Abstract
Anti-donor antibodies cause immunologic injury in transplantation. CD28 blockade with CTLA-4-Ig has the ability to reduce the incidence of these donor-specific antibodies (DSA), but its mechanism is suboptimal for the inhibition of alloimmunity in that CTLA-4-Ig blocks both CD28 costimulation and CTLA-4 coinhibition. Thus selective CD28 blockade that spares CTLA-4 has potential to result in improved inhibition of humoral alloimmunity. To test this possibility, we utilized a full allogeneic mismatch murine transplant model and T follicular helper (Tfh):B cell co-culture system. We observed that selective blockade with an anti-CD28 domain antibody (dAb) compared to CTLA-4-Ig led to superior inhibition of Tfh cell, germinal center and DSA responses in vivo, and better control of B cell responses in vitro. CTLA-4 blockade enhanced the humoral alloresponse, and in combination with anti-CD28 dAb abrogated the effects of selective blockade. This CTLA-4-dependent inhibition was Tfh cell-specific in that CTLA-4 expression by Tfh cells was necessary and sufficient for the improved humoral inhibition observed with selective CD28 blockade. As CD28 blockade attracts interest for control of alloantibodies in the clinic, these data support selective CD28 blockade as a superior strategy to address DSA via the sparing of CTLA-4 and more potent targeting of Tfh cells.
1. Introduction
Kidney transplantation is the treatment modality of choice for the majority of patients suffering from end-stage renal disease (1, 2). Advances in solid organ transplantation have significantly reduced acute rejection rates, leading to significant improvements in short-term kidney allograft survival (3). However, long-term outcomes following kidney transplantation remain suboptimal due in part to allograft injury resulting from HLA antibodies directed against the donor (4). These donor-specific antibodies (DSA) have been increasingly recognized to cause immunologic injury of kidney allografts and present a barrier to improving long-term outcomes. Despite the deleterious role of DSA, large knowledge deficits exist regarding the mechanisms underlying their development and maintenance, and therapeutic strategies to control DSA-mediated allograft dysfunction are lacking (5–7).
Targeting of the CD28 costimulation pathway with belatacept, a second generation CTLA-4-Ig, has achieved translation and improved long-term outcomes following kidney transplantation (8). However, de novo use of belatacept has been limited by acute rejection rates and uncertainty in immunologically high risk patients (9). This has partially been attributed to its mechanism of action. CTLA-4-Ig acts to inhibit T cell activation through binding of the ligands CD80 and CD86, thereby preventing their engagement with the T cell co-stimulator CD28 (10). Undesirably, this mechanism also indiscriminately blocks CD80 and CD86 ligation of the co-inhibitor CTLA-4, depriving T cells of potentially important inhibitory signals. Abundant evidence exists regarding the stimulatory impact associated with the loss of CTLA-4 activity (11), suggesting that the secondary effect of CTLA-4 blockade may detract from the overall goal of attenuating alloreactivity. Accordingly, others and we have demonstrated that next generation costimulation blockade that selectively antagonizes CD28 and preserves CTLA-4 leads to superior control of alloimmune responses (12–16). Therefore selective CD28 blockade holds promise to further improve upon the encouraging long-term outcomes realized with belatacept.
Preclinical and clinical studies have shown that CTLA-4-Ig relative to conventional immunosuppression is more effective at reducing de novo alloantibody formation (8, 17), but antibodies still develop and the efficacy of belatacept on pre-formed antibodies in sensitized recipients and DSA over the long-term is unknown. Results from a clinical trial designed to evaluate the ability of belatacept to prevent DSA formation in kidney transplant recipients with failed allografts indicate that belatacept alone is not sufficient to completely prevent DSA in this setting (18). As more liberal use of belatacept in higher immunologic risk recipients is beginning to occur due to increased recognition of the benefits of CD28 blockade on humoral alloimmunity (6, 19), more potent methods of CD28 blockade will be highly desirable. Moreover, we have shown that selective CD28 blockade is superior to CTLA-4-Ig at preventing alloantibodies in a minor antigen mismatch murine transplant model (20). Thus, optimization of current methods of costimulation blockade (i.e. CTLA-4-Ig) is a promising and intriguing strategy to address the problem of HLA antibodies in the clinic.
Although the mechanisms of antibody inhibition by CD28 costimulation blockade have not been entirely elucidated, it is likely due in large part to the inhibition of T follicular helper (Tfh) cells (21, 22). Tfh cells are a CD28-dependent lineage of CD4+ T cells required for the provision of B cell help to generate mature antibody responses (23) and may also contribute to early extrafollicular (EF) humoral responses (24, 25). Tfh cells have been implicated in many immune processes and their inhibition has been associated with the prevention of DSA in transplantation (20, 26–28). Importantly, several studies have implicated CTLA-4 as a regulator of Tfh cells primarily through regulatory T cell-mediated mechanisms (29, 30), and improved alloimmune inhibition with selective CD28 blockade has often been attributed to enhanced T cell regulation (13, 16). Therefore, improved inhibition of alloantibody responses with selective CD28 blockade relative to CTLA-4-Ig may directly result from the preservation of CTLA-4 co-inhibitory capacity following transplantation. Yet if this is truly the case and whether Tfh or regulatory T cells like T follicular regulatory (Tfr) cells are mediating this effect is unknown.
In this study, we utilized a BALB/c to B6 full MHC mismatch skin allograft model to examine the role of CTLA-4 coinhibition in the setting of selective CD28 blockade. We demonstrate that selective CD28 blockade results in superior inhibition of both Tfh and Tfr cells, germinal center (GC) and DSA responses compared to CTLA-4-Ig. Donor-elicited Tfh cells differentially upregulated the coinhibitor CTLA-4, and CTLA-4 blockade augmented the follicular T cell and GC alloresponses. Anti-CTLA-4 treatment in conjunction with selective CD28 blockade reversed the superior inhibition observed with CD28-specific blockade in vivo after allotransplantation and in in vitro Tfh:B cell co-cultures. This CTLA-4 dependence was Tfh cell-specific in that CTLA-4 expression by Tfh cells but not Tfr cells was necessary and sufficient for the improved humoral inhibition observed with selective CD28 blockade. These findings support the development of next generation costimulation blockers that selectively target CD28 and preserve the inhibitory functions of CTLA-4 as a more potent immunosuppressive strategy to combat HLA antibodies.
2. Materials and Methods
2.1. Mice
B6-Ly5.1/Cr (H2-Kb) and BALB/c (H-2Kd) mice were from the National Cancer Institute and housed in pathogen-free facilities. All studies were approved by the Emory University Institutional Animal Care and Use Committee and conducted in accordance with their guidelines.
2.2. Skin Transplants and Immunosuppression
Bilateral dorsal full-thickness tail and ear skin were transplanted onto recipient mice. Skin graft recipients received no treatment, anti-CD28 domain antibody (dAb) (100 μg, Bristol-Myers Squibb), CTLA-4-Ig (200 μg, Bristol-Myers Squibb), or anti-CTLA-4 mAb (9H10, 250 μg, BioXcell). Treatments were administered intraperitoneally on post-transplant days 0, 2, 4, 6 and 8, and then weekly thereafter. Anti-CD28 dAb and CTLA-4-Ig dosing was based on molecular weight, serum half-life, and murine mixed lymphocyte reaction EC50 (14, 20).
2.3. Flow Cytometry
Graft-draining lymph nodes were processed into single-cell suspensions. Cells were surface stained for indicated markers and pulsed with LIVE/DEAD viability dye (Molecular Probes) before fixation. Intracellular staining was performed with Foxp3 Fixation/Permeabilization Buffer Kit (eBioscience). All antibodies were from BioLegend and BD Biosciences. Samples were run on an LSR Fortessa flow cytometer (BD Biosciences) and analyzed by using FlowJo Software (Flowjo, LLC). CountBright Beads (Invitrogen) were used to determine absolute cell counts.
2.4. Tfh:B Cell Co-Culture
T and B cells from allograft-draining lymph nodes (DLN) were enriched, flow sorted and co-cultured as previously described (31). Briefly, magnetic bead negative selection (Miltenyi Biotec) was used to enrich CD4+ T and CD19+ B cells. T cells from DLNs were flow sorted into Tfh (CD19−CD4+PD-1hiCXCR5+GITR−) and Tfr (CD19−CD4+PD-1hiCXCR5+GITR+) cells on a FACSAria II (BD Biosciences). For proliferation assessment, B cells were stained with eFluor670 proliferation dye (eBioscience). 3×104 Tfh cells were cultured with 5×104 B cells with or without 1.5×104 Tfr cells in 96-well plates in anti-CD3ε (2C11, 2 μg/mL, BioXcell) and anti-IgM (5 μg/mL, Jackson Immunoresearch) containing media for 5 days. Culture media was supplemented with immunosuppression where indicated at the following concentrations: CTLA-4-Ig (50 μg/mL), anti-CD28 dAb (25 μg/mL), anti-CTLA-4 mAb (50 μg/mL). Anti-CD28 dAb and CTLA-4-Ig dosing was based on molecular weight, serum half-life, and murine mixed lymphocyte reaction EC50 (14, 20).
