Thrombopoiesis involves a sequence of complex cellular events in mature bone marrow megakaryocytes, culminating in the generation of proplatelet extensions that release platelets into circulation (1). ETV6 is a member of the ETS family of transcription factors, indispensable for bone marrow hematopoiesis and required for normal megakaryopoiesis. ETV6 encodes the E26 Transformation-Specific (Ets) family transcription repressor and tumor suppressor variant 6. There are 27 members of the Ets family of transcription factors, representing a vast network of inter and intra-molecular interactions to achieve combinatorial regulation of gene expression (2). ETV6 is a canonical member of the Ets family, containing an 85 amino acid ETS DNA binding domain at the C terminal end of the protein, an N terminal PNT (pointed) dimerization domain, and a central linker domain (3). ETV6 maps to chromosome 12p13 and transcribes a 57-kDa protein with these three functional domains. All Ets transcription factors bind to the highly conserved 5’GGA(A/T)3’ motif in the promoter region of target genes (4). While monomeric ETV6 is sterically hindered from the DNA-binding interface, dimerization through the PNT domain facilitates cooperative DNA binding and transcriptional regulatory activity (5). ETV6 has been reported to bind to co-repressors such as HDAC3, NCOR, and Sin3A, forming a multi-protein transcriptional complex that regulates histone acetylation and chromatin condensation at target promoters, thereby influencing gene expression (6, 7).
ETV6 has classically been described as a key transcriptional regulator of hematopoiesis. Global deletion of Etv6 in murine models results in embryonic lethality between E10.5 and E11.5 with yolk sac angiogenic defects (8, 9). Conditional knockout of Etv6 in megakaryocyte erythroid progenitor cells results in mice that are thrombocytopenic (8, 10) with an increased frequency of megakaryocyte colony forming cells. These findings are consistent with a terminal defect in megakaryocyte maturation, and a compensatory increase in megakaryocyte progenitor cells. Etv6 controls the survival of hematopoietic stem cells (8) and is required late in the development of megakaryocytes, where it may be acting in concert with other transcriptional regulators of megakaryopoiesis to bind megakaryocyte specific promoters. ETV6 is typically described as a transcriptional repressor (11), but only a few targets of ETV6 have been reported (12, 13). Interestingly, the genes that encode for megakaryocyte/platelet glycoprotein 1bα (GP1BA) and glycoprotein IX (GP9) are among those transcriptional targets of ETV6 (14). Additionally, it has been reported that ETV6 can interact with another platelet and megakaryocyte specific Ets transcription factor, FLI1, inhibiting its transcriptional activity (14). FLI1, like ETV6, has been implicated in the heritable thrombocytopenia Paris-Trousseau syndrome. (15).
Recently, it has been reported that autosomal dominant variants in ETV6 lead to mild thrombocytopenia with bleeding diathesis, red cell macrocytosis, and predisposition to hematologic malignancies (30.2% risk overall), with B-cell acute lymphoblastic leukemia (B-ALL) being the most common (16–19). The mechanisms responsible for thrombocytopenia and propensity for bleeding in patients with ETV6 variants remain unknown. Missense mutations in the central domain and the ETS DNA binding domain of ETV6 result in aberrant subcellular localization, decreased transcriptional repression, and impaired megakaryocyte maturation (16, 17). Several families carrying these germline mutations have been described, with the overwhelming majority demonstrating heterozygous single-nucleotide changes in the ETS DNA binding domain (19). Five families have been identified with a heterozygous single-nucleotide modification in the central domain of ETV6, c.641C>T, encoding a p.Pro214Leu substitution (19). Deletions have also been described, which have been shown to result in protein truncation as a consequence of alternate splicing (20, 21). The bone marrow of these affected individuals shows erythroid dysplasia and hyperplasia of small, hypolobulated, immature megakaryocytes, suggesting incomplete differentiation and inability to release platelets into circulation (16).
The discovery of these mutations led to additional larger studies that demonstrated a 4.5% prevalence of ETV6 germline mutations in families with known inherited thrombocytopenia (20), confirming the near-complete penetrance of low platelet counts in these families, though some carriers with normal platelet counts have been reported (22). In all patients with thrombocytopenia, 2.6% are estimated to carry ETV6 variants (20). Clinically, the thrombocytopenia is typically mild, with platelet counts >75 × 109/L, and mild bleeding symptoms reported, including epistaxis, mouth bleeding, easy bruising, and menorrhagia (23). A small subset of these patients have large platelets, with the majority of patients exhibiting normal platelet size (23). Complete blood counts in these patients typically demonstrate normal white blood cell counts and hemoglobin concentrations (19). While some patients do not experience bleeding diathesis, others bleed out of proportion to their mildly decreased platelet counts. A portion of patients have abnormal platelet aggregation despite no major difference in platelet membrane receptor distribution suggesting a functional platelet deficit (18). It does not appear to be a correlation between the location of ETV6 mutations and platelet dysfunction, but further studies are needed to confirm this observation.
