Abstract
Introduction:
Low serum magnesium (Mg) is associated with an increased incidence of atrial and ventricular arrhythmias. A richer phenotyping of arrhythmia indices, such as burden or frequency, may provide etiologic insights.
Objectives:
To evaluate cross-sectional associations of serum Mg with burden of atrial arrhythmias [atrial fibrillation (AF), premature atrial contractions (PAC), supraventricular tachycardia (SVT)], and ventricular arrhythmias [premature ventricular contractions (PVC), non-sustained ventricular tachycardia (NSVT)] over 2-weeks of ECG monitoring.
Methods:
We included 2,513 ARIC Study visit 6 (2016-2017) participants who wore the Zio XT Patch—a leadless, ambulatory ECG-monitor—for up to 2-weeks. Serum Mg was modeled categorically and continuously. AF burden was categorized as intermittent or continuous based on the percent of analyzable time spent in AF. Other arrhythmia burdens were defined by the average number of abnormal beats per day. Linear regression was used for continuous outcomes; logistic and multinomial regression were used for categorical outcomes.
Results:
Participants were mean±SD age 79±5 years, 58% were women and 25% black. Mean serum Mg was 0.82±0.08 mmol/F and 19% had hypomagnesemia (<0.75 mmol/F). Serum Mg was inversely associated with PVC burden and continuous AF. The AF association was no longer statistically significant with further adjustment for traditional lifestyle risk factors, only the association with PVC burden remained significant. There were no associations between serum Mg and other arrhythmias examined.
Conclusions:
In this community-based cohort of older adults, we found little evidence of independent cross-sectional associations between serum Mg and arrhythmia burden.
Keywords: serum magnesium, atrial arrhythmias, ventricular arrhythmias, continuous electrographic monitoring, older adults
INTRODUCTION
Magnesium (Mg) plays essential physiologic functions, including having a role in cardiac electrophysiology.1,2 The reference interval for normal serum Mg concentrations is traditionally defined as 0.75-0.95 mmol/L; however, some experts have suggested that individuals with serum Mg concentrations of 0.75-0.85 mmol/L may also exhibit subclinical or chronic latent Mg deficiencies.3 In nutrition sciences, severe Mg deficiency is widely thought to result in dysrhythmias, including atrial fibrillation (AF).4,5
There is growing interest in characterizing arrhythmias as a continuous measure of burden rather than a binary condition.6 While extreme Mg concentrations are thought to lead to adverse electrocardiographic (ECG) changes,7 less is understood about subclinical deficiencies in circulating Mg in relation to burden of atrial and ventricular arrhythmias. Arrhythmias can be asymptomatic and intermittent in nature, which has previously complicated efforts to evaluate the association between Mg and arrhythmia burden. Current ECG technology has evolved to allow continuous monitoring for longer periods, such as 2-weeks,8–10 which leads to the identification of additional arrhythmic events.11 As such, little has been characterized of the association between Mg and burden of atrial arrhythmias [e.g. intermittent AF, premature atrial contractions (PAC) or supraventricular tachycardia (SVT)] or ventricular arrhythmias [e.g. premature ventricular contractions (PVC) or non-sustained ventricular tachycardia (NSVT)] in the community.
Using data from over 2,000 older adult participants of the Atherosclerosis Risk in Communities (ARIC) Study, we characterized cross-sectional associations of serum Mg concentrations across the spectrum of AF burden and other arrhythmias (PAC, SVT, PVC, NSVT) based on up to 2-weeks of continuous ECG recording. We hypothesized that low serum Mg concentrations would be associated with a higher prevalence and burden of atrial and ventricular arrhythmias in this community-dwelling population.
METHODS
Study Design
The multi-center prospective ARIC Study12 began in 1987-89, when the 15,792 participants were aged 45-64 years old. Participants were recruited from 4 communities (suburbs of Minneapolis, Minnesota; Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland). Since the baseline visit, several clinic visits have been conducted. At each ARIC visit, written informed consent was obtained.
Visit 6 occurred in 2016-17 and was attended by 4,003 participants (48% of living participants) when they were aged 75-94 years old. At visit 6, ARIC participants were invited to wear the Zio® XT Patch for 2-weeks provided they did not report a history of an allergic reaction to skin adhesive. Participants completed a brief questionnaire which asked about prior arrhythmias and treatments (e.g. previous arrhythmia diagnosis, anticoagulation status), and the device was applied to the upper left chest. Participants returned the devices by mail, in a pre-paid and labeled envelope to iRhythm (the manufacturer) for processing. Of the 2,650 participants who received a device, 17 devices were lost, and 17 devices were returned without data; thus resulting in 2,616 devices returned with analyzable data. Of the 2,616 with analyzable data on the Zio® XT Patch, we excluded participants missing serum Mg measurements (n=91) at visit 6, and due to small numbers, participants who were neither black nor white (n=6) and black participants at the Minnesota and Maryland field centers (n=6). For analyses of atrial arrhythmias other than AF, we further excluded those with prevalent AF (n=350) as detected during Zio monitoring, or identified by prior ARIC AF diagnosis.13
Biomarker Measures
Prior to ARIC visit 6, participants were asked to fast >8 hours. Blood samples were obtained and frozen until analysis. Serum total Mg was measured using colorimetric methods on the Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics; Indianapolis, Indiana). Serum potassium was measured using an ion selective electrode (Roche C501 Chemistry Analyzer). Serum glucose was measured using a hexokinase assay (Roche Cobas 6000 Chemistry Analyzer). Coefficients of variations (based on duplicate samples) were 1.6%, 2.2%, and 2.0%, for Mg, potassium, and glucose, respectively.
