Abstract
Objective:
People living with HIV/AIDS (PLWHA) are disproportionally exposed to a host of structural, community, and individual-level physical and psychosocial stressors also termed ‘syndemic conditions.’ The current study aimed to examine the association between experiencing syndemic conditions and physiological stress response and be associated with bodily inflammation, including Interlekin-6 (IL-6) and C-reactive protein (CRP) in PLWHA.
Design:
Participants (N = 103) were recruited from a public HIV clinic. They provided saliva samples of IL-6 and CRP and completed psychosocial measures.
Main outcome measures:
Levels of circulating salivary IL-6 and CRP.
Results:
When predictors (birth country, recent housing instability, and incarceration history) were simultaneously entered into a regression model, only incarceration history was negatively associated with IL-6 [b = −.27, t(98) = −3.11, p = .002]. For CRP, the resulting regression model was not significant, [F(3, 98) = 2.23, p = .090].
Conclusion:
Although we had expected higher levels of syndemics to be associated with higher levels of circulating inflammation, in our sample, length of incarceration was associated with lower levels of circulating IL-6. Findings are therefore suggestive of a stress response disruption resulting in a negative feedback loop as the long-term impact of chronic stress on inflammation.
Keywords: HIV, inflammation, cytokines, syndemic
Introduction
In the United States (U.S.), approximately 1.2 million individuals are living with human immunodeficiency virus (HIV). Within the U.S., HIV remains a condition that is overrepresented in socially marginalised groups and underserved populations including individuals living in poverty, racial/ethnic minorities, sexual and gender minorities, and active or former substance users (Centers for Disease Control & Prevention, 2017). Due to belonging to marginalised groups, people living with HIV/AIDS are disproportionally exposed to a host of individual-level psychosocial stressors, community-level circumstances, and structural barriers at a societal level that are present prior to HIV infection and continue to be present afterwards, (Pellowski et al., 2013).
Given the disproportional exposure to such factors, HIV-infected individuals often experience multiple, co-occurring problems that act synergistically to fuel complex HIV/AIDS epidemics within at-risk populations. Such interacting conditions are referred to as syndemics (Singer et al., 2017; Singer & Clair, 2003; Stall et al., 2015). For communities affected by HIV, syndemic conditions often include low socioeconomic status, incarceration, marginal housing, substance use, mental health issues, and physical, emotional, and sexual trauma as well as a host of other psychosocial issues (Sullivan et al., 2015). Syndemic conditions are important to consider for HIV outcomes as a positive dose-response relationship has been found between number of syndemic conditions experienced and poor antiretroviral adherence, lower access to care, higher viral load, and lower CD4 count (Blashill et al., 2015; Glynn et al., 2019; Harkness et al., 2018; Pantalone et al., 2018).
Experiencing syndemic conditions is associated with health outcomes over and above potential associations with self-care (e.g. medication adherence). Social influences create the environment and conditions for poor biological outcomes across a myriad of diseases including HIV (Singer et al., 2017). Experiencing these synergistic problems creates a life of chronic stress that takes a toll on physical health via sustained activation of the physiological stress response (Pearlin et al., 2005; Russell et al., 2018). This prolonged stress response and resulting degradation of the body systems is often referred to as ‘allostatic load’ (McEwen, 1998). Per allostatic load theory, chronic psychological and environmental stressors can overwhelm bodily systems that maintain homeostasis and precipitate a cascade of neural, neuroendocrine, and immune system effects that, ultimately, when dysregulated for too long, lead to a variety of health issues including: coronary heart disease, myocardial infarction, obesity, diabetes, hypertension, cancer, infection, colitis, ulcers, asthma, and cognitive impairment (McEwen, 1998). Thus, allostatic load theory provides a context for how syndemic conditions can affect physiological processes that lead to poorer health outcomes.
The chronic activation of the stress response is pathogenic via prolonged activation of the hypothalamus-pituitary-adrenal-cortical (HPAC) system. Chronic activation of this bodily system is implicated in harmful physiological consequences (Yaribeygi et al., 2017). One specific consequence of continued engagement of the HPAC system includes elevated levels of glucocorticoids (Sapolsky et al., 2000). Glucocorticoids are hormones synthesised in the adrenal cortex that function to maintain homoeostasis post-stress by inducing anti-inflammatory actions (Barnes, 1998). However, increased levels of glucocorticoids, due to repeated stress, result in the suppression of the immune system and lowers body response to acute infection or injury (McEwen, 1998). At the same time chronic glucocorticoid elevation may desensitise glucocorticoid receptors in inflammatory cells, resulting in muted anti-inflammatory control and elevations in circulating pro-inflammatory cytokines (Miller et al., 2002). Levels of bodily inflammation can be detected through salivary cytokines and may provide insight into increased or lowered activation as a result of HPAC disruption due to chronic stress.
The aim of the current study was to explore which syndemic conditions are associated with changes in inflammatory processes, operationalised by the measurement of two biomarkers of inflammation, C-reactive protein (CRP) and Interleukin-6 (IL-6). These two cytokines were chosen with intention due to their association with psychosocial factors and HIV disease. IL-6 is a robust predictor of depression, sleep regulation, inflammation, and is less likely than other biomarkers of allostatic load (e.g. cortisol) to be affected by other body processes and timing (e.g. diurnal sleep curves). IL-6 has been used to study psychosocial stress (Fumaz et al., 2012) and progression of disease (Boulware et al., 2011) in HIV-infected individuals. IL-6 and CRP are both associated with pro-inflammatory processes that signal bodily distress and dysfunction of systems, such as the immune system (Kiecolt-Glaser & Glaser, 2002). In patients with HIV/AIDS who were antiretroviral treatment-naïve or had recently started antiretroviral treatment, elevated IL-6 and CRP have been associated with low CD4, and progression to AIDS and death (Boulware et al., 2011; Nixon & Landay, 2010). Another study found that circulating levels of IL-6 had the strongest, positive association with HIV-1 viral rebound after treatment (Hurst et al., 2015).