2.5. Antibody Assessments
Serum from transplanted animals or supernatant from Tfh:B cell co-cultures were collected to test for anti-donor antibodies. For flow cytometric crossmatch, BALB/c or B6 splenocytes were processed into single-cell suspensions and pre-treated with Fc Block (BioLegend), followed by incubation with recipient serum at 4°C. Splenocytes were then washed and labeled with surface markers and anti-mouse IgG (BioLegend) for quantification of IgG by flow cytometry. For ELISA total IgG measurements from co-culture supernatant, flat-bottom 96-well Immulon 4HBX microtiter plates (VWR) were coated with anti-mouse Ig (5 μg/well; Sigma-Aldrich) overnight at 4°C. Coated plates were blocked with 10% FBS in PBS-T for 1 hour at 37°C, and then incubated with culture supernatant samples for 1.5 hours at 37°C. Total IgG was detected with HRP goat anti-mouse IgG (Poly4053, BioLegend), developed by using the TMB substrate system (Thermo Scientific), and read at 450 nm on a Spectra MAX 340PC Microplate reader (Molecular Devices).
2.6. Statistics
The Mann–Whitney U nonparametric t test was performed for analysis of unpaired groups, and the Holms–Sidak method was used for grouped, multiple t test analyses. All analyses were performed by using GraphPad Prism (GraphPad Software, Inc.). Statistical significance was attributed to p values <0.05 (*<0.05, **<0.01, ***<0.001).
3. Results
3.1. Selective CD28 blockade exhibits superior inhibition of antibody responses compared to CTLA-4-Ig in a full MHC mismatch allotransplantation model
Based on similarities between antigen-specific transgenic and endogenous Tfh cell responses in a surrogate minor antigen mismatch model (20), we tested the impact of selective CD28 blockade on the alloantibody response in a full MHC mismatch murine skin transplant model. Naïve B6 recipients were transplanted with skin grafts from syngeneic (B6) or allogeneic (BALB/c) donor mice and were left untreated or treated with either CTLA-4-Ig or an anti-CD28 (dAb) (Figure 1A). Untreated allograft recipients formed anti-BALB/c alloantibodies by posttransplant day 14, while DSA emerged by day 21 and persisted beyond day 42 in CTLA-4-Ig treated mice (Figure 1B). Conversely and similar to the syngeneic graft recipients, anti-CD28 dAb treated mice did not develop DSA. Donor-elicited Tfh (CXCR5+PD-1hi cells known to reside inside or near the B cell follicle) and more differentiated GC Tfh (the maximally polarized Bcl6hiPD-1hi+ fraction of Tfh cells within GCs) cells (23) were readily identifiable in untreated animals receiving allogeneic grafts as compared to syngeneic grafts (Figures 1C, D). While CTLA-4-Ig resulted in reduced Tfh and GC Tfh cells, the anti-CD28 dAb completely eliminated this cellular alloresponse. Treg (CD4+Foxp3+CD25+) and Tfr (Foxp3+CXCR5+PD-1hi) cells were also differentially reduced by both forms of CD28 blockade (Figures 1E, F). Examination of B cell subsets revealed greater inhibition of GC B cell (GL7+CD95+) and antibody secreting cell (ASC, plasmablast (CD19+B220−CD138+) and plasma cell (CD19−B220−CD138+)) responses by the CD28-specific dAb relative to CTLA-4-Ig (Figures 1G, H). These findings demonstrate that selective CD28 blockade provides superior inhibition of follicular T cell, GC and ASC responses compared to CTLA-4-Ig in a full allogeneic mismatch model, thereby resulting in improved inhibition of DSA.
Figure 1. Selective CD28 blockade exhibits superior inhibition of antibody responses compared to CTLA-4-Ig.
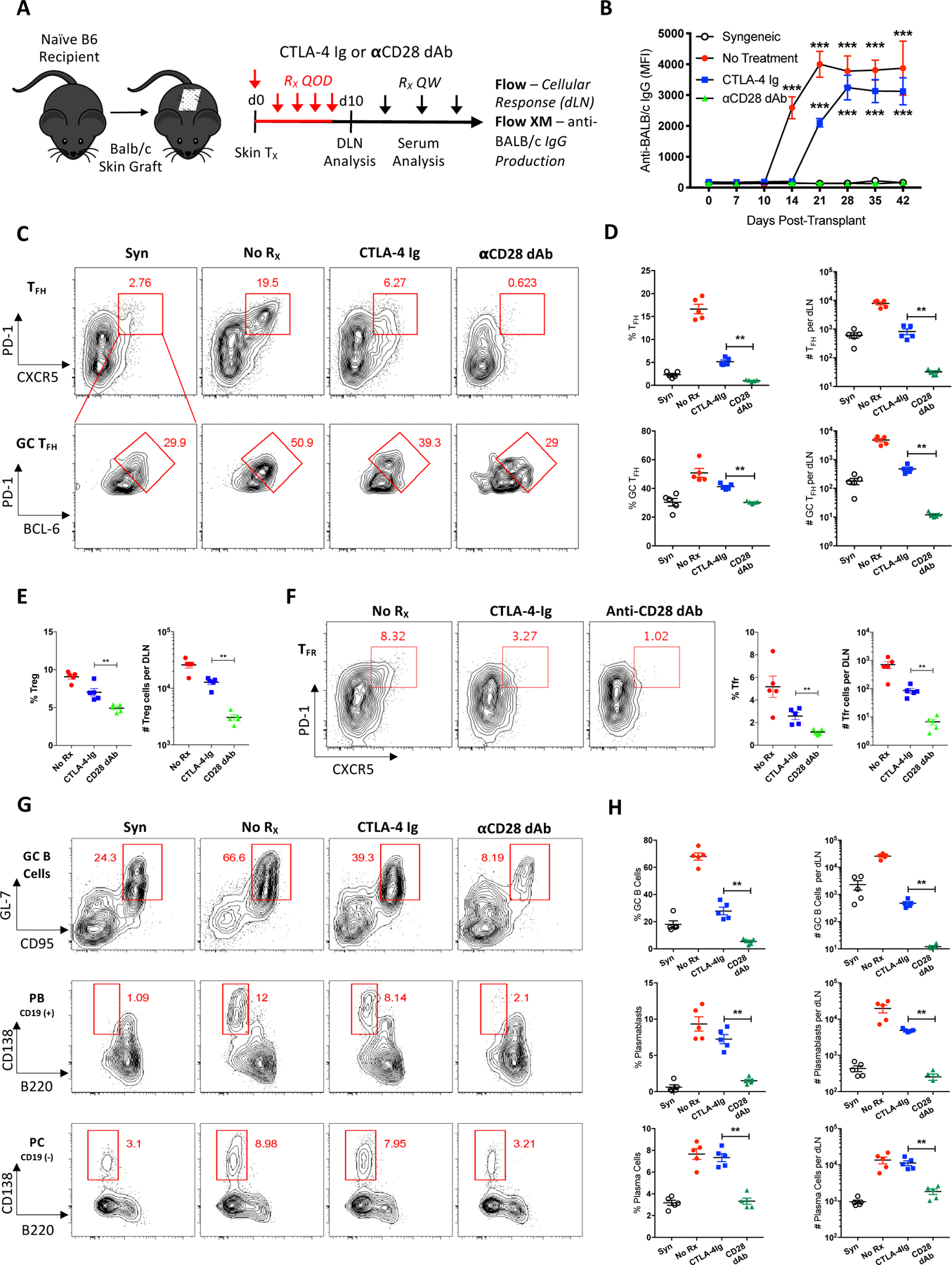
(A) Naïve B6 mice were transplanted with skin from syngeneic (B6) or allogeneic (BALB/c) donors and sacrificed 10 days post-transplant for graft-DLN analysis or serially bled for serum analysis. Transplanted mice were left untreated, or treated with either CTLA-4-Ig or anti-CD28 dAb every other day (QOD, red) the first week and then weekly (QW, black) thereafter. (B) Summary data of anti-donor total serum IgG over time (n=5 per group). (C) Representative flow cytometric plots displaying the frequencies of DLN Tfh (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3− T cells) and GC Tfh (Bcl6hiPD-1hi+, gated on CXCR5+PD-1hi Tfh cells) cells under each treatment condition. (D) Summary data of the frequencies and numbers of Tfh and GC Tfh cells (n=5 per group). (E) Summary data of the frequencies and numbers of Treg (CD4+Foxp3+CD25+) cells (n=5 per group). (F) Representative flow plots and summary data of the frequencies and numbers of Tfr (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3+ T cells) cells. (G) Representative flow plots displaying the frequencies of DLN GC B cells (GL7+CD95+, gated on IgD−CD19+B220+CD138− B cells), plasmablasts (B220−CD138+, gated on IgD−CD19+ B cells), and plasma cells (B220−CD138+, gated on IgD−CD19− B cells). (H) Summary data of the frequencies and numbers of GC B cells, plasmablasts, and plasma cells (n=5 per group). Summary data represent mean (SE) and are representative of at least 2 independent experiments with a total of at least 10 mice per group. **p < 0.01, ***p< 0.001.
