Abstract
Motor challenges are commonly reported in Autism Spectrum Disorder (ASD). Yet, there is substantial heterogeneity in motor ability within ASD, and it is unknown what behavioral characteristics best explain individual differences in motor ability in ASD and related conditions. This observational study examined motor ability as a function of sensory features, attention deficit/hyperactivity symptoms, ASD symptoms, and IQ in 110 children with ASD, typical development, or an intermediate behavioral profile. While motor challenges were more prevalent in the ASD group compared to other groups, sensory symptom severity and IQ across all individuals best predicted motor performance above-and-beyond group status. Therefore, motor challenges may be best characterized by individual variation in sensory features and cognitive abilities rather than diagnostic group.
Keywords: motor, sensory, cognition, autism spectrum disorder
Motor challenges have historically been associated with autism spectrum disorder (ASD) (Kanner 1943) and have been demonstrated in a variety of domains, including balance, postural stability, grip strength, movement planning, gross motor coordination, and fine motor coordination (Ament et al. 2015; Fournier et al. 2010; Travers et al. 2013, 2017). Yet, within these domains there is substantial heterogeneity in motor performance among members of the ASD group (Green et al. 2009; Kopp et al. 2010), and it is unclear what factors underlie this heterogeneity. If we knew the sources of this heterogeneity, we could better understand the nature of motor impairments in ASD in a way that could potentially optimize intervention.
Further, motor challenges are not unique to ASD and have been reported in individuals with conditions such as attention deficit/hyperactivity disorder (ADHD) (Biscaldi et al. 2015) and in first-degree relatives of those with ASD (Mosconi et al. 2010; Wu et al. 2018). While the nature of these motor challenges may be different in ASD compared to ADHD (Mostofsky et al. 2003; MacNeil and Mostofsky 2012), the within-group heterogeneity and the prevalence of motor challenges across ASD and other clinical and subclinical populations raise the question of whether motor challenges in ASD are more accurately represented at the diagnostic group level or at the individual level. Indeed, the majority of the research to date has implemented case-control designs that only have the ability to detect group-level differences between a population of interest and a control group. However, these group-level designs are in stark contrast to clinical best practice in occupational and physical therapy, in which motor challenges are individually assessed and treated in all children with motor difficulties, regardless of whether they have an ASD diagnosis (Campbell 2013; Schaaf 2015). Therefore, the purpose of the present study was to perform and compare both a group-difference analysis and an individual-differences analysis of motor challenges in children with ASD, children with typical development, and children in an intermediate group, comprised of children with diagnoses or first-degree relatives with diagnoses that have been shown to be genetically associated with ASD (Carroll and Owen 2009; Lionel et al. 2011). This intermediate group was selected to represent children who are often excluded from traditional case-control research in ASD but who might represent an intermediate behavioral phenotype between ASD and typical development. Specifically, we sought to determine which combination of behavioral characteristics best predict motor challenges and whether an individual-differences approach would out perform a group-differences approach (Fig 1).
Fig. 1.
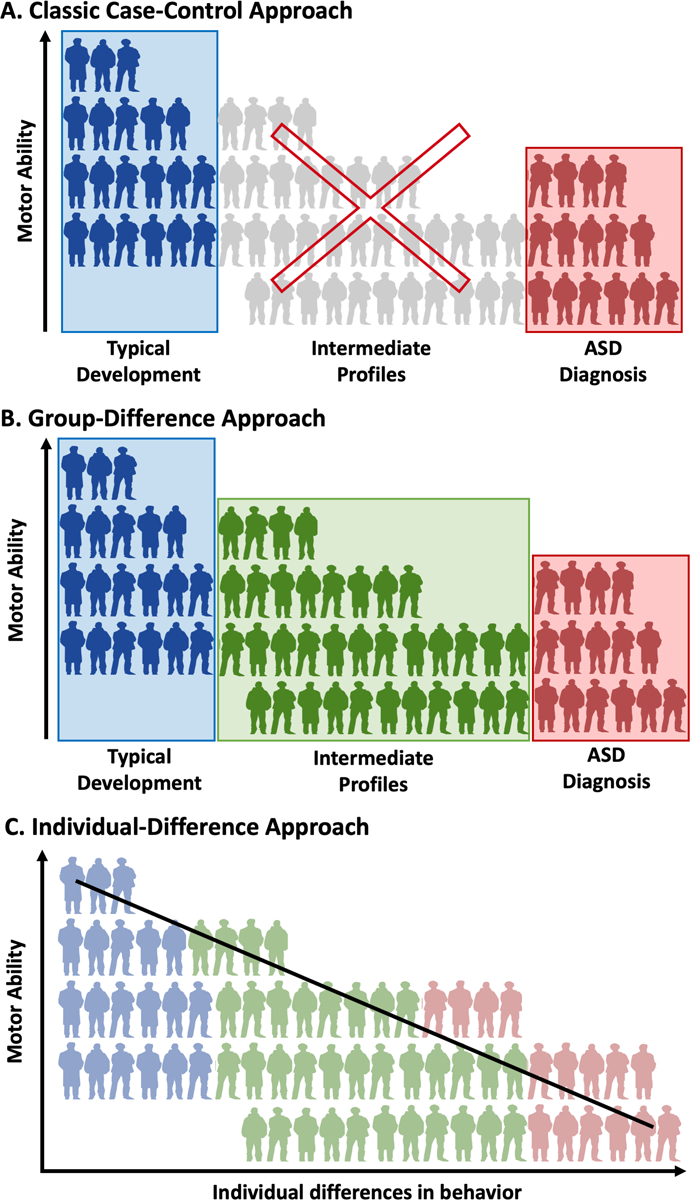
Conceptual representation of the data analysis approaches used to analyze the present data. Blue, green, and red figures represent members of the TD, INT, and ASD groups respectively, that are included in the different analytical designs. (A) The classical case-control model of data analysis that seeks to compare average motor ability between TD and ASD groups and excludes individuals with intermediate behavioral phenotypes or with conditions that are associated with ASD. (B) The group-difference approach which uses group averages to examine motor ability. Unlike the case-control approach, the group-difference approach includes a group of individuals with intermediate behavioral phenotypes or conditions that are associated with ASD. (C) The individual-difference approach seeks to analyze motor ability in relation to specific behavioral parameters across the entire sample. While group status remains, this approach does not group into account but instead assesses individual differences in behavioral characteristics.
