Abstract
Quantitative analysis of modified barium swallow (MBS) imaging is useful to determine the impact of various disease states on pharyngeal swallowing mechanics. In this retrospective proof of concept study, kinematic analysis and computational analysis of swallowing mechanics (CASM) were used to demonstrate how these methods differentiate swallowing dysfunction by dysphagia etiology. Ten subjects were randomly selected from four cohorts of dysphagic patients including COPD, head and neck cancer (HNC), motor neuron disease, and stroke. Each subject was age- and gender-matched with healthy, non-dysphagic controls. MBS videos of 5 ml thin and 5 ml thick bolus trials from each subject were used. A MATLAB tracker tool was adapted and updated to collect and compile data for each video (n = 160). For kinematic measurements, a MANOVA was performed with post-hoc analyses to determine group differences. For CASM measurements, a morphometric canonical variate analysis with post hoc analysis was performed to determine group differences. Kinematic analyses indicated statistically significant differences between HNC cohort and controls in distance measurements for hyolaryngeal approximation (p = .001), laryngeal elevation (p = 0.0001), pharyngeal shortening (p = 0.0002), and stage transition duration timing (p = 0.002). Timing differences were noted between the stroke cohort and controls for pharyngeal transit time (p = 0.007). Multivariate morphometric canonical variate analysis showed significant differences between etiology groups (p < 0.0001) with eigenvectors indicating differing patterns of swallowing mechanics. This study demonstrated that swallowing mechanics among cohorts of dysphagic patients can be differentiated using kinematics and CASM, providing different but complementary quantitative methods for investigating the impact of various disease states on swallowing function.
Keywords: Dysphagia, Swallowing mechanics, Modified barium swallow, Quantitative methods, Kinematics, Morphometrics, Aspiration
Introduction
Multiple interdependent elements of swallowing mechanics underlie the transport of a bolus from the oral cavity through the pharynx into the esophagus while simultaneously protecting the airway. Various disease states can disrupt swallowing mechanics resulting in impaired swallowing performance (dysphagia) [1, 2]. Understanding the complexity and interplay of swallowing mechanics with risk factors, such as a disease state, is important for targeted rehabilitation [3, 4]. The modified barium swallow (MBS) examination, a standard in diagnostic imaging studies of swallowing function, can be utilized to analyze pharyngeal swallowing mechanics [5, 6]. Quantitative analysis of swallowing mechanics, such as kinematic analysis and Computational Analysis of Swallowing Mechanics (CASM), can be achieved using digital tools to reduce data from imaging files [7, 8].
Kinematic analysis has been the standard for determining how various risk factors impact swallowing mechanics. Displacement (kinematic) measurements of various anatomical landmarks during swallowing and timing (temporal) measurements of bolus transit at critical points of the swallowing process can be determined on MBS imaging using open source or commercially available digital tools [6, 8]. These physiological data are widely used to determine differences among various elements of swallowing mechanics in clinical and research settings [9–12].
CASM is a research method that utilizes multivariate morphometric analysis to infer changes in multiple elements of the swallowing mechanism associated with variables of interest, including dysphagic etiologies [13–16]. Data collected for CASM are frame-by-frame coordinates of anatomical landmarks delineating muscle groups and skeletal structures underlying the elements of pharyngeal swallowing mechanics during a swallow (Fig. 1). The resulting shapes of these coordinate data are analyzed using multivariate morphometric (also called geometric) analysis. Shape change is visualized using eigenvectors which show the variance and interaction of each element of pharyngeal swallowing mechanics included in the analysis [17].
Fig. 1.
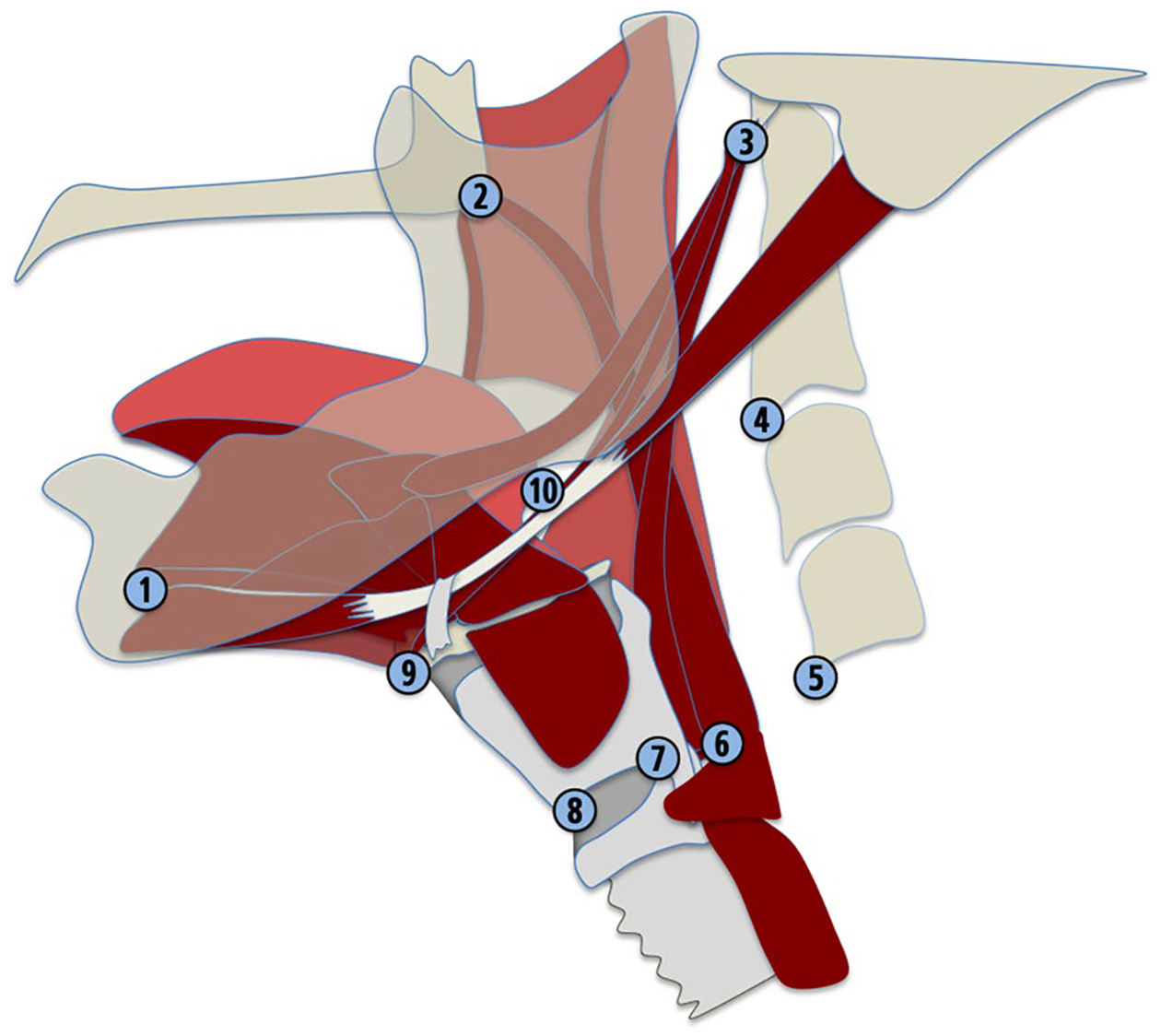
Coordinates map anatomical landmarks relevant to head and neck posture [1–5], proximal attachments of swallowing musculature [1–3] and distal attachments of musculature underlying hyoid movement, laryngeal elevation, pharyngeal shortening, and tongue base retraction [6–10]. Coordinate data are used for kinematic distance measurements and CASM
The purpose of this pilot study was to compare how kinematics and CASM characterize the impact of disease etiology on the swallowing mechanism. This study comprises cohorts from four dysphagic patient populations, including post-treatment squamous cell carcinoma of the oropharynx (HNC), stroke, chronic obstructive pulmonary disease (COPD), and motor neuron disease (MND). Each patient was then age- and sex-matched with a healthy, non-dysphagic control. Each of these patient populations has a well-known comorbidity of dysphagia [18–21]. We used kinematic analysis and CASM independently to test the following hypotheses: swallowing mechanics of each test group differ from an age- and sex-matched control group (H1), swallowing mechanics differ by dysphagia etiology (H2), and impaired swallowing mechanics of each test group is predictive of penetration-aspiration status (H3).
