Abstract
Naturally acquired immunity against clinical malaria is slow to develop, taking years of repeated exposure to parasites to acquire sufficiently broad and potent antibody responses. Increasing evidence suggests that Plasmodium infection and the resulting immune stimulation contribute to changes in the B cell compartment. In particular, accumulation of atypical memory B cells (atMBCs) is common in Plasmodium-exposed individuals. Similarities to B cell subsets present in other acute and chronic disease settings have provided insight into the development and potential function of these cells; however, their contribution to protection against malaria is still poorly understood. Here, we discuss recent findings that have increased our understanding of atMBCs and outline outstanding questions related to their function and development in the protective immune response to malaria.
Keywords: Atypical memory B cell, Plasmodium, Humoral immunity, IFNγ, FcRL5, T-bet
1. Introduction
Malaria is a deadly disease mainly affecting children in southern and southeastern Asia and sub-Saharan Africa. Despite major progress in reducing morbidity and mortality globally, an estimated 228 million cases occurred in 2018, resulting in 405,000 deaths (WHO, 2019). Malaria, caused mainly by the parasites Plasmodium falciparum and Plasmodium vivax, remains a major public health challenge and global financial burden (Shretta et al., 2016; Gunda et al., 2017; Hailu et al., 2017; Tang et al., 2017; WHO, 2019). The development of an effective vaccine therefore has high priority in the fight to eradicate this disease.
Naturally acquired immunity to malaria relies on the development of memory B cells (MBCs) and long-lived plasma cells that serve as the source of antibody responses that inhibit parasite replication and survival (Fig. 1) (Cohen et al., 1961; Riley et al., 1992; Osier et al., 2008). However, these responses are initially short-lived and require repeated parasite exposure over an extended period of time to broaden and develop into a protective response (Baird, 1998; Griffin et al., 2015; Rodriguez-Barraquer et al., 2016, 2018). Over the course of several years, protection against disease is acquired, but sterilising immunity is never reached (Reyburn et al., 2005; Doolan et al., 2009; Roca-Feltrer et al., 2010; Rodriguez-Barraquer et al., 2016). One of the reasons for the slow development of protective immune responses may be the effects of the malaria parasite on the host’s immune system. Increasing evidence suggests P. falciparum infection alters the composition of the B cell compartment in ways that may impair the immune response. A common feature of circulating B cells in Plasmodium-exposed individuals is the accumulation of atypical MBCs (atMBCs). However, it remains unclear whether this expansion is indicative of a productive role in the immune response or a malfunction of the immune system during chronic antigen exposure. Recent efforts have uncovered several potential drivers of atMBC development and provided new insight into their potential role or lack thereof in the immune response to Plasmodium. However, the heterogeneity of this B cell population, coupled with the use of different markers, models, and experimental conditions, has hampered interpretation of these sometimes conflicting studies. Here, we will discuss recent findings and present outstanding questions with respect to the origin and function of atMBCs, their similarities and differences to atypical B cell subsets in other diseases, and their potential role in immunity against malaria.
Fig. 1.
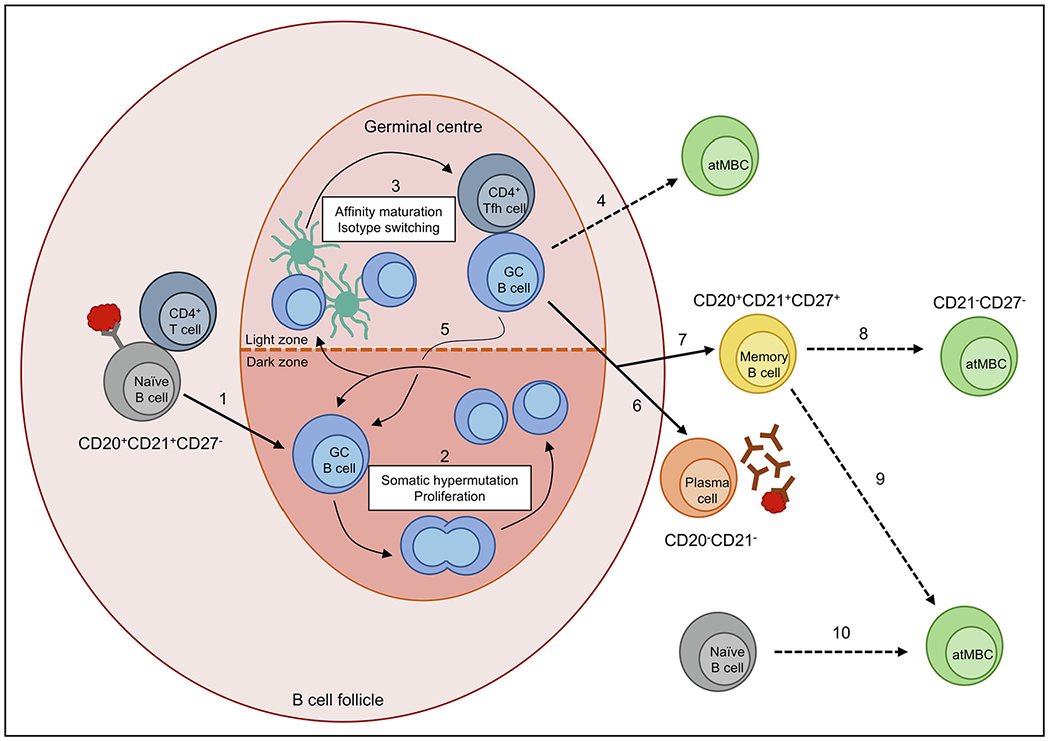