A high degree of individual variability in motor ability is present across the autism spectrum ranging from mild to severe motor deficits (Green et al. 2009; Kopp et al. 2010), such that some individuals with ASD may experience few motor challenges, while others may experience severe motor challenges (as can be seen in data from Kopp et al. 2010). Some motor challenges in ASD may be so severe as to warrant a co-occurring diagnosis of Developmental Coordination Disorder (DCD; Van Waelvelde et al. 2010), a condition characterized by delays to reach early motor milestones and difficulties with coordination of either gross or fine motor skills to the point of interference with activities of daily living (American Psychiatric Association 2013). Based upon the within-group heterogeneity, it appears important to examine motor ability at an individual level as well as at a group level. However, the scientific community has most often employed classic case-control designs (Fig 1A) to understand motor function in ASD (e.g. Biscaldi et al. 2015; Hilton et al. 2012; Pan et al. 2009). While case-control designs elucidate group-averaged differences between ASD and typically developing individuals, they may not effectively convey the heterogeneity of motor challenges within and beyond ASD. Although case-control designs are most common, the nature of motor challenges in individuals with ASD may be most clearly elucidated by using group-difference designs (Fig 1B) (i.e., research designs that include other populations in addition to a typically-developing comparison group when comparing motor ability) and/or individual-difference designs (Fig 1C) (i.e., research designs that cross-diagnostically assess motor ability along a continuum). Individual-difference approaches, in particular, may be powerful in that they align with clinical best practices (Campbell 2013; Schaaf 2015) and with the Research Domain Criteria (RDoC) framework that focuses on using continuously measured variables to understand key behavioral domains across diagnostic groups (Insel et al. 2010).
However, one challenge to using an individual-difference approach is determining which continuously measured characteristics, of many potential candidates, may be the most inter-related with motor performance. Current literature suggests that a variety of behavioral characteristics may be related to motor ability including ASD symptomatology, ADHD symptomatology, cognitive ability, and sensory symptom severity. In terms of ASD symptomatology, several investigations utilizing between-group analyses have shown that children with ASD have poorer motor ability than typically developing peers (Fournier et al. 2010; Fulceri et al. 2019). Additionally, several studies using an individual-differences approach within a group with ASD have demonstrated a relationship between motor ability and core ASD symptoms, such that more severe ASD symptoms correlated with more severe motor impairment (Biffi et al. 2018; Hilton et al. 2012; Radonovich et al. 2013; Travers et al. 2013, 2015). In terms of ADHD symptom severity, the prevalence of motor challenges in ADHD (Blondis 1999; Buderath et al. 2009; Harvey and Reid 2003) suggests that core ADHD symptoms may be associated with motor challenges, although we are unaware of any studies that have looked at this on a continuum. In addition, similarities in motor performance between children with ASD and ADHD have been identified (Biscaldi et al. 2015; Pan et al. 2009).
In terms of cognition, lower IQ has also been associated with more severe motor difficulties in children with ASD (Green et al. 2009; Hilton et al. 2012; Kopp et al. 2010) and their siblings (Green et al. 2009). Further, youth with ASD with lower IQ demonstrated more postural sway on unsteady surfaces than those with higher IQ, suggesting that motor performance may result from an interaction between IQ and task difficulty (Travers et al. 2018). Sensory symptom severity also likely plays a role in motor ability. Not only is there a wealth of neurobiological evidence that link sensory and motor systems (Donoghue 1995; Kaas 2008; Lungarella and Sporns 2006), but both impaired sensory processing and increased variability in sensory input have been linked to poorer motor ability in ASD (Gowen and Hamilton 2013; Liu 2013). While the literature cumulatively suggests links between motor ability and a variety of behavioral characteristics, it is important to note that the majority of studies have employed either a group-difference design to assess average motor ability between populations (Biscaldi et al. 2015; Buderath et al. 2009; Pan et al. 2009) or have used a modified individual-difference design where continuous measures were assessed within a specific clinical population (Fulceri et al. 2019; Green et al. 2009; Liu 2013; Travers et al. 2018; Van Waelvelde et al. 2010; Yu et al. 2018). And while these analyses have indicated relationships between specific behavioral characteristics and motor ability, none have been able to determine which combination of behavioral characteristics best predicts motor ability, nor have they done so across both the ASD population and the population of individuals with conditions or family histories that have been previously found to be genetically associated with ASD. Including a group with a high genetic overlap with ASD (i.e., children with ADHD [Lionel et al. 2011], children with a family history of ASD, schizophrenia, and bipolar disorder [Carroll and Owen 2009]) would likely provide an intermediate motor phenotype and would allow for the dimensional exploration of motor challenges across a spectrum of ability. Therefore, the present study set out to fill these key gaps, using both a group-difference approach to align with prior research and an individual-difference approach to examine behavioral characteristics that best predict motor ability.
Objectives
The first objective of the present study was to characterize group-level differences in motor ability among three different groups: the group with ASD, the group with typical development (TD), and the intermediate group (INT) comprised of individuals with conditions or a family history previously demonstrated to be associated with ASD (Fig 1B). We extended this line of research to also examine the percent of children with more severe motor challenges and the heterogeneity of motor performance within each group. We hypothesized that the ASD, INT, and TD groups would each show differing levels of motor ability, with the ASD group having the most severe motor impairment and the typically developing group having the least severe motor impairment. We also anticipated a great deal of heterogeneity in motor ability within each group but particularly in the ASD and INT groups.
The second objective of the study was to investigate motor ability on a continuum by identifying which combination of behavioral characteristics selected from the literature was most associated with motor ability across children, regardless of diagnosis. This individual-differences approach allowed for more specificity by assessing individual differences rather than group averages (Fig 1C). We hypothesized that this RDoC-aligned (Insel 2010) approach would provide a combination of behavioral characteristics that predicted motor ability in children across the population. Based on the previous literature, we hypothesized that some combination of more severe ASD symptoms, lower IQ, more severe sensory symptoms, and more severe inattention and hyperactivity symptoms associated with ADHD would predict motor impairment.
The third objective of the study was to determine if behavioral characteristics were better predictors of motor ability then diagnostic group by testing if the individual-difference approach out-performed the group-difference approach in predicting motor ability. Given the anticipated heterogeneity within the ASD and INT groups, we hypothesized that the individual-differences approach would more robustly predict motor ability than the group-difference approach.
Methods
Participants
Participants ages 6–10 years were assigned to one of three potential groups: ASD, intermediate (INT), or typically developing (TD). Criteria for ASD, INT, and TD group membership was predefined based upon family history, diagnostic status, and scores on the ADOS-2, SRS, and SCQ (see Online Resource 1 for a diagnostic decision tree). All participants completed the ADOS-2, SRS, and SCQ. Record review by the principal investigator and a licensed clinical psychologist who specializes in autism diagnosis was done for each participant in order to determine final group status. None of the participants had a history of severe head injury or a diagnosis of intellectual disability, tuberous sclerosis, or fragile X.
