Abstract
Objectives:
Prior work in a cohort of youth with functional abdominal pain (FAP) identified patient subgroups (High Pain Dysfunctional, High Pain Adaptive, Low Pain Adaptive) that predicted differences in the course of FAP from childhood into young adulthood. We aimed to replicate these subgroups in a new sample of adolescents with FAP using the original classification algorithm and to extend subgroup characteristics to include parental characteristics and health service use.
Methods:
Adolescents (n = 278; ages 11-17 years, 66% females) presenting to a gastroenterology clinic for abdominal pain and their parents (92% mothers) completed self-report measures; adolescents also completed a 7-day pain diary.
Results:
The replicated patient subgroups exhibited distress and impairment similar to subgroups in the original sample. Moreover, in novel findings, the High Pain Dysfunctional subgroup differed from other subgroups by the predominance of mother-daughter dyads jointly characterized by high levels of anxiety, depressive symptoms, pain behavior, and pain catastrophizing. The High Pain Dysfunctional subgroup used more health care services than Low Pain Adaptive but did not differ from High Pain Adaptive.
Discussion:
Findings replicate and extend the original FAP classification and suggest that the subgroups have unique patient and parent features that may reflect distinct illness mechanisms requiring different treatments.
Keywords: precision medicine, functional gastrointestinal disorders, chronic pain, pediatrics, patient subtypes
Introduction
The Institute of Medicine argued that subgrouping, or phenotyping, patients with pain conditions is a critical prerequisite for personalizing patient care; that is, tailoring treatment according to unique characteristics that differentiate patients with similar pain conditions [1]. Efforts in precision medicine are underway to identify subgroups of pain patients with distinct characteristics and illness mechanisms that could inform individualized treatment [2-4]. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) proposed patient phenotyping as an important initiative to better account for the heterogeneity in patients with chronic pain and their responsiveness to treatment [5]. Functional abdominal pain (FAP) is a common pediatric pain condition in which medical pathology is difficult to identify. Youth are heterogeneous in clinical presentation, course, and outcome [6-9], suggesting that subgrouping may be particularly useful in identifying patient characteristics that reflect unique illness mechanisms.
In a long-term prospective study of FAP [10] we identified three statistically-distinct FAP patient subgroups based on cluster analysis of patient-reported measures of symptoms, pain cognitions, affect, and functional impairment. These subgroups (High Pain Dysfunctional, High Pain Adaptive, and Low Pain Adaptive) identified in childhood predicted differences in the natural course of FAP from childhood into adolescence and young adulthood. At long-term follow-up, members of the High Pain Dysfunctional subgroup, compared to the other subgroups, reported significantly more persistent abdominal pain consistent with a functional gastrointestinal disorder (e.g., irritable bowel syndrome, functional dyspepsia), non-abdominal chronic pain (e.g., headache), comorbid anxiety and depressive disorders, and job loss due to illness. If these FAP patient subgroup classifications are replicable, then these subgroups could be useful for evaluating differential responses to FAP treatment in order to tailor interventions.
As a first step toward tailored treatment, we aimed to replicate the previously identified subgroups in a new sample of pediatric patients with FAP using the classification algorithm derived from the original cohort [10]. To evaluate classification validity and further phenotype these subgroups, we assessed differences among the subgroups on pain-related psychosocial measures not used in our previous work. We hypothesized that, at their initial visit to the pediatric gastroenterology clinic, the High Pain Dysfunctional subgroup would report higher levels of psychological distress (pain catastrophizing, anxiety, depressive symptoms) and impairment (pain interference, sleep disturbance, school absences) than the High Pain Adaptive subgroup, which, in turn, would report higher levels of psychological distress and impairment than the Low Pain Adaptive subgroup.
We also assessed the utility of the patient subgroups in prospectively predicting pain and impairment reported by adolescents on a 7-day diary following their medical evaluation. We expected the diaries to reveal higher daily pain frequency and severity in the High Pain Dysfunctional and High Pain Adaptive subgroups compared to the Low Pain Adaptive subgroup. We further hypothesized greater daily impairment in the High Pain Dysfunctional group compared to the two other subgroups.
Finally, we characterized, for the first time, the relation of FAP patient subgroups to parent characteristics and to health care utilization. We hypothesized that parents of patients in the High Pain Dysfunctional subgroup would report the greatest psychological distress (anxiety and depressive symptoms; catastrophizing about their child’s pain). We also expected that parents of youth in the High Pain Dysfunctional subgroup would endorse the most frequent behavior consistent with modeling and reinforcement of their adolescents’ pain. Moreover, we hypothesized that the High Pain Dysfunctional subgroup would exhibit the highest levels of health service use.
Methods
Participants
Participants were consecutive new adolescent patients (ages 11-17 years) and a parent or legal guardian presenting to a tertiary pediatric gastroenterology clinic for new patient evaluation of abdominal pain that yielded no evidence of organic disease. Participants were enrolled between November 2014 and February 2018. Inclusion criteria for patients were: (1) episodic or chronic abdominal pain for at least the past two months (the pain duration specified in the Rome III criteria for pediatric functional abdominal pain disorders), (2) able to read and write in English at the sixth grade level, and (3) access to the internet for completion of the pain diary. Exclusion criteria for patients were: (1) presence of a chronic disease (e.g., inflammatory bowel disease, diabetes), (2) hospitalization within the month prior to study enrollment, or (3) evidence from the current medical evaluation that abdominal pain could be attributed to an organic disease. Inclusion criteria for caregivers were: (1) able to read and write in English at the sixth-grade level, and (2) access to the Internet.
Research staff contacted parents of potentially eligible pediatric patients by telephone prior to the adolescent’s initial medical appointment for abdominal pain to remind them of their appointment, describe the study, screen for eligibility, and invite participation at the time of the adolescent’s clinic visit. Some patients were introduced to the study at the clinic visit if they could not be reached by phone prior to the visit.
Research staff identified 2028 potentially eligible patients based on referral information for consecutive new patients scheduled for appointments at the clinic. Of the identified patients, research staff made contact with 1253 families (62%), of which 954 expressed interest and were assessed for eligibility. Of the 954 dyads assessed for eligibility, 411 (43%) met eligibility criteria. Of the 411 eligible dyads, 344 dyads (84%) were enrolled during their clinic visit; the remaining 16% did not keep their clinic appointment and were unable to be enrolled. Some dyads (n = 42) did not complete the baseline assessment and were dropped from the study. Based on an examination of medical records for the clinic visit and follow-up appointments, 22 dyads were excluded after enrollment because the adolescent was diagnosed with organic pathology (e.g., inflammatory bowel disease, and peptic ulcers) that could explain the patient’s abdominal pain. Two adolescents were excluded after enrollment due to developmental delay that was not disclosed at the time of screening. Thus, the final sample comprised 278 dyads. Youth were predominately female (66.2%, n = 184) and White (86.0%, n = 239) with a mean age of 14.62 years (SD = 1.88). Parent participants were primarily mothers or maternal guardians (95.3%, n = 265).