Methods
Subjects and Imaging
A database of de-identified video swallows from multiple study cohorts was utilized for this retrospective case-controlled study. Four sets of ten subjects each were randomly selected using a Microsoft Excel randomization function from four cohorts of dysphagic patients including two types of neurogenic dysphagias (Stroke and MND) and two types of structural dysphagias (HNC and COPD). Random sampling was stratified by sex to achieve an even distribution of male and female subjects. For each test subject, an age- and sex-matched control derived from a large normative database was included. Each subject underwent an MBS examination utilizing the Modified Barium Swallow Impairment Profile©™ standardized protocol digitally recorded at 30 frames per second [22]. Swallow tasks from the Modified Barium Swallow Impairment Profile©™ protocol for analysis included the second trial of barium-infused 5-ml thin liquid and the 5-ml pudding task (Varibar®, E-Z-EM, Inc.) administered via teaspoon by the examining speech-language pathologist. For the 5 ml thick bolus swallow, the 5 ml pudding trial was extracted in all patient cohorts except HNC due to missing data. For the HNC test and control group, the 5 ml honey swallow was extracted. The Varibar products offer a 40% w/v ratio of barium sulfate concentration for all bolus types. When mapped onto the International Dysphagia Diet Standardisation Initiative (IDDSI) the Varibar pudding and honey-thick are level 4 (extremely thick) [23]. The shear rate of Varibar pudding is approximately 5000 mPa at 30 s-1 and Varibar honey-thick is approximately 3000 mPa at 30 s − 1 both at 25 degrees Celsius [24]. In sum, 160 single-swallow videos were (i.e. 2 swallows each from 40 patient subjects and 2 swallows each from 40 age- and sex-matched controls). Inclusion criterion included adequate imaging quality to allow for identification of all landmarks required for coordinate mapping. All imaging data were de-identified and shared under Institutional Review Board (IRB) approval from host institutions with ethics permission granted by the author’s IRB to conduct research.
Each video was trimmed using QuickTime and analyzed using a MATLAB tracker tool developed by Natarajan and colleagues [25] which was adapted and reformatted by two authors (YT and PH) to include the requisite functionality for this pilot study. The tracker tool was originally created to map coordinates of anatomical landmarks that represent functional muscle groups underlying pharyngeal swallowing mechanics [25]. These coordinates were designated for each of the 10 anatomical landmarks for each frame of the video and exported to one text file representation.
MATLAB Tracker Tool v5.1
The MATLAB tracker tool was tailored to extract timing and distance kinematic variables commonly reported in the research literature. Additional features include distance measurement tools, standardized unit normalization, valleculae and piriform residue area tracers, pharyngeal area tracers for pharyngeal constriction ratio (PCR) calculation, a robust compiler tool, and an anatomical alignment function for eigenvector visualization of CASM results [26, 27] (Fig. 2). A workflow of how the tracker tool and other MATLAB functions were used is provided in Fig. 3.
Fig. 2.
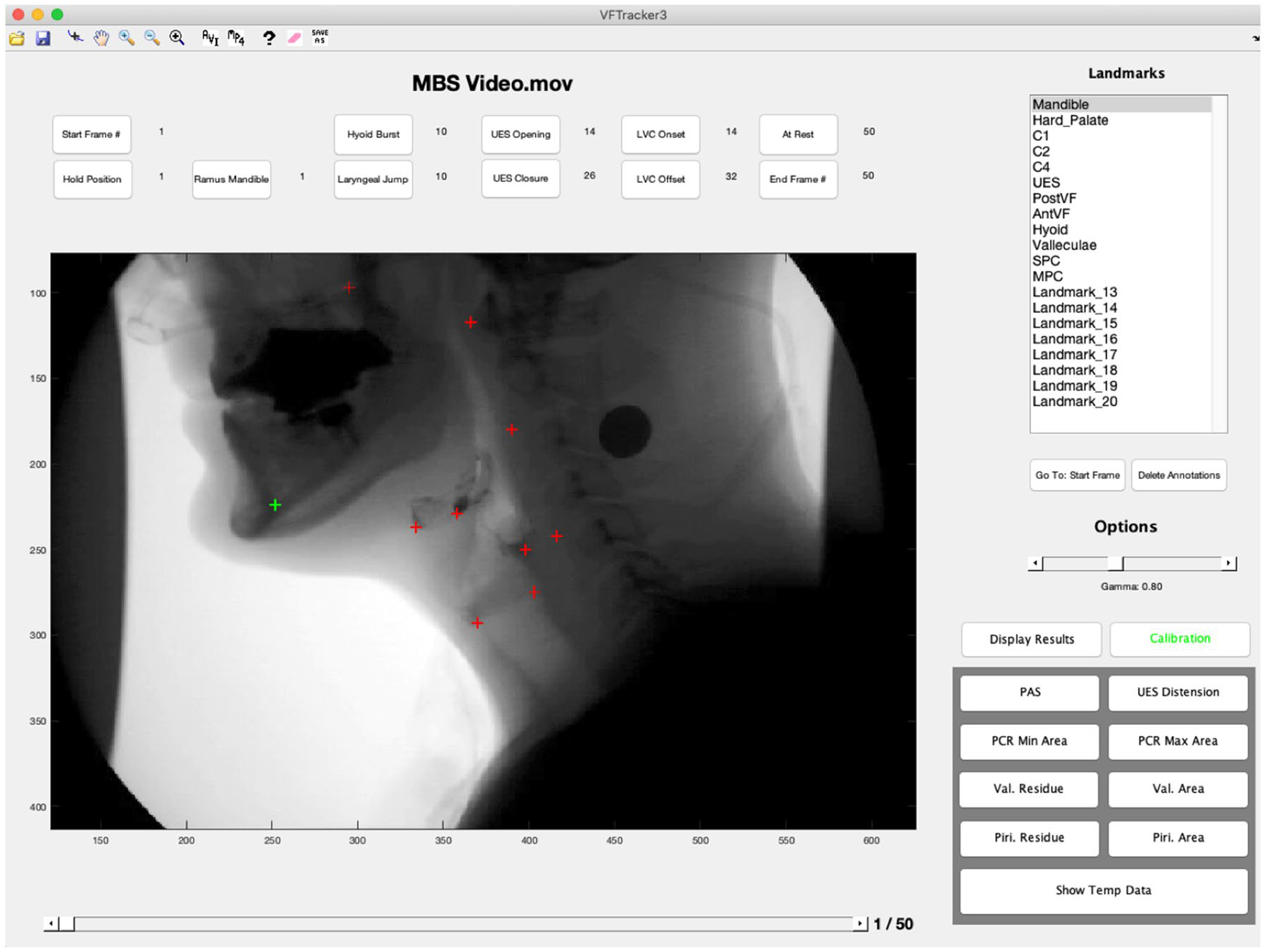
The graphical user interface and functions of the MATLAB tracker tool by Natarajan et al., were expanded to allow for kinematic data to be calculated concurrent with data collection required for morphometric analysis
Fig. 3.
