Abstract
To date, no effective vaccines or therapies are available against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pandemic agent of the coronavirus disease 2019 (COVID-19). Due to their safety, efficacy and specificity, peptide inhibitors hold great promise for the treatment of newly emerging viral pathogens. Based on the known structures of viral proteins and their cellular targets, antiviral peptides can be rationally designed and optimized. The resulting peptides may be highly specific for their respective targets and particular viral pathogens or exert broad antiviral activity. Here, we summarize the current status of peptides inhibiting SARS-CoV-2 entry and outline the strategies used to design peptides targeting the ACE2 receptor or the viral spike protein and its activating proteases furin, transmembrane serine protease 2 (TMPRSS2), or cathepsin L. In addition, we present approaches used against related viruses such as SARS-CoV-1 that might be implemented for inhibition of SARS-CoV-2 infection.
Keywords: Peptide drug, COVID-19, Coronavirus, Fusion, Antiviral
Graphical abstract
1. Introduction
1.1. Peptides as drugs against viral infections
Human pathogenic viruses pose an enormous threat to human health, society and economy. Unlike other pathogens, against most viruses neither specific pharmaceuticals nor vaccines are available. This situation is further complicated by the fact that viruses, such as human immunodeficiency virus type 1 (HIV-1), may develop resistance and that viruses keep (re-)emerging (e.g. Ebola and Zika virus) [1,2]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 2019 (COVID-19), rapidly spread around the world causing many fatalities and devastating consequences for the health sector, the economy and the society in general. Although enormous efforts are made towards the rapid development of a vaccine, no vaccine has been approved so far. In addition, there is no assurance that vaccines against this coronavirus will provide long-lasting effective protection [3,4], and a vaccine will most likely not help those who are already infected. Therefore, the development of therapeutic agents against this pandemic viral pathogen is urgently needed. To date, only convalescent plasma and remdesivir received emergency use authorization for clinical applications because they were reported to shorten the recovery period [5,6]. Repurposing of some small molecule inhibitors such as favipiravir is currently investigated in clinical trials [7,8]. However, no effective therapy against SARS-CoV-2 is available to date.
Peptide and peptide-based inhibitors represent an attractive alternative to small molecules because peptides may be more efficient and specific than small molecule drugs and thus be better tolerated [9,10]. Furthermore, peptide synthesis can be quickly implemented and tuned. In contrast, small molecules often require the development of new time-consuming and costly synthetic strategies. On the other hand, large-scale peptide synthesis may be expensive, depending on the composition of the peptide. Clinical application of the anti-HIV peptide T20 proves that peptidic antivirals can be a safe and effective alternative for the treatment of infectious diseases [11]. Generally, to translate peptide inhibitors into clinical applications these peptides need to be bioavailable, potent, stable, and safe. Their chemical composition makes peptides highly specific and tolerable but due to their relatively large size and proteolytic degradation, peptides frequently have low (oral) bioavailability and short half-lives [9]. However, peptides can be modified to improve activity and stability [12] by shortening their sequences, changing amino acids, or adding moieties that increase their affinities to the respective partner as often determined by computational modeling or amino acid scanning. Notably, such modifications can be introduced in a relatively short time. Typical modifications are polyethylene glycol (PEG)-ylation, which increases the hydrodynamic radius of the peptides [12,13], and the introduction of D- or non-natural amino acids that reduces recognition by proteases.
Antiviral peptides usually target structures essential for viral replication. For instance, they may target viral proteins and interfere with their interactions, tune enzymatic activity, modulate conformational changes, or influence host proteins that are essential for virus infection such as entry receptors or proteases activating viral proteins. Notably, many antiviral peptides naturally occur as part of the innate immune system (e.g. defensins, protegrin, or LL-37 [[14], [15], [16], [17], [18]]). Some of them, including the endogenous peptide inhibitor of CXCR4 EPI-X4 [19] or the virus inhibitory peptide VIRIP [11,150] are released from abundant precursor proteins by proteolytic cleavage. Alternatively, bioactive peptides can be rationally designed based on the structure and function of viral proteins (e.g. T20 against HIV-1 [11] and myrcludex B against hepatitis B virus [20,21]).
For the design of antiviral peptides, interactions sites are often modelled based on the analysis of polarity, charges and steric effects, as well as on mutagenesis studies and biomolecular simulations. A straightforward approach to peptide design is to adopt sequences from the interaction sites of proteins. These peptides have the potential to block key protein – protein interactions. Strategies focusing on targeting viral proteins are usually highly specific to the virus of interest. On the other hand, strategies targeting a cellular factor might result in inhibitors with activity against diverse viruses that depend on the targeted host factor for completion of their replication cycle. However, targeting cellular factors may result in unwanted side effects if the physiological function of the respective target is also inhibited. In many cases, peptides are designed to act extracellularly, i.e. to target early steps of viral replication, such as viral envelope glycoprotein activation, receptor attachment, or fusion. This has the advantage that the therapeutic peptide does not need to penetrate the cell membrane and that potential harmful interactions between the viral pathogen and host cells are minimized. Here, we summarize the status of the research and the strategies used for the development of peptides targeting SARS-CoV-2 entry.
1.2. SARS-CoV-2 entry
SARS-CoV-2 belongs to the family of Coronaviridae. Seven coronaviruses (CoVs) have crossed the species barriers from their bat reservoir via various intermediate hosts to spread and cause disease in humans. These include human α-coronaviruses HCoV-229E and HCoV-NL63 and the β-coronaviruses HCoV-OC43 and HCoV-HKU1, all associated with endemic infections of the upper respiratory tract causing common colds. In recent years, several epidemic β-coronaviruses associated with severe respiratory diseases have emerged: SARS-CoV(-1), the Middle East respiratory syndrome CoV (MERS-CoV), and most recently SARS-CoV-2 [22]. Coronaviruses share considerable sequence homology, also with the viruses found in their reservoir hosts. For instance, SARS-CoV-2 shows 79.6% and 96% nucleotide sequence identity with SARS-CoV-1 and with the bat coronavirus SL-CoV-RaTG13, respectively [23].
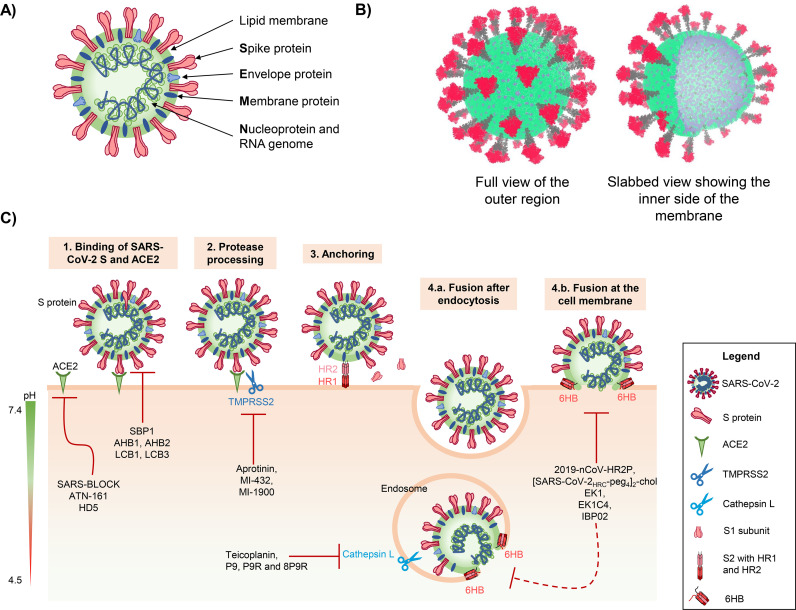
Coronaviruses are enveloped viruses harboring a ~30 kb positive-sense single-stranded RNA genome that encodes structural and accessory genes. The structural nucleocapsid (N) protein is associated with the viral genome, while the membrane (M) and envelope (E) proteins are the main proteins in the shape-giving viral membrane (Fig. 1A, B). The spike (S) protein is exposed at the membrane, which gives the virus particle its corona-like appearance (Fig. 1A, B) [24]. The S protein mediates viral attachment, fusion and entry, and represents the main target for the development of neutralizing antibodies, entry inhibitors and vaccines [25].
Fig. 1.
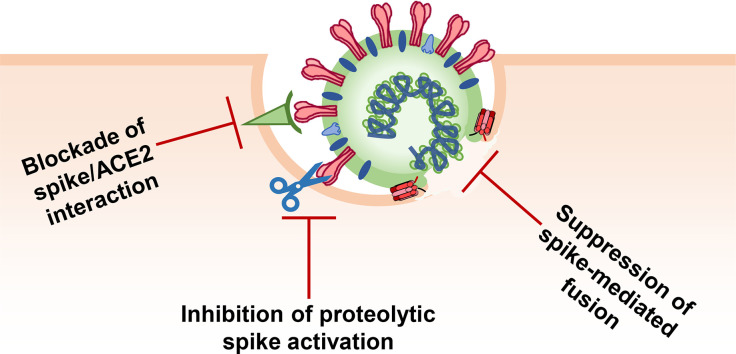
Structure, entry and inhibition of SARS-CoV-2. A) Schematic representation of a SARS-CoV-2 particle. B) Coarse-grained model of the SARS-CoV-2 virion structure [49]. The S1 subunits of the S proteins are depicted in red, the S2 subunits in grey, the lipid bilayer membrane in green and the integrated M and E proteins in cyan. C) Schematic illustration of SARS-CoV-2 entry steps that can be targeted by peptide inhibitors. First, the SARS-CoV-2 S protein binds to the cellular receptor ACE2. Proteolytic processing of the S protein allows rearrangement of the S2 subunit and anchoring by inserting the fusion peptide into the cellular membrane. Conformational reorganization of HR1 and HR2 to form the 6HB mediates fusion between the viral and cellular membrane either at the cell surface or after endocytosis. As soon as both membranes are fused, viral RNA and proteins are released into the cytoplasm.
Entry of SARS-CoV-2 into its target cells involves several steps (Fig. 1C). Initially, the S protein of SARS-CoV-2 binds to the SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) [26]. The S protein needs to be activated by cellular proteases to permit insertion of the viral fusion peptide into the host membrane, the anchoring process. Subsequently, the HR1 and HR2 regions of the trimeric viral transmembrane protein interact to form a six-helix bundle, which pulls the viral and cellular membranes together and mediates fusion, thereby resulting in the release of the viral genome into the cytoplasm [3] (Fig. 1C).
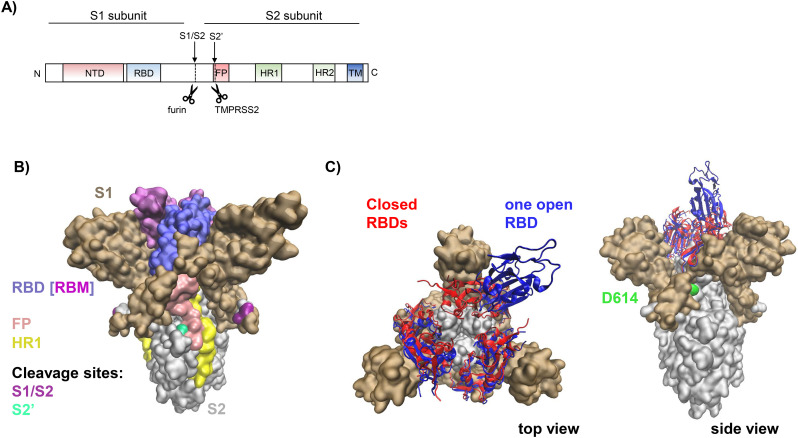
During the maturation process, the S protein is initially cleaved by the host serine protease furin and potentially other proprotein convertases (PCSKs), resulting in a transmembrane S2 subunit and a protruding extracellular S1 subunit that remain non-covalently associated (Fig. 2A, B). While furin cleaves the coronaviral S protein mostly intracellularly, during protein maturation in the trans-Golgi-network, plasma membrane-exposed or secreted furin might also activate viral particles in the extracellular space or during entry [27]. The resulting S1/S2 protomers appear as mushroom-like trimers on the viral membrane. Each of the protomers can adopt an “open/up” or “closed/down” conformation (Fig. 2C) [28]. The “open” conformation exposes the receptor binding domain (RBD) in S1, which includes the receptor binding motif (RBM) that directly interacts with ACE2. Both states, “open” and “closed”, spontaneously and independently rearrange in each protomer, thereby determining the affinity to ACE2. Interestingly, the currently predominating SARS-CoV-2 variant carries a D614G mutation in S1 that favors the “open” conformation, thus rendering the RBD more accessible (Fig. 2B, C). In addition, glycosylation sites N165 and N234 favour the “open” state [29]. Accordingly, the SARS-CoV-2 D614G variant is more infectious in cell culture [30]. The S proteins of SARS-CoV-1 and SARS-CoV-2 share 76% sequence homology while the RBDs of SARS-CoV-1 and SARS-CoV-2 share 75% sequence homology [31]. Both viruses utilize ACE2 as receptor to infect their target cells [23,32,33]. It has been reported that the ACE2-binding affinity of the RBD in the S1 subunit of SARS-CoV-2 is 10- to 20-fold higher than that of SARS-CoV-1 [34]. This may explain the higher transmissibility of SARS-CoV-2 compared to SARS-CoV-1 [23].
Fig. 2.
The SARS-CoV-2 spike protein. A) Schematic representation of the primary structure of the spike (S) protein with cleavage sites S1/S2 and S2′; NTD, N-terminal domain; RBD, receptor binding domain; FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat 2; TM, transmembrane domain. Arrows indicate furin and TMPRSS2 cleavage sites. B) Model of the trimeric ectodomain of the SARS-CoV-2 S protein. The figure is based on the structure reported with PDB ID 6ZGE [50], where missing residues were re-constructed by the SwissModel team [51]. The S1 subunit is shown in ochre with the receptor binding domain (RBD: 319–541) highlighted in violet and the specific receptor binding motif (RBM: 437–508) in light purple. The S2 subunit is shown in grey, with the fusion peptide (FP) region highlighted in pink and the heptad repeat 1 (HR1) region in yellow. S2′ is shown in green and the S1/S2 cleavage site is indicated in purple. C) Ectodomain of the trimeric S protein. All RBDs of S1 subunits in their closed states are shown in red (PDB ID: 6VXX [35]) and one RBD in its open state (PDB ID: 6VYB [35]) is shown in blue. The site associated with the mutation D614G, which favors the open RBD conformation, is indicated as green surface.