Participants in the TD group were required to meet a stringent cutoff of 8 on the Social Communication Questionnaire (SCQ; Rutter et al. 2003) and did not have an existing diagnosis of ASD or ADHD. Participants qualified for ASD group membership by either 1) meeting ASD cutoffs on the Autism Diagnostic Observation Scale, 2nd Edition (ADOS-2; cutoff = 8; Lord et al. 2012) or 2) meeting ASD cutoffs on both the Social Responsiveness Scale, second edition (SRS-2; cutoff = 60; Constantino and Gruber 2012) and the SCQ (cutoff = 15; Rutter et al. 2003). Similar to the ASD and TD groups, the INT group was behaviorally defined. Participants in the INT group met at least one of the following criteria: 1) reported having an existing diagnosis of ADHD or 2) had a first-degree relative with a diagnosis of ASD, bipolar disorder, major depressive disorder, or schizophrenia. The breadth of neuropsychiatric disorders included in the INT group was based upon emerging research that has shown similarities in the genetic makeup of individuals with ASD, schizophrenia, and bipolar disorder (Carroll and Owen 2009; Guan et al. 2019). Additionally, research has indicated similarities in the genes that are thought to increase the risk of ADHD and those thought to increase the risk of ASD (Lionel et al. 2011).
Measures
Bruininks-Oseretsky Test of Motor Proficiency, 2nd Edition Short Form (BOT-2 SF; Bruininks and Bruininks 2005).
The BOT-2 SF was used to assess motor profiles of all participants. This standardized observational assessment measures stability, mobility, strength, coordination and object manipulation. Specifically, analyses included all items from the BOT-2 SF, which have been previously validated (Bruininks and Bruininks 2005). The administration of the BOT-2 SF takes approximately 20–25 minutes and consists of 14 tasks, taken from the eight subtests of the full BOT-2 (fine motor precision, fine motor integration, manual dexterity, bilateral coordination, balance, running speed and agility, upper-limb coordination, and strength). While this measure assesses both gross and fine motor skills, it produces one single score of motor proficiency, where higher scores indicate better motor ability. All scores from the BOT-2 SF are standardized for age and sex. The BOT-2 SF has strong internal consistency reliability (.82−.87), test-retest reliability (.80−.87), and interrater reliability (.98). The BOT-2 SF has also been used in initial evaluations for DCD where potential DCD is marked by a standard score at or below 35.5 (Bruininks and Bruininks 2005). BOT-2 SF standard scores were used in all analyses, with higher scores indicating better motor ability.
Sensory Experiences Questionnaire Version 3.0 (SEQ-3.0; Baranek 2009).
The Sensory Experiences Questionnaire 3.0 (SEQ-3.0) is a parent report measure used to characterize sensory features in children 2–12 years of age. The assessment has been validated for use in children with typical development, ASD, and other developmental disabilities and evaluates sensory experience in both social and non-social contexts (Baranek 2009). This questionnaire contains items that measure the frequency of atypical sensory behaviors across four key sensory patterns (hyporesponsiveness, hyperresponsiveness, repetitions and seeking behaviors, and enhanced perception) and all sensory modalities (auditory, visual, tactile, gustatory/olfactory, and vestibular/proprioceptive; Ausderau et al. 2016). Sensory features were evaluated using the 97 quantitative items on the SEQ-3.0 that utilize a 5-point Likert scale which ranges from 1–5 (never/almost never to always/almost always) with higher scores indicating more severe sensory symptoms. Analyses were done using a composite sensory severity score that was calculated using the summed total score from each sensory pattern.
National Initiative for Children’s Healthcare Quality Vanderbilt Assessment Scales (NICHQ VAS; American Academy of Pediatrics & National Institute for Children’s Health Quality 2002).
The NICHQ VAS was used as a continuous measure of ADHD symptom severity. The NICHQ VAS has two components: symptom assessment and impairment in performance (American Academy of Pediatrics & National Institute for Children’s Health Quality 2002). Analyses were performed using the symptom assessment items of the NICHQ VAS which measures the inattentive and hyperactive behaviors which characterize ADHD (sum of items 1–18). This total score (sum of items 1–18) was used in all analyses, with higher values indicating more prominent ADHD symptoms.
Social Responsiveness Scale, second edition (SRS-2; Constantino and Gruber 2012).
The SRS-2 is a 65-item parent report measure that examines the presence of ASD symptoms in the past six months. Observed reciprocal social behaviors are rated on a scale from “0” (never true) to “4” (almost always true). The SRS-2 has been validated for clinical populations age 4–18 years. Total raw scores from the SRS-2 were used in all analyses, with higher values indicating greater severity of ASD symptoms.
Wechsler Abbreviated Scale of Intelligence-2nd Edition, 2 Subtest (WASI-II; Wechsler and Hsiao-pin 2011).
The WASI-II, 2 Subtest is an abbreviated measure of intelligence designed for individuals aged 6–90 years old which approximates general intellectual ability. Performance on the Vocabulary and Matrix Reasoning subtests of the WASI-II were used to calculate a two-subtest Full-Scale IQ (FSIQ-2) composite score. The reliability coefficients for the Vocabulary and Matrix Reasoning subtests, were high, ranging from 0.86–0.94 in children and the average reliability coefficient for FSIQ-2 is 0.93 for children. The validity of the WASI-II is highly consistent with both the IQ scores of the Wechsler Adult Intelligence Scale, 4th edition (WAIS-IV) and the Wechsler Intelligence Scale for Children, 4th edition (WISC-IV). FSIQ-2 composite scores were used in all analyses, with higher scores representing higher IQ.
Data Analysis
All statistical analyses were performed in R version 3.4.1, and statistical significance was set at a level of .05. Prior to running all general linear models, model assumptions of normality, constant variance, and linearity were confirmed using the ‘lmSupport’ package in R (Curtin, 2018) and graphically shown in Online Resource 3. To determine that our sample size was sufficient to detect the effects of interest, post-hoc power analyses for each set of analyses were performed using G*Power 3.1 with an alpha error probability of .05. These analyses demonstrated an estimated power of .99, suggesting substantial power to detect the effects of interest with the current sample size (Online Resource 2).
Group-difference analyses.
To determine group differences in motor performance among the ASD, INT, and TD groups, we performed a linear regression examining BOT-2 SF scores as a function of group status (Fig. 1B). Tukey’s post-hoc analyses analyzed differences between specific groups. To simulate the traditional case-control analysis, follow-up analyses were performed using two groups (ASD and non-ASD; presented in Online Resource 4).
Individual-difference analyses.
Because a variety of behavioral characteristics have been linked to motor skills, we wanted to narrow the search field and determine which combination of behavioral characteristics best predicted motor performance in this sample (Fig. 1C). To achieve this, we first performed model selection by exhaustive search using the ‘leaps’ package from R (Lumley 2017). The leaps package uses linear regression to model all possible combinations of the independent variables and to rank each statistical model in terms of its Bayesian Information Criterion (BIC). The best fitting model had the lowest BIC value. All independent variables were mean-centered to reduce the chance of multi-collinearity and included ASD symptom severity, IQ, ADHD symptom severity, and the sensory symptom severity composite score. While age has been shown to be a predictor of motor ability, it was not included in the model selection because BOT-2 SF scores are standardized for age. The independent variables selected from the model selection procedures were then used in a linear regression model.
Comparing group-difference and individual-difference analyses.
