Cervical spinal cord injury can cause profound disruption to the nervous system, and impaired cardiovascular autonomic regulation adversely impacts cardiovascular function, which increases morbidity and mortality.[1] Cardiovascular autonomic dysfunction significantly delays therapeutic interventions, limiting functional gains and prolonging inpatient care, thereby diminishing independence and quality of life.[2] Chronically, cardiovascular dysregulation has been associated with cognitive deficits, poor general health and chronic fatigue, and may contribute to significant adverse clinical outcomes including syncope, stroke, seizure, or death.[3] Therapeutic interventions to mitigate effects of cardiovascular autonomic dysregulation and increase quality of life should therefore be a high priority, but functional improvements are limited by damage to spinal sympathetic neurons. Few interventions have proven effective at improving cardiovascular function in chronic spinal cord injury, and individuals are encouraged to adapt to blood pressure instability – recovery is not possible.[3]
However, we found previously spinal cord epidural stimulation targeted for cardiovascular function (CV-scES) can alleviate chronic hypotension by immediately increasing and maintaining blood pressure. Moreover, active CV-scES during postural stress can mitigate orthostatic hypotension. After prolonged daily CV-scES to maintain blood pressure, individuals no longer needed active CV-scES during postural stress to maintain their blood pressure: orthostatic hypotension did not occur. [4,5] This demonstrates improved autonomic cardiovascular regulation, an adaptation that is independent of active CV-scES. In this study, we investigate the mechanism of active CV-scES and the sustained adaption to CV-scES intervention in 4 individuals with chronic spinal cord injury. We hypothesized significant increases in blood pressure during orthostatic stress would be associated with increased surrogates of cardiovascular autonomic control: increased blood pressure variability, increased heart rate variability, increased catecholamine levels, and improved baroreflex responsiveness.
Four individuals with C4 motor complete spinal cord injury participated in the study (Supplemental Table 1). [4,5] All were clinically stable without cardiovascular disease unrelated to spinal cord injury but reported that cardiovascular instability drastically decreased their quality of life. Research participants signed an informed consent approved by the University of Louisville Institutional Review Board in accordance with the Declaration of Helsinki (NCT-02037620).
A 16-electrode array (Specify 5–6-5, Medtronic, Minneapolis, MN) was implanted to span spinal cord segments L1-S1.[6] Individuals used CV-scES two hours daily to maintain systolic blood pressure within 105–120 mmHg, as previously described, for a total of 89 ± 13 days; CV-scES configurations were unique to each individual (Supplemental Figure 1). [4,5] We assessed effects of CV-scES on blood pressure variability, heart rate variability, and baroreflex function with an orthostatic stress test. Assessments were performed as follows: 1) pre-intervention without stimulation; 2) pre-intervention with stimulation; and 3) post-intervention without stimulation. Immediate changes to cardiovascular regulation that occur during CV-scES (1 vs 2, i.e., while individuals were using CV-scES to maintain systolic blood pressure) were assessed prior to beginning daily CV-scES sessions. Changes to cardiovascular regulation that were sustained after daily use of CV-scES (1 vs 3) were assessed over a 2-week period following completion of the CV-scES intervention. At each assessment, data collection was repeated 3 times.
We report significant increases to heart rate variability and baroreceptor effectiveness with immediate CV-scES (Figure 1a versus 1b, 1i versus 1j) and sustained effects after the CV-scES intervention (Figure 1a versus 1c, 1i versus 1k) in association with significantly increased blood pressure during orthostatic stress (Supplemental Table 2, Supplemental Results) [4,5]. These findings demonstrate restoration of cardiovascular regulation can occur even years after a person sustains a severe spinal cord injury.
Figure 1.