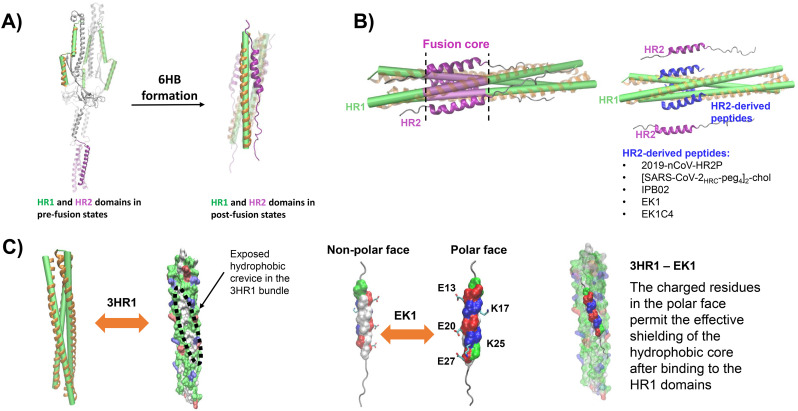
ACE2 binding to S1 induces drastic conformational changes that trigger the S2 subunit to mediate fusion. S2 is a typical viral class I fusion protein [35], which includes a hydrophobic fusion peptide (FP), two α-helical hydrophobic (heptad) repeats (HR1 and HR2), a long linking loop region, and a transmembrane domain. HR2 is located close to the transmembrane anchor, and HR1 is close to the FP (Fig. 2A). After binding of the S1 domain to ACE2, the FP is inserted into the cellular membrane. The HR pair builds an α-helical anti-parallel complex between HR1 and HR2, where the loop acts as a hinge and brings the viral and cellular membranes in close proximity [36]. Finally, the three HR pairs of the S trimer form a six-helix bundle (6HB) motif where the HR1 helices form a central coiled-coil fusion core surrounded by three HR2 helices in an anti-parallel arrangement (Figs. 1 and 6B). This conformation is highly stable and thought to help overcome the large energy barrier involved in membrane fusion [37]. The formation of 6HB in class I fusion proteins is a common process in viral entry and also used e.g. by HIV-1, herpes simplex virus and Ebola virus, among others [10,[38], [39], [40], [41], [42], [43], [44], [45]]. Notably, the HR2 domains of SARS-CoV-2 and SARS-CoV-1 are identical, while the HR1 domains show variations [46]. This might affect the interaction between HR1 and HR2 and consequently explain the superior plasma membrane fusion capacity of SARS-CoV-2 [47].
Fig. 6.
Structure and inhibition of the SARS-CoV-2 S protein fusion core. A) The HR domains of the trimeric S protein in a pre-fusion arrangement (left panel), and in the post-fusion conformation (right panel) building a six-helix bundle (6HB, PDB ID 6LXT [47]). The three heptad repeat (HR1) domains are shown as green cylinders, surrounded by orange helices, HR2 domains are depicted in purple. The long linker loop between HR1 and HR2 is depicted in grey when in the pre-fusion state. B) The HR1 domains form a three-helices bundle that serves as scaffold for the fusion core (left panel). The fusion core, shown in purple, is formed by binding of the three HR2 domains to the HR1 bundle. By binding of HR2-derived peptides (in blue) to the HR1 domains (right panel), the formation of the 6HB is inhibited. The mechanism of activity of these peptides involves their tight binding to HR1, thus preventing the association of the HR2 domains. HR1-derived peptides include IPB02 [145], EK1 [141], EK1C4 [47], 2019-nCoV-HRP2 [46] and [SARS-CoV-2HRC-peg4]2-chol [146]. C) The three HR1 domains in A) are also shown as surface projections (left panel) coloured by residue type (green: polar, red: acidic, blue: basic, white: non-polar). The molecular surface evidences open hydrophobic crevices in the regions between the helices. The EK1 peptide is presented with the molecular surface of the main interaction fragment coloured by residue type, the remaining residues are shown as a grey coil (middle panel). The surface of the interaction motif of EK1 depicts two well-defined faces with inversed polarity. Such distribution of residues allows the non-polar face to effectively couple to a non-polar crevice of the 3HR1 while the polar face shields the hydrophobic core, thus favouring the binding of this peptide (right panel). The side chains of charged residues at the polar face of EK1 are presented with sticks and the residues are labelled. The figure of the 3HR1-EK1 complex was generated using the coordinates reported with PDB ID 5ZVM [141].
For insertion into the cellular membrane, the FP must be accessible, which is achieved through cleavage at the S2′ site by the transmembrane serine protease 2 (TMPRSS2). Alternatively, the pH-dependent enzyme cathepsin L has been proposed to fulfil the processing function after endocytic uptake [3]. Notably, endocytic uptake and activating cleavage by cathepsin L may also overcome the requirement for furin-mediated priming of the S protein [48]. All these steps, S1/S2 and S2′ cleavage, attachment to the cellular receptors, conformational changes of S1 and S2, FP insertion, rearrangement, and 6HB formation, are crucial for SARS-CoV-2 infection and might be targeted by peptide inhibitors.
2. Peptides preventing ACE2 binding of the SARS-CoV-2 spike protein
The first step of SARS-CoV-2 entry depends on binding of the viral S protein to its cellular receptor ACE2 [26] (Fig. 1C). Several strategies to prevent the virus from entering the cell are based on interfering with RBD binding to the ACE2 receptor and range from designing peptides derived from the interacting site of the receptors to de-novo synthesis of RBD-binding peptides or alternatively, generation of peptides binding the S protein outside the RBD (Table 1 ) [52,53].
Table 1.
Inhibition of virus attachment.
| Target protein | Targeted domain | Name | Virus tested | Sequence derived from | Sequence | Ref (preprint) |
|---|---|---|---|---|---|---|
| Spike protein | RBD | P4 (ACE2 mimics) | SARS-CoV-1 | ACE2 (22–44) | EEQAKTFLDKFNHEAEDLFYQSS | Han et al., 2006 [55] |
| P5 (ACE2 mimics) | ACE2 (22–57) | EEQAKTFLDKFNHEAEDLFYQSSLASWNYNTNITEE | ||||
| RBD | SBP1 | – | ACE2 (21–43) | IEEQAKTFLDKFNHEAEDLFYQS | Zhang et al., 2020 (preprint) [52] | |
| unknown | P2 | SARS-CoV-1 | S Protein (259–278) (SARS-CoV-1) | PTT(K)FMLKYDENGTITDAVDC | Zheng et al., 2005 [56] | |
| P6 | S Protein (598–617) (SARS-CoV-1) | YQDVNCTDVS(P)TAIHADQLTP | ||||
| P8 | S Protein (737–756) (SARS-CoV-1) | QYGSFCT(A)QLNRALSGIAA(V)EQ | ||||
| RBD | AHB1 | SARS-CoV-2 | ACE2 | DEDLEELERLYRKAEEVAKEAKDASRRGDDERAKEQMERAMRLFDQVFELAQELQEKQTDGNRQKATHLDKAVKEAADELYQRVRELEEQVMHVLDQVSELAHELLHKLTGEELERAAYFNWWATEMMLELIKSDDEREIREIEEEARRILEHLEELARK | Cao et al., 2020 [53] | |
| AHB2 | ELEEQVMHVLDQVSELAHELLHKLTGEELERAAYFNWWATEMMLELIKSDDEREIREIEEEARRILEHLEELARK | |||||
| LCB1 | SARS-CoV-2 | de-novo design | DKEWILQKIYEIMRLLDELGHAEASMRVSDLIYEFMKKGDERLLEEAERLLEEVER | |||
| LCB3 | NDDELHMLMTDLVYEALHFAKDEEIKKRVFQLFELADKAYKNNDRQKLEKVVEELKELLERLLS | |||||
| ACE2 | RBD-binding site | RBD-11b | SARS-CoV-1, CoV-NL63 | S Protein RBD (438–443) | YKYRYL | Struck et al., 2012 [67] |
| RBD-binding site (ACE2-Spike interaction sites) | SP-4 (S protein mimics) | SARS-CoV-1 | S Protein (192–203) | GFLYVYKGYQPI | Ho et al., 2006 [68] | |
| SP-8 (S protein mimics) | S Protein (483–494) | FYTTTGIGYQPY | ||||
| SP-10 (S protein mimics) | S Protein (668–679) | STSQKSIVAYTM | ||||
| RBD binding site | S471-503 | SARS-CoV-1 | S Protein RBD (471–503) | ALNCYWPLNDYGFTTTGIGYQPYRVVVLSFEL | Hu et al., 2005 [69] | |
| DX600 | SARS-CoV-1 | peptide library | Ac-GDYSHCSPLRYYPWWKCTYPDPEGGG-NH2 | Huang et al., 2003 [70]; Fukushi et al., 2005 [71] | ||
| RBD binding site | SARS-BLOCK | SARS-CoV-2 | S Protein RBD | unknown | Watson et al., 2020 (preprint) [78] | |
| α5β1 integrin | ACE2 and RBD binding domains | ATN-161 | SARS-CoV-2 | fibronectin | Ac-PHSCN-NH2 | Beddingfield et al., 2020 [74] |
Binding to the ACE2 receptor is a characteristic shared by SARS-CoV-2, SARS-CoV-1, and HCoV-NL63 [26,54]. Accordingly, strategies to interfere with this interaction have been previously examined for SARS-CoV-1. Alanine scanning revealed that several charged amino acids between residues 22 and 57 of ACE2 are important for its interaction with the SARS-CoV-1 S protein [55]. On that basis, Han and coworkers synthesized six peptides from this region expected to bind the SARS-CoV-1 S1 RBD and thus to block SARS-CoV-1 pseudoparticle binding. Two of these peptides (P4 and P5, Table 1) have half maximal inhibitory concentration (IC50) values in the micromolar range. Another peptide described in this study (P6), synthesized by linking two fragments with glycine, show higher potency with an IC50 value of 0.1 μM [55], confirming that the RBD is a suitable target for virus inhibition.
Peptides targeting domains in the S protein other than the RBD may also interfere with viral entry (Table 1). In one approach, domains involved in the adaptation to humans were identified by analyzing sequence variations in the SARS-CoV-1 S protein compared to related coronaviruses found in potential intermediate hosts, such as civets, cats, racoons and dogs. On this basis, ten peptides spanning these variation hotspots (some including the RBD), were synthesized and analyzed for their anti-SARS-CoV-1 activity [56]. Four of these peptides, which share a helical structure and are located at the monomers' contact surface of S1 and S2, were identified as antiviral (Table 1). P2, derived from the N-terminal region of the S1 subunit close to the RBD, may hinder the conformational changes involved in ACE2 binding. P6 and P8 are two other peptides identified in this study that target a domain of unknown function in the S1 and S2 subunits. They show IC90 values of 113 μg/ml and 25 μg/ml, respectively, and might bind to exposed monomer interfaces to interfere with receptor-binding, proteinase-cleaving or virion-cell membrane-binding-sites [56]. The high similarity between the S proteins of SARS-CoV-1 and SARS-CoV-2 suggests that these peptides may also inhibit SARS-CoV-2, but this remains to be verified.
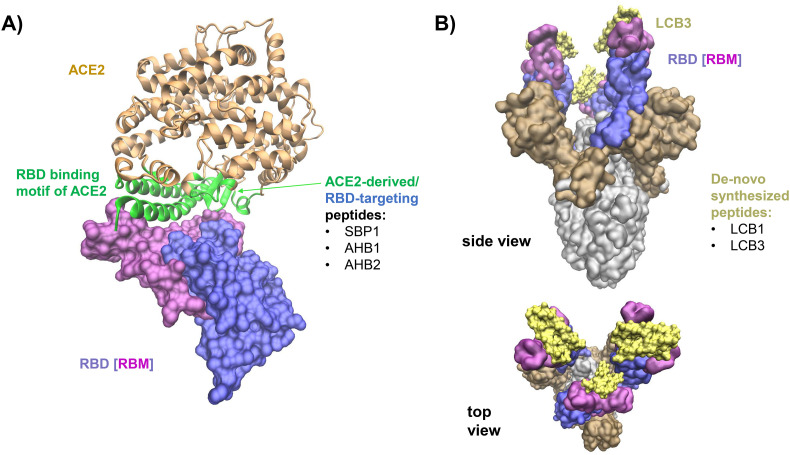
To address the inhibition of SARS-CoV-2, Cao and coworkers designed peptides, also known as miniproteins, using i) ACE2 as scaffold, and ii) de-novo sequencing based on RBD-binding interaction motifs. Following the first approach, they generated two promising agents, AHB1 and AHB2 (Fig. 3A, Table 1), which neutralized SARS-CoV-2 with IC50 values of 35 and 16 nM, respectively [53]. Using the de-novo approach, two candidates, LCB1 and LCB3 (Fig. 3B, Table 1), were identified that bind the RBD with lower dissociation constants and neutralized SARS-CoV-2 in the picomolar range. Interestingly, all miniproteins, except for LCB1, show cross-reactivity with SARS-CoV-1 RBD, although with dissociation constants ~100 times higher compared to SARS-CoV-2. Furthermore, characterization by cryo-electron microscopy of LCB1 and LCB3 in complex with the SARS-CoV-2 S protein trimer reveals the underlying molecular interactions, as shown in Fig. 3B. This technique is a promising step towards identifying highly potent inhibitors of viral RBDs that might also help to combat future pandemics [53].
Fig. 3.
Peptides targeting the SARS-CoV-2 S protein and interfering with ACE2 binding. A) The S protein S1 subunit receptor binding domain (RBD, violet) interacts with ACE2 (gold) via the receptor binding motif (RBM, light purple) (PDB ID: 6M0J [33]). The interface residues of ACE2 interacting with the RBD are highlighted in green. This region was the scaffold for the designed peptides SBP1, AHB1, AHB2, LCB1 and LCB3 targeting the RBD [53]. B) SARS-CoV-2 S protein bound to the high-affinity LCB3 (in yellow) at the RBM (in light purple). The S1 subunit, outside the RBD, is shown as ochre surface and the S2 subunit is indicated in grey.
Zhang and colleagues showed in a preprint that the ACE2 α1-helix is important for the SARS-CoV-2 S1/RBD interaction (indicated in green in Fig. 3A) [52] and designed a 23-mer peptide derived from the α1-helix (SBP1) of the cellular receptor (Fig. 3A, Table 1). Bio-layer interferometry measurements indicated that this peptide specifically associates with the SARS-CoV-2 RBD with low nanomolar affinity. Unfortunately, its antiviral activity has not yet been analyzed [52].
3. Peptides targeting ACE2
ACE2 is a membrane-associated aminopeptidase ubiquitously expressed in the heart, blood vessels, lung, kidney, gut, testis and brain [57]. ACE2 is part of the renin-angiotensinogen system where it antagonizes the function of the angiotensin-converting enzyme (ACE) and supports vasodilation. The coronaviruses SARS-CoV-1, HCoV-NL63 and SARS-CoV-2 utilize ACE2 as entry receptor [58]. Blocking viral entry receptors is a highly promising strategy to prevent virus infection. For example, the small molecule maraviroc that targets the HIV-1 co-receptor CCR5 is approved for clinical use [59], providing proof-of-concept evidence that cellular entry receptors can be targeted for therapy. Another small molecule, AMD3100, interferes with the second major HIV-1 co-receptor CXCR4 [60]. Examples for other receptor-specific peptides are the 16-mer peptide EPI-X4, which binds to CXCR4 and prevents CXCR4-tropic HIV-1 infection [19] or the hepatitis B and D virus inhibitor myrcludex B that binds to the sodium taurocholate co-transporting polypeptide (NTCP) [20,21,61].
