Abstract
A key immunomodulatory cytokine, Interleukin-10 (IL-10), has been shown to be dysregulated in preeclampsia, a pregnancy-specific hypertensive disorder, further characterized by multi-system involvement. However, studies have reported inconsistent findings about circulating IL-10 levels in preeclamptic versus normotensive pregnancies. The aim of the present systematic review and meta-analysis was to assess circulating IL-10 levels in preeclamptic and normotensive pregnancies at two time points: before, and at the time of preeclampsia diagnosis. PubMED, EMBASE and Web of Science databases were searched to include all published studies examining circulating IL-10 levels in preeclamptic and normotensive pregnancies. Differences in IL-10 levels were evaluated by standardized mean differences (SMD). Of 876 abstracts screened, 56 studies were included in the meta-analysis. Circulating IL-10 levels were not different before the time of active disease (SMD: −0.01, 95% confidence interval [CI]: −0.11, 0.08; P = 0.76). At the time of active disease, women with preeclampsia (n=1599) had significantly lower IL-10 levels compared to normotensive controls (n=1998) (SMD: −0.79, 95% CI: −1.22, −0.35; P = 0.0004). IL-10 levels were lower in both early/severe and late/mild forms of preeclampsia. Subgroup analysis revealed that IL-10 measurement methodology (ELISA or multiplex bead array) and the sample type (plasma or serum) significantly influenced the observed differences, with the use of sera paired with ELISA technology providing the best distinction in IL-10 levels between preeclamptic and normotensive pregnancies. These findings support the role of decreased IL-10 levels in the pathophysiology of preeclampsia. Future studies should address the therapeutic potential of IL-10 in preeclampsia.
Keywords: Preeclampsia, Interleukin-10, hypertension, pregnancy, endothelial dysfunction
Graphical Abstract
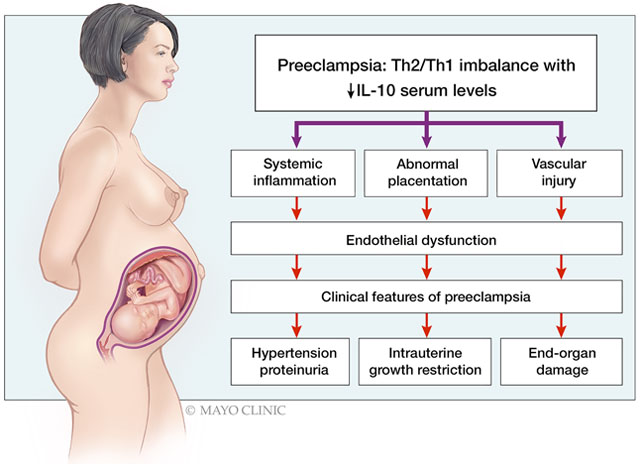
Summary
At the time of active disease, women with preeclampsia, regardless of its severity, have significantly lower IL-10 levels compared to normotensive controls. Our data suggest that the use of sera paired with ELISA technology provides the best distinction between preeclamptic and normotensive pregnancies. Future studies should address the therapeutic potential of IL-10 in the clinical management of preeclampsia.
INTRODUCTION
Preeclampsia (PE) is a hypertensive syndrome of pregnancy characterized by new-onset hypertension (systolic blood pressure [SBP], ≥140 mm Hg and/or diastolic blood pressure [DBP], ≥90 mm Hg) and proteinuria (urinary protein level ≥ 300 mg/24 h) after 20 weeks gestation. [1] It remains a leading cause of maternal and fetal morbidity and mortality, and affects 5–8% of all pregnancies. [2] Two distinct clinical phenotypes have been recognized, namely early-onset PE (before 34 weeks gestation) and late-onset PE (after 34 weeks gestation), which tend to differ in severity, with early PE presenting more commonly with severe features compared to late onset, which tends to be milder.[3]
PE is a multi-systemic disorder with a complex pathophysiology characterized by systemic endothelial dysfunction, which is the common denominator of all potential pathophysiologic pathways. [4,5] Endothelial dysfunction during pregnancy manifests as hypertension, end-organ damage, and proteinuria which are hallmarks of PE and may be mechanistically related to immune system dysregulation. While normal pregnancy represents a state of strictly controlled inflammation with a predominance of a Th2-type immune response, PE is characterized by the predominance of a pro-inflammatory, Th1-type immune response. [6]
Interleukin 10 (IL-10), a key immunomodulatory cytokine in the Th2 immune response, has not only anti-inflammatory, but also significant vaso-protective properties and may promote successful placentation during normal pregnancy.[4] The dysregulation of IL-10 has been associated with the occurrence of PE. The relative or absolute deficiency of IL-10 may contribute to the development of PE through abnormal placentation, systemic inflammation, and vascular dysfunction. An immune imbalance favoring the Th1 response, with decreased IL-10 availability, has been reported in multiple studies including studies using placentas,[7–9] peripheral blood monocytes (PMBC), [10] uterine and circulating NK cells, [11] and fetal macrophages and regulatory T cells.
While studies of placentas and PMBCs in preeclampsia have consistently shown decreased IL-10 levels, studies of circulating IL-10 levels in preeclampsia have provided inconsistent results, with some studies reporting decreased, some increased, and some no differences in IL-10 circulating levels compared to those in normotensive pregnancies. This variability may be caused by several factors, including the heterogeneity of the patient population, and the inclusion of different PE subtypes (eg early vs. late) with distinct underlying mechanisms which may differentially affect circulating IL-10 levels, the timing of IL-10 measurement, patient characteristics (BMI and comorbidities), and the methodology used to detect IL-10. In the current study, we hypothesized that systemic IL-10 levels will be lower at the time of diagnosis in preeclamptic compared to normotensive pregnancies, which, in turn, can contribute to the immune dysregulation, pro-inflammatory milieu, and anti-angiogenic state seen in preeclampsia. To that end, we conducted a systematic review and meta-analysis of studies that reported and compared circulating IL-10 levels in preeclamptic and normotensive pregnancies at the time of diagnosis. Additionally, we analyzed studies that reported IL-10 levels prior to PE diagnosis in order to determine whether circulating IL-10 in early pregnancy may serve as a potential predictor and/or trigger for PE. Finally, we sought to gain insight into factors that may affect IL-10 measurements in the peripheral circulation, and potentially explain the inconsistencies noted in previous reports.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Search Strategy and Selection.