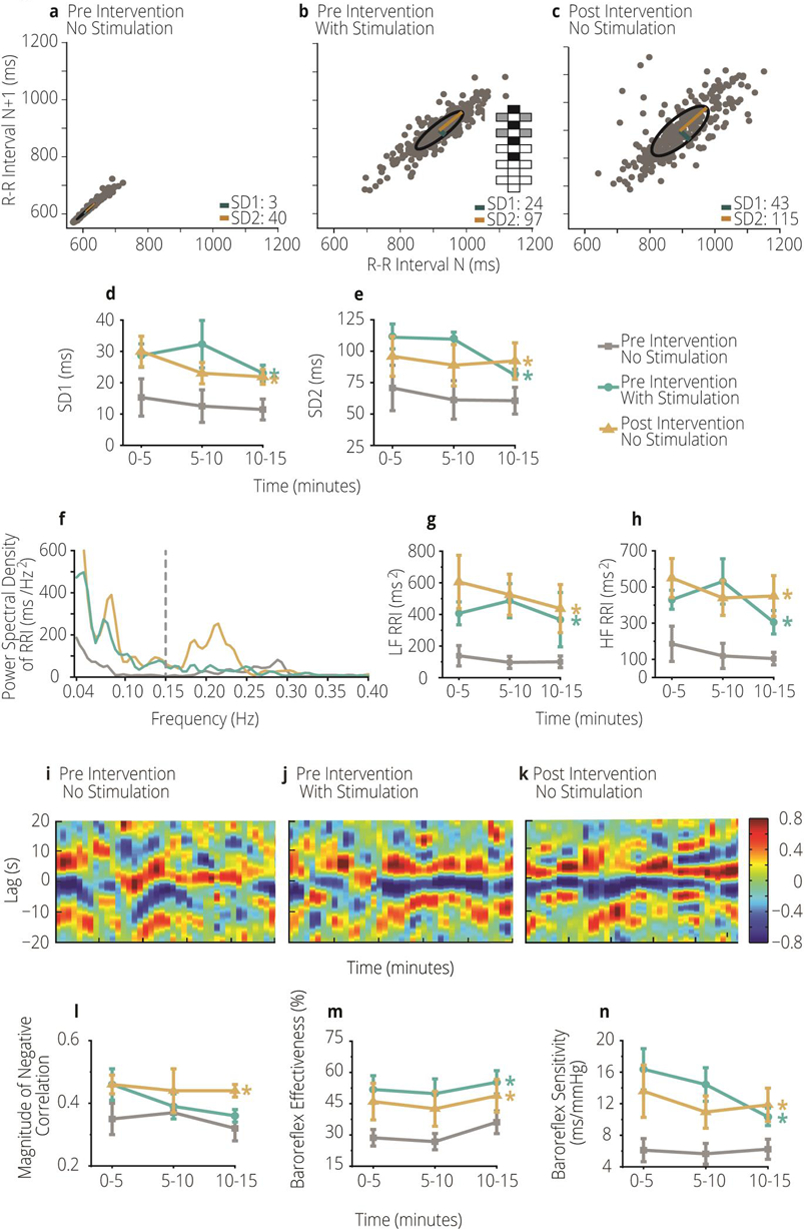
a–c Poincaré plot illustrating nonlinear heart rate variability in one participant of study with C4 motor complete spinal cord injury (A41): pre-intervention without stimulation (a), pre-intervention with stimulation (b), post-intervention without stimulation (c). Configuration of the electrode array is represented in b. Black boxes are anodes, gray boxes are cathodes, and white boxes are inactive. d, e Mean SD1 (d) and SD2 (e) of all four individuals with C4 motor complete spinal cord injury who participated in the study, obtained in the sitting position. SD1 and SD2 increased significantly pre-intervention with stimulation and post-intervention without stimulation compared with pre-intervention without stimulation.f Power spectral density of R-R interval (RRI) in one participant (A41) in the sitting position pre-intervention without stimulation (gray line), pre-intervention with stimulation (turquoise line) and post-intervention without stimulation (yellow line). A dashed, vertical gray line separates the low- (left) and high- (right) frequency bands. g, h Mean spectral power values for all four participants of low-frequency (LF; g) and high-frequency (HF; h) RRI obtained in the sitting position. Spectral power of LF and HF R-R interval oscillations increased significantly pre-intervention with stimulation and post-intervention without stimulation compared with pre-intervention without stimulation (color coding of lines/symbols for f–h is the same as that for d, e).i–k Color map from one participant (A41) illustrating the cross-correlation magnitude (heatmap, right panel) between LF systolic blood pressure and heart rate during orthostatic stress: pre-intervention without stimulation (i), pre-intervention with stimulation (i), and post-intervention without stimulation (k). l–n Mean values for the four participants for peak negative cross-correlation coefficient (l), baroreceptor effectiveness index (m), and baroreceptor sensitivity (n) in the sitting position. Magnitude of the negative correlation increased significantly post-intervention without stimulation compared with pre-intervention without stimulation; baroreflex effectiveness and baroreflex sensitivity increased significantly pre-intervention with stimulation and post-intervention without stimulation compared with pre-intervention without stimulation. Asterisk (*) indicates significant differences at p < 0.05; color coding lines/symbols in l–n is the same as that for d–h. SD1, standard deviation perpendicular to the line of identity; SD2, standard deviation parallel to the line of identity
During orthostatic stress, we demonstrate significant restoration of heart rate variability in contrast to prior reports that suggest an intractable deficit. [3] Others report individuals with cervical spinal cord injury demonstrate significantly diminished heart rate variability in the low- and high-frequency bandwidths during orthostatic stress compared with non-injured individuals. [7] Our data, however, demonstrate significant increases in heart rate variability after CV-scES, illustrated the Poincaré plot (Figure 1a–e; Supplemental Results) and corroborated by significant increases in low- and high-frequency oscillations of R-R interval (Figure 1f–h; Supplemental Results). These increases were immediate upon active CV-scES (turquoise lines) and sustained after the CV-scES intervention (gold lines). This indicates improved cardiac regulation during orthostatic stress.