Given the potential negative consequences of dysregulation in inflammatory processes for overall health, in the current study we were interested in identifying which syndemic factors among structural, psychological, and HIV health indicators were associated with variation in IL-6 and CRP in our sample of patients living with HIV. Because we did not have specific hypotheses as to whether we would see upregulation or downregulation of inflammation or which factors would be associated with dysregulation, we utilised stochastic variable search selection (SVSS) to select variables empirically. SVSS is a method that utilises Bayesian modelling to select a subset of explanatory variables. SVSS uses Markov chain Monte Carlo (MCMC) sampling to sample from a posterior distribution of the possible subsets of predictors to identify the best models. Predictors that are selected more frequently in the MCMC sampling receive higher poster probabilities of inclusion (0.0–1.0) (George & McCulloch, 1993). This approach maximises power and minimises false positives by selecting predictors controlling for uncertainty in which other predictors are included in the model. As such, it was an ideal approach to examine our research question: Which syndemic factors are associated with salivary inflammation as measured by IL-6 and CRP in PLWHA?
Method
Participants and procedures
Participants (N = 103) were recruited from an urban, ambulatory care centre serving the socially marginalised and underserved in Miami, FL, a city with one of the highest HIV prevalence in the U.S. (Centers for Disease Control & Prevention, 2017) as part of a larger study investigating the biobehavioural mechanisms of insomnia in people living with HIV/AIDS. Although the total study sample included 103 participants, one participant’s saliva sample was not detectable. Therefore, 102 saliva samples were used in the analyses presented within this paper.
To be eligible for the parent study, participants had to be patients in the clinic, adults 18–65 years of age, able to read and understand English, and they could have no existing cognitive limitations that would preclude them from the consent process. Informed consent was obtained from all participants in the study. All procedures were approved by the University of Miami and Jackson Memorial Hospital Institutional Review Boards. Participants completed an interview administered assessment battery, which included sociodemographic information and behavioural health questionnaires. Inflammatory cytokines (CRP and IL-6) were collected via saliva samples. Participants also wore an actigraph for one week to collect data on sleep time and patterns. Details of saliva collection, actigraphy, and analyses are included below.
Specimen collection and storage
Salivary specimens were collected using passive drool cryovials and straws from Salimetrics, LLC. Participants were prompted to stay relaxed and think of their favourite foods or sour foods (e.g. lemon) to stimulate saliva production. Participants spit into the tube using a straw, which helped filter the saliva cleanly into the tube. Universal precautions were followed, and all saliva tubes were handled using gloves and placed into a labelled biohazard bag. Vials were labelled and within 20 minutes of collection, were stored in a chilled freezer (−40 degrees Fahrenheit), and, within 24 hours, were placed in a deep freezer chilled to −80 degrees Fahrenheit. Assays were ordered towards the end of data collection and samples were stored immediately in 6 degrees Celsius, per the appropriate requirements (i.e. 2–8 degrees Celsius).
Specimen processing
Both IL-6 and CRP were processed using Salimetrics ELISA assay kits. Assays were run by staff trained specifically in specimen analysis. IL-6 was processed in a 96 well plate. The assay time is approximately 4.5 hours and the sample volume/test is 60 microlitres. Sensitivity of the test is 0.07 pg/millilitre (ml) and the assay range is 0–100 pg/ml. CRP was processed in a 96-well plate. The assay time is approximately 3 hours, and the sample volume/test is 15 microlitres of saliva, 50 microlitres × 10 dilution. Sensitivity of the test is 10 pg/ml, and the assay range is 93.75 picograms − 3000 pg/ml.
Measures
Structural variables
Country of birth.
Individuals were asked for the country of birth. If individuals were born in the U.S., this item was coded yes (yes = 1, no = 0).
Incarceration history.
Participants were asked about their longest length of stay in jail/prison. Length of stay was measured on a scale from 0 to 4 (0 = None; 1 = Less than a week; 2 = 7 days to less than a month; 3 = 1 month to 1 year; 4 = more than a year). For this paper, incarceration history was treated as continuous variable.
Housing instability.
Patients were asked about the stability of their housing over the past year. Individuals were considered to have unstable housing if, at some point over the past year, they did not have housing of their own (this included staying with family or friends temporarily). Individuals who endorsed unstable housing over the past year (1 = yes, 0 = no) were identified as positive for unstable housing if they reported homelessness or temporary/transitional housing in the past 12 months.
Psychological symptoms and behavioural health
Interpersonal violence.
A 9-item adaptation of the Intimate Partner Violence Screening Tool (Kalokhe et al., 2012) was used to assess lifetime childhood abuse, abuse experienced as an adult, and abuse in the context of a romantic relationship. Each item was a yes/no response and a summed score was calculated such that greater scores indicated increased experienced of abuse/violence (range 0 to 9). The SSVS model was run with the summative score.
Post-Traumatic stress disorder (PC-PTSD).
