Abstract
BACKGROUND
The loss of PTEN function presents in up to 50% of late-stage prostate cancers, and is therefore a potential target for therapeutics. PTEN-deficient cells depend on de novo pyrimidine synthesis, a feature which can present a vulnerability.
METHODS
We utilized in vitro growth assays and in vivo xenograft models to test the effect of de novo pyrimidine synthesis inhibition on prostate cell lines.
RESULTS
Here, we demonstrate that PTEN-deficient prostate cancer cell lines are susceptible to inhibition of de novo pyrimidine synthesis by leflunomide. Tumor growth inhibition was observed in vitro and in vivo following leflunomide treatment, and is likely due to an overwhelming accumulation of DNA damage.
CONCLUSIONS
Our work highlights that synthetic lethality arises upon the combination of PTEN loss and leflunomide treatment in prostate cancer, and may present a therapeutic opportunity for this patient population.
Introduction
Phosphatase and tensin homolog on chromosome 10 (PTEN) is a critical regulator of Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling via catalyzing the conversion of phosphatidylinositol (3,4,5)-triphosphate (PIP3) into phosphatidylinositol 4,5-bisphosphate (PIP2) (1–4). The decrease in the PIP3 levels due to PTEN activity has an inhibitory effect on the signaling pathway, which results in slower cellular growth and a shift in the molecular balance in favor of apoptosis (5). Therefore, PTEN is an effective tumor suppressor and its loss of function is observed in wide variety of malignancies including prostate cancer. In primary prostate cancer, genomic alterations of PTEN are observed in around 20% of cases, and in castration resistant prostate cancer these alterations may affect up to 50% of patients (6–9). These numbers indicate a significant opportunity for targeted therapy if we can specifically target PTEN mutant tumor cells.
PTEN is also a regulator of cellular metabolism. A downstream consequence of its canonical effect on the PI3K pathway — including suppression of downstream AKT, mTOR, and RAC signaling — is the regulation of insulin signaling and glucose metabolism (10–12). Furthermore, our recent work demonstrated increased glutamine flux and a dependence on de novo pyrimidine synthesis upon PTEN loss in fibroblasts and breast cancer cells (13). This was consistent with a prior report linking mTOR signaling with the first enzyme in de novo pyrimidine synthesis(14).
A rate limiting step in de novo pyrimidine synthesis is the conversion of dihydroorotate to orotate by the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH). Our previous work showed that the inhibition of DHODH halted cellular growth and caused apoptosis due to the exacerbation of elevated DNA damage in PTEN null fibroblasts and breast cancer cells(13). To achieve a clinically relevant synthetic lethality in PTEN mutant tumors, we utilized FDA-approved leflunomide, a drug used in the treatment of rheumatoid arthritis, to inhibit DHODH (15, 16). Leflunomide-treated breast cancer xenografts showed slower growth and even tumor regression in some cases compared to controls(13). Examination of other PTEN mutant cancer cell lines, including prostate, for sensitivity to leflunomide in vitro also suggested that they may require de novo pyrimidine synthesis to remain viable.
In this study, we show that prostate cancer cell lines deficient for PTEN are significantly more sensitive to leflunomide than prostate cells with functional PTEN, and that this sensitivity is associated with DNA damage, cell death, and tumor growth inhibition in xenograft models. We hope these data provide a rationale for the use of leflunomide in prostate cancer patients and will instigate clinical trials in this field.
Material and Methods
Cell culture
Cell lines were obtained from ATCC. RWPE-1 cells were grown in keratinocyte serum free medium (K-SFM) which was supplemented with bovine pituitary extract (0.05mg/ml) and 5ng/ml epidermal growth factor together with 1% penicillin/streptomycin (PS). PC3 cells were grown in F-12K supplemented with 10% fetal bovine serum (FBS) and PS. 22RV1, Pten−/−; KrasG12D and LNCaP cells were grown in RPMI, 10% FBS and PS. MDA PCa 2b cells were grown in F-12K media supplemented with 20%FBS, 25ng/ml cholera toxin, 10ng/ml EGF, 100pg/ml hydrocortisone, 45nM sodium selenite, 5μg/ml insulin and PS. Cells were confirmed to be negative for mycoplasma using the MycoAlertTM Mycoplasma Detection Kit from Lonza. The authentication of the cell lines were done by Laboratory Corporation of America (LabCorp).