Several peptides that target ACE2 and inhibit SARS-CoV-1 infection have been described (Table 1). Studies of SARS-CoV-1 show that peptides derived from the region comprising amino acids 270–837 of its RBD [[62], [63], [64]] can inhibit viral infection. Crystal structure analyses reveal that the 424–494 region is involved in interactions with ACE2 [65]. The R441A mutation abrogated cell entry by SARS-CoV-1 pseudoparticles [66], suggesting that peptides targeting the region where this residue is found might display inhibitory activity. Accordingly, a peptide library based on residues 318–509 was synthesized and a plasmon resonance screening study yielded RBD-11b (438–443) as best ACE2-binding peptide. However, RBD-11b inhibited infection by SARS-CoV-1 only at millimolar concentrations. The peptide was also active against HCoV-NL63, suggesting conserved activity against viruses using ACE2 as receptor [67]. However, RBD-11b has not been tested against SARS-CoV-2.
In another approach, Ho and coworkers designed a set of peptides derived from the S protein of SARS-CoV-1 based on predicted hydrophilicity, surface probability, and chain flexibility. Three peptides, SP-4(192–203), SP-8(483–494), and SP-10(668–679) (Table 1) abrogated the interaction between a recombinant S protein and ACE2 and SP-10 inhibited SARS-CoV-1 pseudoparticle entry in Vero cells in the nanomolar range [68]. Another peptide S471-503 derived from the RBD region (Table 1) was identified upon screening of a 10mer peptide library of SARS-CoV-1 B cell epitopes. S471-503 also bound to ACE2 and reduced plaque formation of SARS-CoV-1 with an IC50 value of 42 μM [69]. An RBD-independent approach was applied by Fukushi and coworkers. DX600, a peptide identified in a screening for ACE2 inhibitors [70] (Table 1), inhibited VSV-SARS-CoV-1 pseudoparticles only modestly and not in a dose-dependent manner. This result suggests that the enzymatic activity of ACE2 is not important for infection, but that DX600 partially blocks ACE2 as SARS-CoV-1 receptor [71].
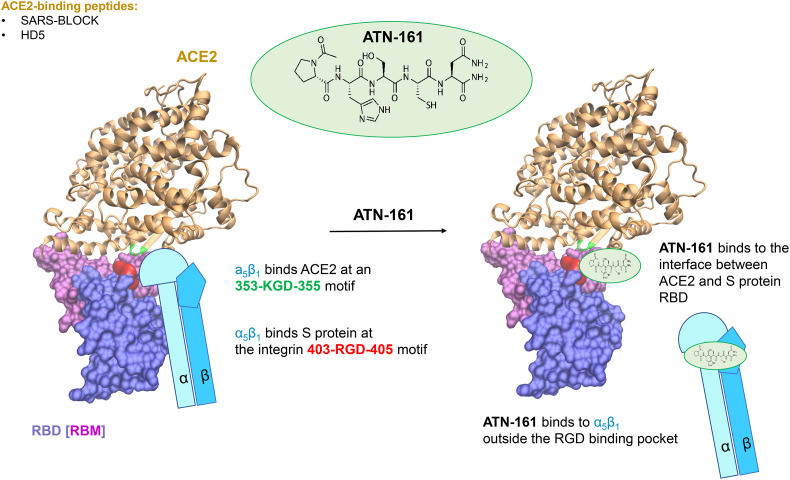
Currently, several groups are working on the development of ACE2-targeting peptides to inhibit SARS-CoV-2 (Fig. 1, Table 1). Wang and coworkers analyzed SARS-CoV-2 infection of intestinal cells and specifically investigated the role of defensins, a well-known family of antimicrobial peptides [18]. Human defensin 5 (HD5) is the most abundant α-defensin in the intestine. Due to its lectin-like activity, HD5 binds lipids and glycosylated proteins [72,73], which makes ACE2 and the SARS-CoV-2 S protein its potential targets (Fig. 4 ). The binding of HD5 to ACE2 was confirmed using biolayer interferometry measurements. Using confocal microscopy, it was shown that pretreatment with HD5 reduced SARS-CoV-2 pseudoparticle infection [18], but antiviral activity against genuine SARS-CoV-2 isolates remains to be demonstrated.
Fig. 4.
Peptidic SARS-CoV-2 inhibitors targeting ACE2. The receptor binding domain (RBD, violet surface) and the RBM (light purple surface) of the SARS-CoV-2 S protein were used to design SARS-BLOCK peptides [78] that target ACE2 (gold). Similarly, human defensin 5 (HD5) is capable to block the interaction between ACE2 and RBD by targeting the binding interface of ACE2 [18]. The SARS-CoV-2 RBD contains an integrin-binding RGD motif (in red) and interacts with integrin α5β1. ACE2 interacts with α5β1 via a KGD binding site (green). This interaction might enhance SARS-CoV-2 binding to ACE2 [74] (left panel). The peptide ATN-161 binds tightly to the S protein and to α5β1, resulting in the disruption of α5β1 binding to ACE2 and to RBD, thus reducing SARS-CoV-2 infection (right panel).
Interestingly, passive interference with ACE2 was achieved by targeting integrin α5β1 [74]. Integrins are receptors of extracellular matrix proteins, like fibronectin. They have several interaction partners and play roles in cell-to-cell adhesion, cell migration, and as entry cofactors for viruses, such as human herpesvirus or HIV-1 [75,76]. Integrins have been shown to bind to a KGD sequence in ACE2 and the RGD motif in the SARS-CoV-2 S protein [60,156]. Therefore, Beddingfield and colleagues analyzed the potential antiviral activity of the fibronectin-derived anticancer peptide ATN-161 [74,77] (Fig. 4, Table 1), which might bind directly to the integrin, as well as to the RGD motif of the S protein, and to the KGD motif in ACE2 [60,156]. They showed that ATN-161 reduces SARS-CoV-2 infection (IC50 ~ 3 μM), suggesting that this integrin may play a role during SARS-CoV-2 entry. In the clinical context, inhibitors of integrins are successfully used for the treatment of various diseases and several studies and clinical trials are underway to find more potent and safe integrin inhibitors. [157,158].
The next report discussed in this section is available as a preprint (Table 1). Watson and coworkers designed peptides, SARS-BLOCK™, that mimic the SARS-CoV-2 RBD domain (Fig. 4) and characterized their binding to ACE2 and to neutralizing antibodies by biolayer interferometry. Peptides 1, 4, 5 and 6 inhibited RBD binding to ACE2 and show activity against SARS-CoV-2 pseudoparticles, with peptide 5 showing up to ∼95% inhibition of infection at the low micromolar range [78]. Yet, the antiviral activity of these peptides against genuine SARS-CoV-2 remains to be demonstrated.
Altogether, these studies show that the ACE2 receptor can be targeted to prevent virus attachment and infection. However, targeting host factors such as ACE2 might induce adverse effects as observed for other receptor-targeting drugs. For example, life-threatening side effects have been reported for maraviroc [59] and AMD3100 was discontinued as HIV-1 treatment due to its severe side effects [79]. The SARS-CoV-2 receptor ACE2 is an important factor in the renin-angiotensin system, where interference might have deleterious consequences. Indeed, the development of the ACE2 inhibitor MLN-4760 was stopped due to toxicity concerns after phase Ib/IIa clinical trials [80,81].
4. Targeting proteolytic S protein activation
Activation of the SARS-CoV-2 S protein requires proteolytic cleavage at two sites. First, the S protein is cleaved and primed at the poly-basic S1/S2 site by the host protease furin, which generates two distinct subunits that remain non-covalently linked (Figs. 2 and 5 ). The second cleavage site is found in the S2 region (S2′) and is processed by the plasma membrane-associated protease TMPRSS2, which releases the FP of S2 [35]. Alternatively, lysosomal cathepsin L can process and activate the S protein independently of furin-mediated priming, after endosomal uptake. However, its role in vivo is not entirely clear [[82], [83], [84]]. Inhibition of these proteases blocks SARS-CoV-2 infection, as described for small molecule inhibitors of TMPRSS2, such as camostat mesylate [26], or, for cathepsin L, e64d [85]. Therefore, identifying and designing peptides that disrupt this process is another strategy to develop therapeutic agents against SARS-CoV-2 (Fig. 1, Table 2). Inhibitory peptides can be designed by mimicking known cleavage motifs.
Fig. 5.
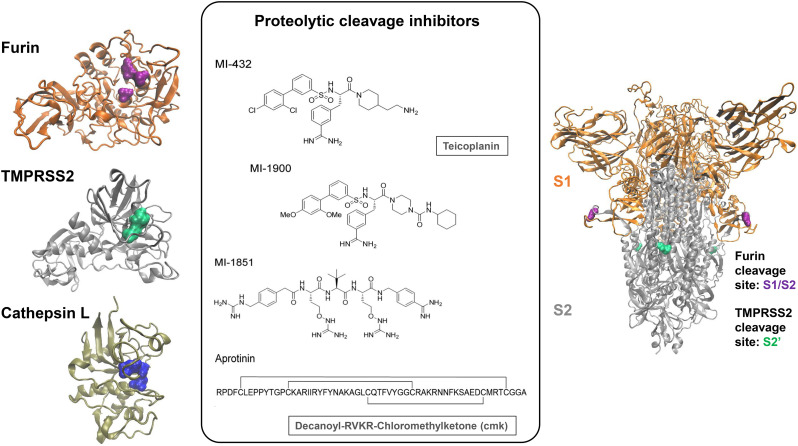
Proteases involved in the activation of the SARS-CoV-2 S protein and inhibitors designed to block these enzymes. The proteases furin, TMPRSS2, and cathepsin L (left panel) cleave the SARS-CoV-2 S protein at different cleavage sites (right panel). The active sites of the proteases are indicated in the surface projection and can be targeted by peptidic or peptidomimetic substrates (middle panel). The peptidomimetics MI-432 and MI-1900 inhibit the protease TMPRSS2 [84]. The inhibitors MI-1852 and dec-RVKR-cmk target furin and related protein convertases, while aprotinin is a broad-range protease inhibitor [84,90]. Teicoplanin targets cathepsin L cysteine proteinase [91]. The structure of TMPRSS2 was obtained from the SwissModel repository with ID code O15393 [92]. This model was built by the SwissModel team based on the structure with PDB ID 1Z8G [93]. The structure of furin corresponds to that reported with PDB ID 4Z2A [94], and the representation of cathepsin L was built based on the structure reported with PDB ID 2YJ9 [95]. The structure of the S protein corresponds to a model with reconstructed loops based on the structure with PDB ID 6ZGE [50,96]. The colour code used to represent the spike protein is: orange, S1 subunit of the S protein; grey, S2 subunit of the S protein; green, S2′ cleavage site; purple, S1/S2 cleavage site. The same colour scheme is used to highlight the active sites of the corresponding enzymes.
Table 2.
Inhibition of S protein processing.
| Target protein | Targeted domain | Name | Virus tested | Sequence derived from | Sequence | Ref (preprint) |
|---|---|---|---|---|---|---|
| TMPRSS2 | Aprotinin | SARS-CoV-2 | Aprotinin | RPDFC LEPPY TGPCK ARIIR YFYNA KAGLC QTFVY GGCRA KRNNF KSAED CMRTC GGA | Bestle et al., 2020 [84] | |
| Catalytic site | MI-432 | SARS-CoV-2 | Protease inhibitor (peptidomimetic) | (S)-3-(3-(4-(2-Aminoethyl)piperidin-1-yl)-2-((2′,4′-dichloro-[1,1′-biphenyl])-3-sulfonamido)-3-oxopropyl)benzimidamide | ||
| MI-1900 | (S)-4-(3-(3-Carbamimidoylphenyl)-2-((2′,4′-dimethoxy-[1,1′-biphenyl])-3-sulfonamido)propanoyl)-N-cyclohexylpiperazine-1-carboxamide | |||||
| Cathepsin L | Enzymatic domain | Teicoplanin | SARS-CoV-2, SARS-CoV-1, MERS-CoV | glycopeptide antibiotic | antibiotic glycopeptide | Zhang et al., 2020 (preprint) [91] |
| Acidification | P9 | SARS-CoV-2 | mouse β-defensin-4 | NGAICWGPCPTAFRQIGNCGHFKVRCCKIR | Zhao et al., 2020 [116] | |
| P9R | SARS-CoV-2 | mouse β-defensin-4 | NGAICWGPCPTAFRQIGNCGRFRVRCCRIR | |||
| 8P9R | SARS-CoV-2, SARS-CoV-1, | mouse β-defensin-4 | 8 x NGAICWGPCPTAFRQIGNCGRFRVRCCRIR | Zhao et al., 2020 [117] | ||
| Furin | Catalytic domain | dec-RVKR-cmk | SARS-CoV-2 | PCSK target motif | dec-RVKR-cmk | Cheng et al., 2020 [90,101,190] |
| Probably catalytic domain | MI-1851 | SARS-CoV-2 | Protease inhibitor (peptidomimetic) | (S)-N-((S)-1-((4-Carbamimidoylbenzyl)amino)-4-(guanidinooxy)-1-oxobutan-2-yl)-2-((S)-2-(2-(4-(guanidinomethyl)phenyl)acetamido)-4-(guanidinooxy)butanamido)-3,3-dimethylbutanamide | Bestle et al., 2020 [84] |
When designing protease inhibitors to prevent viral infection, it has to be considered that viral glycoproteins can frequently be primed and/or activated by several redundant proteases recognizing similar target sequences. For example, hemagglutinin of influenza viruses can be cleaved not only by TMPRSS2, but also by TMPRSS4, human airway trypsin-like protease (HAT) or even bacterial proteases [[86], [87], [88]]. Similarly, TMPRSS4 was recently shown to facilitate SARS-CoV-2 S fusogenic activity thus promoting virus entry into host cells [89] and furin-like proprotein convertases may process its poly-basic S1/S2 site [27]. In addition, other unknown proteases might activate the S protein. Thus, combined targeting of several proteases might be required for efficient inhibition [84].
4.1. Antiviral peptides targeting furin
As mentioned, the SARS-CoV-2 S protein is cleaved by furin at a poly-basic cleavage site into its S1 and S2 subunits. This feature distinguishes SARS-CoV-2 from closely related bat coronaviruses and SARS-CoV-1, which harbor a mono-basic S1/S2 site that cannot be cleaved by furin. Notably, furin plays a major role in the proteolytic activation of a broad range of viruses, including several flavi-, filo-, paramyxo- and retroviruses [27,97]. Furin is a proprotein convertase that is ubiquitously expressed in eukaryotic tissues and physiologically cleaves precursors of multiple proteins, including growth factors, hormones, cell surface receptors, and adhesion molecules during their transport along the secretory pathway. Furin-mediated cleavage not only activates multiple viral glycoproteins, but also a variety of bacterial toxins and cellular oncoproteins. Thus, furin inhibition is already explored in clinical trials for the treatment of cancer [27] and a represents a promising approach to treat infectious diseases.
