Abstract
Isoprenoids are a large class of natural products with myriad applications as bioactive and commercial compounds. Their diverse structures are derived from the biosynthetic assembly and tailoring of their scaffolds, ultimately constructed from two C5 hemiterpene building blocks. The modular logic of these platforms can be harnessed to improve titers of valuable isoprenoids in diverse hosts and to produce new-to-nature compounds. Often, this process is facilitated by the substrate or product promiscuity of the component enzymes, which can be leveraged to produce novel isoprenoids. To complement rational enhancements and even re-programming of isoprenoid biosynthesis, high-throughput approaches that rely on searching through large enzymatic libraries are being developed. This review summarizes recent advances and strategies related to isoprenoid synthetic biology, combinatorial biosynthesis, and chemo-enzymatic synthesis, focusing on the last five years. Emerging applications of cell-free biosynthesis and high-throughput tools are included that culminate in a discussion of the future outlook and perspective of isoprenoid biosynthetic engineering.
Keywords: Isoprenoids, terpenes, synthetic biology, combinatorial biosynthesis, hemiterpenes
Introduction
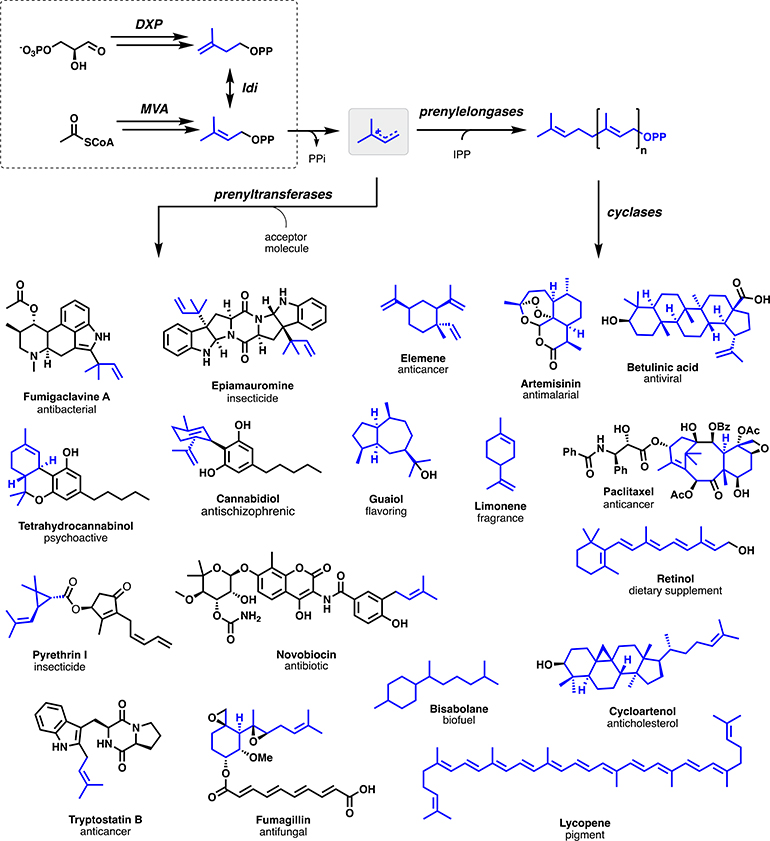
Isoprenoids (also known as terpenoids) are widely distributed in Nature, representing approximately one-third of characterized natural products and comprising >80,000 unique chemical structures [1]. These diverse molecular scaffolds provide a versatile source of potent pharmaceuticals with activities including antibiotic (fumigaclavine A), anticancer (paclitaxel), antiviral (betulinic acid), and antiparasitic (artemisinin) (Fig. 1) [2]. In addition to their pharmaceutical relevance, isoprenoids have utility as commodity and industrial chemicals including fragrances (limonene), flavorings (guaiol), pigments (carotenoids), biofuels (bisabolane), biopolymers (rubber), and pesticides (pyrethrin I) [3].
Fig. 1.
Biosynthesis of diverse isoprenoid natural products.
Notably, the broad members of this class are universally derived from two isomeric five-carbon (C5) hemiterpene diphosphates, dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP), which are natively biosynthesized via the mevalonate (MVA) or 1-deoxy-D-xylulose-5-phosphate (DXP) pathways. These ubiquitous monomeric precursors are subsequently leveraged as small molecule building blocks for intermolecular prenylation by prenyltransferases (PTases). The broad functionality of PTases enables the regioselective alkylation of diverse nucleophilic acceptor molecules including polyketides, non-ribosomal peptides, and amino acids, as well as the polymerization of hemiterpenes to form a variety of elongated linear diphosphates via a subset of PTases referred to as prenylelongases. Elongated prenyl diphosphate moieties can subsequently undergo various intermolecular transfers or can be leveraged as substrates for intramolecular cyclization by terpene cyclases (TCs) wherein the appropriate linear diphosphate chain is leveraged to produce diverse monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20) etc., via the generation and propagation of highly reactive carbocation intermediates. These products are often subsequently tailored and diversified via the action of P450 hydroxylases (CYPs), glycosyltransferases (GTs), acyltransferases, and methyltransferases (MTs).
In order to gain access to bioactive isoprenoid natural products as well as their analogues, synthetic organic chemistry [4, 5] has used starting materials with the chirality and precursor-scaffold built-in. These synthetic scaffolds have leveraged the integrated chirality of the starting material to facilitate chemical diversification yet are often limited in availability for large scale multistep synthesis. Conversely, Nature has evolved a multitude of enzymatic machinery for cascade reactions, but has limited access to chemically diverse starting materials [6]. Interestingly, small modifications and diversification of isoprenoid scaffolds can lead to significant differences in biological activity. For example, while meroterpenoid cannabidiol (CBD) shows prominent activity as an antischizophrenic [7], anti-inflammatory [8], and antiepileptic agent [9], its analogue (−)-trans-Δ9-tetrahydrocannabinol (THC) is a psychoactive drug [10, 11]. Hence, the huge advancement in biomimetic terpenoid synthesis notwithstanding [4], accessing a broad range of non-natural isoprenoid analogues in a facile, scalable, cost effective, and sustainable fashion remains a critical challenge [11].
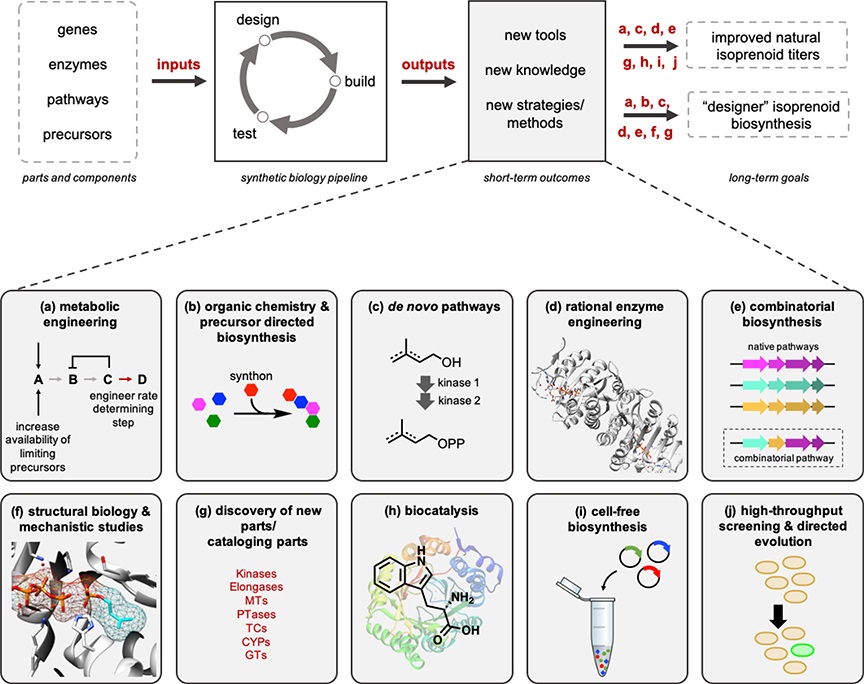
As the demand for industrially relevant isoprenoids [12] and high-value therapeutics continues to increase, improving accessibility of terpenoids and their analogues to access novel drug targets, has become critical [13–15]. Over the past decade, emerging synthetic biology technologies have demonstrated great promise for both enhancing isoprenoid titers and diversifying their structures. Metabolic engineering and novel high-throughput screening technologies have been leveraged to improve natural product titers. Moreover, engineered de novo biosynthetic pathways have been developed to simultaneously improve access to natural building blocks as well as their non-natural analogues. While engineering the native promiscuity of these pathways ushered towards novel biosynthetic avenues of isoprenoid production, promiscuity with non-native substrates has revealed significant potential for regio- and chemoselective diversification, spurring advancements towards novel drug development. This review summarizes the current state-of-the-art in isoprenoid synthetic biology, combinatorial biosynthesis, and chemo-enzymatic synthesis, with special emphasis on key progress over the last five years (Fig. 2).
Fig. 2.
Combinatorial synthetic biology and chemo-enzymatic approaches to isoprenoids and their non-natural designer analogues.
Engineering Isoprenoid Precursor Production
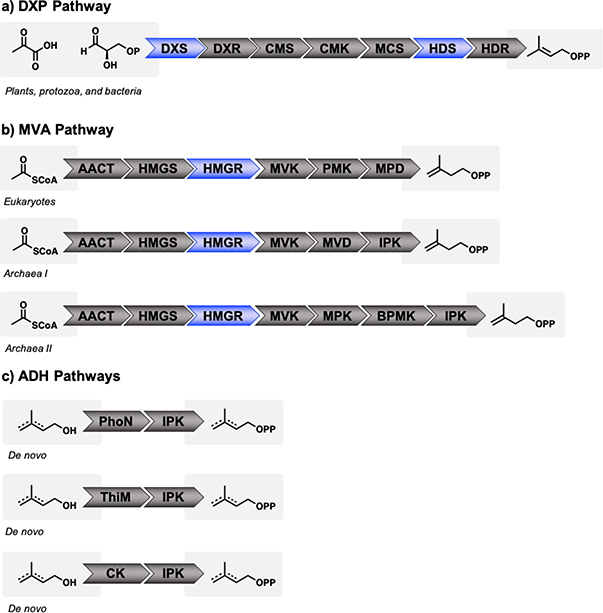
Enhancing isoprenoid titers has often relied on precursor pathway engineering [11, 16], making both the DXP and MVA pathways critical engineering targets. Within plants, protozoa, and most bacteria, the DXP pathway is initiated by the decarboxylative condensation of acetyl-CoA and glyceraldehyde-3-phosphate (G3P) to yield DXP, which is then converted to DMAPP or IPP via an additional six enzymatic steps (Fig. 3a). Engineering efforts for this pathway have been historically met with minimal success as a result of poorly understood regulatory mechanisms [17]. However, recent metabolic control analyses have identified multiple potential enzymatic bottlenecks across the DXP pathway, including DXS and HDS, further complicating engineering efforts [18].
Fig. 3.
Enzymatic biosynthesis pathways to isoprenoid precursors, DMAPP and IPP. a Native DXP pathway. b Native MVA pathway. c de novo pathways. Enzymatic steps highlighted in blue have been identified for critical metabolic engineering described in the text. DXS, DXP synthase; DXR, DXP reductoisomerase; CMS, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; CMK, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; MCS, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase; HDS, HMB pyrophosphate synthase; HDR, HMB pyrophosphate reductase. AACT – acetoacetyl-CoA thiolase; HMGS, HMG-CoA synthase; HMGR, HMG-CoA reductase; MVK, mevalonate kinase; PMK, phosphomevalonate kinase; MVD, mevalonate-5-phosphate decarboxylase; MPK, mevalonate-3-phosphate-5-kinase; MPD, mevalonate phosphate decarboxylase; IPK, isopentenyl phosphate kinase; BPMK, bisphosphate mevalonate; CK, choline kinase.
The MVA pathway has enjoyed substantial success for the overproduction of DMAPP and IPP despite its lower theoretical hemiterpene yield compared to the DXP pathway. Utilized in archaea and higher eukaryotes, the MVA pathway is initiated by the sequential additions of acetyl-CoA to yield 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA). HMG-CoA is subsequently reduced to MVA, which is then funneled to the lower MVA pathway, which has been convergently evolved among archaea and eukaryotes to yield IPP through a series of at least six enzymatic steps (Fig. 3b). IPP is then interconverted to DMAPP via isopentenyl diphosphate isomerase (Idi). Chassis organisms with an endogenous MVA pathway, such as Saccharomyces cerevisiae, have been successfully engineered through the identification and removal of negative regulatory elements like the pyruvate dehydrogenase bypass [19], and the overexpression of enzymatic bottlenecks, both within the pathway such as HMG-CoA reductase [20] and those that act on the MVA pathway including diglycerol kinase 1 [21]. These modifications and combinations thereof have yielded >3-fold titer enhancements of target isoprenoids. Variations of the MVA pathway have also been reconstructed in various heterologous hosts, such as Escherichia coli [22]. With novel strategies based on clustered regularly interspaced short palindromic repeats (CRISPR) interference, balancing carbon flux through this pathway in heterologous hosts has been easily manipulated [23].
While enhancing precursor levels often improves the production of bioactive isoprenoids, elevated levels of IPP have been demonstrated to cause toxicity in E. coli [24]. Indeed, physiological analysis of strains that accumulate IPP indicated decreased cellular viability and reduced plasmid stability as a result of the formation of a toxic isoprenyl-ATP analog. Thus, maintaining a balance between the precursor availability and downstream intermediates is crucial for optimal production.
Recently, novel strategies for the production of DMAPP and IPP from alternative precursors have been explored. For example, the potential for enhancing DMAPP and IPP titers by leveraging conversion of bioethanol to acetyl-CoA in recombinant Pseudomonas putida was recently demonstrated [25]. Another example includes the utilization of methane as a feedstock for the ribulose monophosphate cycle, which feeds into the DXP pathway via the Embden–Meyerhof–Parnas pathway in the methanotroph Methylotuvimicrobium alcaliphilum 20Z [26]. In addition, emerging de novo pathways to the universal prenyl diphosphate precursors DMAPP/IPP offer potentially powerful complements to native biosynthetic pathways. To date, these pathways leverage two sequential phosphorylations of the corresponding alcohol precursors, dimethylallyl alcohol (DMAA) and isoprenol (ISO), to produce DMAPP and IPP [27–30]. Unlike engineering of endogenous metabolism, which is often limited by carbon and energy inefficiencies, these alcohol-dependent hemiterpene (ADH) pathways circumvent native metabolism to enable the decoupling of cellular growth and isoprenoid biosynthesis (Fig. 3c). The de novo pathways described so far leverage the native promiscuity of a kinase or reversible phosphatase to catalyze the first phosphorylation reaction, while the second phosphorylation is catalyzed by isopentenyl phosphate kinase (IPK) from the terminal step of the archaeal MVA pathway. These pathways significantly reduce the number of enzymatic steps that are required for optimization via metabolic engineering and enzyme engineering, allowing for facile engineering for enhancing isoprenoid titers. Notably, lycopene production through these prototype pathways is on par with or exceeds reported production in engineered strains of E. coli that leverage the MVA pathway and can be anticipated to far outpace the native pathways once engineered further. Interestingly, IPK mutants and homologs have also been explored for utility as a single-enzyme ADH pathway that catalyzes both phosphorylations sequentially; however these variants are not as robust as the two-enzyme alternatives when evaluated for lycopene production [31, 32].