Xenograft studies
For the xenograft experiments, 6 week old male nude mice were acquired from The Jackson Laboratory. After one week of acclimation period, mice were subcutaneously injected with 5 million PC-3 or Pten−/−; KrasG12D cells. 200mg/kg leflunomide was given to the mice via oral gavage, the method of leflunomide treatment currently used clinically. Tumor growth was monitored via IVIS machine weekly and photon flux quantification done by Living Image Software.
Flow cytometry
To measure γH2AX, we used the DNA Damage Kit from Millipore (FCCH12542). Cells were fixed, permeabilized and then stained with antiphospho-H2A.X antibody for 1 hour. Fluorescence signal was measured by the Guava flow cytometry machine and mean fluorescence intensity (MFI) was calculated.
Cell death assay
3000 cells were plated in each well of a 96-well plate with DRAQ7 dye which stained nuclei of dead cells in far red wavelength. The number of dead cells were measured by analyzing fluorescent and phase-contrast images acquired with IncuCyte ZOOM (Essen Biosciences) live-cell imaging system.
GI50 calculations
3000 cells were plated in each well of a 96-well plate with serial dilutions of leflunomide (Sigma PHR1378) in triplicates, maintaining constant DMSO in each well to control for solvent effects. By using the IncuCyte ZOOM (Essen Biosciences) live-cell imaging system, growth rates were assessed for 5 days based on phase-contrast confluency readings. The leflunomide concentration that slows the growth rate of a certain cell line by 50% was calculated by linear regression.
Statistics
Student t test was used for statistics and calculations were done by using GraphPad Prism 6 or Microsoft Excel.
Results
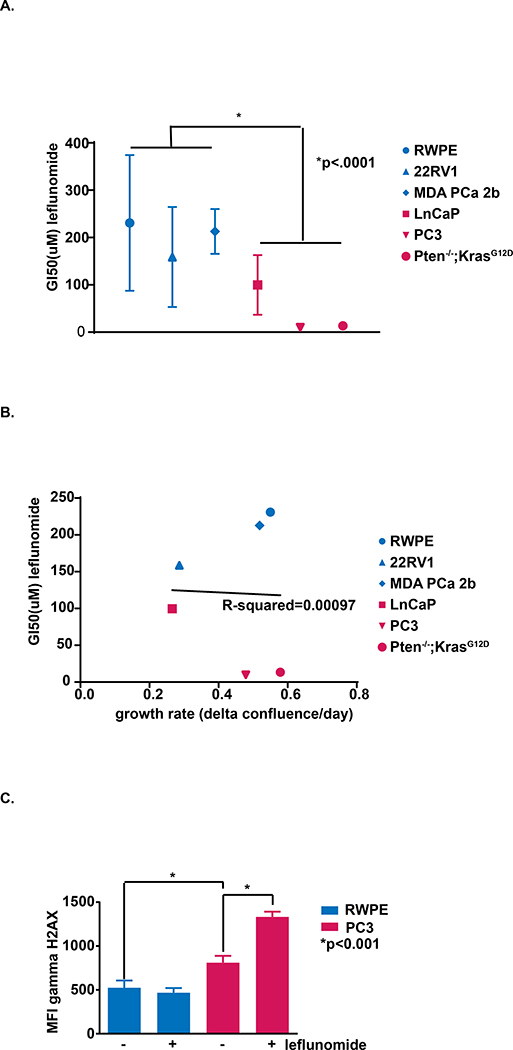
To assess sensitivity to leflunomide in human prostate cells, we treated PTEN wild-type (WT) RWPE-1, 22RV1, and MDA PCa 2b cells with a dose titration of leflunomide, and compared the GI50 values to those of PTEN null LNCaP,PC3, and Pten−/−; KrasG12D cells (Fig. 1a). The average GI50 concentration for leflunomide treatment was 192μM in PTEN WT cells, nearly four times that of the average 49μM GI50 observed for PTEN null prostate cancer cells (Supplementary Fig. 1a).