The poly-basic consensus sequence targeted by furin and related proprotein convertases is R-X-R/K-R↓ [97,98]. Previous studies have shown that the peptidomimetic furin inhibitor decanoyl-RVKR-chloromethylketone (dec-RVKR-cmk) inhibits the activation of different viral glycoproteins [[99], [100], [101]]. Most likely, this ketone-based peptide inhibits furin by a chemical reaction leading to the formation of a hemiketal and thus to an irreversible inhibition of its enzymatic activity [102]. Importantly, dec-RVKR-cmk was shown to block SARS-CoV-2 S processing (Fig. 5) [103] and to inhibit syncytium formation and virus infection with an IC50 of 5 μM (Table 2). Bestle and coworkers analyzed the peptidomimetic furin inhibitor MI-1851 (Fig. 5). Western blotting revealed that MI-1851 prevented SARS-CoV-2 S protein cleavage. Moreover, it reduced SARS-CoV-2 titers released from Calu-3 cells up to 190-fold at a dose of 10 μM [84]. Together, these studies suggest that furin inhibitors might have the potential to be clinically applied not only against cancer but also infectious diseases including SARS-CoV-2.
4.2. Peptides targeting TMPRSS2
TMPRSS2 is a serine protease involved in many physiological and pathological processes and is widely expressed in epithelial cells of the respiratory, gastrointestinal, and urogenital tracts [104]. Moreover, TMPRSS2 is involved in the proteolytic activation of many respiratory viruses, including SARS-CoV-1, SARS-CoV-2 and MERS-CoV [26,97]. Mice deficient in TMPRSS2 showed reduced pathological features after infection with SARS-CoV-1 and MERS-CoV, indicating that inhibition of proteolytic activation may exert beneficial effects [105]. Treatment with the small molecule camostat mesylate confirmed that inhibition of TMPRSS2 suppresses SARS-CoV-2 infection [26].
Bestle and coworkers demonstrated that a knockdown of TMPRSS2 prevents proteolytic activation and spreading of SARS-CoV-2 in human airway epithelial cells (Calu-3) cells [84]. The polypeptide aprotinin (Fig. 5) from bovine lung is a broad-range inhibitor of serine proteases that inhibits TMPRSS2 and thus decreases replication of influenza [106] and Sendai virus [107]. The safety and efficacy of aprotinin was shown in clinical trials, where inhalation reduced symptoms of patients suffering from influenza [106]. When adding aprotinin to SARS-CoV-2 in vitro, it suppressed infectious virus production by 25- to 100-fold at a concentration of 10 μM at 16–48 h post infection. However, after 72 h post-infection the inhibitory effect was abolished [84]. Additionally, the team tested the peptidomimetic inhibitor MI-432 and its analog MI-1900 (Fig. 5, Table 2 ) that have been designed to target serine proteases and shown to inhibit TMPRSS2 and the replication of influenza virus [108]. Peptidomimetics are pseudopeptide sequences that have been tuned to enhance bioavailability, improve transport through the blood-brain barrier, or to exhibit reduced rates of clearance [12]. Both peptidomimetic inhibitors, MI-432 and MI-1900, reduced viral titers more potently than aprotinin [84]. Finally, combinations of TMPRSS2 and furin inhibitors were analyzed. Combining the furin inhibitor MI-1851 (Fig. 5) with either aprotinin, MI-432 or MI-1900 generally increased antiviral efficacy. MI-1851 together with MI-432 is most effective and reduces virus titers to up to 32-fold lower levels compared to each inhibitor alone [84]. Thus, TMPRSS2 and furin inhibitors targeting different steps of S protein processing and activation hold promise for therapeutic development (Table 2).
In a preprint report, Wettstein and coworkers recently showed that α1-antitrypsin, an endogenous serine protease inhibitor, hinders SARS-CoV-2 infection [109]. It was previously shown that α1-antitrypsin inhibits proteolytic activity of TMPRSS2 [110]. Thus, α1-antitrypsin may block SARS-CoV-2 infection by inhibiting TMPRSS2-mediated S protein activation. Notably, plasma purified α1-antitrypsin has been approved as augmentation therapy for α1-antitrypsin deficiency, a hereditary disorder characterized by low plasma concentrations of the serine protease inhibitor. Several clinical studies that aim to evaluate the therapeutic efficacy of α1-antitrypsin in hospitalized COVID-19 patients have been initiated [111].
4.3. Peptides targeting cathepsin L
Cysteine cathepsins are a group of ubiquitously expressed proteases that are localized in endosomes and lysosomes where they activate the SARS-CoV-1 and SARS-CoV-2 S proteins [112] at a pH range of 3.0–6.5 [85,113]. S protein activation by cathepsin L is most likely to occur in the absence of TMPRSS2 and/or furin after endocytosis. However, the sites of cleavage and whether cathepsin L fulfils an important function in vivo remain open questions. In fact, it has been suggested that activation by cathepsins is a mechanism acquired through cell culture and dispensable for SARS-CoV-2 infection in vivo [[82], [83], [84]]. Consistent with this, inhibition of cathepsin L by the small molecule e64d reduces SARS-CoV-2 pseudoparticle infection in TMPRSS2 negative HEK293T and Vero cells [26,84]. However, upon TMPRSS2 expression, the effect of e64d is abolished, but the combination of e64d with camostat mesylate shows synergistic effects [26]. Many FDA-approved cathepsin L inhibitors exist, supporting their clinical safety and highlighting the potential of combination therapies [114].
One agent that interferes with the activity of cathepsin L is peptide P9 (Table 2), which is derived from mouse β-defensin-4 and exerts broad antiviral activity against SARS-CoV-1, MERS-CoV, and influenza virus. P9 directly binds to SARS-CoV-2 particles and inhibits endosome acidification (Fig. 1) thereby indirectly interfering with the activity of cathepsin L [115]. P9 was optimized by increasing the number of proton-accepting amino acids to generate P9R [116] (Table 2). This peptide shows antiviral activity against the coronaviruses MERS-CoV, SARS-CoV-1, and SARS-CoV-2 with IC50 values in the low μg/ml range. P9R was also active against the pH-dependent influenza viruses A(H1N1)pdm09 and A(H7N9), as well as a non-enveloped rhinovirus [116]. Of note, the anti-SARS-CoV-2 effects of P9R were found on TMPRSS2 negative Vero E6 cells, while its activity in TMPRSS2 positive cells is unknown. Altogether, these results show that targeting acidification broadly prevents infection by viruses that depend on endosomal acidification. This effect may be related to the viral glycoprotein that depends on acidification for rearrangement (in case of influenza) or the endosomal cathepsin L that activates the viral glycoprotein (in case of SARS-CoV-1 and SARS-CoV-2). Although targeting acidification might have several side effects in a physiological setting, A(H1N1)pdm09 infected mice showed better survival rates upon intranasal treatment with P9R [116]. A follow-up study, currently available as preprint, suggests that virus-binding P9R-derived oligomers may cross-link viral particles [117]. The authors describe that an eight-branched derivative, 8P9R, is more potent than P9R in blocking SARS-CoV-2 entry. 8P9R also inhibits infection of TMPRSS2 positive Calu-3 cells by aggregating viral particles, evidencing its dual-antiviral mechanism. The antiviral effect of 8P9R was confirmed in vivo in mice and hamsters where its application decreased viral loads. In contrast, the TMPRSS2 inhibitor camostat mesylate was inactive, further illustrating that blocking only one entry pathway might be insufficient [117].
The glycopeptide antibiotic teicoplanin (Table 2), commonly used to treat gram-positive bacterial infections [118], has also been shown to inhibit cathepsin L (Fig. 5) and to reduce infection by SARS-CoV-1 and MERS-CoV [119]. A study currently available as preprint shows that the SARS-CoV-1 and SARS-CoV-2 cathepsin L cleavage sites are conserved and that teicoplanin also prevents infection of SARS-CoV-2 pseudoparticles with an IC50 of 1.66 μM [91]. Additionally, teicoplanin seems to inhibit the viral 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2 that is important for viral spread [120]. In clinics, teicoplanin has been applied to treat Staphylococcus aureus superinfections that may occur as major complications in COVID-19 patients [121] and simultaneously might act as antiviral agent. However, the effect of teicoplanin on SARS-CoV-2 remains to be shown [122].
The fact that the inhibition of cathepsin L does not interfere with infection of TMPRSS2-expressing lung derived Calu-3 cells [84], suggests that it is not a suitable target for monotherapy. However, combinations of furin, TMPRSS2 and cathepsin L inhibitors showed higher activities than the individual agents. Thus, a combination therapy targeting viral fusion at the cell surface as well as in endosomes might be useful against SARS-CoV-2 [26,84]. As with other approaches, unwanted side effects arising from broad interference with the activity of physiologically relevant proteases need to be considered [123].
5. Peptides targeting the fusion mechanism
Another promising antiviral target is the fusion mechanism of viral class I fusion proteins. Research on HIV-1 showed that mutations in the HR regions often result in a fusion-deficient phenotype [124,125], which revealed HR rearrangement as a potential target for drug development. In the 1990s, peptides derived from HR1 and HR2 of the HIV-1 glycoprotein 41 (gp41) were designed. These peptides inhibit HIV-1 entry [[126], [127], [128]], revealing crucial information on the viral fusion mechanism [129]. The peptides designed to mimic one of the HR regions were found to interact with the complementary HR region and to inhibit conformational rearrangements, 6HB formation, and thus membrane fusion (Fig. 6A, B). These so called C peptides were further developed into HIV-1 entry inhibitors [130,131]. The best known C peptide is T20 [11], which found applications in clinics, thus highlighting the potential of peptidic fusion inhibitors [10]. Peptides derived from the HR regions of other class I fusion protein viruses [36,38,[40], [41], [42]], such as parainfluenza [43], Ebola [39], Newcastle disease [45], and respiratory syncytial virus [44], inhibit viral entry with nanomolar to micromolar activities.
The strategy of designing and synthesizing peptides that overlap with HR regions was also applied to members of the Coronaviridae family e.g. HCoV-229E [132], SARS-CoV-1 [133,134], and MERS-CoV [[135], [136], [137]] (Table 3 ). Liu and coworkers identified a HR2-derived peptide from SARS-CoV-1, namely CP-1, that binds to HR1 and blocks 6HB formation and fusion of SARS-CoV-1 [134]. Other SARS-CoV-1 HR1-targeting peptides have been developed in the 2000s by several research groups, and show activities in the nanomolar to low micromolar range [133,134,[138], [139], [140]]. In comparison, peptides derived from HR1 (and thus targeting HR2) are often poorly active [133,134,139], possibly due to their propensity to self-aggregation [129].
Table 3.
Inhibition of membrane fusion.
| Target protein | Targeted domain | Name | Virus tested | Sequence derived from | Sequence | Ref (preprint) |
|---|---|---|---|---|---|---|
| Spike S2 | HR1 | 229E-HR2P | 229E-CoV | HR2 (1053–1102) (229E) | VVEQYNQTILNLTSEISTLENKSAELNYTVQKLQTLIDNINSTLVDLKWL | Xia et al., 2018 [132] |
| HR2 | MERS-5HB | MERS-CoV | HR1 + HR2 (3× HR1 residues 984 to 1062 and 2× of HR2 residues 1245 to 1289) (MERS) | HR1–SGGRGG–HR2–GGSGGSGG–HR1–SGGRGG–HR2–GGSGGSGG–HR1 | Sun et al., 2017 [135] | |
| HR1 | P1 | MERS-CoV | HR2 (I1246 to L1286) (MERS) | LTQINTTLLDLTYEMLSLQQVVKALNESYIDLKEL | Gao et al., 2013 [136] | |
| HR1 | HR2P | MERS-CoV | HR2 (1251–1286) (MERS) | SLTQINTTLLDLTYEMLSLQQVVKALNESYIDLKEL | Lu et al., 2014 [137] | |
| HR1 | CP-1 | SARS-CoV-1 | HR2 (1153–1189) (SARS-CoV-1) | GINASVVNIQKEIDRLNEVAKNLNESLIDLQELGKYE | Liu et al., 2004 [134] | |
| HR1 | HR2-8 (HR peptide) | SARS-CoV-1 | HR2 (1126–1193) (SARS-CoV-1) | ELDSFKEELDKYFKNHTSPDVDLGDISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK | Bosch et al., 2004 [133] | |
| HR2 | HR1-1 | SARS-CoV-1 | HR1 (889–926) (SARS-CoV-1) | NGIGVTQNVLYENQKQIANQFNKAISQIQESLTTTSTA | Yuan et al., 2004 [138] | |
| HR1 | HR2-18 | HR2 (1161–1187) (SARS-CoV-1) | IQKEIDRLNEVAKNLNESLIDLQELGK | |||
| HR1 | HR2 peptide | SARS-CoV-1 | HR2 (1149–1186) (SARS-CoV-1) | GDISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELG | Zhu et al., 2004 [139] | |
| HR2 | HR1-a | SARS-CoV-1 | HR1 (899–926) (SARS-CoV-1) | YENQKQI ANQFNKA ISQIQES LTTTSTA | Chu et al., 2008 [140] | |
| HR1 | GST-removed-HR2 | HR2 (1145–1192) (SARS-CoV-1) | DVDLGD ISGINAS VVNIQKE IDRLNEV AKNLNES LIDLQEL GKYEQYI | |||
| HR1 | HR2 | HR2 (1151–1185) (SARS-CoV-1) | ISGINAS VVNIQKE IDRLNEV AKNLNES LIDLQEL | |||
| HR1 | EK1 | SARS-CoV-2, SARS-CoV-1, 229E, NL63, OC43 | HR2 (OC43) | SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKEL | Xia et al., 2019 [47,141] | |
| HR1 | 2019-nCoV-HR2P | SARS-CoV-2 | HR2 (1150–1185) (SARS-CoV-2) | DISGINASVVNIQKEIDRLNEVAKNLNESLIDLQEL | Xia et al., 2020 [46] | |
| HR1 | [SARSHRC-PEG4]2-chol | SARS-CoV-2 | HR2 (1168–1203) (SARS-CoV-2) | [DISGINASWNIQKEIDRLNEVAKNLNESLIDLQEL -PEG4]2-chol | de Vries1 et al., (preprint) [146] | |
| HR1 | EK1-C4 | SARS-CoV-2, SARS-CoV-1, 229E, NL63, OC43 | HR2 (OC43) | Ac-SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKELGSGSG-amino-PEG3-acetyl-Cys(cholesteryloxycarbonylmethyl)-NH acetate salt | Xia et al., 2020 [47] | |
| HR1 | IPB01-IPB-09 | SARS-CoV-2, SARS-CoV-1, 229E, NL63, OC43 | HR2 (1151–1186) (SARS-CoV-2) | IBP02: ISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELK(Chol) | Zhu et al., 2020 [145] | |
| HR2 | P9 | SARS-CoV-1 | HR1 (890–909) (SARS-CoV-1) | GIGVT(A)QNVLYENQKQIANQF | Zheng et al., 2005 [56] | |
| HR1 | P10 | SARS-CoV-1 | HR1 (1161–1180) (SARS-CoV-1) | IQK(E)EIDRLNEVAKNLNESLI | ||
| HR1 + Hrloops | SARSWW-III | SARS-CoV-1 | SARS-CoV-1 hydrophobic S2 | GYHLMSFPQAAPHGVVFLHVTW | Sainz et al., 2006 [148] | |
| SARSWW-IV | GVFVFNGTSWFITQRNFFS | |||||
| SARSWW-V | AACEVAKNLNESLIDLQELGKYE-QYIKW | |||||
| FP region | SARSWW-I | MWKTPTLKYFGGFNFSQIL | ||||
| SARSWW-II | ATAGWTFGAGAALQIPFAMQMAY |
Usually, HR-derived peptides are specific for their corresponding viruses. In 2019, however, a HR peptide was identified that shows pan-corona virus inhibiting activity. While HR2P peptides from MERS-CoV and SARS-CoV-1 lack broad-spectrum antiviral activity against heterologous human coronaviruses, the peptide OC43-HR2P exhibits broad-spectrum activity against both human α-and β-coronaviruses [141] (Table 3). By specific changes in the amino acid sequence, the antiviral activity and solubility of this peptide was further optimized resulting in the EK1 derivative (Fig. 6B, C). EK1 targets HR1 domains of several human coronaviruses, including SARS-CoV-1 and MERS-CoV [47,141]. Importantly, EK1 displays potent prophylactic and therapeutic in vivo activity against MERS-CoV and HCoV-OC43 infections by intranasal application in mice [141]. Furthermore, EK1 proved to be safe and shows only low immunogenicity in vivo [141]. Thus, EK1 is the first broadly active HR-targeting inhibitor with potential applications against various coronaviruses.