This systematic review was undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta Analysis of Observational Studies in Epidemiology.[12,13] Along with the guidance of an expert librarian, H.C. and N.M.M. determined the search strategies, which focused on MeSH headings and keywords related to preeclampsia and circulating IL-10 (Table S1 in the Data Supplement). The databases Medline, Embase, and Web of Science were examined using these search strategies to identify possibly eligible studies. The criteria for publication dates included all studies through April 16, 2019. The search was restricted to studies written in English.
Article Screening and Selection.
Two reviewers (H.C. and M.C.N.) independently assessed potentially relevant titles and abstracts found within the aforementioned databases. An online technology platform for systematic reviews, Covidence, was used to record the decisions of the reviewers and to help resolve discrepancies.[14] The reviewers included studies for full text screening that they deemed to be potentially eligible or that conveyed inadequate information within the title and abstract. Studies were selected for full text screening if they included circulating plasma or serum IL-10 levels in preeclamptic and normotensive patients during pregnancy. Articles were also selected for full text screening if the associated abstracts did not mention preeclampsia, but discussed patients with pregnancy complications relevant to preeclampsia such as pre-term birth, intrauterine growth restriction, and gestational diabetes. The exclusion criteria for full text selection were as follows: non-English studies, editorials, letters to the editor, review papers, studies examining IL-10 in animal models, conference abstracts, case reports or series, letters to the editor, studies reporting IL-10 levels in tissues/cells other than sera or plasma, studies that reported IL-10 levels in disease settings other than preeclampsia, studies that lacked normotensive controls, studies examining IL-10 levels outside of pregnancy, and studies that included the same patients. Upon completing title and abstract screening, the reviewers independently conducted full text screening. The reviewers based their final decision about the inclusion of each study based on the criteria outlined above. After separate assessments, the reviewers worked together to resolve discrepancies.
Data Abstraction.
The two reviewers specified above abstracted the following data from the selected studies: 1) Circulating IL-10 levels in preeclamptic and normotensive patients, 2) Study design and setting, 3) Source of preeclamptic and normotensive patients, 4) Types of preeclamptic (early-onset, late-onset, severe, mild) and normotensive (pregnant vs. non-pregnant) patients, 5) Number of preeclamptic and normotensive patients, 6) Preeclampsia diagnostic criteria, 7) Patient exclusion criteria, 8) Pregnancy history assessment, 9) Timing of sampling, 10) Gestational age and BMI, 11) Methodology used for measurement of IL-10 levels and its associated coefficient of variation, 12) Sample type (serum or plasma), and 13) Information about blinding. If studies were missing relevant data, the reviewers contacted the authors to request information. Studies were excluded if reviewers were unable to obtain the necessary information upon request. Some studies reported IL-10 levels graphically; the reviewers used plot digitizer software to determine raw IL-10 values.[15] The eligible studies were stratified into two groups based on the timing of a preeclampsia diagnosis among patients. One group was comprised of studies that reported IL-10 levels in pregnant women at the time of or after a preeclampsia diagnosis, while the second group included studies with IL-10 levels in pregnant women prior to preeclampsia diagnosis.
Risk of Bias.
The reviewers independently assessed the risk of bias within each study using an adapted version of the Newcastle-Ottawa tool for observational studies and the guidelines outlined by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) Working Group. [16,17]
Statistical Analyses.
The primary outcome was circulating IL-10 levels, expressed as means with standard deviations. Because the studies employed a variety of methodologies to measure IL-10 levels, our team calculated the standardized mean differences (SMD) between IL-10 levels of the preeclamptic and normotensive groups. This measurement of effect size expresses the difference between group means in units of standard deviations and was assessed by pooling individual trial results employing random-effects models through the DerSimonian-Laird method (Review Manager, version 5.3; Cochrane Collaboration). Heterogeneity was evaluated using the Cochran Q test and the I2 statistic. A forest plot was created for each analysis depicting the SMD (box), 95% CI (lines) and weight (size of box) for each trial. Subgroup analyses were performed to examine the effects of: 1) more and less severe forms of preeclampsia, 2) methodology used for measurement of IL-10 (ELISA vs. Multiplex platforms), and 3) sample type used for measurement of IL-10 (serum vs. plasma). Sensitivity analyses were conducted to examine the effects of: 1) measurements at different time points in cohort studies, 2) the exclusion of studies that observed a very large effect, 3) the exclusion of studies utilizing data obtained from women with chronic hypertension, and 4) the exclusion of studies that presented IL-10 values as median (interquartile range-IQR). One study in each time period reported medians and interquartile ranges (IQR) because IL-10 data were not normally distributed. Although the DerSimonian-Laird method is highly robust against even very severe violations of the assumption of normally distributed effect sizes, we performed sensitivity analyses to verify that excluding these studies would not have changed the results. For the purpose of the analyses, the median was used to approximate the mean, while the SD was estimated as IQR/1.35.
RESULTS
The review process of this systematic review is presented using the PRISMA flow diagram (Figure 1). The database search identified 876 potential articles, from which 97 full text articles were reviewed and 62 were included in the systematic review. A total of 56 studies were included in the meta-analysis. This included studies that measured circulating IL-10 levels in patients before PE diagnosis (n = 9) and at the time of PE diagnosis (n = 49). The online-only Data Supplement includes tables presenting study summaries (Table S2 and S3 in the Data Supplement), diagnostic and exclusion criteria (Table S4 and S5 in the Data Supplement) and quality of studies assessment (Table S6 and S7 in the Data Supplement).
Figure 1.