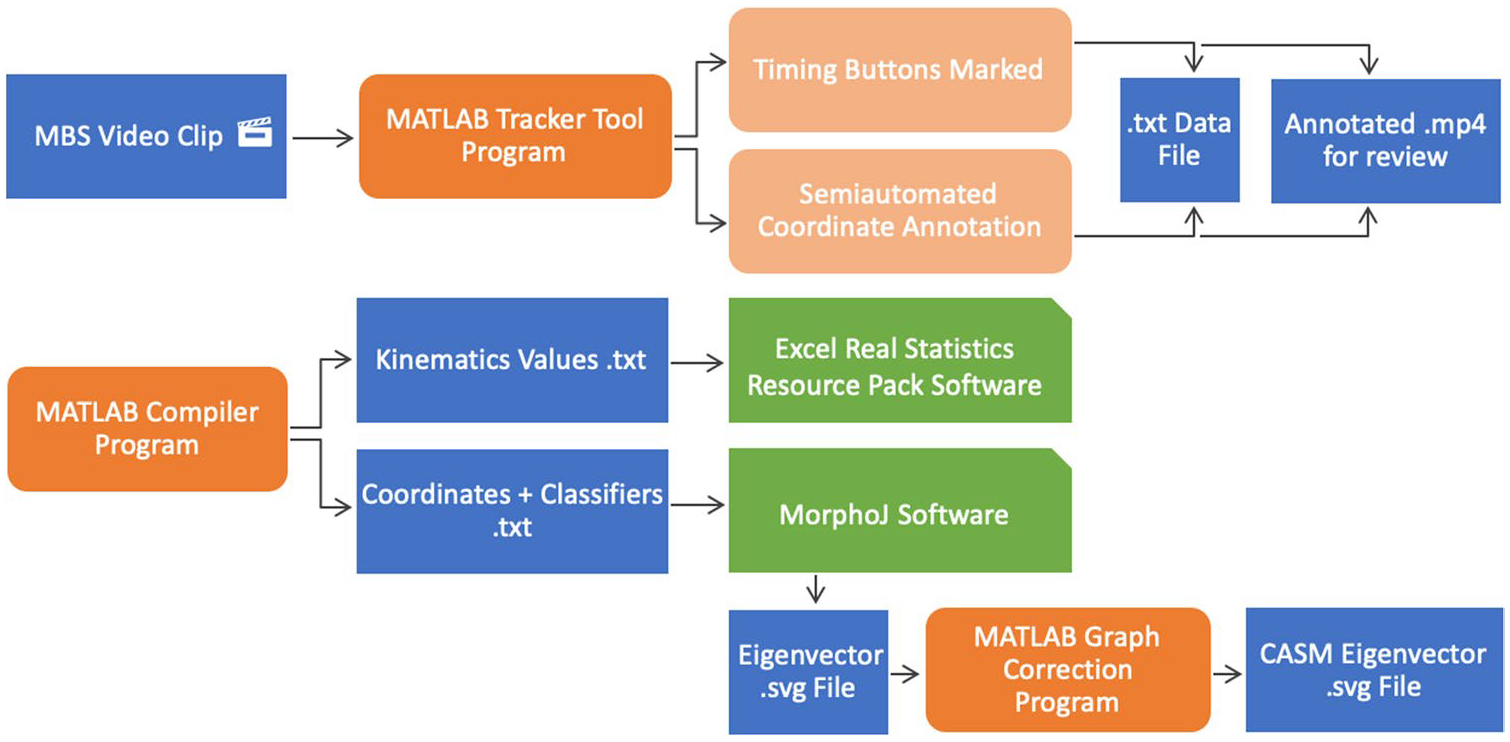
Workflow with data files in blue, MATLAB programs in orange, and analysis software in green. A user annotates individual video swallow files with MATLAB tracker tool to produce a.txt data file and annotated.mp4 video file (useful for visual review). The MATLAB compiler program searches all.txt data files and aggregates a.txt file of kinematic values, and two.txt files required for morphometric analysis in MorphoJ. The MATLAB graph correction program places eigenvectors within anatomical context
The user assigned specific frames as time points throughout the swallow including bolus hold position, bolus crossing of the ramus of the mandible, hyoid burst, and upper esophageal sphincter (UES) closure. Hyoid burst was operationally defined as the first rapid anterosuperior movement of the hyoid associated with a pharyngeal swallow response. Using these designated time points, the tracker tool calculated oral transport time, stage transition duration, and pharyngeal transport time. The semi-automated tracker tool function was used to annotate anatomical landmarks frame-by-frame to map the action of muscles that displace the hyoid, larynx, tongue base, position of the UES, and head and neck posture [7].
After timing buttons were selected and coordinates were annotated, the tracker tool program determined kinematic variables. Hyoid burst and UES closure time frames selected by the user designated each frame related to pharyngeal swallowing. Displacement measurements were calculated using coordinates annotated in every pharyngeal swallowing frame and stored in an array. A search function found the maximum displacement distance in pixels within an array. Pixels were converted to centimeters based on a radiopaque referent in the image (a 1.9 cm penny). Pixels were also converted to C2-C4 vertebrae lengths using a within subject mean of the C2-C4 distance measurements [28]. Displacement measurements included in this study were hyoid excursion in reference to the vertebrae, hyolaryngeal approximation, laryngeal elevation, pharyngeal shortening, and base of tongue retraction ratio. Operational definitions for timing and distance variables in this study are listed in Table 1.
Table 1.
Operational definitions of spatial and temporal variables used in this study (# = coordinates pictured in Fig. 1)
| Operational definitions for kinematic variables | ||
|---|---|---|
| Spatial variables | Hyoid excursion (normalized to vertebrae) | The orthogonal distance between the line connecting C1-C4 (#3, #5) and the hyoid bone (#9) |
| Hyolaryngeal approximation | The distance between hyoid bone (#9) and the anterior vocal fold (#8) | |
| Laryngeal elevation | The distance between the C1 disk (#3) and posterior vocal fold (#7) | |
| Pharyngeal shortening | The distance between the posterior edge of the hard palate (#2) and the upper esophageal sphincter (#6) | |
| Base of tongue retraction ratio | The orthogonal distance between the line connecting C1-C4 and the pit of the valleculae (#10) | |
| Temporal variables | Oral transit time | The time between the start of the swallow and the bolus crossing of the ramus of the mandible |
| Pharyngeal transit time | The time between the bolus crossing of the ramus of mandible and the closure of the upper esophageal sphincter | |
| Stage transition duration | The time between the bolus crossing of the ramus of mandible and hyoid burst | |
The MATLAB tracker tool was tailored to include a compiler function to aggregate data points from selected videos. Three components were included in the compiler: kinematic variable aggregation, coordinate aggregation, and classifier assignment. Coordinate aggregation and classifier assignment were essential steps toward multivariate morphometric analysis, or CASM. Kinematic aggregation provided one text file of five distance measurements and three timing values from each video for subsequent statistical analysis. For this study, the compiler function reported 1280 total kinematic values from 160 videos representing two swallows each from the 80 subjects. Coordinates were compiled from every frame representing pharyngeal swallowing (n = 2718 frames). A data balance function that randomly selects the maximum equal numbers of observations per pharyngeal swallow was performed resulting in 7 frames per swallow (n = 1120). Each set of 10 coordinates was assigned a unique identifier. The classifier assignment function was used to designate categorical variables to each of these unique identifiers. Categorical variables for the present study include: cohort group (e.g. stroke versus healthy control), binary penetration-aspiration score (PAS) status (PAS 1–2 versus PAS 3–8), and bolus type (5 mL thin versus 5 mL thick). The coordinates and classifier variable files were imported into MorphoJ integrated software for multivariate morphometric analysis [29].