Approaches to Non-Natural Isoprenyl Diphosphate Production
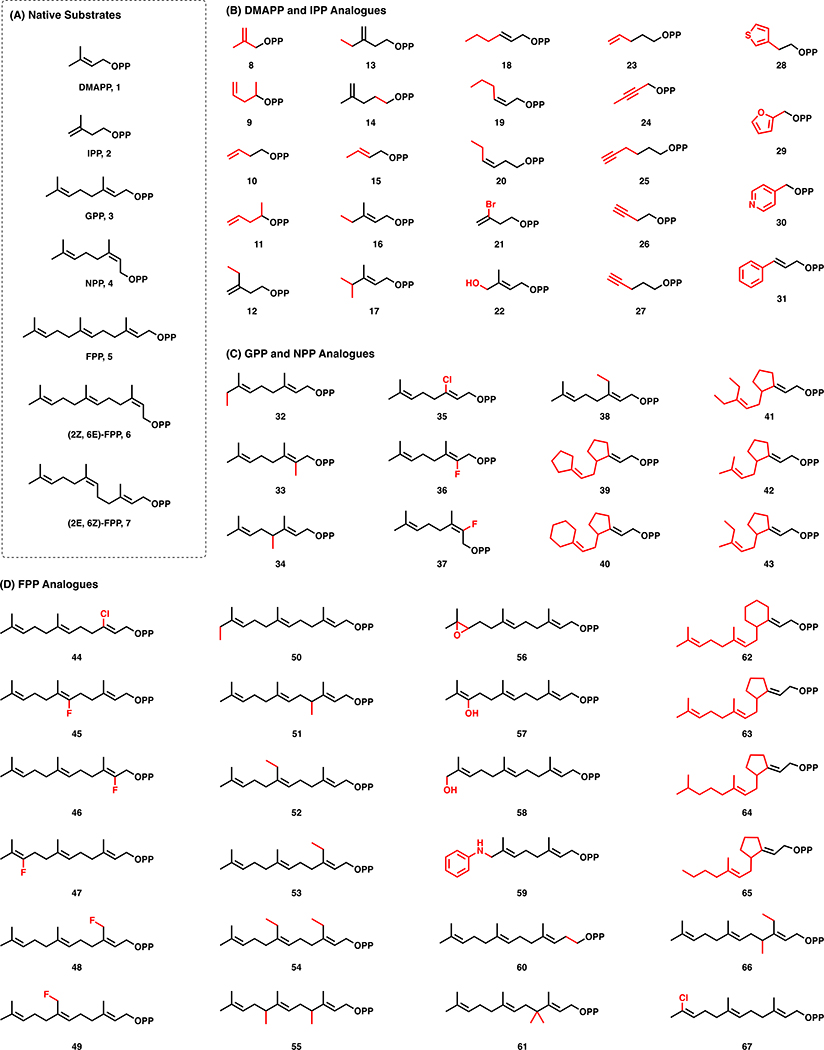
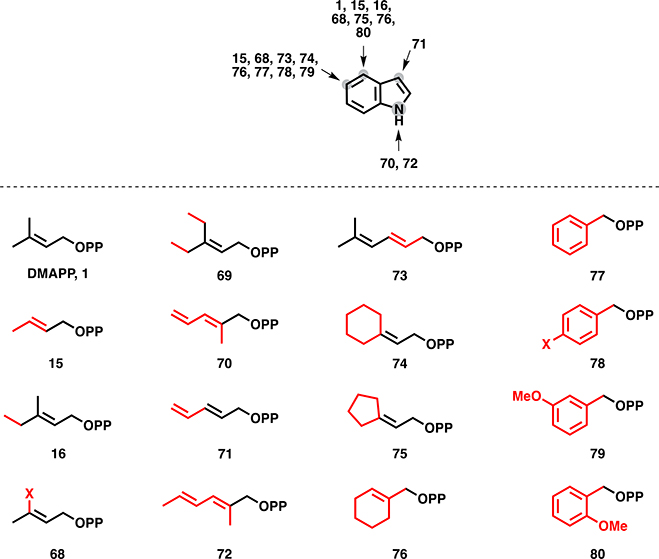
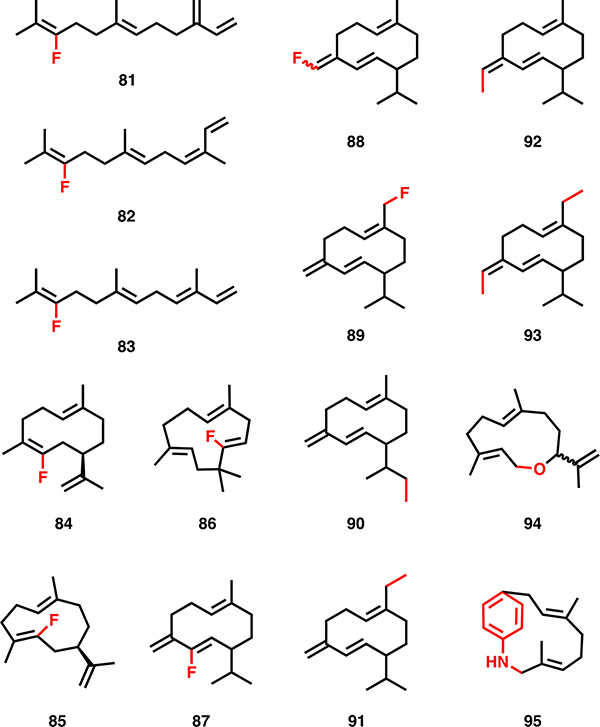
As the building blocks of isoprenoids, prenyl diphosphates present an opportunity for precursor-directed biosynthetic diversification. While de novo ADH pathways offer attractive opportunities to improve titers of natural hemiterpenoids, these same pathways offer an additional benefit: promiscuity of the enzymes towards non-natural substrates with different carbon skeletons. Indeed, twenty-two non-native alkyl monophosphates were utilized by IPK (e.g. 8, 10-11, 18-19, 21, 23-31, Fig. 4b), suggesting a remarkable capacity to produce non-natural prenyl diphosphates [33]. This was also highlighted by a one-pot in vitro cascade reaction, to utilize hydroxylated and methylated analogues of DMAPP and IPP to produce farnesol analogues(e.g. 9, 12-17, 22, Fig. 4b) [34].
Fig. 4.
Biologically and chemoenzymatically derived non-natural prenyl diphosphates. Structural differences from the natural diphosphates are highlighted in red. See text for references.
Native precursor biosynthetic pathways may also have some inherent promiscuity that could be leveraged for diversification. Several naturally occurring pathways produce methylated prenyl diphosphate analogues. For example, an S-adenosylmethionine (SAM)-dependent IPP methyltransferase was recently discovered by Drummond et al [35] in Streptomyces through genome mining. In vitro evaluation of enzymatic activity showed the creation of not only C6-methylated IPP and DMAPP derivatives (e.g. 12, 13, 16, 17, Fig. 4b), but also C7-derivatives, presumably made through two successive methylations of the corresponding hemiterpene. GC-MS analysis of culture headspace showed in vivo production of these same compounds, as well as C11, C12, C16, and C17 isoprenoid alcohols, suggesting that native prenylelongases are able to recognize and incorporate these methylated IPP analogues (e.g. 32-34, 38, 50-55, 61, 66, Fig. 4c/4d). Another strategy uses propionyl-CoA starter units, rather than its native acetyl-CoA precursor, for the MVA pathway to yield C6 hemiterpenoids [36].
Another example combined several promiscuous and engineered enzymes to create a C11 isoprenoid biosynthesis pathway in S. cerevisiae. A 2-methylgeranyl diphosphate (2-MeGPP) methyltransferase was fused with Erg20p—the native geranyl diphosphate (GPP) and farnesyl diphosphate (FPP) production enzyme in yeast—and mutagenized at several positions indicated by previous studies and structural analysis. These changes allowed for greater preference of the fusion enzyme for release and methylation of GPP, creating the linear C11 isoprenoid. This product was paired with two heterologous dedicated C11 terpene synthases; however, other C11 products were also identified, suggesting usage of 33 by native terpene synthases. The synthases were also mutagenized to prefer C11 substrates over their native C10 through saturation mutagenesis at positions chosen through structural and homology data [37].
Non-natural analogues of hemiterpenes are also accessible via organic synthesis. Typically, hemiterpenoid diphosphates are synthetically built via the activation of their respective alcohols (DMAA and ISO) towards a better leaving group, such as alkyl halides, tosylates, mesylates etc., followed by subsequent nucleophilic attack by pyrophosphates and extensive purification of the desired diphosphate [38, 39]. Leveraging this method, a diverse panel of hemiterpenoid analogues have been accessed including deuterated hemiterpene diphosphates for mechanistic probes [40]. Halogenated precursors have also been developed and while they often cannot be leveraged for prenylation or cyclization, they have enabled a range of mechanistic studies [41]. For example, the enantioselectivity of (−)-limonene synthase from Citrus sinensis has been probed with 36 and 37 (Fig. 4c) [42–44]. This subsequently led to co-crystallization of linalyl diphosphate in limonene synthase to further elucidate the mechanism of cyclization [45].
In summary, significant progress has been made towards accessing unnatural prenyl diphosphates (particularly with respect to variation of alkyl groups), the use of these derivatives by in vivo pathways, and the development of de novo pathways to access larger chemical diversity. Further exploration of the ability of these derivatives to be used by downstream pathways and to provide libraries of desired target compounds through precursor-directed biosynthesis is now critical.
Prenylelongase Promiscuity and Engineering
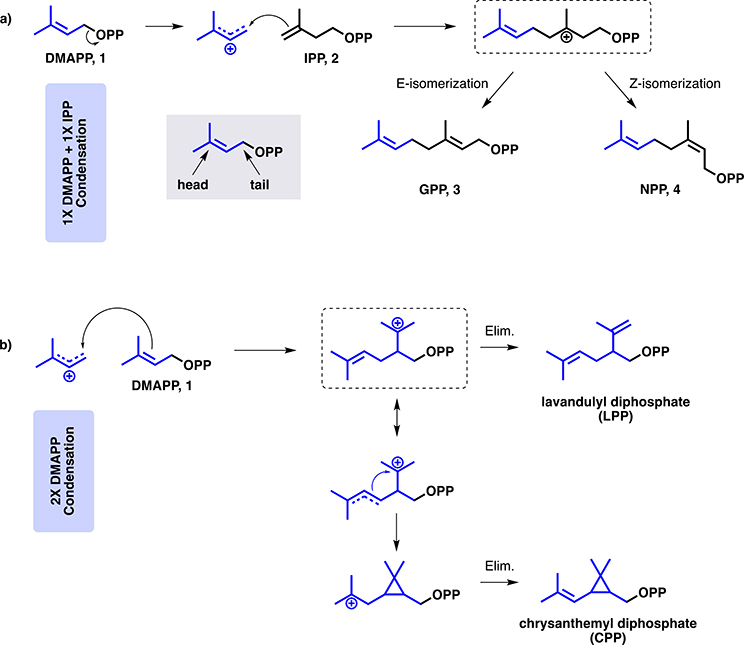
Prenylelongases polymerize the monomeric building blocks DMAPP and IPP into long chain isoprenoids. The simplest product is the C10 geranyl pyrophosphate (GPP), via the condensation of one unit each of DMAPP and IPP, catalyzed by GPP synthase (Fig. 5a). The mechanism of GPP synthase is initiated by the dissociation of the pyrophosphate moiety of DMAPP, facilitated by an active site Mg2+ ion. The resulting stabilized dimethylallyl cation then undergoes nucleophilic attack by the double bond of IPP. The tertiary carbocation subsequently undergoes syn or anti β-hydrogen elimination, yielding GPP [46] or neryl diphosphate (NPP), respectively. The condensation is categorized as “head-to-tail” because it involves the carbon distal to the pyrophosphate of the ‘extender’ unit (“head”) reacting with the carbon atom proximal to the pyrophosphate (“tail”) of the ‘starter’ unit. Reactions are also possible with the secondary “middle” carbon (head-to-middle). In this way, structural diversity can be generated from just the two isomeric monomeric building blocks. For example, there are four possible C10 linear monoterpenes produced from DMAPP/IPP: GPP, NPP (the cis conformer of GPP), lavandulyl pyrophosphate, made from a head-to-middle condensation of two DMAPP units, and chrysanthemyl pyrophosphate, which is also head-to-middle condensation of two DMAPP units with accompanying intramolecular cyclopropanation rather than elimination (Fig. 5b) [47]. Each of these reaction types can also be performed during the formation of longer-chain isoprenoid polymers using IPP as the extender, leading to additional natural diversification and allowing for transfer to other classes of molecules or cyclization into terpenoid natural products.
Fig. 5.
Mechanism of C10 isoprenoid pyrophosphate formation. a GPP and NPP are formed by the condensation of one unit each of DMAPP and IPP, with the formation of an E- or Z- olefin, respectively. b LPP and CPP are formed by the head-to-middle condensation of two units of DMAPP. LPP undergoes a simple elimination, while CPP undergoes an intramolecular cyclopropanation to form the final product.
While prenylelongases are not generally known to be promiscuous, some side reactions producing alternative products are known. For example, a dedicated cis prenylelongase, which had been known to catalyze head-to-tail condensations of long chain polyprenyl pyrophosphates, also produces small quantities of head-to-middle isomers [48]. The limited inherent promiscuity of prenylelongases has been expanded via rational mutagenesis and directed evolution. For instance, a high-throughput screen based on the production of lycopene was leveraged to find mutants of IspA, a farnesyl pyrophosphate (FPP, C15) synthase capable of producing geranylgeranyl pyrophosphate (GGPP, C20). The geranylgeranyl pyrophosphate synthase CrtE from carotenoid biosynthesis endogenous to Pantoea ananatis was substituted with an error-prone library of IspA and screened via absorbance based measurement of tetradehydrolycopene [49]. Most of the productive mutations were in close proximity to two aspartate-rich domains that were previously implicated in chain length determination [50]. Notably, a double mutant was shown to have complete abolition of FPP synthase activity, becoming a dedicated GGPP synthase, while several other mutants showed mixed activity. A yeast FPP synthase from ergosterol biosynthesis was converted to a GGPP synthase through similar means [51]. Other studies have shown that FPP synthases can be interconverted via rational mutagenesis to GPP synthases [52, 53] and that these mutants can be used in cell-free biosynthesis of monoterpenoids [54]. The chain-length specificity of cis-olefin-forming prenylelongases has also been relaxed by enzyme engineering [55].
The product stereochemistry of prenylelongases has also been engineered. In a study attempting to produce Z,E-farnesol (a farnesol isomer with a cis olefinic bond) in E. coli, using the Z,E-FPP synthase Rv1086 from M. tuberculosis, endogenous IspA was found to interfere by competing for precursors. Free geraniol in the cytosol suggested that IspA was releasing and re-uptaking GPP, rather than performing two subsequent condensations in the active site to produce the desired FPP isomer. The authors reasoned that a fusion protein of IspA and Rv1086 may provide the best chance for capturing released GPP and providing a higher proportion of the Z,E isomer of farnesol. The fusion protein resulted in a 15-fold increase of the desired isomer compared to separate expression of each synthase [56].
Prenylelongases have also been shown, in a few cases, to accept unnatural substrates without engineering. One such example established the ability of avian FPP synthase to condense 3-chloro-IPP with DMAPP or GPP to form 35 and 44, respectively (Fig. 4c/d). No products were formed when two chlorinated derivatives were incubated with the enzyme together, suggesting that these enzymes are only able to utilize one unnatural substrate in a given reaction [41]. Other work has shown the ability of FPP synthases to use methylated and cyclized analogues of DMAPP and IPP to construct novel GPP- (39-43, Fig. 4c) and FPP-like compounds (62-65, Fig. 4d) [57]. As described earlier, native prenylelongases have been shown to accept mono- and dimethylated IPP and DMAPP derivatives and incorporate them into up to C17 FPP products [35].
These studies have contributed to our mechanistic understanding of prenylelongases and demonstrate some potential for diversification of isoprenoids during the chain elongation phase of their biosynthesis, albeit being largely limited to naturally-occurring substrates. Yet, the promiscuity of prenylelongases towards non-native and non-natural substrates appears limited, although this may reflect difficulties associated with accessing the necessary pyrophosphates. However, prenylelongases have proven amenable to engineering. Development of methods for high-throughput detection of non-natural chain extensions, as well as additional structural information of prenylelongases to aid rational mutagenesis efforts, will help to expand the diversity introduced during the chain-elongation phase of isoprenoid biosynthesis.
PTase Substrate Scope and Engineering
The best characterized PTases are the ABBA-fold aromatic PTases, composed of an antiparallel β/α barrel-like structure with a central cavity open on both ends. While not catalytically dependent on metals, they are more efficient in the presence of Mg2+ ions. Mechanistic studies of this broad enzymatic class, including by kinetic isotope effect (KIE) and positional isotope exchange (PIX) experiments, have indicated that the mechanism with natural substrates proceeds via the allylic stabilized carbocation intermediate [58, 59] and was thought to be concerted [60]. However, a more recent study suggests that the reaction proceeds via a dissociative mechanism wherein the nucleophilic attack does not participate in the rate determining step [54].
Due to the relatively open nature of their active sites, ABBA-fold aromatic PTases have demonstrated promiscuity towards both their aromatic substrates and the pyrophosphate alkyl donors [61]. Two families of ABBA-fold PTases have been proposed: (1) the dimethylallyltryptophan synthase (DMATS) family are mostly fungal enzymes which generally alkylate indole-containing natural products, and (2) NphB/CloQ-like proteins which are mostly of bacterial origin and alkylate phenols and phenazines [62]. Later studies have expanded this framework to include phylogenetic analysis and further subgroups [63]. Each group has demonstrated high levels of substrate promiscuity (Table 1).
Table 1.