Potential pathways of atypical memory B cell (atMBC) development. In response to Plasmodium infection, antigen-recognising naïve B cells interact with antigen through B cell receptors (BCRs) and receive survival signals from CD4+ T cells, which drive naïve B cells to enter germinal centres (GCs) (1). In the GC, these B cells undergo proliferation and somatically hypermutate the BCR to increase antigen affinity (2). In the light zone of the GC, B cells with high affinity are selected through interactions with antigen-presenting follicular dendritic cells and CD4+ T follicular helper (Tfh) cells before undergoing class switch recombination (3). Poor Tfh cell help during this step may drive B cell differentiation into atMBCs (4). B cells can undergo multiple rounds of affinity maturation (5) before exiting the GC. Outside of the GC, B cells can differentiate into plasma cells (6) which produce high affinity antibodies to control the infection, or differentiate into long-lived memory B cells (7) which remain in circulation to respond to subsequent infections. Upregulation of inhibitory receptors and reduced BCR signalling driven by poorly understood mechanisms may promote the development of atMBCs from classical MBCs (8), representing an abnormal immune response. Alternatively, atMBCs could arise during activation of MBCs in a normal response to infection via an unknown mechanism (9). Finally, similar to DN2 cells in lupus, atMBCs may represent a population of pre-antibody secreting cells originating from the extra-follicular activation of naïve B cells (10).
2. Definition of atMBCs
2.1. AtMBCs in malaria
In the broadest terms, human atMBCs are defined as B cells lacking CD21 and CD27 surface expression (CD19+CD21−CD27−). High expression of the pan-B cell marker CD19, as well as CD20, is also seen in the atMBC population (Sundling et al., 2019). Cells falling into this category frequently have altered expression (compared with other B cell populations) of other surface proteins including CD11c, CXCR3, and CXCR5, as well as transcription factors such as T-bet (Weiss et al., 2010; Muellenbeck et al., 2013; Portugal et al., 2015; Sullivan et al., 2015). Generally, these cells also demonstrate increased expression of inhibitory receptors such as CD72, CD85j, FcRL3, and FcRL5 (Portugal et al., 2015; Sullivan et al., 2015). However, expression of these markers on atMBCs is heterogeneous, and division based on marker status often reveals subpopulations of atMBCs with potentially distinct functions. For example, division based on FcRL5 status reveals two populations with distinct B cell receptor (BCR) signalling and antibody secretion phenotypes (Sullivan et al., 2015). Heterogeneity in surface marker expression among CD21−CD27− B cells may indicate differences in time from antigen exposure, developmental path, or functional status. The general definition of atMBCs as CD21−-CD27− B cells also fails to exclude smaller subsets such as CD21−CD27−CD20−CD38hi plasmablasts that spontaneously secrete antibodies ex vivo (Sullivan et al., 2015) and are likely distinct from true atMBCs. However, it is starting to become clear that atypical B cell markers are not confined to the CD21−CD27− B cell population. While CD11c can be regarded as a marker of an atypical phenotype, Sundling et al. (2019) showed that, following malaria, CD11c expression is not strictly limited to atMBCs, and the expression of surface markers associated with atMBCs is dynamic (Sundling et al., 2019). In addition, FcRL5 was also found to be expressed on a subset of CD21+CD27+ memory B cells in individuals exposed to Plasmodium (Sullivan et al., 2015). In more recent studies, atMBCs are therefore often defined according to different definitions such as CD19hi, CD20hi, and/or expression of CD11c, T-bet, CXCR3, or inhibitory receptors.
Differences between markers used to identify atMBCs may contribute to the varied functional phenotypes observed for these cells, and as more becomes known about the phenotype and characteristics of CD21−CD27− B cells, the definition of atMBCs will require refinement to ensure selective analysis of these cells. Here, unless otherwise specified, the term atMBC refers to the CD21−-CD27− B cell population. We would like to note that it has recently been proposed that the term atMBC may be misleading, since these cells may be part of a normal immune response and might not functionally serve as memory cells (Sanz et al., 2019). In addition, the distinction between unswitched (IgM+/IgD+) and switched atMBCs is important as these cells might have different origins and functions.
