Abstract
Sphingolipids are a class of lipids highly enriched in the central nervous system (CNS), which shows great diversity and complexity, and has been implicated in CNS development and function. Alterations in sphingolipid metabolism have been described in multiple diseases, including those affecting the central nervous system (CNS). In this review, we discuss the role of sphingolipid metabolism in neurodegeneration, evaluating its direct roles in neuron development and health, and also in the induction of neurotoxic activities in CNS-resident astrocytes and microglia in the context of neurologic diseases such as multiple sclerosis and Alzheimer’s disease. Finally, we focus on the metabolism of gangliosides and sphingosine-1-phosphate, its contribution to the pathogenesis of neurologic diseases, and its potential as a candidate target for the therapeutic modulation of neurodegeneration.
Introduction
In 1874, the German scientist Johann Ludwig Wilhelm Thudichum identified sphingolipids, as a class of lipids highly enriched in the central nervous system (CNS). Sphingolipids are the second most abundant membrane lipids after phospholipids (Merrill et al. 1997). This class of lipids shows great diversity and complexity, and has been implicated in CNS development and function. Indeed, sphingolipids are not only structural components of the cell membrane, they play important roles in cellular processes such as endocytosis, intracellular trafficking and signal transduction (Hannun & Obeid 2008).
Sphingolipid structure and metabolism
Structurally, sphingolipids are composed of a long fatty acid chain and a polar head group in position one. Depending on their polar head group, sphingolipids are classified into three groups: (a) ceramides, (b) sphingomyelins, and (c) glycosphingolipids (Lahiri & Futerman 2007). The sphingolipid backbone is composed of a sphingoid base (sphingosine), which is a long chain of amino alcohol, originally represented by dihydrosphingosine and a chain length of 18 carbon atoms. Dihydrosphingosine, also known as sphinganine, and its N-acyl-derivatives are intermediaries in the de novo biosynthesis of ceramide. More complex examples of sphingolipids are phosphosphingolipids and glycosphingolipids with head groups attached to the hydroxyl group on carbon 1. The structural diversity of the latter compounds is highlighted by the hundreds of head group variants in mammals alone (Lahiri & Futerman 2007).
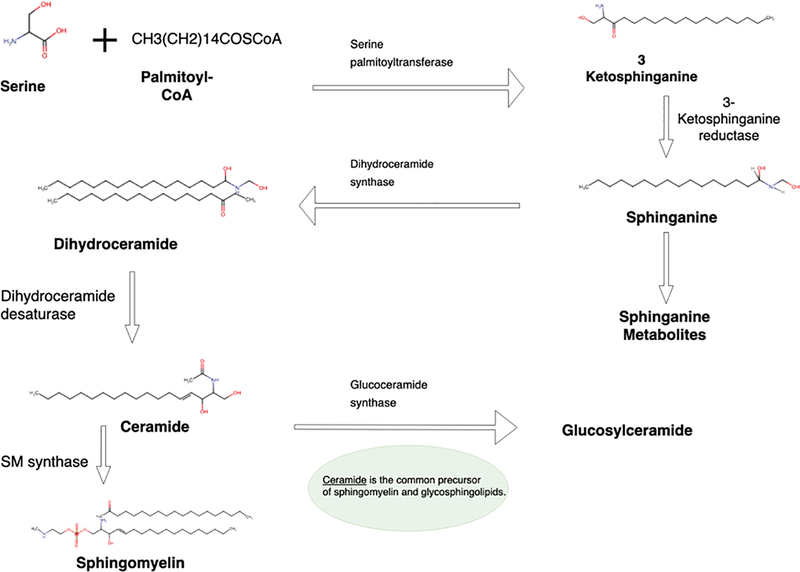
Sphingolipid biosynthesis is initiated when sphinganine is synthesized de novo from serine and a long chain fatty acyl-CoA. Subsequently, sphinganine is N-acylated with a long-chain fatty acid to form N-acyl-sphinganine (dihydroceramide) (Hannun & Obeid 2018). Subsequent addition of a double bond at the 4, 5 carbon–carbon bond of the sphinganine backbone results in the formation of ceramide N-acylsphingosine. More complex sphingolipids are formed when polar head groups are added at position 1 of ceramide such as sphingomyelin. Ceramide produced in the endoplasmic reticulum is transported to the Golgi apparatus (Figure 1) and converted into complex sphingolipids (Hannun & Obeid 2008).
Figure 1.
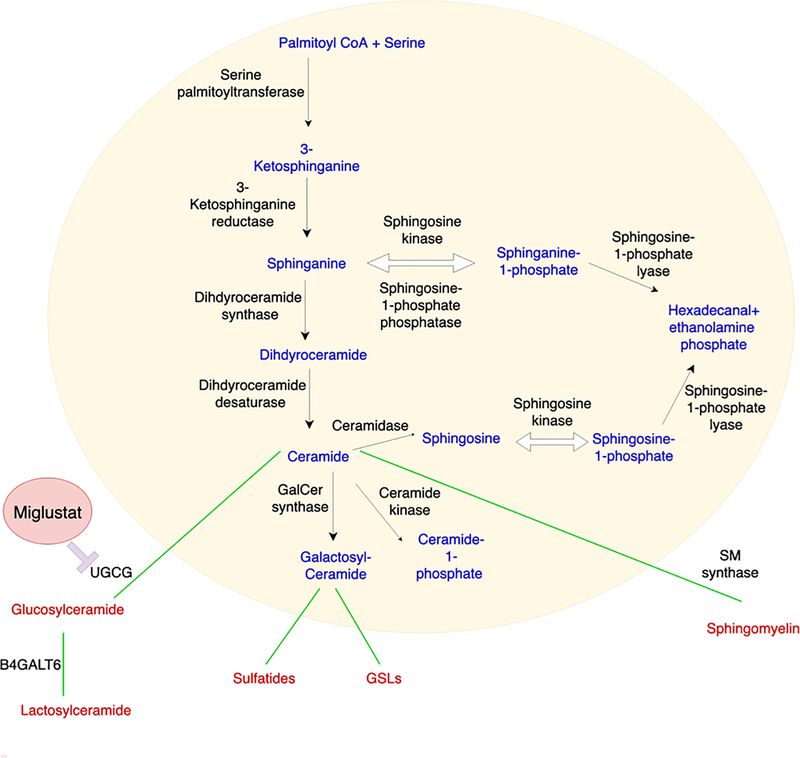
Ceramide is a central point for both biosynthesis and catabolism (Figure 2). First, ceramide is phosphorylated by ceramide kinase to produce ceramide 1-phosphate. Second, phosphocholine is added by sphingomyelin synthase to produce sphingomyelin. Third, a sugar molecule is added by glucosyl- and galactosyl-ceramide synthases to produce glucosylceramide (GluCer) or galactosylceramide (GalCer) (Hannun & Obeid 2018; Gault et al. 2010). While sphingomyelin is not modified any further, glucosyl- and galactosyl-ceramides are further modified to produce complex glycosphingolipids (GSls) (Gault et al. 2010). GalCer is sulphated by cerebroside sulfotransferase to produce sulfatide, which is subsequently galactosylated to produce Gb2 or sialylated to produce GM4. GalCer is galactosylated to produce lactosylceramide (LacCer) by lactosylceramide synthase (LCS) (Yamaji & Hanada 2015). LacCer has multiple fates and is further glycosylated to produce a series of GSLs which are processed by additional glycosyltransferases to produce nearly 500 varieties of GSLs (Chatterjee & Alsaeedi 2012).
