SUMMARY
Mosquito inoculation of humans with arthritogenic alphaviruses results in a febrile syndrome characterized by debilitating musculoskeletal pain and arthritis. Despite an expanding global disease burden, no approved therapies or licensed vaccines exist. Here, we describe human monoclonal antibodies (mAbs) that bind to and neutralize multiple distantly related alphaviruses. These mAbs compete for an antigenic site and prevent attachment to the recently discovered Mxra8 alphavirus receptor. Three cryoelectron microscopy structures of Fab in complex with Ross River (RRV), Mayaro, or chikungunya viruses reveal a conserved footprint of the broadly neutralizing mAb RRV-12 in a region of the E2 glycoprotein B domain. This mAb neutralizes virus in vitro by preventing virus entry and spread and is protective in vivo in mouse models. Thus, the RRV-12 mAb and its defined epitope have potential as a therapeutic agent or target of vaccine design, respectively, against multiple emerging arthritogenic alphavirus infections.
Graphical Abstract

In Brief
Powell et al. describe human antibodies that bind to and neutralize multiple distantly related alphaviruses. These antibodies prevent attachment to the Mxra8 alphavirus receptor. Three cryoelectron microscopy structures of Fab in complex with Ross River (RRV), Mayaro, or chikungunya viruses reveal a conserved footprint of the broadly neutralizing mAb RRV-12.
INTRODUCTION
Alphaviruses are mosquito-transmitted, positive-strand RNA viruses in the Togaviridae family. These viruses are divided into two groups, those causing musculoskeletal disease or encephalitis. Chikungunya (CHIKV), Mayaro (MAYV), Ross River (RRV), Sagiyama (SAGV), Getah (GETV), and O’nyong’nyong (ONNV) viruses are alphaviruses that cause rash, fever, and most prominently, arthralgia that can persistent from months to years. A A226V mutation in the CHIKV E1 protein resulted in adaptation of the virus to the Aedes albopictus mosquito vector, contributing to a dramatic expansion of CHIKV geographic range and increased number of cases beginning in 2006 (Caglioti et al., 2013; Morrison, 2014; Zeller et al., 2016). MAYV is an emerging threat in South America, and outbreaks have been reported in Bolivia, Ecuador, Peru, Venezuela, Suriname, French Guyana, and Mexico (Acosta-Ampudia et al., 2018; Alva-Urcia et al., 2017; Levi and Vignuzzi, 2019). The detection of a case in Haiti in 2015 suggests that MAYV may also circulate in the Caribbean (Lednicky et al., 2016). ONNV caused a large epidemic from 1959–1962 across portions of both eastern and western Africa, resulting in over 2 million cases, and seroprevalence studies have indicated continuous transmission since that time (Levi and Vignuzzi, 2019; Rezza et al., 2017). RRV is endemic to Australia and causes an estimated 5,000 infections per year, although larger outbreaks of up to 80,000 infected individuals have been reported in the Pacific Islands (Harley et al., 2001; Tesh et al., 1981; Tupanceska et al., 2007). SAGV and GETV are distributed widely in Asia and are closely related to RRV, although clinical disease occurs primarily in horses. However, serum antibodies against SAGV and GETV have been reported in humans, birds, and domestic animals (Kumanomido et al., 1988; Shirako and Yamaguchi, 2000).
Alphaviruses have icosahedral virions, comprised 80 copies of a trimer of heterodimeric glycoproteins on the virus surface (Jose et al., 2009; Roussel et al., 2006). This heterodimer is formed from an association between the E1 and E2 glycoproteins, which are targets of mouse and human alphavirus antibodies, including those against CHIKV, RRV, and MAYV (Earnest et al., 2019; Fox et al., 2015; Jin et al., 2015; Pal et al., 2013; Smith et al., 2015). In particular, the E2 protein is a target for neutralizing antibodies that block alphavirus attachment, entry, fusion, or egress. E2 is composed of three domains: the A domain, which is surface-exposed and connects the B and C domains; the B domain, which also is surface-exposed and shields the fusion loop in the E1 protein; and the C domain, located at the base of the trimer (Fox et al., 2015; Jin et al., 2015; Weger-Lucarelli et al., 2015). Recently, mutagenesis, cryoelectron microscopy (cryo-EM), and X-ray crystallography studies have established that a newly discovered shared receptor for arthritogenic alphaviruses, Mxra8, contacts both the A and B domains of the E2 protein as well as sites on the E1 protein (Basore et al., 2019; Song et al., 2019; Zhang et al.,2018).
Because there is no licensed treatment or vaccine for arthritogenic viruses, there is a need to better define the epitopes recognized by protective antibodies. Identifying broadly cross-reactive and neutralizing human antibodies could prove useful for revealing conserved epitopes that may be beneficial for the design of a universal alphavirus vaccine. Previously, murine monoclonal antibodies (mAbs) were identified that broadly neutralized multiple alphaviruses, including CHIKV, MAYV, ONNV, RRV, and Semliki Forest virus (SFV) (Earnest et al., 2019; Fox et al., 2015). These mAbs blocked multiple steps of the viral life cycle, including entry and egress, and one mAb, CHK-265, neutralized by binding to the B domain and possibly by cross-linking it to the A domain of an adjacent viral spike (Fox et al., 2015). However, few human broadly cross-reactive mAbs have been identified, and their mechanism of neutralization is unknown.
Here, we sought to identify cross-reactive alphavirus antibodies from the B cells of immune human subjects and to determine their epitopes and mechanism(s) of inhibition. We screened pre-existing plasma antibodies isolated from individuals with prior history of infection with RRV or CHIKV and identified six mAbs that cross-reacted with two or more arthritogenic alphaviruses. Next, we determined that these mAbs competed for binding to a common antigenic site and defined the molecular basis for recognition of CHIKV, RRV, MAYV, SAGV, GETV, and ONNV by a broadly neutralizing mAb, RRV-12. High resolution cryo-EM structures of RRV-12 in complex with CHIKV, RRV, or MAYV revealed that the epitope for this mAb lies at the distal end of E2 protein B domain in a region with partial sequence conservation across multiple alphaviruses. This mAb showed efficacy in vivo against infection and disease when administered therapeutically in challenge models of RRV or MAYV in mice. Overall, this work provides insight into the humoral immune response to alphaviruses during human infection and identifies a shared epitope targeted by human antibodies. In addition to the use of RRV-12 as a possible therapy against multiple alphaviruses, our molecular and structural understanding of its binding site could inform rational epitope-based vaccine design strategies.
RESULTS
Isolation of Human mAbs with Broad Cross-Reactivity for Multiple Alphaviruses
We isolated a panel of human mAbs from two subjects. The first subject had a history of a laboratory-confirmed case of RRV that was acquired in Australia and the second subject came from Colombo, Sri Lanka with serological evidence of prior natural CHIKV infection. We screened supernatants of Epstein-Barr virus (EBV) transformed B cells from these donors for direct binding to RRV or MAYV by enzyme-linked immunosorbent assay (ELISA). B cells that secreted antibody reactive with either RRV or MAYV were fused to a myeloma line to establish stable hybridoma cell lines, which then were cloned through single-cell flow cytometric sorting.
Binding and Neutralization Profiles for mAbs
RRV-12, which was isolated from cells from the RRV-immune subject, and five mAbs isolated from the CHIKV-immune subject, were tested for binding and neutralization of six alphaviruses: CHIKV, RRV, ONNV, MAYV, GETV, and SAGV, chosen based on close evolutionary distance and virus availability (Figures S1 and S2). Each of the six mAbs studied was genetically distinct from the others (Table S1). Binding was tested with both direct virus and protein ELISAs using CHIKV and MAYV E2 proteins. While most half maximal binding (EC50) values were between 4 and 100 ng/mL for the virus ELISAs, the mAbs bound less well to recombinant soluble forms of the viral proteins, with EC50 values ranging from 27 to 1,259 ng/mL (Table S2). Notably, mAbs did not bind to ONNV in ELISA, although each of the mAbs neutralized this virus (Table S2; Figures 1 and S3). We used a focus reduction neutralization test (FRNT) to assess more quantitatively the inhibitory activity of mAbs against these six viruses, and found reasonable correspondence between binding and neutralization activity, as measured by half maximal inhibitory concentration (IC50). However, whereas CHKV-70 bound to CHIKV, MAYV, and RRV in a virus ELISA, it did not neutralize these viruses efficiently (Table S1 and Figure 1). As described previously (Heidneretal., 1996; Zhang et al., 2011), some mAbs were unable to neutralize infection completely and left a resistant fraction of infectious virus even when tested at high concentrations (Figure 1B and Table S3). In general, ONNV was the most potently neutralized, with only a small amount of unneutralized virus remaining (Figure 1B and Table S3).
Figure 1. Antibodies Generated from Donors Naturally Infected with RRV or CHIKV Bind and Neutralize RRV, MAYV, CHIKV, SAGV, GETV, and ONNV.
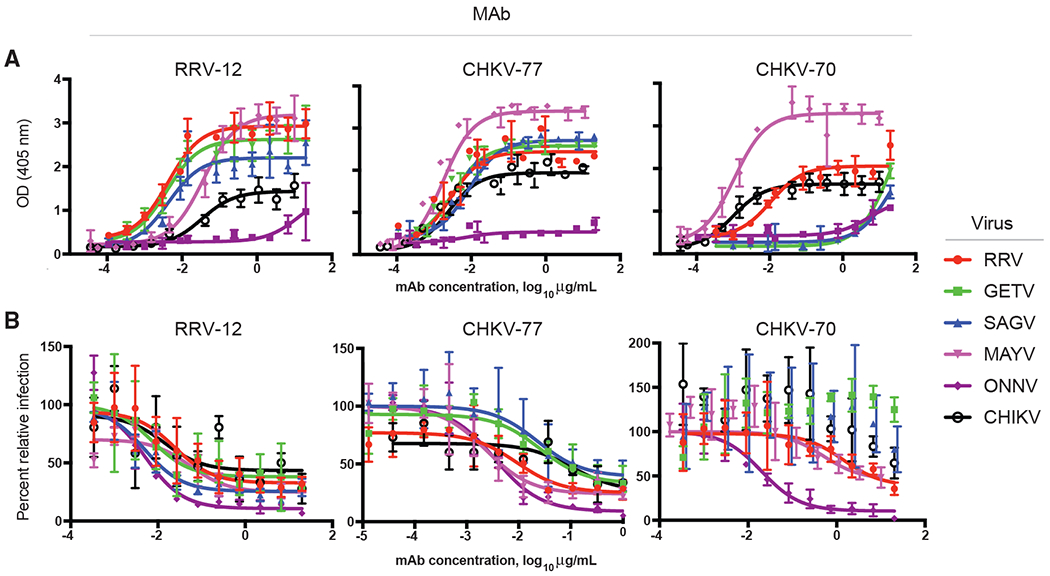
(A and B) (A) Binding or (B) neutralization profiles of three broadly neutralizing mAbs, as determined through ELISA or FRNT. Two independent experiments were performed, and representative binding and neutralization curves are shown, with error bars representing mean ± SD binding was measured using a virus ELISA and neutralization was measured using a FRNT. Both ELISA and FRNT were performed in triplicate, and curves and IC50 or EC50 binding values were obtained using non-linear fit analysis with top of curve constrained to 100 for neutralization, using Prism software version 7 (GraphPad Software).
Biolayer Interferometry Competition-Binding Assays
To determine if these cross-reactive mAbs recognized a similar antigenic site, we performed competition-binding biolayer interferometry (BLI) assay using recombinant protein containing a histidine tag. We first immobilized either CHIKV E2 or MAYV E3E2 recombinant protein on Anti-Penta-HIS (HIS1K) biosensors before adding two antibodies sequentially. The percent binding of the second antibody in the presence of saturating concentrations of the first antibody was compared with non-competed binding. As expected, each antibody competed with itself. A binding value of less than 25% was considered completely blocking, whereas values between 25% and 50% were considered partially blocking. CHKV-66 was excluded from the analysis because it did not bind avidly enough to E2 protein to assess competition. Virtually all of the cross-reactive mAbs competed for binding to a common antigenic site on CHIKV and MAYV E2 protein (Figure 2A).
Figure 2. Cross-Reactive mAbs Compete for the Same Antigenic Site and Inhibit Binding to Mxra8 Receptor Protein.
