High blood pressure (BP) impacts over a billion people and is the leading risk factor for global morbidity and mortality [1–3]. More than half of all cardiovascular events are due to hypertension. In the United States alone, 103 million (45.6%) adults have high BP (≥130/80 mm Hg)3. However, less than half of Americans with hypertension are currently treated to goal [4]. Widespread health disparities persist such that control rates are even worse in minority and vulnerable communities. Across developing and lower-income nations, a dismal percentage (15–20%) achieve adequate BP control [5]. Given these sobering statistics, large-scale initiatives are now more than ever needed to combat hypertension [6]. Our aim is to show that practical strategies that reduce air pollution exposures may be a novel tactic to help in the global battle against hypertension.
Fine particulate matter air pollution <2.5 μm (PM2.5) is a leading risk factor for global morbidity and mortality. It accounts for 8.9 million deaths per year, with the largest proportion from cardiovascular diseases (e.g., myocardial infarctions, strokes) [1, 7, 8]. PM2.5 is an amalgam of compounds (e.g., carbon species, metals) coexisting with other pollutants (e.g., ozone) in a complex mixture. The major cause is fossil fuel combustion (e.g., coal, gasoline) inherent to numerous modern-day processes (e.g., power-generation, traffic) [8]. As such, >90% of the global population faces PM2.5 levels above annual World Health Organization Air Quality Guidelines (AQGs) (10 μg/m3) [1, 7, 8]. At linkage between PM2.5 and high BP may not seem obvious; however, the totality of evidence reveals a pernicious circular relationship (Fig. 1) [9, 10]. Here, we briefly review the studies showing that PM2.5 can raise BP and outline the underlying mechanisms that dictate the temporal nature of the responses. We summarize the evidence showing that reducing PM2.5 lowers BP. Finally, we call for clinical trials to test if personal-level interventions that lower PM2.5 exposures could be a realistic strategy to help combat high BP.
Fig. 1. Circular relationships among fine particulate matter air pollution, high blood pressure, and cardiovascular risk.
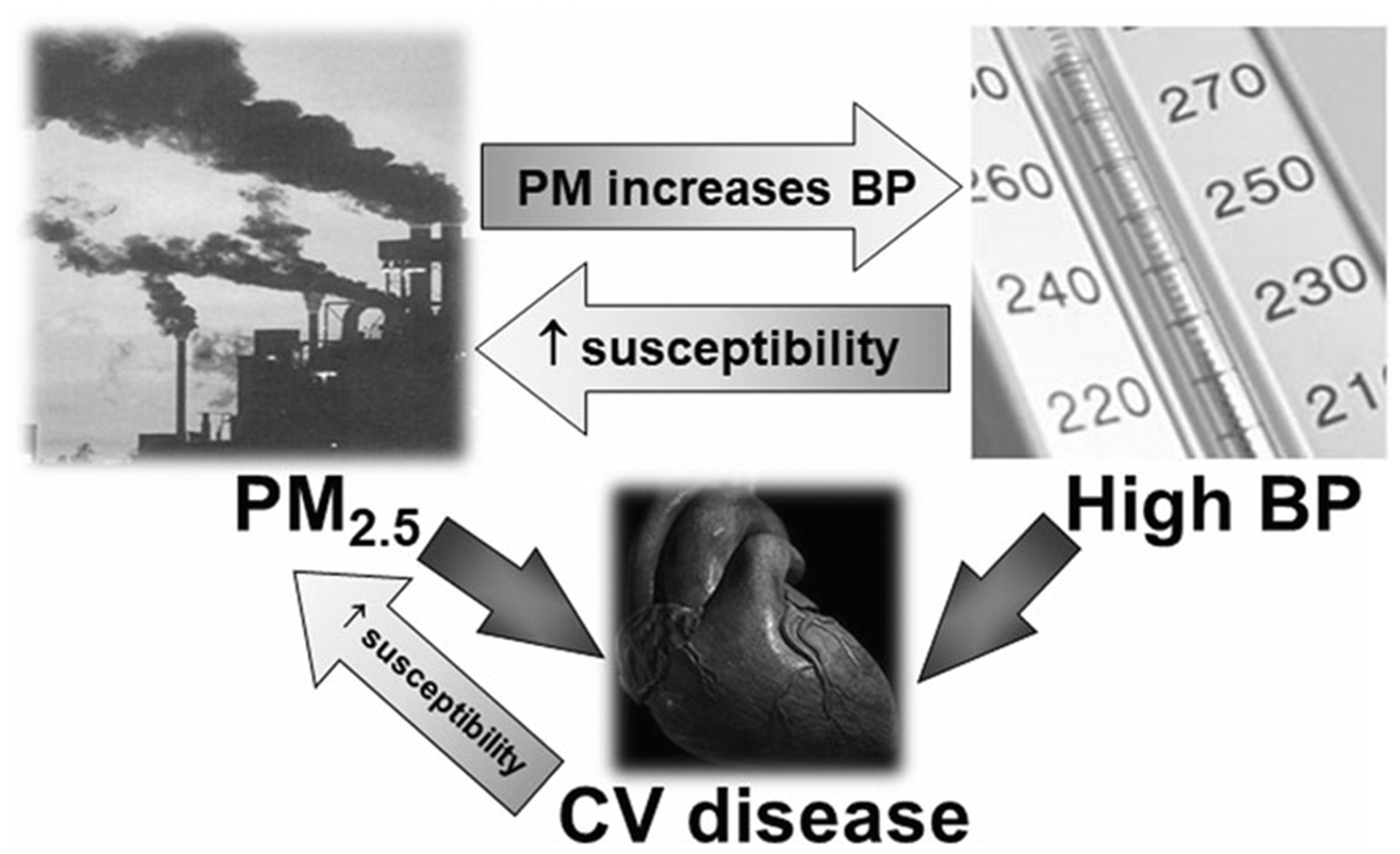
BP blood pressure, CV cardiovascular, PM particulate matter, PM2.5, particulate matter <2.5 μm. Higher BP and ambient PM2.5 levels both directly increase cardiovascular risk. Higher ambient PM2.5 levels also increase BP. Higher BP can increase the susceptibility to cardiovascular events related to PM2.5 exposure. Preexisting cardiovascular diseases increase the susceptiblity to future events due to PM2.5 exposures.
Air pollution and high BP