MorphoJ was used to generate.svg files of eigenvector results indicating the magnitude and direction of the variance at each coordinate associated with a classifier variable of interest. These vectors represented the inferred muscle action underlying hyoid movement, laryngeal elevation, tongue base retraction, pharyngeal shortening, and head and neck posture. However, the vectors were in reference to a mathematically derived centroid, which assumes unlimited degrees of freedom, whereas the movement of these coordinates was constrained by anatomy (i.e. the mandible and cranial base moved in reference to the vertebrae, not a mathematical centroid). To represent this anatomical constraint, a matrix transformation was performed applying a MATLAB Procrustes superimposition to these vector data. First, the shape defined by the tip of eigenvectors 3, 4, & 5 (representing the C1, C2, and C4 vertebrae) was translated, scaled, and rotated such that the average length of eigenvectors 3, 4, and 5 were minimized. Then, the remaining eigenvectors were translated, scaled, and rotated to align within the proper anatomical context.
PAS
A speech-language pathologist (SLP) and author (SR) with six years of experience interpreting MBS studies reviewed each video and assigned a score using the Penetration–Aspiration Scale (PAS) [30]. The PAS is an 8-point scale used to describe the presence or absence of laryngotracheal invasion of ingested bolus material. Ten percent of the videos were randomly selected for intra-rater testing and for inter-rater testing by a second SLP with fourteen years of experience. Intraclass correlation coefficients confirmed acceptable intra- and inter-rater reliability levels of ICC = 0.98 and ICC = 0.99, respectively. In subsequent analyses, these scores were reduced into a categorical variable as follows: scores of 1–2 were considered within functional limits, and scores of 3–8 were indicative of penetration-aspiration.
Statistical Analyses of Kinematic Data
Kinematic variables were analyzed using Excel Real Statistics Resource Pack. To evaluate H1, a MANOVA of distance measurements and timing measurements comparing each test group with the respective control was performed with post-hoc ANOVA to determine p-values and effect sizes using root mean square standardized effect (RMSSE). Significance for the MANOVA was set at p < 0.05 with the post hoc ANOVAs subjected to a Bonferroni correction. To evaluate H2, a MANOVA of kinematic distance measurements and timing measurements of all test groups was performed using Pillai’s trace (V), a positive-valued statistic ranging from 0 to 1, where an increase in value indicates a greater effect contributed to the model. To test H3, logistic regressions were performed using a binary penetration-aspiration status (PAS 1–2 = within normal limits versus PAS 3–8 = penetration-aspiration) as the outcome variable.
Statistical Analyses of CASM Data
Aggregate coordinate and classifier text files were imported in MorphoJ for multivariate morphometric analysis. A Procrustes fit was performed on all coordinates to control for size differences and correct for image rotation and camera movement. No outliers were identified using the MorphoJ outlier function. Discriminant function analysis (DFA) comparing test groups with age- and sex-matched controls was performed to evaluate H1. A canonical variate analysis (CVA) by test group was performed to evaluate H2. In morphometric analysis, a DFA is used to determine pairwise shape differences, whereas a CVA is used to determine the impact of multiple independent variables on shape differences. To test H3, regressions were performed of DFA scores of group vs. control as the independent variable and DFA scores of penetration-aspiration status as the dependent variable. Permutation tests were performed in all analyses.
Results
H1 Kinematics: Differences Between Dysphagia and Control Groups
MANOVA results of kinematic distance measurements between each test group (H1) with respective age- and sex-matched controls resulted in significant differences for HNC group (V = 0.40, F(5,34) = 4.54, p = 0.003, η2 = 0.40). MANOVA results of kinematic timing measurements testing H1 comparing each test group with respective age- and sex-matched controls resulted in significant differences for HNC (V = 0.29, F(3,36) = 4.83, p = 0.006, η2 = 0.29), and stroke (V = 0.27,F(3,36) = 4.51, p = 0.009, η2 = 0.27). All significant results showed a small effect size (η2). P-values and RMSSE effect sizes resulting from post hoc ANOVAs comparing distance and timing variables between each test and control group are reported in Table 2 a–b.
Table 2.
a-b p-values resulting from post-hoc ANOVAs comparing distance and timing variables for test vs. control group
| Hyoid Excursion | HyolaryngealApproximation | Laryngeal Elevation | Pharyngeal Shortening | Base of Tongue Retraction Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| vs. control | p-value | RMSSE | p-value | RMSSE | p-value | RMSSE | p-value | RMSSE | p-value | RMSSE |
| HNC | 0.02 | 0.57 | 0.001 | 0.84 | 0.0001 | 0.99 | 0.0002 | 0.93 | 0.12 | 0.36 |
| Stroke | 0.08 | 0.40 | 0.70 | 0.09 | 0.37 | 0.20 | 0.10 | 0.38 | 0.88 | 0.03 |
| COPD | 0.39 | 0.19 | 0.08 | 0.40 | 0.39 | 0.19 | 0.23 | 0.27 | 0.16 | 0.32 |
| MND | 0.17 | 0.31 | 0.57 | 0.13 | 0.71 | 0.08 | 0.25 | 0.26 | 0.36 | 0.21 |
| Oral Transit Time | Stage Transition Duration | Pharyngeal Transit Time | |||||
|---|---|---|---|---|---|---|---|
| vs. controls | p-value | RMSSE | p-value | RMSSE | p-value | RMSSE | |
| HNC | 0.04 | 0.48 | 0.002 | 0.74 | 0.019 | 0.55 | |
| Stroke | 0.91 | 0.02 | 0.019 | 0.55 | 0.007 | 0.64 | |
| COPD | 0.34 | 0.22 | 0.99 | 0.00 | 0.16 | 0.32 | |
| MND | 0.40 | 0.19 | 0.79 | 0.06 | 0.60 | 0.12 | |
With a Bonferroni correction, alpha = .01 and .0167 for distance and timing variables, respectively. Italic values indicate statistically significant p-values. Root mean square standardized effect (RMSSE) was used to report effect size differences with significant values (≥ .20) in bold
H2 Kinematics: Differences Between Dysphagia Etiology Groups
MANOVA results of kinematic distance measurements testing H2 showed statistically significant differences between test groups with V = 0.65, F(15,222) = 4.13, p < 0.0001, η2 = 0.22. MANOVA results of kinematic timing measurements testing H2 showed statistically significant differences between test groups with V = 0.26, F(9,228) = 2.43, p = 0.01, η2 = 0.09. While the model showed significance, the effect size (η2) indicated a small difference for distance measurements and no difference for timing variables.