In order to determine whether the individual-difference analysis out-performed the group-difference analysis, we compared the BIC values of the individual-difference and group-difference models. BIC was used to compare the group-difference and individual-difference models as they did not have any independent variables in common. A lower BIC indicated a more parsimonious model that had more explanatory power in predicting BOT-2 SF scores. As an additional, more rigorous test, we created a third combined model that contained the independent variables from the group-difference and individual-difference models. Then, we compared all three models (group-difference, individual-difference, and combined models) using BIC. All assumptions for the models were tested and met.
Results
Participants
In all, 110 children ages 6–10 years-old participated in the study (M = 8.65 years, SD = 1.25, Range 6.02–10.79; 32 female). Thirty-six participants were in the TD group (M = 8.52 years, SD = 1.58, Range 6.02–10.88; 12 female).
Forty-two children met criteria to be in the ASD group (M = 8.66 years, SD = 1.47, Range 6.22–10.97; 8 female). Forty participants entered into the study with an existing diagnosis of ASD. Two participants entered the study as members of the INT group (both had a prior ADHD diagnosis and a first degree relative with ASD) and were moved into the ASD group, as record review suggested that they met ASD cutoffs on the ADOS-2, SRS-2, and SCQ (Online Resource 1).
Thirty-two children were in the behaviorally-defined INT group (M = 8.78, SD = 1.54, Range 6.15–10.97; 12 female). Thirty participants entered the study with an existing diagnosis of ADHD or had a first-degree relative with a diagnosis of ASD, bipolar disorder, major depressive disorder, or schizophrenia. Two participants who originally entered into the study in the TD group, were considered to be in the INT group because they did not meet the SCQ cutoff of 8 and had elevated scores on the ADOS-2 and SRS-2. As can be seen in Online Resource 1, 37.5% of those in the INT group were a first-degree relative of someone with ASD and 62.5% of those in the INT group had a parent-reported diagnosis of ADHD.
There were no significant differences in age, F(2,107)=0.24, p=.78, nor sex, χ2 (2, N=110)=3.46, p=.18, across all three groups. The groups, however, were not matched on IQ, F(2,107)=4.59, p=.01, as the ASD group had a significantly lower IQ than the TD group, t(73)=−2.96, p=.004. Table 1 contains means and standard deviations for variables that defined the final sample.
Table 1.
Demographic characteristics for the groups with autism spectrum disorder (ASD), intermediate phenotypes (INT), and typical development (TD). Calculated means and standard deviations (in brackets) reported.
| ASD [n=42] | INT [n=32] | TD [n=36] | |
|---|---|---|---|
| Sex (% female) | 19.0% | 37.5% | 33.3% |
| Age (years) | 8.66[1.47] | 8.78[1.54] | 8.52 [1.58] |
| Range | 6.2–10.9 | 6.2–10.9 | 6.0–10.8 |
| WASI-2, 2 Subtest | 102 [17] | 109 [14] | 112 [12] |
| Range | 62 – 131 | 88 – 142 | 91 – 136 |
| ADOS-2 | 11.8 [4.6] | 5.5 [5.6] | 5.2 [4.6] |
| Range | 3 – 21 | 0 – 18 | 0 – 14 |
| SRS-2 | 96.8 [24.7] | 46 [30] | 21.7 [10.2] |
| Range | 40 – 145 | 5 – 114 | 5 – 47 |
| SCQ | 20.3 [6.8] | 6 [5.4] | 2.1 [1.9] |
| Range | 7 – 33 | 0 – 20 | 0 – 7 |
| NICHQ VAS | 9.93[5.23] | 7.38[5.56] | 1.03[1.89] |
| Range | 2–18 | 0–18 | 0–9 |
Note. WASI-2, Wechsler Abbreviated Scale of Intelligence-Second Edition; ADOS-2, Autism Diagnostic Observation Schedule, Second Edition; SRS-2, Social Responsiveness Scale, Second Edition; SCQ, Social Communication Questionnaire
Group Differences in Motor Performance
Fig. 2 shows the standardized motor performance of participants in the ASD, INT, and TD groups.
Fig. 2.
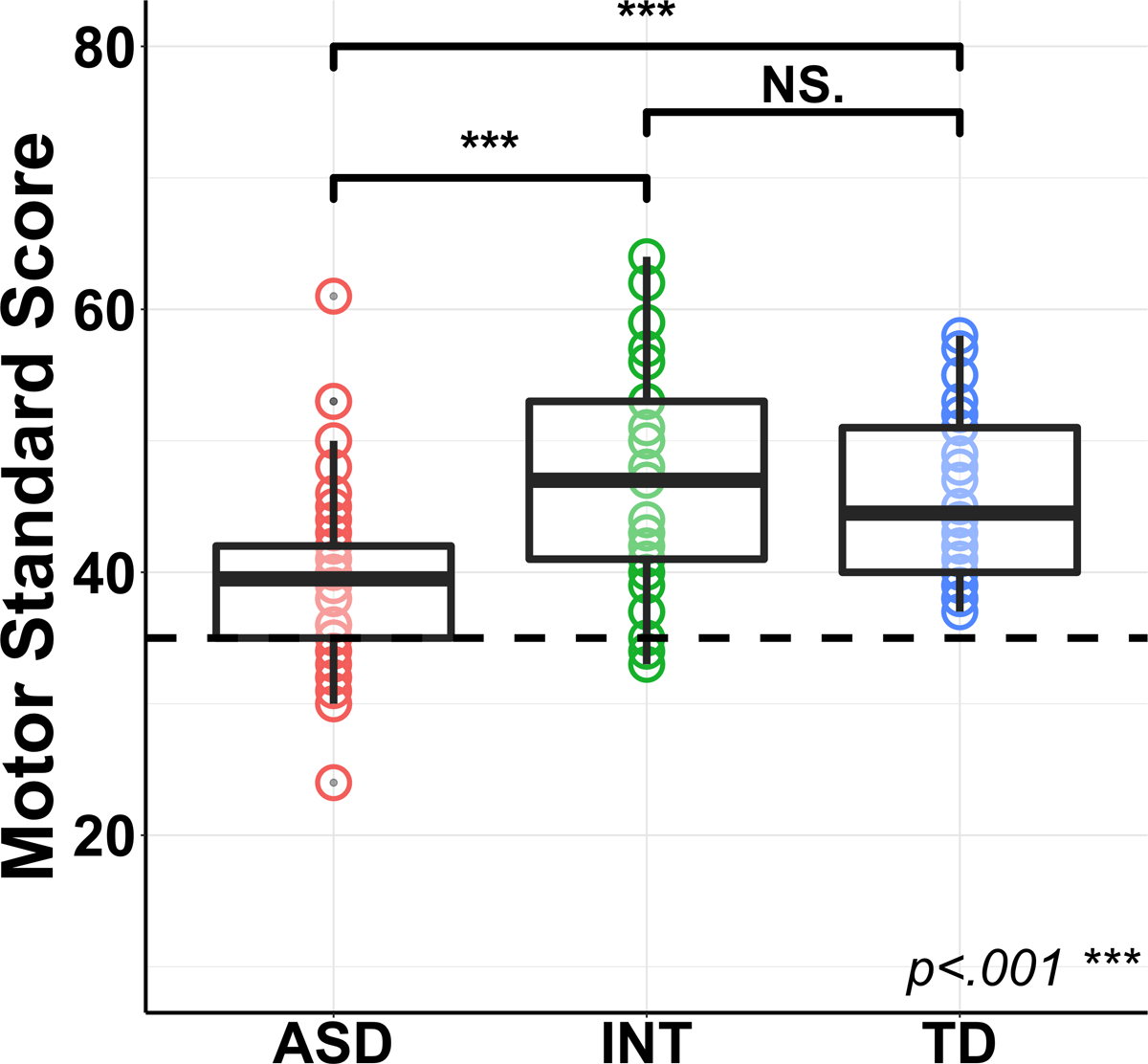
Results of the group difference analytical approach where the box-and-whisker plot with underlying Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2) motor standard scores depicts the differences in motor ability between the ASD, INT, and typically developing (TD) groups. The dashed line indicates the cut-off score for Developmental Coordination Disorder (DCD).