Numerous studies have demonstrated that exposure to higher levels of ambient air pollutants can raise BP [8–10]. On average, a 10 μg/m3 increase in PM2.5 during the previous day increases systolic and diastolic BP by 0.5–1.0 mm Hg [10]. However, there is a wide range of responses with some individuals having much larger elevations (5–10 mm Hg) [11]. The inhalation of extreme concentrations of PM2.5 for only 2-h is capable of acutely raising BP. On the other hand, living in locations facing higher ambient levels increases the risk for developing overt hypertension over a few years. Determinants of susceptibility include sex, age, baseline BP, anti-hypertensive medication use, and certain co-morbidities (e.g., obesity) [9–11]. In addition, the BP responses can be modified by particulate composition and co-pollutants (ozone) as well as other environmental factors (e.g., temperature, barometric pressure) [12, 13]. The acute exposure-response relationship appears to be largely monotonic and independent of chronic concentrations of ambient PM2.5 [9, 10]. This means that people living in heavily-polluted locations (e.g., Asia) are not immune to the acute BP-raising effects provoked by day-to-day increases in PM2.5. Their absolute BP responses are in fact larger compared to individuals residing in less-polluted regions because ambient concentrations and their variations are generally threefold to tenfold higher (50–100 ± 10–50 versus 5–25 ± 2–10 μg/m3) [14].
PM2.5 exposures can raise BP by a several mechanisms (Fig. 2) [8, 9]. The pathways have been divided into (1) initiating responses in the lungs; (2) mediating pathways that transmit signals systemically; and (3) end-organ responses. Some mediating pathways (e.g., autonomic imbalance, inhaled components translocating into the circulation) can trigger responses (e.g., vasoconstriction) within minutes. Other pathways (e.g., release of inflammatory mediators and the generation of secondarily-modified endogenous factors such as oxidized phospholipids that “spill-over” into the circulation) and hypothalamic-pituitary-adrenal axis (HPAA) activation likely increase BP over hours-to-days via several end-organ mechanisms (e.g., vascular dysfunction, arterial stiffness). There is also evidence for renin-angiotensin system (RAS) activation, sodium retention, and impaired sleep/chronobiology [15, 16]. Finally, long-term exposures can worsen renal function promoting chronic kidney disease (CKD) [17]. Common to all pathways (except CKD) is that they are reversible, require ongoing activation to elicit continual BP responses, and are of relatively rapid on- and off-set. As such, the lowering of PM2.5 should promptly decrease BP, regardless of the dose and chronicity of prior exposures. Clinical trials now confirm this to be true.