Figure 1:
The effect of PTEN loss and leflunomide treatment on prostate cells in vitro. a) Cells were treated with dose titrations of leflunomide and GI50s were calculated for each cell line. (Student t test, *, P value on figure, n = 3). b) Linear regression was used to determine the correlation between proliferation rate and leflunomide GI50 among our cell lines. P-value of correlation = .9533. c) The levels of γH2AX were assessed by measuring mean fluorescence intensity in flow cytometry. The average MFI is depicted for PTEN WT RWPE-1 and PTEN null PC3 cells with or without 100μM leflunomide treatment (MFI; Student t test, *, P value on figure, n = 3. Representative of 2 independent experiments).
Importantly, we verified that the effect of leflunomide was not simply due to an antiproliferative effect. We calculated the growth rates of each cell line and plotted growth rate vs. GI50 of leflunomide for each. By fitting a linear regression curve we determined that there is no correlation between proliferation rate and response to leflunomide (p-value = 0.9533) (Fig. 1b). Additionally, we treated our cell lines with antiproliferative chemotherapies, and found no significant difference in response between PTEN WT and mutant groups (Supplementary Fig. 1b–c). To further ascertain whether inhibitory affects were due to cytostatic or cytotoxic effects, we monitored cell death upon treatment with 100μM leflunomide using a cell death marker in conjunction with live-cell imaging. We found significantly higher accumulation of dead cells over time in PTEN null cells compared to PTEN WT, suggesting that PTEN loss confers higher susceptibility to cell death in prostate cancer following leflunomide treatment (Supplementary Fig. 1d).
We hypothesized that, akin to PTEN mutant breast cancer, PTEN null prostate cancer cells would have increased DNA damage compared to WT controls. We assessed γH2AX via flow cytometry with and without leflunomide treatment (Fig. 1c) and observed that PTEN null PC3 prostate cancer cells exhibited significantly more γH2AX compared to PTEN WT RWPE-1 prostate cells. In addition, the γH2AX signal in PC3 cells increased more than 40% following leflunomide treatment whereas DNA damage in RWPE-1 cells was insensitive to leflunomide. Our data suggest a synthetic lethality between PTEN loss and DHODH inhibition in prostate cancer cells due to an overwhelming accumulation of DNA damage and consequent cell death.
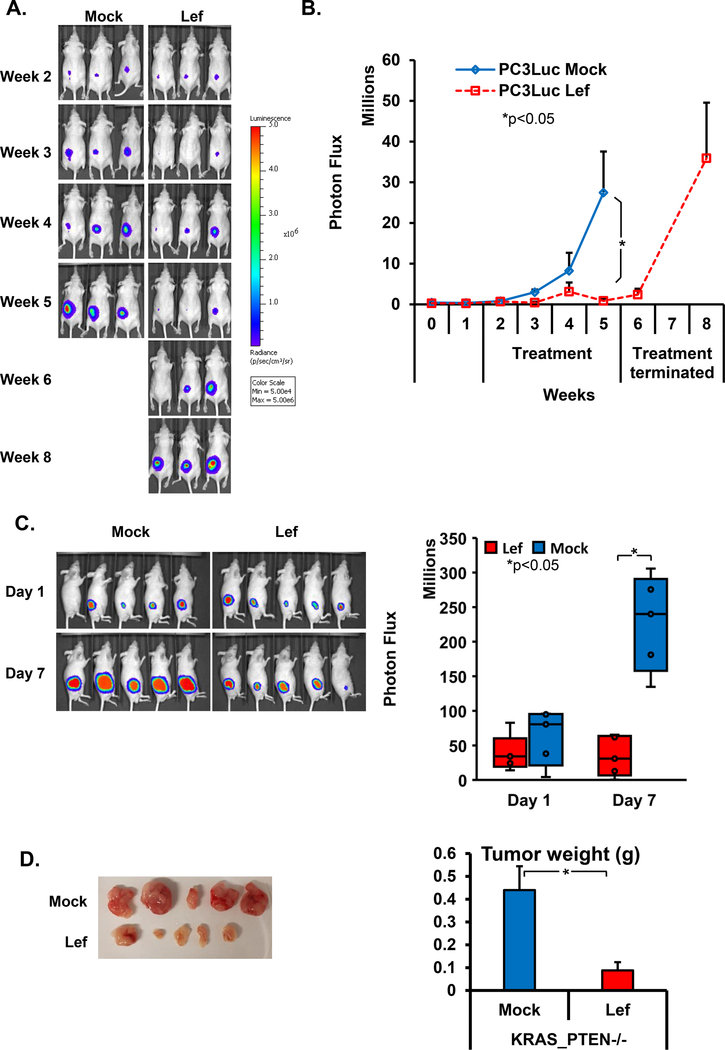
To test the therapeutic potential of leflunomide on PTEN-deficient prostate cancer we monitored its effect on PC3 xenografts in nude mice. We injected 5 million PC3 cells expressing firefly luciferase and monitored tumor growth via the In Vivo Imaging System (IVIS) for an 8 week period. Two weeks after the injections, when palpable tumors formed, we started leflunomide treatments; by treating established tumors rather than immediately following injection, we ensure that we are testing the effects of therapy rather than prevention. After 4 weeks, we observed a significant difference in tumor volume between leflunomide-treated and control groups (Fig. 2a and b). At this point, we had to end the experiment in the control group since the tumor sizes achieved the predetermined end point of 1cm3. We also decided to discontinue treatment in the leflunomide group to observe the effects of treatment