Procedures
Study procedures were approved by the Vanderbilt University Medical Center Institutional Review Board. For eligible dyads, informed consent and assent were obtained. Both adolescents and their parents completed a baseline survey during their clinic visit on REDCap, a secure online survey site [11]. Adolescents were informed that they would be asked questions about pain, mood, sleep, and activities. Adolescents and parents completed their surveys independently and neither saw the others’ answers to reduce bias. Following the initial clinic visit, adolescents completed an online diary that assessed daily pain and functional impairment for 7 days. This diary assessment, and all other data presented in this report, took place prior to randomization to a treatment condition.
Measures
FAP Patient Subgroups.
Adolescent-reported measures included in the classification algorithm for the FAP subgroups were the same measures used in the original classification study [10]: the Abdominal Pain Index (API, [12]; Gastrointestinal (GI) and non-GI symptom subscales of the Children’s Somatic Symptoms Inventory – 24 (CSSI-24, [13]); the Catastrophizing subscale of the Pain Response Inventory (PRI, [14]); the Children’s Depression Inventory (CDI, [15]; the Functional Disability Inventory (FDI, [16]); and subscales of the Pain Beliefs Questionnaire-Short Form (PBQ-SF, [17]) including Pain Threat, Problem-Focused Coping Efficacy (PFCE), and Emotion-Focused Coping Efficacy (EFCE). The FDI was condensed to 10-items based on the most frequently endorsed items in a large database of FAP patients and strongly correlated with the 15-item total score (r = .0.98). The Abdominal Pain Index, Pain Response Inventory, PBQ-SF, and FDI are scored on a 0-4 scale. CDI total scores were converted to a 0-4 scale to match the scaling of the other measures. All measures exhibited adequate internal consistency (α’s ranged from 0.73-0.90). Additional information regarding these measures and development of the FAP patient subgroup classification has been presented elsewhere [10].
Psychological Distress.
Anxiety and Depressive Symptoms.
Adolescents and parents completed self-report measures of their own anxiety and depressive symptoms. Specifically, youth completed the Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Anxiety – Short Form 1 and PROMIS Pediatric Depressive Symptoms – Short Form 1 [18]. Parents completed the PROMIS Anxiety Short Form 7a and PROMIS Depression Short Form 8b [19, 20]. PROMIS measures were developed using rigorous methodology as part of an initiative of the National Institutes of Health to standardize and improve assessment of patient-reported outcomes. Each of these short-forms comprised 7 to 8 items each and asked participants to rate how often they experienced each symptom over the past 7 days on a 5-point Likert response scale ranging from Never to Always. Items were summed and converted to T-scores based on the PROMIS scoring manuals available at http://www.healthmeasures.net/promis-scoring-manuals. A T-score of 50 represents a score consistent with what was reported by the average participant in the centering sample (e.g., a nationally representative sample of youth or adults from the general population in the United States) and has a standard deviation of 10. For these PROMIS measures, higher scores indicate greater anxiety and depressive symptoms. Alpha reliabilities for the PROMIS measures of anxiety and depressive symptoms all exceeded 0.93 in the current sample.
Pain Catastrophizing Scale.
Youth completed the Pain Catastrophizing Scale for Children (PCS-C, [21]) and parents completed the Pain Catastrophizing Scale-Parent Version (PCS-P, [22]). The PCS-C and PCS-P are 13-item self-report measures assessing the extent to which a child or parent has thoughts consistent with magnifying the child’s pain severity, ruminating about the child’s pain, and feeling helpless in response to the child’s pain. Using a 5-point scale ranging from (0) not at all to (4) all the time, youth and parents indicated the degree to which they had each of the 13 thoughts or feelings in response to the child’s pain experiences. Items were summed yielding a total score ranging from 0 to 52 with higher scores indicating greater pain catastrophizing. The PCS-C and PCS-P both exhibited strong alpha reliability in the current sample, α = 0.94.
Impairment.
Pain Interference.
Adolescents completed the PROMIS Pediatric Pain Interference - Short Form 1 that assessed self-reported consequences of pain on the adolescent’s life over the past 7 days, including social, cognitive, emotional, and physical domains [23]. Using the method reported above for anxiety and depressive symptoms, items were summed and converted to T-scores based on the PROMIS scoring manuals. Higher scores indicate greater pain interference. Alpha reliability for the PROMIS Pediatric Pain Interference Short Form was 0.85 in the current sample.
Sleep Disturbance.
Adolescents completed the PROMIS Sleep Disturbance Short-Form v1.0 8b which comprises 8-items assessing sleep quality as well as difficulty falling and staying asleep over the past 7 days. Youth indicated the extent to which they had sleep problems on a scale of 1 to 5 with higher numbers indicating greater sleep disturbance. As in other studies of adolescents [24, 25], raw scores were converted to T-scores based on the centering sample for this measure, which was a nationally representative sample of adults. The present study used the adult version of the PROMIS Sleep Disturbance measure because the pediatric version was not available at the time of data collection for this study. The adult version of the PROMIS Sleep Disturbance measure has demonstrated content validity [26] as well as initial convergent validity with measures of daytime sleepiness as well as actigraphic sleep patterns in healthy adolescent samples [25]. The PROMIS Sleep Disturbance Short-Form exhibited excellent alpha reliability in the current sample of youth (α = 0.94).
School Absences.
Parents reported the number of school days their adolescent missed during the 3 months prior to the clinic visit. During summer months, parents reported the number of days missed during the last 3 months of the school year.
Adolescent Daily Diary.
Adolescents completed a 7-day online diary assessing daily pain and functional impairment. Pain intensity was assessed each day on an 11-point numeric rating scale ranging from (0) no pain to (10) worst pain. Adolescents also completed a daily diary version of the Child Activity Limitations Interview (CALI, [27, 28]) that assesses school-age children’s perceived difficulty in completing their typical daily activities due to pain. Each day, adolescents provided difficulty ratings for eight items using a 5-point scale ranging from (0) no difficulty to (4) extremely difficult. Item ratings were summed to yield total daily ratings that ranged from 0 to 32 with higher scores indicating greater functional limitations. Mean daily ratings were computed across the 7 day diary period for youth who completed at least 4 diary days (n = 270; 97%). In a large multicenter trial of adolescents with mixed idiopathic chronic pain diagnoses, the average score for the CALI over a 7-day diary period was approximately 7 out of 32 [29].
Social Learning.
Parent Pain Behaviors.
