 0.99 (0.92, 1.06) | 0.45 | 0.73 |
| ALB (n = 402) | 3.43 ± 12.04 | 1.01 (0.98, 1.05) | 0.42 | 0.45 |
| ALP (n = 455) | 163.77 ± 128.99 | 0.999 (0.997, 1.000) | 0.51 | 0.18 |
| AST | 115.86 ± 248.31 | 1.00 (0.999, 1.001) | 0.47 | 0.54 |
| ALT | 64.95 ± 155.36 | 1.00 (0.999, 1.001) | 0.46 | 0.77 |
| Cr (n = 454) | 1.54 ± 1.88 | 1.19 (1.03, 1.38) | 0.60 | 0.02 |
Y/N: yes/no; SD: standard deviation; C-statistic: concordance statistic; OR: odds ratio; CI: confidence interval; SIRS: systemic inflammatory response syndrome; DM: diabetes mellitus; CKD: chronic kidney diseases; HD: hemodialysis; TIPS: transjugular intrahepatic portosystemic shunt; Fib-4: fibrosure-4; APRI: AST to platelet ration index; CTP: Child-Turcotte-Pugh; MELD: model for end-stage liver disease; WBC: white blood cell count; Hb: hemoglobin; PT: prothrombin; INR: international normalized ratio; PTT: prothrombin time; TB: total bilirubin; DB: direct bilirubin; ALB: albumin; ALP: alkaline phosphatase test; AST: aspartate aminotransferase; ALT: alanine transaminase; Cr: creatinine.
Table 2 displays the results of this analysis for bacterial infections. Age, sex, SIRS, smoking status, serum lactate, white blood cell count, and Child-Turcotte-Pugh yielded significant ORs. Again, the SIRS criteria yielded the highest C-statistic, 0.63 (95% CI: 0.58, 0.67). The logistic regression model including SIRS criteria and all other significant variables yielded a C-statistic of 0.71 (95% CI: 0.65, 0.77). The improvement in prediction of bacterial infection based on the increase in C-statistic from 0.63 to 0.71 was statistically significant (P < 0.001).
Table 2. Identification of Factors Associated With Bacterial Infection (N = 457).
| Variable | Infection frequency (%)/mean ± SD | OR (95% CI) | C-statistics | P value |
|---|---|---|---|---|
| SIRS criteria, Y/N (n = 455) | 114 (60.32)/92 (34.59) | 2.88 (1.96, 4.23) | 0.63 | < 0.0001 |
| Sex, male/female | 120 (40.82)/86 (52.76) | 0.62 (0.42, 0.91) | 0.56 | 0.01 |
| DM, Y/N (n = 455) | 70 (46.67)/135 (44.26) | 1.10 (0.74, 1.63) | 0.51 | 0.63 |
| CKD, Y/N (n = 456) | 28 (51.85)/177 (44.03) | 1.37 (0.78, 2.42) | 0.52 | 0.28 |
| HD, Y/N | 15 (62.50)/191 (44.11) | 2.11 (0.90, 4.93) | 0.52 | 0.08 |
| Chemotherapy, Y/N (n = 449) | 1 (14.29)/200 (45.25) | 0.20 (0.02, 1.69) | 0.51 | 0.14 |
| Immunotherapy, Y/N (n = 454) | 12 (33.33)/194 (46.41) | 0.58 (0.28, 1.19) | 0.52 | 0.13 |
| History of Clostridium difficile, Y/N | 7 (41.18)/199 (45.23) | 0.85 (0.32, 2.27) | 0.50 | 0.74 |
| History of cocci, Y/N | 5 (35.71)/201 (45.37) | 0.67 (0.22, 2.03) | 0.51 | 0.48 |
| TIPS, Y/N | 25 (58.14)/181 (43.72) | 1.79 (0.95, 3.38) | 0.53 | 0.07 |
| Ever smoker, Y/N (n = 453) | 94 (39.83)/109 (50.23) | 0.66 (0.45, 0.95) | 0.55 | 0.03 |