2.2. Atypical B cells in other contexts
CD21−CD27− B cells are not unique to Plasmodium-exposed individuals. Similarities to atMBCs associated with other chronic infectious and autoimmune diseases may offer clues to the function of atMBCs in the immune response to Plasmodium. CD21lo/− B cells are enriched in autoimmune diseases (Warnatz et al., 2002; Wehr et al., 2004; Isnardi et al., 2010) and share similarities with a population of FcRL4+ B cells identified by Ehrhardt et al. (2005) as tissue-based MBCs that express high levels of CD20, low levels of CD21, and mostly lacked CD27 (Ehrhardt et al., 2005). In HIV-infected individuals, a similar population of CD27−CD21lo B cells expressing FcRL4 was identified (Moir et al., 2008) and referred to as tissue-like MBCs due to their similarity to the tissue-based MBCs discovered by Ehrhardt et al. (2005). Some groups have classified these cells as ‘exhausted’ or ‘anergic’ based on their hypo-responsiveness to BCR stimulation and upregulation of inhibitory receptors (Moir et al., 2008; Isnardi et al., 2010). CD21−CD27− B cells have since been identified in individuals with hepatitis C (HCV), tuberculosis (TB), and autoimmune disorders (Wei et al., 2007; Oliviero et al., 2015; Joosten et al., 2016). CD21−CD27− atMBCs also share features with an atypical B cell population expanded in aged mice (Hao et al., 2011; Rubtsov et al., 2011; Wang et al., 2018).
In the context of HIV infection, CD21−CD27− B cells frequently upregulate the transcription factor T-bet and exhibit reduced antibody production (Moir et al., 2008; Knox et al., 2017). In contrast, T-bet+ atMBCs described in autoimmune diseases, including common variable immunodeficiency (CVID) and systemic lupus erythematosus (SLE), seem to produce inflammatory cytokines and autoantibodies. In SLE, a population of IgD−CD21−CD27−T-bet+CD11c+CXCR5− cells, termed DN2 cells, expands and their abundance is associated with disease severity (Jenks et al., 2018; Wang et al., 2018). These SLE-associated DN2 B cells can localise to sites of inflammation and contribute to autoreactive antibody production (Wang et al., 2018). In these cells, T-bet expression is associated with autoreactivity (Liu et al., 2017; Wang et al., 2018). Additionally, SLE-associated DN2 B cells upregulate FcRL5, although it is unclear if this expression is associated with activating or inhibitory effects (Jenks et al., 2018; Wang et al., 2018). Expression of CD11c, FcRL5, and T-bet is a shared feature between autoimmune-associated atMBCs and malaria-associated atMBCs, but while autoimmune-associated atMBCs appear to play a primary role in the generation of autoantibodies, the role of malaria-associated atMBCs remains elusive.
While malaria-associated atMBCs share characteristics with HIV, HCV, TB, and autoimmune-associated atMBCs, phenotypic distinctions between these populations, such as differences in the expression of specific inhibitory receptors (e.g., FcRL4 versus FcRL5) and responsiveness to different Toll-like receptor (TLR) ligands (e.g., TLR7/8 versus TLR9), suggest their development and function may be impacted by the infectious agent and the resulting immune environment. Nevertheless, insights from studies on atMBCs found in other settings may provide direction for further study of malaria-associated atMBCs.
Finally, while healthy human adults harbour a small population of CD21−CD27− B cells, it is unknown whether atMBCs observed in disease are derived from this population. The function of these cells in healthy individuals remains unknown, although it has been suggested they may act as early responders to viral infections (Knox et al., 2017).
2.3. Atypical B cells in mice
In mice, B cells with characteristics of human atMBCs have been identified in the contexts of autoimmunity, ageing, and disease. Aged female mice have a population of CD21−CD11b+CD11c+ B cells, termed age-associated B cells (ABCs), that are associated with the response to viral infections and the development of autoimmunity (Rubtsov et al., 2011; Rubtsova et al.,2013). This population of B cells arises during the peak of viral infection and produces high titers of IgG2a antibodies (Rubtsova et al., 2013). In a model of SLE, these CD11b+CD11c+ B cells were the major contributors to autoantibody production (Rubtsov et al., 2011). These cells express genes involved in plasma cell differentiation, including Blimp1 and Xbp1, as well as transcription factors used to define human atMBCs, such as T-bet (Rubtsov et al., 2011). In the context of malaria, Kim et al. (2019) identified a population of atypical mouse B cells bearing similarity to human atMBCs (Kim et al., 2019). Defined by expression of FcRL5, this B cell subset transiently expanded following Plasmodium chabaudi infection in mice (Kim et al., 2019). Transcriptional profiling of these cells revealed similarities to human atMBCs, including increased expression of receptors and adaptors with inhibitory functions (Kim et al., 2019). FcRL5+ B cells also increased expression of T-bet, markers defining ABCs (CD11b, CD11c), and markers upregulated on human atMBCs (CD86, CD40) (Kim et al., 2019). Perez-Mazliah et al. (2018) also characterised a population of CD21loCD11b+CD11c+FcRL5hi B cells which increased following P. chabaudi infection in a mouse model of MSP121-specific B cells (Perez-Mazliah et al., 2018). Within this population, cells expressed inhibitory markers and markers of antigen exposure also seen in human atMBCs (Perez-Mazliah et al., 2018). Mouse atypical B cells expressed genes associated with human atMBCs including Cxcr3, Tbx2l, and Fcrl5 (Perez-Mazliah et al., 2018). Mouse atypical B cells also upregulated Blimp1 and had low expression of Cxcr5, suggesting these cells could represent a population of pre-plasmablasts (Perez-Mazliah et al., 2018). While human atMBCs in Plasmodium-exposed individuals showed a modest increase in BLIMP1 expression compared with classical MBCs, much larger upregulation was observed for the BLIMP-1 repressor BCL6 (Sullivan et al., 2015). Despite similarities in transcriptional profiles between mouse atypical B cells and human atMBCs, subtle differences in gene expression between these cell populations suggest that mouse atypical B cells might not be functionally identical to human atMBCs. Since mice do not harbour a B cell marker equivalent to human CD27, it is difficult to infer whether this population is truly representative of the CD21−CD27− atMBCs seen in humans. Moreover, it has been proposed that FcRL5 serves as a memory marker in mice (Perez-Mazliah et al., 2018) and is thus not functionally equivalent to FcRL5 in humans. Due to the difficulties in comparing results about atMBCs from mouse and human studies, we focus this review on atMBCs in humans, unless specifically mentioned otherwise.