H3 Kinematics: Swallowing Mechanics and Penetration‑Aspiration Status
Logistic regressions of a binary penetration-aspiration status on kinematic distance and timing variables by group resulted in significant findings showing an increase in pharyngeal transport time was predictive of penetration-aspiration status in the stroke cohort (X2 = 5.42, p = 0.02, U = 0.22), and reduced hyoid excursion was predictive of penetration-aspiration status in the MND cohort (X2 = 4.61, p = 0.03,U = 0.36). Here, U functions are analogous to an r2 statistic indicating how much of the variance is explained by the variable.
CASM H1: Differences Between Dysphagia and Control Groups
Pairwise DFA results revealed significant differences (p < 0.0001) between each test and control group with Mahalanobis distances ranging from 2.42–2.82. Eigenvectors characterizing the differences in swallow mechanics between test and control groups are visualized in Fig. 4a–d. Eigenvectors for the COPD cohort indicated increased kyphosis of the vertebrae, slight head flexion, increased pharyngeal shortening and anteriorly positioned larynx during pharyngeal swallowing compared with controls. Eigenvectors for the MND cohort showed an elevated hyolaryngeal complex and tongue base with some head and neck extension during pharyngeal swallowing compared with controls. Eigenvectors for the HNC cohort indicated reduced hyolaryngeal excursion and tongue base retraction with some flexion of the head and neck during pharyngeal swallowing compared with controls. Eigenvectors for the stroke cohort showed a reduction in all pharyngeal swallowing mechanics with moderate reduction of hyolaryngeal excursion, pharyngeal shortening, and tongue base retraction with notable extension of the head and neck when compared with controls.
Fig. 4. a.
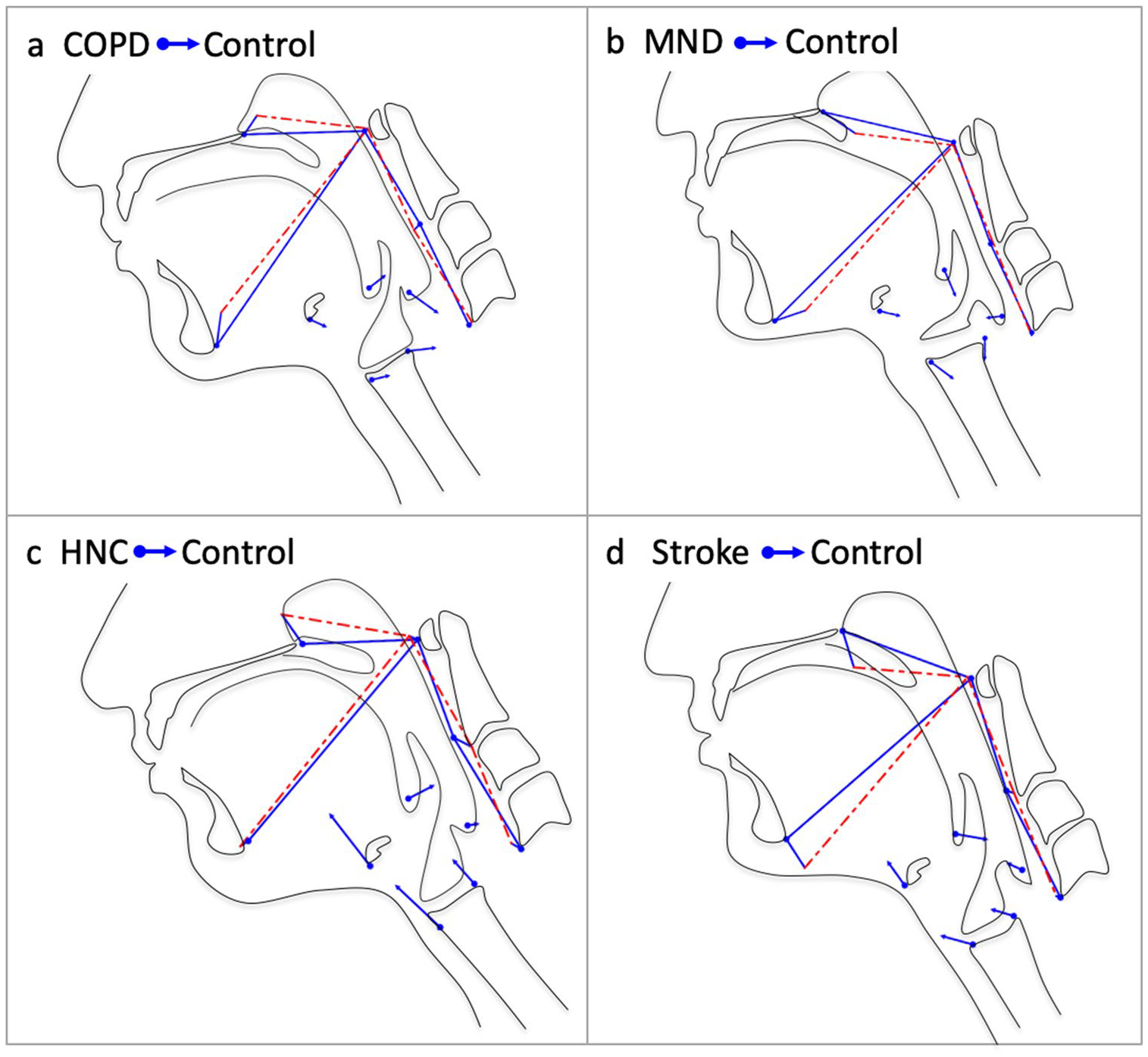
a–d DFA eigenvectors of indicate the impact of dysphagia etiologies on the functional anatomy during swallowing compared with controls (H1). The vectors represent relative changes in multiple elements of swallowing associated with each dysphagia etiology with the dots and blue lines showing the mean position of anatomical landmarks in the dysphagic group and the arrowhead and dashed red lines the control group (e.g. the stroke vectors indicate reduced hyolaryngeal elevation, pharyngeal shortening, and tongue base retraction with head and neck extension as compared to controls)
CASM H2: Differences Between Dysphagia Etiology Groups
CVA results of the test groups showed significant differences (p < 0.0001) between all test groups with Mahalanobis distances ranging from 2.62–3.66 (Fig. 5). The neurogenic etiology mechanics (MND and Stroke) and non-neurogenic etiology mechanics (COPD and HNC) stratified as the first canonical variate describing 55% of the variance. Eigenvectors of the second canonical variate, representing 30% of the variance, indicated stratification by dysphagic etiologies that reduce hyolaryngeal excursion (stroke and HNC), and those with exaggerated hyolaryngeal movements (COPD and MND).
Fig. 5.