Fig. 2. Pathways and end-organ mechanisms whereby fine particulate matter air pollution exposures can increase blood pressure.
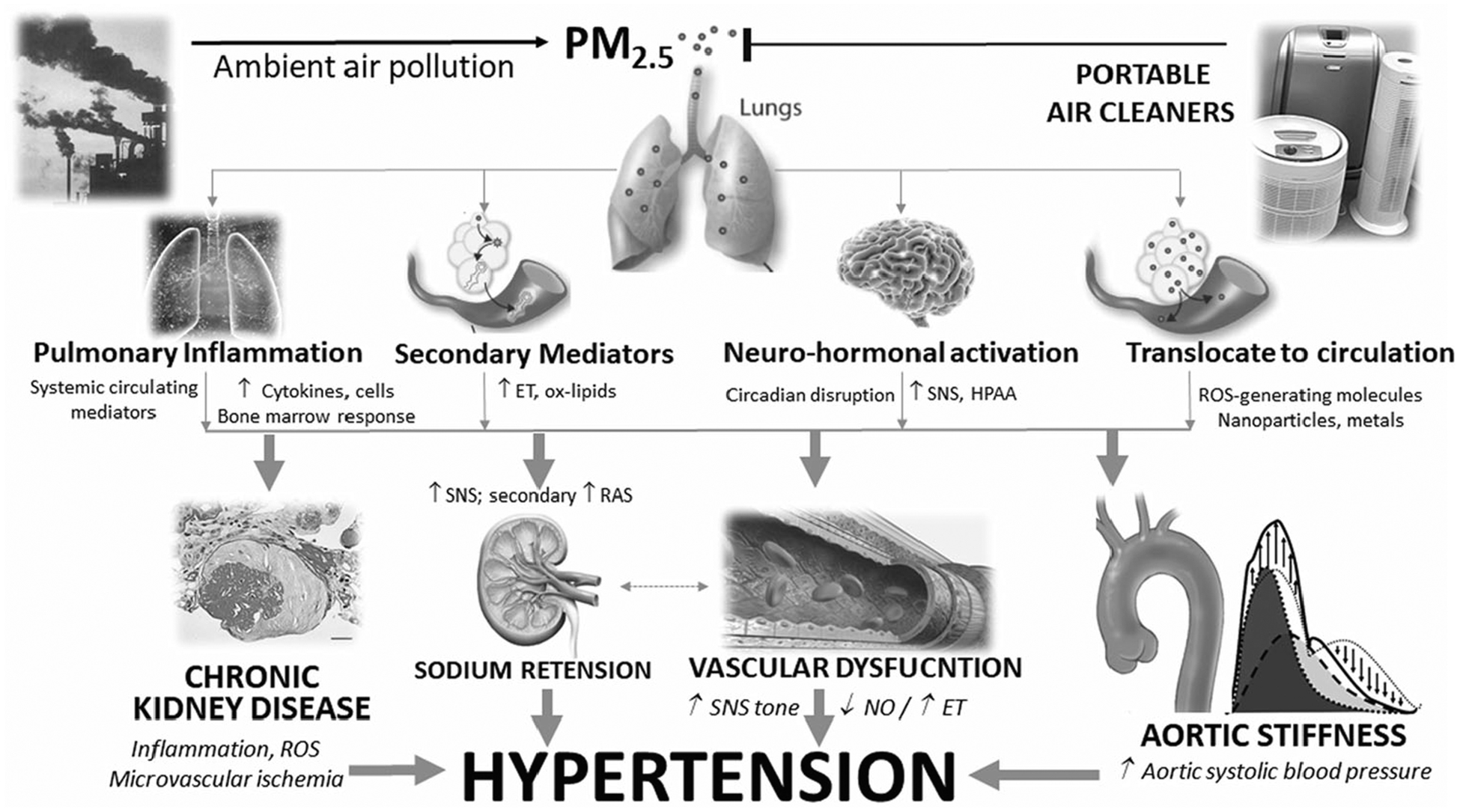
ET endothelins, HPAA hypothalamic pituitary adrenal axis, NO nitric oxide, ox-lipids oxidized lipoproteins/phospholipids; PM2.5, fine particulate matter; ROS reactive oxygen species, SNS sympathetic nervous system.