The primary care post-traumatic stress disorder screener was used to assess for current PTSD symptoms (Prins et al., 2004). The PC-PTSD is a 4-item screen that maps onto DSM-5 criteria for PTSD and is used to identify individuals with PTSD symptoms. Each of the four items are coded (1 = yes, 0 = no). The full score (0–4) was used for these analyses.
Total sleep time.
Total sleep time was calculated using actigraphy data. To objectively assess sleep in the home environment, participants were instructed to wear an actigraph (wGT3X-BT, 2016, Actigraph Corporation) on their non-dominant wrist for 24 hours a day except when bathing. Actigraphy data were scored individually by a trained research assistant using ActiLife software version 6.13.3 and the Cole-Kripke algorithm was applied (Actigraph, LLC, 2009–2015). Each day of data was individually scored and wake after sleep onset (WASO) was removed from the sleep period to obtain total sleep time. The mean, daily total sleep time (in minutes) for each person (over the course of all days of observation) was calculated.
Insomnia symptoms.
To measure insomnia symptoms, we used the Insomnia Severity Index (ISI). The ISI (Bastien et al., 2001) is a seven-item scale assessing night time difficulties (e.g. falling asleep, staying asleep, and waking too early) and daytime impairments (e.g. satisfaction, distress/concern, etc.) associated with insomnia. Each item is rated on a 5-point scale and the total score ranges from 0 to 28; higher scores are associated with more insomnia symptoms and insomnia-related impairment. This measure had high internal reliability within our sample (Cronbach’s α = .86).
Depression.
Depressive symptoms were measured by the Patient Health Questionnaire − 9. The PHQ-9 (Kroenke et al., 2001) is a 9-item depression scale that assesses cognitive, behavioural, and physiological symptoms of depression, with scores ranging from 0 to 27, where higher scores are associated with more depression and depression-related impairment. This measure had high internal reliability within our sample (Cronbach’s α = .82).
Medication adherence.
Participants were to report how many days they had missed taking their HIV medication within in the past week and the past month. This item was taken from a validated 3-item measure of HIV medication adherence (Wilson et al., 2014, 2016).
Health-related quality of life.
The AIDS Clinical Trial Group Quality of Life Measure SF-21 (ACTG-QOL SF-21) general health-related quality of life subscale was used. This includes two items. Each item was used as a separate variable entered into the model. The first item assesses a quality rating of one’s health on a scale from 1 (Poor) to 5 (Excellent). The second item assesses the numeric rating of one’s health on a scale from 0 (death) to 100 (perfect health). Each item was treated as continuous.
Biological health
HIV RNA log viral load.
HIV RNA log viral load was obtained via medical chart extraction. Viral load is a measure of circulating virus in the bloodstream and is expressed as HIV RNA units/millilitre. Lower numbers indicate better health and higher numbers indicate worse health. Individuals who are adherent to their medication are expected to be ‘undetectable,’ which, within our dataset was defined as having a value <20. For values of viral load <20, the number 20 was imputed.
CD4.
CD4 was obtained via medical chart extraction. CD4 is a measure of immune health used to assess HIV/AIDS, with lower numbers indicating worse health and higher numbers indicating better health and immune functioning. Values below 200 cells/microlitre meet the clinical definition of AIDS. Both original CD4 count (i.e. first CD4 count recorded at first HIV visit for patient) and most recent CD4 count was entered into the model.
BMI.
Body mass index (BMI) was calculated using height (feet and inches were converted to meters) and weight (pounds were converted to kilograms) using the following equation: BMI = kg/m2.
Salivary biomarker outcome variables
Interleukin-6 (IL-6).
IL-6 is an interleukin, or type of cytokine, that enables communication between leukocytes and other active cells in the specific immune response or inflammation. Specifically, IL-6 mediates the response to acute injury and infection and plays a role in differentiation of immune cells and functioning; and, when psycho-logically stressed, the body is triggered to produce IL-6 (Tanaka et al., 2014). IL-6 was obtained via passive saliva collection. Saliva samples were analysed using ELISA immunoassay. IL-6 facilitates communication between leukocytes and other active cells in the specific immune response or IL-6. When the body experiences acute physical stress, it also produces IL-6 (Tanaka et al., 2014). The natural log of IL-6 was used in these analyses.
C-Reactive protein.
CRP is associated with circulating levels of inflammation and is associated with various medical comorbidities, and, in particular, cardiovascular events (Ridker et al., 2000). CRP has been used to study progression of disease (Boulware et al., 2011) and risk for myocardial infarction (Triant et al., 2009) in HIV-infected samples. CRP was obtained via passive saliva collection. Saliva samples were analysed using ELISA immunoassay. Analyses were conducted using the natural log of CRP.
Data analysis
Analyses were conducted in SPSS Version 25 (IBM SPSS Statistics for Windows, 2017). Distributions of variables were examined and tests for normality indicated outcome variables were non-normal. Thus, CRP and IL-6 were log-transformed (natural log) and the resulting distributions were relatively normal with no significant outliers. Frequency of missing data was examined. Due to nature of data collection, there was no missing data in behavioural assessments. Specifically, data were collected using tablets with electronic surveys that validate the data and check for missingness in real time. Further, no participant declined to answer any items. However, for the biological data (IL-6 and CRP), there was one saliva sample that was not detected when the assays were run; this person was omitted from the analyses.