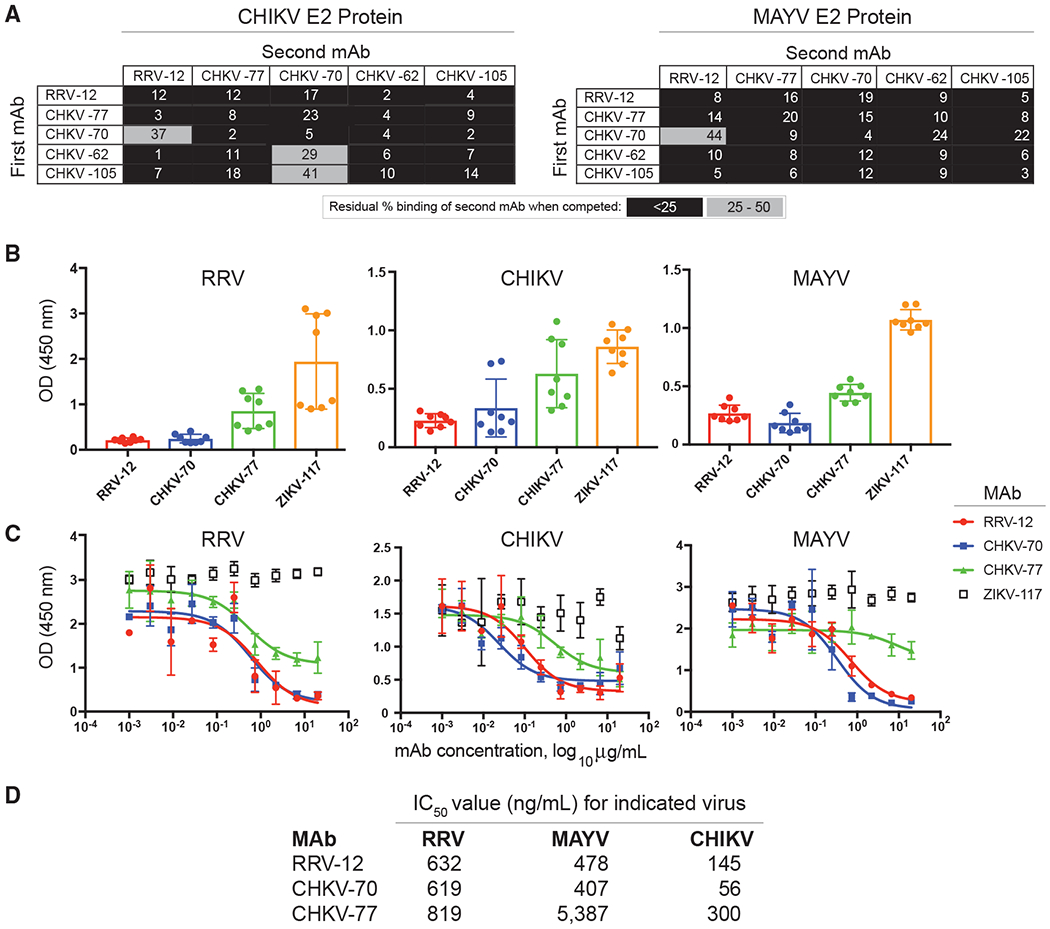
(A) An Octet RED96 instrument (Pall FortéBio) was used to perform epitope-binning studies using competition binding. After a baseline measurement, HIS1K biosensor tips were used to immbolize either CHIKV or MAYV E2 protein containing a histidine tag. After another baseline measurement, biosensors then were transferred to wells containing a first mAb at a concentration of 50 μg/mL for 5 min, before immersion in a solution containing a second mAb, also at a concentration of 50 μg/mL for 5 min. The percent competition of the second mAb in the presence of the first mAb was determined by comparing the maximal signal of binding for the second mAb in the presence of the first antibody to the maximal signal of the second mAb in the absence of competition. Competition was defined by reduction of the maximal binding score to <25% of uncompeted binding (black boxes). A 25% to 50% reduction in maximal binding was considered intermediate competition (gray boxes).
(B) Antibody blocking of virus binding to mouse Mxra8-Fc fusion protein was determined through competition ELISA. MAYV, CHIKV, or RRV were captured on the plate with a human mAb before addition of RRV mAbs followed by Mxra8-mFc (mouse Fc). A loss of signal indicates competition of RRV mAbs with Mxra8-mFc for binding to virus. Two independent experiments were performed in quadruplicate.
(C) Dose-response curve of mAb inhibition of virus binding to mouse Mxra8-Fc fusion protein through competition ELISA. Two independent experiments were performed in triplicate and representative curves are shown.
(D) Half maximal inhibitory values (IC50) values for dose-response curves, calculated based on the average of two independent experiments performed in triplicate.
Blockade of Mxra8 Binding
Recently, mAbs against multiple alphaviruses have been shown to block binding to the Mxra8 receptor (Basore et al., 2019; Zhang et al., 2018). To begin to determine if our cross-reactive human mAbs also blocked attachment to the Mxra8 receptor, we performed a competition-binding ELISA with three of the viruses, RRV, MAYV, and CHIKV. Viral particles were captured onto an ELISA plate, and mAbs were allowed to attach to virus before the addition of purified recombinant mouse Mxra8-Fc fusion protein. At a concentration of 10 μg/mL, RRV-12 and CHKV-70 substantially inhibited each of the three viruses from binding to Mxra8-Fc protein, as compared with a control antibody (Figure 2B). In comparison, CHKV-77 reduced Mxra8-Fc binding only slightly. We also performed titration curves to test various concentrations of mAb and found that RRV-12 and CHKV-70 maximally blocked binding of RRV to Mxra8-Fc protein at a concentration of ~2 μg/mL, whereas for CHIKV, maximal inhibition occurred at <1μg/mL, and for MAYV, maximal inhibition was observed at a higher concentration of ~7 μg/mL (Figure 2C). All mAbs tested more potently blocked Mxra8 binding to CHIKV, as evidenced by IC50 values and inhibition curves (Figure 2D).
RRV-12 Mechanism of Neutralization
We chose one of the receptor-blocking mAbs for further testing to gain insight into the mechanism of neutralization. To determine if RRV-12 blocked a step in the viral entry pathway, we performed a variation on the FRNT in which virus was incubated with antibody at 37°C before addition to Vero cell monolayers, also at 37°C. Virus and antibody then were removed from cells with three washes before addition of a methylcellulose semisolid overlay. 18 h later, the overlay was removed, and cell monolayers were stained for viral antigen. Entry of RRV, MAYV, CHIKV, SAGV, GETV, and ONNV was reduced significantly compared with the isotype control mAb (Figure 3A). We then quantified viral foci size using another variation on the FRNT in which antibody was added directly to the methylcellulose layer after cells were incubated with virus at 37°C. When an ImmunoSpot® plate reader was used to calculate viral foci area after staining, we found that RRV-12 significantly reduced foci size for each of the viruses except ONNV (Figures 3B and 3C). In the absence of the methylcellulose overlay, viral spread occurred more substantially in the presence of the control antibody, whereas only individual antigen-positive cells were observed when RRV-12 was applied (Figure 3C, right). To further probe the mechanism of neutralization, we evaluated the inhibitory activity of RRV-12 against RRV, in pre- and post-attachment assays. In the pre-attachment assay, virus was incubated with antibody at 4°C before addition to Vero cell monolayers, also at 4°C. Unbound virus and antibody were washed out, and attached virus was allowed to internalize during a brief incubation period at 37°C. The post-attachment assay was performed similarly, except excess virus was washed out before mAb was added, also at 4°C. For both assays, after virus internalization, cells were overlaid with methylcellulose, incubated, and then fixed and stained 18 h later. RRV-12 blocked at both pre- and post-attachment steps (Figure 3D). While the pre-attachment inhibition results supported our finding that RRV-12 blocks viral binding to Mxra8, we next tested if the observed post-attachment blockade could include steps downstream from entry, such as fusion. We accomplished this testing by performing a fusion from without (FFWO) assay, which has been used previously to measure alphavirus fusion with the plasma membrane under low-pH conditions as a surrogate assay for endosomal fusion (Jin et al., 2015; Pal et al., 2013; Smith et al., 2015). Virus was absorbed first to cells at 4°C before mAbs were added. After removing unbound virus and antibody, cells were pulsed at 37°C in a low-pH medium to promote plasma membrane-mediated viral fusion. Virus that entered the cells was stained with antibodies 14 h later and detected by flow cytometry. At a concentration of 10 μg/mL, RRV-12 did not inhibit fusion, with virus levels comparable to those of the negative control antibodies (Figure 3E). This result indicates that attachment, entry, and viral spread are the most likely mechanisms of inhibition used by RRV-12.
Figure 3. RRV-12 Blocks Entry and Cell-Cell Spread of RRV, MAYV, CHIKV, SAGV, GETV, and ONNV.
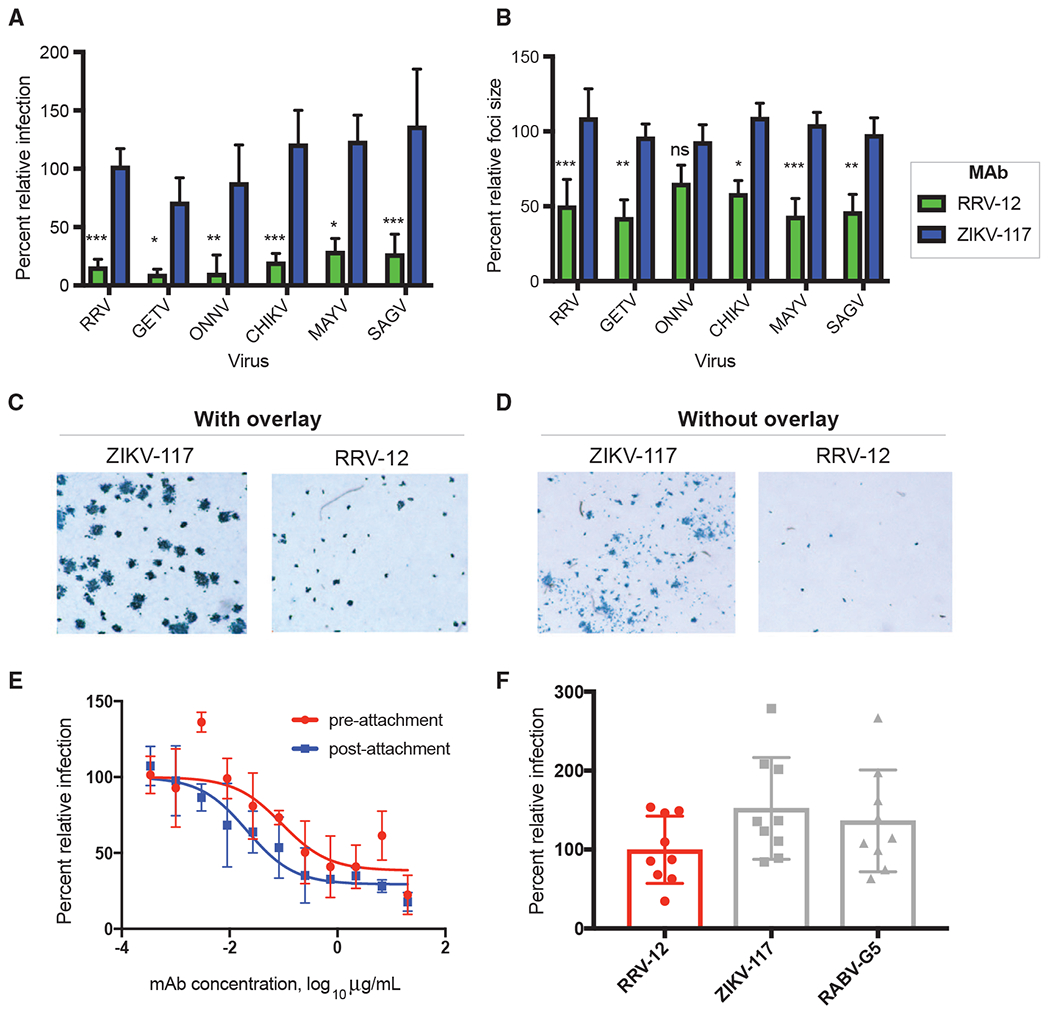
(A) RRV-12 blocks an entry step of RRV, MAYV, CHIKV, SAGV, and GETV. Antibody at a concentration of 20 μg/mL was incubated 1:1 with virus for 1 h at 37°C before addition to Vero cells for 1 h, also at 37°C. Antibody then was removed with 3 washes in medium before addition of a methylcellulose overlay. Cells were incubated at 37°C for 18 h before fixing and immunostaining. Foci were imaged and counted with an automated spot counter. Three independent experiments were performed, with triplicate samples in each experiment. Values were normalized to a virus-only control (One-way ANOVA with Kruskal-Wallis post-test, with mean ± SD compared with ZIKV-117 control; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(B) RRV-12 blocks cell-to-cell spread of RRV, MAYV, CHIKV, SAGV, GETV, and ONNV as measured through reduction of foci size. Virus was added to Vero cells for 1 h at 37°C before addition of 20 μg/mL of antibody diluted in methylcellulose overlay. After 18 h, cells were fixed and immunostained, and foci size was measured with a CTL Biospot reader. Three independent experiments were performed with triplicate samples in each experiment. Values were normalized to a virus-only control (One-way ANOVA with Kruskal-Wallis post-test, with mean ± SD compared with ZIKV-117 control; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(C and D) Representative images of RRV-12 reduction of foci size for RRV infection as compared with a control antibody, with antibody added to methylcellulose overlay (C), or with antibody in the absence of methylcellulose overlay (D). Antibody concentrations were at 20 μg/mL, and immunostaining was performed as in FRNT.