3.2. CTLA-4 blockade augments the follicular T cell and GC B cell alloresponses
The coinhibitory receptor CTLA-4 has been implicated as a critical mediator of Tfh cell differentiation and function (29, 30), and is highly expressed on Tfh cells as well as Treg and Tfr cells (Figure S1). Differential expression of CTLA-4 on donor-elicited Tfh and GC Tfh cells suggests that CTLA-4-mediated coinhibition may play a critical role in Tfh cell-mediated alloreactivity. To test this possibility, skin-grafted mice were treated with a blocking anti-CTLA-4 mAb and graft-DLNs examined 10 days after transplant (Figure 2A). We observed that CTLA-4 antagonism alone significantly increased the frequency and numbers of graft-elicited Tfh, Tfr and GC B cells compared to untreated controls (Figures 2B–D), indicating that blockade of CTLA-4 leads to augmentation of the follicular T cell-mediated GC response following allotransplantation. These data support the hypothesis that CTLA-4 is an important functional inhibitor of Tfh cell-mediated alloreactivity and may underlie the superior humoral inhibition observed with selective CD28 blockade (Figure 1).
Figure 2. CTLA-4 blockade augments the follicular T cell and GC B cell alloresponses.
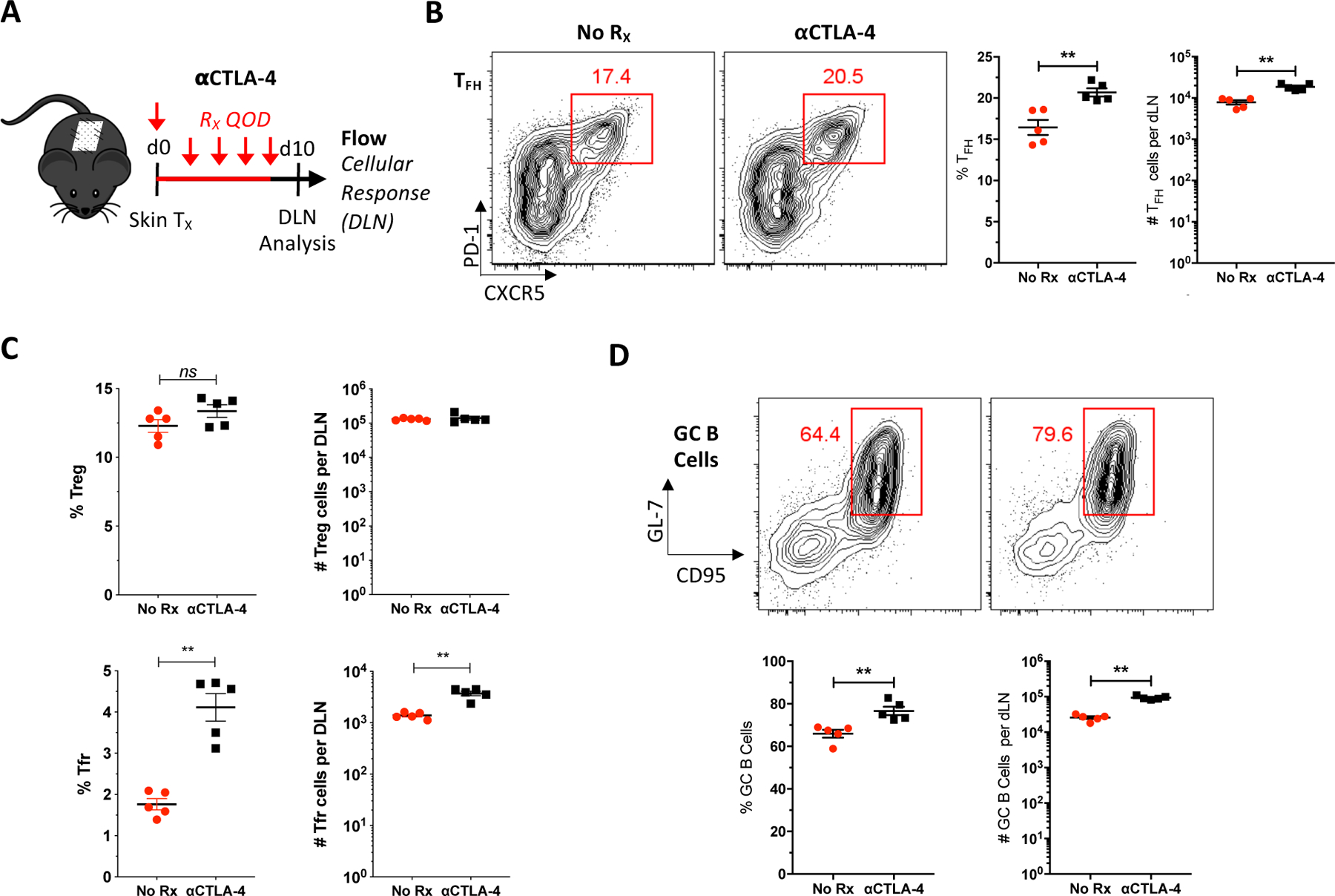
(A) Naïve B6 mice were transplanted BALB/c skin, either left untreated or treated with anti-CTLA-4 mAb every other day (QOD, red), and graft-DLNs examined 10 days posttransplant. (B) Representative flow plots displaying the frequencies of Tfh cells and summary data of the frequencies and numbers of Tfh cells (n=5 per group). (C) Summary data of the frequencies and numbers of Treg and Tfr cells (n=5 per group). (D) Representative flow plots displaying the frequencies of GC B cells (GL7+CD95+, gated on IgD−CD19+B220+CD138− B cells) from untreated and anti-CTLA-4-treated mice and summary data of the frequencies and numbers GC B cells (n=5 per group). Summary data represent mean (SE). **p < 0.01.
3.3. Selective CD28 blockade inhibition of alloantibody formation is CTLA-4-dependent
To determine the role of preserved CTLA-4 coinhibitory capacity in enhancing the immunosuppressive effects of selective CD28 blockade over that of CTLA-4-Ig, naïve B6 mice were transplanted with BALB/c skin and left untreated, or administered either anti-CD28 dAb monotherapy or anti-CD28 dAb in combination with anti-CTLA-4 mAb (Figure 3A). DSA formed in untreated animals but was completely inhibited in the anti-CD28 dAb treated mice (Figures 3B, C). The addition of anti-CTLA-4 mAb to anti-CD28 dAb-treated skin graft recipients resulted in DSA relative to anti-CD28 dAb alone. Thus, CTLA-4 antagonism reversed selective CD28 blockade-mediated inhibition of alloantibody formation. Because peak Tfh response precedes antibody formation by 4 days (32), we next examined the GC response in graft-DLNs 24 days post-transplant. The addition of anti-CTLA-4 mAb significantly increased CXCR5+PD-1hi Tfh and Bcl6hiPD-1hi+ GC Tfh cells over that observed in anti-CD28 dAb alone treated animals (Figures 3D, E). Conversely, CTLA-4 antagonism did not augment the Treg or Tfr cell responses in the presence of CD28 blockade (Figures 3F, G). Examination of B cell subsets revealed that CTLA-4 blockade abrogated the inhibition of GC B cells, plasmablasts and plasma cells by anti-CD28 monotherapy (Figures 3H, I). Overall, blockade of the coinhibitor CTLA-4 reversed selective CD28 blockade-mediated inhibition of alloreactive Tfh cells, B cell subsets, and DSA, but not Treg or Tfr cells.