EK1 was also active against SARS-CoV-2 with an IC50 value of 2.5 μM in TMPRSS2 negative Vero E6 cells [47]. Interestingly, the IC50 value was about 10-fold lower in TMPRSS2 positive Caco-2 cells, suggesting that fusion at the cell surface is more potently inhibited by this peptide [142]. It is known that the conjugation of lipids to fusion inhibitory peptides can increase their activity and in vivo stability. This is likely due to an increased affinity of the peptide for membranes [143], as shown for the peptide LP-19 against HIV-1 [144]. Accordingly, cholesterol was covalently attached to the C-terminus of EK1 linked by a PEG spacer to generate EK1C4 [47] (Table 3). The resulting EK1C4 shows increased solubility and enhanced interactions with the lipid membrane, which results in a prolonged half-life and increased endocytic uptake. Thus, EK1C4 may show increased fusion inhibition not only at the cell surface but also after viral endocytosis. EK1C4 is 19 to 190-fold more potent than EK1 against several coronaviruses and inhibits infection of TMRPSS2 negative Vero E6 cells by SARS-CoV-2 with an IC50 of 37 nM. EK1 and EK1C4 were both tested against HCoV-OC43 in vivo in mice and show prophylactic as well as therapeutic efficacy when applied intranasally, with better stability, antiviral activity and half-life in case of EK1C4 [47].
The S2 subunits of SARS-CoV-1 and SARS-CoV-2 are highly conserved with 92.6% overall identity of the HR1 domain and 100% for the HR2 region [46]. Thus, peptides derived from SARS-CoV-1 HR2 are also expected to inhibit SARS-CoV-2. Two studies show that the HR2 peptides 2019-nCoV-HR2P and IPB-01 (varying in one amino acid, Table 3) inhibit SARS-CoV-2 pseudoparticle infection with IC50 values of 0.98 μM and 22 μM, respectively [46,145] (Fig. 6B). In agreement with previous studies [133,134,139], HR1-derived peptides show no inhibitory activity [46]. The peptide IPB-01 was modified by adding a cholesterol group to its C-terminus (IPB-02), which reduces IC50 values against SARS-CoV-2 and SARS-CoV-1 pseudoparticles to 0.08 μM and 0.25 μM respectively [145]. Additionally, a structure-activity relationship study with truncations of IPB-02 allowed to identify sequences essential for the inhibitory effect [145]. In line with these results, Zheng et al . [56] reported the design of ten peptides based on sequence variations of the S protein (Table 3). Of those, peptide P10 resembles the HR2 region and thus exhibits antiviral activity by targeting HR1. The other active peptides P2, P6, and P8 have been discussed in chapter 2.
A study available as preprint reported the optimization of a peptide, varying only in a single amino acid from 2019-nCoV-HRP2 [46], by PEGylation, lipidation and dimerization [146]. The authors confirmed the observation that the fusion inhibiting peptide [SARSHRC-PEG4]2-chol is highly active on TMPRSS2 positive cells with IC50 values as low as 3.8 nM (Table 3). Most notably, intranasal application in ferrets protected them from viral transmission when co-housed with SARS-CoV-2 infected ferrets [146].
Altogether, these studies show that targeting the HR1 domain by peptides derived from the HR2 region is a promising strategy for generating fusion inhibitors against many class I fusion protein viruses, including SARS-CoV-2 (Fig. 1). The high HR homology in coronaviruses allowed to generate the broadly active and potent fusion inhibitor EK1C4 for potential treatment of infection by different coronaviruses including SARS-CoV-2 (Table 3).
To find other targets relevant to the fusion process, the Wimley and White interfacial hydrophobicity scale [147] was used for the identification of membrane-interacting regions within S2 [148]. Membrane interactions, especially those involving the FP, could be important for viral fusion and thus represent potential antiviral targets [148]. Five peptides analogous to the identified regions were tested for inhibition of SARS-CoV-1 entry (Table 3). All peptides were active, but the most potent inhibition of SARS-CoV-1 plaque formation was found for WW-III and WW-IV with IC50 values between 2 and 4 μM. Since both peptides are derived from the loop domain between HR1 and HR2, the authors speculate that they might sterically hinder extension of the FP or transition to the 6HB conformation [148]. Similarly, the WW-V peptide, which corresponds to the aromatic region overlapping with the C-terminal HR2 domain, might interfere with rearrangement. In comparison, the WW-I and WW-II peptides correspond to the N-terminus of the S2 region containing the FP, suggesting that they might interfere with FP insertion in the cell membrane. Compounds targeting the FP of class I fusion proteins have previously shown to exert antiviral activity. For example, the α1-antitrypsin-derived fragment VIRIP targets the FP of HIV-l and prevents infection [11,150]. An optimized derivative with increased plasma stability and anti-HIV-1 activity reduced viral loads in the treatment of naïve patients demonstrating that viral FPs are druggable viral structures [11,149,150]. Currently, this strategy has not been explored for coronaviruses. Peptides binding the FP of the SARS-CoV-2 S protein might represent an alternative to HR- and RBD- targeting peptides.
6. Computational strategies for the design of new peptide inhibitors against SARS-CoV-2
As it is the case of many disorders, infectious or not, in-silico strategies for the design and screening of active compounds, as well as for studying the dynamics of the involved proteins are key to global efforts aiming to identify novel therapeutics and vaccines [[151], [152], [153]], also in the context of the SARS-CoV-2 pandemic [53,[154], [155], [156], [157], [158], [159], [160], [161], [162]]. In this section, we provide an overview of computational approaches used for delivering timely predictions of peptide and peptide-based inhibitors targeting the virus' entry mechanism.
As discussed, the entry process of SARS-CoV-2 in human cells is mediated by interactions between S proteins at the surface of the viral membrane and the ACE2 receptor in human cells. Consequently, most computational protocols aimed at preventing the protein – protein interaction between the ACE2 receptor and the RBD domain of the SARS-CoV-2 S proteins [53,[155], [156], [157], [158], [159], [160],[163], [164], [165], [166]]. The structure of this complex was elucidated early in this year, 2020, and the first coordinates were reported in the Protein Data Bank under the ID codes: 6VW1 [167], 6M17 [168], 6lZG [169], and 6M0J [33]. Specifically, peptide design strategies mainly focused on extracting candidates based on the ligand binding motif of the ACE2 receptor, and optimizing the sequences of these peptides to obtain potent inhibitors targeting the RBD [53,[155], [156], [157], [158], [159], [160],163,164,166]. Similarly, efforts have been devoted to the in-silico design of peptide inhibitors targeting the ACE2 receptor [165] and the HR1 domain of the S protein [154]. The computational workflow used in these designs usually comprised well-established techniques such as homology modeling, computational mutagenesis, docking protocols, re-scoring methods and molecular dynamics simulations (Table 4 ). The applications of these techniques allowed the exploration of peptide libraries [158,[163], [164], [165]] and to build models of new peptides [53,[154], [155], [156], [157], [158], [159],166], as well as the structural and dynamical characterization of the interactions of ACE2 and the peptides with the interface of the viral S protein [156,160,164,166]. In addition, computational protocols have been used to predict the antiviral activity and the toxicity of peptides with potential inhibitory effect [154,158] (Table 4).
Table 4.
Summary of the computational tools primarily used to design peptides targeting the spike protein and to study the interactions between RBD and the ACE2 receptor.
| Purpose | Tools |
|---|---|
| To build structural models of new peptide sequences, based on the scaffold of the known Spike-RBD structure, as well as for adding missing fragments in the elucidated structures [29,53,154,155,157] | SwissModel [51], MODELLER [191] and I-TASSER [192], and Rosetta [193] with RIF docking [194] |
| To reconstruct missing residues and prepare structures for the simulations. Optimizations of rotamers as well as minimization procedures and alanine scanning analyses [157,159,160] | Schrödinger (Prime) [195], UCSF Chimera software [196], FoldX [197] and the proprietary software BIOVIA Discovery Studio [198], CHARMM-GUI [199] |
| For peptide – protein docking [53,154,155,157,163,164,166] | HADDOCK [200], HPEPDOCK [201], ClusPro [202], Rosetta [193], and ZDOCK [203] |
| Evaluation of the antiviral potential of new peptides and toxicity likelihoods for such peptides [154,158] | AVPpred [204] and AVP-IC50pred [205] as well as as well as ToxinPred[206] |
| Re-scoring of the peptide candidates´ affinity to the Spike protein [157] | PRODIGY [207] |
| Peptide – protein interaction energies and free energy changes [154,156] | NAMD [208] MMGBSA [209] with the VSGB 2.0 solvation model, as implemented in the Schrödinger Prime suite of programs [195] |
| To sample the conformational variability of the S protein and the peptide candidates derived from truncated structures of the Spike – ACE2 interface [29,156,160,166] | NAMD [208], DESMOND [171] and GROMACS [210] |
| Analyses and visualization of the molecular dynamics simulations [29,160] | VMD [211] and and MDtraj [212] |
A key step for the design of inhibitors is to gain knowledge of the structural and dynamical properties of the target and its protein partners. In this respect, a molecular dynamics study of the ACE2 receptor bound to the RBDs of both, SARS-CoV-1 and SARS-CoV-2, was reported [160], revealing specific interactions stabilizing the binding mode of the viruses. Recently, a full-length model of the S protein, including multiple glycosylated sites was presented [29]. Based on this model, obtained from extensive and rigorous simulations, two key glycosylation sites (N165 and N234) that modulate the ratio between the open and closed states of the S protein were characterized [29].
D.E. Shaw Research [162] and the research group of Sergio Pantano at the Pasteur Institute of Montevideo [161] computed and distributed, freely, molecular dynamics simulations in the microseconds timescale of the SARS-CoV-2 proteome, thus providing valuable structural and dynamical insights. These simulations were performed at both, the full-atom resolution (D. E. Shaw Research, DESMOND [171]), and with coarse-grained models (Pantano, SIRAH force field [172], AMBER20[213]). In addition, ongoing efforts delivered the first coarse-grained model of the SARS-CoV-2 virion structure [49]. This model represents a step forward for the study of conformational events and interactions between SARS-CoV-2 proteins, using molecular dynamics simulations.
Although at relatively early stages, these studies evidence the potential of computational tools for the development of new inhibitory peptides against SARS-CoV-2 and future pandemics. The application of these computational tools allowed the rapid screening of peptide candidates and provided structural and dynamical information of interaction patterns key for the rational design of compounds with therapeutic potential.
7. Towards clinical applications of peptide-based inhibitors
The high potency and selectivity as well as the pharmacological profile and ease of synthesis of peptides enable their translation into clinical applications [173]. Frequently, peptides are highly specific while being tolerable and safe. Importantly, their interactions and degradation pathways are often predictable. Thus, absorption, distribution, metabolism and excretion (ADME) properties can be anticipated and improved [173,174]. In this respect, administration and targeted tissues play critical roles in ADME and determine therapeutic efficacy. Peptides are rarely orally available and to achieve systemic distribution, they are often delivered intravenously or subcutaneously [174]. Systemically available peptides face plasma protein binding, proteolytic degradation, metabolization in the liver or free filtration by the kidneys, which may drastically reduce plasma half-life and concentrations at the targeted tissue [174]. Thus, a limitation for the clinical application of peptide inhibitors is their lack of systemic bioavailability [9].
However, the sites of SARS-CoV-2 infection and replication, i.e. the upper and lower airways, are highly accessible [175], which renders the need for oral availability or systemic application obsolete. Respiratory diseases are commonly targeted by nasal or oral sprays or inhalation of small molecules allowing direct delivery to the target organ. Several peptide-based inhalants have already made it into the market. For example, lucinactant is applied as inhalable surfactant replacement to treat the respiratory distress syndrome (RDS) [174,[176], [177], [178]]. Other pulmonary peptide therapeutics such as aviptadil against RDS and pulmonary hypertension, as well as inhalable peptides targeting cystic fibrosis, asthma, and infectious diseases, are currently in clinical trials [176]. Additionally, inhalation can be used to systemically deliver drugs as the lungs have a large surface area, are highly vascularized and the epithelial barrier function is low, which allowed e.g. the development of inhalable insulin [177].
Inefficient passing of the respiratory epithelium after inhaled delivery may even be beneficial because it results in a high concentration directly in the lung instead of systemic distribution thereby increasing local potency and decreasing adverse effects. [179]. Application of peptides in the lung usually leads to long-lasting local activity because proteolytic activity is low [180,181] and the number of antiproteases high [178]. Challenges of pulmonary delivery include the homogenous deposition of peptides throughout the alveoli whilst passing the barriers of the mucous layer and the elimination via mucociliary clearance [182]. Strategies to improve peptide formulation and aerosolization such as optimizing aerodynamic diameters, mucoadhesion, or peptide release are currently investigated [174]. Thus, the oral, nasal and pulmonary mode of delivery is easy and feasible, absorption and distribution occur directly at the targeted site, the metabolization is reduced, and the elimination occurs mainly via mucus flow which reduces potential side effects. Therefore, locally applied extracellular peptides that do not need to enter the blood stream to block SARS-CoV-2 infection might be strong candidates for clinical development.
Of the anti-SARS-CoV-2 peptides reported to date, the HR-analogue EK1C4 is the most promising candidate for clinical development. It directly targets the virus, does not interfere with cellular functions, has already been optimized for potency and stability and was tolerated and effective in vivo in a mouse model for SARS CoV-2 infection [47]. Furthermore, the pan-coronavirus activity of EK1C4 suggests that it might also be effective in future coronavirus outbreaks. Similarly, the optimized peptide based on 2019-nCoV-HRP2 [46] shows lower nanomolar IC50 values in vitro and prevented SARS-CoV-2 transmission in vivo [146]. Alternatively, the miniproteins LCB1 and LCB3 that also target the S protein are interesting for further development, as they show activities in the picomolar range [53]. However, with 56–160 amino acids, these miniproteins are still very large, which might result in high production costs and low output.