Parents completed the PROMIS Pain Behavior Short Form [20] to assess observable pain behaviors exhibited by parents over the past 7 days (e.g., grimacing and moving slowly). Parents indicated the frequency with which they exhibited each behavior using a 6-point scale ranging from (1) had no pain to (6) always. Items were summed with higher scores indicated a higher frequency of pain behaviors. The sum score was converted to T-scores using the conversion table provided in the PROMIS scoring manual. Of note, our prior work has indicated that the adolescent proxy-report of parent pain behaviors exhibited a moderate level of agreement (intra-class correlation = 0.48) with the parent self-report measure [30]. Alpha reliability for the PROMIS Pain Behavior Short Form in the current study was 0.94.
Parent Protective and Solicitous Responses.
The Adult Responses to Child Symptoms (ARCS, [31]) assessed parent behavior in response to their children’s chronic pain complaints. We used an abbreviated version of the original ARCS to reduce participant burden and used the stem, “When your child has a stomachache or abdominal pain, how often do you…” Because our sample comprised adolescents, we referred to the developmental analysis of the factor structure of the ARCS to select items best suited for parents of adolescents [32]. The form comprised three Solicitousness items and four Protectiveness items (Appendix 1). Participants rated each item on a 5-point scale ranging from (0) never to (4) always. Items were averaged to yield a composite score indicating the extent to which parents engaged in solicitous and protective responses to adolescents’ pain. Higher scores indicate more solicitous and protective responses. Alpha reliability for the composite score was adequate in this sample, α = 0.78, and stronger than either the Solicitousness or Protect subscales alone (α = 0.64 and α = 0.73, respectively).
Health Service Use.
Parents provided information regarding their adolescent’s health service use during the 3 months prior to the clinic visit. They indicated the number of visits to outpatient providers (medical doctors, mental health professionals, and alternative medicine providers), and the number and type of prescription medications. The number of visits to each type of health care provider (from “0” to “6 or more”) were summed across all three types to create an index of visits to outpatient providers over the past 3 months (range: 0-18). We coded prescription medications into four classes (GI-related, antidepressant, pain medication, ADHD medication).
Data Analysis
Data analyses were conducted with IBM SPSS version 25, R Version 3.5.3, and R Studio Version 1.0.143. The sample size was determined a priori via power analyses to detect a clinically meaningful effect for the larger study. All variables used in the classification algorithm had complete data. Overall, missing data was low for dependent variables. The highest percentage of missing data was observed for the diary data (3.2%, n = 9) and was handled with pairwise deletion in line with evidence that 5% or less missing data has negligible effects on results [33, 34].
Classification Algorithm.
Three FAP patient subgroups (Low Pain Adaptive, High Pain Adaptive, High Pain Dysfunctional) were identified in our original FAP patient classification study by means of a split-half hierarchical cluster analysis using Ward’s method with squared Euclidian distance measures and clustering at the patient level [10]. The original classification algorithm was based on 117 items from 6 patient-report measures assessing 9 different constructs. Prior to the current study, the length of three of these measures (CSSI-24, PBQ-SF, FDI) was reduced based on psychometric data; thus, 87 of the original 117 items were administered and used in the classification algorithm. Therefore, to create a classification algorithm for the current study, we computed new subgroup centroids using the shortened measures in the original database of 843 patients. Next, for each patient in the current sample, we calculated the squared Euclidian distance from the centroid of each FAP subgroup for the 9 constructs in the classification algorithm. Each patient received a sum score of the squared Euclidian distances for each of the three patient subgroups across the 9 constructs (i.e., sum of squared Euclidian distances from the Low Pain Adaptive centroids, sum of squared Euclidian distances from the High Pain Adaptive centroids, and sum of squared Euclidian distances from the High Pain Dysfunctional centroids). As in our previous work replicating pain coping profiles [35], patients were assigned to the subgroup for which they had the lowest squared Euclidian distance (SPSS syntax is available from the first author).
Evaluation of FAP Patient Subgroups.
We compared the resulting FAP patient subgroups on measures not used in the classification algorithm, including demographic characteristics, adolescent-reported and parent-reported measures obtained at the clinic visit, and subsequent 1-week daily diary reports of pain and impairment completed by adolescents. Depending on whether data were categorical or continuous, differences between patient subgroups were evaluated with either chi-squared analyses or one-way analysis of variance (ANOVA). Chi-squared analyses used follow-up z-tests to compare proportions using p-values adjusted with the Bonferroni method. ANOVA analyses used post-hoc tests adjusted with the Bonferroni method to evaluate pair-wise comparisons. Because FAP patient subgroups differed by age and sex, subsequent analyses included both variables as covariates. One-way analysis of covariance (ANCOVA) evaluated whether FAP patient subgroups differed on adolescent- and parent-reported measures administered at the clinic and on subsequent measures from the adolescent-reported 7-day diary. Effect sizes for ANCOVA analyses are reported as partial eta squared (ηp2). According to Cohen [36], small, medium, and large effects correspond with ηp2 values of 0.01, 0.06, and 0.14, respectively. Post-hoc tests adjusted with the Bonferroni method evaluated pair-wise comparisons when warranted based on a significant omnibus F-test.
To accommodate outcome variables that were in the form of counts (i.e., school absences, number of medications used, and outpatient visits), we first identified the appropriate count data model for each variable. Though Poisson regression is the classic analytic model for counts, it often fails to accommodate overdispersion, a common feature of count data, where the conditional variances around predicted values are greater than those estimated by the model [37, 38]. Thus, we assessed the comparative fit of negative binomial (NB) regression, which can account for overdispersion, using bounded likelihood ratio chi-squared tests. Model fit indices indicated the need for a zero-inflated NB regression for school absences and NB regression for outpatient visits and medications. Each of these models included age and sex as covariates and was run twice, once with the High Pain Adaptive subgroup as the referent group, and once with the Low Pain Adaptive subgroup as the referent group to allow for follow-up comparisons among all three groups. Incident rate ratios and 95% confidence intervals are presented for significant findings.
Results
Demographic Characteristics of FAP Patient Subgroups
Figure 1 presents means on the measures used in the FAP patient subgroup classification algorithm. Of the 278 enrolled participants, 39% were classified in the High Pain Dysfunctional subgroup (n = 109), 41% in the High Pain Adaptive subgroup (n = 114), and 20% in the Low Pain Adaptive subgroup (n = 55). Compared to the original sample used for the development of the classification algorithm [10], the current sample had significantly more youth classified as High Pain Dysfunctional (39% vs 23%), Χ2(1) = 27.15, p < 0.001 and fewer youth classified as Low Pain Adaptive (20% vs 37%), Χ2(1) = 27.39, p < 0.001. The proportion of youth classified as High Pain Adaptive was similar between samples (41% vs 40%). Of note, the original sample included youth ages 7-17 years-old, whereas the current sample focused on older youth, ages 11-17 years-old.