To explore what factors (syndemics) in the lives of people living with HIV/AIDS may contribute to higher levels of inflammation, stochastic search variable selection (SSVS) (George & McCulloch, 1993) was used, which is a Bayesian framework for variable selection. Analyses were performed using an online application that performs SSVS (Bainter et al., 2019; https://ssvsforpsych.shinyapps.io/ssvsforpsych/). SSVS treats the set of predictors in a regression model as unknown and the estimation is aiming to characterise uncertainty in the best values of β and the best set of predictors. This statistical approach samples thousands of regression models selecting among models that best explain variability in the outcome. To start, the model specification builds on a standard regression model for y with some number of predictors, p:
This standard regression model is augmented with a set of binary indicator variables, δj, associated with each predictor, which toggle variables in and out of the model and a set of prior distributions for each parameter in the model (Table 2).
Table 2.
Potential predictor variables entered into stochastic search variable selection.
| M or n | SD or % | |
|---|---|---|
| Incarceration history (0–4) | 2.4 | 1.5 |
| Born in the U.S. | 92 | 89.3% |
| Homeless within the past 12 months | 17 | 16.5% |
| Interpersonal violence (0–9) | 2.9 | 3.1 |
| PTSD symptoms (0–4) | 1.12 | 1.40 |
| Depression symptoms (0–27) | 7.1 | 5.8 |
| Total sleep time (in minutes) | 385.5 | 72.8 |
| Insomnia symptoms (0–28) | 11.7 | 7.4 |
| Missed days of medication over past week | 0.7 | 1.5 |
| Missed days of medication over past month | 1.5 | 3.7 |
| Overall health quality rating (1–5) | 2.3 | 1.2 |
| Overall health numeric rating (0–100) | 76.0 | 22.9 |
| BMI | 29.5 | 6.5 |
| Log10 HIV viral load (units/milliliter) | 1.8 | 1.0 |
| Current CD4 count (cells/microliter) | 511.0 | 309.4 |
| Original CD4 count (cells/microliter) | 325.6 | 239.0 |
After sampling, the proportion of times each predictor is selected into the model is its marginal inclusion probability (MIP). Predictors with higher MIPs are the most reliable predictors, controlling for uncertainty in which other variables are included in the model. The prior for the regression coefficients used in SSVS is sometimes called a ‘spike-and-slab prior’ because it implies the prior belief that many predictors have no effect (i.e. a spike at zero) but a subset of predictors may share a meaningful relationship with the outcome (i.e. a slab of non-zero coefficients). For our analyses, we assigned each δj a prior probability of .5 indicating that each predictor has a 50/50 prior probability of being included the model and a default non-informative prior distribution for the non-zero regression coefficients. For more detail about this SSVS methodology, please see (Bainter et al., 2019). An important advantage of the Bayesian variable approach used here is that SVSS accounts for uncertainty in variable selection and this decreases false-positive results and increases power relative to frequentist approaches (Swartz et al., 2008; Viallefont et al., 2001).
One SSVS was performed using the natural log of IL6 (ln IL6) as the outcome variable and a second SSVS was performed using the natural log of CRP (ln CRP) as the outcome variable. We entered the following set of candidate predictors into the SSVS model: incarceration history, nativity (born U.S. vs. not), homelessness, overall health quality rating, overall health numeric rating, weekly and monthly medication adherence, PTSD symptoms, interpersonal violence history, depression, insomnia, total sleep time, BMI, CD4 count, original CD4 count at first HIV visit, and HIV viral load. Descriptive statistics for each of these potential predictor variables is represented in Table 2. The sampler was run for 20,000 iterations, discarding the first 5,000 to ensure convergence. After running SSVS, we examined plots of the MIPS to determine an appropriate cut-off value. Variables selected based on a .10 cut-off were then entered in linear regression models for CRP and IL-6, respectively.
Bivariate correlations were run between IL-6 and CRP to examine associations. Selected potential predictors were entered in regression models simultaneously. Alpha level was set to .05, two-tailed. For IL-6, there were several (n = 16) individuals with below detectable IL-6 (<1.6 pg/mL). We imputed this lowest detectable value 1.6 and used the natural log of the variable for our analyses. We did a sensitivity test and ran results with and without these individuals. The magnitude and direction of the results did not change. As a result, we have presented the results with the full sample.
Results
The majority of the sample was Black (81.6%), non-Hispanic (89.3%), identified as hetero-sexual (82.5%), and had less than a high school education (41.7%). The sample was mostly split between cisgender male (49.5%) and cisgender female (49.5%) with <1% identifying as transgender female. Additional sample characteristics are shown in Table 1.
Table 1.
Participant characteristics, N = 103.