2.4. T-bet as the defining transcription factor for atypical B cells
First identified as a regulator of CD4+ T cell differentiation, T-bet is a transcription factor expressed in certain populations of B cells where it promotes class switching to IgG3 in humans and IgG2a/IgG2c in mice (Zhang et al., 2012; Obeng-Adjei et al., 2017). Recently, Stone et al. (2019) uncovered a role for T-bet in IFNγ-stimulated B cells in mice (Stone et al., 2019). In these cells, T-bet functions to repress IFNγ-stimulated gene programmes that are incompatible with antibody-secreting cell (ASC) formation. In this way, T-bet promotes ASC development by preventing B cells from acquiring an inflammatory effector cell fate.
IFNγ signalling through the IFNγ receptor can also stimulate expression of T-bet in human B cells (Fig. 2) (Ambegaonkar et al., 2019; Zumaquero et al., 2019). In naïve B cells derived from human tonsil or peripheral blood, IFNγ signalling, BCR crosslinking, and TLR7 or TLR9 engagement all contributed to the induction of high levels of T-bet expression (Fig. 2) (Ambegaonkar et al., 2019; Zumaquero et al., 2019). Under these in vitro conditions, T-bet+ cells also upregulated additional surface markers associated with an atypical phenotype, but expression of these markers appears to be independent of T-bet expression and may be driven by other processes. For example, CD11c expression was shown to be dependent on IL-21 stimulation in both human and mouse T-bet+ B cells (Naradikian et al., 2016; Wang et al., 2018) and did not increase in T-bet+ B cells in the absence of IL-21 stimulation (Ambegaonkar et al., 2019), suggesting IL-21 is a driver of CD11c expression in atMBCs. Other atypical surface markers may also require specific stimulatory conditions to reach maximum expression.
Fig. 2.
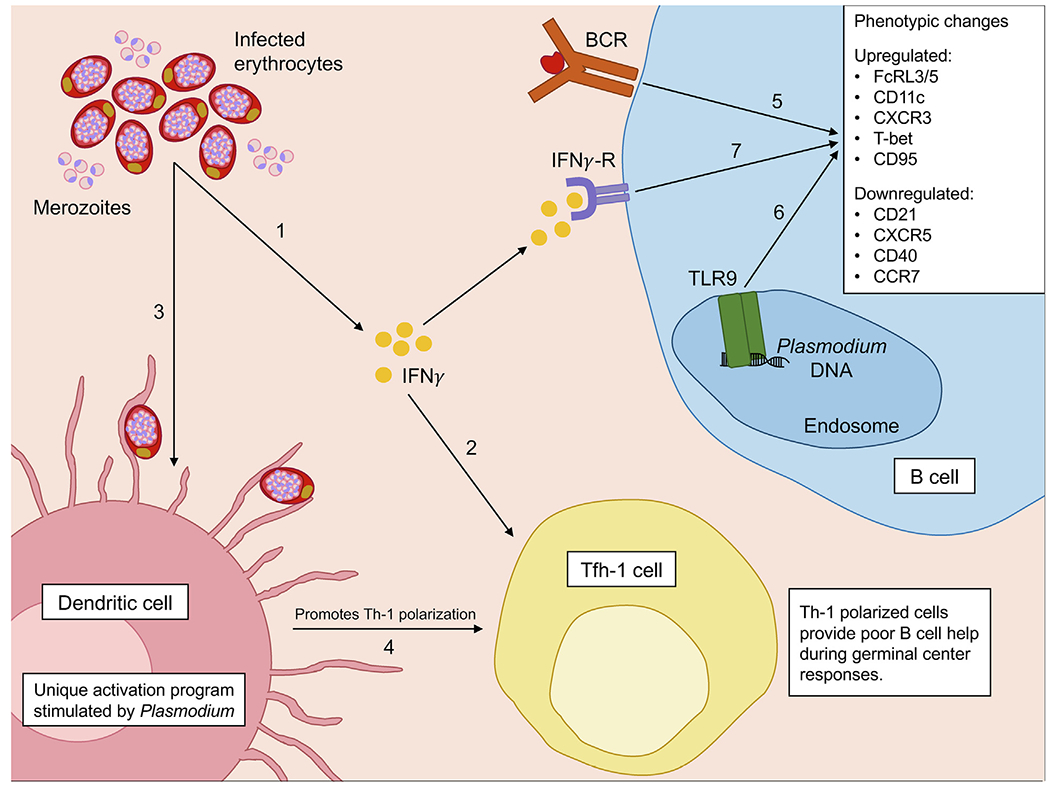
Potential drivers of the atypical memory B cell (atMBC) phenotype in response to Plasmodium infection. Plasmodium infection activates the immune system in several distinct, yet complimentary, ways which seem to promote the development of atMBCs. Infected erythrocytes stimulate increased IFNγ production by innate immune cells (1). High serum levels of IFNγ promote the development of Th-1 polarised T follicular helper (Tfh-1) cells which provide poor B cell help during germinal centre reactions (2). In response to infected erythrocytes, dendritic cells undergo a unique activation programme (3) also resulting in the promotion of Th-1 polarised Tfh-1 cells (4). Crosslinking of the B cell receptor (BCR) by antigen (5) and activation of Toll-like receptor-9 (TLR9) by Plasmodium DNA (6) both contribute to the upregulation of transcription factor T-bet, impaired BCR signalling, and development of an atypical phenotype. T-bet expression in B cells can also be induced by IFNγ signalling through the IFNγ receptor (IFNγ-R) (7).
