Abstract
Inhibiting the cytotoxicity of amyloid aggregation by endogenous proteins is a promising strategy against degenerative amyloid diseases due to their intrinsically high biocompatibility and low immunogenicity. In this study, we investigated the inhibition mechanism of the structured core region of αB-crystallin (αBC) against Aβ fibrillization using discrete molecular dynamics simulations. Our computational results recapitulated the experimentally observed Aβ binding sites in αBC and suggested that αBC could bind to various Aβ aggregate species during the aggregation process – including monomers, dimers, and likely other high molecular weight oligomers, proto-fibrils and fibrils – by capping the exposed β-sheet elongation surfaces. Thus, the nucleation of Aβ oligomers into fibrils and the fibril growth could be inhibited. Mechanistic insights obtained from our systematic computational studies may aid in the development of novel therapeutic strategies to modulate the aggregation of pathological, amyloidogenic protein in degenerative diseases.
Introduction
The abnormal misfolding and aggregation of amyloid-β (Aβ) peptides into β-sheet enriched insoluble amyloid deposits are the major pathological hallmark of Alzheimer’s disease (AD)1–3. Common to most amyloid proteins (e.g. amylin4 and α-synuclein5), the fibrillization process4 usually involves oligomer formation, nucleated conformational conversion, and proto-fibril elongation before forming mature fibrils. Nearly all experimentally-determined amyloid fibril structures to date feature the in-registered cross-β core structures6–8 with β-strand perpendicular to the fibril axis9–11. Increasing evidences reveal that the low molecule weight soluble oligomers formed during the early aggregation stage are the main cytotoxic species12–15. Numerous experimental studies have demonstrated that inhibiting fibrillization is an effective approach to mitigate the aggregation-mediated cytotoxicity2, 16–19. Therefore, the inhibition of amyloid proteins fibrillization is considered as promising strategy for future cure amyloid diseases.
To migrate the cytotoxicity induced by amyloid protein pathological aggregation, the modulation of amyloid aggregation by natural polyphenols (e.g. EGCG, curcumin and resveratrol)17, 20, 21, nanoparticles (e.g., Graphene oxide quantum dot, fullerene derivative)17, 18, 22–24 and small peptides25, 26 has been widely studied by prior experimental and computational studies. Among many anti-amyloid reagents2, 16–19, 27, the endogenous αB-crystallin protein displaying amyloid-inhibiting effects against multiple amyloidogenic proteins and peptides28–30 is of particular interest due to its intrinsic biocompatibility, biological origin and low immunogenicity. The αB-crystallin is a small heat-shock protein (a.k.a. HspB5)31 and widely expressed in the human body, including brain, retina, heart, skeletal muscle, skin, spinal cord, kidneys, lungs and eye lens28, 32–35. Hochberga et. al. found that the αB-crystallin could prevent Aβ fibrillization and reduce its toxicity in the cell assay36. Shammas et. al. found that αB-crystallin could inhibit the seeding effect of preformed Aβ fibrils by stopping the elongation/growth of Aβ fibrils28. Chemical shift perturbation analysis with NMR suggested that the hydrophobic edge of the central β-sandwich of αB-crystallin preferred to bind to the elongation surface of Aβ amyloid fibril, thus hindered Aβ fibril growth via a capping mechanism30. Moreover, αB-crystallin was also observed to inhibit aggregation and mitigate aggregation-mediated cell toxicity associated for other amyloid peptides, such as α-synuclein, Tau and κ-casein29, 36, 37. However, the detailed inhibition mechanism was still unclear. Better understanding the inhibition mechanism at the molecular level will be helpful for designing novel amyloid inhibitors against amyloid diseases.
To investigate the inhibition mechanism of αB-crystallin against Aβ fibrillization, we systematically studied the interactions between Aβ42 and the structured core region of αB-crystallin (αBC) by applying discrete molecular dynamic (DMD) simulation, a rapid and predictive molecular dynamics algorithm widely used to study protein folding and misfolding by both our group9, 10, 27 and others38–42. Our simulation results revealed that the exposed β4&β8 surfaces αBC dimer could bind to the β-sheet elongation surfaces of Aβ monomers and dimers forming parallel or anti-parallel β-sheet. Due to the fact that the elongation surfaces of Aβ oligomers are occupied, their growth to high molecular weight oligomers or nucleation of proto-fibrils should be inhibited. The hot-spot binding regions between Aβ and αBC identified from our simulation is consistent with prior Aβ fibril and αBC NMR chemical-shift perturbation experimental study30, indicating this mechanism is also suitable for the other β-sheet abundant high molecular weight oligomers (e.g. proto-fibrils and fibrils). Overall, the αBC could prevent Aβ oligomers from nucleation into fibrils and also fibril growth by binding on the β-sheet elongation surface of Aβ oligomers. This study reveals a complete picture of the inhibitory mechanism of Aβ aggregation by the endogenous αB-crystallin protein, providing theoretical insights into the development of novel therapeutic strategies against AD.
Materials and Methods
Molecular systems.
The sequence of Aβ and the structured core region of αB-crystallin (αBC, residue 68–153) were shown in Fig. 1a. The initial structure of Aβ (PDB ID: 1zoq) was taken from the NMR structures solved in aqueous solution43. The monomer and dimer structure of αBC were taken from previous experimentally determined 24-mer model structures (PDB ID: 3j07) based on solid-state NMR, small-angle X-ray scattering (SAXS), and EM data44. Molecular systems including an Aβ monomer, an Aβ monomer with an αBC monomer or dimer, two Aβ monomers, two Aβ monomers with one αBC dimer were simulated. For each molecular system, multiple independent simulations were performed starting from different initial states (i.e., coordinates, orientations, and velocities), where Aβ monomer, αBC monomer or dimer were randomly placed in a simulation box with randomly rigid-body rotations and any inter-molecular atomic distance was no less than 1.5 nm. Details of all the simulations were summarized in Table 1.