Figure 2.
Alternatively, ceramide is broken down by ceramidase, which removes the acyl chain to produce lyso-sphingolipid sphingosine. In addition, sphingosine can also be reconverted to ceramide by ceramide synthase or dephosphorylation by sphingosine kinase to produce sphingosine 1-phospate (S1P). S1P is dephosphorylated to become sphingosine or irreversibly catalyzed by S1P lyase to ethanolamine phosphate and hexadecenal.
Sphingolipids show differential biologic activities as a result of variability in their functional groups. The main bioactive sphingolipids are ceramides, ceramide 1-phosphates (C1P), glucosylceramides, sphingosines, and S1P (Hannun & Obeid 2011). Ceramide usually inhibits cell proliferation and promotes apoptosis (van Echten-Deckert & Herget 2006), while S1P stimulates growth and suppresses apoptosis. The induction of apoptosis and growth is linked to ceramides implicated in the action of tumor necrosis factor-α (TNF-α). Ceramides are also involved in cytotoxic responses to amyloid-β (Aβ) and its role in Alzheimer’s disease (AD) and neurodegeneration (Hardy & Selkoe 2002). Moreover, ceramide signaling has been linked to inflammation, free radical generation and oxidative stress in neurons (Maceyka & Spiegel 2014). Because of these and additional biologic activities, slight changes in the metabolism of ceramides and other sphingolipids has profound effects on CNS homeostasis and function (van Echten-Deckert & Herget 2006). Thus, sphingolipid metabolism has been identified as an important player in neurologic diseases and a candidate target for therapeutic intervention, as discussed below.
Sphingolipid metabolism in neurodegenerative diseases
Defects in sphingolipid metabolism have been linked to numerous neurologic diseases including AD, Parkinson’s disease (PD), and multiple sclerosis (MS). Indeed, the activity of sphingolipid-metabolizing enzymes and the levels of sphingolipid metabolites are usually modulated in pathophysiological conditions. For example, plasma sphingolipid concentrations serve as prognostic biomarkers for various diseases (Aoki et al. 2016).
AD is a neurodegenerative disorder linked to Aβ accumulation, characterized by a progressive cognitive decline leading to dementia (Tanzi & Bertram 2005). Sphingolipids have been shown to contribute to the development and progression of AD (van Echten-Deckert & Herget 2006; van Echten-Deckert & Walter 2012). In particular, Aβ accumulation stimulates sphingomyelin hydrolysis, increasing ceramide levels in AD (Haughey et al. 2010). Indeed, Flippov et al detected elevated ceramide species in CNS samples from AD patients (Filippov et al. 2012). One potential mechanism involved is the increased expression of the enzyme ceramide synthase 2 (CerS2), which participates in the de novo synthesis of ceramide (Katsel et al. 2007). The increase in ceramide levels was detected during the mild stage of dementia and decreased afterwards during the course of AD (Katsel et al. 2007), suggesting that it represents an early event in disease pathology. Support for a pathogenic role of sphingolipid metabolism dysregulation in AD was provided by Couttas et al, who reported that the disrupted balance of ceramides and S1P promotes neurodegeneration in the brain cortex (Couttas et al. 2014). Indeed, depletion of glycosphingolipids and decreased synthesis of S1P correlates with AD progression.
Sphingolipids also play a role in PD pathogenesis, characterized by the accumulation of α-synuclein in neurons (Halliday et al. 2005). A link between sphingolipid metabolism and PD was reported in a lipodomic study in which the analysis of PD brain samples detected increased levels of ceramide species, including monohexosylceramide, lactosylceramide and sphingomyelin (Abbott et al. 2014). Ceramide abundance was highest among PD patients with the largest neurologic impairment, suggesting that ceramide and monohexosylceramide metabolism contribute to PD pathophysiology (Mielke et al. 2013). Interestingly, mutations in glucocerebrosidase (GBA), which catalyzes the synthesis of ceramide from glucocerebroside (glucosylceramide), are among the top genetic contributors to PD development (Pchelina et al. 2017; Arkadir et al. 2018). In addition, β-glucocerebrosidase activity is reduced in the cerebrospinal fluid of PD patients independently of GBA mutations (Parnetti et al. 2017), resulting in elevated plasma glucosylceramide levels in PD patients (Mielke et al. 2013).
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS (Reich et al. 2018). In most patients, MS initially shows a relapsing-remitting clinical course (RRMS), which is followed by a secondary progressive phase (SPMS) characterized by the progressive and irreversible accumulation of neurologic disability unresponsive to most available therapies (Reich et al. 2018). Thus, there is an unmet clinical need for efficacious therapies for SPMS (Reich et al. 2018). In its RRMS phase, MS is thought to be driven by recruited peripheral autoreactive immune cells targeting myelin and other antigens in the CNS (Reich et al. 2018). However, inflammation driven by CNS-resident cells such as astrocytes and microglia is thought to be the major contributor to disease pathogenesis in the SPMS phase (Colonna & Butovsky 2017; Fontana et al. 1984; Ousman et al. 2007; Sun & Wekerle 1986; Wheeler & Quintana 2019; Liddelow & Barres 2017; Rothhammer et al. 2018; Rothhammer et al. 2016; Wheeler et al. 2019a; Chao et al. 2019; Wheeler et al. in press).