Native promiscuity of PTases for non-natural substrates.
| Enzyme (organism) | Acceptors | Donors | Reference |
|---|---|---|---|
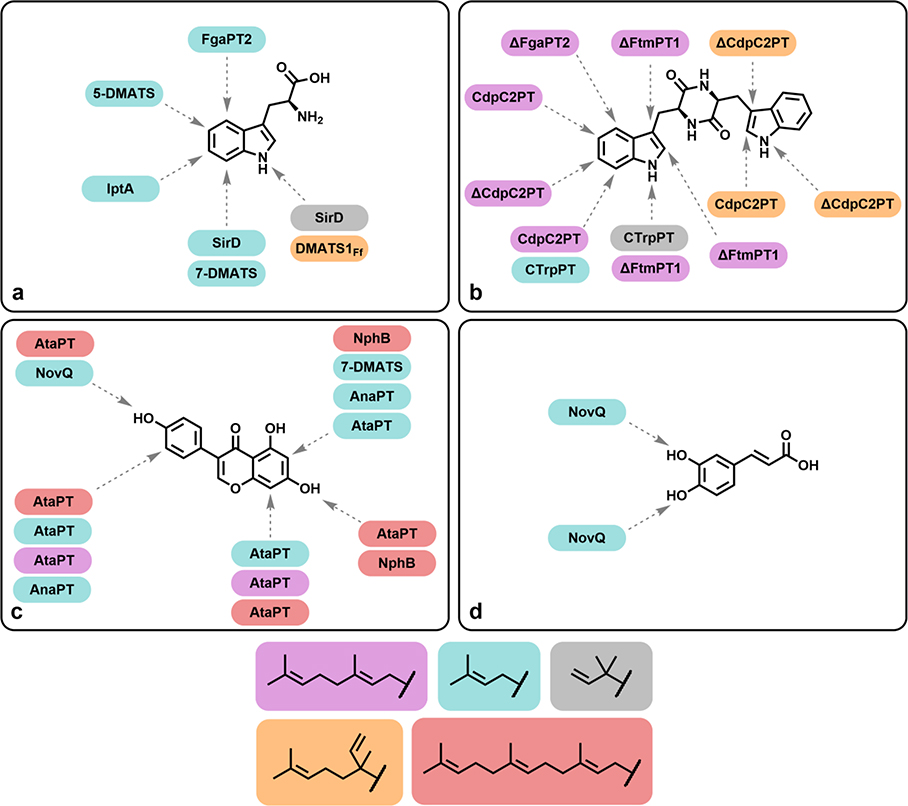
| FgaPT2 (Aspergillus fumigatus) [D] | L-Trp;* D-Trp; altered backbone Trp derivatives, L-Tyr and isomers, indole-containing DKPs and indolocarbazoles, fumiquinazolines | 1*, 15, 16, 68–80 | [64–70] |
| AtaPT (Aspergillus terreus) [D] | lignanoids, indole DKPs, quinolines, xanthones, benzophenones, flavonoids, stilebenes, coumarins | 1, 3, 5, GGPP, PPP | [76] |
| FtmPT1 (Neosartorya fumigata) [D] | Cyclo(Trp-Pro);* Indole DKPs | 1* | [67, 69] |
| CTrpPT (Aspergillus oryzae) [D] | Indole DKPs | 1 | [70, 76] |
| DMATS1Ff (Fusarium fujikuroi) | L- and D-Trp, 5-hydroxytryptophan, L-Tyr | 1 | [77] |
| NphB (Streptomyces spp.) [N] | 2,7-dihyroxynaphalene;* DHN isomers, flavonoids (O- and C-), olivetol, resveratrol, sulfabenzamide | 1, 3*, 5, 15–17, 29, 69, 71–76, 78, methyl, propargyl, o-propargyl, and o-benzyl GPP analogues; alkyl DMAPP analogues; substituted benzyl PP analogues, furan, thiophene, cyclohexene derivatives | [62, 83–86] |
| NovQ (Streptomyces spheroides) [N] | 4-hydroxyphenylpyruvate;* phenylpropanoids (caffeic acid, p-coumaric acid), resveratrol, olivetol, flavonoids (naringenin, genestein) | 1* | [62] |
| CloQ (Streptomyces roseochromogenes) [N] | 4-hydroxyphenylpyruvate;* flavonoids (i.e. luteolin, daidzein, genistein), reseveratrol | 1* | [62, 86] |
| UbiA (Escherichia coli) [X] | 4-hydroxybenzoate* | 3, 5, solanesyl PP (C45) | [89] |
| UbiX (many species) [X] | Flavin mononucleotide* | 1/DMAP,* 3/GP (species dependent) | [91] |
| Protein FTase/GGTase (mammalian) [X] | dansyl GCVLL | 5/GGPP;* 5/GGPP analogues with additional methylene units | [82] |
| KabA (Pseudo-nitzxhia spp.) [X] | L-glutamate* | 1*, crotyl diphosphate | [92] |
| DabA ((Pseudo-nitzxhia spp.) [X] | L-glutamate* | 3*, 5 | [92] |
Denotes the native substrates for the enzyme.
Letter in brackets indicates PTase group: N=NphB-like; D=DMATS-like; X=O-PTase.
The most-studied member of the fungal aromatic PTases, is FgaPT2, a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus (Table 1). While the enzyme shows remarkable regiospecificity for C4 of the indole ring of tryptophan when utilizing DMAPP as an alkyl donor, there is a preference for the C5 position with alternative donors (e.g. 15, 68, 73, 74, 76-79, Fig. 6). For alkyl donors, FgaPT2 has shown a tolerance for a variety of allylic and benzylic (e.g. 77–80, Fig. 6) donors [64, 65]. Of those tested, shorter chain donors were not utilized, including propargylic pyrophosphate [66]. Electron-donating groups on benzylic donors do not appear to increase alkylation. For aromatic acceptors, FgaPT2 natively leverages L-tryptophan (Fig. 7a), but tolerates tryptophan derivatives with altered amino acid backbone moieties, indole-containing DKPs (Fig. 7b), indolocarbazoles, m- and o-tyrosine, and fumiquinazolines [67–70]. Other members of the fungal aromatic PTase family have shown similar levels of promiscuity for both aromatic acceptors and donors, including FtmPT1, CdpNT, 7-DMATS [71], and SirD [72, 73], while others may be as tolerant but are less well-studied, such as 5-DMATS and IptA [74, 75]. More recently, the aromatic PTase AtaPT demonstrated unprecedented promiscuity towards aromatic acceptors and donors, including donors with various alkyl substituents and differing chain lengths, and showing multiple sites of alkylation on the same acceptors [76].
Fig. 6.
Non-native promiscuity and regioselectivity of FgaPT2 with unnatural alkyl diphosphates on indole acceptor.
Fig. 7.
Promiscuity and regioselectivity of PTases. a Alkylation of amino acids. b Alkylation of DKPs. c Alkylation of flavonoids. d Alkylation of phenylpropanoids. PTases shown are wild-type unless labeled with ‘Δ’.
Variations in PTase regioselectivity are likely the result of repositioned carbocation via the tilt of the aromatic acceptor. For example, comparisons between crystal structures of FgaPT2 and FtmPT1 shows that due to change of a tyrosine residue to a glycine in the active site, the indole ring in FtmPT1-substrate brevianamide F is tilted compared to the indole ring of tryptophan in FgaPT2. This tilt puts C-4, C-5, C-6 and C-7 of indole far away from the other substrate DMSPP. This in turn alters position of DMSPP, which indicates towards the observed change in regioselectivity of this two enzymes [77, 78]. Recent studies focused on FtmPT1 have described prenylation of the native C2 position and the anomalous C3 position of the dipeptide. It was suggested that the initial ionization to produce the allylic carbocation is stabilized by cation-π interactions with the aromatic substrate (dipeptide) and Tyr382 in the PTase barrel. Combination of this binding effect with the aromatic acceptor tilt forms the basis of the observed regioselectivity [79]. Interestingly, CTrpPT from Aspergillus oryzae prenylates indole-containing DKPs at C7 and N1 [80]. Despite its broad scope of prenyl acceptors [81], the mechanism for the regioselectivity of CTrpPT prenylation remains unknown. Recently, the reverse N-prenylation by DMATS1Ff was shown to proceed via a unique transition state via direct nucleophilic attack of N1 to the tertiary center of the allylic carbocation or rotation of the carbocation, followed by C3-nucleophilic attack on the primary allylic carbocation and subsequent aza-Cope rearrangement [82].
The prenyltransferase NphB is the canonical example of the second, bacterial family of PTases. NphB is dependent on the presence of Mg2+ ions, though many other members are not, and all lack the (N/D)DXDX motif usually required for magnesium ion chelation. Like its relatives in the DMATS family, NphB tolerates a wide range of acceptors (Table 1), though it prefers phenolic compounds rather than indoles, and catalyzes both C-C (Fig. 7c) and C-O (Fig. 7d) bond formation [83]. NphB has proven useful for the production of cannabinoids in yeast, where it catalyzes the crucial geranylation of olivetolic acid to produce cannabigerolic acid [84]. However, the subsequent landmark heterologous biosynthesis of cannabinoids and unnatural analogues did not utilize NphB, preferring instead a plant PTase, due to issues with selectivity towards the desired reaction [85]. Recent work with NphB has revealed extensive promiscuity towards allylic alkyl donors and the ability to append them to an unnatural acceptor molecule [86]. The related enzymes CloQ and NovQ are part of the pathways forming clorobiocin and novobiocin, respectively, share 84% sequence identity, and accept many phenolic compounds [87, 88]. The donor promiscuity of this group, outside of NphB, has been poorly described; indeed, CloQ and NovQ are only reported to utilize DMAPP in the studies above. This is a notable difference from the DMATS group, which have been shown to utilize unnatural alkyl pyrophosphates of various chain lengths. It is unclear whether this is a true difference, or rather due to a lack of exploration of alternative alkyl donors. The recent demonstration of NphB’s remarkable promiscuity towards alkyl donors suggests that this family of enzymes may be more accommodating than previously thought.
Outside of the soluble ABBA-fold PTases, membrane-bound PTases such as UbiA have been shown to utilize natural alkyl pyrophosphates of varying lengths (Table 1). The E. coli UbiA has shown to use C10, C15, and C45 prenyl groups to alkylate 4-hydroxybenzoate [89]. Analogous plant PTases show similar activity [90]. UbiX, a C5-prenyltransferase, has isoforms that natively use the monophosphate DMAP, but are able to catalyze the addition of a geranyl group to a flavin ring system as well [91]. Mammalian peptide farnesyltranferases and geranylgeranyltransferases have been shown to use ‘frame-shifted’ FPP and GGPP analogues with added methylene units between isoprenes in their backbones, showing some additional chain-length preference flexibility [82]. However, these enzymes appear to be extremely stringent in terms of their aromatic acceptor selectivity. An additional non-ABBA fold example comes from the algal neurotoxin biosynthesis PTases DabA and KabA, which were unable to use a variety of resonance-stabilized unnatural alkyl donors, including alkynyl diphosphates; however, KabA was shown to use crotonyl diphosphate to alkylate its native acceptor, glutamic acid [92].
The considerable inherent promiscuity of PTases has inspired engineering efforts to further expanding the substrate scope of these enzymes (Table 2). These efforts have focused on mutagenesis of residues in the prenyl donor binding pocket and those that alter the aromatic acceptor scope. Previous work suggested that overlapping specificity with L-tryptophan/L-tyrosine of FgaPT2 and SirD may be a common feature of these enzymes [63, 71]. Structural determination of the FgaPT2-catalyzed product with DMAPP and tyrosine revealed that prenylation occurs at the C3 position. Co-crystallization of FgaPT2 with the non-native tyrosine and a sulfur-based non-hydrolyzable DMAPP analogue, dimethylallyl-S-thiolodiphosphate, enabled the selection of candidate residues for mutagenesis in an attempt to direct specificity towards tyrosine [71]. Subsequently the mutant K174F was identified, which lost all activity towards tryptophan but displayed a three-fold increase in activity towards tyrosine compared to the wild type enzyme. It was suggested that Lys174 acts as a base in the rearomatization of the indole ring following alkylation, but that an alternative residue (E89) performs that function when tyrosine is the acceptor. Subsequent mutagenesis of FgaPT2 has altered its substrate scope towards various non-natural acceptors. Indole-containing cyclic dipeptides have been targeted through removal of an arginine involved in Trp side chain binding [93]. Combining K174F and R244Y [94] led to activity at the C3-position of indole-containing cyclic dipeptides; selectivity toward longer chain prenyl donors through a single M328G mutation in the prenyl binding pocket was also achieved [95]. These studies, while limited to one enzyme, showcase a framework for altering the regioselectivity and promiscuity of PTases through single- or multiple-site saturation mutagenesis, guided by structural studies.
Table 2.
Examples of engineered PTases to enhance or alter specificity and activity.
| Enzyme | Mutation | Product Profile | Reference |
|---|---|---|---|
| FgaPT2 | K174F | Loss of activity towards Trp, increase towards Tyr | [93] |
| K174F:R244Y | Increase towards indole DKPs | [94] | |
| M328G | Increase towards GPP and away from DMAPP | [95] | |
| R244Q:M328G | Increase towards GPP and away from DMAPP, accept indole DKPs | [75] | |
| FtmPT1 | M364G | Increase towards GPP and away from DMAPP | [75] |
| CdpC2PT | T351G | Increase towards GPP and away from DMAPP | [75] |
| CdpC3PT | F335G | Increase towards GPP and away from DMAPP | [75] |
| CdpNPT | M349G | Increase towards GPP and away from DMAPP | [75] |
| BrePT | I337G | Increase towards GPP and away from DMAPP | [75] |
| A173M | Increase towards DMAPP and away from GPP | [96] | |
| TleC | W97Y | Increase towards DMAPP | [96] |
| MpnD | Y80W | Increase towards GPP and away from DMAPP | [96] |
| M159A | Increase towards GPP/FPP and away from DMAPP | [96] | |
| PagF | F222A | Increase towards GPP and away from DMAPP | [97] |
| AtaPT | W397A | Altered diversity of many acceptor compounds | [76] |
| E91A/E91Q | Only monoprenylations for all wild-type products | [76] | |
| EpzP | A285Q | 14-fold increase in enzyme velocity | [98] |
Another example of this approach is the engineering of a group of cyclic dipeptide PTases to decorate the indole ring at every position [75]. A total of nine products were isolated: seven normal-geranylated, one at each position, and two additional products which were reverse-geranylated at C2 and C3. These products were accessed by altering the gate-keeping residues to glycine to accommodate and select for GPP. Conversely, substitution at the gatekeeping glycine and alanine residues with larger, more sterically-demanding residues can be leveraged to switch the specificity of enzymes which natively prefer longer chain prenyl donors to DMAPP [95, 96]. This approach is applicable outside of the small molecule aromatic ABBA-fold PTases. For example, the dimethylallyl transferase, PagF, and the geranyl transferase, PirF, post-translationally alkylate peptides on tyrosine residues. Single amino acid switches from phenylalanine to alanine or glycine converted the DMAPP-dependent PagF to a GPP-specific enzyme [97].
The acceptor side of the AtaPT active site has been subjected to alanine mutagenesis at residues that interact with the aromatic acceptor [76]. Several mutants showed different product profiles, including altered number of prenylations, changes in regioselectivity, and increased activity towards poor substrates. With the goal of optimizing enzyme efficiency towards its natural substrate rather than increasing promiscuity, a 14-fold increase in catalytic efficiency was seen in a single point mutant for EpzP; the residue was selected by homology to a related enzyme with better efficiency [98].
Although the field of PTase engineering is relatively young, much progress has been made towards impacting the product profiles. The potential chemical space to be filled by application of these enzymes for the chemoenzymatic synthesis of non-naturally alkylated natural product analogues, is remarkable. Medicinal chemistry studies have pointed to potential opportunities leveraging PTases for development of compounds with enhanced biological properties. For example, arylated brevianamide [99] and aminocoumarin novobiocin [100] analogues have shown increased antitumor activity, a transformation readily feasible using promiscuous aromatic PTases. Moreover, crystallographic evidence has demonstrated the critical role of the dimethylallyl moiety of clorobiocin for its antibiotic activity, and implies that modifications to this side chain may be successful in increasing binding affinity [101, 102]. However, these opportunities for engineering and diversification have yet to be widely applied to PTases outside of the ABBA-fold family, which are generally less well-characterized. Future efforts in this regard may provide access to novel scaffold modifications.
Terpene Cyclase Specificity, Promiscuity, and Engineering
Isoprenoid cyclization cascades are initiated by the generation of highly reactive carbocation intermediates from elongated prenyl diphosphates. The formation of these intermediates is highly dependent on the active site architecture of the cyclase and can be mechanistically subdivided into two types. Metal-dependent type I cyclases, characterized by the conserved DDXX[D/E] motif, cleave the terminal diphosphate to yield an allylic carbocation. Type II cyclases, with a catalytic DXDD motif, protonate the olefin to produce a tertiary carbocation [103, 104]. The cyclization cascade allows the linear precursor to undergo a variety of structural changes before the reaction is terminated via deprotonation or quenching with water to yield a wide range of cyclic isoprenoids. The broad goal of terpene cyclase engineering is to redirect this chemistry to provide mutants with improved catalytic activity, altered product profiles, expanded or narrowed product specificity, or altered substrate specificity.