Fig. 1. Binding of Aβ monomer with αBC monomer.
a) The amino acid sequence and structure of Aβ42 and structured core region of αB-crystallin used in our simulation. b) The time evolution of the number of inter-chain hydrogen bonds formed between Aβ and αBC monomers and secondary structure for each Aβ reside adopted in two representative trajectories, where Aβ42 bound to the β3+β6−7 (up) and β4+β8 (down) strands of αBC. The snapshots at 50, 300 and 500 ns are shown to the right, corresponding to before and after binding. For clarity in the presentation of Aβ-αBC complex, the two β-sheet elongation surfaces β3+β6−7 and β4+β8 of αBC are colored by blue and red, respectively. The other regions of αBC are shown in cyan. Aβ is colored in magenta. c) The inter-molecular contact frequency map between Aβ and αBC were computed based on the last 200 ns trajectories of 50 independent DMD simulations after reaching steady states. For each residue in Aβ and αBC, its total number of inter-chain contacting residues are also computed by integrating the corresponding pair-wise contact frequency map. The representative binding motifs of Aβ (colored in red) and αBC (colored in blue) segments labeled as 1–9 corresponding to the parallel or anti-parallel β-sheet patterns heighted by boxes in the contact frequency map are also presented on the right. The hydrophobic and salt bridge residue-residue pairs are highlight in bold.
Table 1.
The details of simulation systems in our DMD simulations, including the number of αBC monomer (αBCM), αBC dimer (αBCD) and Aβ monomer molecules; the corresponding dimension of the cubic simulation box; the total number of independent DMD trajectories performed; the simulation time of each DMD trajectory.
| System | Box size (nm) | DMD Run | Simulation time (ns) | |
|---|---|---|---|---|
| Molecules | Number | |||
| Aβ | 1 | 6.0 | 50 | 500 |
| 2 | 9.0 | 50 | 500 | |
| αBCM :Aβ | 1:1 | 9.0 | 50 | 500 |
| αBCD :Aβ | 1:1 | 9.0 | 50 | 500 |
| αBCD :Aβ | 1:2 | 9.0 | 40 | 400 |
DMD simulations.
All simulations were performed at 300K by using the united-atom DMD algorithm with implicit solvent45, where the continuous potential functions in classic molecular dynamics (MD) were modeled by discrete step-wise functions46. Similar to the classic MD, both bonded interactions (i.e., covalent bonds, bond angles, and dihedrals) and non-bonded interactions (i.e., van der Waals, solvation, hydrogen bond, and electrostatic terms) were considered in DMD47. The step function potentials are adapted from the Medusa forcefield48. In Medusa forcefield the bonded interactions (including bonds, bond angles, and dihedrals) are modeled as infinite square wells, where covalent bonds and bond angles usually have a single well and dihedrals may feature multiple wells corresponding to cis- or trans-conformations. Non-bonded interactions (i.e., van der Waals, solvation, hydrogen bond, and electrostatic terms) are represented as a series of discrete energetic steps, decreasing in magnitude with increasing distance until reaching zero at the cutoff distance. The van der Waals parameters are taken from the CHARMM force field49 and the EEF1 implicit solvent model was used to model the solvation50. A reaction-like algorithm is used to model hydrogen bond formation51. DMD software is available to academic researchers at Molecules In Action (www.moleculesinaction.com). The units of mass, time, length, and energy used in our united-atom with implicit water model were 1 Da, ~50 femtosecond, 1 Å, and 1 kcal/mol, respectively. With significantly enhanced sampling efficiency, DMD was widely used to study protein folding/aggregation11, 52 and protein-nanoparticle interactions2, 22 by both our group and others38, 53. The difference between DMD and traditional MD approaches is in the form of the interaction potential functions. Interatomic interactions in DMD are approximated by step functions instead of continuous potential functions. The system’s dynamics is, thus, dictated by a series of collision events at which two atoms meet at an energy step and change their velocities according to conservation laws. By iteratively updating only the two colliding atoms, predicting their new collisions with corresponding neighbors, and finding the next collision via quick sort algorithms, the sampling efficiency of DMD is significantly enhanced without frequent calculations of forces and accelerations (e.g., every ~1–2 fs) in MD simulations. At an adequately small step size, the discrete step function approaches the continuous potential function and DMD simulations become equivalent to traditional MD. Following the same physical laws, the dynamics observed in DMD are equivalent to continuous potential MD at timescales longer than picoseconds with differences mainly at short timescales within the sub-picosecond range (i.e., the average time step between two consecutive interatomic collisions where a potential energy step is encountered).
Computational Analysis.
The secondary structure was calculated using the DSSP program54. A hydrogen bond was considered to be formed if the distance between the backbone N and O atoms was ≤3.5 Å and the angle of NH···O ≥ 120°12. Inter-chain peptide interactions were analyzed by the residue-residue contact frequencies, where two residues were in contact if they had at least one heavy atom contacts defined according to a cutoff distance of 0.65 nm.
Results and Discussions
Binding of Aβ monomer with αBC monomer.
