Abstract
Intensive blood pressure control decreases the rate of cardiovascular events by >25% compared to standard blood pressure control. We sought to determine if the decrease in cardiovascular events seen with intensive blood pressure control is associated with an increased rate of other causes of hospitalization. This is a post-hoc analysis of SPRINT (Systolic Blood Pressure Intervention Trial) in 9361 adult participants with hypertension and elevated cardiovascular risk. Participants were randomly assignment to an intensive or standard systolic blood pressure goal (<120mmHg or <140mmHg, respectively). The primary outcome was hospitalization rates per 100 person-years for hospitalizations not associated with SPRINT primary events. After excluding hospitalizations linked to SPRINT primary events, there were 4,678 participants with a rate of 19.70 hospitalizations per 100 person-years, compared to 4,683 participants with a rate of 19.65 (p=0.37). Equivalence testing shows that these hospitalization rates were statistically equivalent at the p=0.05 level. Of those with hospitalizations, more than one hospitalization was seen in 38.8% of intensive arm participants and 41.9% of standard arm participants (p=0.08). The mean cumulative count of non-primary event hospitalizations was comparable between the two arms. The most common causes of hospitalization were cardiovascular (23.6%) followed by injuries, including bone and joint therapeutic procedures (15.7%), infections (12.0%), and nervous systems disorders (10.7%). No categories of hospitalization were statistically more common in the intensive arm compared to the standard arm. Thus, the decrease in cardiovascular events seen with intensive blood pressure control is not associated with an increased rate of other causes of hospitalization.
Keywords: Hypertension, hospitalizations, cardiovascular disease, cost-effectiveness, clinical trials
Introduction
The National Institutes of Health sponsored SPRINT was a randomized multicenter trial of blood pressure control conducted in patients at increased risk of cardiovascular events (1). The primary outcome in SPRINT was a composite outcome of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes.
Participants who were randomized to a systolic blood pressure goal of ≤120 mm Hg (intensive arm), compared to participants randomized to a systolic blood pressure goal of ≤140 mm Hg (standard arm), had a significant decrease in the primary composite outcome (1.65% per year vs. 2.19% per year; hazard ratio with intensive treatment, 0.75; 95% confidence interval [CI], 0.64 to 0.89; P<0.001) and in all-cause mortality (hazard ratio, 0.73; 95% CI, 0.60 to 0.90; P = 0.003). Although the numbers of serious adverse events were similar in the two randomized arms, some adverse events of interest occurred at a higher rate in intensive arm participants compared to standard arm participants, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury (2–4).
We hypothesized that, after excluding those hospitalizations linked to the primary outcomes, that there would be no difference in the hospitalization rates between the intensive and standard arms of the trial; that is, that there was no evidence of significant harms from the intensive blood pressure intervention as ascertained by examining hospitalization rates.
Methods
Anonymized data and materials have been made publicly available at the National Heart, Lung and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and can be accessed at https://biolincc.nhlbi.nih.gov/studies/sprint/. SPRINT was designed to test the hypothesis that aggressive control of systolic blood pressure in hypertensive participants with an increased risk of cardiovascular events would result in a lower rate of cardiovascular morbidity and mortality. Participants eligible for enrollment into SPRINT were assessed in 102 clinical sites in both the mainland United States and Puerto Rico between November 2010 and March 2013. The study was approved by the local IRB at each clinical site and informed consent was obtained from all participants. In this randomized multi-center unblinded clinical trial, participants were randomly assigned to a systolic blood pressure goal of either ≤ 140 mm Hg or ≤ 120 mm Hg. Randomization was stratified by clinical site and utilized an internet-based web browser randomization procedure. Individuals with diabetes mellitus, prior history of stroke, advanced chronic kidney disease (eGFR <25 ml/min/1.73 m2), significant proteinuria (urine albumin excretion ≥ 600 mg/day or urine protein excretion ≥1000 mg/day), or polycystic kidney disease were excluded from enrollment. Details regarding the study design and primary results of SPRINT have been previously published (1, 5). Dose adjustment was based on a mean of three blood-pressure measurements at an office visit while the patient was seated and after 5 minutes of quiet rest as per American Heart Association guidelines; the measurements were made with the use of an automated measurement system (Model 907, Omron Healthcare). The primary outcome specified in SPRINT was a composite of cardiovascular death or first occurrence of myocardial infarction, stroke, heart failure, or non-MI acute coronary syndrome. There were several cardiovascular and renal secondary outcomes, with the latter dependent on the presence or absence of chronic kidney disease at baseline. A structured interview was used in both groups every three months to obtain self-reported clinical cardiovascular and end-stage kidney disease outcomes. On August 20, 2015, the intervention was stopped early on advice from the Data and Safety Monitoring Board due to benefit in the intensive arm on the primary outcome as determined by the use of the Lan-DeMets method with an O’Brien-Fleming type spending function.