Covariates
At ARIC clinic visit 6, participants were interviewed, underwent anthropomorphic measurements and sitting blood pressure measurements, as well as a blood draw. Participants were asked to bring bottles of current medications and nutritional supplements to the visit, where this information was transcribed and coded. Diabetes was defined as a having a fasting glucose level ≥126 mg/dL, non-fasting glucose level ≥200 mg/dL, self-reported use of diabetes medication or self-reported physician diagnosis. Systolic blood pressure was quantified based on the mean of the second and third of three blood pressure measurements. Body mass index (BMI) was calculated based on weight (kg) divided by height (m2) squared. Physical activity (sports index) was quantified using the validated Baecke questionnaire.14 Detailed definitions of coronary heart disease (CHD),15 heart failure (HF)16 and stroke17 have been previously published. Briefly, trained staff abstracted possible hospitalized CHD and stroke events onto standardized forms, which were classified by physicians using computer-assisted classification algorithms. HF was classified based on a prior hospital discharge code including ‘428’ (428.0–428.9) or outpatient HF using previously published criteria.16 CHD, HF, and stroke were also based on self-reported prevalent disease at visit 1 (1987-1989).
Outcomes
AF was defined by an irregularly irregular rhythm with absent P-waves lasting >30 seconds. AF burden was defined by percent of recording time spent in AF, which we categorized as no AF (0%), intermittent AF (0 to <100%) and continuous AF (100%).
SVT was defined by narrow complex tachycardia >4 beats with a rate >100/min, while NSVT was defined by wide complex tachycardia >4 beats with a rate >100/min. PAC count refers to the number of isolated PACs, while PVC count refers to the number of isolated PVCs. PAC, SVT, NSVT and PVC burden refer to the average number of arrhythmic beats per day (e.g. PAC count divided by duration of recording time).
Statistical Analysis
We present unadjusted mean ± standard deviation and proportions for the covariates stratified by serum Mg categories (i.e. ≥0.95 mmol/L hypermagnesemia, 0.85-<0.95 mmol/L normal, 0.75-<0.85 mmol/L subclinical hypomagnesemia, <0.75 mmol/L hypomagnesemia).3 Several statistical models were used. Predicted probabilities of intermittent/continuous AF by serum Mg were plotted using demographic adjusted restricted cubic splines with 5 knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. Linear regression was used for continuous outcomes (PAC and PVC burden). We log-transformed PAC and PVC burden due to the right-skew distribution of these variables. Effect estimates for these outcomes are presented as ratios of PAC or PVC burden geometric means [exp(β0.75 vs 0.85-0.95 mmol/L)]. Logistic regression was used to assess the association between serum Mg and binary measures of arrhythmias (NSVT). Multinomial logistic regression was used for categorical outcomes (AF burden). Confidence intervals were estimated based on model-based standard errors. In Model 1, we adjusted for demographic characteristics: age, sex and race-center (North Carolina-white, North Carolina-black, Maryland-white, Minnesota-white, Mississippi-black). In Model 2, we additionally adjusted for educational attainment (less than high school; high school or GED; more than high school), current smoking status, current drinking status, physical activity, magnesium or calcium supplement use, and BMI (continuous). In Model 3 (our fully adjusted model), we further adjusted for diabetes, systolic and diastolic blood pressure (continuous), and antihypertensive medication use. Because circulating potassium plays an important role in cardiac electrophysiology and hypokalemia can frequently co-occurs with hypomagnesemia, we added serum potassium (continuous) to Model 3 to test whether the serum Mg-arrhythmia associations were independent of serum potassium concentrations (Model 4).
To test the robustness of our findings, we conducted several sensitivity analyses: 1) excluding those taking antiarrhythmic medications (including digoxin), 2) excluding users of ACEI/ARBs and diuretics, 3) excluding users of proton pump inhibitors (PPIs) as well as 4) excluding those with a history of CVD (CHD, HF, stroke). SAS version 9.4 was used for statistical analyses (SAS Institute; Cary, NC).
RESULTS
The 2,513 participants were mean±SD aged 79±5 years, 58% were women and 25% were black. Mean serum Mg was 0.82±0.08 mmol/L and hypomagnesemia (<0.75 mmol/L) prevalence was 19%. Median analyzable time for the Zio® XT Patch monitor was 13.7 days (Q1-Q3=12.7-13.9). As shown in Table 1, participants with low serum Mg tended to take more medications and have a higher prevalence of cardiometabolic diseases as compared to those with normal serum Mg (0.75-0.95 mmol/L).