To investigate the inhibition mechanism of αB-crystallin against Aβ fibrillization, we first studied the interaction between Aβ42 (PDB: 1zoq43, shown in Fig. 1a) and the structured core region of αB-crystallin (αBC, residue 68–153, PDB:3j0744; the amino acid sequences and structures are shown in Fig. 1a). Fifty independent DMD simulations, each of which started from different initial configurations (i.e., orientations, coordinates and velocities) with minimum inter-molecule distance no less than 1.5nm and lasted 500 ns, were performed for understanding detailed interactions between monomeric αBC and Aβ42. Both of the two exposed β-sheet elongation surfaces of αBC β-sandwich (i.e., β3+β6–7 and β4+β8 as highlighted in Fig. 1b) could bind Aβ peptide. The time evolution of the number of inter-molecular backbone hydrogen bonds between Aβ and αBC and secondary structure of each Aβ residue(Fig. 1b) revealed that Aβ could directly bind to the exposed β-strand edges of αBC β-sandwich by forming inter-chain hydrogen bonds and converting into β-sheet rich structures. The inter-chain contact frequencies between Aβ and αBC residues and the representative binding motifs were also analyzed (shown in Fig. 1c). Specifically, the β6–7 strand of αBC displayed strong binding propensity with Aβ1–19 and Aβ29–42, forming parallel (with Aβ1–12) or antiparallel (with Aβ7–19 or Aβ29–42) β-sheets. The β3 strand of αBC formed antiparallel β-sheet with Aβ16–22 and parallel β-sheet with Aβ31–42. The β4 strand of αBC mainly formed antiparallel β-sheets with Aβ9–16 and Aβ27–33. The β8 strand of αBC was observed to form parallel β-sheet with Aβ30–38 and anti-parallel β-sheet with Aβ36–42. The calculation of the total number of inter-chain contacts per residue showed that αBC residues in β6–7 had stronger binding propensities to Aβ than the other αBC strands, especially with the N-terminal Aβ residues 1–20 displaying the strongest inter-molecular binding frequencies (Fig. 1c). The inter-molecular residue-to-residue pairing analysis of these populated motifs (Fig. 1c) reveled that these β-sheets were mainly stabilized by attractive hydrophobic interactions and salt-bridges. Averaged secondary structure propensities for each Aβ residue revealed that those residues with high binding propensity to αBC had strong probabilities to adopt β-sheet structures (Fig. S1a). The residue-pairwise contact frequency and most populated conformation analysis demonstrated that Aβ monomers were structurally dynamic with a highly tendency to adopt β-hairpin conformations (Fig. S1b&c). The observed conformational flexibility of Aβ monomers were consistent with FRET experiments55. While some experiments suggested that the backbone torsion angle distributions of Aβ monomers derived from NMR J-couplings closely resembled those of random coils56, other studies indicated a significant population of β-sheet conformations57. A CD spectra experiment found that the β-sheet content of Aβ monomers was around 24%58. Using experimentally determined J-coupling measurements as the benchmark, the OPLS forcefield was found as the most experimentally consistent to capture Aβ conformational dynamics59, 60 and the corresponding structural ensemble of Aβ42 featured β-hairpin rich conformations as in our simulations59, 60. Additionally, sequence regions with high β-sheet propensities were similar to those observed in the experimentally-determined Aβ amyloid fibrils61 and also in prior all-atom MD simulations 58, 62–64. The residue-pairwise contact map reveled that there were no major averaged conformational changes for Aβ42 in the presence of αBC (Fig. S1b). Together with the representative Aβ-αBC heterodimer structures (Fig. S1d), we could learn that Aβ42 mainly bind to the exposed β-strand edges of the αBC sandwich with un-saturated hydrogen bonding donors and acceptors (i.e. β3&β6–7 and β4&β8) and form inter-molecular β-sheets.
Binding of Aβ monomer with αBC dimer.
The αBC tends to form dimers in solution and previous X-ray crystallography and solution-state NMR spectroscopy studies showed that the β6–7 strand of αBC is buried in the homo-dimer interface by forming β-sheets with each other30, 36, 65. Hence, to investigate the inhibition mechanism of αBC dimer against Aβ amyloid aggregation in solution, we further studied the interaction between Aβ monomer and αBC dimer by performing fifty independent binding simulations. Initially, Aβ42 monomer was randomly placed at least 1.5 nm away from the αBC dimer. Since the β6–7 surface was buried in the αBC dimer19, 25, 32, the Aβ42 monomer predominantly bound to the β4&β8 interface in DMD simulations (Fig. 2a). Similar to our simulations with the αBC monomer, Aβ42 mainly adopted β-sheet conformations after binding the αBC dimer (e.g. a representation trajectory in Fig. 2a). The average number of inter-molecular contacts for each αBC residue revealed that Aβ42 mainly attached on β4 and β8 of αBC. The result agreed well with prior NMR chemical shift perturbation experiments, where residues in β4 and β8 were the most perturbed regions in αBC upon binding Aβ30, 36. The residue-wise inter-molecular contact frequency map along with the representative binding motifs in simulations demonstrated that the β4 and β8 strands of αBC mainly interacted with Aβ14–21 and Aβ28–42 forming either parallel or anti-parallel β-sheets, which were also stabilized by hydrophobic and salt-bridge interactions (Fig. 2b). Additionally, we also observed a weak binding between αBC β3 and Aβ16–26, a small NMR chemical shift exchanges for L79 in αBC β3 was also observed in prior Aβ-αBC experimental study30. Overall, the hydrophobic and charged residues from β4&β8 interface of αBC displayed significant binding propensity to Aβ42. For Aβ42, residues 30–42 in the C-terminus had much higher binding propensities with αBC dimer than the rest of peptide. Secondary structure analysis of Aβ42 monomer before and after binding αBC confirmed that the β-sheet content of residues around Aβ13–21 and Aβ30–42 was enhanced due to binding with the αBC dimer (Fig. S2). Top populated complex structures of Aβ42 monomer and αBC dimer from the root-mean-square deviation (RMSD)-based clustering analysis showed that Aβ binding extended the β-sheets of αBC (Fig. 2c). Hence, the simulation results of Aβ monomer binding with αBC dimer demonstrated that Aβ mainly bound to the β4&β8 interface of αBC dimer in solution.
Fig. 2. Binding of Aβ monomer with αBC dimer.
a) The time evolution of the number of inter-chain hydrogen bonds formed between Aβ monomer and αBC dimer, and secondary structure for each Aβ residue. The snapshots along with the simulation time are shown on the right. The representative trajectory is randomly selected out of 50 independent simulations. b) The inter-molecular contact frequency map between Aβ and αBC. For each residue in Aβ and αBC, its total number of inter-chain contacting residues are also computed by integrating the corresponding pair-wise contact frequency map. Representative binding motifs of Aβ (colored in red) and αBC (colored in blue) labeled as 1–8 are also shown. The hydrophobic and salt bridge residue-residue pairs are highlight in bold. c) The top six most populated representative complex structures are shown. For clarity, the two chains of αBC dimer are colored by orange and cyan, respectively. The hot spot binding regions β4&β8 for Aβ are shown in red. Aβ peptide is colored in magenta.