| M or N | SD or % | ||
|---|---|---|---|
| Sociodemographic variables | |||
| Age | 51.6 | 8.5 | |
| Gender | |||
| Cisgender male | 51 | 49.5% | |
| Cisgender female | 51 | 49.5% | |
| Transgender female | 1 | <1.0% | |
| Race | |||
| Black | 84 | 81.6% | |
| White | 12 | 11.7% | |
| Mixed/Other | 9 | 8.7% | |
| Ethnicity | |||
| Hispanic/Latino | 11 | 10.7% | |
| Sexual Orientation | |||
| Heterosexual | 85 | 82.5% | |
| Gay/Lesbian/Homosexual | 12 | 11.7% | |
| Bisexual | 3 | 2.8% | |
| Other Sexual Minority | 3 | 2.8% | |
| Education | |||
| Did not complete high school | 43 | 41.7% | |
| High school graduate | 37 | 35.9% | |
| Some college | 19 | 18.4% | |
| College graduate | 4 | 3.9% | |
| HIV health status biomarkers | |||
| Log10 HIV viral load (units/milliliter) | 1.8 | 1.0 | |
| Suppressed viral load (<200 copies/mL) | 85 | 82.5% | |
| CD4 count (cells/microliter) | 511.0 | 309.4 | |
| Salivary biomarkers | |||
| IL-6 (picograms/milliliter) | 37.1 | 111.1 | |
| Log IL-6 | 2.7 | 1.2 | |
| CRP (picograms/milliliter) | 6299.2 | 10429.7 | |
| Log CRP | 8.0 | 1.0 | |
We ran bivariate correlation on the two outcomes, IL-6 and CRP. As expected, they were highly correlated with one another, Pearson’s r = .520, n = 102, p < .001. For IL-6, the following factors were identified by SSVS and were included in the prediction model: nativity (born in the US), homelessness, and incarceration history. The full regression model [F(3, 98) = 3.75, p = .013], explained 10.3% of the variance in IL-6. Of the variables entered, only incarceration history was significantly associated with IL-6 [β = −.27, t(98) = −3.11, p = .002], such that more time incarcerated was associated with lower levels of IL-6. For CRP, the following factors were identified by SSVS and included in the prediction model: nativity (born in the US), homelessness, and incarceration history. This model was not significant, [F(3, 98) = 2.23, p = .090]. See Figure 1 for a summary of results.
Figure 1.
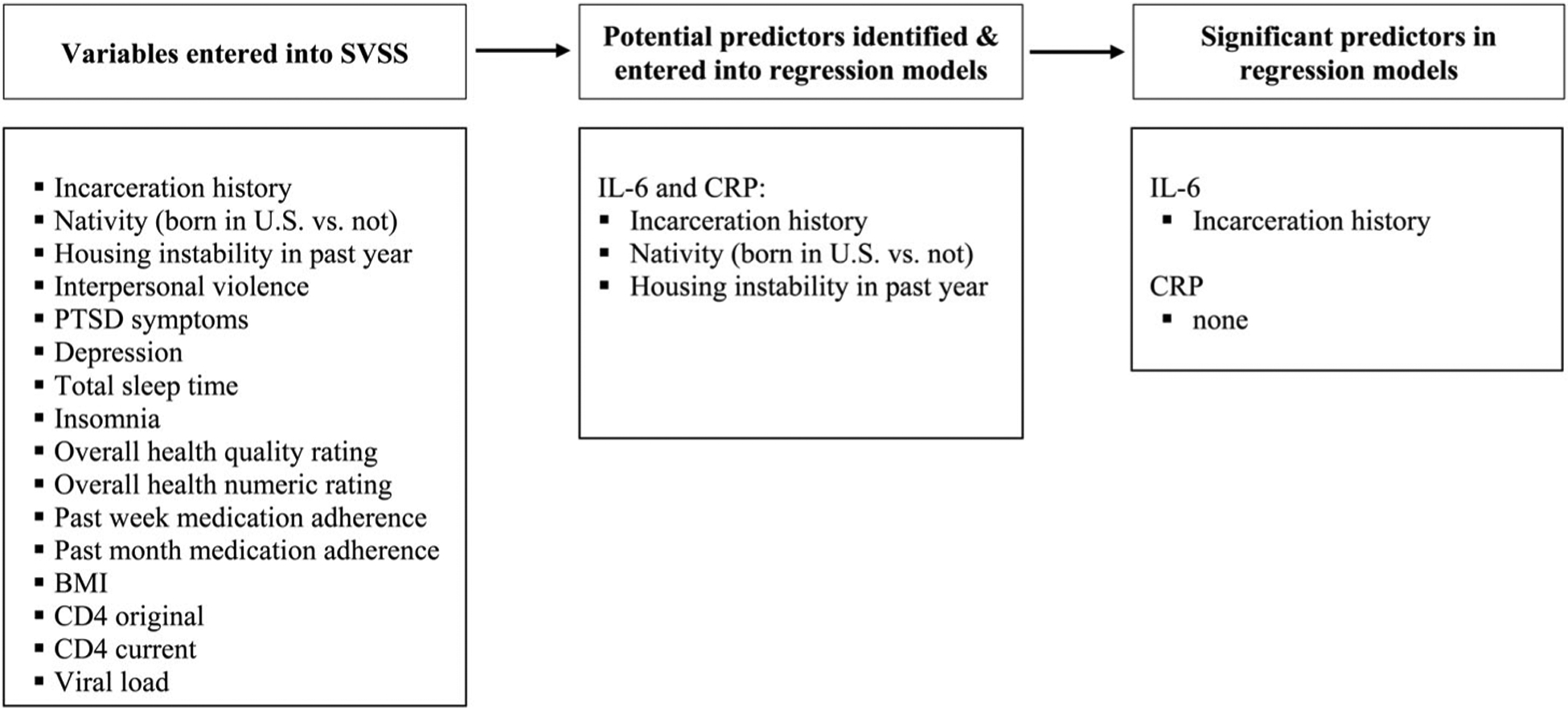
Stochastic search variable selection (SSVS) process for predicting IL-6 and CRP.