Table 1.
Descriptive characteristics by serum magnesium categories, the ARIC study, 2016-2017.
| Serum magnesium, mmol/L† |
|||||
|---|---|---|---|---|---|
| Overall | <0.75 | 0.75-<0.85 | 0.85-<0.95 | ≥0.95 | |
| N | 2513 | 478 | 986 | 1004 | 45 |
| Serum magnesium, mmol/L‡ | 0.82 (0.37-1.11) | 0.70 (0.37-0.74) | 0.82 (0.78-0.82) | 0.86 (0.86-0.95) | 0.99 (0.99-1.11) |
| Age, y | 79.2±4.6 | 79.0±4.6 | 79.2±4.6 | 79.3±4.7 | 79.9±4.5 |
| Women | 1445 (57.5) | 285 (59.6) | 549 (55.7) | 581 (57.9) | 30 (66.7) |
| Race | |||||
| White | 1893 (75.3) | 330 (69.0) | 734 (74.4) | 796 (79.3) | 33 (73.3) |
| Black | 620 (24.7) | 148 (31.0) | 252 (25.6) | 208 (20.7) | 12 (26.7) |
| Educational attaimnent | |||||
| <High school | 311 (12.4) | 70 (14.6) | 121 (12.3) | 116 (11.6) | 4 (8.9) |
| High school or GED | 1048 (41.7) | 212 (44.4) | 389 (39.5) | 424 (42.2) | 23 (51.1) |
| >High school | 1148 (45.7) | 195 (40.8) | 474 (48.1) | 462 (46.0) | 17 (37.8) |
| Current smoker | 173 (6.9) | 34 (7.1) | 69 (7.0) | 65 (6.5) | 5 (11.1) |
| Current drinker | 1247 (49.6) | 207 (43.3) | 491 (49.8) | 529 (52.7) | 20 (44.4) |
| Body mass index, kg/m2 | 28.3±5.3 | 29.7±5.7 | 28.3±5.2 | 27.6±5.0 | 27.0±4.7 |
| Diabetes | 574 (22.8) | 252 (52.7) | 203 (20.6) | 112 (11.2) | 7 (15.6) |
| Systolic blood pressure, mmHg | 135.0±18.9 | 136.7±19.8 | 134.6±18.3 | 134.7±18.9 | 134.7±20.3 |
| Diastolic blood pressure, mmHg | 67.2±10.6 | 67.7±10.6 | 67.2±10.6 | 67.2±10.4 | 61.9±10.6 |
| Antihypertensive medication use | 1922 (76.5) | 435 (91.0) | 745 (75.6) | 702 (69.9) | 40 (88.9) |
| Diuretics | 662 (26.3) | 179 (37.5) | 261 (26.5) | 207 (20.6) | 15 (33.3) |
| ACEi/ARB | 1195 (47.6) | 314 (65.7) | 466 (47.3) | 393 (39.1) | 22 (48.9) |
| Antiarrhytlunic medication use | 43 (1.7) | 7 (1.5) | 15 (1.5) | 20 (2.0) | 1 (2.2) |
| PPI medication use | 642 (25.6) | 166 (34.7) | 256 (26.0) | 213 (21.2) | 7 (15.6) |
| Digoxin medication use | 25 (1.0) | 9 (1.9) | 11 (1.1) | 5 (0.5) | 0 (0.0) |
| Magnesium supplement use | 57 (2.3) | 4 (0.8) | 19 (1.9) | 32 (3.2) | 2 (4.4) |
| Calcium supplement use | 531 (21.1) | 83 (17.4) | 205 (20.8) | 235 (23.4) | 8 (17.8) |
| Serum potassium, mmol/L | 4.1±0.4 | 4.1±0.4 | 4.1±0.4 | 4.2±0.4 | 4.2±0.4 |
| Prevalent coronary heart disease | 204 (8.1) | 43 (9.0) | 75 (7.6) | 79 (7.9) | 7 (15.6) |
| Prevalent heart failure | 193 (7.7) | 49 (10.3) | 72 (7.3) | 65 (6.5) | 7 (15.6) |
| Prevalent stroke | 100 (4.0) | 15 (3.1) | 40 (4.1) | 44 (4.4) | 1 (2.2) |
Abbreviations: Atherosclerosis Risk in Communities, ARIC; general education development, GED; angiotensin converting enzyme inhibitor / angiotensin receptor blocker, ACEi/ARB; proton pump inhibitor, PPI.
N (%) or mean ± standard deviation except where indicated otherwise
Median (Range)
Lower serum Mg tended to be associated with higher model-predicted probability of any AF (Figure 1). In the demographic adjusted model (Model 1), compared to those with normal Mg concentrations (0.85-<0.95 mmol/L) the odds of having continuous AF were higher among those with clinical hypomagnesemia (<0.75 mmol/L) [OR (95% CI) 1.90 (1.17-3.06)] (Table 2). The association was attenuated with adjustment for lifestyle characteristics (Model 2) and particularly after further adjustment for diabetes and other traditional CVD risk factors (Model 3). Similarly, with serum Mg evaluated in a continuous fashion, each 0.1 mmol/L decrement (approximately 1 standard deviation in this sample) of serum Mg was associated with a 1.27 (1.02-1.57) fold higher odds of continuous AF in the demographic-adjusted model. This association was also attenuated with further adjustment to 1.12 (0.85-1.47). Due to small numbers, individuals with hypermagnesemia (≥0.95 mmol/L) were excluded from AF analyses.