Sphingolipids are major components of oligodendrocytes and the myelin sheath. Abnormalities in sphingolipid metabolism have been described in MS (Vidaurre et al. 2014; Wheeler et al. 2008; Cumings & Goodwin 1968). In addition, antibodies to sphingolipids are detected in SPMS patients and correlate with markers of disease progression such as brain atrophy, a feature of MS thought to be driven by astrocytes and microglia (Bakshi et al. 2016; Farez et al. 2009; Kanter et al. 2006). Interestingly, antibodies to sphingolipids and antibodies to other lipids are independently regulated in MS patients (Sadaba et al. In press). Most importantly, sphingolipid metabolism and its products promote inflammation through multiple mechanisms (Maceyka & Spiegel 2014). CerS2 and CerS6, for example, drive neutrophil pro-inflammatory activities in EAE (Barthelmes et al. 2015; Eberle et al. 2015). Moreover, the sphingolipid S1P controls the T-cell response and is a therapeutic target in MS (Blaho et al. 2015; Liu et al. 2010; Mandala et al. 2002; Matloubian et al. 2004; Pappu et al. 2007).
In addition, increased ceramide production and accumulation have been linked to oligodendrocyte damage and demyelination; enhanced levels of S1PR1 have also been reported in active MS lesions (Kim et al. 2012; Van Doorn et al. 2010). Indeed, disturbances in sphingolipid metabolism destabilize myelin structure (Olsen & Faergeman 2017). Potent activators of the sphingomyelin cycle in oligodendrocytes include pro-inflammatory cytokines and oxidative stress which characterize MS pathogenesis. It has also been reported that IL-1β and TNF-α decrease intracellular glutathione and induce the degradation of sphingomyelin to ceramide (Jana & Pahan 2010).
Recently it was reported that LacCer produced by β−1,4-galactosyltransferase 6 (B4GALT6) in astrocytes promotes the recruitment of CNS-infiltrating monocytes, microglial activation, CNS inflammation and neurodegeneration in MS and its model experimental autoimmune encephalomyelitis (EAE) (Mayo et al. 2014). Indeed, through a combination of proteomic, metabolomic, transcriptomic and in vivo cell-specific gene perturbation studies, we recently found that sphingolipid metabolism in astrocytes triggers the interaction of the C2 domain in cytosolic phospholipase A2 (cPLA2) with the CARD domain in mitochondrial antiviral signaling protein (MAVS), boosting NF-κB-driven transcriptional programs that promote CNS inflammation in EAE and MS (Chao et al. 2019). cPLA2 recruitment to MAVS also disrupts MAVS-hexokinase 2 (HK2) interactions, decreasing HK enzymatic activity and the production of lactate involved in the metabolic support of neurons (Chao et al. 2019). Collectively, these findings define a novel immunometabolic mechanism by which sphingolipid metabolism drives pro-inflammatory astrocyte activities, while they outline a new role for MAVS in CNS inflammation. Moreover, since sphingolipid metabolism and cPLA2 have been linked to multiple neurologic disorders in which astrocytes contribute to disease pathogenesis (Stephenson et al. 1999; Sanchez-Mejia et al. 2008; Maceyka & Spiegel 2014; Platt 2014), cPLA2-MAVS signaling may be a common driver of astrocyte pathogenic activities, synergizing with signaling pathways associated to specific astrocyte populations and neurologic disorders to amplify CNS pathology (Liddelow & Barres 2017; Liddelow et al. 2017; Wheeler et al. in press).
Biology of Gangliosides
Gangliosides are a large class of glycosphingolipids that consist of a ceramide and an oligosaccharide backbone, with one or more sialic acid residues present in most, if not all, mammalian tissues. They play a pivotal role in several physiological processes, including cell signaling, neuronal recovery and protection (Mojumdar et al. 2019). Structurally, gangliosides are an essential constituent of the cell surface membrane of almost all animal cells, where they are positioned with the ceramide group anchored in the external face of the lipid bilayer and sialylated glycans protruding from the surface of the plasma membrane (Nobile-Orazio et al. 1994). The heterogeneity of the sialoglycan molecular structure is the molecular hallmark of gangliosides; together with variations in ceramide lipid constituents it results in hundreds of distinct ganglioside structures.
Ganglioside concentration, composition and patterns vary among different animal species, and also between ontogenetic developmental stages and cell types, suggesting that individual gangliosides serve distinct biological functions (Sena 1993). Indeed, gangliosides play a pivotal role in many physiological processes, including cell signaling, neuronal recovery and protection, and apoptosis. They regulate these biochemical and cellular functions by two general molecular strategies: a) The modulation of the interaction of gangliosides with proteins on the same cell membrane (cis regulation), and b) The interaction between the ganglioside in the surface in one cell and glycan binding proteins (receptors) on another cell (trans regulation) which triggers specific signaling responses. In these cases, signal transduction is thought to involve the downstream hydrocarbon moiety of the ganglioside or transmitted to receptor-bearing cells via conformational changes induced in the receptor (Schnaar 2016).
Experimental evidence indicates that the orientation of gangliosides on the cell surface is key to their biological functions. In an Nuclear magnetic resonance and modeling study of gangliosides, Aureli et al. found that the glucose-ceramide bond of GM1, one of the abundant gangliosides in the mammalian brain, is oriented such that the glycan extends perpendicular to the plane of the lipid bilayer of the plasma membrane (Aureli et al. 2016). Additional studies on GM1 also suggest that a dominant conformation displayed by brain gangliosides is the rigid block structure of the branch trisaccharide of GM1 gangliosides (Schnaar 2016).