Study flow chart
Circulating IL-10 before PE diagnosis
Nine studies [18–26] reported circulating IL-10 levels among 781 pregnant women who were later diagnosed with preeclampsia and 1781 normotensive pregnant women (Table S2 in the Data Supplement). Of these studies, 6 were cohort studies [19–24] and 3 were nested case control studies[18,25,26]. All studies were included in both the systematic review and meta-analysis. Circulating IL-10 levels were not significantly different between women who subsequently developed PE and women who did not develop PE (SMD: −0.01, 95% confidence interval [CI]: −0.11, 0.08; P=0.76) (Figure 2). The analysis did not reveal significant heterogeneity among the study results (I2=0%, P=0.76). Two studies included multiple time points. [20,22] Results did not differ between analyses that included second trimester studies only (SMD: −0.05, 95% confidence interval [CI]: [−0.16, 0.06; P=0.37), [18,23,25] the earliest time points from all studies (SMD: −0.00, 95% CI: [−0.10, 0.10; P=0.96) [19–21,24,26] or the latest time points from all studies (SMD: −0.01, 95% CI: −0.10, 0.08; P=0.82). [18,22,23] Results were not different when studies that reported IL-10 as median (interquartile range) [19,21] were excluded (SMD: −0.05, 95% CI: −0.17, 0.06; P=0.36).
Figure 2.

Standardized mean difference in IL-10 levels among the studies performed before preeclampsia diagnosis
Circulating IL-10 at the time of PE diagnosis
A total of 49 studies[8,20,22,27–72] reported circulating IL-10 levels in 1599 preeclamptic and 1998 normotensive pregnant women at the time of a preeclampsia diagnosis (Table S3 in the Data Supplement). Six additional studies were included in the qualitative synthesis. [11,73–77] Of these studies, 42 [8,27–32,34–38,41–44,47–58,60–72,78] were cross sectional studies, five were cohort [20,22,39,40,45] and 2 were nested case control studies. [46,59] Women with preeclampsia had significantly lower circulating IL-10 levels at the time of their diagnosis of PE (SMD: −0.79, 95% CI: −1.22, −0.35; P = 0.0004) (Figure 3). There was significant heterogeneity among the results of the respective studies (I2=97%, P<0.001). The exclusion of two studies that highly affected the level of heterogeneity [28,49] led to a small reduction in the SMD (−0.38, 95% CI: −0.74, −0.03; P=0.04). Preeclampsia superimposed on chronic hypertension is a distinct clinical entity, and, as such, likely different with respect to cytokine abnormalities compared to preeclampsia de novo. The magnitude of the overall effect was attenuated in sensitivity analyses of studies that excluded women with chronic hypertension, [28,31,34,36–38,42,44,45,49,51,53,54,60,61,64,65,67–69,72], but the difference between women with preeclampsia and normotensive pregnancies remained highly statistically significant (SMD: −1.47, 95% CI: −2.37, −0.57; P=0.001). Selecting different time points for one study that included multiple time points [22] did not alter the results. Excluding studies in which IL-10 was reported as median (interquartile range) had no effect.[8,22,27,30,32,39,45,46,52,55,57,58,68–71,79]
Figure 3.

Standardized mean difference in IL-10 levels among the studies performed at the time of preeclampsia diagnosis
Subgroup analyses were performed to examine the effects of more and less severe forms of preeclampsia (diagnostic criteria: Table S5 in the Data Supplement). At the time of PE diagnosis, 20 studies [28,29,34–36,40–42,45,49,56,58,64–67,70–72,78] reported circulating IL-10 levels in 446 patients with more severe forms of PE and 791 normotensive controls, while 20 studies [28,29,33–38,40–42,45,49,58,64–67,70,71] reported IL-10 levels in 632 patients with less severe forms of PE and 905 normotensive controls. At the time of active disease, IL-10 was significantly lower in women with severe forms of PE compared with women who had normotensive pregnancies (SMD: −1.04, 95% CI: −1.69, −0.39; P=0.002) (Figure 4). There was significant heterogeneity among the individual study results (I2=95%, P<0.001). Similar results were observed in studies examining women with less severe forms of preeclampsia [28,29,33–38,40–42,45,49,58,64–67,70,71] compared with women who had normotensive pregnancies (SMD: −1.53, 95% CI: −2.37, −0.70; P=0.003) (Figure 5).
Figure 4.

Standardized mean difference in IL-10 levels among the studies that compared women with severe preeclampsia to those with normotensive pregnancies
Figure 5.

Standardized mean difference in IL-10 levels among the studies that compared women with less severe preeclampsia to those with normotensive pregnancies
Separate analyses were performed to examine the effects of methodology used for measurement of IL-10 (ELISA vs. multiplex technology) (Table S5 in the Data Supplement). At the time of PE diagnosis, 30 studies [8,20,28–31,34,38–41,44–49,51,53–57,60,62–66,72] reported circulating IL-10 levels measured by ELISA in 1079 patients and 1177 normotensive controls, while 17 studies [22,27,32,33,35,37,42,50,52,58,59,61,67–71] reported IL-10 levels measured by multiplex technology in 458 patients and 760 normotensive controls. Analyses of studies that used ELISAs revealed significantly lower IL-10 levels in women with PE compared with women who had normotensive pregnancies (SMD: −1.48, 95% CI: −2.20, −0.76; P<0.001) (Figure S1 in the Data Supplement), while analyses of studies which used multiplex technology showed no significant difference between the groups in circulating IL-10 levels (SMD: 0.16, 95% CI: −0.07, 0.38; P=0.18) (Figure S2 in the Data Supplement).
Additional analyses were performed to examine the effects of sample type used for IL-10 measurement (serum vs. plasma) (Table S5 in the Data Supplement). At the time of PE diagnosis, 29 studies reported circulating IL-10 levels measured from serum in 1050 patients and 1261 normotensive controls, while 20 [20,22,29,31,33,37–39,42,47,52,54,56,62–65,67,70,72] studies reported IL-10 levels measured in plasma in 549 patients and 737 normotensive controls. Analyses of studies that used serum revealed significantly lower IL-10 levels in women with PE compared with women who had normotensive pregnancies (SMD: −1.33, 95% CI: −1.98, −0.68; P<0.001) (Figure S3 in the Data Supplement), while analyses of studies which used plasma revealed no significant difference in circulating IL-10 levels between the groups (SMD: 0.02, 95% CI: −0.50, 0.55; P=0.93) (Figure S4 in the Data Supplement). The use of sera paired with ELISA technology resulted in significantly lower IL-10 levels in PE compared normotensive pregnancies (Figure S5 in the Data Supplement); studies using multiplex technology, irrespective of the sample type, and those using ELISAs for plasma samples, revealed no significant differences in IL-10 levels between preeclamptic and normotensive pregnancies.