Cyclases, such as amorpha-4,11-diene synthase (responsible for the construction of the artemisinin core), often catalyze the rate-determining step in the biosynthesis of the mature natural product and are a critical target for optimization of catalytic efficiency through mutagenesis [105]. Leveraging a mutability map, wherein libraries of mutants were screened to identify positive, negative, and neutral amino acid substitutions, the double mutant T339S/H448A was identified that successfully enhanced the titer of amorpha-4,11-diene ~3-fold compared to that of the wild-type [105]. Phylogeny-based mutagenesis studies have also yielded mutants with significant improvements in catalysis, including those of the ophiobolin synthase, Au8003, a bifunctional terpene synthase that catalyzes both the prenylelongation and the cyclization [106]. Random mutagenesis strategies enabled by high-throughput screening have also been reported for enhancing catalytic activity and thermostability of the botrydial synthase, BcBOT2; however, as explored in a later section, these efforts remain fairly limited [107].
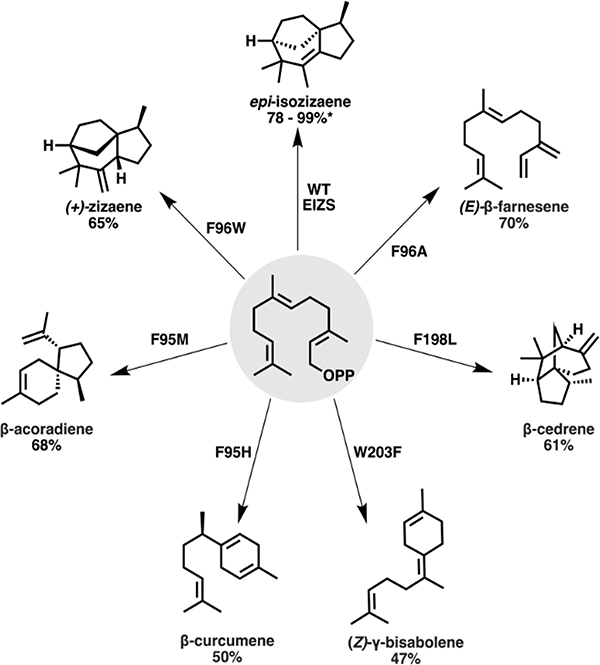
The active site architecture of terpene cyclases is critical in determining the product profile, serving as a ‘cryptic’ template for catalysis using a combination of geometry [108], solvolysis, energetics, and electrostatics [109] to guide the carbocation intermediate. High-fidelity cyclases, such as δ-cadinene synthase [110] and limonene synthase, demonstrate significant preference for the formation of a single cyclic product by providing exquisite chemical control of the carbocation cascade. Recent structural analyses and mutagenesis-guided studies have elucidated the mechanistic basis of chemical control within these product-specific cyclases. Structural studies of 5-epi-aristolochene synthase identified a highly conserved Mg+2 binding domain, which templates product formation via the binding of an active site water molecule [111, 112]. The contour of the enzymatic active site also significantly impacts the cyclization cascade in epi-isozizaene synthase (EIZS), which is largely defined by Phe95, Phe96, Phe198, and Trp203 [113]. Utilizing these structural insights, rational reprogramming of the EIZS active site significantly altered its product profile (Fig. 8) [113–115]. Mechanistic studies of cyclases have led to an improved understanding of the energetics behind these highly controlled reactions. For example, recent simulations of trichodiene synthase (TDS) indicated that the cyclization of FPP is facilitated by raising the free energy of the bisabolyl carbocation which is directed by a sulphur-carbocation bonding interaction with Met73 to provide the chemical control demonstrated by this enzyme [116, 117].
Fig. 8.
Rational engineering of EIZS to produce different product profiles. Shown are the major products that result from some of the individual point mutations evaluated with the percent fraction of the indicated product of the total products shown [113, 115]. *denotes temperature dependence of the product distribution.
Mutagenesis studies have engineered high fidelity cyclases with altered or promiscuous activity. Bacterial cineole synthase (bCinS) is highly specific for cineol (~95% yield) as a result of quenching the highly reactive α-terpinyl carbocation intermediate by an Asn305-stabilized water by [118]. Leveraging a semi-rational approach, Asn305 was targeted for site-directed mutagenesis to afford mutants with dramatically altered product profiles, albeit with concomitant reductions in overall isoprenoid production. For example, N305D produced linalool and α-terpineol exclusively, while N305Q and N305L each produced 100% linalool [119]. Similarly, the single point mutations L454G and L454A of the β-sesquiphellandrene synthase from Persicaria minor (PmSTS) each produced multiple hydroxylated cyclic products that were not produced by the wild-type enzyme [120].
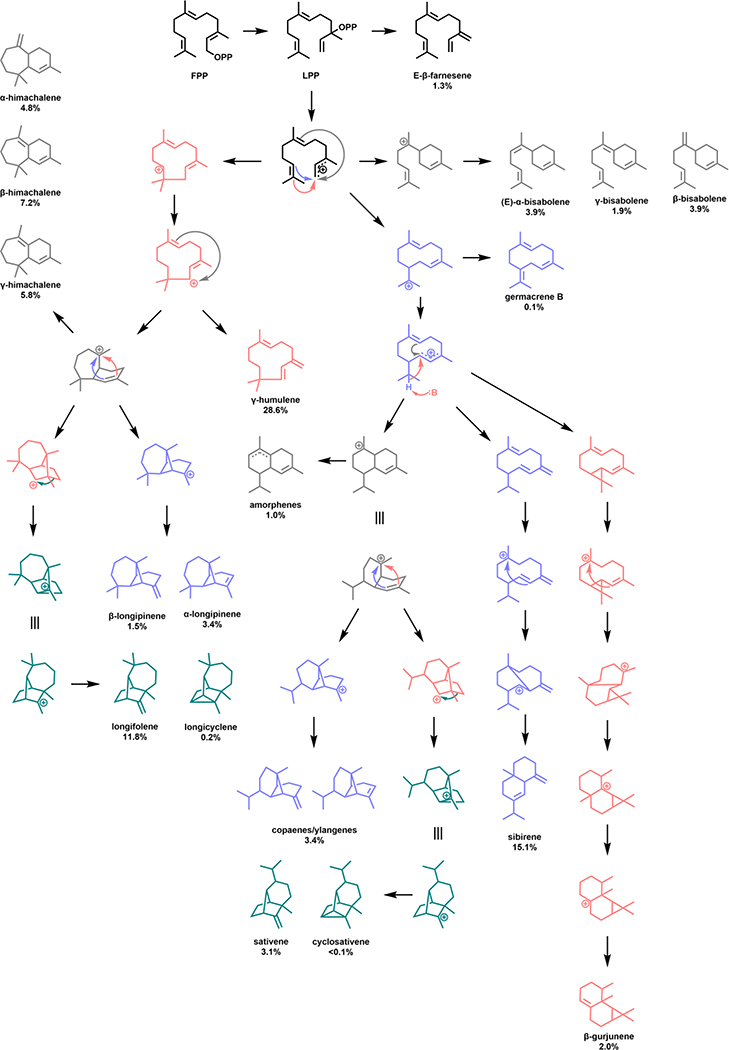
In contrast to the examples above, the majority of terpene cyclases produce multiple cyclic products resulting from the formation and propagation of multiple discrete high-energy carbocation intermediates throughout the cyclization cascade with limited enzymatic direction and control [104]. Enzymes with substantial product promiscuity include δ-selinene synthase and γ-humulene synthase, which produce 34 and 52 sesquiterpene products, respectively (Fig. 9) [121].
Fig. 9.
Proposed mechanism of sesquiterpene formation by γ-humulene synthase [117].
In Nature, production of multiple products may present a significant benefit as isoprenoids broadly produce molecules of significant value to plants including signaling molecules, pollination attractants, insecticides, and antioxidants among other activities [3]. Such broad product specificity may be a natural method to maximize resources, while minimizing required cellular energy; however, for the overproduction of a single valuable product, engineering for improved specificity may be necessary. Only a few published examples have focused on re-engineering these promiscuous cyclases for the production of a single product. Early reports leveraged truncated enzymes in an attempt to alter product profiles with mixed results [122]. More recently however, a semi-rational approach has been leveraged to engineer the product profile of γ-humulene synthase towards a variety of its minor products including β-bisabolene, α-longipinene, longifolene, and siberene, as well as demonstrating dramatic enhancements in both the relative percentage and total titer of γ-humulene [123].
Notably, both product specific and promiscuous terpene cyclases display various levels of substrate promiscuity and are therefore able to accept multiple elongated isoprene diphosphate chains as substrates, including different isomers of the native substrate and those of different chain lengths [124]. Based on a recent ancestral sequence reconstruction of a spiroviolene synthase it has been speculated that this substrate promiscuity is likely an artifact of the general catalysis of ancestral cyclases that have evolved over-time for enhanced specialization [125]. While GPP is the canonical substrate for most monoterpene cyclases, alternative substrates can be utilized [126, 127]. For example, monoterpene synthases that natively use GPP were found to use the cis-isomer NPP in vivo, without significant changes to the product profiles in four of the five synthases evaluated. Remarkably, this indicated that NPP could be leveraged as an orthogonal monoterpenoid production pathway [128]. More commonly, however, alternative substrate isomers can cause non-native cyclization to occur. Indeed, (+)-zizaene synthase, which natively utilizes (2E,6E)-FPP for high yields of (Z)-β-farnesene is able to leverage (2E,6Z)-FPP (7, Fig. 4a) to produce only (Z)-β-farnesene, while the same enzyme produces three products, (+)-zizaene, β-(R)-farnesene, and β-acoradiene, when the isomer (2Z,6E)-FPP (6, Fig. 4a) is provided [129]. Such activities have also been identified in other cyclases [130].
Moreover, as a result of steric accommodation within the active site, some cyclases accept shorter-chain non-native prenyl diphosphates as substrates for cyclization. Notably, the remarkable product promiscuity of terpene cyclases with their native substrate is often mirrored with a non-native substrate. For instance, γ-humulene synthase, which natively leverages FPP to produce numerous sesquiterpene products, also supports the use of GPP as a substrate to yield at least ten monoterpene products [121]. Promiscuity for non-native substrates has also been demonstrated with enzymes that have complete fidelity for a single product with their native substrate. For example, when incubated with NPP or GPP, the sesquiterpene synthase (+)-zizaene synthase produces a variety of monoterpenes including myrcene, limonene, sabinene, terpinolene, terpineol, linalool, and geraniol [129].
Notably, cyclases are able to accept synthetically derived unnatural prenyl diphosphate analogues to produce new-to-nature isoprenoids (Fig. 10, Table 3). One example of this promiscuity is aristolochene synthase, which is natively able to cyclase non-natural FPP analogues including halogenated (45, 49, Fig. 4d) [131] and aza analogues (59) [132]. Enzymes including δ-cadinene synthase [133] and (S)-germacrene D synthase [134] also leverage halogenated substrates; however the ability to successfully utilize [45] and incorporate [135] halogenated diphosphate analogues to produce isoprenoid analogues is not universal among these enzymes. In order to produce heteroatom substituted isoprenoid analogues, FPP analogues with a 10,11-epoxide substitution (56) or an allylic alcohol substitution (57) have been reported to produce 11-membered oxygen-substituted rings when reacted with a wide variety of sesquiterpene synthases including aristolochene synthase, δ- cadinene synthase, amorphadiene synthase, (R)-germacrene A synthase, germacradien-4-ol synthases [136]. Notably, other oxygenated substrates have been leveraged by amorphadiene synthase, such as 58, to yield dihydroartemisinic aldehyde, a key precursor to artemisinin [137]. Recent studies of the highly promiscuous type II triterpene cyclase, squalene hopene cyclase, have demonstrated the promiscuity of these enzymes for acyclic terpene analogues with a variety of functionalization to produce C-O and C-N heteroatom substituted compounds [138, 139] including ambroxan and sclareolide. As the cyclization of non-natural substrates continues to be explored, thus expanding the chemical space of the terpenome, the full scope of isoprenoid diversification will offer a combinatorial and semi-synthetic approach to tailored compounds with potential biological and industrial implications [4, 34].
Fig. 10.
New-to-nature cyclized isoprenoids derived from precursor-directed biosynthesis.
Table 3.
Demonstrated substrate promiscuity of terpene cyclases with native, chemoenzymatically, and synthetically accessible elongated prenyl diphosphates.
| Enzyme (organism) | Native Substrates | Non-Native Substrates | New-to-Nature Products | Reference |
|---|---|---|---|---|
| amorpha-4,11-diene synthase (Artemisia annua) | 5 | 57, 58 | 94 | [137] |
| 5-epi-aristolochene synthase (Nicotiana tabacum) | 5 | 59 | 95 | [132] |
| aristolochene synthase (Penicillium roqueforti) | 5 | 56, 57 | 94 | [136] |
| cineol synthase (Salvia fruticosa) | 3 | 4 | - | [128] |
| δ-selinene synthase (Abies grandis) | 5 | 3 | - | [121] |
| γ-humulene synthase (Abies grandis) | 5 | 3 | - | [121] |
| limonene synthase (Citrus limon) | 3 | 4 | - | [128] |
| camphene synthase (Solanum elaeagnifolium) | 3 | 4 | - | [128] |
| sabinene synthase (Salvia pomifera) | 3 | 4 | - | [128] |
| TPS5 (Medicago truncatula) | 5 | 6 | - | [130] |
| δ-cadinene synthase (Gossypium arboreum) | 5 | 46, 47 | 81–85 | [133] |
| germacradien-4-ol synthase (Streptomyces citricolor) | 5 | 56, 57 | 94 | [136] |
| (+)-zizaene synthase (Chrysopogon zizanioides) | 5 | 3, 4, 6, 7 | - | [129] |
| germacerene D synthase | 5 | 45–50, 52–54 | 87–93 | [134] |
| (E)-β-farnesene synthase (Mentha × piperita) | 5 | 56 | 94 | [136] |
| R-germacene A synthase (Solidago canadensis) | 5 | 56, 57 | 94 | [136] |
Modifications to Isoprenoid Scaffolds
While canonical isoprenoid biosynthesis uses a single terpene cyclase to generate the core terpene scaffold, full bioactivity often relies upon the chemo-, regio-, and stereoselective functionalization by tailoring enzymes. Tailoring enzymes include CYPs, GTs, and MTs that transform hydrocarbon terpene scaffolds to functionalize a wide range of biologically active natural products [140]. Moreover, these oxidized scaffolds can offer reaction handles in downstream modifying steps such as alkylation, peptidylation or glycosylation towards the bioactive final product [141].
Often, investigations into these core scaffold modifications lead to the discovery of new metabolites. For example, bioinformatics based fungal genome mining for NRPS-TC hybrid cluster and heterologous expression of the flv pathway from A. flavus led to the production of an unprecedented natural product, the peptido-terpenoid flavunoidine 1. Moreover, this helped identify FlvE as a type I terpene cyclase that forms the flv tetracyclic core and the CYP FlvD that selectively carries out its oxidation. In addition, NRPS, MTs and PLP-dependent lyases help build the complete alkaloid terpenoid [142].
Notably, newer technologies such as untargeted GC/MS based metabolomics have enabled in vitro scanning of CYP activities for a broad range of oxy-functionalized products. For instance, three CYPs capable of robust activities were tested across 15 acyclic, monocyclic and bicyclic monoterpenoids. Employing multi-site selective mutations, a panel of nine variants successfully utilized monoterpene scaffolds to furnish 53 previously identified and 27 novel oxygenated monoterpenoids. Additionally, leveraging cell-free NADPH recycling systems and high substrate concentrations enabled maximum CYP turnover [143, 144]. In an effort to enhance titers of oxidized isoprenoids, extensive metabolic engineering efforts that focus on CYPs have been explored in a variety of heterologous hosts, including E. coli and S. cerevisiae. For example, Cas9-mediated homologous recombination of the casbene pathway in yeast and subsequent heterologous expression led to >5-fold increased titer of casbene. Additionally, integration of two CYPs genes from Euphorbia lathyris and Jatropha curcas that oxidize the C9 and C5 positions of casbene has led to titers of the lathyrane diterpenoid jolkinol C as high as ~400 mg/L. This was further enhanced to ~800 mg/L upon integration of the E. lathyris alcohol dehydrogenase [145]. Similarly, to increase the titer of (+)-epi-α-bisabolol, a precursor of potential natural sweetener hernandulcin, metabolic engineering was used to tune yeast native metabolism away from the formation of sesquiterpenol (nerolidol and farnesol). Integration and overexpression of two CYP reductase genes with the CYP in a previously engineered S. cerevisiae strainoptimized to produce bisabolol (precursor sesquiterpenol) resulted in 36 mg/L of (+)-epi-α-bisabolol that was further enhanced to 64 mg/L upon integration of an additional copy of bisabolol synthase [146].