Role of Gangliosides in Neurodegeneration
The CNS is enriched with gangliosides, which account for ~15 % of the total lipid content in the neuronal membrane (Mojumdar et al. 2019). The primary gangliosides in neural stem cells are GM3 and GD3 which contain only glucose (Glc), galactose (Gal), and one or two sialic acid moieties, whereas gangliosides containing more complex carbohydrate moieties, GM1a, GD1a, GD1b and GT1b are highly expressed during the generation of neurons and astrocytes (Schengrund 2015). Following the finding that gangliosides affect neural differentiation and maturation, most research focused on the study of the signal transduction mechanisms involved (Bremer et al. 1984). However, a potential role of gangliosides in neurodegeneration was suggested following the detection of CNS deficits in infantile-onset symptomatic epilepsy syndrome as a result of an homozygous loss-of-function mutation in the GM3 synthase (Simpson et al. 2004).
Accumulating evidence suggest a role for gangliosides in the pathology of neurodegenerative diseases such as PD, AD, and Huntington’s disease (HD). GM1 administration reduces neuronal damage as a result of its neurotrophic or neuroprotective roles detected in a variety of neurodegenerative conditions. However, although based on the beneficial effects of GM1 in PD patients and animal models GM1 signaling has been suggested as a potential therapeutic target, the molecular mechanisms underlying GM1 neurotrophic and neuroprotective effects are still unclear (Schneider 2014). In vitro studies have shown that lipid raft-associated GM1 gangliosides bind to α-synuclein in small unilamellar vesicles altering the structure of the α-synuclein and obstructing its ability to aggregate and form insoluble fibrils therefore disrupting normal neuronal function (Schengrund 2015).
GM1 gangliosides have also been implicated in the regulation of Aβ, with potential implications for AD pathology. GM1 gangliosides are thought to contribute to AD pathogenesis by accelerating the extracellular aggregation of Aβ to form the characteristic fibrils of AD in a structurally specific manner (GM1 > GD1b > GD1a, GT1b) (Schnaar 2016). Indeed, recent work suggests that Aβ is preferentially inserted into GM1-rich membrane regions where peptides undergo a conformational change that interrupts membrane stability and induces oligomer formation (Schneider 2014). However, other studies have shown that GM1 alone inhibits the Aβ-induced release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Noteworthy, increased GM1 and GD1 levels were detected when GD3-synthase was deleted in a transgenic mouse model of AD, resulting in improved memory and reduced aggregation and formation of Aβ plaques (Ledeen & Wu 2018).
Altered ganglioside biosynthesis and composition have also been implicated in HD pathology in several studies. Desplats et al. reported reduced expression of genes encoding glycosyltransferases and sialyltransferases involved in ganglioside biosynthesis in the striatum of HD transgeneic mice, concomitant with reduced levels of GM1. Notably, similar results were observed in postmortem caudate nucleus samples from HD patients (Desplats et al. 2007). Di Pardo et al. confirmed previous findings of decreased GM1 levels HD, while showing that GM1 administration by intraventricular infusion in a pre-clinical HD mouse model induces phosphorylation of mutant huntingtin and restores normal motor behavior, increasing the striatal expression of DARP-32 (Di Pardo et al. 2012). Taken together, these findings suggest important roles for ganglioside metabolism in neurodegeneration, and identify GM1 signaling as a candidate therapeutic target.
Sphingosine-1-phosphate in neurodegeneration
Sphingosine-1-phosphate (S1P) signaling is crucial for the regulation of multiple cellular events including cell survival, proliferation, differentiation, and migration of neurons, astrocytes, and microglia (Tham et al. 2003). S1P is a key player implicated in a plethora of biological mechanisms related to neurodegeneration including neurotoxicity, autophagy and neuroinflammation (Mitroi et al. 2016; Moruno Manchon et al. 2015; Hagen et al. 2011). S1P is reported to be neurotoxic (Hagen et al. 2011). Indeed, Moore et al demonstrated that S1P induces apoptosis in hippocampal neurons, and the accumulation of S1P induced apoptosis in neurons lacking S1P lyase (S1PL) (Moore et al. 1999). However, sphingosine kinase 1 (SK1), which catalyzes S1P synthesis, is upregulated in murine brains during development; S1P deficiency results in severe defects in neural cell survival and neurogenesis (Mizugishi et al. 2005). Other studies have also shown crucial roles for S1P on the stability of the presynaptic structure, synaptic function and strength (Brailoiu et al. 2002; Camoletto et al. 2009; Darios et al. 2009; Kanno et al. 2010; Riganti et al. 2016). Moreover, Mitroi et al showed that S1PL ablation impairs presynaptic architecture and function through a ubiquitin-proteasome mediated mechanism (Mitroi et al. 2016). In addition, S1P also affects microglial and astrocyte pro-inflammatory responses known to promote neurodegeneration (Lv et al. 2016; Nayak et al. 2010; Rothhammer et al. 2017).
Based on its important biologic roles, it is not surprising that S1P has been linked to neurodegeneration in multiple diseases. In the context of MS, targeting S1P signaling has shown to interfere with pathologic processes driven by the adaptive immune response (Derfuss et al. 2020). However, S1P may play additional roles in neurodegenerative processes associated to disease progression. Choi et al reported upregulation of the S1P receptors S1P1 and S1P3 in reactive astrocytes present in demyelinating and chronic MS lesions (Choi et al. 2011). S1P alters astrocyte morphology and increases the expression of the markers of astrogliosis (Choi et al. 2011). The same group demonstrated that the downregulation of S1P receptors in astrocytes and microglia using fingolimod reduces demyelination, increases remyelination, and suppresses the production of pro-inflammatory cytokines (Choi et al. 2011). Moreover, S1P receptor modulation suppresses astrocyte-pathogenic activities in murine and human models, and ameliorates disease pathogenesis in an experimental model of SPMS (Rothhammer et al. 2017).
In the context of AD, the role of S1P is still controversial, with seemingly opposing results which have not been reconciled. Takasugi et al reported a direct correlation between S1P levels and Aβ generation in neurons. In line with these findings, it was reported that lowering SK activity or increasing the activity of S1P-degrading enzymes decreased Aβ generation (Takasugi et al. 2011). However, Couttas et al showed that S1P levels in the hippocampus are independent of Aβ generation (Couttas et al. 2014). Moreover, Ceccom et al demonstrated a negative correlation between the expression of SK1 and that of S1PL suggesting a negative correlation between S1P content and Aβ levels (Ceccom et al. 2014). Thus, additional studies are needed to clarify the role of S1P in AD pathology.