| Ever drinker, Y/N (n = 442) | 121 (41.72)/77 (50.66) | 0.70 (0.47, 1.04) | 0.54 | 0.07 |
| Antibiotic, Y/N (n = 441) | 28 (56.00)/172 (43.99) | 1.62 (0.90, 2.93) | 0.52 | 0.11 |
| Supplements, Y/N (n = 456) | 27 (45.76)/179 (45.09) | 1.03 (0.59, 1.78) | 0.50 | 0.92 |
| Lactate (n = 314) | 3.29 ± 2.77 | 1.11 (1.01, 1.21) | 0.58 | 0.02 |
| Platelet | 117.28 ± 78.21 | 1.001 (0.999, 1.004) | 0.52 | 0.23 |
| Fib-4 | 12.09 ± 26.13 | 1.003 (0.996, 1.010) | 0.47 | 0.40 |
| APRI | 5.11 ± 17.57 | 1.01 (0.99, 1.02) | 0.45 | 0.28 |
| CTP | 11.48 ± 1.46 | 1.16 (1.02, 1.32) | 0.57 | 0.02 |
| MELD (n = 428) | 18.73 ± 8.52 | 1.02 (1.00, 1.05) | 0.56 | 0.05 |
| Age (n = 456) | 55.50 ± 10.87 | 1.03 (1.01, 1.05) | 0.59 | < 0.01 |
| WBC (n = 456) | 9.08 ± 6.03 | 1.06 (1.03, 1.10) | 0.58 | < 0.001 |
| Hb | 11.16 ± 2.78 | 0.98 (0.92, 1.05) | 0.51 | 0.58 |
| PT (n = 433) | 21.42 ± 17.95 | 1.01 (0.99, 1.02) | 0.55 | 0.35 |
| INR (n = 433) | 1.69 ± 0.96 | 1.20 (0.97, 1.49) | 0.55 | 0.09 |
| PTT (n = 231) | 38.57 ± 16.80 | 1.01 (0.99, 1.03) | 0.57 | 0.28 |
| TB (n = 453) | 5.00 ± 6.32 | 0.98 (0.95, 1.01) | 0.52 | 0.27 |
| DB (n = 106) | 5.70 ± 5.51 | 1.00 (0.93, 1.07) | 0.51 | 0.95 |
| ALB (n = 402) | 3.43 ± 12.04 | 1.00 (0.98, 1.01) | 0.53 | 0.73 |
| ALP (n = 455) | 163.77 ± 128.99 | 0.999 (0.997, 1.000) | 0.54 | 0.13 |
| AST | 115.86 ± 248.31 | 1.000 (0.999, 1.001) | 0.47 | 0.84 |
| ALT | 64.95 ± 155.36 | 1.000 (0.998, 1.001) | 0.57 | 0.62 |
| Cr (n = 454) | 1.54 ± 1.88 | 1.10 (0.98, 1.22) | 0.59 | 0.09 |
Y/N: yes/no; SD: standard deviation; C-statistic: concordance statistic; OR: odds ratio; CI: confidence interval; SIRS: systemic inflammatory response syndrome; DM: diabetes mellitus; CKD: chronic kidney diseases; HD: hemodialysis; TIPS: transjugular intrahepatic portosystemic shunt; Fib-4: fibrosure-4; APRI: AST to platelet ration index; CTP: Child-Turcotte-Pugh; MELD: model for end-stage liver disease; WBC: white blood cell count; Hb: hemoglobin; PT: prothrombin; INR: international normalized ratio; PTT: prothrombin time; TB: total bilirubin; DB: direct bilirubin; ALB: albumin; ALP: alkaline phosphatase test; AST: aspartate aminotransferase; ALT: alanine transaminase; Cr: creatinine.
The entire cohort was also analyzed for factors predictive of fungal infections. MELD score and hemoglobin (Hb) were associated with fungal infections (Table 3). A logistic regression model including all significant variables (i.e., MELD, Hb, prothrombin, international normalized ratio, and total bilirubin) yielded a C-statistic of 0.74 (95% CI: 0.63, 0.84). A cut-off MELD score of 19 is associated with sensitivity of 82% and specificity of 59% to predict fungal infection (Table 4).