To advance translation of peptide inhibitors into clinical applications these peptides can be optimized in terms of bioavailability, potency, stability, safety and size to allow large-scale manufacturing. Successful translation of a peptide inhibitor with nanomolar IC50 values in vitro into the clinics has been shown for the HIV-1 HR analogue T20 (enfurvitide) [183]. However, T20 suffered from low potency, short half-life, induction of local immune responses and quick development of resistance in vivo [10,184]. Notably, next-generation T20-related peptides have a higher barrier for resistance development and show substantially higher potency and stability [185]. In addition, most of the mentioned drawbacks are specific for HIV-1, which causes life-long and systemic infections and is rapidly mutating. Here, T20 treatment requires constant plasma levels and hence repeated injections over long time periods. In contrast, prevention and treatment of an acute and locally confined infection such as SARS-CoV-2 might benefit from the temporal and topical application of a highly potent peptide solution. The short application time and broad activity of EK1C4 together with the relatively low mutation rates of SARS-CoV-2 might prevent viral escape. The prophylactic potential of HR2-peptide-based nasal sprays has already been shown [146] and more studies in animal models to assess this approach seem highly warranted. The process of developing peptide drugs is usually shorter than for small molecules but might still take years [173,186]. However, even anticipating that vaccination efforts will be successful, the development of therapeutics is essential for the treatment of infected individuals. Here, novel peptide-based agents may be a promising alternative to drug repurposing and small molecules. The early treatment of upper airway infections can be highly beneficial as it potentially prevents immunopathological processes and viral dissemination into the lower airways and other tissues and organs [187,188]. Similarly, reducing viral spread in patients already suffering from COVID-19 might improve recovery. Thus, the short-term application of peptides could be useful as prophylactic or therapeutic intervention and help to gain control over the pandemic.
8. Conclusions
The prevention and treatment of the pandemic SARS-CoV-2 requires the rapid development of effective vaccines and antivirals to limit viral spread and associated fatalities. Fast design and synthesis, high target specificity and reduced side effects make peptides a great scaffold for new drugs [9,10,189]. Previous data on related viruses and the rapidly increasing knowledge of the SARS-CoV-2 entry process allowed the computational modeling, identification, characterization and optimization of antiviral peptides interfering with the proteolytic activation of the S protein, the binding to ACE2, or the membrane fusion process, in record time. Targeting viral entry does not require cell-penetrating peptides and minimizes detrimental effects of infection because it blocks the pathogen at the earliest step of its replication cycle.
Notably, the S protein-targeting peptides EK1C4, [SARSHRC-PEG4]2-chol, LCB1, and LCB3 show promising antiviral potency against SARS-CoV-2 without affecting the function of the host protein. Thus, oral, intranasal or inhalative applications might unfold the activity of these peptides directly at the site of replication without the need for optimization of properties such as tissue-penetration, plasma stability, or half-life. Degradation by proteases in the respiratory tract might even be beneficial as it will reduce immunologic responses and adverse effects. Animal studies are needed to assess the potential of this approach as an alternative to repurposing strategies and small molecule drugs against SARS-CoV-2.
Declaration of Competing Interest
We declare no conflicts of interest.
Acknowledgements
We thank Elisabeth Braun and Daniel Sauter for critically reviewing the manuscript. This work was supported by the German Research Foundation (DFG) under the collaborative research center CRC 1279 “Exploiting the Human Peptidome for Novel Antimicrobial and Anticancer Agents” (JM, ES-G and FK). FK received funding from the BMBF (“Restrict SARS-CoV-2”), JM received funding through the EU 2020 Horizon project “Fight-nCoV”. ES-G. was also supported by the DFG under the collaborative research center 1093 (CRC 1093 “Supramolecular Chemistry and Proteins”) and Germany's Excellence Strategy – EXC 2033–390677874 – RESOLV. JAM was funded by an individual research grant of the DFG. FK, JM, and JAM are funded by grants from the Ministry of Science, Research and the Arts of the State of Baden-Württemberg. JAM is indebted to the Baden-Württemberg Stiftung for the financial support of this research project by the Elite-Program for Postdocs. DS is part of the International Graduate School in Molecular Medicine, Ulm University.
References
- 1.Kuritzkes D.R. Drug resistance in HIV-1. Curr. Opin. Virol. 2011;1:582–589. doi: 10.1016/j.coviro.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourya D.T., Yadav P.D., Ullas P.T., Bhardwaj S.D., Sahay R.R., Chadha M.S., Shete A.M., Jadhav S., Gupta N., Gangakhedkar R.R., Khasnobis P., Singh S.K. Emerging/re-emerging viral diseases & new viruses on the Indian horizon. Indian J. Med. Res. 2019;149:447–467. doi: 10.4103/ijmr.IJMR_1239_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock G.A., Kim H., Xue L. Why are vaccines against many human viral diseases still unavailable; an historic perspective? J. Med. Virol. 2020;92:129–138. doi: 10.1002/jmv.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA, FDA Combating COVID-19 With Therapeutics, (n.d.). https://www.fda.gov/media/136832/download (accessed November 5, 2020).
- 7.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favipiravir in Hospitalized COVID-19 Patients (FIC), (n.d.). https://clinicaltrials.gov/ct2/show/NCT04359615 (accessed November 5, 2020).
- 9.Bruno B.J., Miller G.D., Lim C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013;4:1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer F., Schmidt K., Zahn R.C., Richter C., Radeke H.H., Schmitz J.E., von Laer D., Egerer L. A rationally engineered anti-HIV peptide fusion inhibitor with greatly reduced immunogenicity. Antimicrob. Agents Chemother. 2013;57:679–688. doi: 10.1128/AAC.01152-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggink D., Bontjer I., de Taeye S.W., Langedijk J.P.M., Berkhout B., Sanders R.W. HIV-1 anchor inhibitors and membrane fusion inhibitors target distinct but overlapping steps in virus entry. J. Biol. Chem. 2019;294:5736–5746. doi: 10.1074/jbc.RA119.007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vagner J., Qu H., Hruby V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008;12:292–296. doi: 10.1016/j.cbpa.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arranz-Gibert P., Ciudad S., Seco J., García J., Giralt E., Teixidó M. Immunosilencing peptides by stereochemical inversion and sequence reversal: retro-D-peptides. Sci. Rep. 2018;8:6446. doi: 10.1038/s41598-018-24517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo C., Cong P., He Z., Mo D., Zhang W., Chen Y., Liu X. Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2015;20:573–582. doi: 10.3851/IMP2918. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi S., Wang G., White M., Qi L., Taubenberger J., Hartshorn K.L. Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic Influenza A viruses. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilas Boas L.C.P., Campos M.L., Berlanda R.L.A., de Carvalho Neves N., Franco O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019;76:3525–3542. doi: 10.1007/s00018-019-03138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson S.S., Wiens M.E., Smith J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013;425:4965–4980. doi: 10.1016/j.jmb.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C., Wang S., Li D., Wei D.-Q., Zhao J., Wang J. Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology. 2020;159:1145–1147. doi: 10.1053/j.gastro.2020.05.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buske C., Kirchhoff F., Münch J. EPI-X4, a novel endogenous antagonist of CXCR4. Oncotarget. 2015;6:35137–35138. doi: 10.18632/oncotarget.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze A., Schieck A., Ni Y., Mier W., Urban S. Fine mapping of pre-S sequence requirements for Hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 2010;84:1989–2000. doi: 10.1128/JVI.01902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank A., Markert C., Hohmann N., Carls A., Mikus G., Lehr T., Alexandrov A., Haag M., Schwab M., Urban S., Haefeli W.E. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J. Hepatol. 2016;65:483–489. doi: 10.1016/j.jhep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., C.S.G. of the I.C. on T. of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front. Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. https://www.frontiersin.org/article/10.3389/fmicb.2020.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun E., Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 2019;8 doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gur M., Taka E., Yilmaz S.Z., Kilinc C., Aktas U., Golcuk M. Conformational transition of SARS-CoV-2 spike glycoprotein between its closed and open states. J. Chem. Phys. 2020;153:75101. doi: 10.1063/5.0011141. [DOI] [PubMed] [Google Scholar]
- 29.Casalino L., Gaieb Z., Goldsmith J.A., Hjorth C.K., Dommer A.C., Harbison A.M., Fogarty C.A., Barros E.P., Taylor B.C., McLellan J.S., Fadda E., Amaro R.E. Beyond shielding: the roles of glycans in the SARS-CoV-2 spike protein. ACS Cent. Sci. 2020 doi: 10.1021/acscentsci.0c01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J.B., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N.V., Shen K., Luban J. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020 doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaimes J.A., André N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020;432:3309–3325. doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12 doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 34.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80-. ) 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White J.M., Delos S.E., Brecher M., Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianni T., Menotti L., Campadelli-Fiume G. A heptad repeat in herpes simplex virus 1 gH, located downstream of the α-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 2005;79:7042–7049. doi: 10.1128/JVI.79.11.7042-7049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe S., Takada A., Watanabe T., Ito H., Kida H., Kawaoka Y. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol. 2000;74:10194–10201. doi: 10.1128/jvi.74.21.10194-10201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netter R.C., Amberg S.M., Balliet J.W., Biscone M.J., Vermeulen A., Earp L.J., White J.M., Bates P. Heptad repeat 2-based peptides inhibit avian sarcoma and leukosis virus subgroup a infection and identify a fusion intermediate. J. Virol. 2004;78:13430–13439. doi: 10.1128/JVI.78.24.13430-13439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapaport D., Ovadia M., Shai Y. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 1995;14:5524–5531. doi: 10.1002/j.1460-2075.1995.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bossart K.N., Mungall B.A., Crameri G., Wang L.-F., Eaton B.T., Broder C.C. Inhibition of Henipavirus fusion and infection by heptad-derived peptides of the Nipah virus fusion glycoprotein. Virol. J. 2005;2:57. doi: 10.1186/1743-422X-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Q., Compans R.W. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology. 1996;223:103–112. doi: 10.1006/viro.1996.0459. [DOI] [PubMed] [Google Scholar]
- 44.Wang E., Sun X., Qian Y., Zhao L., Tien P., Gao G.F. Both heptad repeats of human respiratory syncytial virus fusion protein are potent inhibitors of viral fusion. Biochem. Biophys. Res. Commun. 2003;302:469–475. doi: 10.1016/s0006-291x(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 45.Yu M., Wang E., Liu Y., Cao D., Jin N., Zhang C.W.-H., Bartlam M., Rao Z., Tien P., Gao G.F. Six-helix bundle assembly and characterization of heptad repeat regions from the F protein of Newcastle disease virus. J. Gen. Virol. 2002;83:623–629. doi: 10.1099/0022-1317-83-3-623. [DOI] [PubMed] [Google Scholar]
- 46.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleine-Weber H., Elzayat M.T., Hoffmann M., Pöhlmann S. Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci. Rep. 2018;8:16597. doi: 10.1038/s41598-018-34859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu A., Pak A.J., He P., Monje-Galvan V., Casalino L., Gaieb Z., Dommer A.C., Amaro R.E., Voth G.A. A multiscale coarse-grained model of the SARS-CoV-2 virion. BioRxiv. 2020 doi: 10.1101/2020.10.02.323915. 2020.10.02.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), (n.d.). https://swissmodel.expasy.org/repository/species/2697049 (accessed October 7, 2020). [DOI] [PMC free article] [PubMed]
- 51.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., Lepore R., Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang G., Pomplun S., Loftis A.R., Loas A., Pentelute B.L. The first-in-class peptide binder to the SARS-CoV-2 spike protein. BioRxiv. 2020 doi: 10.1101/2020.03.19.999318. 2020.03.19.999318. [DOI] [Google Scholar]
- 53.Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., Chen R.E., Carter L., Walls A.C., Park Y.-J., Strauch E.-M., Stewart L., Diamond M.S., Veesler D., Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science (80-. ) 2020 doi: 10.1126/science.abd9909. eabd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng B.-J., Guan Y., Hez M.-L., Sun H., Du L., Zheng Y., Wong K.-L., Chen H., Chen Y., Lu L., Tanner J.A., Watt R.M., Niccolai N., Bernini A., Spiga O., Woo P.C.Y., Kung H., Yuen K.-Y., Huang J.-D. Synthetic peptides outside the spike protein heptad repeat regions as potent inhibitors of SARS-associated coronavirus. Antivir. Ther. 2005;10:393–403. [PubMed] [Google Scholar]
- 57.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardy W.D., Gulick R.M., Mayer H., Fätkenheuer G., Nelson M., Heera J., Rajicic N., Goodrich J. Two-year safety and virologic efficacy of maraviroc in treatment-experienced patients with CCR5-tropic HIV-1 infection: 96-week combined analysis of MOTIVATE 1 and 2. J. Acquir. Immune Defic. Syndr. 2010;55:558–564. doi: 10.1097/QAI.0b013e3181ee3d82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Clercq E. New developments in anti-HIV chemotherapy. Curr. Med. Chem. 2001;8:1543–1572. doi: 10.2174/0929867013371842. [DOI] [PubMed] [Google Scholar]
- 61.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., Stindt J., Königer C., Nassal M., Kubitz R., Sültmann H., Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. e6. [DOI] [PubMed] [Google Scholar]