(E) Pre-attachment and post-attachment neutralization assay for RRV-12. In the pre-attachment assay, antibody was incubated with virus at 4°C before addition to Vero cells kept at 4°C. For the post-attachment assay, virus was applied to Vero cell monolayer cultures at 4°C before addition of antibody to cells at 4°C. Two independent experiments were performed in triplicate for each antibody, and representative curves are shown.
(F) A FFWO assay was used to measure antibody inhibition of virus fusion with the cell membrane under low-pH conditions. Virus was adsorbed to Vero cell culture monolayers at 4°C for an hour before addition of antibody dilutions, also at 4°C, after removing excess virus. Cells then were exposed to a pH 5.5 medium or a control medium at neutral pH for 2 min and incubated at 37°C. The acidic pH medium was removed, and cells were incubated for an additional 14 h before fixing, permeabilizing, and staining for intracellular virus antigens before flow cytometric analysis. Intracellular virus was quantified by measuring percent PE-positive cells relative to a virus-only control. Three separate experiments were performed in triplicate for each antibody.
RRV-12 Targets a Partially Conserved E2 B Domain Epitope
To gain structural insight into the cross-reactivity of RRV-12, Fab fragments of RRV-12 were bound to RRV, CHIKV, or MAYV virions and analyzed by cryo-EM and single-particle reconstruction. Cryo-EM micrographs of each virus incubated with Fab RRV-12 demonstrated intact particles suitable for further analysis. Following single-particle reconstruction, structures for each virus/Fab RRV-12 complex were determined to a resolution of 6.3 Å for RRV, 5.3 Å for CHIKV, 5.3 Å for MAYV, and 4.8 Å for native MAYV (Figure 4A).
Figure 4. Structural Analysis of Fab RRV-12 Binding to RRV, CHIKV, and MAYV by Cryo-EM.
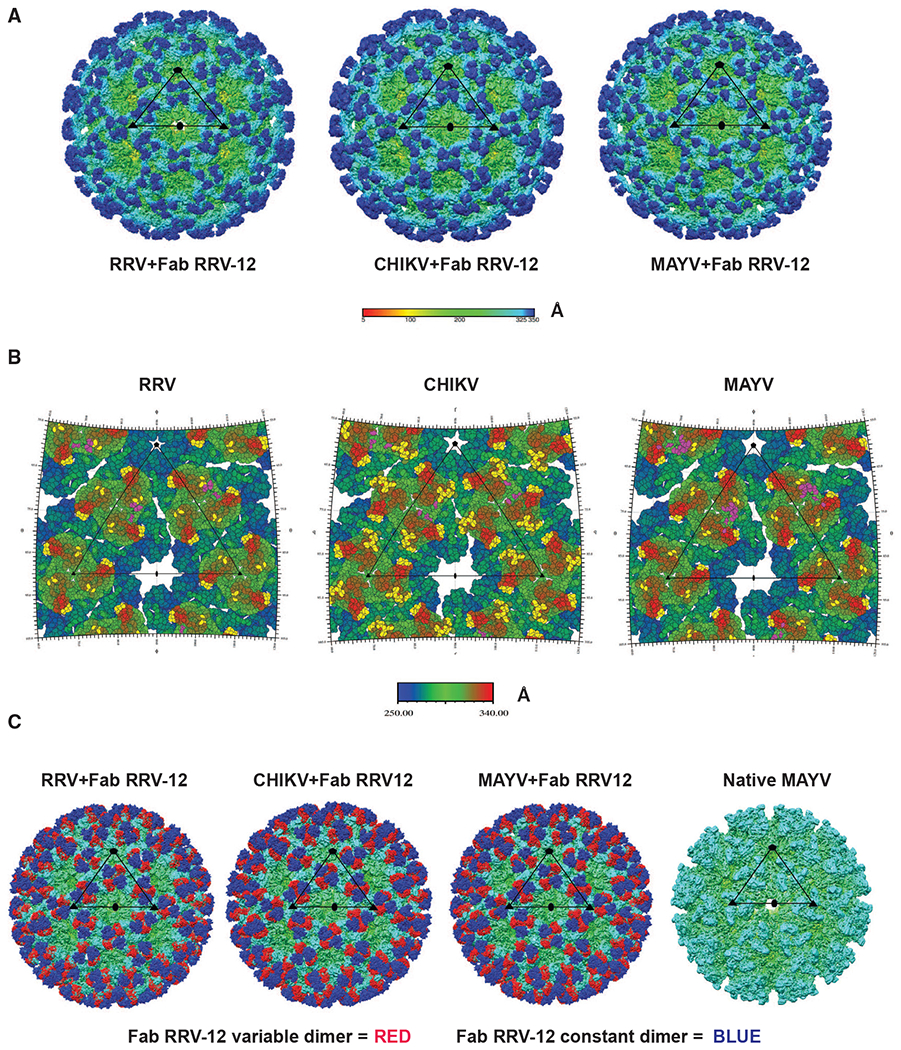
(A) Cryo-EM density maps of RRV, CHIKV, and MAYV bound by Fab RRV-12. Each map was determined by single-particle reconstruction and icosahedral averaging. The scale bar represents the radial distance from the particle center by color in angstroms. The superimposed black triangle represents the asymmetric unit; symmetry axes are indicated by a pentagon for the 5-fold axis, a triangle for the icosahedral 3-fold axis, and an oval for the 2-fold axis. The resolution of each map is 6.3Å for RRV and 5.3Å for both CHIKV and MAYV.
(B) RIVEM road maps of the viral surface. Scale bar, Å, is radial distance from the center of the virus. Residues highlighted in yellow on RRV and MAYV E2 B domain, E2 A domain, and β-ribbon, and CHIKV E2 B domain and E3 and are all located within 6 Å or less to the backbone of the fitted Fab structure. Highlighted residues indicate the B domain of each i3 and q3 trimer is fully occupied. Residues highlighted in purple indicate residues of the viral surface 6 Å or less to the position of the backbone structures of Mxra8. Mxra8 occupies only one position per trimer.
(C) Cryo-density maps of RRV, CHIKV, and MAYV bound with Fab RRV-12 and native MAYV structure. Fab constant or variable domains are colored blue or red, respectively. The antibody variable domain binds the B domain of one trimer and the antibody constant domain extends to cover an adjacent trimer. The native structure is shown for a comparison with an unoccupied trimer. The remaining density of each structure is colored based on radial distance from the center of the particle, see scale bar in (Å).
The location of Fab RRV-12 binding sites on each virus was determined from the fitted coordinates of viral glycoprotein E2 structures and the Fab CHK-265 model (Fox et al., 2015). The position of CHIKV E3 was determined by alignment of a CHIKV E2-E3 structure derived from PDB 6NK7 (Basore et al., 2019) to the E2 ectodomain structure fit of the RRV-12 bound CHIKV density map. In comparison with the other RRV-12 bound viruses, E3 was only found to be present in the CHIKV structure. The positions of RRV and MAYV E2, and CHIKV E2, E3, and Fab structures relative to each other in the context of the density maps were analyzed to map regions of the virus surface within 6 Å of the Fab structure (Figure 4B).
These results revealed that the RRV-12 Fab bound to similar regions of the E2 B domain from all three viruses at both the i3 axis and q3 axis trimers (Figure 4B), with each epitope on the surface being fully occupied. The Fab CDR loops binds a region of the E2 B domain spanning residues: RRV E2 183–187, 218–221, and 223; CHIKV E2 179–184, 198–200, and 213–219; and MAYV E2 184–187 and 219–221 (Figures 4B and 5A; Table S4). A sequence alignment of RRV, CHIKV, MAYV, ONNV, SAGV, and GETV E2 B domain amino acid sequences revealed a partially conserved region on the E2 B domain composed of residues 183–187 and 217–222 shared between all the viruses (Figure 5C).
Figure 5. Fab RRV-12 Variable Domain Binds the B Domain.
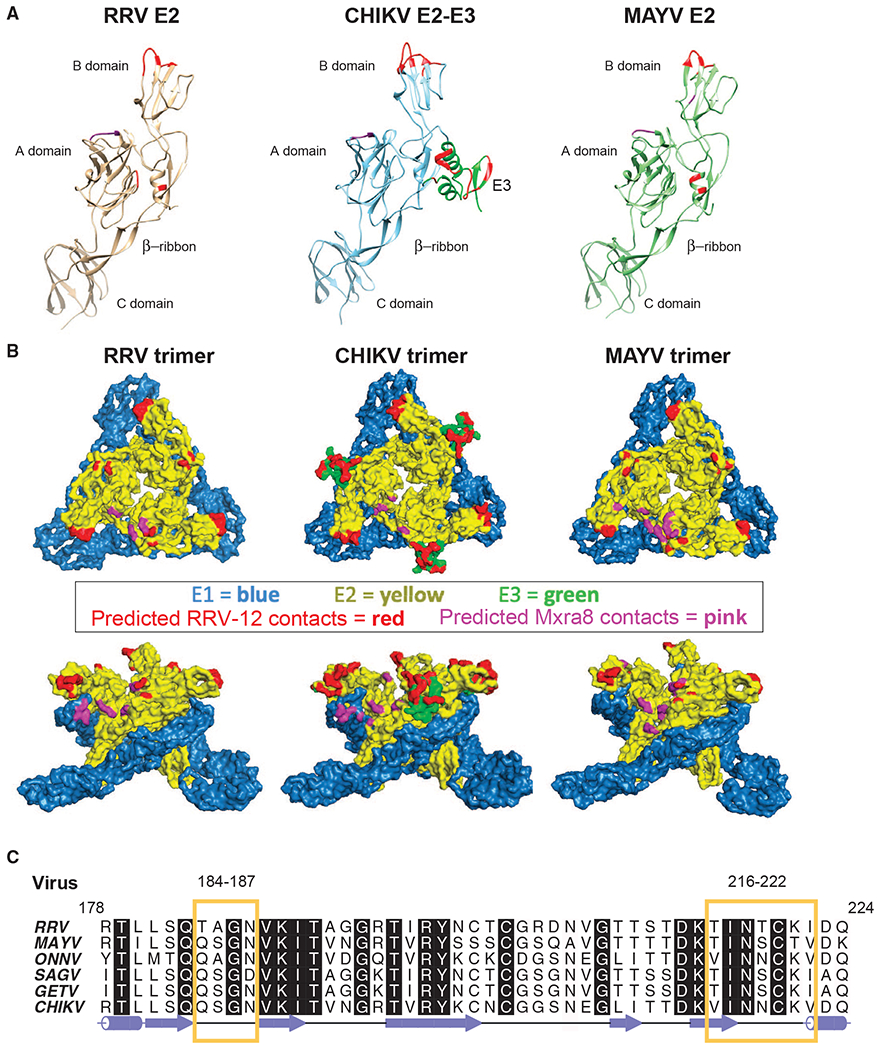
(A) Ribbon diagrams of E2 ectodomain structure from each virus. Regions of the B domain highlighted in red are the predicted epitope. Highlighted regions span residues RRV 183–187, 218–221, and 223; CHIKV E2 179–184, 198–200, and 213–219; and MAYV E2 184–187 and 219–221. Residues highlighted in red on the RRV and MAYV A domain and β-ribbon and on CHIKV E3 are predicted to be in close vicinity of the constant domain due to orientation of the Fab to the viral surface. Residues RRV 25–28 and 61–63, CHIKV 22–24 and 192, and MAYV 25–27 and 192, shown in purple, are predicted to be the Mxra8 binding site.