Table 3. Identification of Factors Associated With Fungal Infections (N = 457).
| Variable | Infection frequency (%)/mean ± SD | OR (95% CI) | C-statistics | P value |
|---|---|---|---|---|
| SIRS criteria, Y/N (n = 455) | 12 (6.35)/13 (4.89) | 1.32 (0.59, 2.96) | 0.53 | 0.50 |
| Sex, male/female | 13 (4.42)/12 (7.36) | 0.58 (0.26, 1.31) | 0.57 | 0.19 |
| DM, Y/N (n = 455) | 11 (7.33)/14 (4.59) | 1.65 (0.73, 3.72) | 0.56 | 0.23 |
| CKD, Y/N (n = 456) | 4 (7.41)/21 (5.22) | 1.45 (0.48, 4.40) | 0.52 | 0.51 |
| HD, Y/N | 1 (4.17)/24 (5.54) | 0.74 (0.10, 5.72) | 0.51 | 0.77 |
| Chemotherapy, Y/N (n = 449) | 1 (14.29)/24 (5.43) | 2.90 (0.34, 25.09) | 0.51 | 0.33 |
| Immunotherapy, Y/N (n = 454) | 3 (8.33)/22 (5.26) | 1.64 (0.47, 5.75) | 0.52 | 0.44 |
| History of Clostridium difficile, Y/N | 0 (0)/25 (5.68) | NA | 0.52 | 0.98 |
| History of cocci, Y/N | 0 (0)/25 (5.64) | NA | 0.52 | 0.98 |
| TIPS, Y/N | 1 (2.33)/24 (5.80) | 0.39 (0.05, 2.93) | 0.53 | 0.36 |
| Ever smoker, Y/N (n = 453) | 13 (5.51)/12 (5.53) | 1.00 (0.44, 2.23) | 0.50 | 0.99 |
| Ever drinker, Y/N (n = 442) | 15 (5.17)/9 (5.92) | 0.87 (0.37, 2.03) | 0.52 | 0.74 |
| Antibiotic, Y/N (n = 441) | 5 (10.00)/20 (5.12) | 2.06 (0.74, 5.76) | 0.55 | 0.17 |
| Supplements, Y/N (n = 456) | 2 (3.39)/23 (5.79) | 0.57 (0.13, 2.49) | 0.53 | 0.45 |
| Lactate (n = 314) | 3.29 ± 2.77 | 1.10 (0.98, 1.25) | 0.62 | 0.11 |
| Platelet | 117.28 ± 78.21 | 0.996 (0.990, 1.002) | 0.55 | 0.21 |
| Fib-4 | 12.09 ± 26.13 | 1.00 (0.99, 1.02) | 0.57 | 0.76 |
| APRI | 5.11 ± 17.57 | 0.99 (0.95, 1.03) | 0.45 | 0.67 |
| CTP | 11.48 ± 1.46 | 1.20 (0.91, 1.60) | 0.55 | 0.20 |
| MELD (n = 428) | 18.73 ± 8.52 | 1.06 (1.01, 1.10) | 0.69 | 0.01 |
| Age (n = 456) | 55.50 ± 10.87 | 1.01 (0.98, 1.05) | 0.53 | 0.53 |
| WBC (n = 456) | 9.08 ± 6.03 | 0.96 (0.89, 1.04) | 0.51 | 0.35 |
| Hb | 11.16 ± 2.78 | 0.84 (0.72, 0.97) | 0.64 | 0.02 |
| PT (n = 433) | 21.42 ± 17.95 | 1.013 (1.001, 1.026) | 0.70 | 0.03 |
| INR (n = 433) | 1.69 ± 0.96 | 1.34 (1.03, 1.74) | 0.71 | 0.03 |
| PTT (n = 231) | 38.57 ± 16.80 | 1.015 (0.996, 1.034) | 0.73 | 0.13 |
| TB (n = 453) | 5.00 ± 6.32 | 1.052 (1.004, 1.102) | 0.63 | 0.03 |
| DB (n = 106) | 5.70 ± 5.51 | 0.91 (0.76, 1.10) | 0.52 | 0.35 |
| ALB (n = 402) | 3.43 ± 12.04 | 1.013 (0.996, 1.031) | 0.41 | 0.13 |
| ALP (n = 455) | 163.77 ± 128.99 | 0.999 (0.995, 1.003) | 0.53 | 0.59 |
| AST | 115.86 ± 248.31 | 0.996 (0.990, 1.003) | 0.52 | 0.27 |
| ALT | 64.95 ± 155.36 | 0.99 (0.98, 1.01) | 0.56 | 0.18 |
| Cr (n = 454) | 1.54 ± 1.88 | 1.01 (0.82, 1.25) | 0.51 | 0.89 |
Y/N: yes/no; SD: standard deviation; C-statistic: concordance statistic; OR: odds ratio; CI: confidence interval; SIRS: systemic inflammatory response syndrome; DM: diabetes mellitus; CKD: chronic kidney diseases; HD: hemodialysis; TIPS: transjugular intrahepatic portosystemic shunt; Fib-4: fibrosure-4; APRI: AST to platelet ration index; CTP: Child-Turcotte-Pugh; MELD: model for end-stage liver disease; WBC: white blood cell count; Hb: hemoglobin; PT: prothrombin; INR: international normalized ratio; PTT: prothrombin time; TB: total bilirubin; DB: direct bilirubin; ALB: albumin; ALP: alkaline phosphatase test; AST: aspartate aminotransferase; ALT: alanine transaminase; Cr: creatinine.