Baseline participant characteristics were obtained from self-report, laboratory data, and available medical records. Baseline blood and urine samples were analyzed at a central laboratory and were collected as fasting specimens. Baseline eGFR in ml/min/1.73 m2 was calculated using the serum creatinine concentration obtained at the baseline visit and the four-variable Modification of Diet in Renal Disease (MDRD) equation. Chronic kidney disease was defined as an eGFR less than 60 ml/min/1.73 m2.
Although cardiovascular and renal outcomes were only assessed at scheduled quarterly study visits, ascertainment of hospitalizations could be reported to study staff spontaneously by participants through telephone calls or emails between study visits or during either drug titration visits or prn visits. Information on hospitalizations was obtained by participant self-report as well as from available medical records. The cause of hospitalization was classified using the MedDRA system. Up to three MedDRA codes were used to classify each hospitalization. The cause of each hospitalization was converted from the MedDRA codes to ICD-10 codes by one individual (MVR). Those hospitalizations that were linked to a primary SPRINT outcome were subsequently excluded from this analysis. Thus, the primary outcome of this study reflects the hospitalization rate after accounting for the beneficial effects of the SPRINT intervention and can be used to determine if the intervention resulted in an increased rate of other causes of hospitalization.
Statistical analysis
For the main SPRINT trial, an enrollment target of 9250 participants would provide an estimated 88.7% power to detect a 20% effect on the primary outcome. For this post-hoc analysis, secondary event hospitalization rates for each participant were expressed as hospitalizations per 100 patient years. Patient years were determined from the date of randomization to the date of the first event of participant death, withdrawal from the study, loss to follow-up or August 20, 2015.
To compare differences in demographics and clinical characteristics, Wilcoxon rank sum tests and chi square tests of independence for contingency tables were computed. To test for differences in secondary event hospitalization rates between the standard and intensive arms of the SPRINT trial, a generalize linear model was computed using the Tweedie distribution for the natural logarithm (plus 1) of the hospitalization rate. The Tweedie distribution is applied to data with zero inflation (i.e., large number of observations at zero). A formal test of treatment arm and patient characteristics were computed. A sensitivity analysis applying the gamma distribution were also computed resulting in comparable inference. Secondary event hospitalization rates of the two arms were tested for equivalence using Wilcoxon rank sum test and the two one-sided test procedure of equivalence testing (6). To estimate the burden of recurrent events in the presence of competing risks (i.e., death, primary events), the mean cumulative count (MCC) for secondary event hospitalizations was estimated (and confidence intervals) for the standard and intervention arms (7). This approach estimates the average number of secondary event hospitalizations per participant over time per arm.
Role of the funding source
The steering committee designed SPRINT, gathered the data (in collaboration with investigators at the clinics and other study units), and approved the decision to submit the manuscript for publication. The writing committee wrote the manuscript and vouches for the completeness and accuracy of the data and analysis. The Southeast Clinic Coordinating Center statisticians were responsible for analyzing these data. Scientists at the National Institutes of Health participated in the design of the study and, as a group, had one vote on the steering committee.
Results
Hospitalization rate comparable between intensive and standard arms of SPRINT
The CONSORT diagram is shown in Figure 1. Hospitalization status was ascertained in 4678 individuals in the intensive arm and 4683 individuals in the standard arm of the trial (Table 1). Those hospitalizations that were linked to a primary SPRINT outcome were excluded from this analysis, in order to determine the effect of the SPRINT intervention on other hospitalizations. These “non-SPRINT primary outcome hospitalizations” provide insight into other potential non-cardiovascular risks and/or benefits of the intensive blood pressure intervention.
Figure 1 -.