Figure 1.
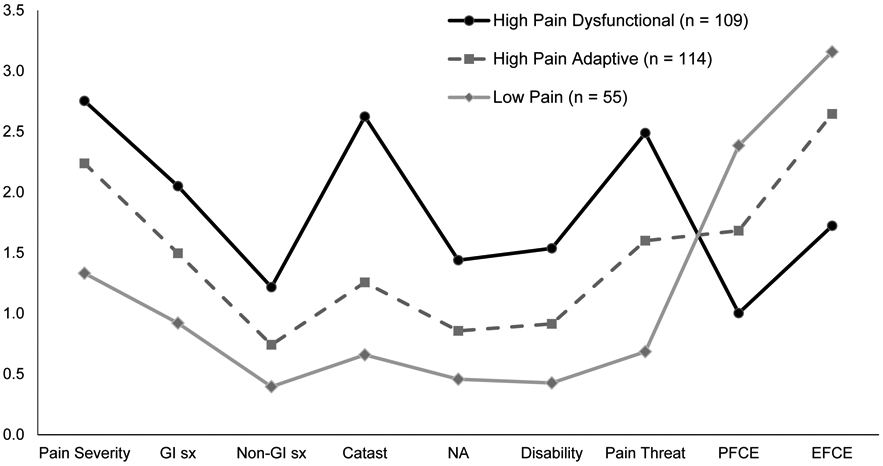
FAP subgroups by pain and pain coping variables used in classification algorithm
Note. All measures are presented on a 0-4 scale. All pairwise comparisons between subgroups significant at the p < 0.001 level for each variable. GI sx = gastrointestinal symptoms; Non-GI sx = non-gastrointestinal symptoms; Catast = catastrophizing from the Pain Response Inventory; NA = negative affect (Children’s Depression Inventory); PFCE = problem focused coping efficacy; EFCE = emotion focused coping efficacy
Table 1 presents demographic characteristics by subgroup. FAP patient subgroups differed significantly by youth age, F(2, 277) = 6.72, p = 0.001, and sex, Χ2(2) = 28.33, p < 0.001. Youth in the High Pain Dysfunctional subgroup were significantly older compared to those in the High Pain Adaptive and Low Pain Adaptive subgroups. The High Pain Dysfunctional subgroup comprised significantly more females than the High Pain Adaptive subgroup, which comprised significantly more females than the Low Pain Adaptive subgroup. Female caregivers constituted 90% or more of parent participants in all three groups. FAP subgroups did not differ significantly by youth race or by parental education, employment, or marital status.
Table 1.
Demographic factors by FAP patient subgroup
| Demographic Factor | High Pain Dysfunctional (n = 109) |
High Pain Adaptive (n = 114) |
Low Pain Adaptive (n = 55) |
Subgroup comparisons |
|---|---|---|---|---|
| Adolescent Age, M ± SD | 15.08 ± 1.89 | 14.48 ± 1.82 | 14.01 ± 1.79 | HPD > HPA & LPA |
| Adolescent Sex, % (n) | ||||
| Female | 79.8% (87) | 66.7% (76) | 38.2% (21) | HPD & HPA > LPA |
| Male | 20.2% (22) | 33.3% (38) | 61.8% (34) | LPA > HPD & HPA |
| Adolescent Race, % (n) | ||||
| White | 80.7% (89) | 88.6% (101) | 92.6% (50) | N.D. |
| Person of Color* | 19.3% (21) | 11.4% (13) | 7.4% (4) | N.D. |
| Parent in study, % (n) | ||||
| Mother or grandmother | 93.6% (102) | 95.6% (109) | 96.4% (53) | N.D. |
| Father | 6.4% (7) | 4.4% (5) | 3.6% (2) | N.D. |
| Parent employment, % (n) | ||||
| Employed | 66.7% (72) | 67.5% (77) | 61.8% (34) | N.D. |
| Unemployed | 33.3% (36) | 32.5% (37) | 38.2% (21) | N.D. |
| Parent education, % (n) | ||||
| High school or less | 26.6% (29) | 16.7% (19) | 21.8% (12) | N.D. |
| Vocational school or some college | 32.1% (35) | 36.8% (42) | 40.0% (22) | N.D. |
| Four-year college | 31.2% (34) | 32.5% (37) | 25.5% (14) | N.D. |
| Graduate or professional school | 10.1% (11) | 14.0% (16) | 12.7% (7) | N.D. |
| Parent marital status, % (n) | ||||
| Married or partnered | 69.7% (76) | 71.9% (82) | 78.2% (43) | N.D. |
| Single, Divorced, or Separated | 30.3% (33) | 21.1% (32) | 21.8% (12) | N.D. |
Note. Adolescent age and sex significantly differed at p < .05 level.
Due to the low frequency of some racial groups, races typically identified by the National Institutes of Health as minority racial groups (Black or African American, American Indian or Alaska Native, Asian, and Mixed Race) were collapsed into a single category. Percentages do not always add up to 100% due to missing data for some demographic variables.
Concurrent Validity of FAP Patient Subgroups
Table 2 presents results from ANCOVA analyses that, controlling for youth age and sex, evaluated differences among the patient subgroups on adolescent-reported concurrent validity measures (not included in the algorithm for subgroup classification). Results indicated FAP patient subgroups differed significantly across all continuous measures of psychological distress (PROMIS Pediatric Anxiety, PROMIS Pediatric Depressive Symptoms, and the Pain Catastrophizing Scale for Children) and impairment (PROMIS Pediatric Pain Interference and PROMIS Sleep Disturbance). As hypothesized, pair-wise comparisons across subgroups indicated stair-wise effects for anxiety, depressive symptoms, pain catastrophizing, pain interference, and sleep disturbance. Specifically, youth in the High Pain Dysfunctional subgroup reported significantly higher scores than youth in the High Pain Adaptive group which, in turn, reported significantly higher scores than youth in the Low Pain Adaptive subgroup. Effect sizes were large for all of these comparisons (see Table 2). Moreover, T-scores for the PROMIS Pediatric measures showed that the High Pain Dysfunctional group scored 1 standard deviation above the mean reported for youth in the general population on PROMIS measures of anxiety, depressive symptoms, and pain interference, whereas youth in the High Pain Adaptive and Low Pain Adaptive groups scored at or below the population mean.
Table 2.