Additional studies have investigated the contribution of S1P signaling to the pathogenesis of other neurologic disorders. For instance, De Pardo et al reported that S1P metabolism is disrupted in Huntington Disease (HD) and some of its pre-clinical models (Di Pardo et al. 2012). S1P metabolism may participate in HD pathology through multiple mechanisms. For example, S1P promotes autophagy in neurons (Mitroi et al. 2017; Moruno Manchon et al. 2015; Nixon 2013). Autophagy plays key roles in neuronal survival, preventing the accumulation of misfolded proteins and dysfunctional organelles (Nixon 2013). Autophagy stimulation triggers the relocation of SK1 to organelles positive for endosomal or autophagosomal markers or both in neurons, whereas, expression of a dominant-negative form of SK1 inhibits autophagosome formation (Moruno Manchon et al. 2015). In addition, using an HD pre-clinical model the same group demonstrated that inhibition of S1PL by 2-acetyl-5-tetrahydroxybutyl imidazole (THI) enhanced neuron survival (Moruno Manchon et al. 2015). However, the relationship between S1P and autophagy is complex, as it has been recently reported that S1PL deficiency leading to increased S1P levels, blocks the autophagic flux in murine brains (Mitroi et al. 2017). Further studies on the role of S1P in neurodegeneration may provide novel therapeutic strategies for diseases in which the S1P pathway is not yet being fully understood.
Therapeutic targeting of sphingolipid metabolism in neurodegenerative disease
Several lines of evidence identify sphingolipid metabolism as a viable therapeutic target for neurodegeneration. Indeed, a number of compounds selectively targeting sphingolipid signaling have been developed for the treatment of neurologic disorders (Stepanovska & Huwiler 2019) (Table I). FTY720 (fingolimod) is a structural analogue of sphingosine. Pharmacologically, FTY720 is a non-selective agonist of all S1PRs (with the exception of S1PR2), which triggers the down-modulation of S1P signaling (Stepanovska & Huwiler 2019). FTY720 inhibits S1P-driven lymphocyte egress from the thymus and lymph nodes to the periphery, and therefore limits lymphocyte recruitment to sites of inflammation. FTY720 also crosses the blood-brain barrier, where it can act directly on neuronal and glial cells (Stepanovska & Huwiler 2019; Rothhammer et al. 2017). Several lines of evidence suggest that FTY720 modulates sphingolipid metabolism-driven processes that contribute to chronic CNS inflammation and progressive neurodegeneration (Brunkhorst et al. 2014). In vitro studies have demonstrated direct effects of FTY720 on human astrocytes, oligodendrocytes and neurons (Wu et al. 2013; Miron et al. 2010; Janssen et al. 2015; Sheridan & Dev 2012; Rothhammer et al. 2017). In addition, recent findings reported that FTY720 ameliorated chronic progressive experimental encephalomyelitis (EAE) in non-obese diabetic mice, an experimental model of CNS chronic inflammation and progressive neurodegeneration in which disease pathology is driven by CNS-resident cells during the progressive phase of the disease (Rothhammer et al. 2017). The same study also reported that FTY720 administration reduced the expression of chemoattractant, pro-inflammatory, and neurotoxic molecules known to drive the pathogenicity of CNS-infiltrating monocytes, astrocytes, and microglia in MS. Moreover, unbiased genome-wide association studies revealed that FTY720 suppresses transcriptional modules that drive disease progression by astrocytes, which may enable the identification of other new therapeutics for the treatment of progressive neurodegenerative disease (Rothhammer et al. 2017). FTY720 is approved by the United States Food and Drug Administration for the treatment of RRMS, and has reached phase III for PPMS (Stepanovska & Huwiler 2019).
Table 1.
Summary of drugs targeting S1P signaling.
| S1PR modulator | Molecular target | Phase of clinical trial |
|---|---|---|
| Fingolimod | S1P1, S1P3, S1P4, S1P5 | Approved for RRMS Phase III for PPMS |
| Siponimod | S1P1, S1P5 | Phase III for SPMS Phase I for RRMS |
| Amiselimod | S1P1, S1P4, S1P5 | Phase II for RRMS |
| Ceralifimod | S1P1, S1P5 | Phase II for RRMS |
| Ozanimod | S1P1, S1P5 | Phase III for RRMS |
| CS-0777 | S1P1 | Phase I for MS |
| Ponesimod | S1P1 | Phase III for RRMS |
Following the development of FTY720 and its clinical success in treating relapsing-remitting MS, ongoing research efforts have focused on the development of FTY720 analogues with increased selectivity for S1PR1 and S1PR5 (Pan et al. 2013). These efforts led to the development of BAF312 (Siponimod), a potent and selective dual agonist at S1PR1 and S1PR5 (Pan et al. 2013). The therapeutic potency of BAF312 in MS has been recently documented in terms of clinical and MRI parameters (Kappos et al. 2016). Currently, BAF312 is undergoing a phase III efficacy and safety clinical trial in patients with SPMS (Stepanovska & Huwiler 2019). Since BAF312 regulates multiple signaling pathways in astrocytes and attenuates demyelination in organotypic slice cultures, it may show therapeutic efficacy beyond MS (O’Sullivan et al. 2016).
As we already mentioned, it was reported that LacCer produced by B4GALT6 in astrocytes promotes disease pathogenesis in MS and EAE. These findings suggest that the modulation of sphingolipid metabolism is a potential therapeutic approach for neurodegenerative diseases such as MS, in which astrocyte activation drives disease pathology (Baecher-Allan et al. 2018; Mayo et al. 2016; Rothhammer et al. 2018; Rothhammer et al. 2016; Wheeler et al. 2019b; Wheeler & Quintana 2019; Itoh et al. 2018). Interestingly, B4GALT6 deletion results in a major reduction in LacCer synthetic activity in the CNS, identifying B4GALT6 as a candidate target for the therapeutic modulation of astrocyte activity (Tokuda et al. 2013). Indeed, Miglustat, a glucosylceramide synthase inhibitor which is currently used to treat Gaucher and Niemann–Pick disease, suppresses astrocyte pathogenic activities and ameliorates chronic progressive EAE in NOD mice, a pre-clinical model of SPMS (Chao et al. 2019). Hence, Miglustat and other drugs developed to modulate sphingolipid metabolism in lysosomal storage disorders (Platt 2014) constitute candidate agents to be repurposed for SPMS treatment.