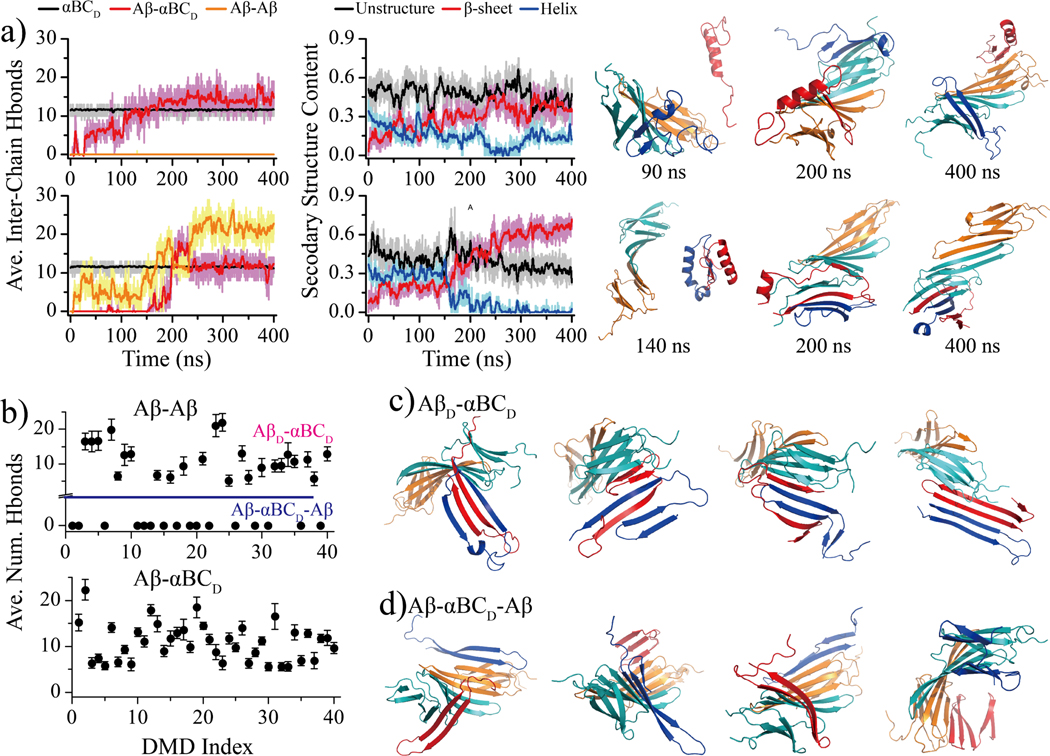
Aβ dimerization in the presence of αBC dimer.
As the first step toward understanding the effect of αBC on the Aβ aggregation, we studied the dimerization process of Aβ by simulating two Aβ monomers in the presence of an αBC dimer. Two Aβ monomers and one αBC dimer were randomly placed in a 9.0 nm cubic simulation box with a periodic boundary condition and a minimum inter-molecule distance greater than 1.5 nm. In our simulations, the number of inter-chain hydrogen bonds between β6–7 segments in the αBC dimer (Fig. 3a) was maintained around 12 without much fluctuations (less than 3), indicating the αBC dimer was stable in solution as observed in prior experimental studies19, 25, 32. As shown in Fig. 3a, two Aβ monomers could separately bind to the two exposed β-sheet elongation surfaces of the αBC dimer and form an Aβ-αBCD-Aβ complex. We also observed the dimerization of Aβ monomers before binding to the αBC dimer and forming an AβD-αBCD complex. These results indicated that the αBC could interact with both Aβ monomers and dimers (i.e., the smallest oligomer), and likely other higher-molecular-weight oligomers should follow a similar manner. The average number of backbone hydrogen bonds between two Aβ monomers during last 100 ns simulation in each independent simulation showed that both types of complexes, Aβ-αBCD-Aβ (with zero inter-Aβ hydrogen bonds formed) and AβD-αBCD (multiple inter-Aβ hydrogen bonds ranging from 5 to 21) were the stable conformations (Fig. 3b). Aβ could bind to the lateral surface of αBC during the early binding stage. But these states were unstable and Aβ spontaneously diffused to the exposed edges of the β-sheets. Aβ and αBC always formed backbone hydrogen bonds in all independent trajectories (Fig. 3b). The analysis of secondary structure propensities of each Aβ residue (Fig. S3) showed that Aβ mainly adopted unstructured coil and β-sheet conformations in both absence and presence of αBC dimer. The presence of αBC dimer didn’t induce much secondary structure changes in terms of β-sheet content. consistent with a prior circular dichroism (CD) spectroscopy experiment demonstrating that co-incubation of Aβ42 with αBC had no effect on Aβ42 β-sheet formation66. However, the time evolution of the inter-Aβ backbone hydrogen bonds that stabilize amyloid fibril structures 6–8 were significantly inhibited by αBC dimer (Fig. S4). Together with the representative conformations of Aβ42 with αBC complexes (Fig. 3c&d), we could learn that αBC dimer could bind Aβ monomers, dimers and likely other high-order oligomers along the β4&β8 interface forming β-sheet conformations.
Fig. 3. Aβ dimerization in the presence of αBC dimer.
a) Two representative trajectories, forming AβM-αBCD- AβM (top) and AβD-αBCD (bottom) complex, are randomly selected from 40 independent DMD simulations. The time evolution of the number inter-molecular hydrogen bonds between αBC and αBC (black), Aβ and Aβ (orange), Aβ and αBC (red) are shown on the left. The snapshots along the noted times are also shown to the right. b) The average number of hydrogen bonds between two Aβ peptides (top) and between Aβ and αBC (bottom) during last 100 ns of each 40 independent simulations. The representative AβM-αBCD-AβM (c) and AβD-αBCD (d) structures are randomly selected from the final snapshots of independent DMD trajectories. For clarity, the two chains of αBC dimer are colored by orange and cyan, respectively. Two Aβ peptides are colored in red and blue.
The inhibition mechanism of αBC against Aβ fibrillization.