Lowering PM2.5 reduces BP
Mounting evidence shows that reducing exposure to PM2.5 results in a decrease in BP within a few days [18, 20]. Large-scale reductions in air pollution, such as the efforts to improve air quality during the Beijing Olympics and secular changes in Taiwan, are associated with decreases in BP and/or a lower incidence of hypertension [18, 20]. Other interventions undertaken at a personal-level, such as wearing an N95-respirator in heavily-polluted cities, have also proven to lower BP within hours-to-days. Another strategy is to use indoor portable air cleaners (PACs) [18]. We recently completed a meta-analysis of 10 studies (n = 604) using PACs over the short-term (median 13.5 days) [19]. Indoor PM2.5 was consistently lowered (56 ± 17%); however, the decreases in absolute concentrations (21 ± 18 μg/m3) were dependent on baseline air quality. On average, the interventions produced a decrease in systolic BP by 3.9 mm Hg (95% confidence interval −7.0–0.9; p = 0.01). This is slightly larger (1.9 mm Hg per 10 μg/m3 of PM2.5) than that predicted from epidemiologic studies, suggesting that the true biological response may have been previously underestimated [10]. There were consistent effects across all ages (college students to elderly), locations, and durations. These findings bolster support that PAC interventions could yield rapid health benefits in a broad array of patients. However, most studies have been limited by a small sample size and short duration.
Another key finding from the meta-analysis was that PACs lowered BP irrespective of background (or achieved on-treatment) PM2.5 levels, such as among people living in heavily-polluted Shanghai China (96.2 μg/m3) [19]. This indicates that it is not necessary to achieve some lower absolute threshold of PM2.5 in order to significantly decrease BP. The fact that BP rapidly reverted to a lower set-point also shows that chronic high-level exposures do not cause fixed (immutable) hypertension. It further indirectly implies that there must be little-to-no attenuation of PM2.5-mediated elevations in BP during chronic periods of high exposure. In sum, people who remain exposed to poor air quality for years will have persistently higher BP levels but intervening with a PAC will nonetheless rapidly lower BP. From a clinical standpoint, these findings support that PACs have the potential to be cardio-protective across the global spectrum of air quality.
Our review of the overall scientific data yields further important implications for epidemiological research [18, 19]. Prior studies relating annual PM2.5 averages with hypertension prevalence or incidence may have reported inaccurate associations due to erroneous assumptions (Fig. 3) [10]. Consider the well-known positive association between body weight (or dietary sodium) averaged over the prior year and BP [3]. It is important to note that this relationship is dependent upon the fact that weight and salt intake are typically static over long periods and therefore the physiological pathways that elevate BP remain continually active. However, when an individual loses weight or reduces dietary salt, BP decreases within a few weeks. As such, their body weight (or salt intake) averaged from 2 to 12 months earlier (as well as year-long averages) will not be biologically (or statistically) related to their BP. On the other hand, air pollution levels are highly variable over days-to-weeks across a biologically-relevant range. Given the rapidly malleable nature of the pathways whereby PM2.5 impacts BP, pollutant concentrations that occurred months beforehand are of little biological relevance (Fig. 2). The precise “operative exposure window” that is causally-linked to BP levels is not well-established. Nonetheless, the totality of evidence supports that it is on the order of a few days to weeks beforehand [9, 19]. We should therefore not expect averages of annual PM2.5 levels, which are highly-influenced by remote exposures months earlier, to be biologically related to BP. Even so, year-long PM2.5 levels are often correlated with those during the prior few days at any given location. This correlation likely explains why annual PM2.5 averages have been associated with BP in large-scale studies [10]. We acknowledge the possibility that even after a few weeks of lowered PM2.5 levels, an unclear fraction of the hypertensive response might persist. This is because cumulative cardiac and vascular damage and/or renal insufficiency (CKD) will not improve in this brief time frame. The contribution of these progressive and “fixed” biological effects to PM-mediated BP elevations remains uncertain; however, we believe the evidence suggests it is relatively modest [19]. In summary, epidemiological studies should be supported by a sound physiological basis. They should thus focus on the effects of PM2.5 levels during the days-to-weeks proximal to the BP measurement. They should also treat BP as a continuous risk factor, rather than as a categorical endpoint. A 3–4 mm Hg increase in BP in a population (at any basal BP level) has large public health effects even if it does not result in the crossing of an arbitrary threshold that produces a diagnosis of hypertension [3].