Because bleeding is mild in the majority of these patients, observation is usually sufficient; however, antifibrinolytics or desmopressin may be considered in cases where bleeding is significant. Beyond preventing excessive bleeding in these patients, management includes identifying related carriers and considering surveillance for the development of hematologic malignancies. Genetic counseling remains a cornerstone for patients with germline ETV6 variants, as family members may carry the same ETV6 variant and are at risk for developing malignancies, or could also be considered as donors for hematopoietic stem cell transplantation in cases of relatives that progress to myelodysplasia or leukemia (19).
Functional studies suggest that decreased ETV6 function leads to megakaryocyte maturation arrest, impaired platelet production, and differentially expressed platelet transcripts among individuals affected with ETV6 mutations when compared to control relatives (16). Furthermore, recent studies describe decreased ability of platelets from individuals with ETV6 mutations to spread on fibrinogen covered surfaces (20) and abnormal clot retraction, suggesting a platelet outside-in signaling defect in these patients (18). Finally, megakaryocytes derived from patients expressing ETV6 variants are smaller and form fewer proplatelets (16). Interestingly, platelet RNA-seq analyses of patients with a mutation in the central domain (ETV6 P214L) show significant decrease of transcripts involved in megakaryocyte and platelet pathways, underscoring the role of ETV6 in megakaryopoiesis, thrombopoiesis and platelet function (Figure 1) (16).
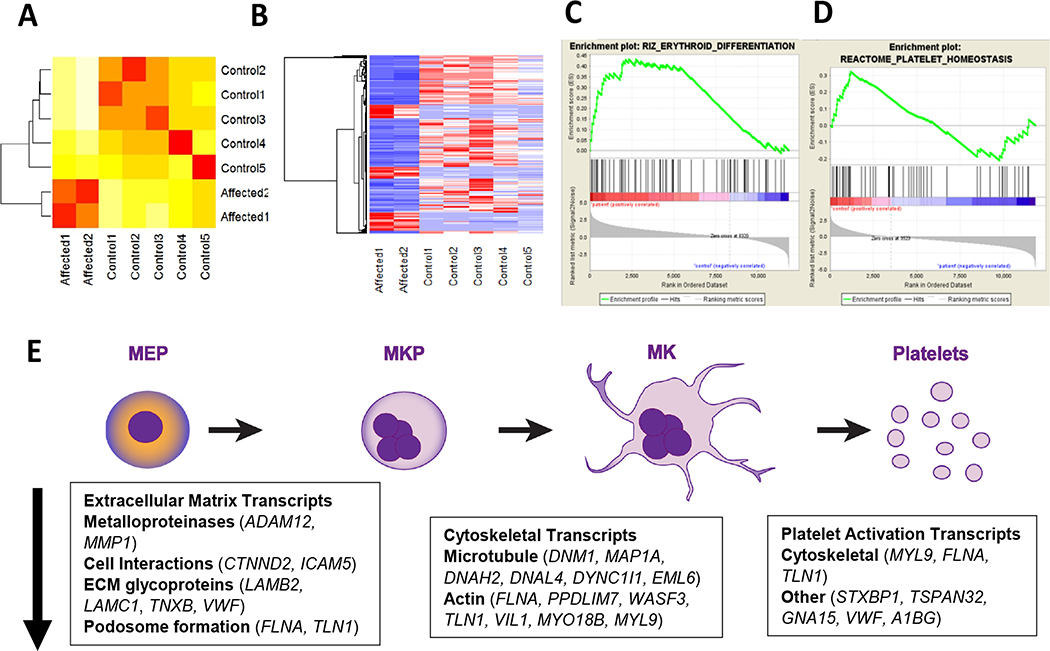
Figure. Transcriptional changes induced by mutant ETV6, overall decreased megakaryocyte and platelet transcripts.