In addition to host and metabolic engineering, in silico methods have facilitated deeper mechanistic insights into CYPs. Following in silico prediction, two CYPs, BezC and BezE, were identified from the biosynthetic gene cluster of benzastatin, a potent antiviral agent. Subsequently, heterologous expression of the benzastatin biosynthetic gene cluster in Streptomyces lividans and gene deletion experiments identified BezC as a methylated-GPP oxidase and BezE as a cytochrome P450-dependent oxidase that catalyzes the cyclization of benzastatin D and E derivatives. Furthermore, in vitro analysis of BezE with BezF (a putative UbiA-type polyprenyltransferase), BezG (a putative N-acetyltransferase), p-hydroxybenzoic acid and GPP in the presence of 18O-labelled water revealed that the cyclization proceeds via a nitrene transfer like reaction mechanism where a putative iron nitrenoid intermediate is leveraged instead of an epoxide. This result was supported by the substrate binding absorption spectrum of BezE where a Soret type II substrate binding shift indicated that the amine of N-acetoxy-geranylated-PABA derivative directly binds to the hemin of BezE [147].
Notably, structure-based and in silico CYP engineering has been complemented by high-throughput approaches that rely on random mutagenesis. For instance, semi-synthetic chromogenic probes were leveraged to map active site size and geometry to develop a P450 fingerprinting method based on first-sphere mutagenesis, semi-synthetic chromogenic probes were leveraged to map the size and geometry of the active site of the P450 [148]. Cleverly, a reporter methoxy group allowed rapid profiling via a 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Pupald) based high-throughput colorimetric assay that generated reliable prediction of activity-substrate structure relationship [149, 150]. This was leveraged in late stage regio- and enantioselective hydroxylation of artemisinin on a preparative scale [151].
In addition to CYP engineering of terpene scaffolds, heterologous expression of glycosyl transferases (GTs) have been another area of active investigation [152]. Recently, the Bacillus licheniformis GT, YjiC, was shown to have a broad substrate promiscuity with various monoterpene alcohols and sugar moieties. Moreover, the conversion with menthol and β-D-glucose was over 40% and the glucosylated terpenes displayed 2–4-fold increased antibacterial potency against MRSA and MSSA strains, compared to the aglycone [153]. Interestingly, the cyclized terpene scaffolds generally undergo oxidation by CYPs prior to methylation [154]. However, cyclization is often initiated by methylation of the prenyl chain to afford an active cationic species. While type II TCs are often activated by protonation by a Bronsted acid to a double bond or epoxide, a SAM dependent MT and the TC TleD can bi-functionally catalyze the cyclization of lyngbyatoxin A by activating the double bond via attachment of a methyl group instead of a proton. A similar mechanism is also employed in the case of sodorifen biosynthesis [155].
Isoprenoid Semi-Synthesis
Chemical modifications to isoprenoid scaffolds via semi-synthetic strategies have enhanced the production of critical natural products with potent bioactivities. The semi-synthesis of artemisinin from artemisinic acid serves as one of the earliest such examples. This leveraged engineered yeast for the biosynthesis of artemisinic acid at industrially relevant levels as a viable synthetic starting point that circumvented its otherwise costly and challenging total synthesis [20, 156]. Similar semi-synthetic efforts have been applied to the construction and optimization of de novo biosynthetic pathways to valuable natural products. For example, a novel pathway to entatiseronic acid was recently produced in yeast at high titers (>500 mg/L) which was subsequently transformed via four additional chemical reactions into serofendic acid, a natural neuroprotective molecule with no known natural biosynthetic route [157].
Semi-synthesis strategies have also been utilized to produce a variety of non-natural analogues of isoprenoids to enhance their native bioactivities. For example, semi-synthetic amide analogues of rupestonic acid, a sesquiterpenoid influenza treatment, have been synthesized to yield compounds with comparable activity to Tamiflu against influenza B and enhanced activity against H1N1 and H3N2 [158]. Aside from the combination of biosynthesis and chemical steps, total synthesis efforts have continued to provide methods for accessing isoprenoids and their analogues and to ultimately inspire further synthetic biology advances to match them. For instance, an elegant two-phase synthetic strategy for the anti-cancer agent, Taxol, furnished multigram quantities of the taxane core, demonstrating a simplified synthetic route to this biosynthetically challenging compound (~20 enzymes), particularly as some enzymes within the pathway have not yet been identified. Furthermore, this approach uniquely enables the potential for structure-activity relationship (SAR) studies for the development of potent pharmaceuticals [159]. Other notable semi-synthetic approaches to isoprenoid analogues of yahazunol [160] and strongylophorine 2 [161], derived from sclareol and isocupressic acid, respectively, have yielded compounds that are significantly more potent than their parent molecules. As synthesis continues to expand the isoprenoid chemical space, it is critical to consider how synthetic biology platforms may be able to provide key precursors in a scalable and sustainable manner, or even replace the synthetic steps entirely.
Cell-Free Isoprenoid Biosynthesis
Cell-free biosynthesis (CFB) has recently emerged as a robust platform for the biosynthesis of isoprenoid natural products. Unlike traditional in vivo production platforms, CFB offers a host of unique benefits including the ability to rapidly prototype pathway variations in a controlled setting, decoupled from cellular growth, that enables the scalable and directed biosynthesis of a single product [162] including toxic isoprenoids, such as limonene [163] and α-pinene [164], without negatively impact overall yields or substantial host engineering.
The glycolysis and MVA pathways have been leveraged by CFB to produce a variety of monoterpenoid products, which have dramatically improved product yields and production rates compared to previous approaches. The production of limonene, α-pinene, and sabinene was demonstrated using a purified protein CFB platform in titers >15 g/L from glucose in >95% yield—a 10-fold and 5-fold improvement, respectively, compared to optimized in vivo platforms [165]. More recently, lysate-based CFB platforms have been developed, which eliminates time-consuming protein purification and reduces the need for other workups required prior to use. These lysate systems have been leveraged to enhance the production of limonene [54, 166] and α-pinene [164], via the modulation of cofactors and protein levels, rather than manipulating transcription and translation and the DNA-level. Notably, despite the higher theoretical yields of DMAPP and IPP produced by the DXP pathway, it has not yet been leveraged for cell-free use in the production of isoprenoids, likely due its complexity.
Variants of the ADH pathway have also been developed for CFB. One purified CFB platform utilized choline kinase (CK) and IPK in combination with various prenylelongases and TCs to rapidly produce limonene, amorphadiene, valencene, and taxadiene from isopentenol [167]. Similar to the benefits that ADH pathways afford in vivo, these CFB platforms provide abridged pathways compared to the MVA and DXP pathways with fewer required cofactors, making the optimization of the system simple.
The full utility of CFB platforms can be exploited via complete cell-free protein synthesis (CFPS) whereby DNA is introduced into the system which is transcribed and translated in vitro to effectively mimic in vivo biosynthesis. While offering the rapid prototyping capabilities of CFB [168], CFPS further enables rapid prototyping of multiple protein homologs for rapid characterization without the need for pre-expression. For example, novel in vitro prototyping and rapid optimization of biosynthetic enzymes (iPROBE) strategy enabled by CFPS was utilized to rapidly test 580 unique pathway variants with various enzyme homologs and cofactor conditions to increase limonene production [169]. Following the multiparameter optimizations, the CFB pathway was subsequently used for the production of the biofuels α- and β-pinene as well as bisabolene.
Considering the future of CFB for isoprenoid compounds, a key facet of these systems has often been overlooked: eliminating concerns regarding cell wall permeability [170]. Indeed, the ability to directly introduce non-natural diphosphates into a cell-free reaction to rapidly probe and prototype isoprenoid biosynthesis for promiscuous non-natural activities has not yet been reported. Moreover, this would essentially eliminate competition from the native substrates, which are otherwise available in vivo. These types of critical prototyping strategies could enable the rapid engineering of downstream machinery as well as providing potentially useful platforms for biological structure-activity relationship screening of non-natural isoprenoid analogues.
High-Throughput Isoprenoid Synthetic Biology
Isoprenoids and their analogues are often not biosynthesized at titers that are industrially viable and require additional optimization. However, the complexities of biological systems and the number of enzymatic transformations can often be challenging to optimally re-engineer isoprenoid biosynthesis through rational or semi-rational approaches. As such, metabolic engineering strategies are moving towards top-down approaches, whereby requirements of the system are identified and obtained via combinatorial and directed-evolution strategies [171]. However, high-throughput application of the design-build-test cycle is throttled by the moderate throughput of chromatography based methods (<103) that are often used to screen the variants, thereby creating a testing bottleneck [171]. While high-throughput screening and selection strategies have been successfully leveraged for other classes of natural products [172], these technologies are just emerging within isoprenoid synthetic biology.
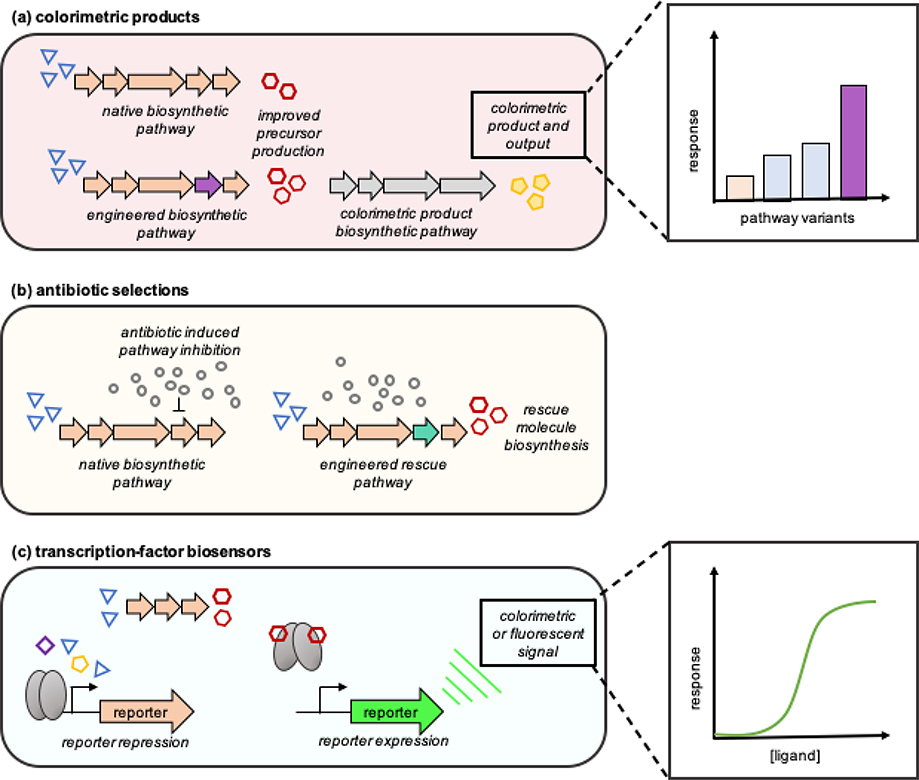
Optimizing precursor levels remains a critical focus of isoprenoid metabolic engineering. However, as the MVA and DXP pathways comprise six or more enzymatic steps, subsequent combinatorial libraries are vast and difficult to screen for optimal DMAPP and IPP production. Colorimetric isoprenoid products (Fig. 11a), such as carotenoids, present a high-throughput opportunity for the qualitative and quantitative analysis of modular precursor engineering strategies [173–175]. Application of these assays have yielded improvements to precursor pathways; however, precursor toxicity is tightly coupled to product formation and improvements in titer may not be transferable to other downstream pathways.
Fig. 11.
High throughput screening platforms for isoprenoids. a Colorimetric product biosynthesis to enhance the production of precursor levels or colorimetric product titers. b Antibiotic (denoted with grey circles) selection markers for optimizing native or bioorthogonal pathways to enable growth. c Transcriptional regulation by a repressor protein prevents the expression of a downstream reporter gene such as green fluorescent protein (GFP). In the presence of the target small molecule, the protein binds the ligand, causing a conformational change in protein structure to yield a quantitative signal. These biosensing platforms have numerous applications related to isoprenoid synthetic biology.
For engineering valuable isoprenoid small-molecules and their analogues, prenyl diphosphates must subsequently undergo intra- or intermolecular prenyltransfer, which universally produces inorganic pyrophosphate. As such, a variety of assays for the rapid detection of pyrophosphate have been developed as surrogate measurements for enzymatic conversion to the desired product. These assays have leveraged a variety of chemical and enzymatic transformations including malachite green [176], 7-methyl-6-thioguanosine (MESG) [177], amplex red [178], and luciferase [179], for the highly sensitive colorimetric, fluorescent, and luminescent quantification of inorganic pyrophosphates. While byproduct assays offer rapid and affordable screening of enzymatic activity, these screening approaches suffer from a number of critical caveats. Background cellular activity often prevents these screens from being leveraged in lysate reactions for high throughput screening of enzyme libraries and are challenging to leverage for large scale screening of enzyme libraries. Moreover, while background hydrolysis can be accounted for through appropriate control reactions, false positives generated via unproductive enzymatic dephosphorylation still represent a potential source of error. Additional methods have also been developed leveraging the cyclization of an oxidized substrate, that upon reacting, releases methanol to interact with Purpald to yield a colorimetric output [107].
Selections can be more efficient than traditional screening approaches as a result of the simultaneous removal of non-active variants from the population [171]. As hemiterpenoids are essential cellular building blocks, inhibition or downregulation of the DXP or MVA pathways negatively affects growth, and thus can be leveraged as a selection for enhancing native pathway activity or engineering non-native pathways (Fig. 11b). In the case of the DXP pathway, a number of inhibitors have been leveraged for high-throughput selections [180]. Fosmidomycin, an antibacterial and antimalarial agent, is a DXR inhibitor [181], and blocks native E. coli DMAPP/IPP biosynthesis. This selectable marker has been utilized for the rapid engineering of a bioorthogonal IPP-bypass pathway to isopentenol with titer improvements ~2.4-fold above the wild-type [182].
Direct-detection of isoprenoid products, such as gas chromatography mass spectroscopy (GC-MS), eliminates concerns related to indirect assays but are often low-throughput and require cumbersome sample preparation. Recent advances in analytical chemistry provide novel opportunities to develop high-throughput screening platforms. For example, liquid handling systems have been leveraged for organic extractions and subsequent assay using GC-QTOF equipped with a 96-well autosampler to screen monoterpene cyclase libraries [144]. In addition, the direct analysis of isoprenoids has been enabled via infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) and secondary electrospray ionization (SESI) [183]. In other studies [184], the high-throughput capabilities of this technology have been demonstrated with other enzymatic reactions and whole-cell samples, suggesting the possibility for the direct detection of isoprenoids from whole-cell reactions with minimal or no workup.
Engineered transcription factor-based biosensors for the detection of isoprenoids are emerging as a useful tool for engineering isoprenoid biosynthesis. These genetic molecular switches operate as activators or repressors of gene expression, whereby the binding of an effector causes a conformational change to the transcription factor protein. This change subsequently promotes or represses the expression of a reporter gene, which transduces the input signal into a qualitative or quantitative output (Fig. 11c). While these systems offer a potentially facile approach to detecting and quantifying the production of a desired isoprenoid or other effector, their selectivity, background-to-noise, and other transfer functions can be challenging to optimize. The complexity of these engineering efforts can be significantly compounded if the biosensor platform does not natively recognize the target small molecule or if a de novo biosensor platform must be constructed.
While no native transcription factors have yet been identified for the detection of DMAPP and IPP, novel biosensor platforms have been developed for enhancing titers of these critical precursors. For example, leveraging AraC, a native E. coli activator of the L-arabinose operon, was engineered for the detection of MVA, a critical precursor to IPP in the MVA pathway. Leveraging this optimized platform to screen a library of >1 million tHMGR RBS variants, the authors reported mutants of the MVA-biosynthetic operon that produced 4-fold higher MVA titers than that of the native pathway [185]. More recently, novel chimeric biosensors were developed wherein well-characterized DNA-binding domains were fused to ligand binding domains from Idi and IspS that recognize DMAPP and IPP to produce fully functional biosensor platforms in E. coli and S. cerevisiae [186, 187]. Unlike assays that measure byproduct formation or production of a colorimetric product, these biosensor platforms may offer significantly more valuable information as these tools detect and report the production of DMAPP and IPP, rather than an enzymatic product that is multiple steps removed from their production with multiple potential bottlenecks.