Figure 3. Selective CD28 blockade inhibition of alloantibody formation is CTLA-4-dependent.
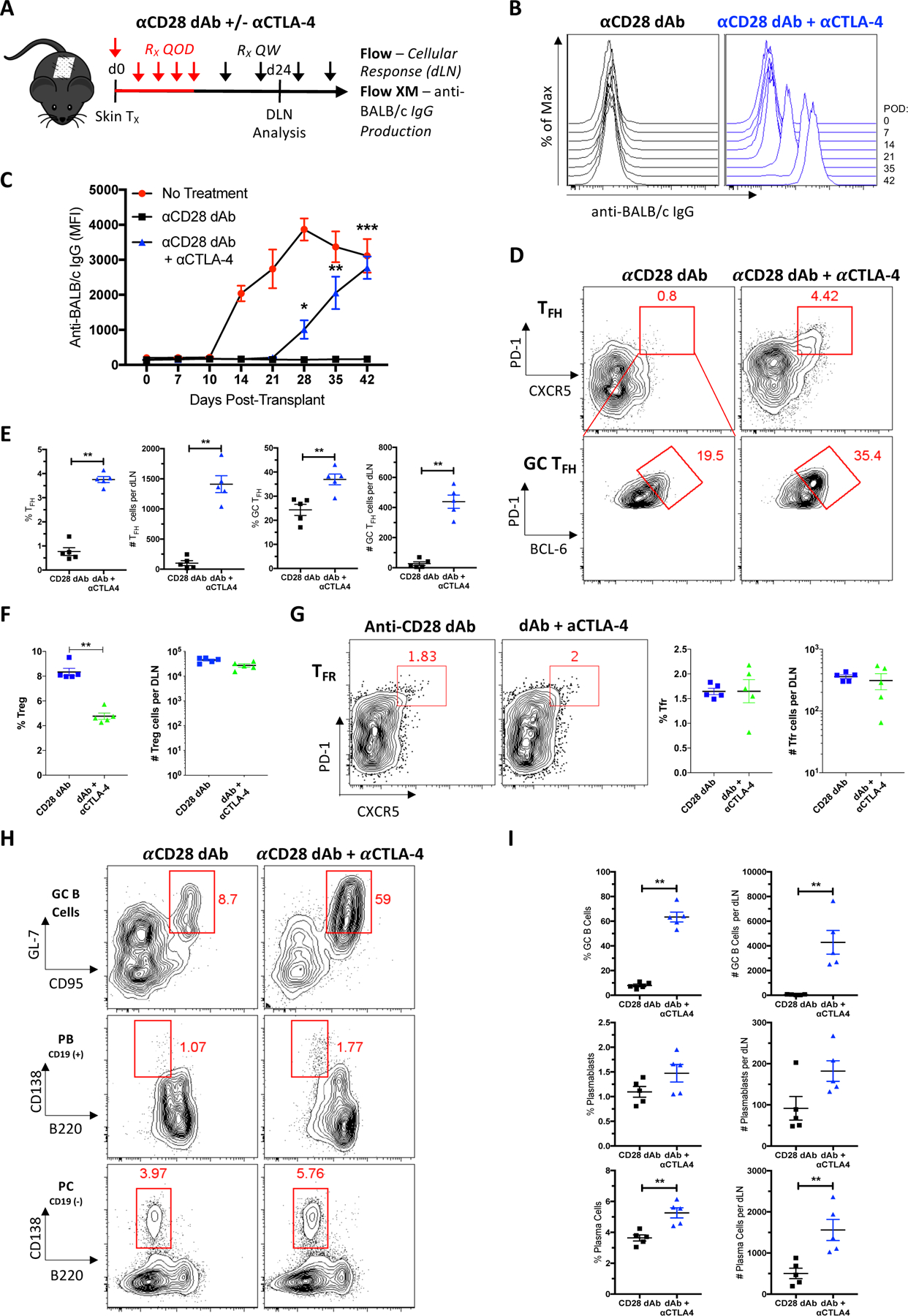
(A) Naïve B6 mice were transplanted with skin from BALB/c donors and sacrificed 24 days post-transplant for graft-DLN analysis or serially bled for serum analysis. Transplanted mice were left untreated, or treated with anti-CD28 dAb alone or anti-CD28 dAb plus anti-CTLA-4 mAb every other day (QOD, red) the first week and then weekly (QW, black) thereafter. (B) Representative histograms depicting anti-donor total serum IgG over time. (C) Summary data of anti-donor total serum IgG over time (n=5 per group). (D) Representative flow plots displaying the frequencies of DLN Tfh (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3− T cells) and GC Tfh (Bcl6hiPD-1hi+, gated on CXCR5+PD-1hi Tfh cells) cells under each treatment condition. Summary data of the frequencies and numbers of (E) Tfh and GC Tfh cells, and (F) Treg cells (n=5 per group). (G) Representative flow plots and summary data of Tfr (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3+ T cells) cells (n=5 per group). (H) Representative flow plots displaying the frequencies of DLN GC B cells (GL7+CD95+, gated on IgD−CD19+B220+CD138− B cells), plasmablasts (B220−CD138+, gated on IgD−CD19+ B cells), and plasma cells (B220−CD138+, gated on IgD−CD19− B cells). (I) Summary data of the frequencies and numbers of GC B cells, plasmablasts, and plasma cells (n=5 per group). Summary data represent mean (SE) and are representative of at least 2 independent experiments with a total of at least 10 mice per group. *p < 0.05, **p < 0.01.
3.4. CTLA-4 is critical for the superior inhibition of selective CD28 blockade at the level of Tfh:B cell cognate interactions
To further test the hypothesis that CTLA-4 coinhibition of cognate Tfh:B cell interactions is responsible for the improved efficacy of selective CD28 blockade to inhibit DSA, we performed alloreactive Tfh:B cell co-cultures from BALB/c skin-grafted mice (Figure 4A). Sorted Tfh and enriched B cells from graft-DLNs were co-cultured for 5 days to assess B cell proliferation, class switch recombination (CSR) and antibody production. Tfh:B cell co-cultures were left untreated or treated with CTLA-4-Ig, anti-CD28 dAb, or anti-CD28 dAb plus anti-CTLA-4 mAb. Co-cultures resulted in the production of donor-specific IgG alloantibodies (Figure 4B). Untreated co-cultures exhibited robust B cell proliferation as measured by proliferation dye and Ki-67 expression (Figures 4C–E), while CTLA-4-Ig partially and anti-CD28 dAb completely inhibited this B cell proliferative burst. Consistent with our in vivo data, the addition of anti-CTLA-4 mAb to selective CD28 blockade resulted in reversal of the inhibition of B cell proliferation observed with the anti-CD28 dAb alone (Figures 4C–E). CTLA-4 blockade had the same impact on the differentiation of B cells into class-switched GC-like GL-7+IgG1+ B cells and their proliferation (Figures 4F, G). Interestingly, Tfh cell proliferation was similarly inhibited with anti-CTLA-4 partially reversing the effects of the anti-CD28 dAb to levels similar to CTLA-4-Ig (Figure 4H). Culture supernatant total IgG was significantly increased when CTLA-4 blockade was combined with selective CD28 blockade (Figure 4I). Hence these data indicate that the co-inhibitor CTLA-4 plays a critical role in the superior inhibition observed with selective CD28 blockade (Figure 1), and that CTLA-4 is acting at the level of Tfh:B cell cognate interaction.
Figure 4. CTLA-4 is critical for the superior inhibition of selective CD28 blockade at the level of Tfh:B cell cognate interactions.