The BOT-2 SF specifies that a standardized score at or below 35.5 is consistent with a diagnosis of DCD (Bruininks and Bruininks 2005). In the ASD group, 24% of participants were below this cutoff, whereas only 6% of participants in the INT group, and no participants in the TD group were below this cutoff. Fig. 2 also demonstrates the variability in motor performance within the ASD and INT groups.
A linear regression model showed group differences in standardized motor performance (Table 2, Model 1).
Table 2.
Regression analysis results from the group difference, individual difference, and combined (individual difference + group difference) analytical approaches.
| Analysis Approach | t | p | β | ηp2 | F | df | p | adj. R2 | BIC | ΔR2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1: Group-Difference | ||||||||||
| Overall Model | 11.17 | 2,107 | <.001 | .16 | 760.5 | 0.17 | ||||
| Intercept | 35.96 | <.001 | 39.64 | |||||||
| Intermediate | 4.28 | <.001 | 7.17 | |||||||
| Typical Development | 3.71 | <.001 | 6.02 | |||||||
| Model 2: Individual-Difference | ||||||||||
| Overall Model | 23.20 | 2,107 | <.001 | .29 | 741.8 | |||||
| Intercept | 69.86 | <.001 | 43.70 | .978 | ||||||
| IQ | 2.86 | <.01 | 0.12 | .071 | 0.05 | |||||
| Sensory Symptom Severity | −4.79 | <.001 | 0.06 | .176 | 0.15 | |||||
| Model 3: Combined (Individual-Difference + Group-Difference) | ||||||||||
| Overall Model | 13.22 | 4,105 | <.001 | .31 | 746.0 | |||||
| Intercept | 33.07 | <.001 | 42.23 | .912 | ||||||
| IQ | 2.91 | <.01 | 0.12 | .053 | 0.05 | |||||
| Sensory Symptom Severity | −3.39 | <.001 | −0.06 | .073 | 0.07 | |||||
| Group | .032 | 0.03 | ||||||||
| Intermediate | 1.38 | .12 | 2.58 | |||||||
| Typical Development | −0.39 | .84 | −0.85 | |||||||
| Model Comparisons | ||||||||||
| Model 1 vs Model 3 | 12.8 | 2,105 | <.001 | 0.16 | ||||||
| Model 2 vs Model 3 | 2.55 | 2,105 | .08 | 0.03 | ||||||
Note. Lower Bayesian Information Criterion (BIC) value is indicative of a preferred model
Tukey’s post-hoc analyses confirmed that the standardized motor scores in the ASD group (M=39.64, SD=7.09) were significantly lower than both the INT group (M=46.81, SD=8.28), b=7.17, t=4.00, p=.00015, and the TD group (M=45.67, SD=6.03), b=6.02, t=4.00, p=.00014, with no significant difference between the INT and TD groups, b=-1.15, t=−0.66, p=.51. Supplemental analyses of this relationship with a two-level diagnostic group parameter (ASD and non-ASD) revealed similar results and are reported in Supplemental Materials (Online Resource 5, Model 1).
Individual Predictors of Motor Profiles across All Participants
Among all the variables tested (i.e., ASD symptom severity [SRS-2], IQ [WASI-II FSIQ-2], ADHD symptom severity [NICHQ-VAS], and the sensory symptom severity composite score [SEQ 3.0]), the model selection procedures found that sensory features and full-scale IQ in combination were the best predictors of motor performance (Online Resource 6).
As can be seen in Fig. 3 and Table 2 (Model 2), more severe sensory symptoms and lower IQ were associated with more motor impairment. Follow-up analyses demonstrated that group status did not significantly moderate the relationship between the predictors and motor ability, however this group moderation effect may be trending toward statistical significance, F(2,101)=2.59, p=0.08 (Online Resource 7). Similarly, continuous measures of ASD symptom severity (i.e., SRS-2 and SCQ) did not significantly moderate the relationship between predictors and motor ability nor did it enhance the statistical model’s predictive power. Addition of or replacement with other factors such as ASD symptoms and/or ADHD symptoms did not lead to stronger statistical models (Online Resource 6).
Fig. 3.
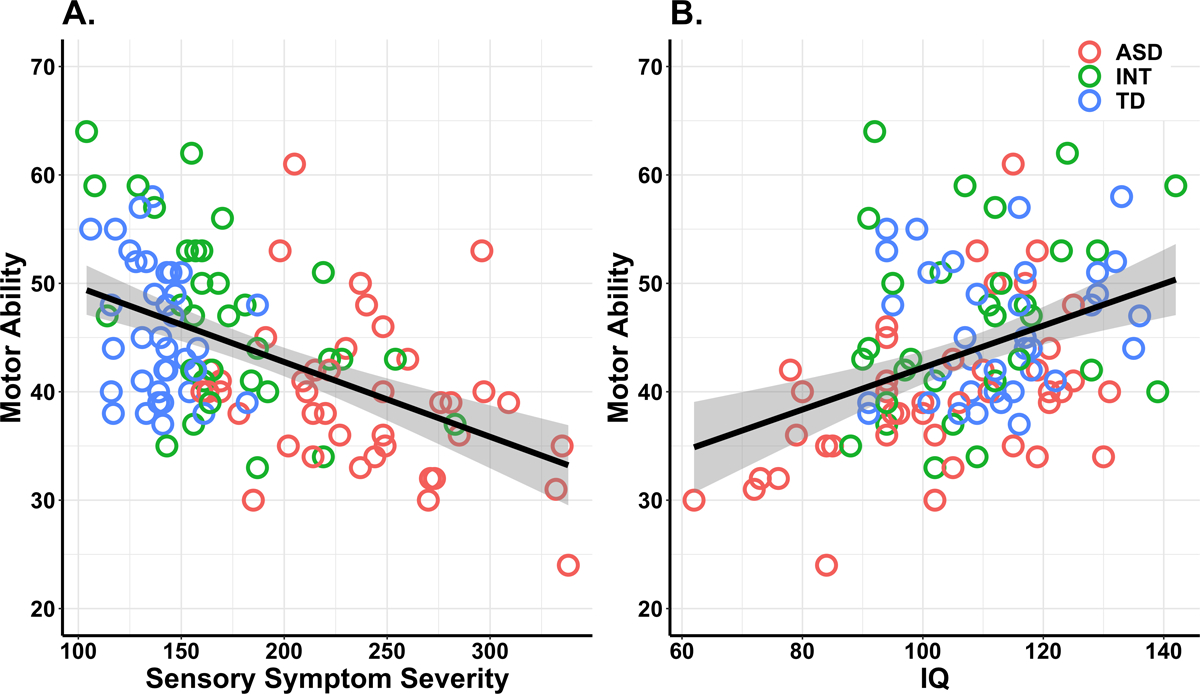
Results of the individual difference analytical approach where scatterplots depict the relationship between motor ability and behavioral characteristics across the ASD, intermediate (INT), and typically developing (TD) groups. (A) More severe sensory symptoms significantly predict poorer motor ability. (B) Higher IQ significantly predicts better motor ability.