Discussion
The current study identified an association between histories of jail or prison time and lower levels of salivary IL-6. Although we had originally hypothesised that we would observe increased circulating inflammatory cytokines, reduced circulating inflammatory cytokines may indicate a form of immunosuppression, which is associated with long-term stress responses. While acute stress is associated with an upregulation of cytokines, chronic stress can lead to a blunted or downregulation that would be observed years later than the initial stressor (Dhabhar, 2009). Specifically, chronic stress reduces the immune response by changing the type 1-type 2 cytokine balance and decreasing leukocyte levels and functioning (Dhabhar, 2008). For these reasons, our findings suggest that incarceration may continue to affect immune response long after release from prison and may result in poorer immune functioning years later. This is a particularly important finding given that this study was conducted in a sample of PLWHA, who already have reduced immune functioning as a result of HIV virus and CD4 depletion.
Prior research has demonstrated a complex and nuanced relationship between stress and inflammation (Del Giudice & Gangestad, 2018). Allostatic load theory (McEwen, 1998) posits that although acute stress is adaptive as it initiates neuroimmune responses to aid the body in defence and healing, chronic stress is pathogenic. Due to chronic stress, the body experiences repeated fluctuating neuroendocrine responses resulting in dysregulated immune responses including glucocorticoid resistance (i.e. dampening or depleting the immune response). Thus, inflammatory markers, such as IL-6 and CRP (Juster et al., 2010) are shown to have varied roles in the body and presence of either higher or lower amounts can signal diverse and different meanings depending on the context. Contextualising the current findings in allostatic load theory, lower IL-6 levels associated with longer incarceration histories may indicate a dysregulated, depleted immune response.
Although, in the current sample, interpersonal trauma was not significantly associated with circulating IL-6 and CRP in the SSVS, more than half of our sample reported physical or sexual abuse at some point in their lifetime - either childhood, adult, or both time points. Adverse childhood experiences have been associated with many behavioural and physical health problems later in life (Felitti et al., 1998). People who experience multiple traumas have an altered physiological response (Russell et al., 2018) and increased disease profile across almost all health conditions (Krause et al., 2004; Pearlin et al., 2005). This may have been observed in our sample as it is likely that there was considerable overlap between those who had experienced lifetime interpersonal trauma and had an incarceration history due to high prevalence of both in our sample.
Another consideration of findings in this clinic sample of PLWH is the association between ART and inflammation. Specifically, use of ART may have restricted the range on the high end of inflammatory biomarkers given that some research has shown a decline in inflammation in those with a supressed viral load on ART (Hunt, 2012; Zicari et al., 2019). However, this may not be the case given that other research has shown sustained inflammation among PLWHA even in the context of ART use and viral suppression (Hunt, 2012; Zicari et al., 2019). In sum, inflammatory responses are multifaceted and nuanced with even greater complexity among PLWHA.
Finally, it is noteworthy that a large portion of our sample (77%) endorsed a history of incarceration - either jail or prison time - at some point in the past with 58.3% endorsing jail/prison time of one month or more. This is a substantial percentage of the participants who were not specifically targeted for incarceration history. This is likely reflective of the larger population of underserved, minority persons living with HIV/AIDS (Pellowski et al., 2013). While almost all individuals in our sample reported this history as being more than a year ago, findings suggest that even experiences that happened years prior can have a significant impact on current levels of inflammation and inflammatory dysregulation, which is congruent with the allostatic load model (Danese & McEwen, 2012).
Although the current study adds to the complex literature of stress and inflammation limitations should be noted. One of the challenges of pursuing this work is that there are currently no universally agreed upon cut points or ranges for evaluating maladaptive levels of salivary cytokines in ‘healthy’ (e.g. individuals without active medical diagnoses) or other populations. This is because cytokine levels fluctuate as a function of several biological, psychological, and environmental factors. It is almost impossible to derive ‘typical values’ for any given population and the manufacturer recommends using within group comparisons to understand values in a given sample. For example, Borges and colleagues (2015) reported that for their sample of PLWHA, plasma IL-6 was elevated for those who were older, did not identify as Black, had higher BMI, lower serum lipid levels, HIV replication, low CD4+ cell count, took protease inhibitors, had comorbid conditions and had poorer kidney function as measured by decreased estimate glomerular filtration rate (eGFR) (Borges et al., 2015). However, compared to data published by Ouellet-Morin and colleagues (2011) (Ouellet-Morin et al., 2011) and information available from the manufacturing company (Salimetrics, LLC) (Salivary Interleukin-6,6, 2020) our sample, on average, had elevated salivary CRP (ref: >1600 pg/mL) and IL-6 (ref: ≥detectable) compared to the general, healthy population, which is consistent with prior research that suggests PLWHA experience greater levels of clinical inflammation (Hunt, 2012). However, more detailed information about how our sample compares to a comparable sample of PLWHA on ART is not available, and to try to draw comparisons to other published data may be misleading. Instead, the aim of this study was to collect a wide variety of psychosocial syndemic variables and examine associations between these variables and salivary CRP and IL-6 as markers of inflammation.
The current study is also limited by the variables we collected as it is possible other unmeasured factors could have been associated with circulating levels of salivary IL-6 and CRP. Additionally, for almost all individuals in our sample, the episode of jail or prison time was over a year ago. Unfortunately, more detailed information about the proximity of their incarceration was not collected, (‘over a year’ was the highest option provided) thus, we are unable to provide more nuanced conclusions about the time since imprisonment and current circulating inflammation. Relatedly, the study did not collect data to quantify the level of chronic stress (e.g. length of time experienced) to measure immune response depletion. Further, biomarkers were not collected to confirm the posited blunting effect of chronic stress; specifically, there was no data assessing hypothalamic-pituitary-adrenal (HPA) axis functioning or glucocorticoid response receptor sensitivity in immune cells. Future research should collect these additional variables to be able to better explore the biological mechanisms underpinning this relationship. The study is also subject to the same limitations as all cross-sectional data collection; namely, that no temporal relationships or conclusions can be drawn from this study.