cessation. Three weeks later, tumor size indicated relapse following the withdrawal of the leflunomide treatment, suggesting that the leflunomide treatment was causal of the observed tumor suppression. To corroborate our findings, we performed an additional xenograft experiment in another PTEN mutant cancer cell line, Pten−/−; KrasG12D. Treatment with leflunomide for just 7 days resulted in smaller tumors compared to mock-treated controls, as determined both by IVIS and endpoint tumor weights (Fig. 2c and d). Overall, our in vivo experiments indicated that the combination of PTEN loss and leflunomide treatment caused significant suppression of tumor growth.
Figure 2:
Leflunomide treatment on PTEN null prostate cancer xenografts. a) IVIS images were collected weekly following the subcutaneous injection of 5 million PC3 cells in mock and leflunomide (200mg/kg) treated groups. Experiments were ended once the predetermined end point of 1cm3 tumor sized was reached. In the leflunomide treated group, after week 4 of the treatment, the drug was withdrawn retreated. b) The quantification of tumors’ sizes based on photon flux (*, P < 0.05 for Student tests, n = 3 per arm). c) IVIS images (left) and quantification (right) following the subcutaneous injection of 5 million Pten−/−; KrasG12D cells in mock and leflunomide (200mg/kg) treated groups (*, P < 0.05 for Student tests, n = 5 per arm). d) Images of tumors (left) and quantification of weights (right) of the endpoint of the experiment in Fig. 2C (*, P < 0.05 for Student tests, n = 5 per arm).
Discussion
In advanced prostate cancer, loss of both functional alleles of PTEN is observed in 50% of patients, rendering it an attractive therapeutic target. In this study, we demonstrated that PTEN-deficient prostate cancer cells were susceptible to the inhibition of de novo pyrimidine synthesis, while PTEN WT prostate cancer cells did not exhibit the same vulnerability. First, we observed that PTEN null cells were more sensitive to growth inhibition by the DHODH inhibitor leflunomide, indicating their dependency on de novo pyrimidine synthesis. Treatment with chemotherapeutics, on the other hand, did not result in differential sensitivity between PTEN WT and deficient groups. Second, we noted that leflunomide caused cell death in PTEN null cells, indicating cytotoxic rather than cytostatic effects. Third, we demonstrated that the loss of PTEN led to an accumulation of DNA damage in PTEN-deficient prostate cancer cells, and that this was exacerbated in the presence of DHODH inhibition. The consistency of these phenotypes with our prior analysis in breast cancer cells suggest that a similar mechanism of action is likely exhibited in prostate cancer cells. These in vitro results provided us with the rationale to perform xenograft assays. PC3 and Pten−/−; KrasG12D xenograft experiments demonstrated effective tumor suppression in mice upon oral leflunomide treatment. These results support that PTEN deficiency in combination with DHODH inhibition leads to synthetic lethality in prostate cancer cells, thereby offering proof of principle for utilizing leflunomide as a potential therapeutic agent against PTEN mutant prostate cancer. Furthermore, given the castration-resistant nature of PC3 cells, it is possible that leflunomide could be effective in late-stage prostate cancer where targeted therapy options are lacking.