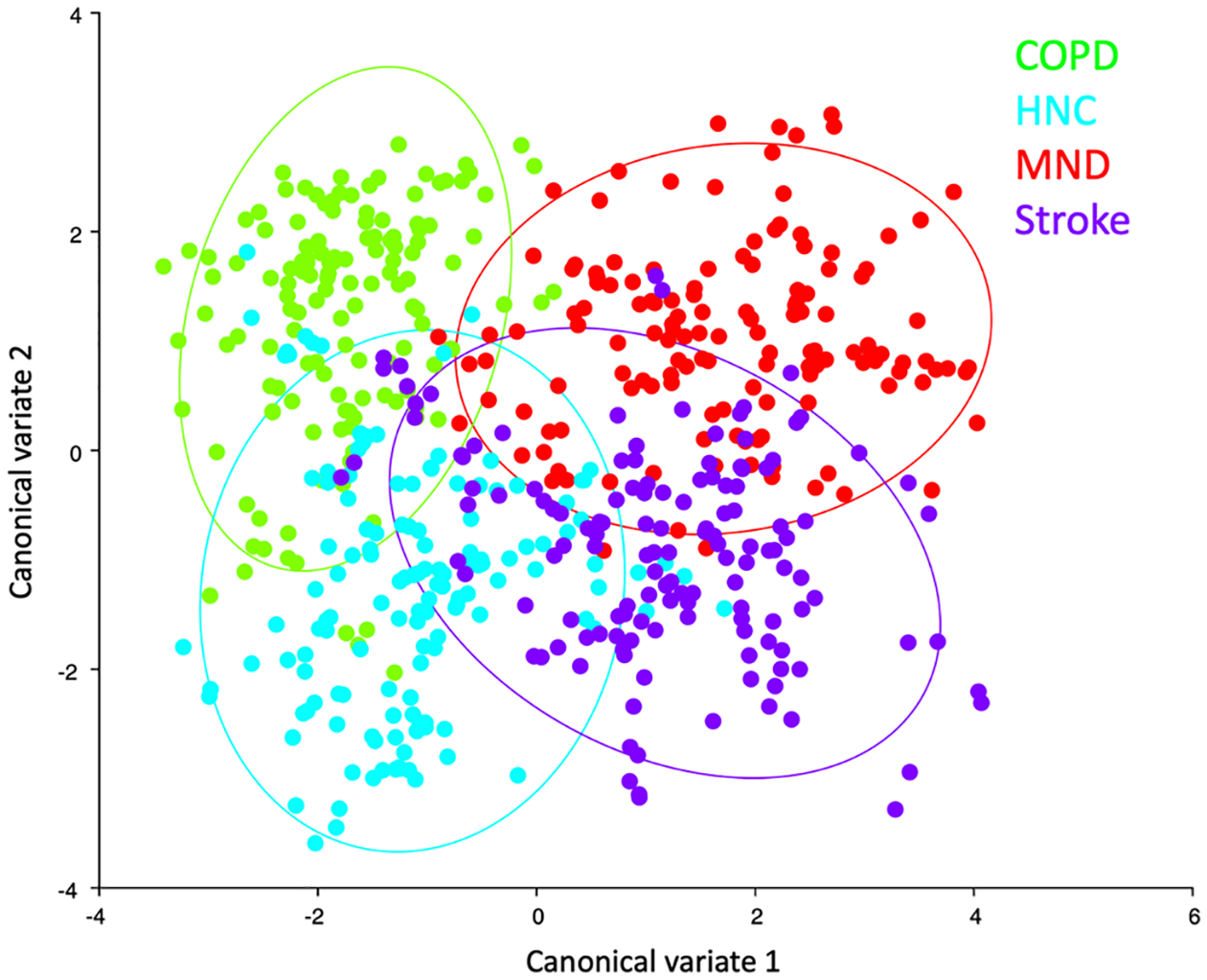
Morphometric CVA results (H2) indicate that pharyngeal swallowing mechanics cluster by dysphagia etiology (see color key) with neurogenic dysphagias (Stroke and MND) and structural dysphagias (HNC and COPD) aligning with canonical variate 1. Each dot represents a configuration of ten coordinates taken from a single video frame. Mathematical differences in geometric configuration, representing changes in swallowing mechanics, are calculated and plotted
CASM H3: Swallowing Mechanics and Penetration‑Aspiration Status
Multivariate regressions of DFA scores of penetration-aspiration status on DFA scores of group vs. controls are visualized in Fig. 6a–d. Results are as follows: stroke, multivariate regression coefficient = 0.70, predicting 11.9% of the variance; MND, multivariate regression coefficient = 0.63, predicting 11.16% of the variance; COPD, multivariate regression coefficient = −0.15, predicting 0.30% of the variance; HNC, multivariate regression coefficient = 0.09, predicting 0.10% of the variance.
Fig. 6. a.
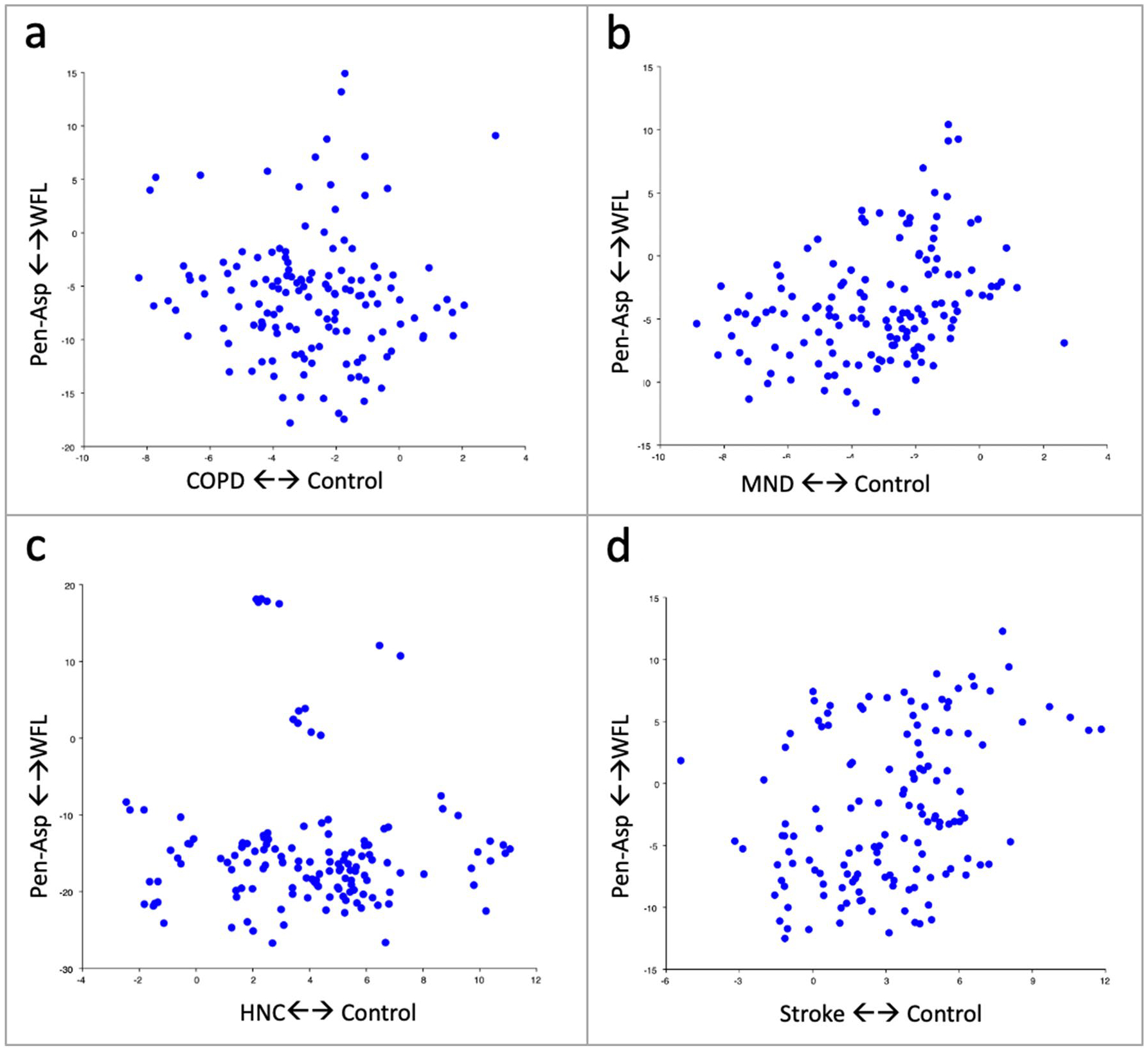
a-d Regressions of morphometric discriminant function scores of penetration-aspiration status (Pen-Asp) vs. within functional limits (WFL) on dysphagic etiologies indicates the variability and relationship of swallowing mechanics to penetration-aspiration status within each group
Discussion
This proof of concept study aimed to demonstrate differences in pharyngeal swallowing mechanics using two types of quantitative analyses: a conventional kinematic approach and a novel multivariate shape change analysis (CASM) to characterize swallowing mechanics. We used these two techniques to document differences across four patient cohorts with swallowing disorders (HNC, stroke, COPD, and MND) against age- and sex-matched healthy, non-dysphagic controls. This study revealed that both kinematic analysis and CASM are useful to differentiate swallowing mechanics by dysphagia etiology.