Notably, some native bacterial transcription factors have been identified to sensitively and selectively detect cyclic monoterpenes, such as cumate and camphor [188, 189]. However, these platforms, as well as other native isoprenoid-detection regulators [190], are often highly specific to oxidized products that do not have particular pharmaceutical or industrial relevance. Similarly, AtuR has been determined to bind acyclic isoprenoids, but has yet to be leveraged for specific biosensing capabilities in situ [191]. Engineering these regulatory proteins by directed evolution and/or rational redesign of the ligand binding domain offer potential sources of designer biosensors for detection of a variety of valuable isoprenoid end-products [192, 193]. Alternatively, non-specific bacterial transcription factors that detect oxidative stress, such as MexR, have been deployed as a means of engineering synthetic feedback loops for the detection of toxic isoprenoids such as α-pinene [194].
Future Outlook and Future Perspectives
The native diversity of the terpenome has yielded an unparalleled abundance of structurally distinct and biologically active small molecules. Yet, the ability to manufacture a given terpene at scale, to tailor the structure of isoprenoids for specific applications, or to access a variety of analogues of a given isoprenoid scaffold remains limited. Databases, such as TeroKit, are also being developed for the identification of the native chemical space as a potential tool to identify isoprenoids suitable as relevant platforms for synthetic biology [195]. Computational tools have also begun to pave the way for facile enzyme engineering for both improved catalytic activity and altered reactivity via machine learning platforms, as well as the identification of novel enzymes with potentially unique activities through genome mining programs. Expanding isoprenoid biosynthesis beyond canonical pathways and host organisms has enabled the potential for further diversification through the use of non-natural precursors. Such “bottom-up” designed pathways are expected to circumvent the stringent or narrow specificity/selectivity of native isoprenoid biosynthesis and to access a variety of isoprenoids that are modified with non-natural chemical functionality, including chemical handles that can be leveraged in downstream modification via semi-synthesis. These engineering efforts are further enhanced by the continued development and deployment of high-throughput screening technologies that offer the ability to rapidly search through large libraries of variants for novel or improved activities. For example, rapidly tailored biosensors that can detect specific terpenes could be leveraged by directed evolution to improve titers of plug-and-play biosynthetic pathways, to increase the catalytic efficiency of terpene cyclases, or to broaden the specificity/selectivity of key biosynthetic enzymes. One vision for the future creates a fully integrated strategy whereby the intersection of organic synthesis, synthetic biology, and directed evolution can be leveraged in tandem for the rapid and selective chemoenzymatic production of designer isoprenoids.
Acknowledgements
Financial support is provided by the National Institutes of Health (award number GM124112) and the Thomas Lord Distinguished Professorship Endowment.
Abbreviations
- ADH
Alcohol-dependent hemiterpene
- CoA
Coenzyme A
- CFB
Cell-free biosynthesis
- CFPS
Cell-free protein synthesis
- CRISPR
Clustered regularly interspaced short palindromic repeats
- CYP
Cytochrome P450
- DKP
Diketopiperazine
- DMAA
Dimethylallyl alcohol
- DMAPP
Dimethylallyl pyrophosphate
- DXP
1-Deoxy-D-xylulose-5-phosphate
- FPP
Farnesyl pyrophosphate
- G3P
Glyceraldehyde-3-phosphate
- GGPP
Geranylgeranyl pyrophosphate
- GPP
Geranyl pyrophosphate
- GT
Glycosyltransferase
- HMG-CoA
3-Hydroxy-3-methyl-glutaryl-CoA
- IPP
Isopentenyl pyrophosphate
- ISO
Isoprenol
- MT
Methyltransferase
- MVA
Mevalonate
- NPP
Neryl pyrophosphate
- PTase
Prenyltransferase
- SAM
(S)-adenosylmethionine
- TC
Terpene cyclase
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Christianson DW (2017) Structural and chemical biology of terpenoid cyclases. Chem. Rev. 117:11570–11648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox-Georgian D, Ramadoss N, Dona C, Basu C (2019) Therapeutic and medicinal uses of terpenes In: Medicinal Plants: From Farm to Pharmacy. Springer International Publishing, pp 333–359 [Google Scholar]
- 3.Tetali SD (2019) Terpenes and isoprenoids: a wealth of compounds for global use. Planta 249:1–8 [DOI] [PubMed] [Google Scholar]
- 4.Harms V, Kirschning A, Dickschat JS (2020) Nature-driven approaches to non-natural terpene analogues. Nat Prod Rep. 10.1039/c9np00055k [DOI] [PubMed] [Google Scholar]
- 5.Brill ZG, Condakes ML, Ting CP, Maimone TJ (2017) Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem Rev 117:11753–11795. 10.1021/acs.chemrev.6b00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jürjens G, Kirschning A, Candito DA (2015) Lessons from the synthetic chemist nature. Nat. Prod. Rep. 32:723–737 [DOI] [PubMed] [Google Scholar]
- 7.Leweke FM, Piomelli D, Pahlisch F, et al. (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94–e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodzki M, Godin B, Rakou L, et al. (2003) Cannabidiol - Transdermal delivery and anti-inflammatory effect in a murine model. J Control Release 93:377–387. 10.1016/j.jconrel.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Cilio MR, Cross H, et al. (2014) Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55:791–802. 10.1111/epi.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanuš LO (2007) Cannabidiol - Recent advances. Chem. Biodivers. 4:1678–1692 [DOI] [PubMed] [Google Scholar]
- 11.Helfrich EJN, Lin G-M, Voigt CA, Clardy J (2019) Bacterial terpene biosynthesis: challenges and opportunities for pathway engineering. Beilstein J Org Chem 15:2889–2906. 10.3762/bjoc.15.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Liwei M, Park J-B Bin, et al. (2018) Microbial platform for terpenoid production: Escherichia coli and yeast. Front Microbiol 9:1–8. 10.3389/fmicb.2018.02460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Tang W, Bidigare RR (2005) Terpenoids as therapeutic drugs and pharmaceutical agents In: Natural Products: Drug Discovery and Therapeutic Medicine. Humana Press, pp 197–227 [Google Scholar]
- 14.Urano E, Ablan SD, Mandt R, et al. (2016) Alkyl amine bevirimat derivatives are potent and broadly active HIV-1 maturation inhibitors. Antimicrob Agents Chemother 60:190–197. 10.1128/AAC.02121-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo CSN, Lam NSK, Yu D, et al. (2017) Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 117:192–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paramasivan K, Mutturi S (2017) Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 37:974–989 [DOI] [PubMed] [Google Scholar]
- 17.Ye L, Lv X, Yu H (2016) Engineering microbes for isoprene production. Metab. Eng. 38:125–138 [DOI] [PubMed] [Google Scholar]
- 18.Volke DC, Rohwer J, Fischer R, Jennewein S (2019) Investigation of the methylerythritol 4-phosphate pathway for microbial terpenoid production through metabolic control analysis. Microb Cell Fact 18:. 10.1186/s12934-019-1235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiba Y, Paradise EM, Kirby J, et al. (2007) Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids. Metab Eng 9:160–168. 10.1016/j.ymben.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 20.Ro DK, Paradise EM, Quellet M, et al. (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943. 10.1038/nature04640 [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan PR, Nawada N, Narayanan AK, Rao DKV (2020) Triglyceride deficiency and diacylglycerol kinase1 activity lead to the upregulation of mevalonate pathway in yeast: A study for the development of potential yeast platform for improved production of triterpenoid. Biochim Biophys Acta - Mol Cell Biol Lipids 1865:158661 10.1016/j.bbalip.2020.158661 [DOI] [PubMed] [Google Scholar]
- 22.Choi BH, Kim JH, Choi SY, et al. (2019) Redesign and reconstruction of a mevalonate pathway and its application in terpene production in Escherichia coli. Bioresour Technol Reports 7:100291 10.1016/j.biteb.2019.100291 [DOI] [Google Scholar]
- 23.Kim SK, Han GH, Seong W, et al. (2016) CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production. Metab Eng 38:228–240. 10.1016/j.ymben.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 24.George KW, Thompson MG, Kim J, et al. (2018) Integrated analysis of isopentenyl pyrophosphate (IPP) toxicity in isoprenoid-producing Escherichia coli. Metab Eng 47:60–72. 10.1016/j.ymben.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Son JH, Kim H, et al. (2019) Mevalonate production from ethanol by direct conversion through acetyl-CoA using recombinant Pseudomonas putida, a novel biocatalyst for terpenoid production. Microb Cell Fact 18:168 10.1186/s12934-019-1213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen AD, Kim D, Lee EY (2020) Unlocking the biosynthesis of sesquiterpenoids from methane via the methylerythritol phosphate pathway in methanotrophic bacteria, using α-humulene as a model compound. Metab Eng 61:69–78. 10.1016/j.ymben.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Lund S, Hall R, Williams GJ (2019) An artificial pathway for isoprenoid biosynthesis decoupled from native hemiterpene metabolism. ACS Synth Biol 8:232–238. 10.1021/acssynbio.8b00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clomburg JM, Qian S, Tan Z, et al. (2019) The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis. Proc Natl Acad Sci U S A 116:12810–12815. 10.1073/pnas.1821004116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatzivasileiou AO, Ward V, Edgar SMB, Stephanopoulos G (2018) Two-step pathway for isoprenoid synthesis. Proc Natl Acad Sci 116:201812935 10.1073/pnas.1812935116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couillaud J, Rico J, Rubini A, et al. (2019) Simplified in vitro and in vivo bioaccess to prenylated compounds. ACS Omega 4:7838–7849. 10.1021/acsomega.9b00561 [DOI] [Google Scholar]
- 31.Rico J, Duquesne K, Petit J-LL, et al. (2019) Exploring natural biodiversity to expand access to microbial terpene synthesis. Microb Cell Fact 18:23 10.1186/s12934-019-1074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Yan Z, Lu X, et al. (2016) Improving the catalytic activity of isopentenyl phosphate kinase through protein coevolution analysis. Sci Rep 6:24117 10.1038/srep24117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund S, Courtney T, Williams GJ (2019) Probing the substrate promiscuity of isopentenyl phosphate kinase as a platform for hemiterpene analogue production. ChemBioChem 20:2217 10.1002/cbic.201900135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson LA, Dunbabin A, Benton JCR, et al. (2020) Modular chemoenzymatic synthesis of terpenes and their analogues. Angew Chemie Int Ed 59:8486–8490. 10.1002/anie.202001744 [DOI] [PubMed] [Google Scholar]
- 35.Drummond L, Kschowak MJ, Breitenbach J, et al. (2019) Expanding the isoprenoid building block repertoire with an IPP methyltransferase from Streptomyces monomycini. ACS Synth Biol 8:1303–1313. 10.1021/acssynbio.8b00525 [DOI] [PubMed] [Google Scholar]
- 36.Eiben CB, De Rond T, Bloszies C, et al. (2019) Mevalonate pathway promiscuity enables noncanonical terpene production. ACS Synth Biol 8:2238–2247. 10.1021/acssynbio.9b00230 [DOI] [PubMed] [Google Scholar]
- 37.Ignea C, Pontini M, Motawia MS, et al. (2018) Synthesis of 11-carbon terpenoids in yeast using protein and metabolic engineering. Nat Chem Biol 14:1090–1098. 10.1038/s41589-018-0166-5 [DOI] [PubMed] [Google Scholar]
- 38.Davisson VJ, Woodside AB, Neal TR, et al. (1986) Phosphorylation of Isoprenoid Alcohols. J Org Chem 51:4768–4779. 10.1021/jo00375a005 [DOI] [Google Scholar]
- 39.Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211 10.15227/orgsyn.066.0211 [DOI] [Google Scholar]
- 40.Gatto N, Vattekkatte A, Kö T, et al. (2015) Isotope sensitive branching and kinetic isotope effects to analyse multiproduct terpenoid synthases from Zea mays. Chem Commun 51:3800 10.1039/c4cc10395e [DOI] [PubMed] [Google Scholar]
- 41.Heaps NA, Poulter CD (2011) Synthesis and evaluation of chlorinated substrate analogues for farnesyl diphosphate synthase. J Org Chem 76:1838–43. 10.1021/jo1024305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyatt DC, Youn B, Zhao Y, et al. (2007) Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc Natl Acad Sci 104:5360–5365. 10.1073/pnas.0700915104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morehouse BR, Kumar RP, Matos JO, et al. (2017) Functional and structural characterization of a (+)-limonene synthase from Citrus sinensis. Biochemistry 56:1706–1715. 10.1021/acs.biochem.7b00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar RP, Morehouse BR, Matos JO, et al. (2017) Structural characterization of early michaelis complexes in the reaction catalyzed by (+)-limonene synthase from Citrus sinensis using fluorinated substrate analogues. Biochemistry 56:1716–1725. 10.1021/acs.biochem.7b00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morehouse BR, Kumar RP, Matos JO, et al. (2019) Direct evidence of an enzymegenerated LPP intermediate in (+)-limonene synthase using a fluorinated GPP substrate analog. ACS Chem Biol 14:2035–2043. 10.1021/acschembio.9b00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oldfield E, Lin FY (2012) Terpene biosynthesis: modularity rules. Angew Chemie - Int Ed 51:1124–1137. 10.1002/anie.201103110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demissie ZA, Erland LAE, Rheault MR, Mahmoud SS (2013) The biosynthetic origin of irregular monoterpenes in Lavandula isolation and biochemical characterization of a novel cis-prenyl diphosphate synthase gene, lavandulyl diphosphate synthase. 10.1074/jbc.M112.431171 [DOI] [PMC free article] [PubMed]
- 48.Ogawa T, Emi K, Koga K, et al. (2016) A cis -prenyltransferase from Methanosarcina acetivorans catalyzes both head-to-tail and nonhead-to-tail prenyl condensation. FEBS J 283:2369–2383. 10.1111/febs.13749 [DOI] [PubMed] [Google Scholar]
- 49.Lee PC, Petri R, Mijts BN, et al. (2005) Directed evolution of Escherichia coli farnesyl diphosphate synthase (IspA) reveals novel structural determinants of chain length specificity. Metab Eng 7:18–26. 10.1016/j.ymben.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 50.Wang K, Ohnuma S ichi (1999) Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution. Trends Biochem. Sci. 24:445–451 [DOI] [PubMed] [Google Scholar]
- 51.Ignea C, Trikka FA, Nikolaidis AK, et al. (2015) Efficient diterpene production in yeast by engineering Erg20p into a geranylgeranyl diphosphate synthase. Metab Eng 27:65–75. 10.1016/j.ymben.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 52.Reiling KK, Yoshikuni Y, Martin VJJ, et al. (2004) Mono and diterpene production in Escherichia coli. Biotechnol Bioeng 87:200–212. 10.1002/bit.20128 [DOI] [PubMed] [Google Scholar]
- 53.Ohnumaf SI, Narita K, Nakazawa T, et al. (1996) A role of the amino acid residue located on the fifth position before the first aspartate-rich motif of farnesyl diphosphate synthase on determination of the final product. J Biol Chem 271:30748–30754. 10.1074/jbc.271.48.30748 [DOI] [PubMed] [Google Scholar]
- 54.Dudley QM, Nash CJ, Jewett MC (2019) Cell-free biosynthesis of limonene using enzyme-enriched Escherichia coli lysates. Synth Biol 4:1–9. 10.1093/synbio/ysz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noike M, Ambo T, Kikuchi S, et al. (2008) Product chain-length determination mechanism of Z,E-farnesyl diphosphate synthase. Biochem Biophys Res Commun 377:17–22. 10.1016/j.bbrc.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, Zhou J, Jang HJ, et al. (2013) Engineered heterologous FPP synthases-mediated Z,E-FPP synthesis in E. coli. Metab Eng 18:53–59. 10.1016/j.ymben.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 57.Koyama T, Saito A, Ogura K, Seto S (1980) Substrate specificity of farnesylpyrophosphate synthetase. Application to asymmetric synthesis. J Am Chem Soc 102:3614–3618. 10.1021/ja00530a050 [DOI] [Google Scholar]