Table 4. Cutoffs for Fungal Infection (N = 457).
| Variable | Best cutoffa | Sensitivityb | Specificityb | Youdenc |
|---|---|---|---|---|
| MELD | 19.33 | 81.8% | 58.6% | 40.4% |
| Total bilirubin | 4.20 | 64.0% | 67.8% | 31.8% |
| International normalized ratio | 1.50 | 79.2% | 54.8% | 33.9% |
| Hemoglobin | 11.30 | 76.0% | 49.8% | 25.8% |
aThe value maximizes Youden statistic. bROC analysis was performed to derive sensitivity and specificity based on a logistic regression model with the continuous variable of interest (e.g., MELD) as the only covariate. cYouden = sensitivity + specificity - 1. MELD: model for end-stage liver disease; ROC: receiver operating characteristic.
To get an idea of how infection types differ, we compared infection subgroups based on microorganism types: GN, GP, fungal infection, and UIO. All four groups were similar, except for prothrombin/international normalized ratio and prothrombin time. UIO had mildly abnormal coagulation parameters with mean international normalized ratio 1.61 and prothrombin time 38.89 s. Patients with fungal infections are more likely to have anemia, elevated bilirubin, and alkaline phosphatase compared to GP or GN infections. After adjusting for all positive variables, elevated MELD was strongly associated with fungal infections (P ≤ 0.01). Overall, patients with fungal infections had higher risk of death (hazard ratio (HR) 2.4, 95% CI 1.35, 4.46, P = 0.003) compared to UIO. Additionally, they were more likely to stay longer in the hospital (P = 0.04) and had higher readmission rates (P = 0.01).
Workup for infection diagnosis and identification
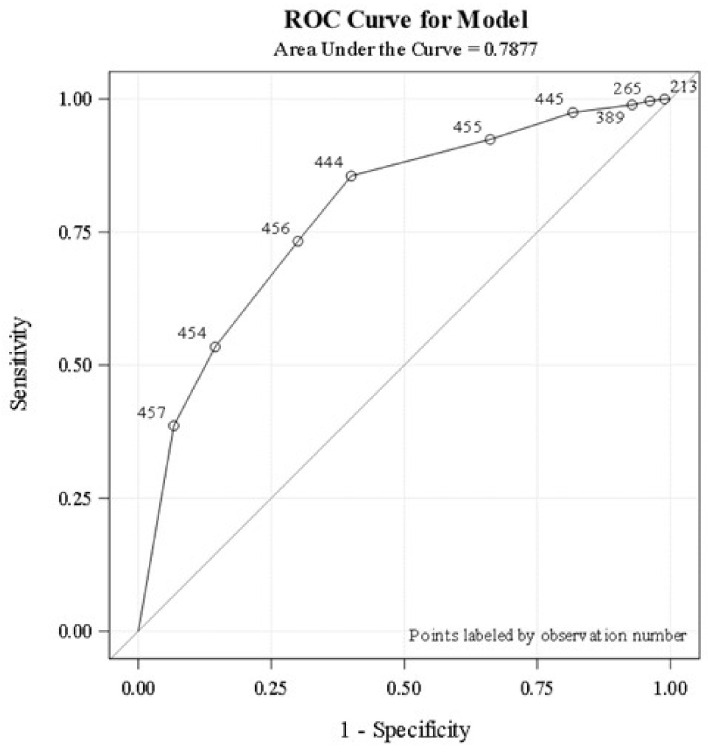
A total of 119/457 (26%) patients completed basic infectious disease workup (A/E ratio 1) during the entire hospital stay. Only 53 patients (12%) completed the workup within the first 24 h of admission. The logistic regression analysis revealed that the higher the proportion of basic infectious workup tests completed (A/E ratio > 0.8), the higher the probability the patient will be classified as having an infection, C-statistic 0.788 (Fig. 1). Basic workup to identify fungal infections such as fungal blood cultures, ascitic fluid for fungus culture, and potassium hydroxide staining were not done in most patients. Ascitic fluid analysis was never performed in about one-third of patients with ascites. Serum lactate (at least one time) was obtained in 67% during acute illness. Compliance to identification and documentation of SIRS criteria upon admission and during the subsequent course was poor. Groups one and two had similar rates of infection identification and were significantly higher compared to group three (90% vs. 50%). Timing and completeness of the basic workup to identify infections did not reveal difference in survival on adjusted regression analysis (P = 0.11).