- 62.Babcock G.J., Esshaki D.J., Thomas W.D.J., Ambrosino D.M. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/jvi.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.-K., Huang I.-C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi C.E., Ba L., Zhang L., Ho D.D., Chen Z. Single amino acid substitutions in the severe acute respiratory syndrome coronavirus spike glycoprotein determine viral entry and immunogenicity of a major neutralizing domain. J. Virol. 2005;79:11638–11646. doi: 10.1128/JVI.79.18.11638-11646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Struck A.-W., Axmann M., Pfefferle S., Drosten C., Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antivir. Res. 2012;94:288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho T.-Y., Wu S.-L., Chen J.-C., Wei Y.-C., Cheng S.-E., Chang Y.-H., Liu H.-J., Hsiang C.-Y. Design and biological activities of novel inhibitory peptides for SARS-CoV spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2006;69:70–76. doi: 10.1016/j.antiviral.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu H., Li L., Kao R.Y., Kou B., Wang Z., Zhang L., Zhang H., Hao Z., Tsui W.H., Ni A., Cui L., Fan B., Guo F., Rao S., Jiang C., Li Q., Sun M., He W., Liu G. Screening and identification of linear B-cell epitopes and entry-blocking peptide of severe acute respiratory syndrome (SARS)-associated coronavirus using synthetic overlapping peptide library. J. Comb. Chem. 2005;7:648–656. doi: 10.1021/cc0500607. [DOI] [PubMed] [Google Scholar]
- 70.Huang L., Sexton D.J., Skogerson K., Devlin M., Smith R., Sanyal I., Parry T., Kent R., Enright J., Wu Q., Conley G., DeOliveira D., Morganelli L., Ducar M., Wescott C.R., Ladner R.C. Novel peptide inhibitors of angiotensin-converting enzyme 2. J. Biol. Chem. 2003;278:15532–15540. doi: 10.1074/jbc.M212934200. [DOI] [PubMed] [Google Scholar]
- 71.Fukushi S., Mizutani T., Saijo M., Matsuyama S., Miyajima N., Taguchi F., Itamura S., Kurane I., Morikawa S. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J. Gen. Virol. 2005;86:2269–2274. doi: 10.1099/vir.0.80955-0. [DOI] [PubMed] [Google Scholar]
- 72.Wehkamp J., Chu H., Shen B., Feathers R.W., Kays R.J., Lee S.K., Bevins C.L. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006;580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 73.Wang C., Shen M., Gohain N., Tolbert W.D., Chen F., Zhang N., Yang K., Wang A., Su Y., Cheng T., Zhao J., Pazgier M., Wang J. Design of a potent antibiotic peptide based on the active region of human defensin 5. J. Med. Chem. 2015;58:3083–3093. doi: 10.1021/jm501824a. [DOI] [PubMed] [Google Scholar]
- 74.Beddingfield B., Iwanaga N., Chapagain P., Zheng W., Roy C., Hu T., Kolls J., Bix G. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. JACC Basic Transl. Sci. 2020 doi: 10.1016/j.jacbts.2020.10.003. S2452-302X(20)30440-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lertjuthaporn S., Cicala C., Van Ryk D., Liu M., Yolitz J., Wei D., Nawaz F., Doyle A., Horowitch B., Park C., Lu S., Lou Y., Wang S., Pan R., Jiang X., Villinger F., Byrareddy S.N., Santangelo P.J., Morris L., Wibmer C.K., Biris K., Mason R.D., Gorman J., Hiatt J., Martinelli E., Roederer M., Fujikawa D., Gorini G., Franchini G., Arakelyan A., Ansari A.A., Pattanapanyasat K., Kong X.-P., Fauci A.S., Arthos J. Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to α4β7. PLOS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussein H.A.M., Walker L.R., Abdel-Raouf U.M., Desouky S.A., Montasser A.K.M., Akula S.M. Beyond RGD: virus interactions with integrins. Arch. Virol. 2015;160:2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lv X., Li Z., Guan J., Zhang J., Xu B., He W., Lan Y., Zhao K., Lu H., Song D., Gao F. ATN-161 reduces virus proliferation in PHEV-infected mice by inhibiting the integrin α5β1-FAK signaling pathway. Vet. Microbiol. 2019;233:147–153. doi: 10.1016/j.vetmic.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 78.Watson A., Ferreira L., Hwang P., Xu J., Stroud R. Peptide antidotes to SARS-CoV-2 (COVID-19) BioRxiv. 2020 doi: 10.1101/2020.08.06.238915. 2020.08.06.238915. [DOI] [Google Scholar]
- 79.Liu T., Li X., You S., Bhuyan S.S., Dong L. Effectiveness of AMD3100 in treatment of leukemia and solid tumors: from original discovery to use in current clinical practice. Exp. Hematol. Oncol. 2016;5:19. doi: 10.1186/s40164-016-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teralı K., Baddal B., Gülcan H.O. Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: insights from a molecular mechanics-assisted structure-based virtual screening experiment. J. Mol. Graph. Model. 2020;100:107697. doi: 10.1016/j.jmgm.2020.107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Safety and Activity of ORE1001 in Subjects With Ulcerative Colitis. 2011. https://clinicaltrials.gov/ct2/show/NCT01039597
- 82.Shirato K., Kawase M., Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. 2018;517:9–15. doi: 10.1016/j.virol.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J. Virol. 2017;91 doi: 10.1128/JVI.01387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bestle D., Heindl M.R., Limburg H., Van Lam T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Klenk H.-D., Garten W., Steinmetzer T., Böttcher-Friebertshäuser E. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Böttcher-Friebertshäuser E., Klenk H.-D., Garten W. Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog. Dis. 2013;69:87–100. doi: 10.1111/2049-632X.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bertram S., Glowacka I., Steffen I., Kühl A., Pöhlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev. Med. Virol. 2010;20:298–310. doi: 10.1002/rmv.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagatake T. Potentiation of infectivity and pathogenesis of influenza virus by host and bacterial proteases. Nihon Rinsho. 2003;61:1892–1896. [PubMed] [Google Scholar]
- 89.Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S., Ciorba M.A., Whelan S.P.J., Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng Y.-W., Chao T.-L., Li C.-L., Chiu M.-F., Kao H.-C., Wang S.-H., Pang Y.-H., Lin C.-H., Tsai Y.-M., Lee W.-H., Tao M.-H., Ho T.-C., Wu P.-Y., Jang L.-T., Chen P.-J., Chang S.-Y., Yeh S.-H., Hoffmann M., Kleine-Weber H., Pöhlmann S. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Mol. Cell. 2020;78:779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. Teicoplanin potently blocks the cell entry of 2019-nCoV. BioRxiv. 2020 doi: 10.1101/2020.02.05.935387. 2020.02.05.935387. [DOI] [Google Scholar]
- 92.Bienert S., Waterhouse A., de Beer T.A.P., Tauriello G., Studer G., Bordoli L., Schwede T. The SWISS-MODEL repository—new features and functionality. Nucleic Acids Res. 2017;45:D313–D319. doi: 10.1093/nar/gkw1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herter S., Piper D.E., Aaron W., Gabriele T., Cutler G., Cao P., Bhatt A.S., Choe Y., Craik C.S., Walker N., Meininger D., Hoey T., Austin R.J. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem. J. 2005;390:125–136. doi: 10.1042/BJ20041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pearce K.H., Overton L.K., Gampe R.T., Barrett G.B., Taylor J.D., McKee D.D., Campobasso N., Nolte R.T., Reid R.A. BacMam production and crystal structure of nonglycosylated apo human furin at 1.89Åresolution. Acta Crystallogr. Sect. F. 2019;75:239–245. doi: 10.1107/S2053230X19001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardegger L.A., Kuhn B., Spinnler B., Anselm L., Ecabert R., Stihle M., Gsell B., Thoma R., Diez J., Benz J., Plancher J.-M., Hartmann G., Isshiki Y., Morikami K., Shimma N., Haap W., Banner D.W., Diederich F. Halogen bonding at the active sites of human cathepsin l and mek1 kinase: efficient interactions in different environments. ChemMedChem. 2011;6:2048–2054. doi: 10.1002/cmdc.201100353. [DOI] [PubMed] [Google Scholar]
- 96.Wrobel A.G., Benton D.J., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020;27:763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Böttcher-Friebertshäuser E. Membrane-anchored serine proteases: host cell factors in proteolytic activation of viral glycoproteins. Act. Virus. Host Proteas. 2018:153–203. doi: 10.1007/978-3-319-75474-1_8. [DOI] [Google Scholar]
- 98.Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 99.Volchkov V.E., Feldmann H., Volchkova V.A., Klenk H.-D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sugrue R.J., Brown C., Brown G., Aitken J., McL Rixon H.W. Furin cleavage of the respiratory syncytial virus fusion protein is not a requirement for its transport to the surface of virus-infected cells. J. Gen. Virol. 2001;82:1375–1386. doi: 10.1099/0022-1317-82-6-1375. [DOI] [PubMed] [Google Scholar]
- 101.Bergeron E., Vincent M.J., Wickham L., Hamelin J., Basak A., Nichol S.T., Chrétien M., Seidah N.G. Implication of proprotein convertases in the processing and spread of severe acute respiratory syndrome coronavirus. Biochem. Biophys. Res. Commun. 2005;326:554–563. doi: 10.1016/j.bbrc.2004.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker G.L., Sielaff F., Than M.E., Lindberg I., Routhier S., Day R., Lu Y., Garten W., Steinmetzer T. Potent inhibitors of furin and furin-like proprotein convertases containing decarboxylated P1 arginine mimetics. J. Med. Chem. 2010;53:1067–1075. doi: 10.1021/jm9012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78:779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vaarala M.H., Porvari K.S., Kellokumpu S., Kyllönen A.P., Vihko P.T. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J. Pathol. 2001;193:134–140. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH743>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 105.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019;93:e01815–e01818. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhirnov O.P., Klenk H.D., Wright P.F. Aprotinin and similar protease inhibitors as drugs against influenza. Antivir. Res. 2011;92:27–36. doi: 10.1016/j.antiviral.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 107.Hayashi T., Hotta H., Itoh M., Homma M. Protection of mice by a protease inhibitor, aprotinin, against lethal Sendai virus pneumonia. J. Gen. Virol. 1991;72(Pt 4):979–982. doi: 10.1099/0022-1317-72-4-979. [DOI] [PubMed] [Google Scholar]
- 108.Meyer D., Sielaff F., Hammami M., Böttcher-Friebertshäuser E., Garten W., Steinmetzer T. Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation. Biochem. J. 2013;452:331–343. doi: 10.1042/BJ20130101. [DOI] [PubMed] [Google Scholar]
- 109.Wettstein L., Conzelmann C., Müller J.A., Weil T., Groß R., Hirschenberger M., Seidel A., Klute S., Zech F., Bozzo C.P., Preising N., Fois G., Lochbaum R., Knaff P., Mailänder V., Ständker L., Thal D.R., Schumann C., Stenger S., Kleger A., Lochnit G., Sparrer K., Kirchhoff F., Frick M., Münch J. Alpha-1 antitrypsin inhibits SARS-CoV-2 infection. BioRxiv. 2020 doi: 10.1101/2020.07.02.183764. 2020.07.02.183764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azouz N.P., Klingler A.M., Rothenberg M.E. Alpha 1 antitrypsin is an inhibitor of the SARS-CoV2–priming protease TMPRSS2. BioRxiv. 2020 doi: 10.1101/2020.05.04.077826. 2020.05.04.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trial of Alpha One Antitrypsin Inhalation in Treating Patient With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), ClinicalTr (n.d.). https://clinicaltrials.gov/ct2/show/NCT04385836.
- 112.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bosch B.J., Bartelink W., Rottier P.J.M. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu T., Luo S., Libby P., Shi G.-P. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213:107587. doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao H., Zhou J., Zhang K., Chu H., Liu D., Poon V.K.-M., Chan C.C.-S., Leung H.-C., Fai N., Lin Y.-P., Zhang A.J.-X., Jin D.-Y., Yuen K.-Y., Zheng B.-J. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci. Rep. 2016;6:22008. doi: 10.1038/srep22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao H., To K.K.W., Sze K.-H., Yung T.T.-M., Bian M., Lam H., Yeung M.L., Li C., Chu H., Yuen K.-Y. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 2020;11:4252. doi: 10.1038/s41467-020-17986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao H., Lam H., Zhou X., To K., Chan J., Lee A.C.Y., Cai J.-P., Chan C., Yeung M.L., Zhang A.J., Yuen K.-Y. Cross-linking peptide and repurposed drugs inhibit both entry pathways of SARS-CoV-2. Nat. Res. 2020 doi: 10.21203/rs.3.rs-87868/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mukhopadhyay J. Teicoplanin for treating MRSA pneumonia. BMJ Br. Med. J. 2014;348:g2317. doi: 10.1136/bmj.g2317. [DOI] [PubMed] [Google Scholar]
- 119.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin l in the late endosome/lysosome and block the entry of ebola virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tripathi P.K., Upadhyay S., Singh M., Raghavendhar S., Bhardwaj M., Sharma P., Patel A.K. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int. J. Biol. Macromol. 2020;164:2622–2631. doi: 10.1016/j.ijbiomac.2020.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., Fernandez-Pittol M., Pitart C., Inciarte A., Bodro M., Morata L., Ambrosioni J., Grafia I., Meira F., Macaya I., Cardozo C., Casals C., Tellez A., Castro P., Marco F., García F., Mensa J., Martínez J.A., Soriano A. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020 doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceccarelli G., Alessandri F., Oliva A., Dell'Isola S., Rocco M., Ruberto F., Pugliese F., d'Ettorre G., Venditti M. Superinfections in patients treated with Teicoplanin as anti-SARS-CoV-2 agent. Eur. J. Clin. Invest. 2020 doi: 10.1111/eci.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lv Z., Chu Y., Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV. AIDS. (Auckl) 2015;7:95–104. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen S.S., Lee C.N., Lee W.R., McIntosh K., Lee T.H. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J. Virol. 1993;67:3615–3619. doi: 10.1128/JVI.67.6.3615-3619.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sergel-Germano T., McQuain C., Morrison T. Mutations in the fusion peptide and heptad repeat regions of the Newcastle disease virus fusion protein block fusion. J. Virol. 1994;68:7654–7658. doi: 10.1128/JVI.68.11.7654-7658.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang S., Lin K., Strick N., Neurath A.R. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 127.Wild C.T., Shugars D.C., Greenwell T.K., McDanal C.B., Matthews T.J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qureshi N.M., Coy D.H., Garry R.F., Henderson L.A. Characterization of a putative cellular receptor for HIV-1 transmembrane glycoprotein using synthetic peptides. AIDS. 1990;4 doi: 10.1097/00002030-199006000-00009. https://journals.lww.com/aidsonline/Fulltext/1990/06000/Characterization_of_a_putative_cellular_receptor.9.aspx [DOI] [PubMed] [Google Scholar]
- 129.Lu M., Blacklow S.C., Kim P.S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 130.Derdeyn C.A., Decker J.M., Sfakianos J.N., Zhang Z., O'Brien W.A., Ratner L., Shaw G.M., Hunter E. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virol. 2001;75:8605–8614. doi: 10.1128/jvi.75.18.8605-8614.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Louis S., Cervia J.S., Smith M.A. Enfuvirtide (T-20): a novel human immunodeficiency virus type 1 fusion inhibitor. Clin. Infect. Dis. 2003;37:1102–1106. doi: 10.1086/378302. [DOI] [PubMed] [Google Scholar]