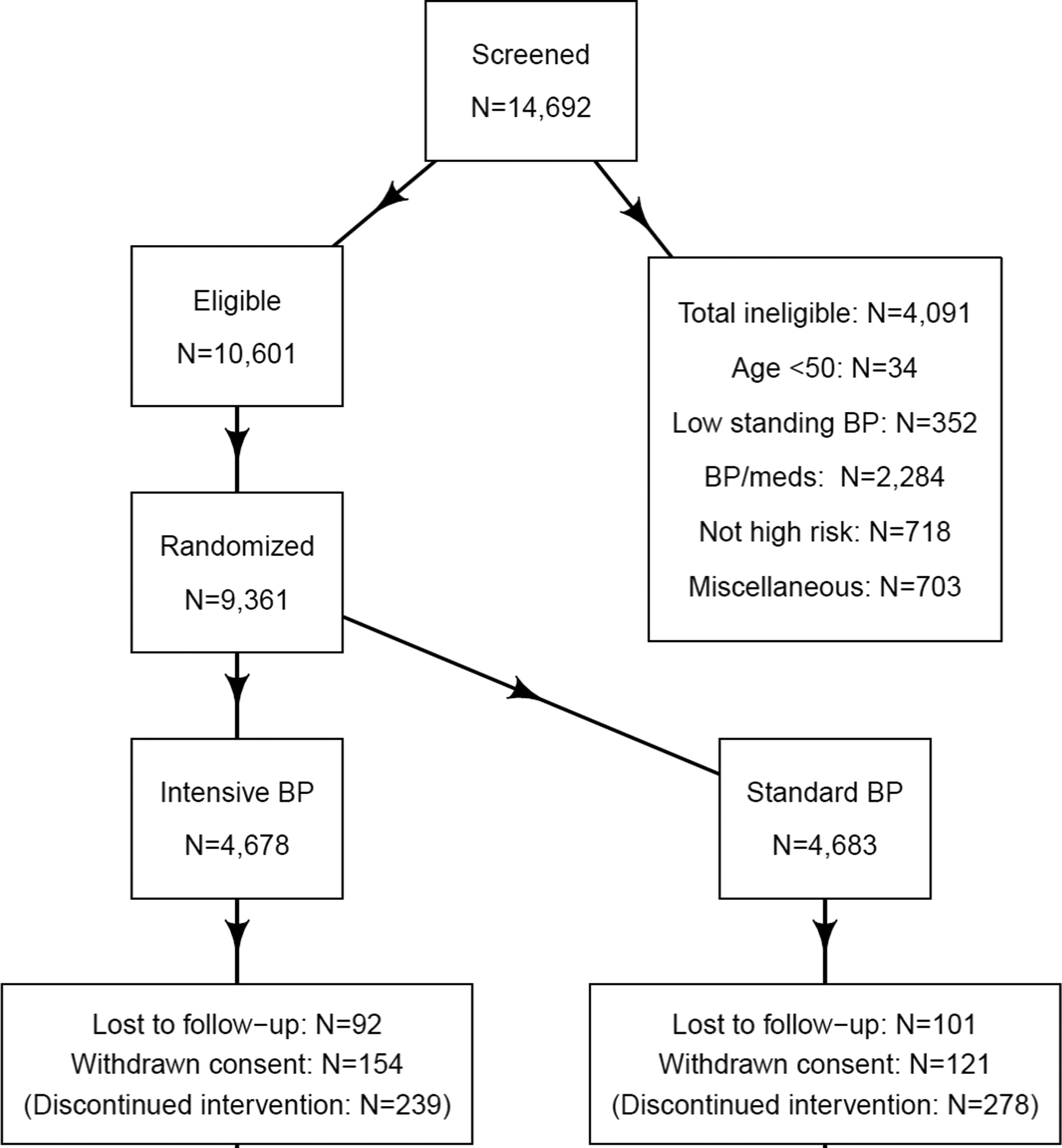
CONSORT diagram. Abbreviation: BP = blood pressure
Table 1 -.