- 58.Qian Q, Schultz AW, Moore BS, Tanner ME (2012) Mechanistic studies on CymD: A tryptophan reverse N-prenyltransferase. Biochemistry 51:7733–7739. 10.1021/bi3009054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luk LYP, Tanner ME (2009) Mechanism of dimethylallyltryptophan synthase: Evidence for a dimethylallyl cation intermediate in an aromatic prenyltransferase reaction. J Am Chem Soc 131:13932–13933. 10.1021/ja906485u [DOI] [PubMed] [Google Scholar]
- 60.Metzger U, Schall C, Zocher G, et al. (2009) The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc Natl Acad Sci U S A 106:14309–14314. 10.1073/pnas.0904897106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steffan N, Grundmann A, Yin W-B, et al. (2008) Indole prenyltransferases from fungi: A new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231. 10.2174/092986709787002772 [DOI] [PubMed] [Google Scholar]
- 62.Haug-Schifferdecker E, Arican D, Brückner R, Heide L (2010) A new group of aromatic prenyltransferases in fungi, catalyzing a 2,7-dihydroxynaphthalene 3-dimethylallyl-transferase reaction. J Biol Chem 285:16487–16494. 10.1074/jbc.M110.113720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kremer A, Li SM (2010) A tyrosine O-prenyltransferase catalyses the first pathway-specific step in the biosynthesis of sirodesmin PL. Microbiology 156:278–286. 10.1099/mic.0.033886-0 [DOI] [PubMed] [Google Scholar]
- 64.Liebhold M, Xie X, Li SM (2012) Expansion of enzymatic Friedel-Crafts alkylation on indoles: Acceptance of unnatural β-unsaturated allyl diphospates by dimethylallyltryptophan synthases. Org Lett 14:4882–4885. 10.1021/ol302207r [DOI] [PubMed] [Google Scholar]
- 65.Liebhold M, Li SM (2013) Regiospecific benzylation of tryptophan and derivatives catalyzed by a fungal dimethylallyl transferase. Org Lett 15:5834–5837. 10.1021/ol4029012 [DOI] [PubMed] [Google Scholar]
- 66.Bandari C, Scull EM, Bavineni T, et al. (2019) FgaPT2, a biocatalytic tool for alkyl-diversification of indole natural products. Medchemcomm 10:1465–1475. 10.1039/c9md00177h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steffan N, Li SM (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466. 10.1007/s00203-009-0467-x [DOI] [PubMed] [Google Scholar]
- 68.Steffan N, Unsöld IA, Li SM (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. ChemBioChem 8:1298–1307. 10.1002/cbic.200700107 [DOI] [PubMed] [Google Scholar]
- 69.Fan A, Xie X, Li SM (2015) Tryptophan prenyltransferases showing higher catalytic activities for Friedel-Crafts alkylation of o- and m-tyrosines than tyrosine prenyltransferases. Org Biomol Chem 13:7551–7557. 10.1039/c5ob01040c [DOI] [PubMed] [Google Scholar]
- 70.Mai P, Zocher G, Ludwig L, et al. (2016) Actions of tryptophan prenyltransferases toward fumiquinazolines and their potential application for the generation of prenylated derivatives by combining chemical and chemoenzymatic syntheses. Adv Synth Catal 358:1639–1653. 10.1002/adsc.201600064 [DOI] [Google Scholar]
- 71.Fan A, Zocher G, Stec E, et al. (2015) Site-directed mutagenesis switching a dimethylallyl tryptophan synthase to a specific tyrosine C3-prenylating enzyme. J Biol Chem 290:1364–1373. 10.1074/jbc.M114.623413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudolf JD, Poulter CD (2013) Tyrosine O -prenyltransferase SirD catalyzes S -, C -, and N -prenylations on tyrosine and tryptophan derivatives. ACS Chem Biol 8:2707–2714. 10.1021/cb400691z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandari C, Scull EM, Masterson JM, et al. (2017) Determination of Alkyl-Donor Promiscuity of Tyrosine- O -Prenyltransferase SirD from Leptosphaeria maculans. ChemBioChem 18:2323–2327. 10.1002/cbic.201700469 [DOI] [PubMed] [Google Scholar]
- 74.Takahashi S, Takagi H, Toyoda A, et al. (2010) Biochemical characterization of a novel indole prenyltransferase from Streptomyces sp. SN-593. J Bacteriol 192:2839–2851. 10.1128/JB.01557-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao G, Mai P, Fan J, et al. (2018) Complete decoration of the indolyl residue in cyclo- l -Trp- l -Trp with geranyl moieties by Using Engineered Dimethylallyl Transferases. Org Lett 20:7201–7205. 10.1021/acs.orglett.8b03124 [DOI] [PubMed] [Google Scholar]
- 76.Chen R, Gao B, Liu X, et al. (2017) Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase. Nat Chem Biol 13:226–234. 10.1038/nchembio.2263 [DOI] [PubMed] [Google Scholar]
- 77.Wollinsky B, Ludwig L, Xie X, Li SM (2012) Breaking the regioselectivity of indole prenyltransferases: Identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270. 10.1039/c2ob26149a [DOI] [PubMed] [Google Scholar]
- 78.Schuller JM, Zocher G, Liebhold M, et al. (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99. 10.1016/j.jmb.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 79.Jost M, Zocher G, Tarcz S, et al. (2010) Structure-function analysis of an enzymatic prenyl transfer reaction identifies a reaction chamber with modifiable specificity. J Am Chem Soc 132:17849–17858. 10.1021/ja106817c [DOI] [PubMed] [Google Scholar]
- 80.Zou HX, Xie XL, Linne U, et al. (2010) Simultaneous C7- and N1-prenylation of cyclo-l-Trp-l-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem 8:3037–3044. 10.1039/c002850a [DOI] [PubMed] [Google Scholar]
- 81.Wunsch C, Zou HX, Linne U, Li SM (2015) C7-prenylation of tryptophanyl and O-prenylation of tyrosyl residues in dipeptides by an Aspergillus terreus prenyltransferase. Appl Microbiol Biotechnol 99:1719–1730. 10.1007/s00253-014-5999-6 [DOI] [PubMed] [Google Scholar]
- 82.Burkhardt I, Ye Z, Janevska S, et al. (2019) Biochemical and mechanistic characterization of the fungal reverse N-1-dimethylallyltryptophan synthase DMATS1Ff. ACS Chem Biol 14:2922–2931. 10.1021/acschembio.9b00828 [DOI] [PubMed] [Google Scholar]
- 83.Kumano T, Richard SB, Noel JP, et al. (2008) Chemoenzymatic syntheses of prenylated aromatic small molecules using Streptomyces prenyltransferases with relaxed substrate specificities. Bioorganic Med Chem 16:8117–8126. 10.1016/j.bmc.2008.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zirpel B, Degenhardt F, Martin C, et al. (2017) Engineering yeasts as platform organisms for cannabinoid biosynthesis. J Biotechnol 259:204–212. 10.1016/j.jbiotec.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 85.Luo X, Reiter MA, d’Espaux L, et al. (2019) Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567:123–126. 10.1038/s41586-019-0978-9 [DOI] [PubMed] [Google Scholar]
- 86.Johnson BP, Scull EM, Dimas DA, et al. (2020) Acceptor substrate determines donor specificity of an aromatic prenyltransferase: expanding the biocatalytic potential of NphB. Appl Microbiol Biotechnol 104:4383–4395. 10.1007/s00253-020-10529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Araya-Cloutier C, Martens B, Schaftenaar G, et al. (2017) Structural basis for non-genuine phenolic acceptor substrate specificity of Streptomyces roseochromogenes prenyltransferase CloQ from the ABBA/PT-barrel superfamily. PLoS One 12:e0174665 10.1371/journal.pone.0174665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozaki T, Mishima S, Nishiyama M, Kuzuyama T (2009) NovQ is a prenyltransferase capable of catalyzing the addition of a dimethylallyl group to both phenylpropanoids and flavonoids. J Antibiot (Tokyo) 62:385–392. 10.1038/ja.2009.48 [DOI] [PubMed] [Google Scholar]
- 89.Melzer M, Heide L (1994) Characterization of polyprenyldiphosphate: 4-hydroxybenzoate polyprenyltransferase from Escherichia coli. Biochim Biophys Acta (BBA)/Lipids Lipid Metab 1212:93–102. 10.1016/0005-2760(94)90193-7 [DOI] [PubMed] [Google Scholar]
- 90.Ohara K, Yamamoto K, Hamamoto M, et al. (2006) Functional characterization of OsPPT1, which encodes P-hydroxybenzoate polyprenyltransferase involved in ubiquinone biosynthesis in Oryza sativa. Plant Cell Physiol 47:581–590. 10.1093/PCP/PCJ025 [DOI] [PubMed] [Google Scholar]
- 91.Marshall SA, Payne KAP, Fisher K, et al. (2019) The UbiX flavin prenyltransferase reaction mechanism resembles class I terpene cyclase chemistry. Nat Commun 10:1–10. 10.1038/s41467-019-10220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chekan JR, McKinnie SMK, Noel JP, Moore BS (2020) Algal neurotoxin biosynthesis repurposes the terpene cyclase structural fold into an N-prenyltransferase. Proc Natl Acad Sci U S A 117:12799–12805. 10.1073/pnas.2001325117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan A, Li SM (2016) Saturation mutagenesis on Arg244 of the tryptophan C4-prenyltransferase FgaPT2 leads to enhanced catalytic ability and different preferences for tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 100:5389–5399. 10.1007/s00253-016-7365-3 [DOI] [PubMed] [Google Scholar]
- 94.Zheng L, Mai P, Fan A, Li SM (2018) Switching a regular tryptophan C4-prenyltransferase to a reverse tryptophan-containing cyclic dipeptide C3prenyltransferase by sequential site-directed mutagenesis. Org Biomol Chem 16:6688–6694. 10.1039/c8ob01735b [DOI] [PubMed] [Google Scholar]
- 95.Mai P, Zocher G, Stehle T, Li SM (2018) Structure-based protein engineering enables prenyl donor switching of a fungal aromatic prenyltransferase. Org Biomol Chem 16:7461–7469. 10.1039/C8OB02037J [DOI] [PubMed] [Google Scholar]
- 96.Mori T, Zhang L, Awakawa T, et al. (2016) Manipulation of prenylation reactions by structure-based engineering of bacterial indolactam prenyltransferases. Nat Commun 7:1–11. 10.1038/ncomms10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Estrada P, Morita M, Hao Y, et al. (2018) A single amino acid switch alters the isoprene donor specificity in ribosomally synthesized and post-translationally modified peptide prenyltransferases. J Am Chem Soc 140:8124–8127. 10.1021/jacs.8b05187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zocher G, Saleh O, Heim JB, et al. (2012) Structure-based engineering increased the catalytic turnover rate of a novel phenazine prenyltransferase. PLoS One 7:e48427 10.1371/journal.pone.0048427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Preciado S, Mendive-Tapia L, Torres-García C, et al. (2013) Synthesis and biological evaluation of a post-synthetically modified Trp-based diketopiperazine. Medchemcomm 4:1171–1174. 10.1039/c3md20353k [DOI] [Google Scholar]
- 100.Hall JA, Seedarala S, Zhao H, et al. (2016) Novobiocin analogues that inhibit the MAPK pathway. J Med Chem 59:925–933. 10.1021/acs.jmedchem.5b01354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galm U, Heller S, Shapiro S, et al. (2004) Antimicrobial and DNA gyrase-inhibitory activities of novel clorobiocin derivatives produced by mutasynthesis. Antimicrob Agents Chemother 48:1307–1312. 10.1128/AAC.48.4.1307-1312.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsai FT, Singh OM, Skarzynski T, et al. (1997) The high-resolution crystal structure of a 24-kDa gyrase B fragment from E. coli complexed with one of the most potent coumarin inhibitors, clorobiocin. Proteins 28:41–52 [PubMed] [Google Scholar]
- 103.Wendt KU, Schulz GE (1998) Isoprenoid biosynthesis: Manifold chemistry catalyzed by similar enzymes. Structure 6:127–133. 10.1016/S0969-2126(98)00015-X [DOI] [PubMed] [Google Scholar]
- 104.Zhang Q, Catti L, Syntrivanis LD, Tiefenbacher K (2019) En route to terpene natural products utilizing supramolecular cyclase mimetics. Nat. Prod. Rep. 36:1619–1627 [DOI] [PubMed] [Google Scholar]
- 105.Abdallah II, Van Merkerk R, Klumpenaar E, Quax WJ (2018) Catalysis of amorpha-4,11-diene synthase unraveled and improved by mutability landscape guided engineering. Sci Rep 8:1–11. 10.1038/s41598-018-28177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan W, Lv S, Chen L, et al. (2019) Production of sesterterpene ophiobolin by a bifunctional terpene synthase in Escherichia coli. Appl Microbiol Biotechnol 103:8785–8797. 10.1007/s00253-019-10103-x [DOI] [PubMed] [Google Scholar]
- 107.Lauchli R, Rabe KS, Kalbarczyk KZ, et al. (2013) High-throughput screening for terpene-synthase-cyclization activity and directed evolution of a terpene synthase. Angew Chemie Int Ed 52:5571–5574. 10.1002/anie.201301362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baer P, Rabe P, Fischer K, et al. (2014) Induced-fit mechanism in class I terpene cyclases. Angew Chemie Int Ed 53:7652–7656. 10.1002/anie.201403648 [DOI] [PubMed] [Google Scholar]
- 109.Major DT, Freud Y, Weitman M (2014) Catalytic control in terpenoid cyclases: Multiscale modeling of thermodynamic, kinetic, and dynamic effects. Curr. Opin. Chem. Biol. 21:25–33 [DOI] [PubMed] [Google Scholar]
- 110.Loizzi M, Miller DJ, Allemann RK (2019) Silent catalytic promiscuity in the high-fidelity terpene cyclase δ-cadinene synthase. Org Biomol Chem 17:1206–1214. 10.1039/c8ob02821d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Felicetti B, Cane DE (2004) Aristolochene synthase: Mechanistic analysis of active site residues by site-directed mutagenesis. J Am Chem Soc 126:7212–7221. 10.1021/ja0499593 [DOI] [PubMed] [Google Scholar]
- 112.Chen M, Chou WKW, Al-Lami N, et al. (2016) Probing the role of active site water in the sesquiterpene cyclization reaction catalyzed by aristolochene synthase. Biochemistry 55:2864–2874. 10.1021/acs.biochem.6b00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aaron JA, Lin X, Cane DE, Christianson DW (2010) Structure of epi-isozizaene synthase from streptomyces coelicolor A3(2), a platform for new terpenoid cyclization templates. Biochemistry 49:1787–1797. 10.1021/bi902088z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blank PN, Barrow GH, Christianson DW (2019) Crystal structure of F95Q epi-isozizaene synthase, an engineered sesquiterpene cyclase that generates biofuel precursors β- and γ-curcumene. J Struct Biol 207:218–224. 10.1016/j.jsb.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li R, Chou WKW, Himmelberger JA, et al. (2014) Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 53:1155–1168. 10.1021/bi401643u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vedula LS, Jiang J, Zakharian T, et al. (2008) Structural and mechanistic analysis of trichodiene synthase using site-directed mutagenesis: Probing the catalytic function of tyrosine-295 and the asparagine-225/serine-229/glutamate-233-Mg2 +B motif. Arch Biochem Biophys 469:184–194. 10.1016/j.abb.2007.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dixit M, Weitman M, Gao J, Major DT (2017) Chemical control in the battle against fidelity in promiscuous natural product biosynthesis: The case of trichodiene synthase. ACS Catal 7:812–818. 10.1021/acscatal.6b02584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karuppiah V, Ranaghan KE, Leferink NGH, et al. (2017) Structural basis of catalysis in the bacterial monoterpene synthases linalool synthase and 1,8-cineole synthase. ACS Catal 7:6268–6282. 10.1021/acscatal.7b01924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leferink NGH, Ranaghan KE, Battye J, et al. (2020) Taming the reactivity of monoterpene synthases to guide regioselective product hydroxylation. ChemBioChem 21:985–990. 10.1002/cbic.201900672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ker DS, Chan KG, Othman R, et al. (2020) Site-directed mutagenesis of β sesquiphellandrene synthase enhances enzyme promiscuity. Phytochemistry 173:112286 10.1016/j.phytochem.2020.112286 [DOI] [PubMed] [Google Scholar]
- 121.Steele CL, Crock J, Bohlmann J, Croteau R (1998) Sesquiterpene synthases from grand fir (Abies grandis): Comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J Biol Chem 273:2078–2089. 10.1074/jbc.273.4.2078 [DOI] [PubMed] [Google Scholar]
- 122.Little DB, Croteau RB (2002) Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases δ-selinene synthase and γ-humulene synthase. Arch Biochem Biophys 402:120–135. 10.1016/S00039861(02)00068-1 [DOI] [PubMed] [Google Scholar]
- 123.Yoshikuni Y, Ferrin TE, Keasling JD (2006) Designed divergent evolution of enzyme function. Nature 440:1078–1082. 10.1038/nature04607 [DOI] [PubMed] [Google Scholar]