(B) Surface representations of RRV, CHIKV, and MAYV trimer generated from the fitted asymmetric unit. E1 and E2 are blue and yellow, respectively. E3 on the CHIKV trimer is shown in green. The surface region highlighted in red on the B domain of each trimer is the predicted epitope. Positions highlighted in red on the RRV and MAYV A domain and β-ribbon are part of the constant domain footprint. Surfaces of CHIKV E3 highlighted in red are part of both the variable and constant domain footprint. Areas highlighted in purple are the predicted position of Mxra8.
(C) Amino acid sequence alignment of E2 B domain residues 178–224 from viruses CHIKV, ONNV, MAYV, RRV, SAGV, GETV, generated using ALINE program (Bond and Schüttelkopf, 2009). Yellow boxes outline semi-conserved regions between the viruses containing residues of the Fab RRV-12 epitope.
Density associated with the constant region of the Fab appears to cross-link neighboring spikes (Figure 4C). Analysis of fitted E2, E3, and Fab constant domain positions in the context of the density maps were used to map residues to the three virus surfaces. Residues RRV 7 (E2 β-ribbon), 59 and 60 (A domain), MAYV 7 and 10 (E2 β-ribbon), and 60 (A domain) are within 6 Å of the constant domain (Figures 4B, 5A, and 5B). These contacts are primarily due to the angle of Fab binding, which brings the constant domain near the virus surfaces (Figure S4). A gap exists in the density between the RRV and MAYV surface and the constant domain (Figure S4) suggesting at least a weak and/or transient interaction not observable with single-particle reconstructions. For these reasons, residues on RRV and MAYV surfaces are most likely not a primary factor in Fab binding but may participate in transiently positioning the Fab.
For CHIKV, areas of E3 density contact the Fab density near the junction of the antibody variable and constant domains (Figure S4). E3 residues 8–10, 12, 14–17, and 19–25 are within 6Å of the antibody variable domain, and residues 52–53 are within 6Å of the antibody constant domain (Figures 4B, 5A, and 5B). Like RRV and MAYV, a gap is present between the CHIKV viral surface and the Fab constant domain, and the angle of Fab binding is similar (Figure S4). The contacts on the CHIKV surface result from occupation by E3 on a position that is empty on RRV and MAYV (Figure S4). E3 appears to lie directly underneath the Fab (Figure S4). Likely, these contacts on the surface of CHIKV are not critical residues in the cross-reactive epitope since the Fab binds similar regions on the E2 B domain on RRV and MAYV in the absence of E3 compared to CHIKV.
The position of Mxra8 in the asymmetric unit relative to CHIKV E2 (Basore et al., 2019) PDB 6NK7) was mapped to the asymmetric units of RRV-12-bound virions in the RRV, MAYV, or CHIKV structures. Residues of the virus surface within 6Å of the Mxra8 position in the asymmetric unit were mapped (Figure 4B; Table S4). Residues 25–28 and 61–63 of RRV, 22–24 and 115 of CHIKV, and 25–27 and 192 of MAYV are located on the A domain of E2 near the B domain Fab contacts and do not overlap the region of the RRV-12 epitope (Figures 5A and 5B). Residues 61–63 and 158–160 of RRV, 142, 155, and 156 of CHIKV, and 55, 59–65, 157–161, and 262–2640 of MAYV are located on the β strand of an adjacent E2 within the trimer. E1 residues 85–87 RRV, 83–85, 97, 98, and 226 CHIKV are within 6 Å of the position of Mxra8 in the asymmetric unit of each virus (Figures 5A and 5B). A comparison of the structures of Mxra8 or RRV-12 bound to the CHIKV E2 B domain reveals that Mxra8 and RRV-12 do not bind to the same regions of E2; however, binding of one protein may occlude the binding of the other (Figure S4).
RRV-12 Therapeutic Activity In Vivo
Next, in order to determine if RRV-12 may have efficacy in vivo, we first tested its protective activity in an immunocompetent mouse model of RRV infection. 4-week-old male wild-type (WT) C57BL/6 mice were treated with 100 μg of mAbs one day after inoculation with 103 focus-forming units (FFU) of RRV strain T48. The gastrocnemius (calf) muscle, ankle, spleen, and quadriceps muscle were harvested at 3 days post-infection (dpi), and viral RNA levels were measured by qRT-PCR. RRV-12 significantly reduced viral burden in all tissues except for the ipsilateral ankle (Figure 6A). We next tested the importance of Fc effector functions by comparing the therapeutic efficacy of RRV-12 IgG and a variant antibody with diminished capacity for FcγR binding (IgG-LALA-Fc; Hessell et al., 2007), in immunocompetent mice (Arduin et al., 2015; Saunders, 2019). Notably, we did not observe significant differences in RRV infection with animals treated with intact or LALA-Fc forms of RRV-12 (Figure 6B). We then tested the therapeutic efficacy of RRV-12 in a stringent immunocompromised mouse model of RRV infection and disease. 4-week-old male WT C57BL76 mice were treated with 0.2 mg of MAR1–5A3 (Sheehan et al., 2006), an anti-lfnar1 blocking mAb, prior to inoculation with 103 FFU of RRV. We administered 100 μg of RRV mAb via the intraperitoneal route one day after virus inoculation. When mice were given a control mAb, death was observed after 7 days, whereas RRV-12 protected 40% of mice from death over the 3-week time course (Figure 6C). We also tested the therapeutic efficacy of RRV-12 in an immunocompetent mouse challenge model of MAYV (Earnest et al., 2019). RRV-12 reduced MAYV viral burden in all tissues examined, including in the ipsilateral ankle, which was not seen in the RRV model. Finally, we used a clinical scoring system to measure severity of RRV disease in WT immunocompetent juvenile mice. 3-week-old WT C57BL/6 mice were inoculated with 103 FFU of RRV strain T48, and 100 μg of RRV-12 or an isotype control mAb was administered via intraperitoneal injection one day later. Mice were weighed and monitored over the course of 18 days and a clinical score was assigned based on grip strength, gait, and righting reflex, as previously described (Haist et al., 2017). Mice that received RRV-12 had marked improvement in clinical score compared with mice treated with the control antibody (Figure 7A). In addition, a significant reduction in viral RNA was observed in all tissues examined, including the spleen, ipsilateral and contralateral gastrocnemius, quadriceps, and ankle tissues harvested at 18 dpi. (Figure 7B).
Figure 6. Protective Activity of RRV-12 In Vivo against RRV and MAYV.
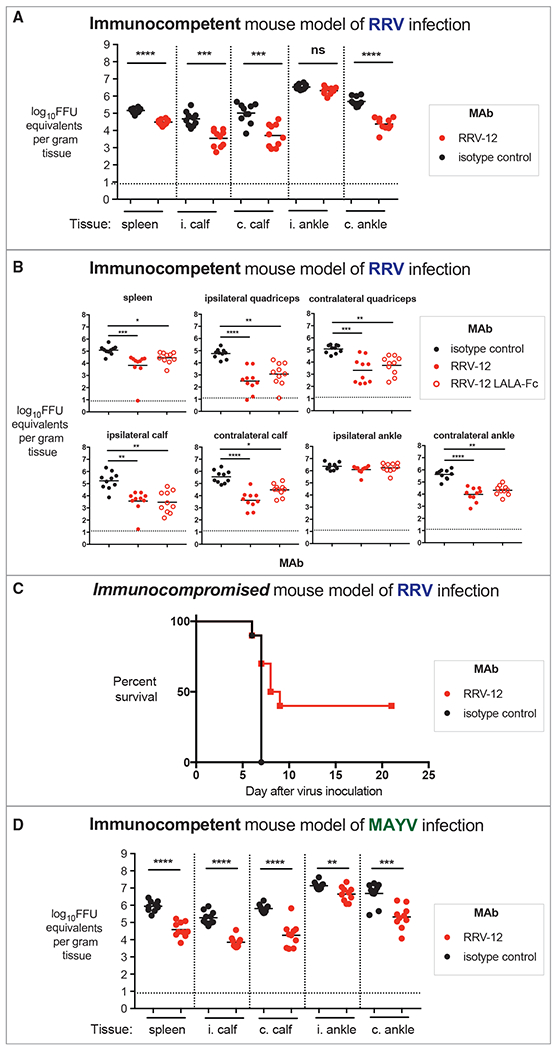
(A) RRV-12 reduces viral infection in immunocompetent model of RRV infection. RRV-12 (100 μg) was administered at 1 dpi to 4-week-old WT C57BL/6J mice, and ipsilateral and contralateral gastrocnemius, quadriceps, ankle, or spleen tissues were collected 3 dpi for measurement of viral RNA through qRT-PCR. Two independent experiments were performed, with ten mice per antibody for each group (one-way ANOVA with a Dunnett’s post-test comparing each group to the isotype control; **p < 0.01, ***p < 0.001, ****p < 0.0001).
(B) RRV-12 LALA mutant and RRV-12 intact mAb have similar therapeutic effect in immunocompetent mouse model of RRV infection. Intact or LALA variant of RRV-12 (100 μg) was administered at 1 dpi to WT mice, and ipsilateral and contralateral gastrocnemius, quadriceps, ankle, or spleen tissues were collected 3 dpi for measurement of viral RNA through qRT-PCR. Two independent experiments were performed, with ten mice per antibody for each group (one-way ANOVA with a Dunnett’s post-test comparing each group to the isotype control; **p < 0.01, ***p < 0.001, ****p < 0.0001).
(C) RRV-12 increases survival when administered therapeutically after RRV infection in an immunocompromised mouse model. Mice were given 0.2 mg of anti-Ifnar1 antibody, and subsequently inoculated with 103 FFU of RRV in the footpad. At 1 dpi, 100 μg of RRV antibody was administered, and mice survival was monitored for 3 weeks. Two independent experiments were performed, with 10 mice per antibody for each group. Statistical analysis was performed using a log rank test with Bonferroni correction, p = 0.0057.
(D) RRV-12 reduces viral burden in an immunocompetent model of MAYV infection in mice. As described above, RRV-12 (100 μg) was administered 1 dpi with 103 FFU of MAYV in WT mice, and ipsilateral and contralateral gastrocnemius, quadriceps, ankle, or spleen tissues were collected 3 dpi for measurement of viral RNA by qRT-PCR. Two independent experiments were performed, with a total of ten mice for each antibody group (one-way ANOVA with a Dunnett’s post-test comparing each group to the isotype control; **p < 0.01, ***p < 0.001, ****p < 0.0001).
Figure 7. RRV-12 Improves RRV-Induced Clinical Disease and Reduces Viral RNA Burden When Given Therapeutically in WT Mice.
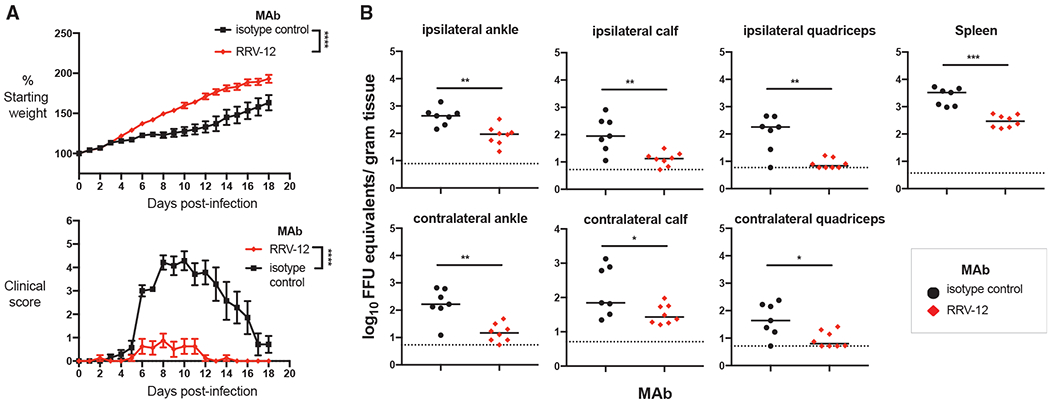
(A) 3-week-old WT C57BL/6J mice were inoculated with 103 FFU of RRV strain T48 before administration of 100-μg antibody by intraperitoneal injection at 24 hpi. Mice were then weighed each day over the course of 18 days and assigned a clinical score based on grip strength, gait, and righting reflex. Two independent experiments were performed, for a total of n = 7–8 mice in each antibody group. Statistical analysis was performed using a Student’s t test of area under the curve analysis (****p < 0.0001).