Means and standard deviations for adolescent factors by FAP patient subgroup
| Adolescent factors | High Pain Dysfunctional (n = 109) |
High Pain Adaptive (n = 114) |
Low Pain Adaptive (n = 55) |
F (2, 273) |
ηp2 | Subgroup comparisons |
|---|---|---|---|---|---|---|
| Psychological Distress | ||||||
| Pain Catastrophizing | 33.85 (8.98) | 17.96 (8.03) | 10.21 (6.23) | 163.60 | 0.55 | HPD > HPA > LPA |
| Anxiety | 59.19 (10.77) | 50.37 (10.69) | 41.45 (8.32) | 43.02 | 0.24 | HPD > HPA > LPA |
| Depressive Symptoms | 56.42 (12.50) | 46.95 (10.26) | 39.83 (6.91) | 36.19 | 0.21 | HPD > HPA > LPA |
| Impairment | ||||||
| Sleep Disturbance | 57.72 (10.70) | 52.40 (10.37) | 42.67 (7.94) | 27.49 | 0.17 | HPD > HPA > LPA |
| Pain Interference | 57.16 (7.24) | 50.92 (6.79) | 43.21 (5.82) | 62.67 | 0.32 | HPD > HPA > LPA |
| School Absences (Past 3 Months) | 7.84 (7.23) | 6.30 (7.34) | 4.88 (4.22) | HPD > HPA > LPA | ||
| Adolescent Daily Diary |
F (2, 264) |
ηp2 | ||||
| Number of days reporting pain | 5.51 (1.92) | 5.49 (1.73) | 3.25 (1.98) | 23.18 | 0.15 | HPD & HPA > LPA |
| Average daily pain intensity | 5.64 (1.62) | 4.70 (1.58) | 3.29 (1.90) | 29.84 | 0.18 | HPD > HPA > LPA |
| Functional impairment | 7.28 (4.81) | 5.09 (3.49) | 2.14 (2.03) | 24.24 | 0.15 | HPD > HPA > LPA |
Note. All f-tests were statistically significant at the p < .001 level. Subgroup comparisons indicate significant pairwise comparisons at the p < .05 level. Differences in school absences were tested with zero-inflated count models. See text for test statistics. Raw means are reported in tables. ANCOVA analyses included youth age and sex as covariates.
Regarding school absences, results from zero-inflated NB regression indicated that, controlling for age and sex, youth in the High Pain Dysfunctional subgroup missed significantly more days of school in the 3 months prior to their clinic visit (mean = 7.84, SD = 7.23) than the High Pain Adaptive (mean = 6.30 days, SD = 7.34) and Low Pain Adaptive (mean = 4.88 days, SD = 4.22) subgroups. The incident rate for school absence in the High Pain Dysfunctional subgroup was 1.38 times (95% CI: 1.05, 1.81) that of the High Pain Adaptive subgroup and 1.68 times (95% CI: 1.19, 2.36) that of the Low Pain Adaptive subgroup. The High Pain Adaptive and Low Pain Adaptive groups did not differ significantly from each other on school absences.
Short-term Predictive Validity of FAP Patient Subgroups
Results from ANCOVA analyses of diary data, controlling for youth age and sex, indicated that the three FAP patient subgroups differed significantly on the number of pain days, average daily pain intensity, and functional impairment during the 7-day diary period following the clinic visit (see Table 2). Specifically, the High Pain Dysfunctional and High Pain Adaptive subgroups both experienced pain on approximately 5 days during the week, significantly more than the 3 days of pain reported by the Low Pain Adaptive subgroup. Youth in the High Pain Dysfunctional subgroup reported significantly greater pain intensity and functional impairment during the 7-day diary compared to youth in the High Pain Adaptive subgroup who, in turn, reported significantly greater pain intensity and functional impairment compared to youth in the Low Pain Adaptive subgroup. Thus, FAP patient subgroup membership significantly predicted patients’ reports of pain and functional impairment during the 7 days following initial assessment.
Convergent Validity of FAP Patient Subgroups
Parent Characteristics.
Results from ANCOVA analyses indicated that the three FAP patient subgroups differed significantly on parent psychological distress (anxiety, depressive symptoms, and catastrophizing about adolescent’s pain) and social learning factors (parents’ reports of their own pain behaviors and protective and solicitous responses to adolescent pain) with effect sizes ranging from small to medium (see Table 3). Parents of youth in the High Pain Dysfunctional subgroup compared to parents of youth in both the High Pain Adaptive and Low Pain Adaptive subgroups reported significantly greater anxiety and catastrophizing about their adolescent’s pain. Parents of youth in the High Pain Dysfunctional subgroup, compared to parents of youth in the High Pain Adaptive subgroup, reported significantly greater depressive symptoms and pain behavior, as well as more frequent protective and solicitous responses to their adolescent’s pain. Parents of youth in the Low Pain Adaptive subgroup did not differ significantly from parents of youth in the other subgroups on depressive symptoms, pain behaviors, or protective and solicitous responses to adolescent’s pain.
Table 3.
Means and standard deviations for parent factors by FAP patient subgroup
| Parent factors | High Pain Dysfunctional (n = 109) |
High Pain Adaptive (n = 114) |
Low Pain Adaptive (n = 55) |
F (2, 273) |
ηp2 | Subgroup comparisons |
|---|---|---|---|---|---|---|
| Parent Psychological Distress | ||||||
| Anxiety | 55.89 (10.64) | 51.86 (9.40) | 50.78 (9.73) | 8.21*** | 0.06 | HPD > HPA & LPA |
| Depressive symptoms | 49.72 (10.31) | 46.40 (8.97) | 45.70 (8.97) | 3.49* | 0.03 | HPD > HPA |
| Catastrophizing about adolescent’s pain | 26.20 (10.94) | 21.57 (10.99) | 19.71 (8.70) | 8.21*** | 0.06 | HPD > HPA & LPA |
| Social Learning | ||||||
| Parent pain behaviors (modeling) | 55.08 (9.87) | 50.25 (11.13) | 51.33 (10.55) | 6.39** | 0.05 | HPD > HPA |
| Protective/solicitous responses to pain | 1.83 (0.77) | 1.56 (0.67) | 1.67 (0.65) | 4.20* | 0.03 | HPD > HPA |
Note.
p < 0.05
p < 0.01
p < .001. Subgroup comparisons indicate significant pairwise comparisons of means at the p < .01 level. Raw means are reported in tables. ANCOVA analyses included youth age and sex as covariates.
Health Services and Prescription Medication.
Parents reported their children’s use of health services and prescription medication during the three months prior to the clinic visit. The number of outpatient visits significantly correlated with the number of prescription medications used (Spearman’s rho = 0.28). All analyses controlled for youth age and sex.
Outpatient Visits.
Controlling for age and sex, results from NB regression showed that individuals in the High Pain Dysfunctional subgroup had a significantly greater number of outpatient visits than individuals in the Low Pain Adaptive group. Specifically, the incident rate of outpatient visits for the High Pain Dysfunctional subgroup was 1.29 times (95% CI: 1.01, 1.65) that of the Low Pain Adaptive subgroup. Number of outpatient visits for the High Pain Adaptive (mean = 3.6, SD = 2.52) subgroup did not differ significantly from the Low Pain Adaptive (mean = 3.02, SD = 2.19) or High Pain Dysfunctional (mean = 4.04, SD = 2.87) subgroups.