Fig. 3. Temporal relationships between fine particulate matter air pollution exposures and blood pressure changes.
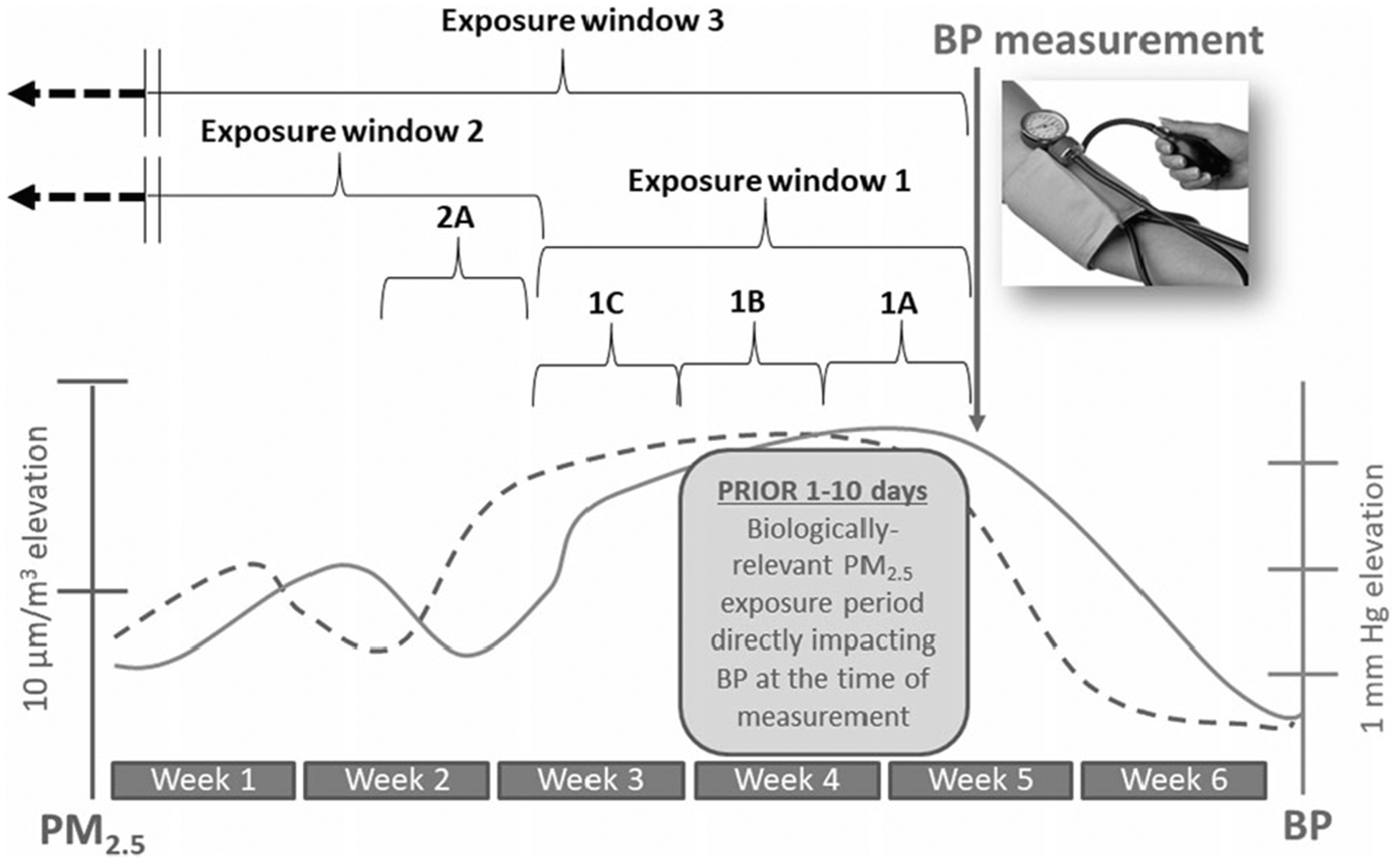
BP increases starting 1 day after higher 24-h long PM2.5 exposures and persists elevated for a few (2–14?) days afterward. BP will remain higher for as long as PM2.5 remains elevated but will start to decrease within 1 (to a few) days after a reduction in PM2.5 levels occur. This occurs at all background levels of PM2.5; therefore, what is illustrated is the increase in systolic BP per increase in PM2.5 over set periods of time. Exposure window 1 captures the “biologically-operative” exposure-response relationship. Exposures during windows 1A and 1B directly play a causal role in changing the BP level at the time of measurement and therefore they accurately predict BP. Window 1C predicts BP simply because the PM2.5 levels are strongly correlated with (and did not change from) windows 1A and 1B. Exposure window 2 represents PM2.5 levels that occurred in a time that is remote from the biologically-operative period. Exposures in this window are biologically unrelated to the BP during the measurement time. If they show a statistical association with BP, it is only because the exposure (i.e., window 2A) is stable and/or highly-correlated to the exposure during window 1. Exposure window 3 represents the chronic period. This includes the biologically-operative period but also exposures that occurred from weeks to months or years earlier. Including longer periods in the average exposure therefore involves many exposure windows that are not biologically relevant (i.e., play no role in determining BP level at the point of measurement). Exposure window 3 may not be predictive of BP because the predominant period of the time that is averaged includes window 2 (and could be months in duration and therefore determines the average level), which is not biologically relevant in relation to the measured BP. Therefore, simply averaging longer exposure periods may produce a more stable chronic exposure estimate but also it produces a worse estimation of the actual “operatively relevant exposure” period (window 1) and can yield a falsely null association with BP levels at the time of measurement. Window 3 might predict BP as a statistical artifact because it might be correlated with the PM2.5 levels during window 1. In comparisons between study locations on a spatial dimension, window 3 might predict BP between sites because they are arranged in an ordinal manner whereby they correlate with exposures during window 1 across sites. In this example, window 3 and 1 are not correlated, and therefore window 3 is not associated with BP.
A call for clinical trials