- 124.Pazouki L, Niinemetst U (2016) Multi-substrate terpene synthases: Their occurrence and physiological significance. Front Plant Sci 7:1019 10.3389/fpls.2016.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hendrikse NM, Charpentier G, Nordling E, Syrén P (2018) Ancestral diterpene cyclases show increased thermostability and substrate acceptance. FEBS J 285:4660–4673. 10.1111/febs.14686 [DOI] [PubMed] [Google Scholar]
- 126.Zhang M, Liu J, Li K, Yu D (2013) Identification and characterization of a novel monoterpene synthase from soybean restricted to neryl diphosphate precursor. PLoS One 8:e75972 10.1371/journal.pone.0075972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schilmiller AL, Schauvinhold I, Larson M, et al. (2009) Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci U S A 106:10865–10870. 10.1073/pnas.0904113106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ignea C, Raadam MH, Motawia MS, et al. (2019) Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate. Nat Commun 10:1–15. 10.1038/s41467-019-11290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aguilar F, Hartwig S, Scheper T, Beutel S (2019) Catalytical specificity, reaction mechanisms, and conformational changes during catalysis of the recombinant SUMO (+)-zizaene synthase from Chrysopogon zizanioides. ACS Omega 4:6199–6209. 10.1021/acsomega.9b00242 [DOI] [Google Scholar]
- 130.Vattekkatte A, Garms S, Boland W (2017) Alternate cyclization cascade initiated by substrate isomer in multiproduct terpene synthase from Medicago truncatula. J Org Chem 82:2855–2861. 10.1021/acs.joc.6b02696 [DOI] [PubMed] [Google Scholar]
- 131.Miller DJ, Yu F, Knight DW, Allemann RK (2009) 6- and 14-Fluoro farnesyl diphosphate: mechanistic probes for the reaction catalysed by aristolochene synthase. Org Biomol Chem 7:962 10.1039/b817194g [DOI] [PubMed] [Google Scholar]
- 132.Rising KA, Crenshaw CM, Koo HJ, et al. (2015) Formation of a novel macrocyclic alkaloid from the unnatural farnesyl diphosphate analogue anilinogeranyl diphosphate by 5-epi-aristolochene synthase. ACS Chem Biol 10:1729–1736. 10.1021/acschembio.5b00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Faraldos JA, Miller DJ, González V, et al. (2012) A 1,6-ring closure mechanism for (+)-δ-cadinene synthase? J Am Chem Soc 134:5900–5908. 10.1021/ja211820p [DOI] [PubMed] [Google Scholar]
- 134.Touchet S, Chamberlain K, Woodcock CM, et al. (2015) Novel olfactory ligands via terpene synthases. Chem Commun 51:7550–7553. 10.1039/c5cc01814e [DOI] [PubMed] [Google Scholar]
- 135.Köksal M, Chou WKW, Cane DE, Christianson DW (2013) Unexpected reactivity of 2-fluorolinalyl diphosphate in the active site of crystalline 2-methylisoborneol synthase. Biochemistry 52:5247–5255. 10.1021/bi400797c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huynh F, Grundy DJ, Jenkins RL, et al. (2018) Sesquiterpene synthase-catalysed formation of a new medium-sized cyclic terpenoid ether from farnesyl diphosphate analogues. ChemBioChem 19:1834–1838. 10.1002/cbic.201800218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Demiray M, Tang X, Wirth T, et al. (2017) An efficient chemoenzymatic synthesis of dihydroartemisinic aldehyde. Angew Chemie Int Ed 56:4347–4350. 10.1002/anie.201609557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seitz M, Syrén P-O, Steiner L, et al. (2013) Synthesis of heterocyclic terpenoids by promiscuous squalene-hopene cyclases. ChemBioChem 14:436–439. 10.1002/cbic.201300018 [DOI] [PubMed] [Google Scholar]
- 139.Hammer SC, Syrén PO, Seitz M, et al. (2013) Squalene hopene cyclases: Highly promiscuous and evolvable catalysts for stereoselective CC and CX bond formation. Curr Opin Chem Biol 17:293–300. 10.1016/j.cbpa.2013.01.016 [DOI] [PubMed] [Google Scholar]
- 140.Chang MCY, Eachus RA, Trieu W, et al. (2007) Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat Chem Biol 3:274–277. 10.1038/nchembio875 [DOI] [PubMed] [Google Scholar]
- 141.Huang JH, Lv JM, Wang QZ, et al. (2019) Biosynthesis of an anti-tuberculosis sesterterpenoid asperterpenoid A. Org Biomol Chem 17:248–251. 10.1039/c8ob02832j [DOI] [PubMed] [Google Scholar]
- 142.Yee DA, Kakule TB, Cheng W, et al. (2020) Genome mining of alkaloidal terpenoids from a hybrid terpene and nonribosomal peptide biosynthetic pathway. J Am Chem Soc 142:710–714. 10.1021/jacs.9b13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hernandez-Ortega A, Vinaixa M, Zebec Z, et al. (2018) A Toolbox for Diverse Oxyfunctionalisation of Monoterpenes OPEN. 8:14396 10.1038/s41598-018-32816-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leferink NGH, Dunstan MS, Hollywood KA, et al. (2019) An automated pipeline for the screening of diverse monoterpene synthase libraries. Sci Rep 9:. 10.1038/s41598-019-48452-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wong J, de Rond T, d’Espaux L, et al. (2018) High-titer production of lathyrane diterpenoids from sugar by engineered Saccharomyces cerevisiae. Metab Eng 45:142–148. 10.1016/j.ymben.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 146.Sarrade-Loucheur A, Ro D-K, Régis R, et al. (2020) Synthetic derivatives of (+)-epi-α-bisabolol are formed by mammalian cytochromes P450 expressed in a yeast reconstituted pathway. ACS Synth Biol 2020:380 10.1021/acssynbio.9b00399 [DOI] [PubMed] [Google Scholar]
- 147.Tsutsumi H, Katsuyama Y, Izumikawa M, et al. (2018) Unprecedented cyclization catalyzed by a cytochrome P450 in benzastatin biosynthesis. J Am Chem Soc 140:6631–6639. 10.1021/jacs.8b02769 [DOI] [PubMed] [Google Scholar]
- 148.Reymond J, Wahler D (2002) Substrate arrays as enzyme fingerprinting tools. ChemBioChem 3:701–708. [DOI] [PubMed] [Google Scholar]
- 149.Fessner ND (2019) P450 monooxygenases enable rapid late‐stage diversification of natural products via C−H bond activation. ChemCatChem 11:2226–2242. 10.1002/cctc.201801829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang K, El Damaty S, Fasan R (2011) P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J Am Chem Soc 133:3242–3245. 10.1021/ja109590h [DOI] [PubMed] [Google Scholar]
- 151.Zhang K, Shafer BM, Demars MD, et al. (2012) Controlled oxidation of remote sp 3 C−H bonds in artemisinin via P450 catalysts with fine-tuned regio-and stereoselectivity. 134:18695 10.1021/ja3073462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rivas F, Parra A, Martinez A, Garcia-Granados A (2013) Enzymatic glycosylation of terpenoids. Phytochem Rev 12:327–339. 10.1007/s11101-013-9301-9 [DOI] [Google Scholar]
- 153.Bashyal P, Pandey RP, Samir ∥, et al. (2019) Biocatalytic synthesis of non-natural monoterpene O-glycosides exhibiting superior antibacterial and antinematodal properties. ACS Omega 4:9367 10.1021/acsomega.9b00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hou A, Lauterbach L, Dickschat JS (2020) Enzymatic synthesis of methylated terpene analogues using the plasticity of bacterial terpene synthases. Chem – A Eur J 26:2178–2182. 10.1002/chem.201905827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rudolf JD, Chang CY (2020) Terpene synthases in disguise: Enzymology, structure, and opportunities of non-canonical terpene synthases. Nat Prod Rep 37:425–463. 10.1039/c9np00051h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355 10.1038/nrmicro3240 [DOI] [PubMed] [Google Scholar]
- 157.Hsu SY, Perusse D, Hougard T, Smanski MJ (2019) Semisynthesis of the neuroprotective metabolite, serofendic acid. ACS Synth Biol 8:2397–2403. 10.1021/acssynbio.9b00261 [DOI] [PubMed] [Google Scholar]
- 158.Li G, Obul M, Zhao J yu, et al. (2019) Novel amides modified rupestonic acid derivatives as anti-influenza virus reagents. Bioorganic Med Chem Lett 29:126605 10.1016/j.bmcl.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 159.Kanda Y, Nakamura H, Umemiya S, et al. (2020) Two-phase synthesis of Taxol®. ChemRxiv 27–31. 10.26434/CHEMRXIV.12061620.V1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang S, Wang X, Hao J, et al. (2018) Expediently scalable synthesis and antifungal exploration of (+)-yahazunol and related meroterpenoids. J Nat Prod 81:2010–2017. 10.1021/acs.jnatprod.8b00310 [DOI] [PubMed] [Google Scholar]
- 161.Yu W, Hjerrild P, Overgaard J, Poulsen TB (2016) A concise route to the strongylophorines. Angew Chemie Int Ed 55:8294–8298. 10.1002/anie.201602476 [DOI] [PubMed] [Google Scholar]
- 162.Karim AS, Jewett MC (2018) Cell-free synthetic biology for pathway prototyping. Methods Enzymol 608:31–57. 10.1016/bs.mie.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 163.Chubukov V, Mingardon F, Schackwitz W, et al. (2015) Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl Environ Microbiol 81:4690–4696. 10.1128/AEM.01102-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Niu FX, Huang Y Bin, Shen YP, et al. (2020) Enhanced production of pinene by using a cell-free system with modular cocatalysis. J Agric Food Chem 68:2139–2145. 10.1021/acs.jafc.9b07830 [DOI] [PubMed] [Google Scholar]
- 165.Korman TP, Opgenorth PH, Bowie JU (2017) A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat Commun 8:15526 10.1038/ncomms15526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dudley QM, Anderson KC, Jewett MC (2016) Cell-Free mixing of Escherichia coli crude extracts to prototype and rationally engineer high-titer mevalonate synthesis. ACS Synth Biol 5:1578–1588. 10.1021/acssynbio.6b00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ward VCA, Chatzivasileiou AO, Stephanopoulos G (2019) Cell free biosynthesis of isoprenoids from isopentenol. Biotechnol Bioeng 116:3269–3281. 10.1002/bit.27146 [DOI] [PubMed] [Google Scholar]
- 168.Liu WQ, Zhang L, Chen M, Li J (2019) Cell-free protein synthesis: Recent advances in bacterial extract sources and expanded applications. Biochem Eng J 141:182–189. 10.1016/j.bej.2018.10.023 [DOI] [Google Scholar]
- 169.Dudley QM, Karim AS, Nash CJ, Jewett MC (2020) Cell-free prototyping of limonene biosynthesis using cell-free protein synthesis. Metab Eng. 10.1016/j.ymben.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 170.Khambhati K, Bhattacharjee G, Gohil N, et al. (2019) Exploring the potential of cell-free protein synthesis for extending the abilities of biological systems. Front. Bioeng. Biotechnol. 7:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rogers JK, Taylor ND, Church GM (2016) Biosensor-based engineering of biosynthetic pathways. Curr Opin Biotechnol 42:84–91. 10.1016/J.COPBIO.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 172.Zeng W, Guo L, Xu S, et al. (2020) High-throughput screening technology in industrial biotechnology. Trends Biotechnol. [DOI] [PubMed] [Google Scholar]
- 173.Li C, Swofford CA, Sinskey AJ (2020) Modular engineering for microbial production of carotenoids. Metab Eng Commun 10:e00118 10.1016/j.mec.2019.e00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Furubayashi M, Ikezumi M, Kajiwara J, et al. (2014) A high-throughput colorimetric screening assay for terpene synthase activity based on substrate consumption. PLoS One 9:e93317 10.1371/journal.pone.0093317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmidt-Dannert C, Umeno D, Arnold FH (2000) Molecular breeding of carotenoid biosynthetic pathways. Nat Biotechnol 18:750–753. 10.1038/77319 [DOI] [PubMed] [Google Scholar]
- 176.Vardakou M, Salmon M, Faraldos JA, O’Maille PE (2014) Comparative analysis and validation of the malachite green assay for the high throughput biochemical characterization of terpene synthases. MethodsX 1:e187–e196. 10.1016/j.mex.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barja MV, Rodríguez-Concepción M (2020) A simple in vitro assay to measure the activity of geranylgeranyl diphosphate synthase and other short-chain prenyltransferases. Methods Mol Biol 2083:27–38. 10.1007/978-1-4939-9952-1_2 [DOI] [PubMed] [Google Scholar]
- 178.Vazquez MJ, Rodriguez B, Zapatero C, Tew DG (2003) Determination of phosphate in nanomolar range by an enzyme-coupling fluorescent method. Anal Biochem 320:292–298. 10.1016/S0003-2697(03)00400-7 [DOI] [PubMed] [Google Scholar]
- 179.Helenius M, Jalkanen S, Yegutkin GG (2012) Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta - Mol Cell Res 1823:1967–1975. 10.1016/j.bbamcr.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 180.Testa CA, Johnson LJ (2012) A whole-cell phenotypic screening platform for identifying methylerythritol phosphate pathway-selective inhibitors as novel antibacterial agents. Antimicrob Agents Chemother 56:4906–4913. 10.1128/AAC.00987-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang B, Watts KM, Hodge D, et al. (2011) A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50:3570–3577. 10.1021/bi200113y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kang A, Meadows CW, Canu N, et al. (2017) High-throughput enzyme screening platform for the IPP-bypass mevalonate pathway for isopentenol production. Metab Eng 41:125–134. 10.1016/J.YMBEN.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 183.Nazari M, Malico AA, Ekelöf M, et al. (2017) Direct analysis of terpenes from biological buffer systems using SESI and IR-MALDESI. Anal Bioanal Chem 1–10. 10.1007/s00216-017-0570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nazari M, Ekelöf M, Khodjaniyazova S, et al. (2017) Direct screening of enzyme activity using infrared matrix-assisted laser desorption electrospray ionization. Rapid Commun Mass Spectrom 31:1868–1874. 10.1002/rcm.7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tang SY, Cirino PC (2011) Design and application of a mevalonate-responsive regulatory protein. Angew Chemie - Int Ed 50:1084–1086. 10.1002/anie.201006083 [DOI] [PubMed] [Google Scholar]
- 186.Liu C-L, Cai J-Y, Bi H-R, Tan T-W (2018) A novel DMAPP-responding genetic circuit sensor for high-throughput screening and evolving isoprene synthase. Appl Microbiol Biotechnol 102:1381–1391. 10.1007/s00253-017-8676-8 [DOI] [PubMed] [Google Scholar]
- 187.Chou HH, Keasling JD (2013) Programming adaptive control to evolve increased metabolite production. Nat Commun 4:2595 10.1038/ncomms3595 [DOI] [PubMed] [Google Scholar]
- 188.Choi YJ, Morel L, Le François T, et al. (2010) Novel, versatile, and tightly regulated expression system for Escherichia coli strains. Appl Environ Microbiol 76:5058–66. 10.1128/AEM.00413-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aramaki H, Kabata H, Takeda S, et al. (2011) Formation of repressor-inducer-operator ternary complex: negative cooperativity of d-camphor binding to CamR. Genes to Cells 16:1200–1207. 10.1111/j.1365-2443.2011.01563.x [DOI] [PubMed] [Google Scholar]
- 190.Zhu D, Wang Y, Zhang M, et al. (2013) Product-mediated regulation of pentalenolactone biosynthesis in Streptomyces species by the marR/SlyA Family Activators PenR and PntR. J Bacteriol 195:1255–1266. 10.1128/JB.02079-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Förster-Fromme K, Jendrossek D (2010) AtuR is a repressor of acyclic terpene utilization (Atu) gene cluster expression and specifically binds to two 13 bp inverted repeat sequences of the atuA-atuR intergenic region. FEMS Microbiol Lett 308:166–174. 10.1111/j.1574-6968.2010.02005.x [DOI] [PubMed] [Google Scholar]
- 192.Kasey C, Zerrad M, Li Y, et al. (2018) Development of transcription factor-based designer macrolide biosensors for metabolic engineering and synthetic biology. ACS Synth Biol 7:227–239. 10.1021/acssynbio.7b00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meyer AJ, Segall-Shapiro TH, Glassey E, et al. (2019) Escherichia coli “Marionette” strains with 12 highly optimized small-molecule sensors. Nat Chem Biol 15:196–204. 10.1038/s41589-018-0168-3 [DOI] [PubMed] [Google Scholar]
- 194.Siu Y, Fenno J, Lindle JM, Dunlop MJ (2018) Design and Selection of a Synthetic Feedback Loop for Optimizing Biofuel Tolerance. ACS Synth Biol 7:16–23. 10.1021/acssynbio.7b00260 [DOI] [PubMed] [Google Scholar]
- 195.Zeng T, Liu Z, Zhuang J, et al. (2020) TeroKit: A Database-Driven Web Server for Terpenome Research. J Chem Inf Model 60:2082–2090. 10.1021/acs.jcim.0c00141 [DOI] [PubMed] [Google Scholar]