Dependence of heart rate on blood pressure, illustrated by cross-correlation, is represented (Figure 1i–k) which indicates immediate (turquoise lines) and sustained (gold lines) increases in baroreflex sensitivity (Figure 1m) and effectiveness (Figure 1n) in response to CV-scES. (Figure 1i–n; Supplemental Results). These increases reflect increased stimulation of baroreceptors in response to systolic blood pressure changes; this led to the significant decreases in heart rate during orthostatic stress, thereby illustrating the increased activity of the baroreflex arc. Restoration of the baroreflex arc may contribute to the significant increases in blood pressure via feed-forward vasopressor reflexes. [8]
We were unable to detect increased surrogates of sympathetic cardiovascular control (i.e., low frequency oscillations of systolic blood pressure, circulating norepinephrine, etc.) (Supplemental Figure 2; Supplemental Results) in response to CV-scES, a limitation possibly related to the scope of this study, small sample size, and/or our chosen methodology. Further investigation into vessel morphology and hemodynamic responses to CV-scES are warranted to illuminate the mechanism by which CV-scES leads to sustained adaptations in cardiovascular autonomic regulation post-intervention.
The improvements to heart rate variability and baroreflex activity that persist post-intervention, without active CV-scES, illustrate improvements to cardiovascular regulation during orthostatic stress and suggest the spinal cord retains the potential for adaptive plasticity in persons with long-standing spinal cord injury. Restoration of cardiovascular function and improved blood pressure stability can ultimately increase independence and improve quality of life for individuals with spinal cord injury [9]. In addition, these beneficial improvements in cardiovascular autonomic regulation have implications for long-term cardiovascular health in the spinal cord injury population, because it is appreciated that blunted baroreflex responsiveness and decreased heart rate variability are significant predictors of cardiac events and development of cardiovascular disease. [10] The use of CV-scES to maintain blood pressure within a normotensive range therefore has the potential to improve participation in daily activities, promote independence and quality of life, and decrease risk of developing cardiovascular disease in the spinal cord injury population.
Supplementary Material
Acknowledgements
We are indebted to our research participants for their courage and perseverance that made these findings possible. We are grateful to Christie Ferreira, Richard Seither, Brittany Logsdon, Katelyn Smith, and Sean Stills for their participant support; Dr. Camilo Castillo, Dr. Darryl Kaelin, Dr. Glenn Hirsch, Courtney Ware, Yukishia Austin, Lynn Robbins, and Hye Conner for medical oversight; Dengzhi Wang and Hanna Martin for data analysis.
Funding
This study was supported by the Craig H. Neilsen Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, University of Louisville Hospital, Christopher and Dana Reeve Foundation, and Medtronic plc.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Author Disclosure Statement
B.L.D, S.A., S.W., B.U., G.A.H, J.M.W, and S.J.H. declare no competing financial interests exist.
Data Sharing
Deidentified data and study-related documents will be made available upon reasonable request.
REFERENCES:
- 1.Teasell RW, Arnold JM, Krassioukov A, Delaney GA (2000) Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. ArchPhysMedRehabil 81 (4):506–516 [DOI] [PubMed] [Google Scholar]
- 2.Furlan JC, Fehlings MG (2008) Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurgical focus 25 (5):E13. doi: 10.3171/foc.2008.25.11.e13 [DOI] [PubMed] [Google Scholar]
- 3.Mills PB, Fung CK, Travlos A, Krassioukov A (2015) Nonpharmacologic management of orthostatic hypotension: a systematic review. Archives of Physical Medicine and Rehabilitation 96 (2):366–375 [DOI] [PubMed] [Google Scholar]
- 4.Harkema SJ, Wang S, Angeli CA, Chen Y, Boayke M, Ugiliweneza B, Hirsch GA (2018) Normalization of Blood Pressure with Spinal Cord Epidural Stimulation after Severe Spinal Cord Injury. Frontiers in human neuroscience 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkema SJ, Legg Ditterline B, Wang S, et al. (2018) Epidural spinal cord stimulation training and sustained recovery of cardiovascular function in individuals with chronic cervical spinal cord injury. JAMA Neurology. doi: 10.1001/jamaneurol.2018.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377 (9781):1938–1947. doi: 10.1016/S0140-6736(11)60547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claydon VE, Krassioukov AV (2008) Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. AmJPhysiol Heart CircPhysiol 294 (2):H668–H678 [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki F, Ushida T, Yokoyama T, Ando M, Yamashita K, Sato T (2006) Artificial baroreflex: clinical application of a bionic baroreflex system. Circulation 113 (5):634–639. doi: 10.1161/circulationaha.105.587915 [DOI] [PubMed] [Google Scholar]
- 9.Piatt JA, Nagata S, Zahl M, Li J, Rosenbluth JP (2016) Problematic secondary health conditions among adults with spinal cord injury and its impact on social participation and daily life. J Spinal Cord Med 39 (6):693–698. doi: 10.1080/10790268.2015.1123845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rovere MT, Bigger JT Jr., Marcus FI, Mortara A, Schwartz PJ (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351 (9101):478–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