Baseline characteristics of SPRINT participants by randomization arm
| Characteristic | Intensive (N=4678) | Standard (N=4683) | P-value† |
|---|---|---|---|
| Inclusion criteria – no. (%) | |||
| Age ≥75 years | 1317 (28.2) | 1319 (28.2) | 0.99 |
| Chronic kidney disease† | 1329 (28.4) | 1316 (28.1) | 0.74 |
| Cardiovascular disease | 940 (20.1) | 937 (20.0) | 0.92 |
| Clinical | 779 (16.7) | 783 (16.7) | 0.93 |
| Subclinical | 247 (5.3) | 246 (5.3) | 0.95 |
| Framingham 10-year cardiovascular disease risk score ≥15% | 3556 (76.0) | 3547 (75.7) | 0.76 |
| Female – no. (%) | 1684 (36.0) | 1648 (35.2) | 0.41 |
| Age – years | 67.9±9.4 | 67.9±9.5 | 0.82 |
| Age among those ≥75 years – years | 79.8±3.9 | 79.9±4.1 | 0.72 |
| Race/ethnicity‡ – no. (%) | |||
| African American | 1379 (29.5) | 1423 (30.4) | 0.33 |
| Hispanic | 503 (10.8) | 481 (10.3) | |
| European American | 2698 (57.7) | 2701 (57.7) | |
| Other | 98 (2.1) | 78 (1.7) | |
| African American‡ – no. (%) | 1454 (31.1) | 1493 (31.9) | 0.40 |
| Baseline blood pressure – mm Hg | |||
| Systolic | 139.7±15.8 | 139.7±15.4 | 0.96 |
| Diastolic | 78.2±11.9 | 78.0±12.0 | 0.45 |
| Systolic blood pressure tertiles – no. (%) | |||
| Systolic blood pressure ≤ 132 mm Hg | 1583 (33.8) | 1553 (33.2) | 0.95 |
| 132 mm Hg < Systolic blood pressure < 145 mm Hg | 1489 (31.8) | 1549 (33.1) | |
| Systolic blood pressure ≥ 145 mm Hg | 1606 (34.3) | 1581 (33.8) | |
| Serum creatinine – mg/dL | 1.07±0.34 | 1.08±0.34 | 0.22 |
| Estimated glomerular filtration rate – ml/min/1.73m2 | |||
| Overall | 71.8±20.7 | 71.7±20.5 | 0.88 |
| Estimated glomerular filtration rate ≥ 60 ml/min/1.73m2 | 81.3±15.5 | 81.1±15.5 | 0.50 |
| Estimated glomerular filtration rate < 60 ml/min/1.73m2 | 47.8±9.5 | 47.9±9.5 | 0.88 |
| Urine albumin/creatinine – mg/g | 44.1±178.7 | 41.1±152.9 | 0.43 |
| Total cholesterol – mg/dL | 190.2±41.4 | 190.0±40.9 | 0.98 |
| Fasting high density lipoprotein cholesterol – mg/dL | 52.9±14.3 | 52.8±14.6 | 0.44 |
| Fasting total triglycerides – mg/dL | 124.8±85.8 | 127.1±95.0 | 0.52 |
| Fasting plasma glucose – mg/dL | 98.8±13.7 | 98.8±13.4 | 0.74 |
| Statin use – no. (%) | 1978/4646 (42.6) | 2076/4640 (44.7) | 0.04 |
| Aspirin use – no. (%) | 2406/4662 (51.6) | 2350/4666 (50.4) | 0.23 |
| Smoking – no. (%) | |||
| Never smoker | 2051 (43.8) | 2072 (44.2) | 0.51 |
| Former smoker | 1977 (42.3) | 1996 (42.6) | |
| Current smoker | 639 (13.7) | 601 (12.8) | |
| Missing | 11 (0.2) | 14 (0.3) | |
| Framingham 10-year cardiovascular disease risk score – % | 24.8±12.6 | 24.8±12.5 | 0.82 |
| Body mass index – kg/m2 | 29.9±5.8 | 29.8±5.7 | 0.61 |
| Antihypertension agents – count | 1.8±1.0 | 1.8±1.0 | 0.54 |
| Not using antihypertension agents – n (%) | 432 (9.2) | 450 (9.6) | 0.54 |
P-values were computed using Wilcoxon rank sum test and chi square tests for contingency tables. The Mantel-Haenszel chi square test for ordered alternatives was used for systolic blood pressure tertiles.
Race/ethnic was self-reported. African American race includes Hispanic ethnicity reporting African ancestry, African American, and African American as part of a multiracial identification.
After excluding SPRINT primary outcome hospitalizations, there was no difference in the hospitalization rates between the two treatment arms (Table 2). Specifically, there were 2907 hospitalizations in the intensive arm and 2887 in the standard arm, for a hospitalization rate of 19.70 versus 19.65 hospitalizations per 100 patient years, respectively (p=0.37). A multivariable model that adjusted for age, gender, ethnicity, previous CKD, previous CVD, and systolic blood pressure also showed no evidence of a difference in secondary event hospitalization rates (p=0.27). In addition, there was no evidence of a statistical interaction between arm of the SPRINT trial and these covariates (Table 2; p>0.05). Finally, of those with hospitalizations, more than one hospitalization was seen in 38.8% of intensive arm participants and 41.9% of standard arm participants (p=0.08).