Figure 1.
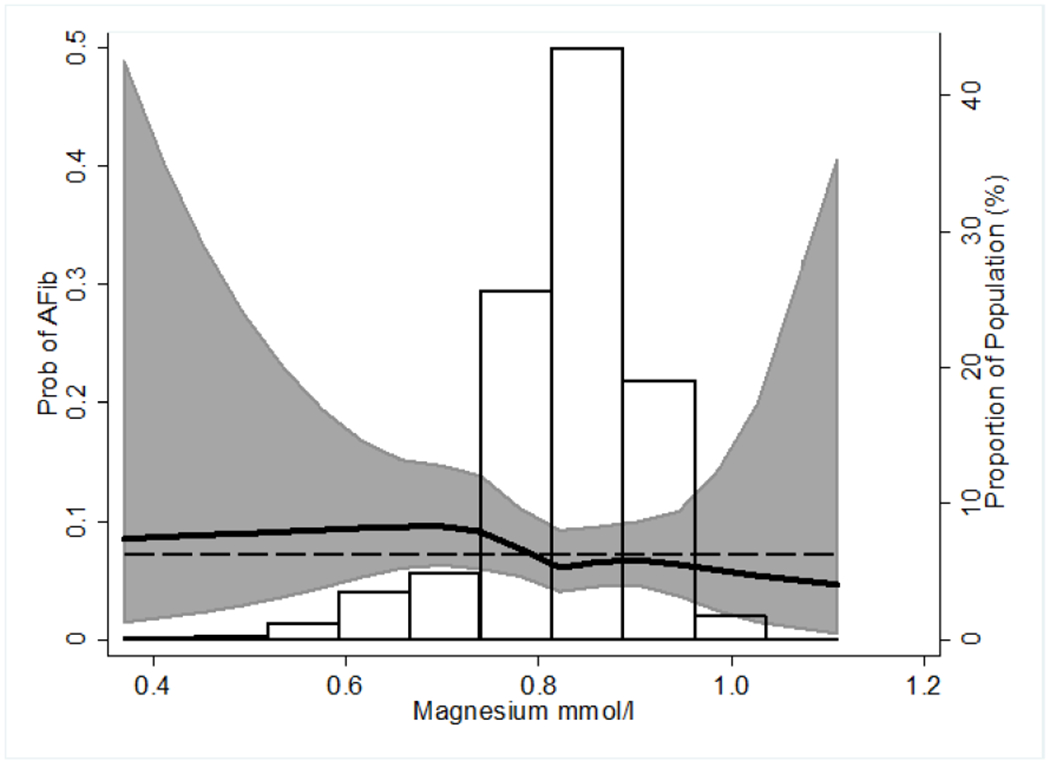
Predicted probability of atrial fibrillation (intermittent or continuous) detected over 2-weeks ambulatory ECG monitoringa across serum magnesium with distribution of serum magnesium superimposed.
a Predicted probabilities calculated from logistic regression modeling serum magnesium using restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles and with adjustment for age (mean 79 years), race-center (white-Minnesota), sex (male). Dashed line reflects average probability of atrial fibrillation equal to 0.07. Confidence intervals are pointwise prediction bands based on model-based standard errors.
Table 2.