Additional studies have documented the potential therapeutic benefit of targeting sphingolipids in other neurologic disorders. For instance, Pirhaji et al reported neuroprotective effects of S1P signaling inhibition in Huntington’s disease models (Pirhaji et al. 2017). S1P signaling inhibition is also reported to offer a possible therapeutic strategy to protect neurons from mutant huntingtin-induced neurotoxicity (Moruno Manchon et al. 2015). S1P receptor targeting also stimulates the production of pro-survival pathways and the generation of neurotrophins, and eventually suppresses disease progression in HD animal models (Miguez et al. 2015; Di Pardo et al. 2014).
Although limited information is still available, there is evidence suggesting that S1P receptor modulation also has beneficial effects in AD models. For example, S1P receptor modulation inhibits cognitive impairment and promotes spatial learning (Asle-Rousta et al. 2013), while it also prevents Aβ-induced alterations in the expression of pro-inflammatory and pro-apoptotic markers in AD mouse models (Asle-Rousta et al. 2014). Moreover, S1P receptor modulators decrease Aβ levels in in vivo and in vitro AD models (Takasugi et al. 2013). Taken together, these data support the role of sphingolipid metabolism as a target for the development of novel therapeutics for neurologic disorders.
Conclusions
Targeting sphingolipid metabolic pathways in the context of neurodegenerative disease is a viable strategy for arresting CNS inflammation and neurodegeneration. Furthermore, the identification of specific pathways in astrocytes and microglia that are regulated by sphingolipid metabolism warrants further investigation as critical modulators of neurodegeneration and CNS pathology. Hence, sphingolipid metabolism may offer novel therapeutic targets to arrest disease progression in neurodegenerative disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health, the National Multiple Sclerosis Society, the International Progressive MS Alliance and the King Abdulaziz City for Science and Technology.
Abbreviations used
- THI
acetyl-5-tetrahydroxybutyl imidazole
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- B4GALT6
β−1,4-galactosyltransferase 6
- CNS
central nervous system
- C1P
ceramide 1-phosphates
- CerS2
ceramide synthase 2
- CSF
cerebrospinal fluid
- cPLA2
cytosolic phospholipase A2
- EAE
experimental autoimmune encephalomyelitis
- Gal
galactose
- GalCer
galactosylceramide
- Glc
glucose
- GBA
glucocerebrosidase
- GluCer
glucosylceramide
- GSls
glycosphingolipids
- HK2
hexokinase 2
- HD
Huntington’s disease
- LacCer
lactosylceramide
- LCS
lactosylceramide synthase
- MAVS
mitochondrial antiviral signaling protein
- MS
Multiple sclerosis
- PD
Parkinson’s disease
- MS
multiple sclerosis
- RRMS
relapsing-remitting clinical course
- SPMS
secondary progressive phase
- S1PL
S1P lyase
- S1P
sphingosine 1-phospate
- SK1
sphingosine kinase 1
- TNF-α
tumor necrosis factor-α
Footnotes
Competing financial interests
The other authors declare no competing interests.
References
- Abbott SK, Li H, Munoz SS, Knoch B, Batterham M, Murphy KE, Halliday GM and Garner B (2014) Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov Disord 29, 518–526. [DOI] [PubMed] [Google Scholar]
- Aoki M, Aoki H, Ramanathan R, Hait NC and Takabe K (2016) Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm 2016, 8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkadir D, Dinur T, Mullin S, Mehta A, Baris HN, Alcalay RN and Zimran A (2018) Trio approach reveals higher risk of PD in carriers of severe vs. mild GBA mutations. Blood Cells Mol Dis 68, 115–116. [DOI] [PubMed] [Google Scholar]
- Asle-Rousta M, Kolahdooz Z, Dargahi L, Ahmadiani A and Nasoohi S (2014) Prominence of central sphingosine-1-phosphate receptor-1 in attenuating abeta-induced injury by fingolimod. J Mol Neurosci 54, 698–703. [DOI] [PubMed] [Google Scholar]
- Asle-Rousta M, Kolahdooz Z, Oryan S, Ahmadiani A and Dargahi L (2013) FTY720 (fingolimod) attenuates beta-amyloid peptide (Abeta42)-induced impairment of spatial learning and memory in rats. J Mol Neurosci 50, 524–532. [DOI] [PubMed] [Google Scholar]
- Aureli M, Mauri L, Ciampa MG, Prinetti A, Toffano G, Secchieri C and Sonnino S (2016) GM1 Ganglioside: Past Studies and Future Potential. Mol Neurobiol 53, 1824–1842. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Kaskow BJ and Weiner HL (2018) Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 97, 742–768. [DOI] [PubMed] [Google Scholar]
- Bakshi R, Yeste A, Patel B et al. (2016) Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurology(R) neuroimmunology & neuroinflammation 3, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmes J, de Bazo AM, Pewzner-Jung Y et al. (2015) Lack of ceramide synthase 2 suppresses the development of experimental autoimmune encephalomyelitis by impairing the migratory capacity of neutrophils. Brain, behavior, and immunity 46, 280–292. [DOI] [PubMed] [Google Scholar]
- Blaho VA, Galvani S, Engelbrecht E et al. (2015) HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523, 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Cooper RL and Dun NJ (2002) Sphingosine 1-phosphate enhances spontaneous transmitter release at the frog neuromuscular junction. Br J Pharmacol 136, 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S, Bowen-Pope DF, Raines E and Ross R (1984) Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem 259, 6818–6825. [PubMed] [Google Scholar]
- Brunkhorst R, Vutukuri R and Pfeilschifter W (2014) Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci 8, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camoletto PG, Vara H, Morando L, Connell E, Marletto FP, Giustetto M, Sassoe-Pognetto M, Van Veldhoven PP and Ledesma MD (2009) Synaptic vesicle docking: sphingosine regulates syntaxin1 interaction with Munc18. PLoS One 4, e5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccom J, Loukh N, Lauwers-Cances V et al. (2014) Reduced sphingosine kinase-1 and enhanced sphingosine 1-phosphate lyase expression demonstrate deregulated sphingosine 1-phosphate signaling in Alzheimer’s disease. Acta Neuropathol Commun 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gutierrez-Vazquez C, Rothhammer V et al. (2019) Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell 179, 1483–1498.e1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S and Alsaeedi N (2012) Lactosylceramide synthase as a therapeutic target to mitigate multiple human diseases in animal models. Adv Exp Med Biol 749, 153–169. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR et al. (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108, 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M and Butovsky O (2017) Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttas TA, Kain N, Daniels B et al. (2014) Loss of the neuroprotective factor Sphingosine 1-phosphate early in Alzheimer’s disease pathogenesis. Acta Neuropathol Commun 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumings JN and Goodwin H (1968) Sphingolopids and phospholipids of myelin in multiple sclerosis. Lancet (London, England) 2, 664–665. [DOI] [PubMed] [Google Scholar]