DISCUSSION
This study, to the best of our knowledge, represents the first major systematic review and meta-analysis of the literature reporting circulating IL-10 levels in PE and normal pregnancies. It included 56 relevant studies published to date, providing IL-10 measurements in approximately 6000 patients, from more than 25 countries and six continents. In this systematic review and meta-analysis, we present several novel findings. First, we found that preeclampsia is associated with decreased circulating IL-10 levels at the time of active disease. The association was present in the general PE population, in patients with severe/early forms of disease, as well among those with mild/moderate/late forms of disease, and was preserved after multiple sensitivity analyses. Second, subgroup analyses showed that IL-10 levels were lower in PE patients when serum rather than plasma samples were analyzed, and/or when studies used the ELISA method versus multiplex bead arrays for IL-10 detection. Third, there were no differences in plasma/serum levels of IL-10 in early pregnancy prior to active disease.
The significance of our findings is of particular clinical interest in several ways. First, this study underscores the role of IL-10 deficiency in the pathophysiology of PE. Our meta-analysis illustrates that IL-10 levels are decreased at the time of active disease in all forms of preeclampsia. This suggests that the state of systemic inflammation and endothelial dysfunction seen in PE is associated with IL-10 deficiency, which may ultimately result in impaired immunomodulation, a pro-inflammatory milieu, and impaired angiogenesis. The possible role of IL-10 in the pathogenesis of preeclampsia was further supported by the observation that IL-10 levels were lower both in mild and severe forms of PE. However, IL-10 levels were not downregulated in early pregnancy in women who ultimately developed PE. This finding implies that IL-10 dysregulation is a relatively late event and that other mediators may be involved in the early disease process. Thus, our results suggest that IL-10 is not a suitable marker for the early detection of preeclampsia. While our findings suggest that methods of increasing IL-10 levels may be a potential treatment option for PE, its therapeutic role needs to be reviewed in the context of currently available preclinical studies.
Animal studies have similarly demonstrated dysregulation of IL-10 pathways in various PE animal models.[80–86] In preclinical studies, the use of recombinant IL-10 (rIL-10) in animal models of PE ameliorated PE symptoms in experimental animals,[81,87–89] without reported adverse effects in their offspring. These studies thus offer evidence of the potential of IL-10 as a therapeutic agent. While the use of rIL-10 in humans may be limited due to its short half-life,[90,91] other modes of increasing IL-10 are also available and have already been reported in preclinical studies. These include the promising use of mesenchymal stem cells [92–95] or Treg stimulation [96] that have been demonstrated to be effective in PE animal models, as well as IL-10 gene therapy in animal models of neuro-immune disease.[90,97,98] Recombinant human IL-10 (rhuIL-10) has been used in several clinical trials for treating inflammatory bowel disease,[99] psoriasis,[100] rheumatoid arthritis and Wegener granulomatosis.[101–103] The use of rhulL-10 was reported to be safe and well tolerated with dose-dependent and reversible anemia and thrombocytopenia.[99] Higher doses have been demonstrated to be pro-inflammatory by increasing interferon gamma (IFN-γ) secretion.[104] There is a pressing need to expand the limited treatment options for PE; currently, aspirin is used to prevent PE,[105] while treatment includes anti-hypertensive drugs and expedited delivery. [106] Thus, the delivery/increase of IL-10 for preeclamptic patients with lower systemic IL-10 values may be a promising avenue of investigation in both pre-clinical and clinical studies.
Our results differ from those of two published meta-analyses of circulating IL-10 levels in PE that found increased IL-10 levels in PE patients. Both of these meta-analyses included far fewer studies compared to our meta-analysis,[107,108]; specifically, Xie et al. (2011) examined 4 studies, and Lau et al. (2013) reported 12 relevant studies. Of greater relevance, decreased circulating IL-10 levels in PE are consistent with multiple studies showing decreased local IL-10 production in placentas/placental explants,[55,109–111] peripheral blood mononuclear cells,[112–115] suggesting a state of overall deficiency of anti-inflammatory defenses in preeclampsia.
The variability in results of individual studies may be caused by several factors, including differences in methodology to quantify IL-10, patient population heterogeneity, body mass index, and differences in the pathophysiologic trigger for preeclampsia. In order to try to control for potential sources of variability, we controlled for gestational age and performed subgroup analyses based on the employed methodology. Two observations related to the methodological differences among studies that were included are noteworthy. First, studies using serum (which does not contain clotting factors) demonstrated lower IL-10 levels in PE versus normotensive pregnancies, while studies using plasma (which contains high concentrations of clotting factors, such as fibrinogen) showed no differences between the groups. This is consistent with previous studies which indicated higher concentrations of multiple metabolites in serum compared to plasma. The increased concentration of these metabolites in serum allowed for more sensitive results in biomarker detection and better discrimination between the study groups, type 2 diabetics and non-diabetic controls.[116] Previous studies that analyzed circulating cytokine levels have reported the correlation of serum versus plasma to be cytokine specific, with good correlation for some cytokines and poor for others including IL-10. Sample handling and the number of freeze-thaw cycles also influenced the measured IL-10 values.[117] The best sample type appears to be metabolite specific, with some performing better in serum and others performing better in EDTA plasma. [118] Consequently, our results support the use of serum in future studies evaluating IL-10 levels in preeclampsia.