A) RNA clustering among affected individuals with the ETV6 p.P214L mutation compared to unaffected relatives. B) differentially expressed transcripts between affected and unaffected relatives. Gene Set Enrichment Analysis of differentially expressed transcripts in p.P214L platelets compared to control C) erythroid transcripts enriched in patients D) platelet transcripts enriched in controls. E) Genes significantly associated with gene ontology terms. All the genes and pathways in boxes are downregulated in patients with the ETV6 p.P214L mutation when compared to related controls and are overlapped to the megakaryocyte erythroid progenitor to megakaryocyte/platelet pathway as potential sites of action.
The clinical relevance of ETV6 has been established by the discovery of germline mutations resulting in thrombocytopenia, bleeding diathesis, and megakaryocyte abnormalities. However, the disease mechanisms and overall biology of ETV6 are incompletely understood. Further research and better understanding of ETV6 is needed to define the function of ETV6 in hematopoiesis, shedding light on its master role in megakaryocyte development, platelet production, and platelet function.
Acknowledgments
This work was supported by R01 HL120728 and R01 HL139825 (JDP), T32GM008497 and 5T32GM008730-19 (MHF).
Footnotes
Declaration of Interests
The authors have no conflicts to declare.
References
- 1.Machlus KR, Italiano JE, Jr., The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–96. Epub 2013/06/12. doi: 10.1083/jcb.201304054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2(11):827–37. Epub 2001/11/21. doi: 10.1038/35099076 [DOI] [PubMed] [Google Scholar]
- 3.Bohlander SK. ETV6: a versatile player in leukemogenesis. Seminars in cancer biology. 2005;15(3):162–74. doi: 10.1016/j.semcancer.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 4.Poirel H, Oury C, Carron C, Duprez E, Laabi Y, Tsapis A, Romana SP, Mauchauffe M, Le Coniat M, Berger R, Ghysdael J, Bernard OA. The TEL gene products: nuclear phosphoproteins with DNA binding properties. Oncogene. 1997;14(3):349–57. doi: 10.1038/sj.onc.1200829 [DOI] [PubMed] [Google Scholar]
- 5.Green SM, Coyne HJ, 3rd,, McIntosh LP, Graves BJ. DNA binding by the ETS protein TEL (ETV6) is regulated by autoinhibition and self-association. J Biol Chem. 2010;285(24):18496–504. Epub 2010/04/20. doi: 10.1074/jbc.M109.096958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Hiebert SW. TEL contacts multiple co-repressors and specifically associates with histone deacetylase-3. Oncogene. 2001;20(28):3716–25. doi: 10.1038/sj.onc.1204479 [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti SR, Nucifora G. The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSin3A. Biochemical and biophysical research communications. 1999;264(3):871–7. doi: 10.1006/bbrc.1999.1605 [DOI] [PubMed] [Google Scholar]
- 8.Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18(19):2336–41. Epub 2004/09/17. doi: 10.1101/gad.1239604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. The EMBO journal. 1997;16(14):4374–83. doi: 10.1093/emboj/16.14.4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LC, Swat W, Fujiwara Y, Davidson L, Visvader J, Kuo F, Alt FW, Gilliland DG, Golub TR, Orkin SH. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes & development. 1998;12(15):2392–402. doi: 10.1101/gad.12.15.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez RG, Carron C, Oury C, Gardellin P, Bernard O, Ghysdael J. TEL is a sequence-specific transcriptional repressor. The Journal of biological chemistry. 1999;274(42):30132–8. doi: 10.1074/jbc.274.42.30132 [DOI] [PubMed] [Google Scholar]
- 12.Boily G, Larose J, Langlois S, Sinnett D. Identification of transcripts modulated by ETV6 expression. British journal of haematology. 2007;136(1):48–62. Epub 2006/10/27. doi: 10.1111/j.1365-2141.2006.06377.x [DOI] [PubMed] [Google Scholar]