Using kinematic analyses, we demonstrated significant differences in distance measurements in the HNC cohort and timing variables in both stroke and HNC to address H1. However, significant effect sizes (RMSSE > 0.2) in Table 2 a–b indicate some comparisons were underpowered. Statistical significance was achieved in cohorts with larger effect size changes (RMSSE = 0.84–0.99). For the remaining variables with a significant effect size, a post hoc two-tailed pairwise power analysis on the basis of means was performed. To obtain 80% statistical power, a sample size of n = 60 would be needed for the stroke and HNC cohorts and a sample size of n = 175 for the MND and COPD cohorts. Interestingly, even with a small sample, we did find a difference in the distance measurements among dysphagic etiologies (H2). Finally, using logistic regression by group, we identified that increases in pharyngeal transport time in the stroke cohort and reductions in hyoid excursion in the MND cohort were predictive of PAS status supporting H3.
Using CASM, we demonstrated significant differences in swallowing mechanics for each test and control group comparison in support of H1. Eigenvectors allowed for visualization of differences and interactions of the multiple elements of swallowing mechanics for each group. CASM also showed that pharyngeal swallowing mechanics differ among dysphagic etiologies (H2). Regressions of morphometric discriminant function scores agree with the kinematic results showing that mechanics associated with stroke and MND were predictive of penetration-aspiration status than mechanics associated with other dysphagia etiologies in the present sample.
Kinematic measurements of distance and time are more easily understood as dependent variables than geometric morphologies in morphospace. It is easier to comprehend the mean difference in laryngeal elevation in centimeters versus results that are mathematically abstracted such as a Mahalanobis distance. Furthermore, while timing variables can be factored into CASM, it is not reported as a discrete variable. In studies where a measured time interval is important, such as assessing sensory deprivation (i.e. in post-treatment oropharyngeal cancer), kinematic temporal variables are preferred.
A challenge in kinematic analysis is whether to include all elements of swallowing mechanics since including more variables invites a statistical penalty. A further challenge is assessing how these multiple elements of swallowing mechanics function together under various conditions (i.e., risk factors, patient characteristics, and bolus types) to achieve swallowing performance goals. Thus, while a result of a dependent variable in kinematics is more easily understood, the results of how multiple kinematic variables interact within the context of patient anatomy under various conditions is less evident.
On the other hand, CASM utilizes a geometric representation of the swallowing mechanism to compute how multiple variables of interest impact multiple elements of swallowing mechanics. Geometric analysis compares differences in shapes, but not sizes. Consider, for example, the shape difference between a 3 mm equilateral triangle and a 3 km equilateral triangle. While this approach controls for size differences between subjects, results cannot be translated into actual distance measurements. Eigenvectors indicate the relative magnitude and direction of variance in gestalt mechanics, in other words the shape representing the interaction of multiple elements of swallowing mechanics. Further, multivariate morphometric analysis can show how these elements of swallowing mechanics covary with independent variables such as risk factors, bolus condition, or swallowing performance outcomes. For example, CASM can depict how hyolaryngeal movement, tongue base retraction, pharyngeal shortening, and head and neck posture covary together in relation to penetration-aspiration status while controlling for other known variables [13]. So, while the dependent variable in CASM may be more difficult to comprehend, the results of CASM provide visualization of how multiple elements of swallowing mechanics interact associated with a variable of interest, such as dysphagia etiologies.
A potential limitation of CASM is that statistically significant p-values are readily found requiring the investigator to determine whether these differences are functionally significant or not. It is important to note the Mahalanobis distance (or D-statistic), which in morphometrics, functions like a z-score. In the present study the Mahalanobis distances, all above 2.0, were significant. In addition, the eigenvector results revealed functional differences in swallowing mechanics that were obscured using univariate kinematic methods. Visualization of how disease etiology impacts the interaction of multiple elements of swallowing mechanics provides substantial opportunity for hypothesizing structure to function relationships. For example, in a previous CASM study of COPD, vectors led to a new hypothesis about the impact of uncoupled swallowing and respiratory function [31]. CASM has also been used in a stroke population to document the impact of focal stroke lesions on the swallowing mechanism [13]. In the current study, the vectors characterizing swallowing in the MND sample may indicate a hyperreflexive response to the bolus stimulus. A future investigation using CASM to investigate HNC could determine the impact of different tumor sites or treatment options on the swallowing mechanism. Additionally, CASM has been used to quantitatively test patient-specific interventions [31].
It should also be noted that DFA assumes independent observations, whereas CASM requires multiple observations per subject. It has been shown in other studies that this can lead to false positives [32]. Methods in this study were patterned after a proposed solution involving permuting a balanced data set to mitigate the problem [32]. Positive findings in a cohort CASM study should be approached cautiously.
Another limitation to address in a future study is to increase the number and diversity of swallows per subject to reduce within-subject variability. Combining honey and pudding into a “thick” bolus classification also needs to be justified before future use. Further, some cohorts had far fewer instances of penetration-aspiration to provide meaningful results for H3. Specifically, the HNC cohort had only one subject with a penetration-aspiration status, which explains the unusual scatter plot in Fig. 4c. Other timing measurements, such as laryngeal vestibular closure time, or additional CASM coordinates that represent the function of the pharyngeal constrictors in the pharyngeal stripping wave, could be included [33]. Additionally, penetration-aspirations status could be stratified by timing of aspiration events relative to the swallow, or by using other swallowing performance variables such as the Normalized Residue Ratio Scale [27]. These data capture capabilities are programed in the MATLAB tracker tool. In spite of these limitations, both approaches were useful to differentiate mechanics by dysphagia etiology on a small sample of patients per cohort.
Built into the MATLAB tracker tool is a strategy to organize and manage large data sets. Future directions include improving semi-automated landmark tracking through machine learning. This coupled with coordination of multisite usage sets the stage for big data analysis using both kinematics and CASM that could effectively characterize swallowing mechanics associated with multiple dysphagia etiologies. Automating CASM to allow for faster, and thus, more feasible analysis in a clinical setting, may even aid the healthcare provider in clinical decision-making and rehabilitation of swallowing impairment.
The number of subjects per group and incidence of penetration-aspiration swallows in the present study is far too small to draw generalizable conclusions. The present pilot study was not intended to definitively phenotype swallowing mechanics associated with dysphagia etiologies but to demonstrate the capacity of kinematics and CASM to address such research questions. This study shows that both approaches are useful for comparing swallowing mechanics by group or associated swallowing performance variables, with each method having advantages and limitations (Table 3).