Prior experimental and computational simulation studies revealed that Aβ monomers first formed oligomers while undergo nucleated conformational conversion into β-sheet rich oligomers before forming proto-fibrils and mature fibrils12, 13, 63, 64, 67–72(Fig. 4). For example, prior all-atom replica-exchange simulation studies demonstrated the Aβ dimer featured extend β-hairpin and β-strand abundant23, 73. Our simulation results demonstrated that αBC dimer in solution could bind Aβ monomers, dimers, possibly other high-order oligomers by forming inter-molecular β-sheets with β4 and β8 strands of αBC. Different from the most of immunoglobulin β-sandwich proteins with edge-protection74, where the charged side-chain located in the middle of the hydrophobic side of the edge β-strand to avoid self-aggregation, the exposed β4&β8 edges of αBC were favorable to interact with the β-strands they encountered. For example, numerous experimental evidences suggested the β4&8 surfaces of αBC dimeric subunit were buried in the αBC complex model30, 75. While β4&8 strands of both monomers in αBC dimer can bind Aβ and potentially form larger intercalated Aβ/αBC complexes, the highly curved β-sheet structure of the αBC dimer is not compatible with long fibers, as shown by the bended structure of the AβM-αBCD-AβM complex in Fig. 3d and also as suggested by the formation of closed hexamers and subsequent high-order 24-mer of αBC. Therefore, the binding of αBCD with Aβ monomers and small aggregates in the early stage Aβ aggregation via β-sheet pairing interrupts the continuous growth into long straight fibrils, comprised of parallel in-register β-sheets between neighboring Aβ peptides. Similarly, our results also suggest that the binding of αBCD with pre-formed or newly-formed Aβ fibrils could also inhibit the further elongation into longer fibrils. Indeed, prior experimental studies revealed that αBC not only could inhibit the amyloid aggregation of Aβ29, 30, 36, 76, but also prevented the growth of preformed Aβ fibrils – i.e., mitigating the seeding effect28, 36. A similar inhibition mechanism was also reported by Hoyer et. al, where the fibrillization of Aβ was inhibited when the β-hairpin structure was stabilized by affibody protein ZAβ377. Although the binding of αBC with Aβ fibrils was not included in the current study due to the large system size, we expect the binding of αBC with Aβ fibrils because the αBC-binding hot-spot residues – e.g. Aβ16–26 and Aβ30–42 – are already in the αBC-binding compatible β-sheet conformations and solvent-exposed at the fibril elongation ends. Hence, the capping of Aβ proto-fibrils and fibrils at the elongation interface by binding with β4 and β8 strands of αBC could inhibit the further fibril growth (Fig. 4).
Fig. 4. The inhibition mechanism of αBC against Aβ fibrillization.
In the absence of αBC, Aβ monomers aggregated into β-sheet rich oligomers before forming proto-fibrils and mature fibrils. In the presence of αBC, the dimer (αBCD) in solution could directly bind the β-sheet elongation surface of Aβ oligomers or proto-fibrils, and prevent them from growth and elongation into mature fibril structure via capping.
Conclusion
In sum, we investigated the inhibition mechanism of endogenous αBC against Aβ amyloid aggregation using united-atom DMD simulations with implicit solvent. We identified the hot-spot regions in Aβ and αBC that are important for their cross-interactions in agreement with NMR chemical shift perturbation studies30, 36. Upon binding αBC dimer in solution, Aβ formed β-sheets with exposed β4 and β8 strands of αBC β-sandwich. Our results suggested that αBC could bind to various Aβ aggregate species during the aggregation process - including monomers, dimers, and likely other high molecular weight oligomers, proto-fibrils and fibrils – by capping the exposed β-sheet elongation surfaces. Mechanistic insights obtained from our systematic computational studies may aid in the development of novel therapeutic strategies to modulate the aggregation of pathological, amyloidogenic protein in degenerative diseases.
Supplementary Material
Acknowledgement
This work was supported in part by the National Natural Science Foundation of China under the Grant No. 11904189 (Sun), K.C.Wong Magna Fund in Ningbo University, China (Sun), NSF CBET-1553945 (Ding), and NIH R35GM119691 (Ding). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSFC, NIH and NSF.
Footnotes
Electronic supplementary information (ESI) available: Supporting Figures.
Conflicts of interest
There are no conflicts to declare.
References
- 1.Wang Z; Chen Y; Li X; Sultana P; Yin M; Wang Z, Amyloid-beta1–42 dynamically regulates the migration of neural stem/progenitor cells via MAPK-ERK pathway. Chemico-biological interactions 2019, 298, 96–103. [DOI] [PubMed] [Google Scholar]
- 2.Ke PC; Pilkington EH; Sun Y; Javed I; Kakinen A; Peng G; Ding F; Davis TP, Mitigation of Amyloidosis with Nanomaterials. Advanced materials 2019, e1901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamley IW, The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chemical reviews 2012, 112 (10), 5147–92. [DOI] [PubMed] [Google Scholar]