While T-bet is generally seen as a hallmark of atMBCs in both humans and mice, the atypical phenotype is not dependent on the induction of T-bet. In a mouse model of SLE, the elimination of T-bet from B cells did not prevent disease, although the generation of auto-antibodies and resulting pathology was delayed (Rubtsova et al., 2017). In mice with T-bet-deficient B cells, Ehrlichia muris infection or Plasmodium infection gave rise to B cells with atypical characteristics, including FcRL5 or CD11c expression, at the same frequencies as seen in the presence of T-bet (Kim et al., 2019; Levack et al., 2020). These results indicate that T-bet is not the defining transcription factor of this lineage. Rather, the main function of T-bet seems to be the promotion of appropriate classswitching (Levack et al., 2020). In addition, with the discovery of other (non-atMBC) subsets of human B cells that express atypical markers, such as FcRL5 and CD11c, it has become clear that these markers are not confined to one subpopulation and that the function of subsets with atypical markers may be highly context-specific. T-bet should thus be used in combination with other markers to distinguish atMBCs.
3. Expansion and contraction of atMBC populations
On average, atMBCs account for up to 30% of the circulating mature B cell population of individuals living in malaria-endemic regions, while this is less than 10% in malaria-naïve adults (Weiss et al., 2009, 2011; Portugal et al., 2012, 2015). As detailed below, recent studies suggest Plasmodium parasites stimulate the immune system in ways that may promote the development of atMBCs. In particular, the increased levels of pro-inflammatory cytokines induced by Plasmodium infection may contribute to the observed atMBC expansion through several distinct but complementary mechanisms (Fig. 1). In subjects infected with either P. falciparum or P. vivax, atMBCs showed higher levels of proliferation than classical MBCs in vivo (Scholzen et al., 2014; Changrob et al., 2018). Furthermore, in people who experienced a single episode of P. falciparum malaria, the atMBC population expanded and peaked 10 days after malaria diagnosis, whereas the fraction of classical MBCs did not increase until 1 month after diagnosis (Sundling et al., 2019). The atMBC compartment showed greater expansion in individuals who had previously experienced Plasmodium infections than in subjects with primary infections (Sundling et al., 2019), suggesting that atMBCs may be derived from both activated naïve and memory B cells.
Additional observations in humans also suggest that the atMBC population contracts after clearance of infection. Following peak levels of atMBCs during natural P. falciparum infection, the compartment gradually contracted to background levels with an estimated half-life of 295 days (Sundling et al., 2019). Similarly, Ayieko et al. (2013) showed that in the absence of infection, the percentage of class-switched atMBCs in malaria-exposed adults in Kenya decreased from an average of 7.7% to 2.8% over the course of 1 year (Ayieko et al., 2013). However, despite the gradual decline of atMBC numbers over time, elevated fractions of atMBCs following natural P. vivax infection have been observed 3 years postinfection (Changrob et al., 2018). Taken together, these results suggest that the atMBC population expands during infection and that the maintenance of this population is dependent on continued exposure to antigen, but that a population of long-lived atMBCs can persist for years in the absence of antigen exposure.
3.1. Drivers of atMBC expansion
Plasmodium infection can selectively induce a pro-inflammatory, Th-1 polarised, immune response resulting in the activation and differentiation of specific cell types. When activated by Plasmodium-infected erythrocytes, dendritic cells can induce CD4+ T cells to differentiate into Th-1 cells (Fig. 2) (Gotz et al., 2017). The infection also selectively induces the differentiation of CD4+ T cells into Th-1 polarised T follicular helper cells (Tfh-1) (Figueiredo et al., 2017). In the germinal centre, B cells require help from Tfh cells during the process of affinity maturation (Fig. 1). Th-2 polarised Tfh cells provide superior B cell help compared with Tfh-1 cells in the form of survival signals (Morita et al., 2011; He et al., 2013; Yap et al., 2019). However, during Plasmodium infection, high levels of the pro-inflammatory cytokine IFNγ, together with higher levels of IL-10 and IL-6, promote the development of Tfh-1 cells, which are poor B cell helpers and contribute to impaired germinal centre responses (Figueiredo et al., 2017). This combination of abnormal dendritic cell activation, resulting in the stimulation of a Th-1 polarised response, and reduced B cell help from Tfh-1 cells, may promote the expansion of atMBCs (Fig. 2).