(B) 18 dpi, the spleen, ipsilateral and contralateral gastrocnemius, quadriceps, and ankle tissues were collected following extensive perfusion with PBS. Viral RNA was quantified through qRT-PCR and statistical analysis was performed using a Mann-Whitney test (*p < 0.05, **p < 0.01, ***p < 0.001).
DISCUSSION
Here, we identified cross-reactive neutralizing antibodies generated from two individuals from different geographic areas with prior history of infection with the arthritogenic alphaviruses CHIKV or RRV. Two of these antibodies broadly neutralized six different arthritogenic alphaviruses: RRV, CHIKV, MAYV, ONNV, SAGV, and GETV. These cross-reactive mAbs competed for binding to a similar antigenic site and blocked binding of virus to Mxra8-Fc fusion protein, a surrogate for the receptor on human cells. Cryo-EM analysis of Fab fragments of RRV-12 bound to CHIKV, MAYV, and RRV revealed recognition of a conserved epitope at the distal end of the B domain. In addition to blocking attachment to the Mxra8 receptor, we found that RRV-12 blocked viral entry and prevented cell-cell spread of virus. Finally, when tested in murine models of RRV and MAYV infection, RRV-12 reduced viral titer in multiple tissues and improved clinical outcome.
Previously, two human antibodies reactive against several alphaviruses, desinated 8I4 and 2H1, were reported, although these mAbs neutralized infection of only CHIKV, RRV, MAYV, and SFV, and with moderate potency (Fox et al., 2015). A cross-reactive murine antibody, CHK-265, also was described previously that potently neutralizes CHIK and MAYV, but it inhibited RRV, SFV, and ONNV to a lesser extent (Fox et al., 2015). A cryo-EM structure of CHK-265 Fab in complex with CHIKV revealed that CHK-265 binds principally to the B domain at residues 182–189, 203–206, and 214–218 (Fox et al., 2015). The structure for CHK-265-bound-CHIKV also suggested that the mechanism of neutralization might involve a tethering of the B domain to an additional contact site in the A domain at residues 71–72 on an adjacent spike. Although the CHK-265 epitope overlaps the RRV-12-binding site defined here at residues 184–187 and 216–222, the footprint for RRV-12 includes fewer residues and does not appear to include the A domain, indicating a slightly different orientation of engagement. The location of both RRV-12- and CHK-265-binding sites within the same B domain region suggests that this epitope is important for recognition of diverse alphaviruses by broadly neutralizing mAbs. For the viruses we tested, complete sequence conservation of the B domain was observed within the footprint of RRV-12 at residues 186,217–218, and 220 within the footprint of RRV-12. While a region of high sequence conservation among alphaviruses also is observed at residues 95–101 in the A domain, it is possible that broadly neutralizing mAbs may target the B domain more frequently, since its position at the distal end of the E2 protein allows for higher accessibility on the viral spike.
RRV-12 and CHKV-70 blocked binding to Mxra8 protein in a competition-binding ELISA. Mxra8 contacts residues within the A and B domains, as well as arch regions of the E2 protein. The Mxra8 footprint on the B domain includes residues 178–182, 189, 191–193, 212–214, and 221–223 (Basore et al., 2019; Zhang et al., 2018). These residues overlap with, or are adjacent to, the binding site we determined for RRV-12 at residues 184–187 and 216–222. Of note, CHKV-77 did not inhibit binding to Mxra8-Fc protein in a competition-binding ELISA, even though its neutralization potency was similar to that of RRV-12. We speculate that CHKV-77 most likely recognizes a spatially distinct epitope in the E2 B domain, even though it competes with RRV-12 in both the MAYV and CHIKV protein BLI competition-binding assays. The neutralization mechanism of CHKV-77 may be similarto other B domain neutralizing antibodies, such as those inhibiting entry, fusion, and egress (Earnest et al., 2019; Fox et al., 2015; Lee et al., 2011). Our finding that RRV-12 inhibits entry is consistent with the action of several other alphavirus antibodies that block infection through this mechanism (Fox et al., 2015; Jin et al., 2015; Pal et al., 2013). RRV-12, however, did not inhibit plasma membrane-mediated fusion, an assay that is a surrogate for endosomal fusion (Pal et al., 2013). This result agrees with the finding that broadly neutralizing murine mAbs primarily block alphavirus infection principally by preventing attachment and entry (Fox et al., 2015).
We discovered a unique focus reduction phenotype for RRV-12, suggesting that it also limits cell-to-cell spread of virus. This phenotype also has been described in other virus systems, for example the case of the influenza heterosubtypic HA-specific human mAb FluA-20 that is not neutralizing in vitro but is protective in vivo (Bangaru et al., 2019). There are several means by which cell-to-cell alphavirus spread may be inhibited by antibodies. During CHIKV infection, neutralizing mAbs against the E2 protein can block envelopment of nucleocapsids driven by the E1/E2 glyocoprotein, thus, preventing viral budding (Jin et al., 2018). Alternatively, viral particles may be released from the cell, yet have reduced infectivity. Mutations in the B domain of RRV, including a cysteine-to-serine mutation at residue 220, were shown to reduce levels of E2 incorporation in viral particles, thereby leading to fewer infectious particles, possibly through destabilization of E2-nucleocapsid interactions (Snyder et al., 2012). Finally, antibodies binding to the B domain may inhibit cell-to-cell spread of alphaviruses (Lee et al., 2011). During the budding process, alphaviruses form actin- and tubulin-containing protrusions that protect virions from inhibitory membrane-associated proteins and prevent superinfection of the same cell (Brown et al., 2018). These structures do not fuse with the neighboring cell but instead concentrate virus near the cell membrane. E2-nucleocapsid interactions are important for formation of these protrusions, and it is, therefore, possible that conformational changes induced by antibody binding to the distal region of the B domain may affect interactions of the transmembrane portion of the B domain with the nucleocapsid (Brown et al., 2018; Martinez and Kielian, 2016). Further study will be required to define which of these inhibition mechanisms RRV-12 utilizes.
Our in vivo results demonstrate that RRV-12 is protective in both RRV and MAYV mouse models of infection and reduces RRV-induced clinical disease in immunocompetent mice when administered therapeutically. Previously, CHIKV and RRV mAbs have demonstrated promising therapeutic activity in WT or immunocompromised mouse models (Fox et al., 2015; Jin and Simmons, 2019; Pal et al., 2013; Smith et al., 2015). The in vivo protection observed with human RRV-12 is comparable to that observed in prior studies with murine CHK-265. Following homologous virus challenge (RRV for RRV-12 or CHIKV for CHK-265) both mAbs reduced clinical disease and viral burden in tissues distal from the inoculation site. During the heterologous MAYV challenge, while CHK-265 treatment had a greater fold reduction in viral burden compared with RRV-12, both mAbs reduced viral burden in all tissues examined. Several factors may explain the differences in efficacy in vivo between RRV-12 and CHK-265 including timing of antibody administration and differences in measurement (viral RNA versus infectious virus). Unlike RRV-12, CHK-265 and the other protective mAbs exhibited nearly complete elimination of virus in vitro. Therefore, it was surprising that RRV-12 demonstrated comparable levels of protection despite a resistant fraction of ~40% of virus in the FRNT. This phenomenon has been reported previously for alphavirus mAbs, and while the exact cause is unknown, particle heterogeneity of virions produced in cell culture due to maturation differences or partial retention of cleaved E3 precursor protein has been suggested as contributing factors (Heidner et al., 1996; Zhang et al., 2011). When a LALA-Fc variant of RRV-12 was tested in vivo, we did not observe diminished protection as compared with intact IgG, suggesting that Fc effector functions are not essential for protection. Our results suggest that incompletely neutralizing mAbs still should be considered as therapeutic candidates.
In addition to intraperitoneal administration of antibodies in mouse models, other forms of antibody delivery have shown promise for alphaviruses. A single dose of a potently neutralizing human anti-CHIKV mAb delivered as lipid-encapsulated mRNA conferred protection against viral infection and arthritis in mice and macaques (Kose et al., 2019; Roussel et al., 2006). Our description of a broadly neutralizing human mAb that protects against disease in multiple mouse models could provide another such candidate for mRNA delivery. Additionally, the knowledge we provide of antibody mechanism and binding epitope on the E2 B domain could aid efforts to design a broadly protective alphavirus vaccine.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, James E. Crowe, Jr. (james.crowe@vumc.org).
Materials Availability
Materials described in this paper are available for distribution for nonprofit use using templated documents from Association of University Technology Managers “Toolkit MTAs”, available at: https://autm.net/surveys-and-tools/agreements/material-transfer-agreements/mta-toolkit.
Data and Code availability
The cryoEM structures are deposited at the Electron Microscopy Data Bank (EMDB). The ID numbers of Fab bound structures are RRV: EMD 21473 PDB 6VYV, CHIKV: EMD 21496 PDB 6W09, MAYV: EMD 21509 PDB 6WIC and the native is MAYV: EMD 21532 PDB 6W2U. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplemental Information. The CHIKV antibodies in this study are available by Material Transfer Agreement with Vanderbilt University Medical Center.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Source of Human B Cells
The RRV-immune subject was a 50-year old woman living in the U.S. who had a history of laboratory-confirmed infection in Australia in 1987, as described in Powell et al (in submission). Peripheral blood was obtained from this subject in the U.S. in 2015 (28 years after infection) after written informed consent with approval from the Vanderbilt University Medical Center Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation on Ficoll and were cryopreserved in liquid nitrogen until used in the experiments. The other blood sample was collected in 2015 from an adult subject in Colombo, Sri Lanka with serological evidence of prior CHIKV infection (>1/5,000 serum neutralizing antibody titer to CHIKV using virus replicon particles based on the Sri Lankan strain SL15649 (Morrison et al., 2011). CHIKV infection was common in Colombo during the years 2006 to 2008. The studies in Sri Lanka were approved by the Ethics Review Committee of the Medical Faculty, University of Colombo, Sri Lanka [serving as the National Institutes of Health (NIH)–approved Institutional Review Board (IRB) for Genetech Research Institute] and the IRB of Vanderbilt University Medical Center. The blood sample was a discarded buffy coat from a routine blood donation at the National Blood Center in Colombo, Sri Lanka. The sample was de-identified before removal from the National Blood Center. PBMCs and a plasma sample were separated at Genetech Research Institute by density gradient centrifugation and then cryopreserved and stored on liquid nitrogen until transfer to Vanderbilt using a liquid nitrogen dry shipper.
Cell Lines
Vero (monkey, sex unspecified), cell lines were obtained from the American Type Culture Collection (ATCC CCL-81). Vero cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (ThermoFisher Scientific) supplemented with 5% fetal bovine serum (FBS; HyClone) at 5% CO2, 37°C. BHK21 cell lines were obtained from (ATCC CCL-10) and cultured in 10% FBS (Sigma) and Minimal Essential Media (MEM, Thermo Fisher Scientific).
Viruses
Ross River virus strain T48, chikungunya virus strain 181/25, O’nyong’nyong virus strain MP30, Mayaro virus strain TR VL-4675, Sagiyama virus strain M6-Mag 132, and Getah virus strain MM 2021 were obtained from the World Reference Center for Emerging Viruses and Arboviruses at UTMB.
Mouse Models
Three-week-old male and female WT C57BL/6J were used for clinical disease model studies and four-week-old male WT C57BL/6J mice were used for acute virological and survival studies. Mice were housed in microisolator cages and provided food and water ad libitum. All animal experiments and procedures were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance number A3381-01). Injections were performed under anesthesia that was induced and maintained with ketamine hydrochloride (80 mg/kg) and xylazine (15 mg/kg), and all efforts were made to minimize animal suffering.
METHOD DETAILS
Generation of Human Hybridomas
Approximately 107 cryopreserved PBMCs were thawed and transformed with Epstein-Barr virus obtained from the supernatant of B95.8 cells in a suspension also containing a Chk2 inhibitor, cyclosporin A, and CpG, and the mixture was plated in a 384-well cell culture plate. After 7 days, transformed cells were transferred to 96-well plates containing a feeder layer of irradiated cells that were PBMCs obtained from discarded leukofiltration devices (Nashville Red Cross). After an additional 5 days, the supernatants of expanded cells were screened for the presence of RRV- or MAYV-reactive antibodies using an enzyme-linked immunosorbent assay (ELISA), described below. Transformed B cells from wells containing supernatant with antibodies reactive to RRV or MAYV were fused to the HMMA2.5 non-secreting myeloma cell line using an established electrofusion technique (Smith and Crowe, 2015). Subsequently, the resulting mixture of hybridoma cells was resuspended in medium containing hypoxanthine, aminopterin, thymidine, and oubain to select for hybrids of B cells and myeloma cells.