- 4.Ke PC; Sani MA; Ding F; Kakinen A; Javed I; Separovic F; Davis TP; Mezzenga R, Implications of peptide assemblies in amyloid diseases. Chem Soc Rev 2017, 46 (21), 6492–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttle MD; Comellas G; Nieuwkoop AJ; Covell DJ; Berthold DA; Kloepper KD; Courtney JM; Kim JK; Barclay AM; Kendall A; Wan W; Stubbs G; Schwieters CD; Lee VMY; George JM; Rienstra CM, Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat Struct Mol Biol 2016, 23 (5), 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao YL; Ma BY; McElheny D; Parthasarathy S; Long F; Hoshi M; Nussinov R; Ishii Y, A beta(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol 2015, 22 (6), 499–U97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colvin MT; Silvers R; Ni QZ; Can TV; Sergeyev I; Rosay M; Donovan KJ; Michael B; Wall J; Linse S; Griffin RG, Atomic Resolution Structure of Monomorphic A beta(42) Amyloid Fibrils. J Am Chem Soc 2016, 138 (30), 9663–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walti MA; Ravotti F; Arai H; Glabe CG; Wall JS; Bockmann A; Guntert P; Meier BH; Riek R, Atomic-resolution structure of a disease-relevant A beta(1–42) amyloid fibril. P Natl Acad Sci USA 2016, 113 (34), E4976–E4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y; Kakinen A; Xing Y; Faridi P; Nandakumar A; Purcell AW; Davis TP; Ke PC; Ding F, Amyloid Self-Assembly of hIAPP8–20 via the Accumulation of Helical Oligomers, alpha-Helix to beta-Sheet Transition, and Formation of beta-Barrel Intermediates. Small 2019, 15 (18), e1805166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y; Kakinen A; Xing Y; Pilkington EH; Davis TP; Ke PC; Ding F, Nucleation of beta-rich oligomers and beta-barrels in the early aggregation of human islet amyloid polypeptide. Biochimica et biophysica acta. Molecular basis of disease 2019, 1865 (2), 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y; Wang B; Ge X; Ding F, Distinct oligomerization and fibrillization dynamics of amyloid core sequences of amyloid-beta and islet amyloid polypeptide. Physical chemistry chemical physics : PCCP 2017, 19 (41), 28414–28423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bieschke J; Herbst M; Wiglenda T; Friedrich RP; Boeddrich A; Schiele F; Kleckers D; del Amo JML; Gruning BA; Wang QW; Schmidt MR; Lurz R; Anwyl R; Schnoegl S; Fandrich M; Frank RF; Reif B; Gunther S; Walsh DM; Wanker EE, Small-molecule conversion of toxic oligomers to nontoxic beta-sheet-rich amyloid fibrils. Nat Chem Biol 2012, 8 (1), 93–101. [DOI] [PubMed] [Google Scholar]
- 13.Bemporad F; Chiti F, Protein misfolded oligomers: experimental approaches, mechanism of formation, and structure-toxicity relationships. Chemistry & biology 2012, 19 (3), 315–27. [DOI] [PubMed] [Google Scholar]
- 14.Iadanza MG; Jackson MP; Hewitt EW; Ranson NA; Radford SE, A new era for understanding amyloid structures and disease. Nature reviews. Molecular cell biology 2018, 19 (12), 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y; Ge X; Xing Y; Wang B; Ding F, beta-barrel Oligomers as Common Intermediates of Peptides Self-Assembling into Cross-beta Aggregates. Sci Rep 2018, 8 (1), 10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belluti F; Rampa A; Gobbi S; Bisi A, Small-molecule inhibitors/modulators of amyloid-beta peptide aggregation and toxicity for the treatment of Alzheimer’s disease: a patent review (2010 – 2012). Expert opinion on therapeutic patents 2013, 23 (5), 581–96. [DOI] [PubMed] [Google Scholar]
- 17.Ehrnhoefer DE; Bieschke J; Boeddrich A; Herbst M; Masino L; Lurz R; Engemann S; Pastore A; Wanker EE, EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol 2008, 15 (6), 558–66. [DOI] [PubMed] [Google Scholar]
- 18.Wang M; Sun Y; Cao X; Peng G; Javed I; Kakinen A; Davis TP; Lin S; Liu J; Ding F; Ke PC, Graphene quantum dots against human IAPP aggregation and toxicity in vivo. Nanoscale 2018, 10 (42), 19995–20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doig AJ; Derreumaux P, Inhibition of protein aggregation and amyloid formation by small molecules. Current opinion in structural biology 2015, 30, 50–56. [DOI] [PubMed] [Google Scholar]
- 20.Syarifah-Noratiqah SB; Naina-Mohamed I; Zulfarina MS; Qodriyah HMS, Natural Polyphenols in the Treatment of Alzheimer’s Disease. Current drug targets 2018, 19 (8), 927–937. [DOI] [PubMed] [Google Scholar]
- 21.Kakinen A; Adamcik J; Wang B; Ge X; Mezzenga R; Davis TP; Ding F; Ke PC, Nanoscale inhibition of polymorphic and ambidextrous IAPP amyloid aggregation with small molecules. Nano research 2018, 11 (7), 3636–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faridi A; Sun Y; Okazaki Y; Peng G; Gao J; Kakinen A; Faridi P; Zhao M; Javed I; Purcell AW; Davis TP; Lin S; Oda R; Ding F; Ke PC, Mitigating Human IAPP Amyloidogenesis In Vivo with Chiral Silica Nanoribbons. Small 2018, 14 (47), e1802825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y; Qian Z; Wei G, The inhibitory mechanism of a fullerene derivative against amyloid-beta peptide aggregation: an atomistic simulation study. Physical chemistry chemical physics : PCCP 2016, 18 (18), 12582–91. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y; Kakinen A; Zhang C; Yang Y; Faridi A; Davis TP; Cao W; Ke PC; Ding F, Amphiphilic surface chemistry of fullerenols is necessary for inhibiting the amyloid aggregation of alpha-synuclein NACore. Nanoscale 2019, 11 (24), 11933–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z; Krause G; Reif B, Structure and orientation of peptide inhibitors bound to beta-amyloid fibrils. Journal of molecular biology 2005, 354 (4), 760–76. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T; Mihara H, Peptide and protein mimetics inhibiting amyloid beta-peptide aggregation. Accounts of chemical research 2008, 41 (10), 1309–18. [DOI] [PubMed] [Google Scholar]
- 27.Sun YX; Kakinen A; Zhang C; Yang Y; Faridi A; Davis TP; Cao WG; Ke PC; Ding F, Amphiphilic surface chemistry of fullerenols is necessary for inhibiting the amyloid aggregation of alpha-synuclein NACore. Nanoscale 2019, 11 (24), 11933–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shammas SL; Waudby CA; Wang SY; Buell AK; Knowles TPJ; Ecroyd H; Welland ME; Carver JA; Dobson CM; Meehan S, Binding of the Molecular Chaperone alpha B-Crystallin to A beta Amyloid Fibrils Inhibits Fibril Elongation. Biophys J 2011, 101 (7), 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehle FC; Ecroyd H; Musgrave IF; Carver JA, alphaB-Crystallin inhibits the cell toxicity associated with amyloid fibril formation by kappa-casein and the amyloid-beta peptide. Cell stress & chaperones 2010, 15 (6), 1013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainz A; Peschek J; Stavropoulou M; Back KC; Bardiaux B; Asami S; Prade E; Peters C; Weinkauf S; Buchner J; Reif B, The chaperone alphaB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat Struct Mol Biol 2015, 22 (11), 898–905. [DOI] [PubMed] [Google Scholar]