Table 2 –
Number of non-primary SPRINT outcome hospitalizations by randomized arm and pre-specified SPRINT subgroups*
| SPRINT group | Intensive arm | Standard arm | p-value† | ||||
|---|---|---|---|---|---|---|---|
| Number of hospitalizations | Number of person years | Hospitalizations per 100 person years | Number of hospitalizetions | Number of person years | Hospitalizations per 100 person years | ||
| Overall | 2907 | 14756.2 | 19.70 | 2887 | 14688.8 | 19.65 | 0.37 |
| Previous CKD | |||||||
| Yes | 1133 | 4229.2 | 26.79 | 1197 | 4161.0 | 28.77 | 0.34 |
| No | 1774 | 10526.9 | 16.85 | 1690 | 10527.9 | 16.05 | 0.06 |
| Age | |||||||
| < 75 years | 1861 | 10774.2 | 17.27 | 1721 | 10749.0 | 16.01 | 0.11 |
| >= 75 years | 1046 | 3981.9 | 26.27 | 1166 | 3939.8 | 29.60 | 0.59 |
| Sex | |||||||
| Male | 1878 | 9393.5 | 19.99 | 1929 | 9463.1 | 20.38 | 0.90 |
| Female | 1029 | 5362.6 | 19.19 | 958 | 5225.7 | 18.33 | 0.16 |
| Race | |||||||
| African American | 840 | 4524.4 | 18.57 | 956 | 4646.7 | 20.57 | 0.31 |
| Non-African American | 2067 | 10231.7 | 20.20 | 1931 | 10042.1 | 19.23 | 0.08 |
| Hispanic | 168 | 1380.6 | 12.17 | 120 | 1344.2 | 8.93 | 0.01 |
| European American | 1864 | 8565.4 | 21.76 | 1787 | 8511.9 | 20.99 | 0.25 |
| Other | 35 | 285.6 | 12.25 | 24 | 186.0 | 12.90 | 0.38 |
| Previous CV disease | |||||||
| Yes | 879 | 2931.0 | 29.99 | 892 | 2871.3 | 31.07 | 0.89 |
| No | 2028 | 11825.1 | 17.15 | 1995 | 11817.5 | 16.88 | 0.25 |
| Systolic BP | |||||||
| <=132 mm Hg | 972 | 5038.0 | 19.29 | 949 | 4841.3 | 19.60 | 0.89 |
| > 132 to < 145 mmHg | 966 | 4714.9 | 20.49 | 874 | 4892.7 | 17.86 | 0.03 |
| >= 145 mm Hg | 969 | 5003.2 | 19.37 | 1064 | 4954.8 | 21.47 | 0.52 |
Excludes hospitalizations linked to a primary SPRINT outcome. These primary SPRINT outcome events include the first occurrence of myocardial infarction, stroke, heart failure, or non-MI acute coronary syndrome.
P-values computed using the Tweedie distribution; analyses were repeated with the gamma distribution which yielded similar results.
There was no evidence of a difference in secondary event hospitalization rates after adjusting for age, gender, ethnicity, previous CKD, previous CVD, and systolic blood pressure (p=0.27).
Formal test of interaction with the treatment arm p-value: Previous CKD p=0.058; Age p=0.157; Sex p=0.294; Race p=0.219; Previous CV disease p=0.469; Systolic BP p=0.564.
A secondary analysis was undertaken to determine within what bounds the non-SPRINT primary event hospitalization rates for the intensive and standard arms were equivalent, excluding those linked to primary cardiovascular outcomes. Using the two one-sided hypothesis approach and the Wilcoxon rank sum test (P<0.05 criteria), the overall hospitalization rates of the two arms are considered to be statistically equivalent within 3.64 hospitalizations per 100 person years (1.74% of the range defined by the 1st percentile and 99th percentile). The actual difference in hospitalization rates of 0.05 hospitalizations per 100 patient years between the two arms of the trial was well within these bounds.
The secondary event hospitalization mean cumulative counts, a method designed for recurrent events while accounting for competing risks (i.e., death and SPRINT primary events), increased linearly over the first three years. Over the entire range of the number of days in the study, the intensive and standard exhibited a comparable rate of increase (Figure 2).
Figure 2 -.