Associations of serum magnesium with atrial arrhythmias: the ARIC study, 2016-2017†
| Serum magnesium, mmol/L |
|||||
|---|---|---|---|---|---|
| <0.75 | 0.75-<0.85 | 0.85->0.95 | ≥0.95 | Per 0.1 mmol/L decrement | |
| AF Burden | OR (95% CI) | ||||
| N | 478 | 986 | 1004 | 45 | |
| Continuous AF vs. no AF | |||||
| Continuous AF, N | 33 | 46 | 43 | 1 | |
| Model 1 | 1.90 (1.17-3.06) | 1.12 (0.73-1.73) | 1 (Ref) | - | 1.27 (1.02-1.57) |
| Model 2 | 1.70 (1.01-2.86) | 0.96 (0.60-1.54) | 1 (Ref) | - | 1.18 (0.93-1.50) |
| Model 3 | 1.48 (0.81-2.68) | 0.90 (0.55-1.49) | 1 (Ref) | - | 1.11 (0.84-1.46) |
| Model 4 | 1.51 (0.83-2.75) | 0.91 (0.55-1.51) | 1 (Ref) | - | 1.12 (0.85-1.47) |
| Intermittent AF vs. no AF | |||||
| Intermittent AF, N | 17 | 32 | 32 | 0 | |
| Model 1 | 1.30 (0.71-2.38) | 1.07 (0.65-1.77) | 1 (Ref) | - | 1.09 (0.83-1.43) |
| Model 2 | 1.11 (0.59-2.12) | 0.96 (0.57-1.62) | 1 (Ref) | - | 1.03 (0.77-1.37) |
| Model 3 | 1.24 (0.63-2.47) | 0.94 (0.56-1.59) | 1 (Ref) | - | 1.07 (0.78-1.47) |
| Model 4 | 1.29 (0.64-2.58) | 0.98 (0.57-1.66) | 1 (Ref) | - | 1.10 (0.80-1.51) |
| Ratio of Geometric Means (95% CI)‡,§ | |||||
| Nc | 400 | 851 | 870 | 42 | |
| Isolated PAC burden | |||||
| Geometric Mean | 226 | 234 | 192 | 159 | |
| Model 1 | 1.20 (0.98-1.48) | 1.21 (1.03-1.43) | 1 (Ref) | 0.82 (0.48-1.40) | 1.09 (0.99-1.19) |
| Model 2 | 1.16 (0.94-1.45) | 1.24 (1.05-1.47) | 1 (Ref) | 0.81 (0.46-1.42) | 1.07 (0.97-1.18) |
| Model 3 | 1.18 (0.93-1.49) | 1.26 (1.06-1.49) | 1 (Ref) | 0.85 (0.48-1.51) | 1.08 (0.97-1.19) |
| Model 4 | 1.16 (0.91-1.48) | 1.26 (1.06-1.50) | 1 (Ref) | 0.86 (0.48-1.53) | 1.07 (0.96-1.18) |
| SVT burden | |||||
| Geometric Mean | 1.9 | 2.2 | 2.2 | 1.9 | |
| Model 1 | 0.90 (0.82-0.99) | 1.00 (0.92-1.07) | 1 (Ref) | 0.85 (0.66-1.08) | 0.96 (0.92-1.00) |
| Model 2 | 0.91 (0.82-1.00) | 1.01 (0.94-1.09) | 1 (Ref) | 0.89 (0.69-1.16) | 0.97 (0.93-1.01) |
| Model 3 | 0.95 (0.85-1.06) | 1.02 (0.95-1.11) | 1 (Ref) | 0.92 (0.71-1.20) | 0.99 (0.94-1.04) |
| Model 4 | 0.95 (0.85-1.06) | 1.02 (0.95-1.11) | 1 (Ref) | 0.92 (0.71-1.20) | 0.99 (0.94-1.04) |
Abbreviations: Atherosclerosis Risk in Communities, ARIC; standard deviation, SD; confidence interval, CI; atrial fibrillation, AF; premature atrial contraction, PAC; supraventricular tachycardia, SVT.
Model 1=age, sex, race-center
Model 2=Model 1+educational attaimnent, smoking status, drinking status, physical activity, magnesium or calcium supplement use, body mass index
Model 3=Model 2+diabetes, systolic and diastolic blood pressure, antihypertensive medication use
Model 4=Model 3+serum potassium
PAC and SVT burden were log-transformed due to right-skew distribution. The ratio of geometric mean refers to the exponentiated beta coefficient from linear regression, e.g., exp(β<0.75 vs 0.85-0.95 mmol/L)· An example interpretation, in Model 1, the geometric mean PAC burden among individuals with serum Mg <0.75 mmol/L was 1.20 fold the geometric mean PAC burden among those with serum Mg 0.85-0.95 mmol/L.
Excluding those with AF detected on the ECG patch monitor or AF identified by prior ARIC ascertainment
Effect estimates for intermittent versus no AF were in the hypothesized direction but smaller in magnitude than for continuous AF (Table 2). No statistically significant associations were observed for serum Mg when modeled categorically or continuously in relation to intermittent versus no AF. Among those with no AF, we also found little evidence of an association of serum Mg with either PAC burden or SVT burden.
Across all models for the association between serum Mg and ventricular arrhythmias, there was a monotonically inverse association between serum Mg categories in relation to PVC burden (Table 3). Similarly, when Mg was modeled continuously, each standard deviation increment in our sample was associated with a lower PVC burden. There was no evidence of an association between serum Mg and the presence of NSVT.
Table 3.