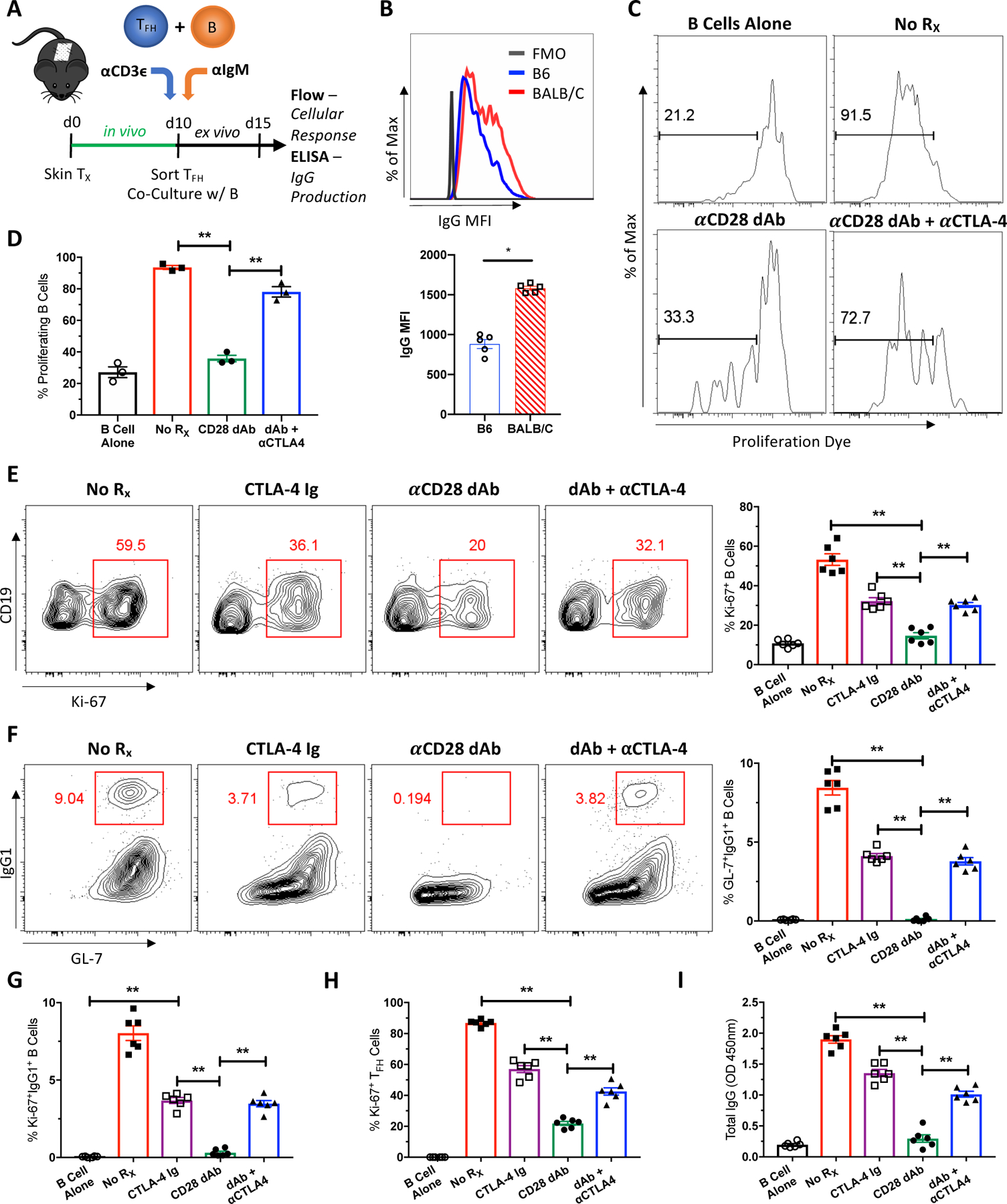
(A) Naïve B6 mice were transplanted with BALB/c skin, left untreated, and sacrificed 10 days post-transplant. Tfh and B cells from graft-DLNs were isolated and co-cultured for 5 days. Tfh:B cell co-cultures were left untreated, or treated with CTLA-4-Ig, anti-CD28 dAb, or anti-CD28 dAb plus anti-CTLA-4 mAb. (B) Untreated co-culture supernatants were crossed against B6 and BALB/c splenocytes. Representative histogram depicting IgG MFI against B6 or BALB/c splenocytes and summary data of IgG MFI (n=5 per group) (C) Representative histograms depicting B cell proliferation by eFluor670 proliferation dye under the indicated conditions. (D) Summary data of the frequencies of proliferating B cells (n=3 per group). (E) Representative flow plots of B cell proliferation as measured by Ki-67 and summary data of the frequencies of proliferating Ki-67+ B cells (n=6 per group). (F) Representative flow plots of class-switched GC-like GL7+IgG1+ B cells and summary data of the frequencies of these class-switched B cells (n=6 per group). Summary data of the frequencies of (G) proliferating class-switched B cells (n=6 per group), (H) proliferating Tfh cells (n=6 per group), and (I) supernatant total IgG levels (n=6 per group). Summary data represent mean (SE) and are representative of at least 2 independent experiments, with cells pooled from 10 mice per experiment and 3–6 co-culture wells per treatment group. **p < 0.01.
3.5. CTLA-4-dependent superior inhibition of selective CD28 Blockade is Tfh cell-specific
Because CD28 and CTLA-4 have been reported to be expressed and functional on B cells (33–35), we next investigated whether the CTLA-4-dependent superior inhibition observed with selective CD28 blockade is Tfh cell-specific. Sorted Tfh or enriched B cells from graft-DLNs were individually pre-treated with anti-CTLA-4 mAb and then co-cultured in the presence of anti-CD28 dAb for 5 days (Figure 5A). As such, this approach enabled us to determine whether Tfh cell- or B cell-derived CTLA-4 is driving improved inhibition with selective CD28 blockade. Selective blockade with the anti-CD28 dAb alone inhibited B cell proliferation, and this inhibition was reversed when both Tfh and B cells were exposed to anti-CTLA-4 mAb (Figures 5B–D). Interestingly, anti-CTLA-4 pre-treated Tfh cells, but not pre-treated B cells, abrogated the inhibition of B cell proliferation with selective CD28 blockade alone. Similarly, only anti-CTLA-4 pre-treated Tfh cells and not pre-treated B cells reversed anti-CD28 dAb-mediated inhibition of B cell differentiation, Tfh cell proliferation, and antibody production (Figures 5E–H). Thus CTLA-4 antagonism reverses the increased efficacy of selective CD28 blockade to control humoral alloresponses relative to CTLA-4-Ig in a Tfh cell-specific manner. Namely, CTLA-4 expression by Tfh cells is necessary and sufficient for the improved inhibition observed with selective CD28 blockade.
Figure 5. CTLA-4-dependent superior inhibition of selective CD28 Blockade is Tfh cell-specific.
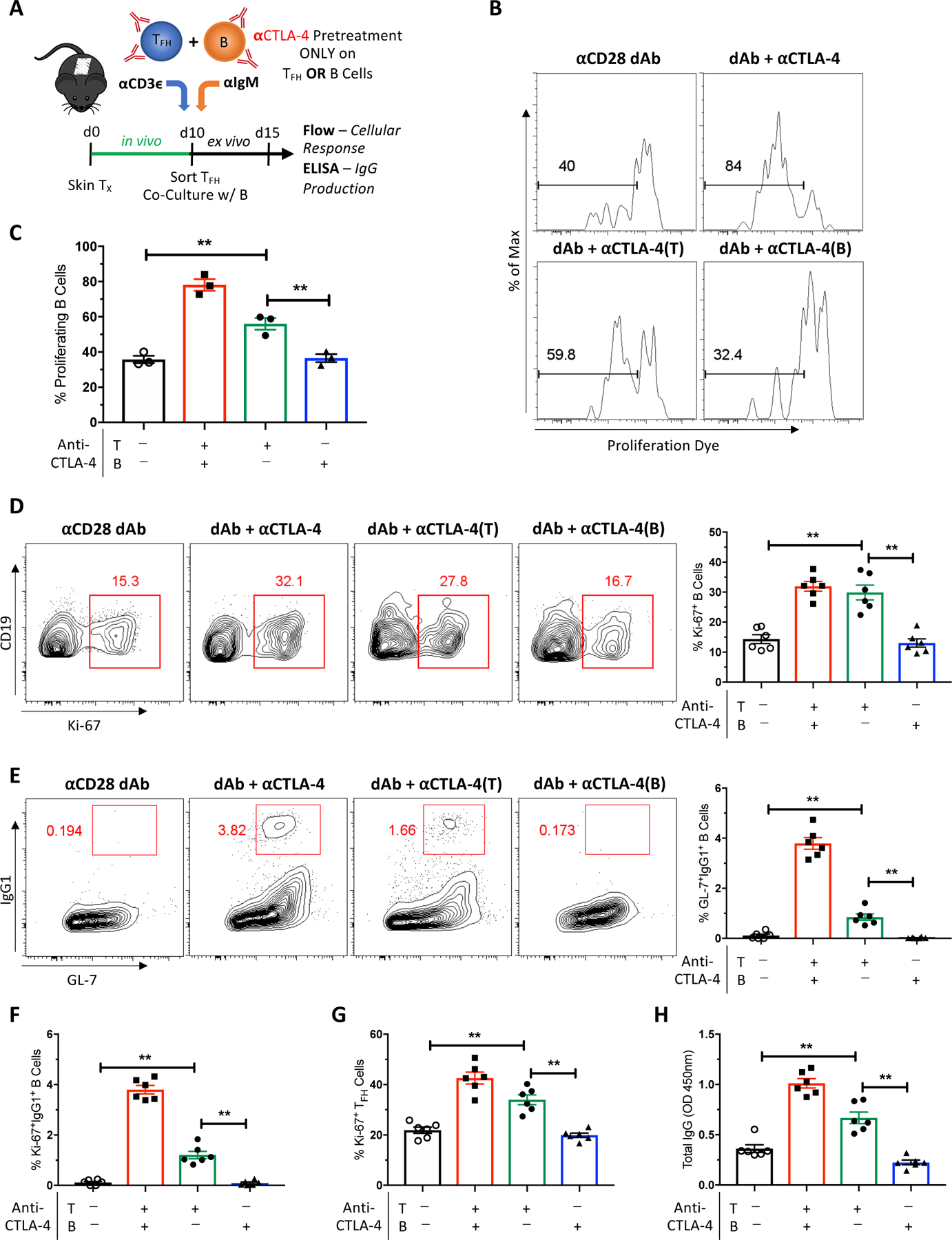
(A) Naïve B6 mice were transplanted with BALB/c skin, left untreated, and sacrificed 10 days post-transplant. Tfh and B cells from graft-DLNs were isolated and co-cultured for 5 days. Tfh:B cell co-cultures were treated with anti-CD28 dAb alone, anti-CD28 dAb plus anti-CTLA-4 mAb, or anti-CD28 dAb plus individual pre-treatment of either Tfh or B cells with anti-CTLA-4 mAb. (B) Representative histograms depicting B cell proliferation by eFluor670 proliferation dye under the indicated conditions. (C) Summary data of the frequencies of proliferating B cells (n=3 per group). (D) Representative flow plots of B cell proliferation as measured by Ki-67 and summary data of the frequencies of proliferating Ki-67+ B cells (n=6 per group). (E) Representative flow plots of class-switched GC-like GL7+IgG1+ B cells and summary data of the frequencies of these class-switched B cells (n=6 per group). Summary data of the frequencies of (F) proliferating class-switched B cells (n=6 per group), (G) proliferating Tfh cells (n=6 per group), and (H) supernatant total IgG levels (n=6 per group). Summary data represent mean (SE) and are representative of at least 2 independent experiments, with cells pooled from 10 mice per experiment and 3–6 co-culture wells per treatment group. **p < 0.01.