Prescription Medications.
NB regression controlling for age and sex showed that the number of prescription medications reported for the High Pain Dysfunctional subgroup (M = 2.08, SD = 1.68) was significantly greater than that for the Low Pain Adaptive subgroup (M = 1.16, SD = 1.30), such that the incident rate of prescription medication for the High Pain Dysfunctional subgroup was 1.58 times (95% CI: 1.09, 2.29) that of the Low Pain Adaptive subgroup. The number of prescription medications for the High Pain Adaptive subgroup (Mean = 1.66, SD = 2.29) did not differ significantly from that for the Low Pain Adaptive or High Pain Dysfunctional subgroups.
Classes of medication by FAP patient subgroup are presented in Supplementary Table 1. Overall, prescription medication use in the sample was primarily GI-related (e.g., proton pump inhibitors, histamine 2 blockers, anti-spasmodics, and anti-nausea) with 54% of the sample (n = 150) reporting that they had at least 1 GI-related prescription medication in the three months prior to their clinic visit. Less than a tenth of the sample reported taking an antidepressant (e.g., SSRI, SNRI, DNRI, and tricyclic; n = 27, 9.7%) or ADHD medication (n = 16, 5.8%). Of the 27 parents reporting their child tried an antidepressant, approximately one-fourth (n = 7) indicated pain as the reason for the prescription. A small proportion of the sample reported taking pain-specific prescription medications (e.g., NSAID, opioid analgesic, antiepileptic medications for pain, and migraine medications; n = 24, 8.6%). Although FAP subgroups did not differ significantly on prescription GI-medications or ADHD medications, controlling for age and sex, the High Pain Dysfunctional subgroup was significantly more likely to report a prescription antidepressant compared to the High Pain Adaptive subgroup (OR = 3.62, 95% CI = 1.37, 9.57) and the Low Pain Adaptive subgroup (OR = 4.91, 95% CI = 1.04, 23.20). Additionally, the High Pain Dysfunctional subgroup was significantly more likely to report a prescription pain medication compared to the Low Pain Adaptive subgroup (OR = 8.35, 95% CI = 1.03, 67.98).
Discussion
This study replicated, in a new sample of adolescents with FAP, the classification of patients into three distinct subgroups based on multidimensional pain assessment [10]. We validated the classification with measures of psychological distress and impairment not included in the classification algorithm. Importantly, we also further characterized the FAP subgroups by assessing, for the first time, clinically important phenotypic domains including parent characteristics and patients’ use of health services and prescription medication. Results provide further evidence that FAP patients are a heterogeneous population comprising subgroups that may reflect distinct illness mechanisms and may benefit from different treatment approaches.
The most striking finding was identification of features distinguishing the High Pain Dysfunctional subgroup from the High Pain Adaptive and Low Pain Adaptive subgroups in our adolescent sample. In our earlier prospective study [10], we followed FAP patients for nine years after medical evaluation and found considerably worse outcomes for the High Pain Dysfunctional subgroup compared to the others. For example, at follow-up in adolescence and young adulthood, patients in the High Pain Dysfunctional subgroup were significantly more likely than those in the High Pain Adaptive and Low Pain Adaptive subgroups to meet Rome III symptom criteria [39] for pain-related functional gastrointestinal disorders (FGID) (63.6% vs. 35.7% and 32.8%) and to have FGID comorbid with non-abdominal chronic pain (41% vs. 11% and 17%) and comorbid with a DSM-IV TR [40] affective disorder (32% vs.13% and 13%; [10]). Further, the High Pain Dysfunctional subgroup compared to the Low Pain Adaptive subgroup exhibited significantly greater temporal summation of pain, suggesting that central sensitization may be one specific mechanism characterizing this subgroup [10]. These earlier findings demonstrated that the High Pain Dysfunctional subgroup was at particular risk for poor long-term outcomes and alerted us to the importance of further characterizing youth in this subgroup.
In this study, we identified subgroup characteristics not included in the original FAP subgroup classification that distinguished the High Pain Dysfunctional subgroup, and focused on a slightly older adolescent population. Youth in the High Pain Dysfunctional subgroup had significantly higher anxiety, sleep disturbance, and school absence than those in other subgroups. These characteristics, in combination with high pain threat, pain catastrophizing, and low pain coping efficacy that define the High Pain Dysfunctional subgroup, are consistent with fear-avoidance mechanisms that drive persistent pain and disability [41], and are consistent with the higher levels of pain-related impairment seen in a broader range of activities in adolescents compared to younger children. An escalating cycle of dysfunction may be associated with high pain threat appraisal and activity avoidance with deteriorating school performance and peer relationships. These, in turn, may impede personal and social development, thereby further escalating anxiety, pain threat, and impairment [42]. Thus, treatment of adolescents in the High Pain Dysfunctional subgroup should target fear-avoidance mechanisms including pain cognitions, anxiety, and avoidance of regular activities.
We also identified family variables that distinguished the High Pain Dysfunctional subgroup. Parents of patients in the High Pain Dysfunctional subgroup, compared to parents of those in the High Pain Adaptive subgroup, reported significantly greater catastrophizing about their adolescent’s pain and significantly more frequent protective and solicitous responses. These characteristics are consistent with social learning mechanisms and operant conditioning that may reinforce patients’ pain and impairment [43, 44]. Thus, the High Pain Dysfunctional subgroup may benefit especially from interventions, such as those by Levy and colleagues [45, 46], that focus on decreasing parents’ protective/solicitous responding to their children’s pain.
Results further suggest that focus of parent treatment should not be limited to parents’ responses to their child’s pain. Parents of youth in the High Pain Dysfunctional subgroup, compared to parents of those in the High Pain Adaptive subgroup, endorsed significantly more pain behaviors themselves and greater anxiety and depressive symptoms. Thus, consistent with Cordts and colleagues [47], parents’ own chronic pain characteristics and psychological functioning represent important and potentially modifiable factors associated with child pain and functioning, and should be included in family-based interventions. Interventions focused on reducing parent distress such as problem solving skills training (e.g., [48]) may be particularly relevant in this population.
Individualizing treatment by sex may be another important consideration for FAP interventions for adolescents. Notably, mother-daughter dyads predominated in the High Pain Dysfunctional subgroup, as this subgroup had significantly more female patients than the other subgroups (80% vs. 67% in High Pain Adaptive and 38% in Low Pain Adaptive), and 94% of participating parents were mothers. Both mothers and daughters in the High Pain Dysfunctional subgroup exhibited high levels of psychological distress and catastrophizing about the daughters’ pain, both reported high levels of pain behavior, and mothers reported high levels of solicitous/protective parenting. This constellation of features, particularly pain catastrophizing by both mothers and daughters, is consistent with evidence that mother-daughter co-rumination about distress is associated with higher levels of internalizing symptoms in adolescent girls [49-51] and should be explored further with research on concordance of pain behavior in mother-daughter dyads.