Figure 1.
Logistic regression analysis demonstrates the association between the proportion of basic infectious workup tests completed and infection status. Analysis is based on the area under the ROC curve. ROC: receiver operating characteristic.
Discussion
Patients with cirrhosis can deteriorate acutely as a result of intercurrent medical problems including infections, drug-induced liver injury, alcohol consumption, and gastrointestinal bleeding with infection being the most common [7]. Diagnosing and treating infections in cirrhotic patients is challenging, both under-treatment and over-treatment can result in higher mortality [7]. As we already know, infections can be diagnosed with isolation of microorganisms, serological tests, or other clinical tools such as urinalysis, chest X-ray, ascites fluid analysis, and clinical examination. Our study clearly indicates that compliance to infection diagnosis guidelines is extremely poor among ACLF patients. We found that six basic infectious tests, if performed efficiently, are good enough to diagnose infection. Serological tests could be added to identify fungal or viral infections (less common).
The liver performs several immune functions, such as clearing bacteria and endotoxins from circulation, and these functions decline as the severity of liver damage progresses, leading to the development of an immune suppressed state [4, 11], in which infection indicators may not be accurate. Our study indicates that SIRS and serum lactate are not sufficient infection indicators. Both SIRS-positive and SIRS-negative patients have high rates of infection; bacterial infections were detected in 60.32% of SIRS-positive patients and 34.59% of SIRS-negative patients. Within our any infection cohort 58% were SIRS-positive and 42% were SIRS-negative. Therefore, infection cannot be ruled out for a SIRS-negative patient. Moreover, cirrhotic patients who meet SIRS criteria do not respond to antibiotic treatment and their mortality risk remains high [7]. Other studies have associated SIRS with poor outcomes independent of other factors including infection [12]. This makes SIRS a prognostic determinant rather than a screening tool for infection at an early stage.
Our data did not suggest that serum lactate alone is an accurate predictor of infection within cirrhotic patients. However, including additional variables in a multivariable logistic model increased predictive accuracy significantly. C-statistics increased to 0.83 for any infection (P < 0.0001), and to 0.71 for bacterial infections. Unfortunately, these C-statistics are not acceptable in diagnostic practice.
There has been promising research on the diagnostic value of additional biochemical and clinical variables not available within our study. Gur et al determined that procalcitonin above 0.217 was associated with an 11 times risk of infection (P < 0.05) [13]. Other studies detected diagnostic correlation with serum C-reactive protein with a cut-off value of greater than 55.8 mg/L [14] and quick sequential organ failure assessment (qSOFA) score [15]. In post hoc analyses researchers found that an onset of infection correlates with an increase in qSOFA score [15].
A recent overview of the treatment of cirrhotic patients advises against the prophylactic use of antibiotics except in special circumstances such as gastrointestinal bleeding, history of spontaneous bacterial peritonitis, or ascites fluid protein concentration < 1.5 g/dL [16]. Despite this recommendation, we know that with septic shock each hour of delay in antibiotic treatment following documented hypotension can decrease patient survival by up to 7.6% for the first 6 h [17]. We lose vital time while waiting the result of a bacterial culture, but we also do not want to put unnecessary stress on the liver by treating a non-existent infection. Further data are needed to identify readily available diagnostics to use within this patient population.