In Plasmodium-exposed individuals, B cells upregulate T-bet and display an atMBC-like phenotype including increased surface expression of FcRL5 and CD11c (Obeng-Adjei et al., 2017). These T-bet+ atMBCs demonstrate reduced BCR signalling compared with naïve and MBCs, which has been interpreted as an impaired capacity to produce antibodies (Obeng-Adjei et al., 2017). Repeated parasite exposure seems to drive the upregulation of T-bet, resulting in an increase in T-bethi atMBCs in children who have experienced five or more episodes of malaria (Obeng-Adjei et al., 2017). In vitro, this phenotype was recapitulated by stimulating tonsillar or peripheral naïve B cells with supernatants of peripheral blood mononuclear cells (PBMCs) co-cultured with infected erythrocytes, in combination with anti-IgM (Obeng-Adjei et al., 2017). This activation of naïve B cells could be inhibited by neutralising IFNγ in the supernatant or by blocking the IFNγ receptor, supporting the essential role of IFNγ in this process.
Parasite-specific B cells have been detected among atMBCs from Plasmodium-exposed individuals (Muellenbeck et al., 2013; Krishnamurty et al., 2016; Aye et al., 2020), indicating that antigen exposure is a factor in the expansion of these cells. However, Aye et al. (2020) recently reported increased levels of tetanus toxoid-specific atMBCs in individuals with continuous, high exposure to Plasmodium compared with individuals with previously high exposure but current low exposure, suggesting that the inflammatory environment of a Plasmodium infection can drive bystander B cell activation and the development of atMBCs (Aye et al., 2020). Collectively, these findings suggest that both antigen exposure and prolonged stimulation within the highly inflammatory environment of a Plasmodium infection contribute to the development of an atypical phenotype in B cells.
4. Potential functions of atMBCs
Studies of atMBC function in immunity to malaria are conflicting, and their exact role remains a point of debate. In general, there are two main theories regarding the role of atMBCs. First, expression of inhibitory receptors and reduced in vitro proliferation and antibody secretion suggest atMBCs may be a population of B cells expanded and hyper-activated as a result of chronic immune stimulation that have upregulated inhibitory receptors as a mechanism to downregulate this immune activation. Conversely, atMBCs may have a role as a class of functional B cells that actively contribute to the immune response during chronic infection, potentially as antigen-presenting cells or pre-antibody secreting cells. These two theories are not necessarily mutually exclusive, and we will explore recent evidence in support of both, as well as several other proposed functions of atMBCs, by comparing the phenotypes of atMBCs associated with malaria with those of related subsets found in other contexts (Table 1).
Table 1.
Potential roles of atypical memory B cells in the immune response to Plasmodium.
| Potential Role | Experimental Observations in Human Studies Supporting Potential Role | Antigen-specific | AtMBC a Definition | Reference |
|---|---|---|---|---|
| Immune Regulators | BCR crosslinking by FcRL5 and immune complexes may reduce BCR signalling | No | CD19+CD10−CD21−CD27−IgD−IgG+ | Sullivan et al., 2015 |
| T-bet+ atMBCs upregulated markers important for CD4+ T cell interactions | No | CD19+CD21−CD27−Tbethi | Obeng-Adjei et al., 2017 | |
| No | CD19+Tbet+ | Ambegaonkar et al., 2019 | ||
| Dysfunctional B Cells | Reduced antibody production in vitro | No | CD19+CD21−CD27− | Portugal et al., 2015 |
| No | CD19+CD10−CD21−CD27−IgD−IgG+ | Sullivan et al., 2015 | ||
| Downregulation of BCR signalling pathways compared with cMBCs | No | CD19+CD21−CD27−− | Portugal et al., 2015 | |
| No | CD19+CD21−CD27−Tbethi | Obeng-Adjei et al., 2017 | ||
| No | CD19+Tbet+ | Ambegaonkar et al., 2019 | ||
| Reduced proliferation | No | CD19+CD21−CD27− | Portugal et al., 2015 | |
| Reduced cytokine production | No | CD19+CD21−CD27− | Portugal et al., 2015 | |
| High frequency of extrafollicular cells in lymph node associated with low HIV-1 serum neutralisation | No | CD19hiT-bethi | Austin et al., 2019 | |
| Activated B Cells | CD11c+ B cell expansion and contraction observed in malaria patients | No | CD19+CD21−CD27− | Sundling et al., 2019 |
| Atypical markers induced in vitro by stimulation with IFNγ | No | CD19+Tbet+ | Ambegaonkar et al., 2019 | |
| No | IgD-CD27-CD11c+ CXCR5− | Zumaquero et al., 2019 | ||
| AtMBCs transiently arise in response to influenza vaccination | No | CD21loCD27−FcRL5+CD85jhiCD62Llo | Andrews et al., 2019 | |
| Pre-antibody Secreting Cells | pBLNK increased compared with unstimulated cells following BCR engagement | No | IgD−CD21−CD27−CXCR5−CD11c+IgA− | Jenks et al., 2018 |
| No | CD19+CD21−CD27−Tbethi | Obeng-Adjei et al., 2017 | ||
| No | CD19+Tbet+ | Ambegaonkar et al., 2019 | ||
| DN2 B cells differentiated into ASCs in the absence of BCR crosslinking | No | IgD−CD27−CD11c+CXCR5− | Zumaquero et al., 2019 | |
| Secretory Ig transcripts found in atMBCs but not in cMBCs | No | CD19+CD21−CD27−Ig+ | Muellenbeck et al., 2013 | |
| Autoreactive B Cells | Stimulated DN2 B cells differentiated into autoreactive plasma cells | No | IgD −CD21−CD27−CXCR5−CD11c+ | Jenks et al., 2018 |
| Antibodies from malaria-associated atMBCs have increased self- and polyreactivity | GMZ2 | CD19+CD21−CD27−Ig+ | Muellenbeck et al., 2013 | |
| atMBCs secrete autoantibodies upon in vitro stimulation | No | CD19+FcRL5+T-bet+ | Rivera-Correa et al., 2019 |
atMBCs, atypical memory B cells; BCR, B cell receptor; cMBCs, classical memory B cell; ASCs, antibody-secreting cells.