Regarding health service utilization in the three months prior to medical evaluation, the High Pain Dysfunctional subgroup, as compared to the Low Pain Adaptive subgroup, reported significantly more outpatient visits and total prescription medications but did not differ from the High Pain Adaptive subgroup. High health service and prescription medication use can put patients at risk for iatrogenic illness and medicalization of relatively benign symptoms [52]. Addressing these problems will require close collaboration between behavioral and medical providers.
Results of the 7-day pain diary were consistent with retrospective pain reports and differed by subgroups; both the High Pain Dysfunctional and High Pain Adaptive subgroups averaged 5.5 days of pain with an average intensity of approximately 5 on an 11-point scale. Even the Low Pain Adaptive subgroup reported pain on 3 days of the week, but their average pain intensity was low -- 3 on an 11-point scale. Additionally, pain-related impairment during the week was significantly higher in the High Pain Dysfunctional subgroup than the High Pain Adaptive subgroup which, in turn, was significantly higher than the Low Pain Adaptive subgroup. Thus, pain diary data prospectively validate the patient subgroups and highlight the need for follow-up after subspecialty medical evaluation.
Weak treatment effects and inconsistent results observed to date across RCTs for FAP [46, 53-59] may be due in part to differences in the characteristics of FAP patients across RCT samples. Numerous studies have documented high rates of anxiety, chronic pain, and disability in the general population of patients with FAP and their parents [60-63], and prospective studies have shown continued abdominal pain in FAP patients weeks, months, and even years following medical evaluation and treatment [6, 64-66]. Based on the current findings, it may be that RCTs that included relatively few patients with the High Pain Dysfunctional profile had little room for improvement in patient outcomes. Stronger treatment effects may be found for future RCT’s that match treatment to the needs of a particular FAP subgroup (e.g., tailored treatment designs or enriched enrollment designs) or that assess FAP subgroup as a potential moderator of treatment outcomes.
The FAP patient classification algorithm used in this study has several strengths. First, it is grounded in current conceptualizations of pain [67, 68] as including not only a sensory component (i.e, pain intensity and frequency) but also emotional and cognitive components (e.g., pain catastrophizing and pain beliefs). Inclusion of these components in the classification of patient subgroups may help to identify potential mechanisms of pain chronification and related treatment targets. Second, the classification algorithm was empirically derived from a large cohort of patients (n = 873) and predicted long-term (~9 years) pain-related outcomes in a prospective study of these patients [10]. Of note, the sample for the original cohort, like the present sample, was recruited from consecutive new patients and therefore not subject to selection bias associated with self- or physician referral. Third, in addition to individual characteristics, parent characteristics of the subtypes have been identified in the present study and enrich our understanding of the social context of pain in the subgroups.
More work is needed to translate our findings to clinical settings. Assessment tools used for classification of FAP patient subgroups must be shortened for application in the pediatric clinic. Practical clinical challenges, such as identifying appropriate referral resources for patients classified as high risk, must also be addressed [7]. Our results suggest that the High Pain Dysfunctional subgroup, representing 40% of adolescents with FAP in our study, may require the greatest resources for clinical intervention by mental health professionals to address patient and parent distress and family interaction patterns that contribute to pain persistence. Further research may show that the High Pain Adaptive and Low Pain Adaptive subgroups, which exhibited significantly lower distress and impairment than patients and parents in the High Pain Dysfunctional subgroup, may be effectively served within the pediatric gastroenterology clinic with brief interventions for pain education and management that do not require a referral for psychological treatment.
Limitations of the study highlight directions for future research. First, results were based on adolescent patients from a single pediatric subspecialty clinic in the Southeastern United States with minimal representation of minority groups and may not apply in other settings or across other age, ethnic, and racial groups. Second, data on parent characteristics were gathered primarily from mothers as they most often accompanied their children to the clinic; additional effort will be needed to recruit fathers as participants in future work. Third, the PROMIS sleep measure used in the study was validated in adults rather than adolescents; now that the pediatric item banks are available for sleep assessment they may be used in future research. Nonetheless, our results provide support for a reliable and distinct clinical FAP patient subgroup, labeled High Pain Dysfunctional, that is most likely to require multidisciplinary care to achieve optimal outcomes.
The ultimate goal of our investigations of FAP patient subgroups is to evaluate whether subgroup membership moderates responses to psychological treatment and to inform the development of tailored interventions for the subgroups. Although the current study focuses on pain-related psychosocial patterns that may meaningfully differentiate FAP patient subgroups, a similar approach could evaluate patterns of biomarkers that reflect underlying biological mechanisms of pain and related treatment targets. Because our classification system focuses on pain characteristics, it might be adapted and evaluated for application to pain conditions other than FAP. Ultimately, our hope is that matching pediatric pain patient subgroups to targeted interventions will improve patient outcomes and reduce health care costs.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (NIH) R01 HD76983 (PI: Walker), P30 HD15052 (Vanderbilt Kennedy Center), DK058404 (Vanderbilt Digestive Disease Research Center), UL 1 RR024975 (Vanderbilt CTSA), and T32 MH018921 (PI: Garber), and T32 GM 108554. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Institute of Medicine Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington D.C: Institute of Medicine of the National Academies, 2011. [PubMed] [Google Scholar]
- 2.Bäckryd E, et al. Chronic pain patients can be classified into four groups: Clustering-based discriminant analysis of psychometric data from 4665 patients referred to a multidisciplinary pain centre (a SQRP study). PLoS One 2018;13:e0192623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Ami N, et al. Outcomes in distressed patients with chronic low back pain: Subgroup analysis of a clinical trial. J Orthop Sports Phys Ther 2018:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl S, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain 2002;95:119–24. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RR, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157:1851–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulvaney S, et al. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 2006;45:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham NR, et al. Risk categorization predicts disability in pain-associated functional gastrointestinal disorders (FGIDs) after 6 months. J Pediatr Gastroenterol Nutr 2017;64:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Størdal K, et al. Recurrent abdominal pain: a five‐year follow‐up study. Acta Pædiatrica 2005;94:234–236. [DOI] [PubMed] [Google Scholar]
- 9.Horst S, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol 2014;12:2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker LS, et al. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012;153:1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird KT, et al. Validation of the abdominal pain index using a revised scoring method. J Pediatr Psychol 2015;40:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone AL, et al. Somatic symptoms in pediatric patients with chronic pain: Proposed clinical reference points for the Children’s Somatic Symptoms Inventory (formerly Children’s Somatization Inventory). The Journal of Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker LS, et al. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain 2008;137:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs M The Children’s Depression, Inventory (CDI). Psychopharmacol Bull 1985;21:995–8. [PubMed] [Google Scholar]