In conclusion, our study revealed that SIRS criteria alone moderately predict infections in acutely decompensated cirrhotic patients. The addition of other factors moderately improves predictive ability. Elevated MELD score is associated with fungal infections, which resulted in high mortality. More comprehensive tools are needed to predict infection upon admission while awaiting the results of an infection workup. Current practice to identify infections is extremely poor but can be predicted using a set of six basic infectious tests. A standardized approach consisting of some of the parameters mentioned here, evaluated in a prospective study, is necessary to improve the care and outcomes of patients with acutely decompensated cirrhosis.
Acknowledgments
This research was supported by Liver Institute PLLC. We appreciate the editorial assistance of Mohammad Habib and Farah Alsafar.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Informed Consent
The informed consent was waived by the University of Arizona’s Institutional Review Board.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Author Contributions
Shahid Habib: study idea, design, data collection, statistical analysis and manuscript writing. Sandeep Yarlagadda: protocol writing and data collection. Chiu-Hsieh Hsu: statistical analysis. Teresia A. Carreon: manuscript writing, editing, and submission. Lindsey Schader: manuscript writing and statistical analysis.
Abbreviations
- ACLF
acute-on-chronic liver failure
- OR
odds ratio
- SIRS
systemic inflammatory response syndrome
- MELD
model for end-stage liver disease
- C-statistic
concordance statistic
- ROC
receiver operating curve
- A/E
actual to expected
- DM
diabetes mellitus
- HD
hemodialysis
- WBC
white blood cell count
- HR
hazard ratio
- qSOFA
quick Sequential Organ Failure Assessment
References
- 1.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20(10):2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V. et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Baijal R, Amarapurkar D, Praveen Kumar HR, Kulkarni S, Shah N, Doshi S, Gupta D. et al. A multicenter prospective study of infections related morbidity and mortality in cirrhosis of liver. Indian J Gastroenterol. 2014;33(4):336–342. doi: 10.1007/s12664-014-0461-3. [DOI] [PubMed] [Google Scholar]
- 4.Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8(6):307–321. doi: 10.4254/wjh.v8.i6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Lewis JH. Hepatotoxicity of antibiotics: a review and update for the clinician. Clin Liver Dis. 2013;17(4):609–642. doi: 10.1016/j.cld.2013.07.008. ix. [DOI] [PubMed] [Google Scholar]
- 6.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib S, Patel N, Yarlagadda S, Hsu CH, Patel S, Schader L, Walker C. et al. Safety and efficacy of antibiotics among acutely decompensated cirrhosis patients. J Gastroenterol Hepatol. 2018;33(11):1882–1888. doi: 10.1111/jgh.14267. [DOI] [PubMed] [Google Scholar]
- 8.Liao MM, Lezotte D, Lowenstein SR, Howard K, Finley Z, Feng Z, Byyny RL. et al. Sensitivity of systemic inflammatory response syndrome for critical illness among ED patients. Am J Emerg Med. 2014;32(11):1319–1325. doi: 10.1016/j.ajem.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo DY, Jo S, Lee JB, Jin YH, Jeong T, Yoon J, Park B. Diagnostic performance of initial serum lactate for predicting bacteremia in female patients with acute pyelonephritis. Am J Emerg Med. 2016;34(8):1359–1363. doi: 10.1016/j.ajem.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 10.Vassiliou AG, Mastora Z, Jahaj E, Koutsoukou A, Orfanos SE, Kotanidou A. Does serum lactate combined with soluble endothelial selectins at ICU admission predict sepsis development? In Vivo. 2015;29(2):305–308. [PubMed] [Google Scholar]
- 11.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(9):727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D. et al. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54(5):718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gur A, Oguzturk H, Kose A, Turtay MG, Ersan V, Bayindir Y, Ince V. et al. Prognostic value of procalcitonin, CRP, serum amyloid A, lactate and IL-6 markers in liver transplant patients admitted to ED with suspected infection. In Vivo. 2017;31(6):1179–1185. doi: 10.21873/invivo.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiakalos A, Karatzaferis A, Ziakas P, Hatzis G. Acute-phase proteins as indicators of bacterial infection in patients with cirrhosis. Liver Int. 2009;29(10):1538–1542. doi: 10.1111/j.1478-3231.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 15.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G. et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375(8):767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.