Virus ELISA Screen
RRV strain T48 was propagated in monolayer cultures of Vero cells. The cell line was authenticated and tested monthly during culture for the presence of mycoplasma and found to be negative in all cases. Infected cell supernatants containing virus with a titer of approximately 5 x 106 FFU/mL were harvested when cytopathic effect was maximal, filtered through a 0.45 μm filter, then frozen and stored at −80°C until use. 384-well ELISA plates were directly coated with 25 μL of RRV strain T48 harvested directly from cell supernatants, diluted 1:100 in PBS, and incubated for 1 h at 37°C. Plates were washed 5 times with PBS containing Tween (PBST) using an EL406 combination washer dispenser instrument (BioTek) and blocked for 1 h at room temperature with 5% milk powder and 2% goat serum, diluted in PBS. After washing 2 times with PBST, 25 μL of supernatants from hybridoma cultures or EBV-transformed cell lines were added to plates, which then were incubated for 1 h at room temperature. Plates were washed 4 times, and 25 μL of goat anti-human alkaline phosphatase-conjugated secondary antibodies (Meridian Life Science) diluted 1:5,000 in PBS was added to plates. After a 45-min incubation period, plates were washed 5 times and 25 μL of alkaline phosphatase substrate tablets (Sigma) diluted in Tris buffer with 1M MgCl2 was added. Optical density was read at 405 nm after 1 h using a Biotek plate reader.
BLI Competition-Binding Studies
An Octet RED96 BLI instrument (Pall FortéBio) was used to perform epitope binning studies using competition binding. Anti-Penta-HIS (HIS1K) biosensor tips were used to immbolize either CHIKV or MAYV E2 protein containing a hexahistidine tag. After measuring the baseline signal, the biosensor tips were immersed into wells containing mAb for 2 min. After another baseline measurement, biosensors then were transferred to wells containing a first mAb at a concentration of 50 μg/mL for 5 min, before immersion in a solution containing a second mAb, also at a concentration of 50 μg/mL for 5 min. The percent competition of the second mAb in the presence of the first mAb was determined by comparing the maximal signal of binding for the second mAb in the presence of the first antibody to the maximal signal of that mAb alone when separately tested uncompeted. Competition was defined by reduction of the maximal binding score to <25% of un-competed binding. An intermediate competing mAb was identified when a 25 to 50% reduction in maximal binding was observed.
Hybridoma Cell Line Clone Production
Two weeks after fusion, hybridoma cell lines were cloned by single-cell sorting using fluorescence-activated cell sorting on a BD FACSAria™ III sorting cytometer with aerosol containment, in the Vanderbilt University Medical Center Flow Cytometry Core. Approximately 2 weeks later, an ELISA screen was performed, and wells containing cloned cells secreting antibodies reactive to RRV or MAYV were selected for expansion.
Purification of mAb IgG Protein
Clonal cells secreting mAbs were grown in 75 cm2 flasks to 70% confluency in hybridoma growth medium (ClonaCell-HY medium E from STEMCELL Technologies, 03805). The cells were expanded equally to four 225 cm2 flasks for antibody expression in serum-free medium (GIBCO Hybridoma-SFM, Invitrogen, 12045084). The supernatant was harvested after 3 weeks and purified by affinity chromatography using protein G columns (GE Life Sciences, Protein G HP Columns). Purified IgG from hybridoma cell expression was used for all assays.
Focus Reduction Neutralization Test
Vero cells (American Type Culture Collection [ATCC] Cat. No. CCL-81) were seeded in 96-well plates at 30,000 cells/well the day before use. Antibodies were diluted in 96-well U-bottom plates, with a 1:3 dilution series across the plate and a virus-only control in the left column. A solution containing infectious virus (RRV, MAYV, CHIKV, ONNV, SAGV, or GETV) was diluted to a concentration of 100 focus-forming units (FFU)/mL and mixed 1:1 by volume in a 96-well plate with antibody suspensions. The virus/antibody mixture was incubated for 1 h at 37°C before transfer to Vero cell monolayer cultures. Infection was allowed to proceed for 1 h and then 1% methylcellulose overlay prepared in DMEM with 2% FBS was added to cells. After 18 h, 1% paraformaldehyde (PFA) prepared in PBS was used to fix cells for at least 1 h. Plates were washed 3 times with PBS before addition of a 1:6,000 dilution of anti-RRV mouse ascites fluid (ATCC Cat. No. VR-1246AF), anti-MAYV mouse ascites fluid (ATCC Cat. No. [V-507-701-562) or anti-CHIKV mouse ascites fluid (ATCC Cat. No. V-548-701-562), prepared in cell permeabilizing buffer (PBS with 0.1% saponin and 0.1% bovine serum albumin). After incubation for at least 2 h at room temperature, plates were washed 3 times in permeabilizing buffer, and anti-mouse HRP-conjugated secondary (Kirkegaard & Perry Laboratories [KPL]) was added at a 1:2,000 dilution in permeabilizing buffer. Plates were incubated for 1 h at room temperature and washed 3 times before addition of TrueBlue Peroxidase substrate (KPL) for 20 min. Plates were rinsed with dH2O, and then plates were imaged with an ImmunoSpot® plate reader (Cellular Technology Limited [CTL]). Foci were counted with BioSpot 5.1 software (CTL), and the percent relative infection was calculated based on the virus-only control. Triplicate tests were performed for each antibody, and the results were averaged.
Entry and Fusion Inhibition, and Foci Reduction Assays
Entry inhibition was performed as a variation of the FRNT. As described above, Vero cells were seeded in 96-well plates at 30,000 cells/well the day before use. Virus and antibody at a concentration of 20 μg/mL were mixed 1:1 and incubated together for 1 h at 37°C before transfer to Vero cell monolayer cultures. Infection was allowed to proceed for 1 h and importantly, antibody and virus were removed with three washes in medium. A 1% methylcellulose overlay prepared in DMEM with 2% FBS was then added to cells and staining was performed similarly to the FRNT. Plates were imaged with an ImmunoSpot® plate reader (Cellular Technology Limited [CTL]). Foci were counted with BioSpot 5.1 software (CTL), and the percent relative infection was calculated based on the virus-only control. Triplicate tests were performed for each antibody and each virus, and the results were averaged.
A fusion from without (FFWO) assay was performed in which Vero cells were seeded at 30,000 cells/well in 96-well plates the day before use in the assay. Cells were washed once with binding medium ((RPMI 1640, 0.2% BSA, 10 mM HEPES pH 7.4, and 20 mM NH4Cl) at 4°C, and incubated for 15 min at 4°C. The T48 strain of RRV was concentrated to 108 FFU/mL using 100 kDa centrifugal filters (Amicon) Centricon. Virus was prepared in binding medium and added to cells at an multiplicity of infection (MOI) of 15 for 1 h at 4°C. Any remaining free virus was removed with two washes in binding medium. Antibodies were prepared in DMEM containing 2% FBS at 10 μg/mL concentrations and added to cells for 1 h at 4°C. Antibody was removed and fusion with the plasma membrane was initiated by the addition of fusion media (RPMI 1640, 0.2% BSA, 10 mM HEPES, and 30 mM succinic acid at pH 5.5) for 2 min at 37°C. Binding medium (RPMI 1640, 0.2% BSA, 10 mM HEPES at pH 7.4) was used in place of low-pH fusion medium in controls wells to ensure that virus entry into cells only occurred due to pH-dependent plasma membrane fusion. After a 2-min incubation, medium was removed and cells were incubated in DMEM supplemented with 5% FBS, 10 mM HEPES, and 20 mM NH4Cl (pH 7.4). 14 h later, cells were detached with trypsin, fixed with 1% PFA in PBS for 1 h, and permeabilized with 0.1% saponin detergent solution. For staining prior to flow cytometry analysis, cells were incubated sequentially with RRV mouse ascites fluid (1:6000 dilution ATCC Cat. No. VR-1246AF) for 1 h and PE conjugated goat anti-mouse IgG secondary antibody for 1 h (Thermo Fisher). Cells were analyzed on BD Fortessa flow cytometer with FlowJo software.
AN FRNT was performed in which antibody was added at a concentration of 20 μg/mL to the methylcellulose semisolid overlay. Virus first was allowed to infect cells for 1 h at 37°C before addition of antibody in the overlay. Staining was performed 18 h later, as in the FRNT. A variation of this assay also was performed in which antibody was added after viral infection of cells for 1 h at 37°C. Virus was washed out with 3 washes in medium, and mAb at a concentration of 20 μg/mL was added to cells without the addition of methylcellulose. Images were taken post-staining using the CTL reader.
Virus Purification and cryo-EM
RRV, CHIKV, and MAYV were propagated in BHK21 cell cultures. For each purification, approximately 2 x 108 BHK21 cells were inoculated with a MOI of approximately 0.1. Infected cell supernatant medium was collected between 24 and 36 h after inoculation, and was clarified by centrifugation at 2,744 x g for 30 min at 4°C. Virions were concentrated through a 28% sucrose cushion in TNE buffer (50 mM Tris pH 7.4, 200 mM NaCl, 1.0 mM EDTA) by ultra-centrifugation at 178,305 x g for 2 h at 4°C. Particles were resuspended in residual supernatant by rocking gently for 1 h at room temperature. Virus particles were concentrated further and purified based on size and density with a 5 to 55% continuous OptiPrep gradient (Sigma, catalog number D1655) and ultracentrifugation was performed at 178,305 x g for 2 h at 4°C. The virus-containing band was extracted, buffer exchanged, and concentrated into TNE buffer with an Amicon Ultra-4 100 kDa molecular weight cutoff centrifugal filter (Millipore-Sigma, catalog number Z648043-24EA) to a final volume of 0.05 mL. Fab fragments of mAb RRV-12 IgG were generated with the Pierce Fab Preparation Kit (ThermoFisher, catalog number 44985) according to the manufacturer’s recommendations. Purified virus and Fab fragment complexes were incubated at a 2:1 molar ratio of Fab to E2 glycoprotein overnight at 4° C. Virus-Fab samples and native MAYV samples were flash frozen on Ultrathin carbon Lacey grids, 400 mesh copper (Ted Pella, catalog number 01824) in liquid ethane with a Gatan CP3 cryo-plunge.
Virus-Fab complexes and native MAYV were imaged with a ThermoFisher Scientific Titan Krios, magnification 18,000x, and a pixel size of 1.6Å for RRV and 0.81Å for CHIKV, and both native and Fab bound MAYV. Images were collected with a Gatan K2 Summit direct electron detector and Leginon software package (Suloway et al., 2005). Micrographs were processed with MotionCor2 (Zheng et al., 2017), and the CTF function was calculated with CTFFIND4 (Rohou and Grigorieff, 2015). Template-based particle selection was done with FindEM (Roseman, 2004), and non-reference 2D classification was performed with RELION (Scheres, 2012). The final particle numbers used in single particle reconstructions were as follows: 9,559 RRV-Fab, 10,395 CHIKV-Fab, 18,410 MAYV-Fab and 20,592 native MAYV. Single particle reconstructions were performed according to the ‘gold standard’ method using jspr (Guo and Jiang, 2014). Briefly, particles were divided equally into two randomly selected independent particle sets. Two de novo models were generated from random sets of 1,000 particles. One de novo model was assigned to one of the particle sets. The other de novo model was assigned to the other particle set. Each independent dataset was refined iteratively assuming icosahedral symmetry. Refinement resulted in two independent models that converge on the same structure. Following corrections for astigmatism, elliptical distortion, defocus, and the masking of the disordered nucleocapsid core, the final models of each independent data set were combined into a single final model. The average resolution of each map calculated at 0.143 from FSC curves was 6.3Å for RRV, 5.3Å for CHIKV, 5.3Å for MAYV, and 4.8 Å for native MAYV.