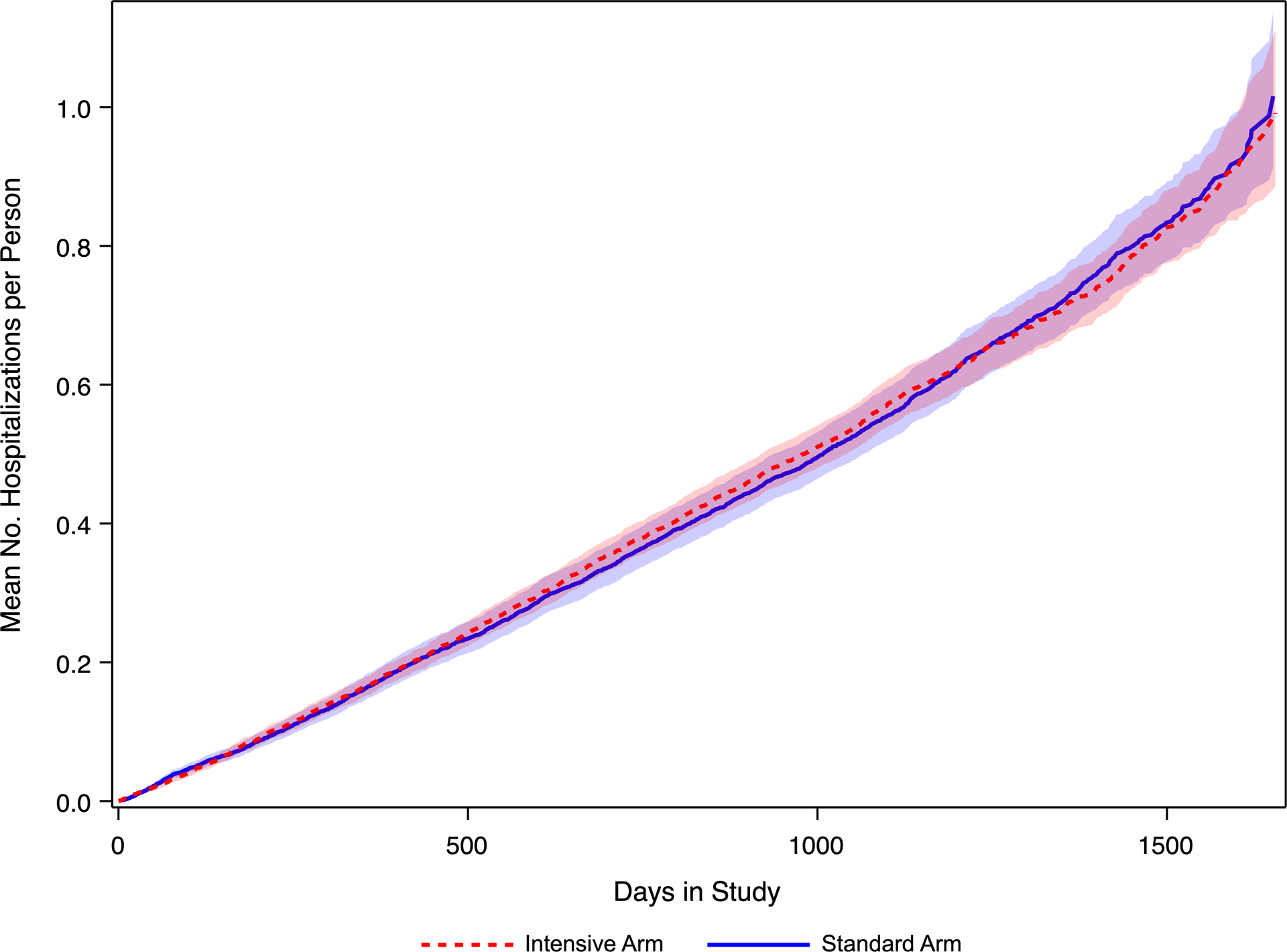
Estimated mean count of hospitalizations using the mean cumulative count method. Using the method of mean cumulative count (ref), the estimated mean number of hospitalizations on the y-axis, with days in study on the x-axis is displayed. The mean count and 95% confidence interval appears in red for the intensive arm, and in blue for the standard arm. This method takes into account the competing risks of death, primary events, and prior hospitalizations.