4.1. Regulators of immune responses
As discussed in section 3, atMBCs expand in response to Plasmodium infection, show signs of hyper-activation and have more recently undergone proliferation than classical MBCs in vivo. However, these cells are resistant to further stimulation in vitro. Downregulation of the PI3K, phospholipase C, and BCR signalling pathways, all necessary for orchestrating B cell responses (Kurosaki et al., 2010; Pieper et al., 2013), suggests these cells are less readily activated in response to BCR signalling (Portugal et al., 2015). Antigen-stimulated proliferation and cytokine production are also reduced in atMBCs compared with other B cell populations (Portugal et al., 2015).
The expression of inhibitory receptors such as FcRL3, FcRL5, and CD85j by atMBCs has been proposed to be a means to downregulate the hyper-activated state of these cells. In this scenario, FcRL5 may function as an IgG receptor, binding all subclasses of IgG with varying affinities (Wilson et al., 2012; Franco et al., 2013). During Plasmodium infection, polyclonal B cell activation in response to blood-stage parasites leads to hypergammaglobulinemia (Gilles and McGregor, 1959; McGregor and Gilles, 1960). In the presence of high quantities of IgG, crosslinking of BCR and FcRL5 by immune complexes may lead to reduced BCR signalling. FcRL5 expression by atMBCs may represent a regulatory mechanism aimed at reducing plasma antibody levels by inhibiting BCR signalling in the presence of high quantities of IgG (Sullivan et al., 2015). Given that atMBCs derived from individuals with different chronic infections express different surface receptors, the selective expression of specific FcRL proteins could be dependent on the antibody response stimulated by each pathogen and reflective of an attempt by the immune system to limit antibody responses during chronic immune activation.
Finally, atMBCs that express T-bet have been shown to upregulate markers – including HLA-DR, ICOS-L, and CD86 – that play important roles in antigen presentation to CD4+ T cells (Muellenbeck et al., 2013; Obeng-Adjei et al., 2017; Ambegaonkar et al., 2019). This indicates that interactions with T cells may be important for atMBC development or their function. Plasmodium infection drives a Th-1 polarised cytokine response which promotes the expansion of Tfh-1 cells with reduced helper activity compared with Tfh-2 cells (Obeng-Adjei et al., 2015). The development of an atypical phenotype could be partially driven by a lack of T cell help at crucial developmental stages. It has been proposed that atMBCs regulate these “impaired” Tfh cells to reduce their functionality over the course of infection (Ambegaonkar et al., 2019).
4.2. Dysfunctional B cells as a result of chronic immune activation
As discussed in Section 4.1, atMBCs show signs of hyper-activation in vivo and are resistant to further activation in vitro. An alternative explanation for the less responsive state of atMBCs in vitro is that these cells have become exhausted as a result of overstimulation by antigen exposure or the inflammatory environment during Plasmodium infections, as has also been suggested for phenotypically similar B cell subsets in the context of HIV infection and autoimmunity (Moir et al., 2008). A potential mechanism driving atMBCs into this less responsive state may be related to the function of T-bet in IFNγ-stimulated cells. It was shown in mice that T-bet plays an essential role in repressing the inflammatory gene programme that is induced by IFNγ signalling in B cells (Stone et al., 2019). Cells that were unable to downregulate this inflammatory gene programme, for example as a result of excessive TLR7 or TLR9 stimulation, remained effector B cells and did not differentiate into antibody-secreting cells (Stone et al., 2019). Given that Plasmodium DNA can serve as a TLR9 ligand (Rivera-Correa et al., 2017), it is conceivable that a subset of B cells can get stuck in the pre-antibody secreting stage during Plasmodium infection and become dysfunctional or poor contributors to humoral immunity.