Based upon resolution values between 5-6Å for all three of the Fab bound virus density maps, the alpha carbon backbone of the alphavirus E1, E2 structural glycoproteins and the Fab model CHK-265 (Fox et al., 2015) were used to identify and interpret the maps. The crystal structure of the CHIKV E1/E2 dimer, RCSB PDB 3N42, (Voss et al., 2010) was used to analyze the CHIKV-Fab-bound model. Homology models of both RRV and MAYV E1 and E2 glycoproteins generated with I-TASSER (Roy et al., 2010; Yang et al., 2015; Zhang, 2008), were used to analyze the RRV and MAYV Fab-bound models. The alpha carbon backbone of the RRV and MAYV E1 and E2 structures were aligned to the alpha carbon backbone of the CHIKV E1 and E2 crystal structures. The RMSD calculated in PyMOL for each alignment is 1.6 Å3 for E1 RRV:CHIKV and 2.0 Å3 for E2 RRV:CHIKV. The RMSD values for MAYV E1 and E2 aligned to CHIKV E1 and E2 are 1.6 Å3for E1 and 2.1 Å3 for E2. The alpha carbon backbone of a model of both the constant and variable domains of Fab CHK-265 was fitted to all three virus-Fab complexes. Homology models of the variable light (VL) and variable heavy (VH) domains of RRV-12 were generated from the primary amino acid sequence of each domain with I-TASSER. In order to confirm Fab CHK-265 was a suitable substitute for RRV-12, the alpha carbon backbone of the RRV-12 variable domains were aligned to the Fab CHK-265 alpha carbon variable domain backbone with PyMOL. The RMSD is 0.94Å3 for the VH domain alignment and 0.51Å3 for the VL domain alignment. The constant region of RRV-12 was not available at the time of this study.
The protein structures of E1, E2, E3 and Fab were fit to the density maps sequentially using Chimera (Pettersen et al., 2004) and EMfit (Rossmann et al., 2001). Based upon the domain structures defined for the CHIKV E1/E2 dimer crystal structure (Voss et al., 2010), E1 was fit as a three-domain structure: domains I, II, and III, and E2 was fit as a four-domain structure using domains A, B, C and β-ribbon. Fab CHK-265 was fit as a four-domain structure using heavy chain constant and variable domains, and light chain constant and variable domains. Fitting was performed assuming icosahedral symmetry for a T=4 virus particle. Goodness of fit of each domain of each protein was analyzed by average density heights, sumf, at each T number 1-4 and average sumf value for all T numbers. Each fitting produced a difference map and coordinates of the protein structure’s position relative to the density. The backbone of CHIKV E3 structure was positioned in the CHIKV RRV-12 bound density map using a backbone E2-E3 structure from PDB 6NK7 aligned to the EMfit CHIKV E2 structure with Chimera and COOT (Emsley et al., 2010). The RMSD, calculated in PyMOL, of the E2 region of the E2-E3 structure aligned to the CHIKV density map is 1.6Å3. Using the fitted coordinates of the Fab and E2 relative to one another for RRV and MAYV and E2 and E3 for CHIKV, residues of E2 for RRV and MAYV and residues of E2 and E3 for CHIKV located 6Å or less from the Fab structure were identified with PyMOL. The residues in this region of the E2 B domain were mapped to the surface of each of the three viruses with RIVEM (Xiao and Rossmann, 2007) and predicted to be the epitope.
The position of Mxra8 was mapped to the RRV-12 bound structures of RRV, MAYV, and CHIKV based upon its position in the asymmetric unit in PDB 6NK7 and EMDB 9395 (Basore et al., 2019). A combined E2, E3, and Mxra8 model was extracted from the asymmetric unit of the CHIKV virus structure bound with Mxra8. The combined structure was converted to backbone atoms only with PyMOL. The position of Mxra8 from that virus structure was mapped to the asymmetric unit of RRV-12 bound viruses RRV and MAYV relative to CHIKV with Chimera, PyMOL, and COOT. An asymmetric unit of each virus with both Mxra8 and RRV-12 bound was generated and used as the template for determining and mapping the residues of the virus surface within 6Å of either the Fab or Mxra8 with RIVEM.
ELISA-Based Mxra8-Fc Competition-Binding Assay
RRV-12 (2 μg/mL) was diluted in PBS and immobilized onto a 384-well ELISA plate before incubation for 1 h at 37°C. The plate was washed four times with PBS containing Tween (PBST) using an EL406 combination washer dispenser instrument (BioTek) and blocked for 1 h at room temperature with 5% milk powder and 2% goat serum, diluted in PBS. RRV, MAYV, or CHIKV were diluted to approximately 107 FFU/mL in PBS and 25 μL per well was added for 1 h at room temperature. After washing five times with PBST, RRV mAbs were diluted to 20 μg/mL in PBS and 25 μL of mAb was added to each well, except for control wells where just PBS was added. Blocking mAbs were incubated for 30 min at room temperature and 25 μL of Mxra8-Fc (mouse Fc region) (Zhang et al., 2018) fusion protein at a concentration of 10 μg/mL was then added to each well. After incubation at room temperature for an hour, the plate was washed four times with PBST and 25 μL per well of a goat anti-mouse HRP-conjugated anti-mouse Fc secondary antibody (SeraCare) was added at a 1:2,000 dilution. After five washes with PBST, plates were developed with TMB Substrate (ThermoFisher Scientific) and the reaction was stopped with H2SO4. Absorbance was read at 450 nm with a Biotek plate reader. A similarly prepared human mAb specific for Zika virus (ZIKV-117 (Sapparapu et al., 2016) was included as a negative control antibody. Titration curves for antibody blockage of Mxra8 were generated similarly.
Mouse Studies
Survival Studies
Four-week-old male WT C57BL/6J mice were treated with 0.2 mg of MAR1-5A3 (anti-Ifnar1 antibody) prior to inoculation with 103 FFU of WT RRV T48 strain in the footpad. The following day, 100 μg of RRV antibody or an isotype control antibody to an unrelated viral target was administered to mice by intraperitoneal injection. Mice were observed over the course of 21 days for survival and moribund mice were euthanized.
Virological Studies
Four-week-old male WT C57BL/6J mice were inoculated with 103 FFU of RRV strain T48 or MAYV strain BeH407 and then 24 h post-infection were given 100 μg antibody by intraperitoneal injection. Three days post-infection, the ipsilateral and contralateral gastrocnemius and quadriceps muscles, and ankle tissues were collected as well as the spleen following extensive perfusion with PBS. RNA was isolated from tissues using the RNeasy mini kit (Qiagen). Viral RNA was quantified by qRT-PCR using the TaqMan RNA to CT one-step kit (Applied Biosystems) with RRV nsp3 specific primers (Forward: 5′- GTG TTC TCC GGA GGT AAA GAT AG -3′, Reverse: 5′- TCG CGG CAA TAG ATG ACT AC -3′) and probe (5′- /56-FAM/ACC TGT TTA/ZEN/CCG CAA TGG ACA CCA/ 3IABkFQ/ -3′) or MAYV specific primers and probe (Earnest et al., 2019) and compared to RNA isolated from viral stocks as a standard curve to determine FFU equivalents.
Clinical Scoring Studies
Three-week-old male and female WT C57BL/6J mice were inoculated with 103 FFU of RRV strain T48 and then 24 hpi were given 100 μg antibody by intraperitoneal injection. Mice were weighed and assigned a clinical score based on grip strength, gait, and righting reflex, as previously described (Haist et al., 2017). Mice were scored blinded and as follows: 0, no disease; 1, mild defect in ipsilateral hind paw gripping; 2, mild defect in bilateral hind paw gripping; 3, bilateral loss in hind paw gripping; 4, bilateral loss in hind paw gripping with moderate hind limb weakness, observable mild altered gait, and difficulty or failure to right self; 5, bilateral loss in hind paw gripping with severe hind limb weakness, moderate altered gait, and loss of righting reflex; 6, bilateral loss in hind paw gripping with severe hind limb weakness, severely altered gait with possible dragging hind paw, and loss of righting reflex; 7, moribund. No mice received a score of 7 throughout the course of the experiment. Eighteen days post-infection, the spleen, ipsilateral and contralateral gastrocnemius, quadriceps, and ankle tissues were collected following extensive perfusion with PBS. Viral RNA was quantified as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses are described in the figure legends. All analyses were performed using Prism software (GraphPad Software). For quantification of viral RNA in RRV and MAYV WT mouse models, statistical analysis was performed using a one-way ANOVA with a Dunnett’s post-test comparing each group to the isotype control (**p < 0.01, ***p < 0.001, ****p < 0.0001). Two independent experiments were performed, with ten mice per antibody for each group. For clinical disease studies in mice, two independent experiments were performed, with a total of n=7-8 mice in each antibody group. Statistical analysis was performed using a student’s t-test of area under the curve analysis (****p < 0.0001) for clinical disease scoring, and a Mann Whitney test (*p < 0.05, **p < 0.01, ***p < 0.001) for analysis of viral RNA. For the lethal challenge immunocompromised RRV mouse model, two independent experiments were performed, with n=10 mice per antibody for each group, and statistical analysis was performed using a log rank test with Bonferroni correction, p = 0.0057.
For mechanistic assays determining reduction in foci number and size due to RRV-12, three independent experiments were performed with triplicate samples in each experiment. A one-way ANOVA with Kruskal-Wallis post-test was used for statistical analysis, with mean ± S.D. compared to a ZIKV-117 control (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat Anti-Human IgG-AP | Meridian Life Science | W99008A |
| ZIKV-117 (recombinant CHO-produced IgG1) | Sapparapu et al., 2016 | N/A |
| rRRV-12 IgG (recombinant CHO-produced) | This paper | N/A |
| rRRV-12 IgG1-LALA (recombinant CHO-produced) | This paper | N/A |
| Goat anti-mouse IgG HRP | SeraCare | 5220-0341 |
| Anti-RRV mouse ascites fluid | ATCC | VR-1246AF |
| Anti-MAYV mouse ascites fluid | ATCC | V-507-701-562 |
| Anti-CHIKV mouse ascites fluid | ATCC | V-548-701-562 |
| Bacterial and Virus Strains | ||
| RRV strain T48 | ATCC | VR-373 |
| Chikungunya virus strain 181/25 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| O’nyong’nyong virus strain MP30 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| Mayaro virus strains TR VL-4675 and BeH407 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| Sagiyama virus strain M6-Mag 132 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| Getah virus strain MM 2021 | World Reference Center for Emerging Viruses and Arboviruses at UTMB | N/A |
| Biological Samples | ||
| PBMCs from RRV-immune donor (laboratory-confirmed infection in 1989) | This paper | Donor ID #974 |
| PBMCs from CHIKV-immune donor (naturally infected in Colombo, Sri Lanka outbreak from 2006-2008) | This paper | Donor ID #899 |
| Chemicals, Peptides, and Recombinant Proteins | This paper | N/A |
| TrueBlue Peroxidase Substrate | SerCare | 5510-0030 |
| Mxra8-Fc Fusion Protein | Zhang et al., 2018 | N/A |
| 1-Step Ultra TMB-ELISA | ThermoFisher | Cat# 34029 |
| ExpiCHO Expression Medium | ThermoFisher | Cat# A2910001 |
| Fetal Bovine Serum, ultra-low IgG | ThermoFisher | Cat# 16250078 |
| Fetal Bovine Serum | Sigma | Cat# F0926 |
| Minimal Essential Medium, NEAA, powder | Thermofisher | Cat# 415000-018 |
| ClonaCell-HY Medium E | Stem Cell Technologies | Cat# 03805 |
| ClonaCell-HY Medium A | Stem Cell Technologies | Cat# 03801 |
| Mayaro virus E3E2 recombinant protein | Meridian Life Sciences | Cat# R01780 |
| Chikungunya virus E2 recombinant protein | Meridian Life Sciences | Cat# R01702 |
| Pierce Fab Preparation Kit | ThermoFisher | 44985 |
| OptiPrep | Sigma | D 1655 |
| Deposited Data | ||
| RRV + RRV-12 cryo-EM structure | This paper | EMD 21473 PDB 6VYV |
| CHIKV + RRV-12 cryo-EM structure | This paper | EMD 21496 PDB 6W09 |
| MAYV + RRV-12 cryo-EM structure | This paper | EMD 21509 PDB 6WIC |
| MAYV cryo-EM structure | This paper | EMD 21532 PDB 6W2U |
| Recombinant DNA | ||
| Plasmid: RRV-12 rIgG1 heavy chain | This paper | N/A |
| Plasmid: RRV-12 light chain | This paper | N/A |
| Plasmid: RRV-12 rIgG1-LALA heavy chain | This paper | N/A |
| Software and Algorithms | ||
| GraphPad Prism 7.2 | GraphPad Software, Inc. | https://www.graphpad.com |
| FlowJo version 10 | Tree Star Inc. | https://www.flowjo.com/solutions/flowjo/downloads |
| PyMOL | Schrödinger | https://www.pymol.org/ |
| BioSpot 5.1 software | CTL | N/A |
| Leginon | Suloway et al., 2005 | N/A |
| MotionCor2 | Zheng et al., 2017 | N/A |
| CTFFIND4 | Rohou and Grigorieff et al., 2015 | N/A |
| FindEM | Roseman et al., 2004 | N/A |
| RELION | Scheres, 2012 | N/A |
| jspr | Guo and Jiang, 2014 | N/A |
| I-TASSER | Yang et al., 2015 | N/A |
| Chimera | Pettersen et al., 2004 | N/A |
| EMfit | Rossmann et al., 2001 | N/A |
| RIVEM | Xiao and Rossmann, 2007 | N/A |
| Coot | Emsley et al., 2009 | N/A |
| Other | ||
| BD Fortessa flow cytometer | BD Biosciences | N/A |
| BD FACSAria III sorting cytometer | BD Biosciences | N/A |
| ÄKTA pure chromatography system | GE Healthcare | N/A |
| Synergy H1 microplate reader | BioTek | N/A |
| Synergy 2 microplate reader | BioTek | N/A |
| EL406 washer dispenser | BioTek | N/A |
| Biostack microplate stacker | BioTek | N/A |
| HiTrap Protein G High Performance | GE Healthcare | Cat# 28-9075-48 |
| Octet RED96 BLI instrument | Pall FortéBio | |
| Anti-Penta-HIS (HIS1K) biosensor tips | Molecular Devices | N/A |
| ImmunoSpot plate reader | CTL | |
| Ultrathin Carbon Film on Lacey Carbon, 400 mesh, Cu grid | Ted Pella | 01824 |
Highlights.