3.6. Tfr suppression of Tfh:B cell interactions is not CTLA-4-dependent in the presence of selective CD28 blockade
Regulatory T cell control of humoral immune responses has been demonstrated to occur via CTLA-4 (29, 30) and more potent CD28 costimulation blockade with selective blockers has been attributed to enhanced Treg activity (13, 16). To test whether the superior inhibition of Tfh:B cell interactions with the anti-CD28 dAb is influenced by Tfr expression of CTLA-4, Tfr cells known to suppress cognate Tfh:B cell interactions were introduced to the co-culture system (Figure 6A). Sorted Tfh and B cells were first co-cultured with and without Tfr cells and administered anti-CTLA-4 mAb. Consistent with in vivo findings (Figure 2), CTLA-4 blockade significantly augmented Tfh-driven formation of GL-7+IgG1+ B cells and IgG antibody production (Figures 6B, C). Tfr cell-mediated suppression of Tfh cell-driven B cell differentiation and antibody production was modestly reversed with anti-CTLA-4 mAb (Figures 6D, E). We next performed co-cultures with Tfr cells and the anti-CD28 dAb to evaluate the ability of anti-CTLA-4 mAb to reverse Tfr-mediated suppression in the presence of selective blockade. Although CTLA-4 antagonism led to moderate reversal of Tfr-mediated suppression of Tfh:B cell co-cultures in the absence of CD28 blockade, anti-CTLA-4 mAb did not overcome Tfr-induced inhibition of B cell differentiation or antibody production in the presence of the anti-CD28 dAb (Figures 6D, E). Thus these findings suggest that CTLA-4-dependent superior inhibition of alloantibody with selective CD28 blockade is not occurring through enhanced Tfr cell control of cognate Tfh:B cell interactions.
Figure 6. Tfr suppression of Tfh:B cell interactions is not CTLA-4-dependent in the presence of selective CD28 blockade.
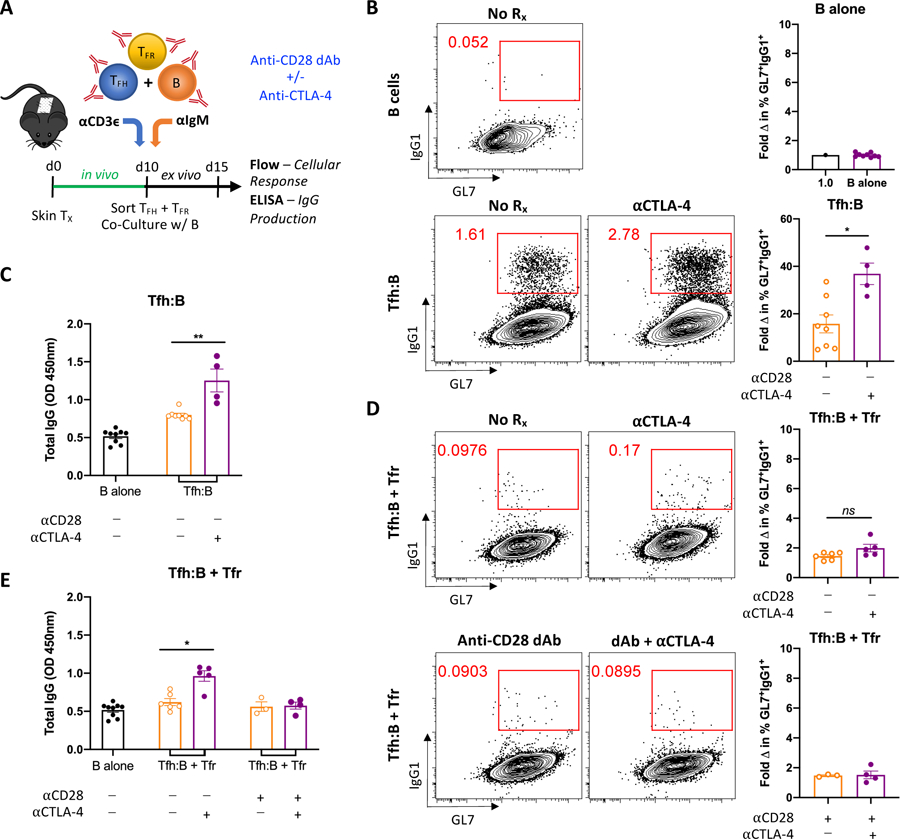
(A) Naïve B6 mice were transplanted with BALB/c skin, left untreated, and sacrificed 10 days post-transplant. Tfh, Tfr and B cells from graft-DLNs were isolated and co-cultured for 5 days. Tfh:B and Tfh:B + Tfr cell co-cultures were left untreated or treated with anti-CTLA-4 mAb alone, anti-CD28 dAb alone, or anti-CD28 dAb plus anti-CTLA-4 mAb. (B) Representative flow plots from B cell alone (control) and Tfh:B cell co-cultures depicting class-switched GC-like GL7+IgG1+ B cells under the indicated conditions. Summary data of the fold change in frequency of these class-switched B cells relative to B cell alone controls (n=4–9 per group). (C) Summary data of supernatant total IgG levels (n=4–9 per group). (D) Representative flow plots from Tfh:B + Tfr cell co-cultures depicting GL7+IgG1+ B cells under the indicated conditions. Summary data of the fold change in frequency of these class-switched B cells relative to B cell alone controls (n=3–6 per group). (E) Summary data of supernatant total IgG levels (n=3–6 per group). Summary data represent mean (SE) and are combined from 2 independent experiments, with cells pooled from 10 mice per experiment and 3–9 co-culture wells per treatment group. *p < 0.05, **p < 0.01.