Second, studies that used ELISAs revealed significantly lower IL-10 levels in PE compared to normotensive pregnancies, while studies using multiplex technology showed no differences between the groups. Previous studies have compared performance characteristics between multiplex bead array assays, which provide quantitative measurements of large numbers of analytes, to the use of ELISA assays. While ELISA assays are considered to be the current gold standard for the quantitative analysis of numerous cytokines, they are not suited for high throughput multiplex analyses.[119] Even though ELISA and multiplex techniques have good correlation for detecting cytokine levels, the correlation coefficients for certain metabolites (TNF-α, IL-1β and IL-6) may be significantly different between the techniques.[120] Certain elements of these assays, such as antibody affinity and specificity, antigen cross reactivity, interference with heterophile antibodies, and the use of different antibody pairs and species of origin as well as sample matrix interference could critically influence whether these assays obtain similar results. As performance multiplex platforms may vary from one to another, prior to the replacement of ELISA assays with multiplex technology, these two methods should be compared head-to-head for quantitative analyses. One study comparing the cytokine values measured by multiplex versus ELISA concluded that certain multiplex immunoassays can be applied to large-scale epidemiological studies for cytokines that are present at relatively large concentrations (ng/ml). In contrast, the use of the ELISA technique seems to remain a more accurate method for cytokines that are present in much smaller amounts, such as TNF-α, IL-1β or IL-6.[120] In light of the fact that IL-10 has very low circulating concentrations, the ELISA detection method may be more specific for the differential analysis of IL-10, which is also supported by the findings in our study. Finally, we did not notice any consistent findings with respect to other cytokines that were reported in studies based on multiplex technology. However, to fully assess these cytokines, an approach similar to ours, which would include not only studies that used multiplex methodology, but those utilizing ELISA methodology for each cytokine of interest as well, would be needed.
Limitations
The limitations of our study include publication bias due to the fact that negative studies are less likely to be published. Furthermore, there is a possibility that our search strategy has missed some relevant studies. The quality of studies that reported the IL-10 values at the time of PE diagnosis was poorer compared to the quality of studies that reported IL-10 values before the onset of the disease, mainly because they were cross sectional as opposed to cohort studies. However, the number of the patients in the studies was significantly higher. Furthermore, we did not take into account differences in the types of antibodies used (e.g. monoclonal vs. polyclonal or their heavy chain class), their species of origin, and potential cross reactivity with related antigens. .
Despite these limitations, we were able to collect relevant data that met the goals of our study by identifying the pattern of IL-10 levels in preeclamptic pregnancies and by defining optimal assay conditions.
Perspectives
The results of this meta-analysis indicate that circulating IL-10 levels are decreased in preeclamptic compared to normotensive pregnancies. Circulating IL-10 levels were not different before the time of active disease. At the time of diagnosis, IL-10 levels were lower in preeclamptic pregnancies, irrespective of clinical signs that indicated the disease severity. Based on our results, exploring the roles of both sample types (plasma vs. serum) and the methodology (ELISA vs. multiplex technology), we suggest that use of serum paired with ELISA technology is likely to give the best discrimination in future studies of IL-10 levels in preeclampsia. Multiplex technology should be considered only after an appropriate comparative performance evaluation of these two tests. Together with preclinical studies indicating that upregulation of IL-10 in animal models attenuates the preeclampsia-like phenotype, our study suggests that a therapeutic approach targeting IL-10 may offer a novel treatment option for preeclampsia.
Supplementary Material
Novelty and Significance.
1). What Is New?
Our study addresses conflicting data in the literature and indicates that circulating IL-10 levels are decreased in preeclampsia compared to normotensive pregnancies.
2). What Is Relevant?
Our study supports the role of IL-10 in the pathophysiology of preeclampsia. Decreased IL-10 levels may contribute to several injurious pathways that are activated in preeclampsia, resulting in immune system dysregulation, excessive inflammation, and anti-angiogenesis.
Source of Funding:
R01HL 136348 (VDG)
Footnotes
Disclosures: None
REFERENCES
- 1.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25. [DOI] [PubMed] [Google Scholar]
- 2.George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert Rev Obstet Gynecol. 2010;5(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. [DOI] [PubMed] [Google Scholar]
- 4.Cubro H, Kashyap S, Nath MC, Ackerman AW, Garovic VD. The Role of Interleukin-10 in the Pathophysiology of Preeclampsia. Curr Hypertens Rep. 2018;20(4):36. [DOI] [PubMed] [Google Scholar]
- 5.Harmon AC, Cornelius DC, Amaral LM, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Colch). 2016;130(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86(2):165–170. [DOI] [PubMed] [Google Scholar]
- 7.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and −8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12(6):428–432. [DOI] [PubMed] [Google Scholar]
- 8.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163(6):3491–3495. [PubMed] [Google Scholar]
- 9.Rein DT, Breidenbach M, Honscheid B, et al. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine. 2003;23(4–5):119–125. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros LT, Peracoli JC, Bannwart-Castro CF, et al. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am J Reprod Immunol. 2014;72(1):5–13. [DOI] [PubMed] [Google Scholar]
- 11.Bachmayer N, Rafik Hamad R, Liszka L, Bremme K, Sverremark-Ekstrom E. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol. 2006;56(5–6):292–301. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 14.Covidence. Covidence Accelerate your systematic review. . https://www.covidence.org. Published 2019. Accessed May 20.
- 15.SourceForge. Plot Digitizer. http://plotdigitizer.sourceforge.net. Published 2019. Accessed May 5, 2019.
- 16.Wells GASB, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis: Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2014. Accessed April 30, 2019.