Excluding those hospitalizations linked to the SPRINT primary cardiovascular events, the most common cause of hospitalizations was cardiovascular (ICD-10 codes I00 - I99; 23.6%), followed by injuries (S00 – T88; 15.7%), infectious diseases (A00 – B99; 12.0%), diseases of the nervous system (G00 – G99; 10.7%), and diseases of the digestive system (K00 – K95; 10.6%) (Table 3). After adjustment for multiple comparisons by the Bonferroni correction (p=0.0028), none of the ICD-10 categories were more common in either the intensive or randomized arms of the trial. It should be noted that many of the cardiovascular causes of hospitalization captured in this analysis that were not adjudicated were secondary to chest pain that did not result in either a cardiovascular procedure or development of a myocardial infarction (data not shown). In addition, most of the injuries were bone and joint therapeutic procedures (data not shown).
Table 3 –
Primary Events and non-SPRINT primary outcome hospitalizations by ICD-10 categories
| SPRINT group | Intensive arm | Standard arm | |||
|---|---|---|---|---|---|
| Number of Events | Events per 100 person years† | Number of Events | Events per 100 person years† | P-value** | |
| All primary events* | 369 | 2.50 | 498 | 3.39 | 0.0006 |
| G00-G99 Diseases of the nervous system - adjudicated | 73 | 0.49 | 83 | 0.57 | 0.72 |
| I00-I99 Diseases of the circulatory system - adjudicated | 296 | 2.01 | 415 | 2.83 | 0.0002 |
| Number of Hospitalizations | Hospitalizations per 100 person years† | Number of Hospitalizations | Hospitalizations per 100 person years† | p-value | |
| All hospitalizations not tied to primary events | 2907 | 19.70 | 2887 | 19.65 | 0.37 |
| A00-B99 Certain infectious and parasitic diseases | 370 | 2.51 | 327 | 2.23 | 0.19 |
| C00-D49 Neoplasms | 94 | 0.64 | 150 | 1.02 | 0.41 |
| D50-D89 Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 34 | 0.23 | 43 | 0.29 | 0.99 |
| E00-E89 Endocrine, nutritional and metabolic diseases | 133 | 0.90 | 107 | 0.73 | 0.11 |
| F01-F99 Mental, Behavioral and Neurodevelopmental disorders | 87 | 0.59 | 61 | 0.42 | 0.02 |
| G00-G99 Diseases of the nervous system - not adjudicated | 338 | 2.29 | 283 | 1.93 | 0.01 |
| H00-H59 Diseases of the eye and adnexa | 6 | 0.04 | 9 | 0.06 | ‡ |
| H60-H95 Diseases of the ear and mastoid process | 22 | 0.15 | 19 | 0.13 | 0.57 |
| I00-I99 Diseases of the circulatory system - not adjudicated | 640 | 4.34 | 730 | 4.97 | 0.10 |
| J00-J99 Diseases of the respiratory system | 157 | 1.06 | 188 | 1.28 | 0.39 |
| K00-K95 Diseases of the digestive system | 303 | 2.05 | 309 | 2.10 | 0.77 |
| L00-L99 Diseases of the skin and subcutaneous tissue | 10 | 0.07 | 2 | 0.01 | ‡ |
| M00-M99 Diseases of the musculoskeletal system and connective tissue | 57 | 0.39 | 63 | 0.43 | 0.74 |
| N00-N99 Diseases of the genitourinary system | 169 | 1.15 | 163 | 1.11 | 0.78 |
| R00-R99 Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 7 | 0.05 | 5 | 0.03 | 0.62 |
| S00-T88 Injury, poisoning and certain other consequences of external causes | 480 | 3.25 | 428 | 2.91 | 0.27 |
| V00-Y99 External causes of morbidity such as accidents and falls | 0 | - | 0 | - | - |
Primary events, as specified in SPRINT, included a composite of cardiovascular death or first occurrence of myocardial infarction, stroke, heart failure, or non-MI acute coronary syndrome
Number of person years was 14,756.2 for the intensive arm, and 14,688.8 for the standard arm.
Model failed to converge due to low numbers.
Note: Columns will not sum to total hospitalizations because all primary events are counted and there were instances with >1 primary event per day; in addition, some primary events did not map to a hospitalization.
After adjustment for multiple comparisons by the Bonferroni correction (p=0.0028), none of the ICD-10 categories were more common in either the intensive or randomized arms of the trial.
CKD – chronic kidney disease; CV – cardiovascular; BP blood pressure
Discussion
The results from SPRINT demonstrated a significant decrease in cardiovascular events and all-cause mortality with intensive blood pressure control in non-diabetic hypertensive individuals at increased risk of cardiovascular disease (1, 5). These benefits are counterbalanced by an increased risk of certain pre-specified adverse events, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury (2, 4).