- 16.Walker LS and Greene JW. The Functional Disability Inventory: Measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16:39–58. [DOI] [PubMed] [Google Scholar]
- 17.Stone AL, et al. Pediatric Pain Beliefs Questionnaire: Psychometric properties of the short form. J Pain 2016;17:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin DE, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res 2010;19:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella D, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone AA, et al. PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. J Clin Epidemiol 2016;74:194–206. [DOI] [PubMed] [Google Scholar]
- 21.Crombez G, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003;104:639–46. [DOI] [PubMed] [Google Scholar]
- 22.Goubert L, et al. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): A preliminary validation. Pain 2006;123:254–263. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain 2010;11:1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian J, et al. Exploring the association between self-reported asthma impact and fitbit-derived sleep quality and physical activity measures in adolescents. JMIR mHealth and uHealth 2017;5:e105–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanish AE, et al. PROMIS Sleep Disturbance and Sleep-Related Impairment in adolescents: Examining psychometrics using self-report and actigraphy. Nurs Res 2017;66:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kooten JAMC, et al. Content validity of the Patient-Reported Outcomes Measurement Information System Sleep Disturbance and Sleep Related Impairment item banks in adolescents. Health Qual Life Outcomes 2016;14:92–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palermo TM, et al. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain 2004;109:461–470. [DOI] [PubMed] [Google Scholar]
- 28.Palermo TM, et al. Validation of a self-report questionnaire version of the Child Activity Limitations Interview (CALI): The CALI-21. Pain 2008;139:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palermo TM, et al. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial. Pain 2016;157:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone AL and Walker LS. Adolescents’ observations of parent pain behaviors: Preliminary measure validation and test of social learning theory in pediatric chronic pain. J Pediatr Psychol 2017;42:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Slyke DA and Walker LS. Mothers’ responses to children’s pain. Clin J Pain 2006;22:387–391. [DOI] [PubMed] [Google Scholar]
- 32.Noel M, et al. A developmental analysis of the factorial validity of the parent-report version of the Adult Responses to Children’s Symptoms in children versus adolescents with chronic pain or pain-related chronic illness. J Pain 2015;16:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 34.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health 2001;25:464–9. [PubMed] [Google Scholar]
- 35.Claar RL, et al. Pain coping profiles in adolescents with chronic pain. Pain 2008;140:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
- 37.Hilbe JM. Negative binomial regression. New York: Cambridge University Press, 2011. [Google Scholar]
- 38.Hilbe JM. Modeling count data. New York: Cambridge University Press, 2014. [Google Scholar]
- 39.Drossman DA and Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237–241. [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM IV-TR. Washington D.C: American Psychiatric Association, 2000. [Google Scholar]
- 41.Asmundson GJ, et al. Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res Manag 2012;17:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker LS The evolution of research on recurrent abdominal pain: History, assumptions, and a conceptual model In: McGrath P and Finley G eds. Chronic and recurrent pain in children and adolescents. Seattle: IASP, 1999. [Google Scholar]
- 43.Stone AL, et al. Social learning pathways in the relation between parental chronic pain and daily pain severity and functional impairment in adolescents with functional abdominal pain. Pain 2018;159:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy RL. Exploring the intergenerational transmission of illness behavior: from observations to experimental intervention. Ann Behav Med 2011;41:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law EF, et al. Longitudinal change in parent and child functioning after internet-delivered cognitive-behavioral therapy for chronic pain. Pain 2017;158:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy RL, et al. Brief telephone-delivered cognitive behavioral therapy targeted to parents of children with functional abdominal pain: a randomized controlled trial. Pain 2017;158:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poppert Cordts KM, et al. The (parental) whole is greater than the sum of its parts: A multifactorial model of parent factors in pediatric chronic pain. J Pain 2019;20:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palermo TM, et al. Problem solving skills training for parents of children with chronic pain: A pilot randomized controlled trial. Pain 2016;157:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hankin BL, et al. Corumination, interpersonal stress generation, and internalizing symptoms: accumulating effects and transactional influences in a multiwave study of adolescents. Dev Psychopathol 2010;22:217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spendelow JS, et al. The relationship between co-rumination and internalizing problems: A systematic review and meta-analysis. Clin Psychol Psychother 2017;24:512–527. [DOI] [PubMed] [Google Scholar]
- 51.Waller EM and Rose AJ. Adjustment trade-offs of co-rumination in mother-adolescent relationships. J Adolesc 2010;33:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barsky AJ and Borus JF. Somatization and medicalization in the era of managed care. JAMA 1995;274:1931–1934. [PubMed] [Google Scholar]
- 53.Levy RL, et al. Twelve-month follow-up of cognitive behavioral therapy for children with functional abdominal pain. JAMA Pediatr 2013;167:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy RL, et al. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol 2010;105:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Veek SM, et al. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics 2013:peds. 2013–0242. [DOI] [PubMed] [Google Scholar]
- 56.Korterink JJ, et al. Yoga therapy for abdominal pain-related functional gastrointestinal disorders in children: A randomized controlled trial. J Pediatr Gastroenterol Nutr 2016;63:481–487. [DOI] [PubMed] [Google Scholar]
- 57.Vlieger AM, et al. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology 2007;133:1430–1436. [DOI] [PubMed] [Google Scholar]
- 58.van Tilburg MA, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 2009;124:e890–7. [DOI] [PubMed] [Google Scholar]
- 59.Saps M, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137:1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campo JV, et al. Physical and emotional health of mothers of youth with functional abdominal pain. Arch Pediatr Adolesc Med 2007;161:131–7. [DOI] [PubMed] [Google Scholar]
- 61.Sherman AL, et al. Individual and additive effects of mothers’ and fathers’ chronic pain on health outcomes in young adults with a childhood history of functional abdominal pain. J Pediatr Psychol 2013;38:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schurman JV, et al. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings 2008;15:241–251. [DOI] [PubMed] [Google Scholar]
- 63.Shelby GD, et al. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics 2013;132:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker LS, et al. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain 2010;150:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotopf M, et al. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. BMJ 1998;316:1196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campo JV, et al. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics 2001;108:E1. [DOI] [PubMed] [Google Scholar]
- 67.International Association for the Study of Pain (IASP) Task Force on Taxonomy Classification of Chronic Pain, Second Edition IASP Press, Seattle, 1994. [Google Scholar]
- 68.Williams AC and Craig KD. Updating the definition of pain. Pain 2016;157:2420–2423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