- 31.Basha E; O’Neill H; Vierling E, Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends in biochemical sciences 2012, 37 (3), 106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhat SP; Nagineni CN, alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochemical and biophysical research communications 1989, 158 (1), 319–25. [DOI] [PubMed] [Google Scholar]
- 33.Bhat SP; Horwitz J; Srinivasan A; Ding L, Alpha B-crystallin exists as an independent protein in the heart and in the lens. European journal of biochemistry 1991, 202 (3), 775–81. [DOI] [PubMed] [Google Scholar]
- 34.Nagineni CN; Bhat SP, Alpha B-crystallin is expressed in kidney epithelial cell lines and not in fibroblasts. FEBS letters 1989, 249 (1), 89–94. [DOI] [PubMed] [Google Scholar]
- 35.May CA; Welge-Lussen U; Junemann A; Bloemendal H; Lutjen-Drecoll E, AlphaB-crystallin in lacrimal gland duct and tears. Current eye research 2000, 21 (1), 588–94. [PubMed] [Google Scholar]
- 36.Hochberg GKA; Ecroyd H; Liu C; Cox D; Cascio D; Sawaya MR; Collier MP; Stroud J; Carver JA; Baldwin AJ; Robinson CV; Eisenberg DS; Benesch JLP; Laganowsky A, The structured core domain of alpha B-crystallin can prevent amyloid fibrillation and associated toxicity. P Natl Acad Sci USA 2014, 111 (16), E1562–E1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waudby CA; Knowles TP; Devlin GL; Skepper JN; Ecroyd H; Carver JA; Welland ME; Christodoulou J; Dobson CM; Meehan S, The interaction of alphaB-crystallin with mature alpha-synuclein amyloid fibrils inhibits their elongation. Biophys J 2010, 98 (5), 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proctor EA; Dokholyan NV, Applications of Discrete Molecular Dynamics in biology and medicine. Current opinion in structural biology 2016, 37, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodie NI; Popov KI; Petrotchenko EV; Dokholyan NV; Borchers CH, Solving protein structures using short-distance cross-linking constraints as a guide for discrete molecular dynamics simulations. Sci Adv 2017, 3 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voelker MJ; Barz B; Urbanc B, Fully Atomistic Abeta40 and Abeta42 Oligomers in Water: Observation of Porelike Conformations. Journal of chemical theory and computation 2017, 13 (9), 4567–4583. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y; Bunce SJ; Radford SE; Wilson AJ; Auer S; Hall CK, Thermodynamic phase diagram of amyloid-beta (16–22) peptide. Proc Natl Acad Sci U S A 2019, 116 (6), 2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunce SJ; Wang YM; Stewart KL; Ashcroft AE; Radford SE; Hall CK; Wilson AJ, Molecular insights into the surface-catalyzed secondary nucleation of amyloid-beta(40) (A beta(40)) by the peptide fragment A beta(16–22). Sci Adv 2019, 5 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomaselli S; Esposito V; Vangone P; van Nuland NAJ; Bonvin AMJJ; Guerrini R; Tancredi T; Temussi PA; Picone D, The alpha-to-beta conformational transition of Alzheimer’s A beta-(1–42) peptide in aqueous media is reversible: A step by step conformational analysis suggests the location of beta conformation seeding. Chembiochem 2006, 7 (2), 257–267. [DOI] [PubMed] [Google Scholar]
- 44.Jehle S; Vollmar BS; Bardiaux B; Dove KK; Rajagopal P; Gonen T; Oschkinat H; Klevit RE, N-terminal domain of alpha B-crystallin provides a conformational switch for multimerization and structural heterogeneity. P Natl Acad Sci USA 2011, 108 (16), 6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng S; Ding F; Urbanc B; Buldyrev SV; Cruz L; Stanley HE; Dokholyan NV, Discrete molecular dynamics simulations of peptide aggregation. Phys Rev E 2004, 69 (4). [DOI] [PubMed] [Google Scholar]
- 46.Ding F; Tsao D; Nie HF; Dokholyan NV, Ab initio folding of proteins with all-atom discrete molecular dynamics. Structure 2008, 16 (7), 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Javed I; Sun YX; Adamcik J; Wang B; Kakinen A; Pilkington EH; Ding F; Mezzenga R; Davis TP; Ke PC, Cofibrillization of Pathogenic and Functional Amyloid Proteins with Gold Nanoparticles against Amyloidogenesis. Biomacromolecules 2017, 18 (12), 4316–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding F; Dokholyan NV, Emergence of protein fold families through rational design. PLoS computational biology 2006, 2 (7), e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks Bernard R., B. RE, Olafson Barry D., States David J. Swaminathan S, Karplus Martin, CHARMM: a program for macromolecular energy, minimization, and dynamics calculations[J]. Journal of computational chemistry. Journal of computational chemistry 1983, 4 (2), 187–217. [Google Scholar]
- 50.Lazaridis T; Karplus M, Effective energy functions for protein structure prediction. Current opinion in structural biology 2000, 10 (2), 139–45. [DOI] [PubMed] [Google Scholar]
- 51.Ding F; Borreguero JM; Buldyrey SV; Stanley HE; Dokholyan NV, Mechanism for the alpha-helix to beta-hairpin transition. Proteins 2003, 53 (2), 220–8. [DOI] [PubMed] [Google Scholar]