Finally, the study had many null findings. Although sample size is often cited as the main source of null findings as it relates to power, given the Bayesian approached used, the sample size was likely adequate and increasing the sample size only slightly most likely would have not changed null findings. The majority of predictors that were not included in the final models (i.e. not selected by the SVSS) had very low associations with the outcomes (r < .01). A more likely alternative explanation is that the participants were medically and psychiatrically complex patients; and, as such, there was likely noise introduced to the data as a result of numerous health conditions and medications. However, this is the challenge of conducting research within clinical samples - there are many factors that may have contributed to inflammation and cytokine levels. Moreover, our participants are representative of HIV patients in general, in that they are not only living with HIV, but also, several other chronic physical and mental health conditions and take a host of medications to manage those conditions. Therefore, our results likely represent what would have been observed for a similar, HIV positive sample. It is possible, however, that a very large sample size (N = 10,000+) might have even been able to detect these small effects and future research should seek to examine these relationships in larger data sets.
Our findings suggest an important role of significant life stressors in inflammatory levels for people living with HIV/AIDS. Future studies should continue to explore these mechanisms to better understand how psychosocial stressors can contribute to immune dysregulation and should extend our findings by looking collecting more information about the length of and time since imprisonment to promote a better understanding of how inflammation varies with stressful life events over time. Future research should also consider collecting biomarkers of both up- and down-relegation of the immune system given the diverse roles inflammatory markers play and in order to clarify the complex relationship between stress and the immune processes.
Funding
Research reported in this paper was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number F31MH113481 (Rogers) and some author time came from 9K24DA040489 (Safren). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the Department of Psychology at the University of Miami.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bainter S, McCauley TG, Wager TD, & Losin ER (2019). Improving practices for selecting a subset of important predictors in psychology: An application to predicting pain. [Preprint]. PsyArXiv. 10.31234/osf.io/j8t7s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ (1998). Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clinical Science, 94(6), 557–572. 10.1042/cs0940557 [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, Mimiaga MJ, & Safren SA (2015). Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS and Behavior, 19(6), 981–986. 10.1007/s10461-014-0925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges ÁH, O’Connor JL, Phillips AN, Rönsholt FF, Pett S, Vjecha MJ, French MA, Lundgren JD, & INSIGHT SMART and ESPRIT Study Groups and the SILCAAT Scientific Committee. (2015). Factors associated with plasma IL-6 levels during HIV infection. Journal of Infectious Diseases, 212(4), 585–595. 10.1093/infdis/jiv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, Bohjanen PR, Novak RM, Neaton JD, & Sereti I, & for the INSIGHT Study Group. (2011). Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. The Journal of Infectious Diseases, 203(11), 1637–1646. 10.1093/infdis/jir134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). HIV surveillance report, 2016 (Vol. 28). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106(1), 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, & Gangestad SW (2018). Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain, Behavior, and Immunity, 70, 61–75. 10.1016/j.bbi.2018.02.013 [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2008). Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection versus immunopathology. Allergy, Asthma & Clinical Immunology, 4(1), 2–11. 10.1186/1710-1492-4-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS (2009). Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation, 16(5), 300–317. 10.1159/000216188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fumaz CR, Gonzalez-Garcia M, Borras X, Muñoz-Moreno JA, Perez-Alvarez N, Mothe B, Brander C, Ferrer MJ, Puig J, Llano A, Fernandez-Castro J, & Clotet B (2012). Psychological stress is associated with high levels of IL-6 in HIV-1 infected individuals on effective combined antiretroviral treatment. Brain, Behavior, and Immunity, 26(4), 568–572. 10.1016/j.bbi.2012.01.001 [DOI] [PubMed] [Google Scholar]
- George EI, & McCulloch RE (1993). Variable selection via Gibbs sampling. Journal of the American Statistical Association, 88(423), 881–889. 10.1080/01621459.1993.10476353 [DOI] [Google Scholar]
- Glynn TR, Safren SA, Carrico AW, Mendez NA, Duthely LM, Dale SK, Jones DL, Feaster DJ, & Rodriguez AE (2019). High Levels of syndemics and their association with adherence, viral non-suppression, and biobehavioral transmission risk in Miami, a U.S. City with an HIV/AIDS epidemic. AIDS and Behavior, 23(11), 2956–2965. 10.1007/s10461-019-02619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness A, Bainter SA, O’Cleirigh C, Mendez NA, Mayer KH, & Safren SA (2018). Longitudinal effects of syndemics on ART non-adherence among sexual minority men. AIDS and Behavior, 22(8), 2564–2574. 10.1007/s10461-018-2180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW (2012). HIV and inflammation: Mechanisms and consequences. Current HIV/AIDS Reports, 9(2), 139–147. 10.1007/s11904-012-0118-8 [DOI] [PubMed] [Google Scholar]
- Hurst J, Hoffmann M, Pace M, Williams JP, Thornhill J, Hamlyn E, Meyerowitz J, Willberg C, Koelsch KK, Robinson N, Brown H, Fisher M, Kinloch S, Cooper DA, Schechter M, Tambussi G, Fidler S, Babiker A, Weber J, Frater J (2015). Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nature Communications, 6(1), 8495. 10.1038/ncomms9495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM SPSS Statistics for Windows. (2017). IBM SPSS Statistics for Windows, Version 25.0 (Version 25) [Computer software]. IBM Corp. [Google Scholar]