- 132.Xia S., Xu W., Wang Q., Wang C., Hua C., Li W., Lu L., Jiang S. Peptide-based membrane fusion inhibitors targeting HCoV-229E spike protein HR1 and HR2 domains. Int. J. Mol. Sci. 2018;19:487. doi: 10.3390/ijms19020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bosch B.J., Martina B.E.E., van der Zee R., Lepault J., Haijema B.J., Versluis C., Heck A.J.R., de Groot R., Osterhaus A.D.M.E., Rottier P.J.M. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., Xiong H., Farmar J., Debnath A.K., Tien P., Jiang S. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet (London, England) 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun Y., Zhang H., Shi J., Zhang Z., Gong R. Identification of a novel inhibitor against middle east respiratory syndrome coronavirus. Viruses. 2017;9 doi: 10.3390/v9090255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao J., Lu G., Qi J., Li Y., Wu Y., Deng Y., Geng H., Li H., Wang Q., Xiao H., Tan W., Yan J., Gao G.F. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the s protein of middle east respiratory syndrome coronavirus. J. Virol. 2013;87:13134–13140. doi: 10.1128/JVI.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu L., Liu Q., Zhu Y., Chan K.-H., Qin L., Li Y., Wang Q., Chan J.F.-W., Du L., Yu F., Ma C., Ye S., Yuen K.-Y., Zhang R., Jiang S. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuan K., Yi L., Chen J., Qu X., Qing T., Rao X., Jiang P., Hu J., Xiong Z., Nie Y., Shi X., Wang W., Ling C., Yin X., Fan K., Lai L., Ding M., Deng H. Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein. Biochem. Biophys. Res. Commun. 2004;319:746–752. doi: 10.1016/j.bbrc.2004.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu J., Xiao G., Xu Y., Yuan F., Zheng C., Liu Y., Yan H., Cole D.K., Bell J.I., Rao Z., Tien P., Gao G.F. Following the rule: formation of the 6-helix bundle of the fusion core from severe acute respiratory syndrome coronavirus spike protein and identification of potent peptide inhibitors. Biochem. Biophys. Res. Commun. 2004;319:283–288. doi: 10.1016/j.bbrc.2004.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu L.-H.M., Chan S.-H., Tsai S.-N., Wang Y., Cheng C.H.-K., Wong K.-B., Waye M.M.-Y., Ngai S.-M. Fusion core structure of the severe acute respiratory syndrome coronavirus (SARS-CoV): in search of potent SARS-CoV entry inhibitors. J. Cell. Biochem. 2008;104:2335–2347. doi: 10.1002/jcb.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.-T.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conzelmann C., Gilg A., Groß R., Schütz D., Preising N., Ständker L., Jahrsdörfer B., Schrezenmeier H., Sparrer K.M.J., Stamminger T., Stenger S., Münch J., Müller J.A. An enzyme-based immunodetection assay to quantify SARS-CoV-2 infection. Antivir. Res. 2020;181:104882. doi: 10.1016/j.antiviral.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pessi A. Cholesterol-conjugated peptide antivirals: a path to a rapid response to emerging viral diseases. J. Pept. Sci. 2015;21:379–386. doi: 10.1002/psc.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chong H., Xue J., Xiong S., Cong Z., Ding X., Zhu Y., Liu Z., Chen T., Feng Y., He L., Guo Y., Wei Q., Zhou Y., Qin C., He Y. A lipopeptide HIV-1/2 fusion inhibitor with highly potent in vitro, ex vivo, and in vivo antiviral activity. J. Virol. 2017;91 doi: 10.1128/JVI.00288-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu Y., Yu D., Yan H., Chong H., He Y. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J. Virol. 2020;94 doi: 10.1128/JVI.00635-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Vries R.D., Schmitz K.S., Bovier F.T., Noack D., Haagmans B.L., Biswas S., Rockx B., Gellman S.H., Alabi C.A., de Swart R.L., Moscona A., Porotto M. Intranasal fusion inhibitory lipopeptide prevents direct contact SARS-CoV-2 transmission in ferrets. BioRxiv. 2020 doi: 10.1101/2020.11.04.361154. 2020.11.04.361154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wimley W.C., White S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 148.Sainz B.J., Mossel E.C., Gallaher W.R., Wimley W.C., Peters C.J., Wilson R.B., Garry R.F. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) infectivity by peptides analogous to the viral spike protein. Virus Res. 2006;120:146–155. doi: 10.1016/j.virusres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou X., Liu Z., Zhang J., Adelsberger J.W., Yang J., Burton G.F. Alpha-1-antitrypsin interacts with gp41 to block HIV-1 entry into CD4+ T lymphocytes. BMC Microbiol. 2016;16:172. doi: 10.1186/s12866-016-0751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Münch J., Ständker L., Adermann K., Schulz A., Schindler M., Chinnadurai R., Pöhlmann S., Chaipan C., Biet T., Peters T., Meyer B., Wilhelm D., Lu H., Jing W., Jiang S., Forssmann W.-G., Kirchhoff F. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell. 2007;129:263–275. doi: 10.1016/j.cell.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 151.Nandy A., Basak S.C. A Brief Review of Computer-Assisted Approaches to Rational Design of Peptide Vaccines. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ojha P.K., Kar S., Krishna J.G., Roy K., Leszczynski J. Therapeutics for COVID-19: from computation to practices-where we are, where we are heading to. Mol. Divers. 2020:1–35. doi: 10.1007/s11030-020-10134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Keshavarzi Arshadi A., Webb J., Salem M., Cruz E., Calad-Thomson S., Ghadirian N., Collins J., Diez-Cecilia E., Kelly B., Goodarzi H., Yuan J.S. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front. Artif. Intell. 2020;3:65. doi: 10.3389/frai.2020.00065. https://www.frontiersin.org/article/10.3389/frai.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ling R., Dai Y., Huang B., Huang W., Yu J., Lu X., Jiang Y. In silico design of antiviral peptides targeting the spike protein of SARS-CoV-2. Peptides. 2020;130:170328. doi: 10.1016/j.peptides.2020.170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang X., Pearce R., Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging (Albany. NY) 2020;12:11263–11276. doi: 10.18632/aging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han Y., Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14:5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Basit A., Ali T., Rehman S.U. Truncated human angiotensin converting enzyme 2; a potential inhibitor of SARS-CoV-2 spike glycoprotein and potent COVID-19 therapeutic agent. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1768150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barh D., Tiwari S., Silva Andrade B., Giovanetti M., Almeida Costa E., Kumavath R., Ghosh P., Góes-Neto A., Alcantara L. Carlos Junior, Azevedo V. Potential chimeric peptides to block the SARS-CoV-2 spike receptor-binding domain. F1000Research. 2020;9:576. doi: 10.12688/f1000research.24074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baig M.S., Alagumuthu M., Rajpoot S., Saqib U. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs R. D. 2020;20:161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ali A., Vijayan R. Dynamics of the ACE2–SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms. Sci. Rep. 2020;10:14214. doi: 10.1038/s41598-020-71188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.The SIRAH-CoV2 Initiative Sergio Pantano at Pasteur Institute of Montevideo & ClusterUY. https://www.cluster.uy/web-covid/ (accessed October 7, 2020).
- 162.Molecular Dynamics Simulations Related to SARS-CoV-2. D. E. Shaw Research Technical Data. 2020. http://www.deshawresearch.com/resources_sarscov2.html (n.d.) (accessed October 7, 2020)
- 163.Wong F.-C., Ong J.-H., Chai T.-T. Identification of putative cell-entry-inhibitory peptides against SARS-CoV-2 from edible insects: an in silico study. EFood. 2020;1:357–368. doi: 10.2991/efood.k.200918.002. [DOI] [Google Scholar]
- 164.Chowdhury S.M., Talukder S.A., Khan A.M., Afrin N., Ali M.A., Islam R., Parves R., Al Mamun A., Sufian M.A., Hossain M.N., Hossain M.A., Halim M.A. Antiviral peptides as promising therapeutics against SARS-CoV-2. J. Phys. Chem. B. 2020;124:9785–9792. doi: 10.1021/acs.jpcb.0c05621. [DOI] [PubMed] [Google Scholar]
- 165.Yu Z., Kan R., Ji H., Wu S., Zhao W., Shuian D., Liu J., Li J. Identification of tuna protein-derived peptides as potent SARS-CoV-2 inhibitors via molecular docking and molecular dynamic simulation. Food Chem. 2020;128366 doi: 10.1016/j.foodchem.2020.128366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jaiswal G., Kumar V. In-silico design of a potential inhibitor of SARS-CoV-2 S protein. PLoS One. 2020;15:e0240004. doi: 10.1371/journal.pone.0240004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (80-. ) 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bowers K.J., Chow E., Xu H., Dror R.O., Eastwood M.P., Gregersen B.A., Klepeis J.L., Kolossvary I., Moraes M.A., Sacerdoti F.D., Salmon J.K., Shan Y., Shaw D.E. Proc. 2006 ACM/IEEE Conf. Supercomput. Association for Computing Machinery; New York, NY, USA: 2006. Scalable algorithms for molecular dynamics simulations on commodity clusters; p. 84. es. [DOI] [Google Scholar]
- 172.Machado M.R., Barrera E.E., Klein F., Sóñora M., Silva S., Pantano S. The SIRAH 2.0 force field: altius, fortius, citius. J. Chem. Theory Comput. 2019;15:2719–2733. doi: 10.1021/acs.jctc.9b00006. [DOI] [PubMed] [Google Scholar]
- 173.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 174.Kovalainen M., Mönkäre J., Riikonen J., Pesonen U., Vlasova M., Salonen J., Lehto V.-P., Järvinen K., Herzig K.-H. Novel delivery systems for improving the clinical use of peptides. Pharmacol. Rev. 2015;67:541–561. doi: 10.1124/pr.113.008367. [DOI] [PubMed] [Google Scholar]
- 175.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 176.Bodier-Montagutelli E., Mayor A., Vecellio L., Respaud R., Heuzé-Vourc'h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expert Opin. Drug Deliv. 2018;15:729–736. doi: 10.1080/17425247.2018.1503251. [DOI] [PubMed] [Google Scholar]
- 177.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patton J.S., Fishburn C.S., Weers J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 179.Fellner R.C., Terryah S.T., Tarran R. Inhaled protein/peptide-based therapies for respiratory disease. Mol. Cell. Pediatr. 2016;3:16. doi: 10.1186/s40348-016-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Patton J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996;19:3–36. doi: 10.1016/0169-409X(95)00113-L. [DOI] [Google Scholar]
- 181.Dombu C.Y., Betbeder D. Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 2013;34:516–525. doi: 10.1016/j.biomaterials.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 182.Veuillez F., Kalia Y.N., Jacques Y., Deshusses J., Buri P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001;51:93–109. doi: 10.1016/s0939-6411(00)00144-2. [DOI] [PubMed] [Google Scholar]
- 183.Zhu X., Zhu Y., Ye S., Wang Q., Xu W., Su S., Sun Z., Yu F., Liu Q., Wang C., Zhang T., Zhang Z., Zhang X., Xu J., Du L., Liu K., Lu L., Zhang R., Jiang S. Improved pharmacological and structural properties of HIV fusion inhibitor AP3 over enfuvirtide: highlighting advantages of artificial peptide strategy. Sci. Rep. 2015;5:13028. doi: 10.1038/srep13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Greenberg M.L., Cammack N. Resistance to enfuvirtide, the first HIV fusion inhibitor. J. Antimicrob. Chemother. 2004;54:333–340. doi: 10.1093/jac/dkh330. [DOI] [PubMed] [Google Scholar]
- 185.Cai L., Jiang S. Development of peptide and small-molecule HIV-1 fusion inhibitors that target gp41. ChemMedChem. 2010;5:1813–1824. doi: 10.1002/cmdc.201000289. [DOI] [PubMed] [Google Scholar]
- 186.Lau J.L., Dunn M.K. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 187.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loffet A. Peptides as drugs: is there a market? J. Pept. Sci. 2002;8:1–7. doi: 10.1002/psc.366. [DOI] [PubMed] [Google Scholar]
- 190.de Haan C.A.M., Stadler K., Godeke G.-J., Bosch B.J., Rottier P.J.M. Cleavage inhibition of the murine coronavirus spike protein by a furin-like enzyme affects cell-cell but not virus-cell fusion. J. Virol. 2004;78:6048–6054. doi: 10.1128/JVI.78.11.6048-6054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 192.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rohl C.A., Strauss C.E.M., Misura K.M.S., Baker D. Protein structure prediction using Rosetta. Methods Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- 194.Dou J., Vorobieva A.A., Sheffler W., Doyle L.A., Park H., Bick M.J., Mao B., Foight G.W., Lee M.Y., Gagnon L.A., Carter L., Sankaran B., Ovchinnikov S., Marcos E., Huang P.-S., Vaughan J.C., Stoddard B.L., Baker D. De novo design of a fluorescence-activating β-barrel. Nature. 2018;561:485–491. doi: 10.1038/s41586-018-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jacobson M.P., Pincus D.L., Rapp C.S., Day T.J.F., Honig B., Shaw D.E., Friesner R.A. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55:351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 196.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 197.Schymkowitz J., Borg J., Stricher F., Nys R., Rousseau F., Serrano L. The FoldX web server: an online force field. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gki387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Systèmes, D., BIOVIA Discovery Studio . Dassault Syst mes; 2016. Dassault Syst mes BIOVIA, Discovery Studio Modeling Environment, Release 2017. (n.d.) [Google Scholar]
- 199.Jo S., Kim T., Iyer V.G., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 200.van Zundert G.C.P., Rodrigues J.P.G.L.M., Trellet M., Schmitz C., Kastritis P.L., Karaca E., Melquiond A.S.J., van Dijk M., de Vries S.J., Bonvin A.M.J.J. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 201.Zhou P., Jin B., Li H., Huang S.-Y. HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018;46:W443–W450. doi: 10.1093/nar/gky357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kozakov D., Hall D.R., Xia B., Porter K.A., Padhorny D., Yueh C., Beglov D., Vajda S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017;12:255–278. doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen R., Li L., Weng Z. ZDOCK: an initial-stage protein-docking algorithm. Proteins. 2003;52:80–87. doi: 10.1002/prot.10389. [DOI] [PubMed] [Google Scholar]
- 204.Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42:D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qureshi A., Tandon H., Kumar M. AVP-IC50 Pred: Multiple machine learning techniques-based prediction of peptide antiviral activity in terms of half maximal inhibitory concentration (IC50) Biopolymers. 2015;104:753–763. doi: 10.1002/bip.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gupta S., Kapoor P., Chaudhary K., Gautam A., Kumar R., Raghava G.P.S. In silico approach for predicting toxicity of peptides and proteins. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xue L.C., Rodrigues J.P., Kastritis P.L., Bonvin A.M., Vangone A. PRODIGY: a web server for predicting the binding affinity of protein-protein complexes. Bioinformatics. 2016;32:3676–3678. doi: 10.1093/bioinformatics/btw514. [DOI] [PubMed] [Google Scholar]
- 208.Phillips J.C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R.D., Kalé L., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li J., Abel R., Zhu K., Cao Y., Zhao S., Friesner R.A. The VSGB 2.0 model: a next generation energy model for high resolution protein structure modeling. Proteins. 2011;79:2794–2812. doi: 10.1002/prot.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- 211.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 212.McGibbon R.T., Beauchamp K.A., Harrigan M.P., Klein C., Swails J.M., Hernández C.X., Schwantes C.R., Wang L.-P., Lane T.J., Pande V.S. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 2015;109:1528–1532. doi: 10.1016/j.bpj.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Case D.A., Belfon K., Ben-Shalom I.Y., Brozell S.R., Cerutti D.S., Cheatham, III T.E., Cruzeiro V.W.D., Darden T.A., Duke R.E., Giambasu G., Gilson M.K., Gohlke H., Goetz A.W., Harris R., Izadi S., Izmailov S.A., Kasavajhala K., Kovalenko A., Krasny R., Kurtzman T., Lee T.S., LeGrand S., Li P., Lin C., Liu J., Luchko T., Luo R., Man V., Merz K.M., Miao Y., Mikhailovskii O., Monard G., Nguyen H., Onufriev A., Pan F., Pantano S., Qi R., Roe D.R., Roitberg A., Sagui C., Schott-Verdugo S., Shen J., Simmerling C.L., Skrynnikov N.R., Swails J., Walker R.C., Wang J., Wilson L., Wolf R.M., Wu X., Xiong Y., Xue Y., York D.M., Kollman P.A. AMBER 2020. University of California; San Francisco: 2020. [Google Scholar]