Because the sensory composite measure was significantly related to motor performance, follow-up correlations were performed with the sensory experience patterns. Motor scores were associated with severity of all four sensory patterns: hyporesponsivity, r = −0.47, p < .001, hyperresponsivity, r = -0.44, p < .001, seeking behaviors, r = −0.49, p < .001, and enhanced perception, r = −0.31, p < 0.01 (Online Resource 8). This result suggests that the relation between the sensory composite score and motor performance was driven by all four patterns.
Comparison of Individual-Differences and Between-Groups Approaches
In order to determine if the individual-difference approach was able to predict motor ability above-and-beyond the group-difference approach, we compared three different statistical models to predict motor ability: Model 1 was the group-difference model reported above (BOT-2 SF scores as a function of ASD, INT, or TD group status); Model 2 was an individual-difference model reported above (BOT-2 SF scores as a function of sensory symptom severity, and IQ); Model 3 was a more rigorous test, combining the group- and individual-difference approaches (BOT-2 SF scores as a function of sensory symptom severity, IQ, and group status). The results of these analyses can be seen in Table 2. Specifically, the results showed that the individual-difference model (Table 2, Model 2) had the lowest BIC of all three models, suggesting that the individual-difference model was the most predictive and parsimonious of the three models. Supplemental analyses of this relationship with a two-level diagnostic group parameter (ASD and non-ASD) revealed similar results (Online Resource 4).
Discussion
The present study characterized overall motor ability in a large sample of children with ASD, children in an intermediate group (INT) (i.e., having a family history of ASD, schizophrenia, or bipolar disorder, or a current diagnosis of ADHD), and children with typical development using both group-difference and individual-difference approaches. As a group, the children with ASD exhibited poorer motor skills compared to the other two groups without ASD, but there was substantial heterogeneity in motor ability within the groups, especially in the ASD and INT groups. At the individual level, sensory symptom severity and IQ were found to most robustly predict motor performance among all the variables tested, suggestive of an important inter-relationship among sensory, cognitive, and motor domains. Comparison of the group-difference and individual-difference approaches found that the individual-difference approach out-performed the group-difference approach when predicting the severity of motor challenges. As discussed in more detail below, these findings suggest that motor challenges are common in ASD. However, a child’s sensory symptoms and cognitive functioning will more accurately predict the severity of motor challenges rather than diagnosis. These findings have important implications regarding the utility of the classical case-control analyses often used by researchers.
Consistent with previous research (Ament et al. 2015; Fournier et al. 2010; Hilton et al. 2012; Pan et al. 2009), our group-difference analysis showed that the ASD group exhibited poorer motor skills than the typically developing group and the INT group. Moreover, 24% of participants with ASD had DCD-consistent motor scores, a finding which aligns with previous records of 25% co-occurring DCD and ASD in a sample of school-aged females (Kopp et al. 2010). Therefore, nearly one in four school-aged children with ASD appeared to have severe motor challenges, which suggests a subgroup within the ASD population that may particularly benefit from interventions that target motor skills. In comparison, only 6% of the INT group and 0% of the typically developing group had scores in this severe range.
While the present group-difference findings were consistent with previous research, an innovation of the present study was that we additionally explored behavioral characteristics most associated with motor ability using an individual-differences approach. Among a number of behavioral characteristics previously linked to motor challenges (i.e., ASD symptoms, ADHD symptoms, cognition, and sensory symptom severity), more severe sensory symptoms and lower IQ were found to be the best predictors of motor ability. The role of sensory symptoms in motor performance was expected, given the well-documented overlap in sensory and motor systems and given the relationship between sensory and motor behavior in children with ASD, ADHD, and typical development (Hannant et al. 2016; Liu 2013; Piek and Dyck 2004; Tomchek et al. 2015). Further, this finding is consistent with the relationship between IQ and motor ability previously reported in ASD, DCD, and ADHD (Green et al. 2009; Hilton et al. 2012; Karatekin et al. 2003; Kopp et al. 2010; Sumner et al. 2016; Travers et al. 2018; Yu et al. 2018). Anecdotally, all children were able to attempt the motor tasks of the BOT-SF, suggesting that they had a baseline understanding of the task instructions. Therefore, the link between IQ and motor ability is unlikely to be solely due to lack of comprehension of the instructions. Because our results support the notion of a strong relationship among sensory symptom severity, cognitive ability, and motor ability, we postulate that a multidimensional approach to motor intervention that integrates sensory-based therapy with adaptive strategies to meet cognitive needs may optimally impact motor performance, an important possibility that should be tested in future research.
Another key aim was to determine whether a group-difference approach (commonly implemented in research) or an individual-differences approach (aligned with clinical best practice and the RDoc framework) better predicted motor challenges in our sample. Importantly, the results suggest that motor challenges within and beyond the ASD population are more accurately represented at the individual level rather than at the diagnostic group level. This means that sensory symptom severity and IQ likely provide a more nuanced and complete characterization of motor ability than diagnostic group, which may help to explain why motor challenges are not unique to ASD but rather span multiple populations that often experience sensory and cognitive symptoms (Mulligan and White 2012; Parke et al. 2015; Piek and Dyck 2004). Additionally, results indicate the possibility of a trending group moderation effect on the relationship between the predictors and the motor ability. This may suggest that taking into account the specific relationship between the IQ, sensory symptom severity, and motor ability within a diagnostic group may additionally provide information about motor ability on an individual level. In the past, the case-control approach of the research literature and the individual-differences approach of clinical best practice has led to a bit of a mismatch between research and practice. Our finding of the power of the individual-difference approach validates clinical best practice, such that assessment and treatment of motor challenges are optimally based on the needs and characteristics of the child rather than on an ASD diagnosis. Further, this finding suggests that if research were able to more frequently implement an individual-differences approach in the study of motor ability in ASD and other neurodevelopmental disorders, the bridge between research findings and clinical practice would be strengthened, thereby enhancing the ability to translate this body of knowledge into optimized, evidence-based interventions.