Associations of serum magnesium with ventricular arrhythmias: the ARIC study, 2016-2017†
| Serum magnesium, mmol/L | |||||
|---|---|---|---|---|---|
| <0.75 | 0.75-<0.85 | 0.85-<0.95 | ≥0.95 | Per 0.1 mmol/L decrement | |
| N | 478 | 986 | 1004 | 45 | |
| OR (95% CI) | |||||
| NSVT (yes/no) | |||||
| NSVTN | 150 | 311 | 281 | 13 | |
| Model 1 | 1.21 (0.95-1.54) | 1.18 (0.97-1.43) | 1 (Ref) | 1.09 (0.55-2.13) | 1.07 (0.96-1.19) |
| Model 2 | 1.14 (0.87-1.48) | 1.17 (0.95-1.44) | 1 (Ref) | 1.07 (0.52-2.23) | 1.06 (0.94-1.19) |
| Model 3 | 1.09 (0.82-1.44) | 1.16 (0.94-1.43) | 1 (Ref) | 1.09 (0.52-2.27) | 1.05 (0.92-1.18) |
| Model 4 | 1.08 (0.82-1.44) | 1.16 (0.94-1.43) | 1 (Ref) | 1.08 (0.52-2.26) | 1.04 (0.92-1.18) |
| Ratio of Geometric Means (95% CI)‡ | |||||
| Isolated PVC burden | |||||
| Geometric Mean | 94.6 | 68.6 | 64.6 | 44.3 | |
| Model 1 | 1.54 (1.20-1.97) | 1.05 (0.86-1.28) | 1 (Ref) | 0.72 (0.37-1.40) | 1.22 (1.10-1.36) |
| Model 2 | 1.40 (1.08-1.82) | 1.01 (0.82-1.24) | 1 (Ref) | 0.73 (0.35-1.49) | 1.17 (1.04-1.31) |
| Model 3 | 1.56 (1.18-2.06) | 1.04 (0.84-1.28) | 1 (Ref) | 0.74 (0.36-1.51) | 1.23 (1.09-1.39) |
| Model 4 | 1.53 (1.16-2.03) | 1.03 (0.83-1.27) | 1 (Ref) | 0.75 (0.36-1.53) | 1.22 (1.08-1.38) |
Abbreviations: Atherosclerosis Risk in Communities, ARIC; standard deviation, SD; confidence interval, CI; non-sustained ventricular tachycardia, NSVT; premature ventricular contraction, PVC.
Model 1=age, sex, race-center
Model 2=Model 1+educational attainment, smoking status, drinking status, physical activity, magnesium or calcium supplement use, body mass index
Model 3=Model 2+diabetes, systolic and diastolic blood pressure, antihypertensive medication use
Model 4=Model 3+serum potassium
PVC burden was log-transfonned due to right-skew distribution. The ratio of geometric mean refers to the exponentiated beta coefficient from linear regression, e.g., exp(β<0.75 vs 0.85-0.95 mmol/L). An example interpretation, in Model 1, the geometric mean PVC burden among individuals with serum Mg <0.75 mmol/L was 1.54 fold higher than the geometric mean PAC burden among those with serum Mg 0.85-0.95 mmol/L.
In sensitivity analyses, the non-statistically significant estimates for serum Mg and atrial arrhythmias were similar after we excluded participants taking anti-arrhythmic medications (Supplemental Table 1a), participants taking ACEi, ARB, and diuretics (Supplemental Table 1b), as well as participants taking PPIs (Supplemental Table 1c). The association was also similar when examined among those without a history of CVD (Supplemental Table 2). A similar set of sensitivity analyses were conducted for ventricular arrhythmias outcomes as shown in Supplemental Tables 3–4, and these results were similar to those in the main analyses.
DISCUSSION
In this community-based study of older adults, we found that higher serum Mg was cross-sectionally associated with a lower burden of PVCs based on 2-week ambulatory ECG monitoring. We also found that participants with low Mg concentrations were more likely to have continuous and intermittent AF with adjustment for demographics; however, this association was attenuated and no longer statistically significant with further adjustment for lifestyle and CVD risk factors. These findings were similar even among those without a history of CVD.
Magnesium & Atrial Arrhythmias
The association between serum Mg and atrial arrhythmia burden has not been fully characterized; evaluation of this relationship – with richer phenotyping of arrhythmia indices – may provide etiologic context for prior studies which have examined Mg and clinically recognized AF (as a binary outcome). Three prospective observational studies, including a prior ARIC publication,18 have documented associations between low serum Mg and an increased risk of developing AF.18–20 In ARIC, serum Mg was examined in relation to incident AF (hospital discharge, study visit ECGs and death certificates). Those in the lowest serum Mg quintile (<0.78 mmol/L) had a hazard ratio (HR) of 1.34 (95% CI: 1.16-1.54) compared to those in the middle quintile (0.80-0.83 mmol/L) after multivariable adjustment.18 At ARIC visit 5 (2011-2013), in a pilot study, a subset of participants were invited to wear a Zio® patch monitor for up to 2 weeks. We found that concentrations of serum Mg (and other electrolytes) were not robustly associated with PAC or SVT burden. Notably, the prior analysis was based on only n~300 participants.21 In the Framingham Offspring study, participants in the lowest serum Mg quartile (≤0.73 mmol/L) had a higher AF risk compared to participants in the highest quartile (≥0.82 mmol/L) [HR=1.52 (95% CI: 1.00-2.31)].19 In an Israeli HMO, both mild and moderate hypomagnesemia were associated with higher AF risk over a follow-up period of about 2 years but not with AF risk over a short-term (3-month) followup.20 However, outcomes in these studies were based on clinically recognized AF and/or shorter term ECG monitoring (e.g. 12-lead ECG), which might not capture those with intermittent AF episodes.22,23 Additionally, an experimental feeding study lends support to these epidemiologic findings. Of 14 healthy women who were fed an extremely low diet in Mg, 3 of the women developed AF. Their AF resolved quickly after Mg repletion.24 Furthermore, intravenous (high dose) Mg is used in the context of cardiac surgery to prevent post-operative AF.25
The pathophysiology linking circulating Mg and supraventricular arrhythmias is uncertain. Mg is involved in hundreds of enzymatic reactions throughout the body.26 Ionic flow of Mg, as well as calcium and potassium, are important for generating action potentials and maintaining the membrane potential of cardiac cells.27 Mg is also considered a natural calcium antagonist, as Mg competes with calcium for membrane–binding sites to the L-type Ca2+ current.28,29 Oxidative stress is another potential explanation. In animal studies, Mg has been shown to exert antioxidant effects,30 and oxidative stress is thought to perpetuate AF.31 Additionally, it is possible that low circulating Mg could act through known AF risk factors (namely hypertension32,33 or diabetes33,34) to promote arrhythmogenesis; hence, accounting for these risk factors would attenuate the association of interest (as occurred in our analyses). The lack of a robust association in the present analysis is rather surprising, but may be related to the agedness of our study population when they wore the Zio® XT Patch. Prospective evaluations of Mg and AF began when participants were middle-aged; the present study population was on average 79 years old.