- Darios F, Wasser C, Shakirzyanova A et al. (2009) Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 62, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen JA and Kappos L (2020) Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol 19, 336–347. [DOI] [PubMed] [Google Scholar]
- Desplats PA, Denny CA, Kass KE, Gilmartin T, Head SR, Sutcliffe JG, Seyfried TN and Thomas EA (2007) Glycolipid and ganglioside metabolism imbalances in Huntington’s disease. Neurobiol Dis 27, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pardo A, Amico E, Favellato M, Castrataro R, Fucile S, Squitieri F and Maglione V (2014) FTY720 (fingolimod) is a neuroprotective and disease-modifying agent in cellular and mouse models of Huntington disease. Hum Mol Genet 23, 2251–2265. [DOI] [PubMed] [Google Scholar]
- Di Pardo A, Maglione V, Alpaugh M et al. (2012) Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A 109, 3528–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle M, Ebel P, Mayer CA et al. (2015) Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol Cell Biol 93, 825–836. [DOI] [PubMed] [Google Scholar]
- Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M and Weiner HL (2009) Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nature immunology 10, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, Yang J and Duerksen-Hughes PJ (2012) Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J Alzheimers Dis 29, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Fierz W and Wekerle H (1984) Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307, 273–276. [DOI] [PubMed] [Google Scholar]
- Gault CR, Obeid LM and Hannun YA (2010) An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol 688, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen N, Hans M, Hartmann D, Swandulla D and van Echten-Deckert G (2011) Sphingosine-1-phosphate links glycosphingolipid metabolism to neurodegeneration via a calpain-mediated mechanism. Cell Death Differ 18, 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Ophof A, Broe M et al. (2005) Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain 128, 2654–2664. [DOI] [PubMed] [Google Scholar]
- Hannun YA and Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9, 139–150. [DOI] [PubMed] [Google Scholar]
- Hannun YA and Obeid LM (2011) Many ceramides. J Biol Chem 286, 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA and Obeid LM (2018) Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19, 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J and Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Bandaru VV, Bae M and Mattson MP (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta 1801, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Itoh Y, Tassoni A et al. (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc Natl Acad Sci U S A 115, E302–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A and Pahan K (2010) Sphingolipids in multiple sclerosis. Neuromolecular Med 12, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Schlegel C, Gudi V, Prajeeth CK, Skripuletz T, Trebst C and Stangel M (2015) Effect of FTY720-phosphate on the expression of inflammation-associated molecules in astrocytes in vitro. Mol Med Rep 12, 6171–6177. [DOI] [PubMed] [Google Scholar]
- Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T and Nakamura S (2010) Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience 171, 973–980. [DOI] [PubMed] [Google Scholar]
- Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L and Robinson WH (2006) Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med 12, 138–143. [DOI] [PubMed] [Google Scholar]
- Kappos L, Li DK, Stuve O et al. (2016) Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis: Dose-Blinded, Randomized Extension of the Phase 2 BOLD Study. JAMA Neurol 73, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Katsel P, Li C and Haroutunian V (2007) Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res 32, 845–856. [DOI] [PubMed] [Google Scholar]
- Kim S, Steelman AJ, Zhang Y, Kinney HC and Li J (2012) Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol 22, 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S and Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64, 2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R and Wu G (2018) Gangliosides of the Nervous System. Methods Mol Biol 1804, 19–55. [DOI] [PubMed] [Google Scholar]
- Liddelow SA and Barres BA (2017) Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46, 957–967. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yang K, Burns S, Shrestha S and Chi H (2010) The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nature immunology 11, 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Zhang D, Dai D, Zhang W and Zhang L (2016) Sphingosine kinase 1/sphingosine-1-phosphate regulates the expression of interleukin-17A in activated microglia in cerebral ischemia/reperfusion. Inflamm Res 65, 551–562. [DOI] [PubMed] [Google Scholar]
- Maceyka M and Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J et al. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL and Cyster JG (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360. [DOI] [PubMed] [Google Scholar]
- Mayo L, Cunha AP, Madi A et al. (2016) IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 139, 1939–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L, Trauger SA, Blain M et al. (2014) Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 20, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH Jr., Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA and Wang E (1997) Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol 142, 208–225. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Maetzler W, Haughey NJ et al. (2013) Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: a pilot study. PLoS One 8, e73094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez A, Garcia-Diaz Barriga G, Brito V, Straccia M, Giralt A, Gines S, Canals JM and Alberch J (2015) Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75NTR up-regulation and astrocyte-mediated inflammation. Hum Mol Genet 24, 4958–4970. [DOI] [PubMed] [Google Scholar]
- Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE and Antel JP (2010) Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 176, 2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroi DN, Deutschmann AU, Raucamp M et al. (2016) Sphingosine 1-phosphate lyase ablation disrupts presynaptic architecture and function via an ubiquitin- proteasome mediated mechanism. Sci Rep 6, 37064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroi DN, Karunakaran I, Graler M, Saba JD, Ehninger D, Ledesma MD and van Echten-Deckert G (2017) SGPL1 (sphingosine phosphate lyase 1) modulates neuronal autophagy via phosphatidylethanolamine production. Autophagy 13, 885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S and Proia RL (2005) Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25, 11113–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumdar EH, Grey C and Sparr E (2019) Self-Assembly in GangliosidePhospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AN, Kampfl AW, Zhao X, Hayes RL and Dash PK (1999) Sphingosine-1-phosphate induces apoptosis of cultured hippocampal neurons that requires protein phosphatases and activator protein-1 complexes. Neuroscience 94, 405–415. [DOI] [PubMed] [Google Scholar]