- 13.Fenrick R, Wang L, Nip J, Amann JM, Rooney RJ, Walker-Daniels J, Crawford HC, Hulboy DL, Kinch MS, Matrisian LM, Hiebert SW. TEL, a putative tumor suppressor, modulates cell growth and cell morphology of ras-transformed cells while repressing the transcription of stromelysin-1. Molecular and cellular biology. 2000;20(16):5828–39. doi: 10.1128/mcb.20.16.5828-5839.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski BA, Bastian LS, Bauer TR, Jr.,, Tsai S, Zielinska-Kwiatkowska AG, Hickstein DD. The ets family member Tel binds to the Fli-1 oncoprotein and inhibits its transcriptional activity. The Journal of biological chemistry. 1998;273(28):17525–30. doi: 10.1074/jbc.273.28.17525 [DOI] [PubMed] [Google Scholar]
- 15.Stevenson WS, Rabbolini DJ, Beutler L, Chen Q, Gabrielli S, Mackay JP, Brighton TA, Ward CM, Morel-Kopp MC. Paris-Trousseau thrombocytopenia is phenocopied by the autosomal recessive inheritance of a DNA-binding domain mutation in FLI1. Blood. 2015;126(17):2027–30. Epub 2015/09/01. doi: 10.1182/blood-2015-06-650887 [DOI] [PubMed] [Google Scholar]
- 16.Noetzli L, Lo RW, Lee-Sherick AB, Callaghan M, Noris P, Savoia A, Rajpurkar M, Jones K, Gowan K, Balduini C, Pecci A, Gnan C, De Rocco D, Doubek M, Li L, Lu L, Leung R, Landolt-Marticorena C, Hunger S, Heller P, Gutierrez-Hartmann A, Xiayuan L, Pluthero FG, Rowley JW, Weyrich AS, Kahr WHA, Porter CC, Di Paola J. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet. 2015;47(5):535–8. Epub 2015/03/26. doi: 10.1038/ng.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang MY, Churpek JE, Keel SB, Walsh T, Lee MK, Loeb KR, Gulsuner S, Pritchard CC, Sanchez-Bonilla M, Delrow JJ, Basom RS, Forouhar M, Gyurkocza B, Schwartz BS, Neistadt B, Marquez R, Mariani CJ, Coats SA, Hofmann I, Lindsley RC, Williams DA, Abkowitz JL, Horwitz MS, King MC, Godley LA, Shimamura A. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet. 2015;47(2):180–5. Epub 2015/01/13. doi: 10.1038/ng.3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poggi M, Canault M, Favier M, Turro E, Saultier P, Ghalloussi D, Baccini V, Vidal L, Mezzapesa A, Chelghoum N, Mohand-Oumoussa B, Falaise C, Favier R, Ouwehand WH, Fiore M, Peiretti F, Morange PE, Saut N, Bernot D, Greinacher A, BioResource N, Nurden AT, Nurden P, Freson K, Tregouet DA, Raslova H, Alessi MC. Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD34+ progenitors. Haematologica. 2017;102(2):282–94. Epub 2016/09/25. doi: 10.3324/haematol.2016.147694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Paola J, Porter CC. ETV6-related thrombocytopenia and leukemia predisposition. Blood. 2019;134(8):663–7. Epub 2019/06/27. doi: 10.1182/blood.2019852418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melazzini F, Palombo F, Balduini A, De Rocco D, Marconi C, Noris P, Gnan C, Pippucci T, Bozzi V, Faleschini M, Barozzi S, Doubek M, Di Buduo CA, Kozubik KS, Radova L, Loffredo G, Pospisilova S, Alfano C, Seri M, Balduini CL, Pecci A, Savoia A. Clinical and pathogenic features of ETV6-related thrombocytopenia with predisposition to acute lymphoblastic leukemia. Haematologica. 2016;101(11):1333–42. Epub 2016/11/02. doi: 10.3324/haematol.2016.147496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topka S, Vijai J, Walsh MF, Jacobs L, Maria A, Villano D, Gaddam P, Wu G, McGee RB, Quinn E, Inaba H, Hartford C, Pui C-H, Pappo A, Edmonson M, Zhang MY, Stepensky P, Steinherz P, Schrader K, Lincoln A, Bussel J, Lipkin SM, Goldgur Y, Harit M, Stadler ZK, Mullighan C, Weintraub M, Shimamura A, Zhang J, Downing JR, Nichols KE, Offit K. Germline ETV6 Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia. PLoS genetics. 2015;11(6):e1005262–e. doi: 10.1371/journal.pgen.1005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirse V, Norvilas R, Gineikiene E, Matuzevičienė R, Griskevicius L, Preiksaitiene E. ETV6 and NOTCH1 germline variants in adult acute leukemia. Leukemia & lymphoma. 2018;59(4):1022–4. Epub 2017/08/04. doi: 10.1080/10428194.2017.1359742 [DOI] [PubMed] [Google Scholar]
- 23.Hock H, Shimamura A. ETV6 in hematopoiesis and leukemia predisposition. Seminars in hematology. 2017;54(2):98–104. Epub 2017/04/07. doi: 10.1053/j.seminhematol.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]