Magnesium & Ventricular Arrhythmias
There has been little characterization of the associations of serum Mg concentrations and burden of ventricular arrhythmias in the community. Prior studies have been based on shorter term ECG monitoring. For instance, a small oral Mg supplement RCT reported decreased PVC intensity on a 24-hour Holter monitor among patients without prior cardiac diseases.35 In the Framingham Offspring Study, each SD (0.08 mmol/L) decrement was associated with greater odds of having a PVC identified over 1 hour ECG monitoring.36
Aside from the aforementioned studies, much of the research on Mg and ventricular arrhythmias has been conducted in populations with existing medical conditions (e.g. congestive HF, MI, diabetes), or based on the presence or absence of ventricular arrhythmias. For example, among 750 obese Canadian participants with type 2 diabetes, participants with serum Mg ≤0.70 mmol/L had a 2.5-fold higher prevalence of PVC—as measured using a Holter monitor—than those with serum Mg >0.70 mmol/L (50% vs 21%).37 This is similar to our findings, among individuals either with or without prior cardiometabolic disease, as detected over 2-weeks ECG monitoring.
Intravenous Mg is commonly used to manage torsade de pointes, a type of ventricular arrhythmia, in the setting of long QT-interval syndrome.38 However, similar to atrial arrhythmias, potential mechanisms underlying the association between low Mg and ventricular arrhythmia burden are not fully understood. There is some overlap in the potential pathophysiologic mechanisms of circulating Mg to both atrial and ventricular arrhythmia burden, as described in the previous section. Specific to ventricular arrhythmias, intravenous Mg administration may lead to suppression of ventricular ectopic activity.29 Mg serves as a cofactor to Na/K ATPase, which, during the action potential, acts to aid transport of potassium into cardiac cells. When cellular Mg is deficient, this results in a less efficient Na/K ATPase system and influences the membrane potential.7,26 As such, it is possible that QT-interval prolongation could arise, and could promote abnormal ventricular rhythm.26
Strengths & Limitations
There are limitations to the present study. First, given the small number of participants with continuous or intermittent AF, precision was limited to detect an association (if one truly exists). Second, as this analysis was cross-sectional the temporality of the association cannot be established, particularly considering the complexity of Mg homeostasis and cardiac electrophysiology. Third, residual confounding is another possibility given the observational nature of our study. It is plausible that those with arrhythmias are sicker and have other confounding characteristics shared by those with low circulating Mg. ECG abnormalities also may not be specific to Mg. For example, abnormal Mg homeostasis may coexist with (and/or exacerbate) other electrolyte abnormalities, particularly calcium and potassium, which themselves are known to be involved in cardiac electrophysiology.39 While we adjusted for serum potassium and numerous other covariates, serum calcium was not measured at visit 6. Therefore, we were neither able to adjust for serum calcium nor evaluate associations of the serum calcium-Mg ratio with arrhythmias. Fourth, late-life may not be the optimal time to characterize associations between Mg and arrhythmias, as the association may differ at different ages. For example, serum Mg may be a more robust risk factor for arrhythmia burden in younger adults, as opposed to older adults such as the population studied herein (mean age 79 years). However, it is important to examine this relationship as older adults are at high-risk for both arrhythmias and Mg deficiency. Lastly, like virtually all studies of Mg and arrhythmias, we did not measure ionized Mg, which may be the more physiologically relevant form of circulating Mg.40 Nevertheless, there are important strengths to these findings. Major strengths of this study were the community-based population and the rigorous characterization of arrhythmia burden using a novel ECG monitor worn for up to 2-weeks.
Conclusions
In conclusion, we found that low serum Mg was associated with greater PVC burden as measured over 2-weeks of ECG monitoring. We found little evidence of a cross-sectional association between serum Mg and atrial arrhythmias in this older community-dwelling population. Future research should test whether serum Mg may be a more robust risk factor for arrhythmia burden in younger adults and further explore the possible Mg-PVC association prospectively.