4. Discussion
It has become well recognized that alloantibodies are an important immunologic cause of allograft injury and kidney graft loss (4, 36). Rapid advances in our understanding of Tfh cells, the important role this subset plays in mediating T-dependent antibody responses, and their dependence on CD28 and CTLA-4 has made them an increasingly relevant therapeutic target to combat DSA (23). Belatacept has reduced the incidence of de novo DSA (8), but its mechanism of action is suboptimal for the purpose of inhibiting alloimmunity in that CTLA-4-Ig not only blocks CD28 costimulation but also prevents critical coinhibitory and regulatory activity mediated via CTLA-4 (10). As evidence supporting antagonism of the CD28 pathway to combat alloantibodies mounts (19), it is foreseeable that more potent forms of CD28 blockade will be desired in cases of highly sensitized patients or potential costimulation blockade-based desensitization protocols (6). Here, we demonstrate that selective CD28 blockade is better than CTLA-4-Ig at inhibiting the humoral alloresponse in a clinically relevant full allogeneic mismatch model, and that this superior inhibition is mechanistically CTLA-4-dependent and Tfh cell-specific. These data support next generation selective CD28 blockade as a more efficacious strategy of addressing the clinical burden of DSA via the sparing of CTLA-4 and more potent targeting of Tfh cells.
Given the ubiquitous roles of CD28 and CTLA-4, distinct T cell subsets have not surprisingly been shown to exhibit differential responses to selective CD28 blockade versus CTLA-4-Ig (13, 14, 16, 37, 38). Our group and others have demonstrated that selective blockade results in better inhibition of alloantibody responses (12, 15, 20), but whether the superior impact of selective CD28 blockade on DSA is CTLA-4-dependent or Tfh cell-specific remains of interest to the field and has not been elucidated. In this study, we provide in vivo data indicating that anti-CD28 dAb-mediated alloantibody inhibition is CTLA-4-dependent, and in vitro Tfh:B cell co-culture data demonstrating that CTLA-4 expression by Tfh cells is necessary and sufficient for the superior humoral inhibition observed with selective CD28 blockade. While the global effects of CD28-specific blockade may contribute to the superior anti-humoral results observed with the anti-CD28 dAb, our data strongly support that selective blockade is functioning primarily on Tfh cells and not Tfr cells at the cognate Tfh:B cell level to enhance DSA inhibition through the coinhibitor CTLA-4.
Antibody responses arise from EF interactions as well as Tfh cell-driven GC responses (23, 39), and are both likely to be inhibited by CD28 blockade. Early T-dependent EF antibody responses have been shown to depend on Bcl-6 and ICOS expression and a Tfh-like peripheral T cell subset that drives B cells in rheumatoid arthritis has also been identified (24, 25, 40, 41). Along these lines, we did not observe early antibody formation with CD28 blockade (Figures 1B, 3C) and early CD138+ ASCs (PBs and PCs) were inhibited (Figures 1G,H and 3H, I). While EF antibody responses are potentially harmful (42), high level titers of GC-independent alloantibodies capable of rejection are not maintained at stable levels over time and fail to induce differentiation of long-lived plasma cells and memory B cells (28, 43). Here we observed the differential impact of selective CD28 blockade on DSA to occur later post-transplant (day 21) and persist beyond 35 days (Figures 1B and 3C), suggesting that the mechanistic impact of selective CD28 blockade on DSA is primarily a result of inhibition of the Tfh cell-driven GC response.
In considering the determinants of T-dependent antibody production, Tregs and Tfr cells have been shown to regulate Tfh cell and humoral immune responses via CTLA-4 (29, 30). Because they too depend on CD28 signals (44), not unexpectedly examination of the graft-elicited Treg and Tfr cell populations revealed reductions in the quantity of both subsets and CTLA-4 blockade in the absence of anti-CD28 therapy augmented Tfr cell responses (Figures 1, 2). However in contrast to Tfh cells, reductions in Treg and Tfr cells in the presence of CD28 blockade were not reversed by CTLA-4 antagonism in vivo (Figure 3). Additionally, CTLA-4 antagonism abrogated anti-CD28 dAb-mediated inhibition of B cell proliferation, CSR and antibody production in vitro independent of Treg and Tfr cells (Figure 4), and anti-CTLA-4 therapy did not rescue Tfr-mediated suppression of cognate Tfh:B cell reactivity in the presence of selective CD28 blockade (Figure 6). Together, these observations make it unlikely that Tfr cells are driving the improved humoral inhibition observed in our transplant model with CD28 blockade. Alternatively, sustained antibody responses have been reported to depend on CD28 function in plasma cells (35), and rare reports have identified CTLA-4 on B cells (33, 34). As such, we were able to isolate the functional role of CTLA-4 during selective CD28 blockade to the Tfh cell and not B cells (Figure 5). Therefore, preserving CTLA-4 coinhibitory capacity on Tfh cells is likely the chief mechanism conferring improved control of DSA responses under selective CD28 blockade in this study. Further examination of how sparing CTLA-4 activity on Tfh cells is influencing germinal center biology and affinity maturation is of interest and warrants future study.
GCs do not develop in the absence of CD28 due to a lack of Tfh cells (45). Tfh differentiation and function has been modeled to depend on CD28-B7 interactions at both the priming T:DC stage and subsequent Tfh:B cell stage to form GCs (23). Prevailing paradigms hold that CD80/86 ligation of CD28 are critical for both DC and B cell interactions with Tfh cells. However, a recent study showed that B7 expression was required on DCs but not B cells for Tfh cell differentiation, GC formation and antibody responses (46). These data suggest that CD28 blockade of Tfh-driven antibody production would impair early stage Tfh cell responses from T:DC priming, but not ongoing or later stage Tfh:B cell conjugate interactions that lead to antibody formation. Furthermore, delayed costimulation blockade with frequent high dose CTLA-4-Ig has been shown to reverse alloantibody responses mostly independent of graft-specific CD4+ T cells and Tfh cells (47, 48). While CD28 blockade is indeed excellent at inhibiting early Tfh cell responses (20), our findings demonstrating the ability of selective CD28 blockade to inhibit maturely differentiated effector Tfh:B cell conjugate interactions in a CTLA-4-dependent manner offer a mechanistic explanation to prior data demonstrating in vivo reversal of alloantibody formation with delayed costimulation blockade (32) and present an alternative perspective on the significance of CD28-B7 engagement between Tfh and B cells in the context of a GC response. These conflicting observations could result from differences in animal model, type of allograft, or mode of CD28 costimulation blockade. Nonetheless, our data indicate that effector Tfh cell-driven cognate B cell interactions and the alloantibody responses that result from them do in fact depend on CD28.
Susceptibility of mature effector Tfh cells engaged in cognate B cell interactions and not just naïve Tfh precursor CD4+ T cells to CD28 blockade has therapeutic implications. There has been resurgent interest in the use of CD28 costimulation blockade to attenuate alloantibody responses (6, 19). Several recent studies have reported a modest ability of belatacept to control nascent or pre-existing HLA antibodies (49–51), and a belatacept-based desensitization regimen helped mediate reductions in DSA in nonhuman primates (52). These studies showing modest efficacy with CTLA-4-Ig represent the growing concept of utilizing costimulation blockade to target CD28-mediated humoral immunity beyond initial CD4+ T cell priming and licensing of Tfh cell differentiation, and highlight a predictable need for more potent CD28 blockers. Our findings provide rationale for the continued application of CD28 blockade and that selective agents may be more potent at inhibiting memory Tfh cell-driven or more terminal GC alloreactivity due to prior HLA humoral sensitization.
In summary, this study provides evidence that superior inhibition of DSA responses through selective CD28 blockade therapy is CTLA-4-dependent and mediated via Tfh cells. Our data highlight the potential for next generation selective CD28 costimulation blockade to provide superior control of alloantibody responses through the preservation of CTLA-4 on Tfh cells. The observed susceptibility of effector Tfh cells to anti-CD28 therapy supports a shift in therapeutic focus to targeting Tfh cells as a means of controlling ongoing or pre-existing humoral alloresponses. Overall, these findings promise to inform therapeutic efforts aimed at controlling both de novo DSA responses and pre-existing alloantibodies to improve long-term outcomes in kidney transplantation.
Supplementary Material
Figure S1. CTLA-4 is differentially expressed on follicular T cells
Naïve B6 mice were transplanted with skin from BALB/c donors and sacrificed 10 days post-transplant for graft-DLN analysis. (A) Representative flow plots of Tfh (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3− T cells) and GC Tfh (Bcl6hiPD-1hi+, gated on CXCR5+PD-1hi Tfh cells) cells, and representative histograms depicting CTLA-4 expression on naïve CD4+ T cells (CD44loCD62L+), CD4+CD44hiCXCR5− T cells, Tfh cells and GC Tfh cells. (B) Summary data of CTLA-4 expression levels and frequencies of CTLA-4+ cells amongst the indicated subsets (n=5 per group). (C) Representative histograms depicting CTLA-4 expression on naïve CD4+ T cells, Treg (CD4+Foxp3+CD25+) cells and Tfr (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3+ T cells) cells, along with summary data of CTLA-4 expression amongst the indicated subsets (n=5 per group).