- Juster R-P, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kalokhe AS, Paranjape A, Bell CE, Cardenas GA, Kuper T, Metsch LR, & del Rio C (2012). Intimate partner violence among HIV-infected crack cocaine users. AIDS Patient Care and STDs, 26(4), 234–240. 10.1089/apc.2011.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, & Glaser R (2002). Depression and immune function: Central pathways to morbidity and mortality. Journal of Psychosomatic Research, 53(4), 873–876. 10.1016/S0022-3999(02)00309-4 [DOI] [PubMed] [Google Scholar]
- Krause N, Shaw BA, & Cairney J (2004). A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychology and Aging, 19(4), 637–648. 10.1037/0882-7974.19.4.637 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9 validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load (1st ed. Annals of the New York Academy of Sciences, 840(1), 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology: Official Journal of the Division of Health Psychology. Health Psychology, 21(6), 531–541. 10.1037/0278-6133.21.6.531 [DOI] [PubMed] [Google Scholar]
- Nixon DE, & Landay AL (2010). Biomarkers of immune dysfunction in HIV. Current Opinion in HIV and Aids, 5(6), 498–503. 10.1097/COH.0b013e32833ed6f4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, & Arseneault L (2011). Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behavior, and Immunity, 25(4), 640–646. 10.1016/j.bbi.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Pantalone DW, Valentine SE, Woodward EN, & O’Cleirigh C (2018). Syndemic indicators predict poor medication adherence and increased healthcare utilization for urban HIV-positive men who have sex with men. Journal of Gay & Lesbian Mental Health, 22(1), 71–87. 10.1080/19359705.2017.1389794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Schieman S, Fazio EM, & Meersman SC (2005). Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior, 46(2), 205–219. 10.1177/002214650504600206 [DOI] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC, Matthews KA, & Adler N (2013). A pandemic of the poor: Social disadvantage and the U.S. HIV epidemic. American Psychologist, 68(4), 197–209. 10.1037/a0032694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins A, Ouimette P, Kimerling R, Camerond RP, Hugelshofer DS, Shaw-Hegwer J, Thrailkill A, Gusman FD, & Sheikh JI (2004). The primary care PTSD screen (PC-PTSD): Development and operating characteristics. Primary Care Psychiatry, 9(1), 9–14. 10.1185/135525703125002360 [DOI] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, & Rifai N (2000). C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine, 342(12), 836–843. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- Russell AL, Tasker JG, Lucion AB, Fiedler J, Munhoz CD, Wu T-YJ, & Deak T (2018). Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. Journal of Neuroendocrinology, 30(10), e12641. 10.1111/jne.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salivary Interleukin-6 (2020). Salimetrics LLC. https://salimetrics.com/analyte/salivary-interleukin-6/
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Singer MC, Bulled N, Ostrach B, & Mendenhall E (2017). Syndemics and the biosocial conception of health. The Lancet, 389(10072), 941–950. 10.1016/S0140-6736(17)30003-X https://doi.org/10.1016/S0140–6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- Singer MC, & Clair S (2003). Syndemics and public health: Reconceptualizing disease in biosocial context. Medical Anthropology Quarterly, 17(4), 423–441. 10.1525/maq.2003.17.4.423 [DOI] [PubMed] [Google Scholar]
- Stall R, Coulter RWS, Friedman MR, & Plankey MW (2015). Commentary on “Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept” by A. Tsai and B. Burns. Social Science & Medicine (1982), 145, 129–131. 10.1016/j.socscimed.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Messer LC, & Quinlivan EB (2015). Substance abuse, violence, and HIV/AIDS (SAVA) syndemic effects on viral suppression among HIV positive women of color. AIDS Patient Care and Stds, 29(S1), S42–S48. 10.1089/apc.2014.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MD, Yu RK, & Shete S (2008). Finding factors influencing risk: Comparing Bayesian stochastic search and standard variable selection methods applied to logistic regression models of cases and controls. Statistics in Medicine, 27(29), 6158–6174. 10.1002/sim.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, & Kishimoto T (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology, 6(10), a016295–a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Meigs JB, & Grinspoon SK (2009). Association of C-reactive protein and hiv infection with acute myocardial infarction. JAIDS Journal of Acquired Immune Deficiency Syndromes, 51(3), 268–273. 10.1097/QAI.0b013e3181a9992c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallefont V, Raftery AE, & Richardson S (2001). Variable selection and Bayesian model averaging in case-control studies. Statistics in Medicine, 20(21), 3215–3230. 10.1002/sim.976 [DOI] [PubMed] [Google Scholar]
- Wilson IB, Fowler FJ, Cosenza CA, Michaud J, Bentkover J, Rana A, Kogelman L, & Rogers WH (2014). Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS and Behavior, 18(12), 2349–2358. 10.1007/s10461-013-0610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IB, Lee Y, Michaud J, Fowler FJ, & Rogers WH (2016). Validation of a new three-item self-report Measure for Medication Adherence. AIDS and Behavior, 20(11), 2700–2708. 10.1007/s10461-016-1406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, & Sahebkar A (2017). The impact of stress on body function: A review. EXCLI Journal, 16, 1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, & Palma P (2019). Immune activation, inflammation, and non-AIDS Co-morbidities in HIV-infected patients under long-term ART. Viruses, 11(3), 200. 10.3390/v11030200 [DOI] [PMC free article] [PubMed] [Google Scholar]