Supplementary Material
Acknowledgements:
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding:
The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). This work was also supported by grants from the NIH/NHLBI [R01HL126637-01A1 (LYC), T32HL007779 (MRR), T32HL007024 (MRR), K24HL148521 (AA)] and by American Heart Association grant 16EIA26410001 (AA). Dr. Selvin was supported by NIH/NIDDK grant K24DK106414. Reagents for magnesium and potassium were donated by Roche Diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Tweet: “Serum Magnesium and Burden of Atrial and Ventricular Arrhythmias in the Atherosclerosis Risk in Communities (ARIC) Study #JECG”
References
- 1.Swaminathan R Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24(2):47–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem. 1991;28(Pt 1): 19–26. [DOI] [PubMed] [Google Scholar]
- 3.Costello RB, Elin RI, Rosanoff A, et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv Nutr. 2016;7(6):977–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryrd-Bredbenner C, Moe G, Beming I, Kelley D. Wardlaws Perspectives in Nutrition Updated with 2015 2020 Dietary Guidelines for Americans. 10 ed. New York NY: McGraw-Hill Education; 2016. [Google Scholar]
- 5.Ross AC. Modern nutrition in health and disease. 11th ed ed. Philadelphia: Kluwer Health/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 6.Chen LY, Chung MK, Allen LA, et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstratiadis G, Sarigianni M, Gougourelas I. Hypomagnesemia and cardiovascular system. Hippokratia. 2006;10(4): 147–152. [PMC free article] [PubMed] [Google Scholar]
- 8.Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis. 2013;56(2):224–229. [DOI] [PubMed] [Google Scholar]
- 9.Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol. 2015;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal S, Movsowitz C, Steinberg JS. Ambulatory External Electrocardiographic Monitoring: Focus on Atrial Fibrillation. Journal of the American College of Cardiology. 2011;58(17): 1741–1749. [DOI] [PubMed] [Google Scholar]
- 11.Heckbert SR, Austin TR, Jensen PN, et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. Journal of Electrocardiology. 2018;51(6):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 13.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1): 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. [DOI] [PubMed] [Google Scholar]
- 15.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2): 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. [DOI] [PubMed] [Google Scholar]
- 18.Misialek JR, Lopez FL, Lutsey PL, et al. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans--Atherosclerosis Risk in Communities (ARIC) study. Circ J. 2013;77(2):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markovits N, Kurnik D, Halkin H, et al. Database evaluation of the association between serum magnesium levels and the risk of atrial fibrillation in the community. International journal of cardiology. 2016;205:142–146. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A, Rooney MR, Chen LY, et al. Circulating electrolytes and the prevalence of atrial fibrillation and supraventricular ectopy: The Atherosclerosis Risk in Communities (ARIC) study. Nutrition, Metabolism and Cardiovascular Diseases. 2020;30(7): 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. International journal of cardiology. 2013;167(5): 1807–1824. [DOI] [PubMed] [Google Scholar]
- 23.Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. Journal of the American College of Cardiology. 2011;58(17):1741–1749. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr. 2007;26(2): 121–132. [DOI] [PubMed] [Google Scholar]
- 25.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database of Systematic Reviews. 2013(1):CD003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238(l-2): 163–179. [DOI] [PubMed] [Google Scholar]
- 27.White RE, Hartzell HC. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem Pharmacol. 1989;38(6):859–867. [DOI] [PubMed] [Google Scholar]
- 28.El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011; 18(3):233–245. [PubMed] [Google Scholar]
- 29.Ho KM. Intravenous magnesium for cardiac arrhythmias: jack of all trades. Magnes Res. 2008;21(l):65–68. [PubMed] [Google Scholar]
- 30.Liu M, Jeong E-M, Liu H, et al. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight. 2019;4( 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sovari AA, Dudley SC Jr. Reactive oxygen species-targeted therapeutic interventions for atrial fibrillation. Front Physiol. 2012;3:311–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9. [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48(7):927–940. [DOI] [PubMed] [Google Scholar]
- 34.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999; 159( 18):2151–2159. [DOI] [PubMed] [Google Scholar]
- 35.Falco CN, Grupi C, Sosa E, et al. Successful improvement of frequency and symptoms of premature complexes after oral magnesium administration. Arq Bras Cardiol. 2012;98(6):480–487. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji H, Venditti FJ Jr., Evans JC, Larson MG, Levy D. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study). Am J Cardiol. 1994;74(3):232–235. [DOI] [PubMed] [Google Scholar]
- 37.Del Gobbo LC, Song Y, Poirier P, Dewailly E, Elin RJ, Egeland GM. Low serum magnesium concentrations are associated with a high prevalence of premature ventricular complexes in obese adults with type 2 diabetes. Cardiovasc Diabetol. 2012; 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Journal of the American College of Cardiology. 2006;48(5):e247–346. [DOI] [PubMed] [Google Scholar]
- 39.Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2): 153–160. [DOI] [PubMed] [Google Scholar]
- 40.Costello RB, Nielsen F. Interpreting magnesium status to enhance clinical care: key indicators. Curr Opin Clin Nutr Metab Care. 2017;20(6):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