- 52.Nedumpully-Govindan P; Kakinen A; Pilkington EH; Davis TP; Chun Ke P; Ding F, Stabilizing Off-pathway Oligomers by Polyphenol Nanoassemblies for IAPP Aggregation Inhibition. Sci Rep 2016, 6, 19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brodie NI; Popov KI; Petrotchenko EV; Dokholyan NV; Borchers CH, Solving protein structures using short-distance cross-linking constraints as a guide for discrete molecular dynamics simulations. Sci Adv 2017, 3 (7), e1700479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kabsch W; Sander C, Dictionary of Protein Secondary Structure - Pattern-Recognition of Hydrogen-Bonded and Geometrical Features. Biopolymers 1983, 22 (12), 2577–2637. [DOI] [PubMed] [Google Scholar]
- 55.Meng F; Bellaiche MMJ; Kim JY; Zerze GH; Best RB; Chung HS, Highly Disordered Amyloid-beta Monomer Probed by Single-Molecule FRET and MD Simulation. Biophys J 2018, 114 (4), 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roche J; Shen Y; Lee JH; Ying J; Bax A, Monomeric Abeta(1–40) and Abeta(1–42) Peptides in Solution Adopt Very Similar Ramachandran Map Distributions That Closely Resemble Random Coil. Biochemistry 2016, 55 (5), 762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ball KA; Phillips AH; Wemmer DE; Head-Gordon T, Differences in beta-strand populations of monomeric Abeta40 and Abeta42. Biophys J 2013, 104 (12), 2714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ono K; Condron MM; Teplow DB, Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A 2009, 106 (35), 14745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sgourakis NG; Yan Y; McCallum SA; Wang C; Garcia AE, The Alzheimer’s peptides Abeta40 and 42 adopt distinct conformations in water: a combined MD / NMR study. Journal of molecular biology 2007, 368 (5), 1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenman DJ; Connors CR; Chen W; Wang C; Garcia AE, Abeta monomers transiently sample oligomer and fibril-like configurations: ensemble characterization using a combined MD/NMR approach. Journal of molecular biology 2013, 425 (18), 3338–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gremer L; Scholzel D; Schenk C; Reinartz E; Labahn J; Ravelli RBG; Tusche M; Lopez-Iglesias C; Hoyer W; Heise H; Willbold D; Schroder GF, Fibril structure of amyloid-beta(1–42) by cryo-electron microscopy. Science 2017, 358 (6359), 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou Y; Qian Z; Chen Y; Qian H; Wei G; Zhang Q, Norepinephrine Inhibits Alzheimer’s Amyloid-beta Peptide Aggregation and Destabilizes Amyloid-beta Protofibrils: A Molecular Dynamics Simulation Study. Acs Chem Neurosci 2019, 10 (3), 1585–1594. [DOI] [PubMed] [Google Scholar]
- 63.Tarus B; Tran TT; Nasica-Labouze J; Sterpone F; Nguyen PH; Derreumaux P, Structures of the Alzheimer’s Wild-Type Abeta1–40 Dimer from Atomistic Simulations. The journal of physical chemistry. B 2015, 119 (33), 10478–87. [DOI] [PubMed] [Google Scholar]
- 64.Sun YX; Qian ZY; Wei GH, The inhibitory mechanism of a fullerene derivative against amyloid-beta peptide aggregation: an atomistic simulation study. Physical Chemistry Chemical Physics 2016, 18 (18), 12582–12591. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z; Wang C; Li Y; Zhao C; Li T; Li D; Zhang S; Liu C, Mechanistic insights into the switch of alphaB-crystallin chaperone activity and self-multimerization. The Journal of biological chemistry 2018, 293 (38), 14880–14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilhelmus MM; Boelens WC; Otte-Holler I; Kamps B; de Waal RM; Verbeek MM, Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain research 2006, 1089 (1), 67–78. [DOI] [PubMed] [Google Scholar]
- 67.Lee J; Culyba EK; Powers ET; Kelly JW, Amyloid-beta forms fibrils by nucleated conformational conversion of oligomers. Nat Chem Biol 2011, 7 (9), 602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Press-Sandler O; Miller Y, Distinct Primary Nucleation of Polymorphic A beta Dimers Yields to Distinguished Fibrillation Pathways. Acs Chem Neurosci 2019, 10 (10), 4407–4413. [DOI] [PubMed] [Google Scholar]
- 69.Benseny-Cases N; Cocera M; Cladera J, Conversion of non-fibrillar beta-sheet oligomers into amyloid fibrils in Alzheimer’s disease amyloid peptide aggregation. Biochemical and biophysical research communications 2007, 361 (4), 916–921. [DOI] [PubMed] [Google Scholar]
- 70.Urbanc B; Betnel M; Cruz L; Bitan G; Teplow DB, Elucidation of amyloid beta-protein oligomerization mechanisms: discrete molecular dynamics study. J Am Chem Soc 2010, 132 (12), 4266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barz B; Liao Q; Strodel B, Pathways of Amyloid-beta Aggregation Depend on Oligomer Shape. J Am Chem Soc 2018, 140 (1), 319–327. [DOI] [PubMed] [Google Scholar]
- 72.Barz B; Olubiyi OO; Strodel B, Early amyloid beta-protein aggregation precedes conformational change. Chemical communications 2014, 50 (40), 5373–5. [DOI] [PubMed] [Google Scholar]
- 73.Jin Y; Sun Y; Chen Y; Lei J; Wei G, Molecular dynamics simulations reveal the mechanism of graphene oxide nanosheet inhibition of Abeta1–42 peptide aggregation. Physical chemistry chemical physics : PCCP 2019, 21 (21), 10981–10991. [DOI] [PubMed] [Google Scholar]
- 74.Richardson JS; Richardson DC, Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci U S A 2002, 99 (5), 2754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jehle S; Rajagopal P; Bardiaux B; Markovic S; Kuhne R; Stout JR; Higman VA; Klevit RE; van Rossum BJ; Oschkinat H, Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Nat Struct Mol Biol 2010, 17 (9), 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santhoshkumar P; Sharma KK, Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem 2004, 267 (1–2), 147–155. [DOI] [PubMed] [Google Scholar]
- 77.Hoyer W; Gronwall C; Jonsson A; Stahl S; Hard T, Stabilization of a beta-hairpin in monomeric Alzheimer’s amyloid-beta peptide inhibits amyloid formation. Proc Natl Acad Sci U S A 2008, 105 (13), 5099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.