Approved therapies or licensed vaccines do not exist for arthritogenic alphaviruses
Characterization of human antibodies that bind to and neutralize diverse alphaviruses
Identified mAbs prevent attachment to the Mxra8 alphavirus receptor
Cryo-EM shows mAb RRV-12 has a conserved footprint on three different alphaviruses
ACKNOWLEDGMENTS
We thank R. Nargi and R. Irving at Vanderbilt University Medical Center for laboratory management support. This work was supported by NIH grants R01 AI114816 and R01 AI1095366 (to M.G.R. and R.J.K.). U19 AI142790 (M.S.D., J.E.C., and R.J.K.), R01 AI143673 (M.S.D.), and NIH contract AI201800001 (J.E.C. and M.S.D.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.chom.2020.07.008.
DECLARATION OF INTERESTS
M.S.D. is a consultant for Inbios and Emergent BioSolutions and on the Scientific Advisory Board of Moderna. J.E.C. has served as a consultant for Takeda Vaccines, Sanofi Pasteur, Pfizer, and Novavax; is on the Scientific Advisory Boards of CompuVax, GigaGen, and Meissa Vaccines; and is the Founder of IDBiologics Inc. All other authors declare no competing interests.
REFERENCES
- Acosta-Ampudia Y, Monsalve DM, Rodríguez Y, Pacheco Y, Anaya JM, and Ramírez-Santana C (2018). Mayaro: an emerging viral threat? Emerg. Microbes Infect 7, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alva-Urcia C, Aguilar-Luis MA, Palomares-Reyes C, Silva-Caso W, Suarez-Ognio L, Weilg P, Manrique C, Vasquez-Achaya F, Del Valle LJ, and Del Valle-Mendoza J (2017). Emerging and reemerging arboviruses: a new threat in Eastern Peru. PLoS One 12, e0187897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduin E, Arora S, Bamert PR, Kuiper T, Popp S, Geisse S, Grau R, Calzascia T, Zenke G, and Kovarik J (2015). Highly reduced binding to high and low affinity mouse Fc gamma receptors by L234A/L235A and N297A Fc mutations engineered into mouse IgG2a. Mol. Immunol 63, 456–463. [DOI] [PubMed] [Google Scholar]
- Bangaru S, Lang S, Schotsaert M, Vanderven HA, Zhu X, Kose N, Bombardi R, Finn JA, Kent SJ, Gilchuk P, et al. (2019). A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell 177, 1136–1152.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basore K, Kim AS, Nelson CA, Zhang R, Smith BK, Uranga C, Vang L, Cheng M, Gross ML, Smith J, Diamond MS, and Fremont DH (2019). Cryo-EM structure of chikungunya virus in complex with the Mxra8 receptor. Ceil 177, 1725–1737.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CS, and SchGtteikopf AW (2009). ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr. D Biol. Crystallogr 65, 510–512. [DOI] [PubMed] [Google Scholar]
- Brown RS, Wan JJ, and Kielian M (2018). The Alphavirus exit pathway: what we know and what we wish we knew. Viruses 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, and Bordi L (2013). Chikungunya virus infection: an overview. New Microbiol. 36, 211–227. [PubMed] [Google Scholar]
- Earnest JT, Basore K, Roy V, Bailey AL, Wang D, Alter G, Fremont DH, and Diamond MS (2019). Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J. Exp. Med 216, 2282–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JM, Long F, Edeling MA, Lin H, van Duijl-Richter MKS, Fong RH, Kahle KM, Smit JM, Jin J, Simmons G, et al. (2015). Broadly neutralizing Alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 163, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, and Jiang W (2014). Single particle cryo-electron microscopy and 3-D reconstruction of viruses. Methods Mol. Biol 1117, 401–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist KC, Burrack KS, Davenport BJ, and Morrison TE (2017). Inflammatory monocytes mediate control of acute Alphavirus infection in mice. PLoS Pathog. 13, e1006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley D, Sleigh A, and Ritchie S (2001). Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin. Microbiol. Rev 14, 909–932, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidner HW, Knott TA, and Johnston RE (1996). Differential processing of Sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J. Virol 70, 2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal DN, Parren PWHI, et al. (2007). Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–104. [DOI] [PubMed] [Google Scholar]
- Jin J, Galaz-Montoya JG, Sherman MB, Sun SY, Goldsmith CS, O’Toole ET, Ackerman L, Carlson LA, Weaver SC, Chiu W, and Simmons G (2018). Neutralizing antibodies inhibit Chikungunya virus budding at the plasma membrane. Cell Host Microbe 24, 417–428.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Liss NM, Chen DH, Liao M, Fox JM, Shimak RM, Fong RH, Chafets D, Bakkour S, Keating S, et al. (2015). Neutralizing monoclonal antibodies block Chikungunya virus entry and release by targeting an epitope critical to viral pathogenesis. Cell Rep. 13, 2553–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, and Simmons G (2019). Antiviral functions of monoclonal antibodies against Chikungunya virus. Viruses 11, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose J, Snyder JE, and Kuhn RJ (2009). A structural and functional perspective of Alphavirus replication and assembly. Future Microbiol. 4, 837–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose N, Fox JM, Sapparapu G, Bombardi R, Tennekoon RN, de Silva AD, Elbashir SM, Theisen MA, Humphris-Narayanan E, Ciaramella G, et al. (2019). A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol 4, eaaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanomido T, Kamada M, Wada R, Kenemaru T, Sugiura T, and Akiyama Y (1988). Pathogenicity for horses of original Sagiyama virus, a member of the Getah virus group. Vet. Microbiol 17, 367–373. [DOI] [PubMed] [Google Scholar]
- Lednicky J, De Rochars VMB, Elbadry M, Loeb J, Telisma T, Chavannes S, Anilis G, Celia E, Ciccozzi M, Okech B, et al. (2016). Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerging Infect. Dis 22, 2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Kam YW, Frio J, Malleret B, Koh EGL, Prakash C, Huang W, Lee WWL, Lin C, Lin RTP, et al. (2011). Chikungunya virus neutralization antigens and direct cell-to-cell transmission are revealed by human antibody-escape mutants. PLoS Pathog. 7, e1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi LI, and Vignuzzi M (2019). Arthritogenic alphaviruses: a worldwide emerging threat? Microorganisms 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MG, and Kielian M (2016). Intercellular extensions are induced by the Alphavirus structural proteins and mediate virus transmission. PLoS Pathog. 12,e1006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE (2014). Reemergence of Chikungunya virus. J. Virol 88, 11644–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, and Heise MT (2011). A mouse model of Chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol 178,32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MKS, et al. (2013). Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog. 9, e1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Rezza G, Chen R, and Weaver SC (2017). O’nyong-nyong fever: a neglected mosquito-borne viral disease. Pathog. Glob. Health 111, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohou A, and Grigorieff N (2015). CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol 192, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman AM (2004). FindEM–a fast, efficient program for automatic selection of particles from electron micrographs. J. Struct. Biol 145, 91–99. [DOI] [PubMed] [Google Scholar]
- Rossmann MG, Bernal R, and Pletnev SV (2001). Combining electron microscopic with x-ray crystallographic structures. J. Struct. Biol 136, 190–200. [DOI] [PubMed] [Google Scholar]
- Roussel A, Lescar J, Vaney MC, Wengler G, Wengler G, and Rey FA (2006). Structure and interactions at the viral surface of the envelope protein E1 of Semliki Forest virus. Structure 14, 75–86. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, and Zhang Y (2010). I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc 5, 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Bin Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. (2016). Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KO (2019). Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol 10, 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH (2012). RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol 180, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KCF, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, and Schreiber RD (2006). Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res 26, 804–819. [DOI] [PubMed] [Google Scholar]
- Shirako Y, and Yamaguchi Y (2000). Genome structure of Sagiyama virus and its relatedness to other alphaviruses. J. Gen. Virol 81, 1353–1360. [DOI] [PubMed] [Google Scholar]
- Smith SA, and Crowe JE (2015). Use of human hybridoma technology to isolate human monoclonal antibodies In Antibodies for infectious diseases, Boraschi D, Crowe JE, and Rappuoli R, eds. (American Society of Microbiology; ), pp. 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, et al. (2015). Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against Chikungunya virus. Cell Host Microbe 18, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AJ, Sokoloski KJ, and Mukhopadhyay S (2012). Mutating conserved cysteines in the Alphavirus e2 glycoprotein causes virus-specific assembly defects. J. Virol 86, 3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Zhao Z, Chai Y, Jin X, Li C, Yuan F, Liu S, Gao Z, Wang H, Song J, et al. (2019). Molecular basis of arthritogenic Alphavirus receptor Mxra8 binding to Chikungunya virus envelope protein. Cell 177, 1714–1724.e12. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, and Carragher B (2005). Automated molecular microscopy: the new Leginon system. J. Struct. Biol 151, 41–60. [DOI] [PubMed] [Google Scholar]
- Tesh RB, McLean RG, Shroyer DA, Calisher CH, and Rosen L (1981). Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in American Samoa. Trans. R. Soc. Trop. Med. Hyg 75, 426–431. [DOI] [PubMed] [Google Scholar]
- Tupanceska D, Zaid A, Rulli N, Thomas S, Lidbury B, Matthaei K, Ramirez R, and Mahalingam S (2007). Ross River virus: an arthritogenic Alphavirus of significant importance in the Asia Pacific, Emerging viral diseases of Southeast Asia In Issues in Infectious Diseases, 4 (Karger Publishers; ), pp. 94–111. [Google Scholar]
- Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, and Rey FA (2010). Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468,709–712. [DOI] [PubMed] [Google Scholar]
- Weger-Lucarelli J, Aliota MT, Kamlangdee A, and Osorio JE (2015). Identifying the role of E2 domains on Alphavirus neutralization and protective immune responses. PLoS Negl. Trop. Dis 9, e0004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, and Rossmann MG (2007). Interpretation of electron density with stereographic roadmap projections. J. Struct. Biol 158, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Huang Y, Gnanadurai CW, Cao S, Liu X, Cui M, and Fu ZF (2015). The inability of wild-type rabies virus to activate dendritic cells is dependent on the glycoprotein and correlates with its low level of the de novo-synthesized leader RNA. J. Virol 89, 2157–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller H, Van Bortel W, and Sudre B (2016). Chikungunya: its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis 214, S436–S440. [DOI] [PubMed] [Google Scholar]
- Zhang R, Hryc CF, Cong Y, Liu X, Jakana J, Gorchakov R, Baker ML, Weaver SC, and Chiu W (2011). 4.4 Å cryo-EM structure of an enveloped Alphavirus Venezuelan equine encephalitis virus. EMBO J. 30, 3854–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kim AS, Fox JM, Nair S, Basore K, Klimstra WB, Rimkunas R, Fong RH, Lin H, Poddar S, et al. (2018). Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 557, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2008). I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cryoEM structures are deposited at the Electron Microscopy Data Bank (EMDB). The ID numbers of Fab bound structures are RRV: EMD 21473 PDB 6VYV, CHIKV: EMD 21496 PDB 6W09, MAYV: EMD 21509 PDB 6WIC and the native is MAYV: EMD 21532 PDB 6W2U. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplemental Information. The CHIKV antibodies in this study are available by Material Transfer Agreement with Vanderbilt University Medical Center.