- 17.Becker LAOA. Overviews of reviews . In: Higgins JPTGS, eds, ed. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2008:607–631. [Google Scholar]
- 18.Djurovic S, Clausen T, Wergeland R, Brosstad F, Berg K, Henriksen T. Absence of enhanced systemic inflammatory response at 18 weeks of gestation in women with subsequent pre-eclampsia. Bjog. 2002;109(7):759–764. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. American Journal of Obstetrics and Gynecology. 2017;216(5):527.e521–527.e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–714. [DOI] [PubMed] [Google Scholar]
- 21.Salazar Garcia MD, Mobley Y, Henson J, et al. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reprod Immunol. 2018;125:25–31. [DOI] [PubMed] [Google Scholar]
- 22.Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK, Allen J, Knudsen UB. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90(7):791–796. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL-10, TNF-alpha & IFN-gamma: potential early biomarkers for preeclampsia. Cell Immunol. 2013;283(1–2):70–74. [DOI] [PubMed] [Google Scholar]
- 24.Tangeras LH, Austdal M, Skrastad RB, et al. Distinct First Trimester Cytokine Profiles for Gestational Hypertension and Preeclampsia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(11):2478–2485. [DOI] [PubMed] [Google Scholar]
- 25.Taylor BD, Tang G, Ness RB, et al. Mid-pregnancy circulating immune biomarkers in women with preeclampsia and normotensive controls. Pregnancy Hypertens. 2016;6(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor BD, Ness RB, Klebanoff MA, et al. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens. 2016;6(4):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adela R, Borkar RM, Mishra N, et al. Lower Serum Vitamin D Metabolite Levels in Relation to Circulating Cytokines/Chemokines and Metabolic Hormones in Pregnant Women with Hypertensive Disorders. Front. 2017;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal. 2019:e22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J Soc Gynecol Investig. 2005;12(5):335–342. [DOI] [PubMed] [Google Scholar]
- 30.Bakheit KH, Bayoumi NK, Eltom AM, Elbashir MI, Adam I. Cytokines profiles in Sudanese women with preeclampsia. Hypertens. 2009;28(2):224–229. [DOI] [PubMed] [Google Scholar]
- 31.Benian A, Madazli R, Aksu F, Uzun H, Aydin S. Plasma and placental levels of interleukin-10, transforming growth factor-beta1, and epithelial-cadherin in preeclampsia. Obstet Gynecol. 2002;100(2):327–331. [DOI] [PubMed] [Google Scholar]
- 32.Bersani I, De Carolis MP, Foell D, et al. Interleukin-22: Biomarker of maternal and fetal inflammation? Immunol Res. 2014;61(1–2):4–10. [DOI] [PubMed] [Google Scholar]
- 33.Boij R, Berg G, Ernerudh J, et al. Biomarkers of coagulation,inflammationandangiogenesis are independently associated with preeclampsia. J Reprod Immunol. 2012;94 (1):109. [DOI] [PubMed] [Google Scholar]
- 34.Borekci B, Aksoy H, Al RA, Demircan B, Kadanali S. Maternal serum interleukin-10, interleukin-2 and interleukin-6 in pre-eclampsia and eclampsia. Am J Reprod Immunol. 2007;58(1):56–64. [DOI] [PubMed] [Google Scholar]
- 35.Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):21–26. [DOI] [PubMed] [Google Scholar]
- 36.Celik H, Avci B, Alper T. Comparison of maternal serum levels of interleukin-10, interleukin-12, and interleukin-2 in normal and preeclamptic pregnancies. Pregnancy Hypertens. 2012;2(1):39–42. [DOI] [PubMed] [Google Scholar]
- 37.Charkiewicz K, Jasinska E, Goscik J, et al. Angiogenic factor screening in women with mild preeclampsia - New and significant proteins in plasma. Cytokine. 2018;106:125–130. [DOI] [PubMed] [Google Scholar]
- 38.Chen JY, Zhao LJ, Wang DC, et al. Contribution of regulatory T cells to immune tolerance and association of microRNA-210 and Foxp3 in preeclampsia. Mol Med Report. 2019;19(2):1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102–111. [DOI] [PubMed] [Google Scholar]
- 40.Cui S, Gao Y, Zhang L, et al. Combined use of serum MCP-1/IL-10 ratio and uterine artery Doppler index significantly improves the prediction of preeclampsia. Clin Chim Acta. 2017;473:228–236. [DOI] [PubMed] [Google Scholar]
- 41.Daneva AM, Hadzi-Lega M, Stefanovic M. Correlation of the system of cytokines in moderate and severe preeclampsia. Clin Exp Obstet Gynecol. 2016;43(2):220–224. [PubMed] [Google Scholar]
- 42.Davila RD, Julian CG, Browne VA, et al. Role of cytokines in altitude-associated preeclampsia. Pregnancy Hypertens. 2012;2(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong M, Wang Z, He J. Serum T helper 1- and 2-type cytokines in preeclampsia. International Journal of Gynecology and Obstetrics. 2005;89(3):288–290. [DOI] [PubMed] [Google Scholar]
- 44.Elhawary TM, Demerdash HD, Sweilam MA. Relationship between interleukin-10 polymorphism and maternal serum leptin level in preeclampsia. Clin Exp Hypertens. 2013;35(5):367–372. [DOI] [PubMed] [Google Scholar]
- 45.Ellis J, Wennerholm UB, Bengtsson A, et al. Levels of dimethylarginines and cytokines in mild and severe preeclampsia. Acta Obstet Gynecol Scand. 2001;80(7):602–608. [PubMed] [Google Scholar]
- 46.Gratacos E, Filella X, Palacio M, Cararach V, Alonso PL, Fortuny A. Interleukin-4, interleukin-10, and granulocyte-macrophage colony stimulating factor in second-trimester serum from women with preeclampsia. Obstet Gynecol. 1998;92(5):849–853. [DOI] [PubMed] [Google Scholar]
- 47.Hao H, He M, Li J, et al. Upregulation of the Tim-3/Gal-9 pathway and correlation with the development of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2015;194:85–91. [DOI] [PubMed] [Google Scholar]
- 48.Jabalie G, Ahmadi M, Koushaeian L, et al. Metabolic syndrome mediates proinflammatory responses of inflammatory cells in preeclampsia. Am J Reprod Immunol. 2019;81(3):e13086. [DOI] [PubMed] [Google Scholar]
- 49.Jiang L, Tang C, Gong Y, et al. PD-1/PD-L1 regulates Treg differentiation in pregnancy-induced hypertension. Braz J Med Biol Res. 2018;51(8):e7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1–2):83–91. [DOI] [PubMed] [Google Scholar]