We believe that the most feasible personal-level strategy to achieve wide-spread reductions in PM2.5 exposures is to use PACs [18–20]. Many devices are low cost ($100–200 plus $10–25 per year for filters and electricity) [18]. There are several types, but devices using barrier filtration with highly-efficient particle arrestance (HEPA) filters are widely-available and do not generate potentially-harmful ozone. HEPA filters capture particles at 0.3-μm diameter by 99.97% with larger and smaller sizes filtered at an even greater effectiveness. Each model has a clean air delivery rate making it appropriate for usage in specific room sizes. Due to room air exchanges, PACs decrease indoor PM2.5 levels in the real-world by roughly 50–80%. HEPA filters do not loose efficiency with continued use (i.e., particle loading) but they should be replaced every 3–12 months. As such, PACs do not require any day-to-day behavioral changes and long-term persistence should not be a problem. Adverse events (e.g., noise nuisance) and patient discontinuation were also very uncommon in published studies [18, 21]. Limitations are that they require electricity and their efficacy can be reduced in households with air leaks (e.g., poor construction, open windows). This could be a particular problem for hot-climate, highly-polluted, developing nations (e.g., India) [22]. However, studies in Delhi and China have shown that PACs can remain effective [18, 22]. PACs also only improve in-home air quality. If individuals spend a major portion of time outdoors or traveling, achieved exposure reduction could be limited. Two studies we conducted help to lessen this concern. Among 40 elderly adults living in a senior facility in midtown Detroit, PACs lowered personal-level PM2.5 exposures by 50% for a full 24-h [21]. Even among free-living cardiac patients residing in their own homes, PACs reduced 24-h personal-level PM2.5 exposures by 44% [23].
The average decrease in BP (3.9 mm Hg) following PAC use may seem modest [19]. However, it is on-par with formally-recommended lifestyle interventions (e.g., exercise, weight loss, salt reduction) that suffer from poor long-term patient persistence [3]. If this BP reduction proves to persist, it should translate into the lowering of cardiovascular events by 20–25% at the population level [3]. Unfortunately, it is premature to conclude if this is indeed the case because studies performed to date have been relatively small and of short duration. As a prelude to large clinical outcome trials [24], we propose that a reasonable next step is to launch more definitive (i.e., larger-scale and longer-duration) trials in a broad spectrum of patients that focus on lowering BP as the primary endpoint. Given the enormous health toll of hypertension and the fact that BP is a universally-accepted surrogate biomarker, positive results would be of major clinical relevance [3]. In addition, >90% of people are exposed to PM2.5 above AQGs [1]. Many vulnerable populations suffer from both poor air quality and BP control [3–5, 25]. PACs are therefore well-positioned to help improve global public health as well as address health disparities and environmental justice. In conclusion, lowering PM2.5 exposures using PACs could be a low-cost, low-burden, low-risk intervention to help in the worldwide battle against hypertension.