Acknowledgments
The authors would like to thank Drs. S. G. Nadler and S. J. Suchard at Bristol-Myers Squibb for providing the anti-CD28 dAb, along with Robert Karaffa and Kametha Fife for their assistance with FACS cell sorting. Grants T32 AI070081 (G.M.L), F31 AI145178 (G.M.L), R01 AI073707 (M.L.F.), R01 AI104699 (M.L.F.), and K08 AI132747 (I.R.B.) supported this work.
Abbreviations:
- ASC
antibody secreting cell
- CSR
class switch recombination
- dAb
domain antibody
- DLN
draining lymph node
- DSA
donor-specific antibody
- EF
extrafollicular
- GC
germinal center
- Tfh
T follicular helper
- Tfr
T follicular regulatory
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. [DOI] [PubMed] [Google Scholar]
- 2.Evans RW, Manninen DL, Garrison LP Jr., Hart LG, Blagg CR, Gutman RA, et al. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312(9):553–9. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant. 2019;19 Suppl 2:19–123. [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8(6):348–57. [DOI] [PubMed] [Google Scholar]
- 5.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14(2):255–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan SC, Ammerman N, Choi J, Huang E, Peng A, Sethi S, et al. Novel Therapeutic Approaches to Allosensitization and Antibody-mediated Rejection. Transplantation. 2019;103(2):262–72. [DOI] [PubMed] [Google Scholar]
- 7.Chong AS, Rothstein DM, Safa K, Riella LV. Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant. 2019;19(8):2155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–43. [DOI] [PubMed] [Google Scholar]
- 9.Heher E, Markmann JF. The Clearer BENEFITS of Belatacept. N Engl J Med. 2016;374(4):388–9. [DOI] [PubMed] [Google Scholar]
- 10.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2010;2(17):17ra0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, et al. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211(2):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ville S, Poirier N, Branchereau J, Charpy V, Pengam S, Nerriere-Daguin V, et al. Anti-CD28 Antibody and Belatacept Exert Differential Effects on Mechanisms of Renal Allograft Rejection. J Am Soc Nephrol. 2016;27(12):3577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaitsu M, Issa F, Hester J, Vanhove B, Wood KJ. Selective blockade of CD28 on human T cells facilitates regulation of alloimmune responses. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–53. [DOI] [PubMed] [Google Scholar]
- 18.Badell IR, Elbein R, Bray RA, Gebel HM, Adams AB, Larsen CP. Belatacept Monotherapy in Kidney Transplant Recipients with Failed Allografts Reduces Humoral Sensitization in a Single Center Randomized Controlled Trial. Am J Transplant. 2019;19(S3):450. [DOI] [PubMed] [Google Scholar]
- 19.Parsons RF, Larsen CP, Pearson TC, Badell IR. Belatacept and CD28 Costimulation Blockade: Preventing and Reducing Alloantibodies over the Long Term. Curr Transplant Rep. 2019;6(4):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badell IR, La Muraglia GM 2nd, Liu D, Wagener ME, Ding G, Ford ML. Selective CD28 Blockade Results in Superior Inhibition of Donor-Specific T Follicular Helper Cell and Antibody Responses Relative to CTLA4-Ig. Am J Transplant. 2018;18(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badell IR, Ford ML. T follicular helper cells in the generation of alloantibody and graft rejection. Curr Opin Organ Transplant. 2016;21(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walters GD, Vinuesa CG. T Follicular Helper Cells in Transplantation. Transplantation. 2016;100(8):1650–5. [DOI] [PubMed] [Google Scholar]
- 23.Crotty S Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63. [DOI] [PubMed] [Google Scholar]
- 24.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208(7):1377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, et al. Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J Immunol. 2013;191(7):3705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S, et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol. 2012;188(6):2643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB 3rd, Iwakoshi NN, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhabra M, Alsughayyir J, Qureshi MS, Mallik M, Ali JM, Gamper I, et al. Germinal Center Alloantibody Responses Mediate Progression of Chronic Allograft Injury. Front Immunol. 2018;9:3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–25. [DOI] [PubMed] [Google Scholar]
- 31.Sage PT, Sharpe AH. In vitro assay to sensitively measure T(FR) suppressive capacity and T(FH) stimulation of B cell responses. Methods Mol Biol. 2015;1291:151–60. [DOI] [PubMed] [Google Scholar]
- 32.La Muraglia GM 2nd, Wagener ME, Ford ML, Badell IR. Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor-specific antibody formation after transplantation. Am J Transplant. 2020;20(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper HM, Brouwer M, Linsley PS, van Lier RA. Activated T cells can induce high levels of CTLA-4 expression on B cells. J Immunol. 1995;155(4):1776–83. [PubMed] [Google Scholar]
- 34.Quandt D, Hoff H, Rudolph M, Fillatreau S, Brunner-Weinzierl MC. A new role of CTLA-4 on B cells in thymus-dependent immune responses in vivo. J Immunol. 2007;179(11):7316–24. [DOI] [PubMed] [Google Scholar]
- 35.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, et al. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208(7):1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. [DOI] [PubMed] [Google Scholar]
- 37.Krummey SM, Cheeseman JA, Conger JA, Jang PS, Mehta AK, Kirk AD, et al. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. Am J Transplant. 2014;14(3):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins BK, Tkachev V, Furlan SN, Hunt DJ, Betz K, Yu A, et al. CD28 blockade controls T cell activation to prevent graft-versus-host disease in primates. J Clin Invest. 2018;128(9):3991–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. [DOI] [PubMed] [Google Scholar]
- 40.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205(12):2873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alsughayyir J, Chhabra M, Qureshi MS, Mallik M, Ali JM, Gamper I, et al. Relative Frequencies of Alloantigen-Specific Helper CD4 T Cells and B Cells Determine Mode of Antibody-Mediated Allograft Rejection. Front Immunol. 2018;9:3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabant M, Gorbacheva V, Fan R, Yu H, Valujskikh A. CD40-Independent Help by Memory CD4 T Cells Induces Pathogenic Alloantibody But Does Not Lead to Long-Lasting Humoral Immunity. American Journal of Transplantation. 2013;13(11):2831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linterman MA, Denton AE. Treg cells and CTLA-4: the ball and chain of the germinal center response. Immunity. 2014;41(6):876–8. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156(12):4576–81. [PubMed] [Google Scholar]
- 46.Watanabe M, Fujihara C, Radtke AJ, Chiang YJ, Bhatia S, Germain RN, et al. Co-stimulatory function in primary germinal center responses: CD40 and B7 are required on distinct antigen-presenting cells. J Exp Med. 2017;214(9):2795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9):2280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young JS, Chen J, Miller ML, Vu V, Tian C, Moon JJ, et al. Delayed Cytotoxic T Lymphocyte-Associated Protein 4-Immunoglobulin Treatment Reverses Ongoing Alloantibody Responses and Rescues Allografts From Acute Rejection. Am J Transplant. 2016;16(8):2312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018;18(7):1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018;18(7):1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leibler C, Matignon M, Moktefi A, Samson C, Zarour A, Malard S, et al. Belatacept in renal transplant recipient with mild immunologic risk factor: A pilot prospective study (BELACOR). Am J Transplant. 2019;19(3):894–906. [DOI] [PubMed] [Google Scholar]
- 52.Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M, et al. Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant. 2019;19(3):724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CTLA-4 is differentially expressed on follicular T cells
Naïve B6 mice were transplanted with skin from BALB/c donors and sacrificed 10 days post-transplant for graft-DLN analysis. (A) Representative flow plots of Tfh (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3− T cells) and GC Tfh (Bcl6hiPD-1hi+, gated on CXCR5+PD-1hi Tfh cells) cells, and representative histograms depicting CTLA-4 expression on naïve CD4+ T cells (CD44loCD62L+), CD4+CD44hiCXCR5− T cells, Tfh cells and GC Tfh cells. (B) Summary data of CTLA-4 expression levels and frequencies of CTLA-4+ cells amongst the indicated subsets (n=5 per group). (C) Representative histograms depicting CTLA-4 expression on naïve CD4+ T cells, Treg (CD4+Foxp3+CD25+) cells and Tfr (CXCR5+PD-1hi, gated on CD4+CD44hiFoxp3+ T cells) cells, along with summary data of CTLA-4 expression amongst the indicated subsets (n=5 per group).