This analysis was designed to test for any additional benefits or risks from the intensive blood pressure intervention after accounting for the cardiovascular benefits of intensive blood pressure control that were identified in SPRINT. The results of these analyses are reassuring, as after accounting for the benefits seen for the primary cardiovascular outcome, there was no significant difference in the overall rate of hospitalizations between the two intervention groups. In fact, the two arms show equivalence in SPRINT secondary-event hospitalization rates. In addition, there was no significant difference in the pre-specified categories of hospitalizations, as defined by ICD-10 codes. Thus, the decrease in cardiovascular events seen with intensive blood pressure control is not associated with an increased rate of other causes of hospitalization. We believe these findings are, in part, related to the careful measurement of blood pressure in the SPRINT trial, using American Heart Association guidelines. (8–9) This method of blood pressure measurement was implemented in order to ensure that the blood pressure measure obtained was not falsely elevated, leading to the increased risk of overtreatment of hypertension. (10) This method of careful blood pressure measurement is reflected in the observation that the incidence of orthostatic hypotension was similar in both arms of the trial (about 5 – 6 %) and the lack of association of orthostatic hypotension with a number of adverse events, including syncope, electrolyte abnormalities, injurious falls, and acute renal failure. (11) These results further supports the paradigm that intensive blood pressure intervention is clinically important and cost effective (8) (9) (10, 11). Our findings thus add to the growing evidence from other trials and meta-analyses that support the rationale for the intensive control of blood pressure in patients at high risk for cardiovascular disease. (12–14)
Strengths of the present study include the design where hospitalizations were ascertained in a standard format at each routine 3-month visit. In addition, the cause of hospitalization was captured in a standard format using MedDRA coding and the analyses explicitly accounted for recurrent events and competing risks. Study limitations include the lack of data on length of stay and costs of hospitalizations.
Supplementary Material
Perspectives.
After accounting for the cardiovascular benefits of the SPRINT intensive blood pressure arm, there was no difference in hospitalization rates and specific causes of hospitalizations. Thus, hospitalization rates were not adversely impacted by intensive control of blood pressure.
Novelty and Significance.
What is New?
Intensive control of hypertension does not result in an increased risk of non-cardiovascular hospitalizations.
What is Relevant?
Hypertension is a major contributor for increased risk of cardiovascular morbidity and mortality
SPRINT was one of the largest studies of intensive blood pressure control (systolic blood pressure of <120 mm Hg compared to systolic blood pressure of <140 mm Hg)
All-cause hospitalizations were obtained in all participants in this study
Summary
Intensive blood pressure control does not lead to adverse events that result in an increased rate of non-cardiovascular hospitalizations.
No categories of hospitalization were statistically more common in the intensive arm compared to the standard arm.
Acknowledgments
The authors thank the participants in the SPRINT trial as well as the study staff in the 102 clinics in the SPRINT trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of members of the SPRINT Research Group, please see the supplementary acknowledgement list at: https://www.sprinttrial.org/public/dspScience.cfm
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the U.S. government.
The full protocol can be assessed at: https://biolincc.nhlbi.nih.gov/studies/sprint/
Primary funding source: National Institutes of Health
Sources of Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.a
Footnotes
Footnote: Disclosures
Michael V Rocco, MD, MSCE - none
Mary E. Comeau, MA - none
Miranda C. Marion, MA - none
Barry I. Freedman, MD - none
Amret T. Hawfield, MD - none
Carl D. Langefeld, PhD – none
Registration: ClinicalTrials.gov number, NCT01206062
References
- 1.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, et al. Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey). Circulation. 2017;135(17):1617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. Jama. 2016;315(24):2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2018;71(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin. Trials 2014;11(5):532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15(6):657–80. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SPRINT Protocol. https://www.sprinttrial.org/public/dspScience.cfm. Accessed August 15, 2020. .
- 9.SPRINT Manual of Operations - Blood Pressure Measurement. https://www.sprinttrial.org/public/dspHome.cfm. Accessed August 15, 2020
- 10.Myers MG. A Short History of Automated Office Blood Pressure – 15 Years to SPRINT. J Clin Hypertension. 2016;18(8), 721–724. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juraschek SP, Taylor AA, Wright JT et al. Orthostatic Hypotension, Cardiovascular Outcomes, and Adverse Events: Results From SPRINT. Hypertension. 2020;75(3):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–43. [DOI] [PubMed] [Google Scholar]
- 13.Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017;2(7):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