Limitations
A goal of the present study was to emphasize the importance of looking beyond diagnostic category in order to determine common behavioral factors that predict motor ability with more specificity than diagnostic group status. However, a potential limitation was that it was not feasible to include all clinical populations that share genetic variance with ASD. Future research in this domain would benefit from including individuals with fragile X, Rett syndrome, cerebral palsy, or Down syndrome, as these conditions frequently co-occur with ASD (Budimirovic and Kaufmann 2011; Capone et al. 2005; Craig et al. 2019; Wulffaert et al. 2009).
Another limitation was the observational and correlational nature of this study. As such, our results cannot inform us of the directionality among these relations. We are only able to suggest that there is a relationship among sensory features, IQ, and motor ability in this population. Perhaps sensory and cognitive features causally affected understanding, planning, or execution of motor tasks. Alternatively, it is possible that all three of these domains appear to be associated because of an upstream neurobiological variable. Further, by using the BOT-2 short form, we were able to determine an overall, normed motor score in a tight timeframe. While we found that both IQ and sensory features were associated with overall motor ability, it is possible that these relationships are stronger or weaker in specific areas of motor functioning (i.e., fine versus gross motor functions), which will be a key avenue for future research. Use of the full version of the BOT-2, which provides specific measures for both fine motor ability, gross motor ability, manual coordination and strength and agility, would be able to elucidate some of these more nuanced relationships in future research. Further, the present study focused on children 6–10 years-old. Given the unique developmental trajectory of motor skills that has been reported in ASD (e.g. Travers et al., 2017), it is possible that the relations among motor difficulties, sensory symptoms, and IQ may change with age, which should be investigated longitudinally.
Conclusions
The present findings reiterate the prevalence of motor impairments in ASD, extending the previous literature to highlight and explain within-group variability. Specifically, these data demonstrate that the wide variability in motor performance (both within and beyond the ASD diagnosis) can be more robustly explained by individual differences in sensory symptom severity and cognition than by diagnostic group status. Our findings highlight the importance of taking an individualized approach to identifying and characterizing motor ability in children rather than basing analysis on diagnostic status alone.
Supplementary Material
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Health Sciences Institutional Review Board, protocol #2016–0441) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, & Wodka E (2015). Evidence for Specificity of Motor Impairments in Catching and Balance in Children with Autism. Journal of Autism and Developmental Disorders, 45(3), 742–751. 10.1007/s10803-014-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics & National Institute for Children’s Health Quality. (2002). NICHQ Vanderbilt Assessment Scales. https://www.nichq.org/sites/default/files/resource-file/NICHQ_Vanderbilt_Assessment_Scales.pdf
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition.). American Psychiatric Association; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, & Baranek GT (2016). Sensory subtypes and associated outcomes in children with autism spectrum disorders: Sensory subtypes and outcomes in children with ASD. Autism Research, 9(12), 1316–1327. 10.1002/aur.1626 [DOI] [PubMed] [Google Scholar]
- Baranek GT (2009). Sensory experiences questionnaire version 3.0. Unpublished manuscript. [Google Scholar]
- Biffi E, Costantini C, Ceccarelli SB, Cesareo A, Marzocchi GM, Nobile M, et al. (2018). Gait Pattern and Motor Performance During Discrete Gait Perturbation in Children With Autism Spectrum Disorders. Frontiers in Psychology, 9, 2530 10.3389/fpsyg.2018.02530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaldi M, Rauh R, Müller C, Irion L, Saville CWN, Schulz E, & Klein C (2015). Identification of neuromotor deficits common to autism spectrum disorder and attention deficit/hyperactivity disorder, and imitation deficits specific to autism spectrum disorder. European Child & Adolescent Psychiatry, 24(12), 1497–1507. 10.1007/s00787-015-0753-x [DOI] [PubMed] [Google Scholar]
- Blondis TA (1999). Motor disorders and attention-deficit/hyperactivity disorder. Pediatric Clinics of North America, 46(5), 899–913, vi–vii. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, & Bruininks BD (2005). Bruininks-Oseretsky Test of Motor Proficiency, Second Edition American Psychological Association; 10.1037/t14991-000 [DOI] [Google Scholar]
- Buderath P, Gärtner K, Frings M, Christiansen H, Schoch B, Konczak J, et al. (2009). Postural and gait performance in children with attention deficit/hyperactivity disorder. Gait & Posture, 29(2), 249–254. 10.1016/j.gaitpost.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, & Kaufmann WE (2011). What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience, 33(5), 379–394. 10.1159/000330213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P (2013). Occupational and physical therapy In Children with Disabilities (7th ed.). Baltimore: Paul H. Brookes Pub. [Google Scholar]
- Capone GT, Grados MA, Kaufmann WE, Bernad-Ripoll S, & Jewell A (2005). Down syndrome and comorbid autism-spectrum disorder: characterization using the aberrant behavior checklist. American Journal of Medical Genetics. Part A, 134(4), 373–380. 10.1002/ajmg.a.30622 [DOI] [PubMed] [Google Scholar]
- Carroll LS, & Owen MJ (2009). Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Medicine, 1(10), 102 10.1186/gm102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2012). Social Responsiveness Scale-Second Edition (SRS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Craig F, Savino R, & Trabacca A (2019). A systematic review of comorbidity between cerebral palsy, autism spectrum disorders and Attention Deficit Hyperactivity Disorder. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society, 23(1), 31–42. 10.1016/j.ejpn.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Curtin J (2018). lmSupport: Support for Linear Models. https://CRAN.R-project.org/package=lmSupport
- Donoghue JP (1995). Plasticity of adult sensorimotor representations. Current Opinion in Neurobiology, 5(6), 749–754. 10.1016/0959-4388(95)80102-2 [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- Fulceri F, Grossi E, Contaldo A, Narzisi A, Apicella F, Parrini I, et al. (2019). Motor Skills as Moderators of Core Symptoms in Autism Spectrum Disorders: Preliminary Data From an Exploratory Analysis With Artificial Neural Networks. Frontiers in Psychology, 9, 2683 10.3389/fpsyg.2018.02683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen E, & Hamilton A (2013). Motor Abilities in Autism: A Review Using a Computational Context. Journal of Autism and Developmental Disorders, 43(2), 323–344. 10.1007/s10803-012-1574-0 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51(4), 311–316. 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Guan J, Cai JJ, Ji G, & Sham PC (2019). Commonality in dysregulated expression of gene sets in cortical brains of individuals with autism, schizophrenia, and bipolar disorder. Translational Psychiatry, 9(1), 152 10.1038/s41398-019-0488-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith A, Jackson CP, Miall RC, & Vijayakumar S (n.d.). Unifying the Sensory and Motor Components of Sensorimotor Adaptation, 8. [Google Scholar]