Table 3.
Overview comparison of kinematic analysis and computational analysis of swallowing mechanics (CASM) research methods
| Kinematic analysis | CASM | |
|---|---|---|
| Dependent variables | Univariate physical measurements of distance and time | Eigenvector-based mathematical representation of geometric morphology. Time can be factored in as a covariate |
| Multivariate analysis and results | Indicates statistical associations of multiple variables | Indicates structural changes in swallowing mechanics associated with multiple independent variables of interest |
| Applications | Can be used to show statistical differences in cohort or population studies | Can be used to visualize swallowing mechanics in patient-specific, cohort, or population studies |
| Limitations | Does not visually assess covariance of the multiple elements of swallowing mechanism | Temporal variables cannot be extracted to calculate time differences in swallowing events |
| Key advantage(s) | Results reported in actual time and distances are easy to comprehend. (Table 2a–b) | Vectors representing differences in multiple swallowing mechanics are visualized within anatomical context (Fig. 4a–d) |
In summary, kinematic analysis and CASM are useful quantitative methods to determine differences in swallowing mechanics among various dysphagic populations. Kinematic analysis has the advantage of measuring time as a continuous variable and should be used where this is important for research. The advantage of CASM lies in visualizing the association of various risk factors, treatment plans or swallowing performance with multiple elements of swallow mechanics with a cohort or an individual patient. Use of this MATLAB tracker tool provides a means to collect data for kinematic and CASM big data analyses.
Acknowledgements
Medical Scholars Program at the Medical College of Georgia provided funding to medical student authors. The following data sets were funded in part by various grants as indicated: Stroke, National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number R01DC012584 (Kumar); COPD, Entera Health, Inc. (EH5220) (PI: Paoletti); HNC, National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number R21DC010480 (PI: Martin-Harris); Normals, Veterans Affairs RR&D under award number CDA-1 1IK1RX001628-01A1 (PI: Garand), the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number K24DC12801 (PI: Martin-Harris), and the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant number TL1 TR000061 (PI: Brady; Project PI: Garand), and the American Speech-Language-Hearing Foundation (PI: Garand). The content is solely the responsibility of the authors and does not necessarily represent the official views the funding agencies listed above.
References
- 1.Seo HG, Oh B-M, Han TR. Swallowing kinematics and factors associated with laryngeal penetration and aspiration in stroke survivors with dysphagia. Dysphagia. 2016;31:160–8. [DOI] [PubMed] [Google Scholar]
- 2.Starmer HM, Tippett D, Webster K, Quon H, Jones B, Hardy S, Gourin CG. Swallowing outcomes in patients with oropharyngeal cancer undergoing organ-preservation treatment. Head Neck. 2014;36:1392–7. [DOI] [PubMed] [Google Scholar]
- 3.German RZ, Crompton A, Gould FD, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia. 2017;32:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molfenter SM, Steele CM. Kinematic and temporal factors associated with penetration–aspiration in swallowing liquids. Dysphagia. 2014;29:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendall KA, McKenzie S, Leonard RJ, Gonçalves MI, Walker A. Timing of Events in Normal Swallowing: A Videofluoroscopic Study. Dysphagia. 2000;15:74–83. [DOI] [PubMed] [Google Scholar]
- 6.Leonard RJ, Kendall KA, McKenzie S, Goncalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146–52. [DOI] [PubMed] [Google Scholar]
- 7.Thompson TZ, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, Sokolove RL, Taylor BK, Tuck JM, Pearson WG Jr. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014;87:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WH, Chun C, Seo HG, Lee SH, Oh B-M: STAMPS: development and verification of swallowing kinematic analysis software. Biomed Eng Online 16: no.120, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, McCullough GH. Maximal hyoid excursion in poststroke patients. Dysphagia. 2010;25:20–5. [DOI] [PubMed] [Google Scholar]
- 10.Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck. 2000;22:120–31. [DOI] [PubMed] [Google Scholar]
- 11.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and Biomechanical Characteristics of Oropharyngeal Swallow in Younger and Older Men. J Speech Lang Hear Res. 2000;43:1264–74. [DOI] [PubMed] [Google Scholar]
- 12.Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DCBH. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May NH, Pisegna JM, Marchina S, Langmore SE, Kumar S, Pearson WG Jr. Pharyngeal Swallowing Mechanics Secondary to Hemispheric Stroke. J Stroke Cerebrovasc Dis. 2017;26:952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garand KL, Schwertner R, Chen A, Pearson WG Jr. Computational Analysis of Pharyngeal Swallowing Mechanics in Patients with Motor Neuron Disease: A Pilot Investigation. Dysphagia. 2018;33:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietsch AM, Rowley CB, Solomon NP, Pearson WG Jr. Swallowing Mechanics Associated With Artificial Airways, Bolus Properties, and Penetration-Aspiration Status in Trauma Patients. J Speech Lang Hear Res. 2017;60:2442–511. [DOI] [PubMed] [Google Scholar]
- 16.Pearson WG Jr, Davidoff AA, Smith ZM, Adams DE, Langmore SE. Impaired swallowing mechanics of post radiation therapy head and neck cancer patients: A retrospective videofluoroscopic study. World J Radiol. 2016;8:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson WG, Zumwalt AC. Visualising Hyolaryngeal Mechanics in Swallowing Using Dynamic MRI. Comput Methods Biomech Biomed Eng Imaging Vis. 2014;2:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–43. [DOI] [PubMed] [Google Scholar]
- 20.Heffernan C, Jenkinson C, Holmes T, Feder G, Kupfer R, Leigh PN, McGowan S, Rio A, Sidhu P. Nutritional management in MND/ALS patients: an evidence based review. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:72–83. [DOI] [PubMed] [Google Scholar]
- 21.Steidl E, Ribeiro CS, Gonçalves BF, Fernandes N, Antunes V, Mancopes R. Relationship between dysphagia and exacerbations in chronic obstructive pulmonary disease: a literature review. Int Arch Otorhinolaryngol. 2015;19:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment–MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cichero JA, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, Duivestein J, Kayashita J, Lecko C, Murray J, Pillay M. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia. 2017;32:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa Nita S, Murith M, Chisholm H, Engmann J. Matching the rheological properties of videofluoroscopic contrast agents and thickened liquid prescriptions. Dysphagia. 2013;28:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natarajan R, Stavness I, Pearson W. Semi-automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Comput Methods Biomech Biomed Eng Imaging Vis. 2015;5:379–89. [Google Scholar]
- 26.Pearson WG Jr, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the normalized residue ratio scale. Dysphagia. 2013;28:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard R, Rees CJ, Belafsky P, Allen J. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR). Dysphagia. 2011;26:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. Journal of speech, language, and hearing research : J Speech Lang Hear Res. 2014;57:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–7. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- 31.Tran TTA, Martin Harris B, Pearson WG. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Comput Methods Biomech Biomed Eng Imaging Vis. 2016;6:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundry R, Sommer C. Discriminant function analysis with non-independent data: consequences and an alternative. Anim Behav. 2007;74:965–76. [Google Scholar]
- 33.Schwertner RW, Garand KL, Pearson WG Jr. A Novel Imaging Analysis Method for Capturing Pharyngeal Constriction During Swallowing. J Imaging Sci. 2016;1:1–6. [PMC free article] [PubMed] [Google Scholar]