- 51.Kalantar F, Rajaei S, Heidari AB, et al. Serum levels of tumor necrosis factor-alpha, interleukin-15 and interleukin-10 in patients with pre-eclampsia in comparison with normotensive pregnant women. Iran J Nurs Midwifery Res. 2013;18(6):463–466. [PMC free article] [PubMed] [Google Scholar]
- 52.Krasnyi AM, Gracheva MI, Sadekova AA, et al. Complex Analysis of Total and Fetal DNA and Cytokines in Blood Plasma of Pregnant Women with Preeclampsia. Bull Exp Biol Med. 2018;164(6):721–725. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Xie Z, Wang YN, Hu HY. Macrophage M1/M2 polarization in patients with pregnancy-induced hypertension. Canadian Journal of Physiology and Pharmacology. 2018;96(9):922–928. [DOI] [PubMed] [Google Scholar]
- 54.Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82(9):797–802. [DOI] [PubMed] [Google Scholar]
- 55.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27(4–5):445–451. [DOI] [PubMed] [Google Scholar]
- 56.Mangione S, Giarratano A. The role of antithrombin III in critical patients in obstetrics. Minerva Anestesiol. 2002;68(5):449–453. [PubMed] [Google Scholar]
- 57.Mansouri R, Akbari F, Vodjgani M, Mahboudi F, Kalantar F, Mirahmadian M. Serum cytokines profiles in Iranian patients with preeclampsia. Iran J Immunol. 2007;4(3):179–185. [DOI] [PubMed] [Google Scholar]
- 58.Moreno-Eutimio MA, Tovar-Rodriguez JM, Vargas-Avila K, et al. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. Biomed Res Int. 2014;2014:413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosimann B, Wagner M, Poon LCY, Bansal AS, Nicolaides KH. Maternal serum cytokines at 30–33weeks in the prediction of preeclampsia. Prenat Diagn. 2013;33(9):823–830. [DOI] [PubMed] [Google Scholar]
- 60.Mundim GJ, Paschoini MC, Araujo Junior E, Da Silva Costa F, Rodrigues Junior V. Assessment of angiogenesis modulators in pregnant women with pre-eclampsia: a case-control study. Arch Gynecol Obstet. 2016;293(2):369–375. [DOI] [PubMed] [Google Scholar]
- 61.Noyan T, Kolusari A, Kamaci M. Serum mast cell tryptase, eosinophil cationic protein, endothelin-1 and cytokine levels in preeclampsia and healthy pregnancy. Turkish Journal of Biochemistry. 2009;34(1):19–24. [Google Scholar]
- 62.Olusi SO, Diejomaoh M, Omu A, Abdulaziz A, Prabha K, George S. Interleukins in preeclampsia. Ann Saudi Med. 2000;20(1):4–7. [DOI] [PubMed] [Google Scholar]
- 63.Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon-gamma, SOCS3 and TGF-beta levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med. 2014;27(15):1513–1517. [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro VR, Romao-Veiga M, Romagnoli GG, et al. Association between cytokine profile and transcription factors produced by T-cell subsets in early- and late-onset pre-eclampsia. Immunology. 2017;152(1):163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahin S, Ozakpinar OB, Eroglu M, et al. The impact of platelet functions and inflammatory status on the severity of preeclampsia. J Matern Fetal Neonatal Med. 2015;28(6):643–648. [DOI] [PubMed] [Google Scholar]
- 66.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58(1):21–30. [DOI] [PubMed] [Google Scholar]
- 67.Silva DM, Marreiro Ddo N, Moita Neto JM, et al. Oxidative stress and immunological alteration in women with preeclampsia. Hypertens. 2013;32(3):304–311. [DOI] [PubMed] [Google Scholar]
- 68.Szarka A, Rigo J Jr., Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valencia-Ortega J, Zarate A, Saucedo R, Hernandez-Valencia M, Cruz JG, Puello E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol Obstet Invest. 2019;84(1):12–19. [DOI] [PubMed] [Google Scholar]
- 70.Xie F, von Dadelszen P, Nadeau J. CMV infection, TLR-2 and −4 expression, and cytokine profiles in early-onset preeclampsia with HELLP syndrome. Am J Reprod Immunol. 2014;71(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu JX, Qian L, Wu FH, Li M, Chen W, Wang HY. Decreased frequency of peripheral blood CD8(+)CD25(+)FoxP3(+)regulatory T cells correlates with IL-33 levels in pre-eclampsia. Hypertens. 2017;36(2):217–225. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Liu H, Shi Y, et al. Increased circulating Th22 cells correlated with Th17 cells in patients with severe preeclampsia. Hypertens. 2017;36(1):100–107. [DOI] [PubMed] [Google Scholar]
- 73.Azizieh F, Dingle K, Raghupathy R, Johnson K, VanderPlas J, Ansari A. Multivariate analysis of cytokine profiles in pregnancy complications. Am J Reprod Immunol. 2018;79 (3):e12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res. 2008;34(6):948–951. [DOI] [PubMed] [Google Scholar]
- 75.Eneroth E, Remberger M, Vahlne A, Ringden O. Increased serum concentrations of interleukin-2 receptor in the first trimester in women who later developed severe preeclampsia. Acta Obstet Gynecol Scand. 1998;77(6):591–593. [DOI] [PubMed] [Google Scholar]
- 76.Pinheiro MB, Martins-Filho OA, Mota AP, et al. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine. 2013;62(1):165–173. [DOI] [PubMed] [Google Scholar]
- 77.Xie F, Hu Y, Turvey SE, et al. Toll-like receptors 2 and 4 and the cryopyrin inflammasome in normal pregnancy and pre-eclampsia. Bjog. 2010;117(1):99–108. [DOI] [PubMed] [Google Scholar]
- 78.Berg G, Boij R, Svensson J, et al. Biomarkers of coagulation, inflammation and angiogenesis are independently associated with preeclampsia. Acta Obstet Gynecol Scand. 2012;159):67. [DOI] [PubMed] [Google Scholar]
- 79.Boij R, Svensson J, Nilsson-Ekdahl K, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68(3):258–270. [DOI] [PubMed] [Google Scholar]
- 80.Kalkunte S, Boij R, Norris W, et al. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177(5):2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57(3):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orange S, Rasko JE, Thompson JF, et al. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine. 2005;29(4):176–185. [DOI] [PubMed] [Google Scholar]
- 83.Heyward CY, Sones JL, Lob HE, et al. The decidua of preeclamptic-like BPH/5 mice exhibits an exaggerated inflammatory response during early pregnancy. J Reprod Immunol. 2017;120:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dong X, Shi D. Simvastatin Alleviates Pathology in a Rat Model of Preeclampsia Involving ERK/MAPK Pathway. Reprod Sci. 2017;24(7):1053–1061. [DOI] [PubMed] [Google Scholar]