- Hannant P, Tavassoli T, & Cassidy S (2016). The Role of Sensorimotor Difficulties in Autism Spectrum Conditions. Frontiers in Neurology, 7 10.3389/fneur.2016.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey WJ, & Reid G (2003). Attention-Deficit/Hyperactivity Disorder: A Review of Research on Movement Skill Performance and Physical Fitness. Adapted Physical Activity Quarterly, 20(1), 1–25. 10.1123/apaq.20.1.1 [DOI] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. 10.1177/1362361311423018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250. [PubMed] [Google Scholar]
- Karatekin C, Markiewicz SW, & Siegel MA (2003). A preliminary study of motor problems in children with attention-deficit/hyperactivity disorder. Perceptual and Motor Skills, 97(3 Pt 2), 1267–1280. 10.2466/pms.2003.97.3f.1267 [DOI] [PubMed] [Google Scholar]
- Kaas JH (2008). The evolution of the complex sensory and motor systems of the human brain. Brain Research Bulletin, 75(2–4), 384–390. 10.1016/j.brainresbull.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S, Beckung E, & Gillberg C (2010). Developmental coordination disorder and other motor control problems in girls with autism spectrum disorder and/or attention-deficit/hyperactivity disorder. Research in Developmental Disabilities, 31(2), 350–361. 10.1016/j.ridd.2009.09.017 [DOI] [PubMed] [Google Scholar]
- Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, et al. (2011). Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Science Translational Medicine, 3(95), 95ra75 10.1126/scitranslmed.3002464 [DOI] [PubMed] [Google Scholar]
- Liu T (2013). Sensory Processing and Motor Skill Performance in Elementary School Children with Autism Spectrum Disorder. Perceptual and Motor Skills, 116(1), 197–209. 10.2466/10.25.PMS.116.1.197-209 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Lumley T (2017). leaps: Regression Subset Selection. https://CRAN.R-project.org/package=leaps
- Lungarella M, & Sporns O (2006). Mapping Information Flow in Sensorimotor Networks. PLoS Computational Biology, 2(10), e144 10.1371/journal.pcbi.0020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil LK, & Mostofsky SH (2012). Specificity of dyspraxia in children with autism. Neuropsychology, 26(2), 165–171. 10.1037/a0026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz A-M, Guter S, Kapur K, Macmillan C, et al. (2010). Neurobehavioral Abnormalities in First-Degree Relatives of Individuals With Autism. Archives of General Psychiatry, 67(8), 830 10.1001/archgenpsychiatry.2010.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, & Denckla MB (2003). Overflow Movements Predict Impaired Response Inhibition in Children with ADHD. Perceptual and Motor Skills, 97(3_suppl), 1315–1331. 10.2466/pms.2003.97.3f.1315 [DOI] [PubMed] [Google Scholar]
- Mulligan S, & White BP (2012). Sensory and Motor Behaviors of Infant Siblings of Children With and Without Autism. American Journal of Occupational Therapy, 66(5), 556–566. 10.5014/ajot.2012.004077 [DOI] [PubMed] [Google Scholar]
- Pan C-Y, Tsai C-L, & Chu C-H (2009). Fundamental Movement Skills in Children Diagnosed with Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Journal of Autism and Developmental Disorders, 39(12), 1694–1705. 10.1007/s10803-009-0813-5 [DOI] [PubMed] [Google Scholar]
- Parke EM, Thaler NS, Etcoff LM, & Allen DN (2015). Intellectual Profiles in Children With ADHD and Comorbid Learning and Motor Disorders. Journal of Attention Disorders, 108705471557634. 10.1177/1087054715576343 [DOI] [PubMed] [Google Scholar]
- Piek JP, & Dyck MJ (2004). Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Human Movement Science, 23(3–4), 475–488. 10.1016/j.humov.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Fournier KA, & Hass CJ (2013). Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in Integrative Neuroscience, 7, 28 10.3389/fnint.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey AJ, & Lord C (2003). The Social Communication Questionnaire: Manual. Western Psychological Services. [Google Scholar]
- Schaaf RC (2015). Creating Evidence for Practice Using Data-Driven Decision Making. American Journal of Occupational Therapy, 69(2), 6902360010p1 10.5014/ajot.2015.010561 [DOI] [PubMed] [Google Scholar]
- Sumner E, Pratt ML, & Hill EL (2016). Examining the cognitive profile of children with Developmental Coordination Disorder. Research in Developmental Disabilities, 56, 10–17. 10.1016/j.ridd.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Little LM, & Dunn W (2015). Sensory Pattern Contributions to Developmental Performance in Children With Autism Spectrum Disorder. American Journal of Occupational Therapy, 69(5), 6905185040p1 10.5014/ajot.2015.018044 [DOI] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, et al. (2017). Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental Science, 20(4), e12401 10.1111/desc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Destiche D, Samsin D, et al. (2015). Brainstem White Matter Predicts Individual Differences in Manual Motor Difficulties and Symptom Severity in Autism. Journal of Autism and Developmental Disorders, 45(9), 3030–3040. 10.1007/s10803-015-2467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Mason AH, Gruben KG, Dean DC, & McLaughlin K (2018). Standing balance on unsteady surfaces in children on the autism spectrum: The effects of IQ. Research in Autism Spectrum Disorders, 51, 9–17. 10.1016/j.rasd.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Powell PS, Klinger LG, & Klinger MR (2013). Motor Difficulties in Autism Spectrum Disorder: Linking Symptom Severity and Postural Stability. Journal of Autism and Developmental Disorders, 43(7), 1568–1583. 10.1007/s10803-012-1702-x [DOI] [PubMed] [Google Scholar]
- Van Waelvelde H, Oostra A, Dewitte G, Van Den Broeck C, & Jongmans MJ (2010). Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder: Motor Problems among Children with ASD, ADHD, and or DCD. Developmental Medicine & Child Neurology, 52(8), e174–e178. 10.1111/j.1469-8749.2009.03606.x [DOI] [PubMed] [Google Scholar]
- Wechsler D, & Hsiao-pin C (2011). Wechsler abbreviated scale of intelligence. San Antonio, TX: Pearson. [Google Scholar]
- Wu D, José JV, Nurnberger JI, & Torres EB (2018). A Biomarker Characterizing Neurodevelopment with applications in Autism. Scientific Reports, 8(1), 614 10.1038/s41598-017-18902-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulffaert J, Van Berckelaer-Onnes IA, & Scholte EM (2009). Autistic disorder symptoms in Rett syndrome. Autism: The International Journal of Research and Practice, 13(6), 567–581. 10.1177/1362361309338184 [DOI] [PubMed] [Google Scholar]
- Yu T-Y, Chou W, Chow JC, Lin C-H, Tung L-C, & Chen K-L (2018). IQ discrepancy differentiates levels of fine motor skills and their relationship in children with autism spectrum disorders. Neuropsychiatric Disease and Treatment, 14, 597–605. 10.2147/NDT.S153102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