- Moruno Manchon JF, Uzor NE, Dabaghian Y, Furr-Stimming EE, Finkbeiner S and Tsvetkov AS (2015) Cytoplasmic sphingosine-1-phosphate pathway modulates neuronal autophagy. Sci Rep 5, 15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA and Dheen ST (2010) Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience 166, 132–144. [DOI] [PubMed] [Google Scholar]
- Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19, 983–997. [DOI] [PubMed] [Google Scholar]
- Nobile-Orazio E, Carpo M and Scarlato G (1994) Gangliosides. Their role in clinical neurology. Drugs 47, 576–585. [DOI] [PubMed] [Google Scholar]
- O’Sullivan C, Schubart A, Mir AK and Dev KK (2016) The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflammation 13, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ASB and Faergeman NJ (2017) Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH and Steinman L (2007) Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 448, 474–479. [DOI] [PubMed] [Google Scholar]
- Pan S, Gray NS, Gao W et al. (2013) Discovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator. ACS Med Chem Lett 4, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I et al. (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Paciotti S, Eusebi P et al. (2017) Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in parkinson’s disease patients. Mov Disord 32, 1423–1431. [DOI] [PubMed] [Google Scholar]
- Pchelina S, Emelyanov A, Baydakova G et al. (2017) Oligomeric alpha-synuclein and glucocerebrosidase activity levels in GBA-associated Parkinson’s disease. Neurosci Lett 636, 70–76. [DOI] [PubMed] [Google Scholar]
- Pirhaji L, Milani P, Dalin S et al. (2017) Identifying therapeutic targets by combining transcriptional data with ordinal clinical measurements. Nat Commun 8, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM (2014) Sphingolipid lysosomal storage disorders. Nature 510, 68–75. [DOI] [PubMed] [Google Scholar]
- Reich DS, Lucchinetti CF and Calabresi PA (2018) Multiple Sclerosis. N Engl J Med 378, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti L, Antonucci F, Gabrielli M et al. (2016) Sphingosine-1-Phosphate (S1P) Impacts Presynaptic Functions by Regulating Synapsin I Localization in the Presynaptic Compartment. J Neurosci 36, 4624–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC et al. (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Kenison JE, Tjon E et al. (2017) Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A 114, 2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaba MC, Rothhammer V, Munoz U et al. (In press) Serum antibodies to phosphatidylcholine in multiple sclerosis. Neurology(R) neuroimmunology & neuroinflammation. [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S et al. (2008) Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nature neuroscience 11, 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schengrund CL (2015) Gangliosides: glycosphingolipids essential for normal neural development and function. Trends Biochem Sci 40, 397–406. [DOI] [PubMed] [Google Scholar]
- Schnaar RL (2016) Gangliosides of the Vertebrate Nervous System. J Mol Biol 428, 3325–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS (2014) Gangliosides and glycolipids in neurodegenerative disorders. Adv Neurobiol 9, 449–461. [DOI] [PubMed] [Google Scholar]
- Sena A (1993) [Gangliosides in neurobiology]. Acta Med Port 6, 341–346. [PubMed] [Google Scholar]
- Sheridan GK and Dev KK (2012) S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia 60, 382–392. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Cross H, Proukakis C et al. (2004) Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet 36, 1225–1229. [DOI] [PubMed] [Google Scholar]
- Stepanovska B and Huwiler A (2019) Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res, 104170. [DOI] [PubMed] [Google Scholar]
- Stephenson D, Rash K, Smalstig B et al. (1999) Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia 27, 110–128. [DOI] [PubMed] [Google Scholar]
- Sun D and Wekerle H (1986) Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature 320, 70–72. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Ebinuma I, Osawa S, Isshiki H, Takeo K, Tomita T and Iwatsubo T (2013) FTY720/fingolimod, a sphingosine analogue, reduces amyloid-beta production in neurons. PLoS One 8, e64050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Suzuki K et al. (2011) BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J Neurosci 31, 6850–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE and Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120, 545–555. [DOI] [PubMed] [Google Scholar]
- Tham CS, Lin FF, Rao TS, Yu N and Webb M (2003) Microglial activation state and lysophospholipid acid receptor expression. Int J Dev Neurosci 21, 431–443. [DOI] [PubMed] [Google Scholar]
- Tokuda N, Numata S, Li X et al. (2013) beta4GalT6 is involved in the synthesis of lactosylceramide with less intensity than beta4GalT5. Glycobiology 23, 1175–1183. [DOI] [PubMed] [Google Scholar]
- Van Doorn R, Van Horssen J, Verzijl D et al. (2010) Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 58, 1465–1476. [DOI] [PubMed] [Google Scholar]
- van Echten-Deckert G and Herget T (2006) Sphingolipid metabolism in neural cells. Biochim Biophys Acta 1758, 1978–1994. [DOI] [PubMed] [Google Scholar]
- van Echten-Deckert G and Walter J (2012) Sphingolipids: critical players in Alzheimer’s disease. Prog Lipid Res 51, 378–393. [DOI] [PubMed] [Google Scholar]
- Vidaurre OG, Haines JD, Katz Sand I et al. (2014) Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 137, 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A and Haughey NJ (2008) A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131, 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Clark IC, Tjon EC et al. (in press) Genomic control of astrocyte heterogeneity in multiple sclerosis. Nature. [Google Scholar]
- Wheeler MA, Jaronen M, Covacu R et al. (2019a) Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 176, 581–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Jaronen M, Covacu R et al. (2019b) Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 176, 581–596.e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA and Quintana FJ (2019) Regulation of Astrocyte Functions in Multiple Sclerosis. Cold Spring Harbor perspectives in medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Leong SY, Moore CS et al. (2013) Dual effects of daily FTY720 on human astrocytes in vitro: relevance for neuroinflammation. J Neuroinflammation 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji T and Hanada K (2015) Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic 16, 101–122. [DOI] [PubMed] [Google Scholar]