Footnotes
Conflict of interest This work was partially supported by grants from the National Institutes of Health: R01-ES019616 (Brook and Rajagopalan) and 2R01-NR014484 (Brook). The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–82. [DOI] [PubMed] [Google Scholar]
- 3.2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:1269–324. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyseni L, Elliot-Green A, Lloyd-Williams F, Kypridemos C, O’Flaherty M, McGill R, et al. Systematic review of dietary salt reduction policies: Evidence for an effectiveness hierarchy? PLoS ONE. 2017;12:e0177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. 2018;115:9592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–70. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Newby DE, Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens. 2017;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut. 2018;235:576–88. [DOI] [PubMed] [Google Scholar]
- 11.Dvonch JT, Kannan S, Schulz AJ, Mentz G, House J, Benjamin A, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009; 53:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, et al. Particulate matter air pollution and ambient temperature: Opposing effects on blood pressure in high risk cardiac patients. J Hypertens. 2015;33:2032–8. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Brook RD. Echoes from Gaea, Poseidon, Hephaestus, and Prometheus: environmental risk factors for high blood pressure. J Hum Hypertens. 2018;32:594–607. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl J Med 2019;381:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberzettl P Circadian toxicity of environmental pollution. Inhalation of polluted air to give a precedent. Curr Opin Physiol. 2018;5:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai DH, Riediker M, Wuerzner G, Maillard M, Marques-Vidal P, Paccaud F, et al. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension. 2012;60:1061–9. [DOI] [PubMed] [Google Scholar]
- 17.Wu MY, Lo WC, Chao CT, Wu MS, Chiang CK. Association between air pollutants and development of chronic kidney disease: a systematic review and meta-analysis. Sci Total Environ. 2020; 706(Mar):135522. [DOI] [PubMed] [Google Scholar]
- 18.Bard RL, Ijaz MK, Zhang JJ, Li Y, Bai C, Yang Y, et al. Interventions to reduce personal exposures to air pollution: a primer for health care providers. Glob Heart 2019;14:47–60. [DOI] [PubMed] [Google Scholar]
- 19.Walzer D, Gordon T, Thorpe L, Thurston G, Xia Y, Zhong H, et al. The effects of home particulate air filtration on blood pressure: a systematic review. Hypetension. 2020. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bo Y, Guo C, Lin C, Chang LY, Chan TC, Huang B, et al. Dynamic changes in long-term exposure to ambient particulate matter and incidence of hypertension in adults. Hypertension. 2019;74:669–77. [DOI] [PubMed] [Google Scholar]
- 21.Morishita M, Adar SD, D’Souza J, Ziemba RA, Bard RL, Spino C, et al. Portable air filtration systems, personal-level exposure to fine particulate matter, and blood pressure levels among residents in a low-income senior facility in Detroit. JAMA Intern Med. 2018;178:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyas S, Srivastav N, Spears D. An experiment with air purifiers in Delhi during winder 2015–6. PLos ONE. 2016;11: e0167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bard RL, Rubenfire M, Bryant J, Fink S, Wang L, Speth K, et al. Reduced fine particulate matter air pollution exposures using in-home portable air cleaners: pilot results of the Cardiac Rehabilitation Air Filter Trial (CRAFT.)J Cardiopulm Rehab Prev. 2020. (In press). [DOI] [PubMed] [Google Scholar]
- 24.Brook RD, Newby DE, Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health time for intervention. JAMA Cardiol. 2017;2:353–4. [DOI] [PubMed] [Google Scholar]
- 25.Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of Cause-Specific Mortality Associated With PM2.5 Air Pollution in the United States. JAMA Netw Open. 2019;2:e1915834. [DOI] [PMC free article] [PubMed] [Google Scholar]