- 85.Chatterjee P, Chiasson VL, Kopriva SE, et al. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;58(3):489–496. [DOI] [PubMed] [Google Scholar]
- 86.Kemse NG, Kale AA, Joshi SR. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot Essent Fatty Acids. 2016;104:25–32. [DOI] [PubMed] [Google Scholar]
- 87.Harmon A, Cornelius D, Amaral L, et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens. 2015;34(3):291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R713–719. [DOI] [PubMed] [Google Scholar]
- 89.Chatterjee P, Chiasson VL, Seerangan G, et al. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 2015;28(1):135–142. [DOI] [PubMed] [Google Scholar]
- 90.Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96(Pt A):55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kastin AJ, Akerstrom V, Pan W. Interleukin-10 as a CNS therapeutic: the obstacle of the blood-brain/blood-spinal cord barrier. Brain Res Mol Brain Res. 2003;114(2):168–171. [DOI] [PubMed] [Google Scholar]
- 92.Grimes S, Bombay K, Lanes A, Walker M, Corsi DJ. Potential biological therapies for severe preeclampsia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang D, Fu L, Wang L, et al. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection. Hypertens Pregnancy. 2017;36(3):247–258. [DOI] [PubMed] [Google Scholar]
- 94.Wang LL, Yu Y, Guan HB, Qiao C. Effect of Human Umbilical Cord Mesenchymal Stem Cell Transplantation in a Rat Model of Preeclampsia. Reprod Sci. 2016;23(8):1058–1070. [DOI] [PubMed] [Google Scholar]
- 95.Fu L, Liu Y, Zhang D, Xie J, Guan H, Shang T. Beneficial effect of human umbilical cord-derived mesenchymal stem cells on an endotoxin-induced rat model of preeclampsia. Exp Ther Med. 2015;10(5):1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibrahim T, Przybyl L, Harmon AC, et al. Proliferation of endogenous regulatory T cells improve the pathophysiology associated with placental ischaemia of pregnancy. Am J Reprod Immunol. 2017;78(5):e12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Croxford JL, Feldmann M, Chernajovsky Y, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J Immunol. 2001;166(6):4124–4130. [DOI] [PubMed] [Google Scholar]
- 98.He Z, Guo Q, Xiao M, He C, Zou W. Intrathecal lentivirus-mediated transfer of interleukin-10 attenuates chronic constriction injury-induced neuropathic pain through modulation of spinal high-mobility group box 1 in rats. Pain Physician. 2013;16(5):E615–625. [PubMed] [Google Scholar]
- 99.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119(6):1473–1482. [DOI] [PubMed] [Google Scholar]
- 100.Roberti ML, Ricottini L, Capponi A, et al. Immunomodulating treatment with low dose interleukin-4, interleukin-10 and interleukin-11 in psoriasis vulgaris. J Biol Regul Homeost Agents. 2014;28(1):133–139. [PubMed] [Google Scholar]
- 101.Maini. Hu lL-10 in subjects with active rheumatoid arthritis (I&A): phase I and cytokine response study Paper presented at: RNPH BP; 1997, 1997; Washington. [Google Scholar]
- 102.Phase I Trial of Recombinant Human Interleukin-10 (SCH 52000) in Patients With Wegener’s Granulomatosis. https://ClinicalTrials.gov/show/NCT00001761. Accessed.
- 103.Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tilg H, van Montfrans C, van den Ende A, et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50(2):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293.e281. [DOI] [PubMed] [Google Scholar]
- 106.Magee LA, von Dadelszen P. State-of-the-Art Diagnosis and Treatment of Hypertension in Pregnancy. Mayo Clin Proc. 2018;93(11):1664–1677. [DOI] [PubMed] [Google Scholar]
- 107.Xie C, Yao MZ, Liu JB, Xiong LK. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56(3):550–559. [DOI] [PubMed] [Google Scholar]
- 108.Lau SY, Guild SJ, Barrett CJ, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2013;70(5):412–427. [DOI] [PubMed] [Google Scholar]
- 109.Xu J, Gu Y, Lewis DF, Wang Y. Reduced CD200 expression contributes to altered th1/th2 cytokine production in placental trophoblasts from preeclampsia. Reprod Sci. 2017;24 (1 Supplement 1):159A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peixoto AB, Araujo Junior E, Ribeiro JU, et al. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med. 2016;29(1):75–79. [DOI] [PubMed] [Google Scholar]
- 111.Kamali-Sarvestani E, Kiany S, Gharesi-Fard B, Robati M. Association study of IL-10 and IFN-gamma gene polymorphisms in Iranian women with preeclampsia. J Reprod Immunol. 2006;72(1–2):118–126. [DOI] [PubMed] [Google Scholar]
- 112.Chen W, Qian L, Wu F, Li M, Wang H. Significance of Toll-like Receptor 4 Signaling in Peripheral Blood Monocytes of Pre-eclamptic Patients. Hypertens. 2015;34(4):486–494. [DOI] [PubMed] [Google Scholar]
- 113.Campos-Canas J, Romo-Palafox I, Albani-Campanario M, Hernandez-Guerrero C. An imbalance in the production of proinflammatory and anti-inflammatory cytokines is observed in whole blood cultures of preeclamptic women in comparison with healthy pregnant women. Hypertens. 2014;33(2):236–249. [DOI] [PubMed] [Google Scholar]
- 114.Medeiros LT, Peracoli JC, Bannwart-Castro CF, et al. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am J Reprod Immunol. 2014;72(1):5–13. [DOI] [PubMed] [Google Scholar]
- 115.Cristofalo R, Bannwart-Castro CF, Magalhaes CG, et al. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic Res. 2013;47(4):268–275. [DOI] [PubMed] [Google Scholar]
- 116.Yu Z, Kastenmuller G, He Y, et al. Differences between human plasma and serum metabolite profiles. PLoS ONE. 2011;6(7):e21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parkitny L, McAuley JH, Kelly PJ, Di Pietro F, Cameron B, Moseley GL. Multiplex cytokine concentration measurement: how much do the medium and handling matter? Mediators Inflamm. 2013;2013:890706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O’Neal WK, Anderson W, Basta PV, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38(4):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods. 2009;350(1–2):125–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