Leflunomide is an FDA approved drug against rheumatoid arthritis and psoriatic arthritis (16–19). It is an immunosuppressive drug which was tested in wide variety of diseases at clinical trials including a phase-II prostate cancer study (20). In that study, leflunomide (SU101) was given to patients with hormone-refractory prostate cancer, and 21% of patients had tumor responses or disease stabilization upon treatment. This study did not stratify patients based on their PTEN status; it is possible that a new study in which PTEN status is one of the main criteria for patient selection may yield a higher response rate in light of our findings reported here, and could be an important step in personalized medicine.
Supplementary Material
Acknowledgements
We thank all the Parsons’s lab members for their critical input during the planning, execution and reporting phases of the study.
Grant Support: Grant support for this work was provided by Prostate Cancer Foundation 2016 PCF Challenge Award 16CHAL14 and National Cancer Institute R35 CA220491.
Footnotes
Conflict of Interest: Ramon Parsons is a shareholder of Therapten, Inc., a company focused on using an isoform of PTEN protein as a treatment for disease.
References cited
- 1.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–7. [DOI] [PubMed] [Google Scholar]
- 4.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27(41):5477–85. [DOI] [PubMed] [Google Scholar]
- 5.Tian XX, Pang JC, To SS, Ng HK. Restoration of wild-type PTEN expression leads to apoptosis, induces differentiation, and reduces telomerase activity in human glioma cells. Journal of neuropathology and experimental neurology. 1999;58(5):472–9. [DOI] [PubMed] [Google Scholar]
- 6.Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nature reviews Urology. 2018;15(4):222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Kornblum M, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. The American journal of pathology. 2012;181(2):401–12. [DOI] [PubMed] [Google Scholar]
- 8.Leinonen KA, Saramaki OR, Furusato B, Kimura T, Takahashi H, Egawa S, et al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(12):2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotan TL, Heumann A, Rico SD, Hicks J, Lecksell K, Koop C, et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget. 2017;8(39):65566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mense SM, Barrows D, Hodakoski C, Steinbach N, Schoenfeld D, Su W, et al. PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Science signaling. 2015;8(370):ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong JT, Kim PT, Peacock JW, Yau TY, Mui AL, Chung SW, et al. Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia. 2007;50(2):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell. 2016;164(3):433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur D, Stratikopoulos E, Ozturk S, Steinbach N, Pegno S, Schoenfeld S, et al. PTEN Regulates Glutamine Flux to Pyrimidine Synthesis and Sensitivity to Dihydroorotate Dehydrogenase Inhibition. Cancer discovery. 2017;7(4):380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339(6125):1323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene S, Watanabe K, Braatz-Trulson J, Lou L. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochemical pharmacology. 1995;50(6):861–7. [DOI] [PubMed] [Google Scholar]
- 16.Munier-Lehmann H, Vidalain PO, Tangy F, Janin YL. On dihydroorotate dehydrogenases and their inhibitors and uses. Journal of medicinal chemistry. 2013;56(8):3148–67. [DOI] [PubMed] [Google Scholar]
- 17.Shawver LK, Schwartz DP, Mann E, Chen H, Tsai J, Chu L, et al. Inhibition of platelet-derived growth factor-mediated signal transduction and tumor growth by N-[4-(trifluoromethyl)-phenyl]5-methylisoxazole-4-carboxamide. Clinical cancer research : an official journal of the American Association for Cancer Research. 1997;3(7):1167–77. [PubMed] [Google Scholar]
- 18.Hail N Jr., Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia. 2010;12(6):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fragoso YD, Brooks JB. Leflunomide and teriflunomide: altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expert review of clinical pharmacology. 2015;8(3):315–20. [DOI] [PubMed] [Google Scholar]
- 20.Ko Y-J, Small EJ, Kabbinavar F, Chachoua A, Taneja S, Reese D, et al. A multi-institutional phase II study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. 2001;7(4):800–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.