Recently, Ly et al. (2019) demonstrated that T-bet expression in germinal centre B cells during Plasmodium berghei infection in mice drives localization to the dark zone where these cells undergo affinity maturation (Ly et al., 2019). T-bet expression appears to prevent premature exit of germinal centre B cells, helping to drive the development of high affinity antibodies (Ly et al., 2019). In the setting of primary Plasmodium infection, T-bet may thus be important for the development of a potent humoral immune response. In contrast, chronic immune activation and antigen exposure may interfere with the normal role of T-bet in germinal centre reactions. In HIV-infected individuals, CD19hiT-bethi B cells were mainly extrafollicular, but shared high clonal overlap with germinal centre B cells, which suggests these two groups may share a common precursor (Austin et al., 2019). The CD19hiT-bethi B cells in HIV-infected individuals may represent recent germinal centre emigrants or a population of cells that remain outside the germinal centre during the immune response, fitting with the low expression of the germinal centre homing receptor CXCR5 on these T-bet+ B cells. High frequencies of CD19hi B cells in lymph nodes were associated with low serum neutralising activity against HIV, suggesting that the abundant presence of these cells hampered the development of high-affinity B cells in the germinal centre. These contrasting findings may represent the difference between the important function of T-bet in B cell development during an optimal immune response and an impaired response of T-bet+ B cells in conditions of chronic immune activation.
4.3. Activated B cells as part of the normal immune response
The atMBC compartment expands during and shortly after infection, followed by a long contraction phase (Sundling et al., 2019). Andrews et al. (2019) identified three populations of activated memory B cells that transiently arise in response to influenza vaccination (Andrews et al., 2019). These populations were defined by low expression of CD21 and high expression of FcRL5, and could be distinguished from each other by the expression of CD85j, CD62L and CD27 (Andrews et al., 2019). The AM3 population (CD21loCD27−FcRL5+CD11c+CD85jhiCD62LloT-bet+) was the most phenotypically similar to atMBCs identified in Plasmodium-exposed individuals, with the notable exception that AM3 cells did not express CXCR3. Of the three activated memory B cell populations, AM3 cells were the last to peak in frequency, approximately 28 days after vaccination, followed by a return to baseline over the course of several months (Andrews et al., 2019). AM3 cells almost exclusively recognised epitopes present in the vaccine that individuals had not previously been exposed to. Based on these observations, the authors proposed these cells represent a population of new antigen-specific cells that were distinct from B cells activated as part of the recall response (Andrews et al., 2019). These observations suggest that atMBCs are part of the normal B cell response to vaccination, and possibly infection, and that these cells decline in numbers over the course of several months in the absence of antigen or other stimuli, similar to the kinetics observed for malaria-associated atMBCs.
The AM1 and AM2 cell populations described by Andrews et al. (2019) shared similarities with CD21lo B cell subsets described by others (Ellebedy et al., 2016; Lau et al., 2017; Kim et al., 2019). Lau et al. (2017) defined a population of CD21loCD27+ B cells with elevated T-bet, CD11c and FcRL5 expression. Based on the upregulation of key genes related to plasma cell differentiation (including BLIMP1), the authors proposed that these cells represent a transitional stage of recent germinal centre emigrants that are primed to become long-lived plasma cells (Lau et al., 2017). Kim et al. (2019) found that B cells expressing both FcRL5 and CD11c were enriched among B cells specific for tetanus toxoid C fragment (TTCF), suggesting these markers delineate long-lived, functional antigen-specific MBCs. However, despite FcRL5 and CD11c expression, TTCF-specific B cells were almost exclusively found in the classical (CD21+CD27+) and activated (CD21−CD27+) memory B cell compartments, not among atypical (CD21−CD27−) MBCs (Kim et al., 2019). This could be indicative of functional differences between antigen-specific MBCs and atMBCs, possibly as a consequence of different stimuli that mediate B cell activation upon vaccination versus during chronic infection. These studies point out that the ‘atypical’ B cell markers T-bet, CD11c, and FcRL5 are not sufficient to distinguish atMBCs and highlight the need for careful phenotyping of B cell subsets. Future studies will have to determine whether different T-bet+FcRL5+CD11C+ B cell subsets have different functions and origins, and to what extent these subsets are clonally related.
4.4. Pre-antibody secreting cells
AtMBCs identified in Plasmodium-exposed individuals share phenotypic and functional similarities with a population of double negative (IgD−CD27−) B cells that lack CXCR5 expression but do express CD11c and T-bet, termed DN2 cells (Jenks et al., 2018; Scharer et al., 2019). Similar to malaria-associated atMBCs, DN2 cells also express FcRL5, but not FcRL4 (Jenks et al., 2018). Furthermore, DN2 cells and atMBCs share key transcriptional features including expression of the BLIMP-1 repressor BCL6, the cytokine receptor IL21R, and the inhibitory protein CD72 (Sullivan et al., 2015; Jenks et al., 2018).
It has been proposed that atMBCs are dysfunctional as a result of decreased B cell signalling and limited antibody secretion upon stimulation compared with classical MBCs. In both DN2 and atMBCs, BCR crosslinking induced an approximately 1.5-fold increase in phosphorylated BLNK (pBLNK) compared with unstimulated cells, which was significantly lower than the increase in pBLNK in classical MBCs (Portugal et al., 2015; Jenks et al., 2018; Ambegaonkar et al., 2019). In the case of DN2 cells, the higher level of pBLNK compared with unstimulated cells has been interpreted as a sign of functionality (Jenks et al., 2018), while the reduced level of pBLNK in atMBCs compared with classical MBCs led to the conclusion that this represents a lack of responsiveness (Portugal et al., 2015; Ambegaonkar et al., 2019). In line with limited BCR signalling, atMBCs failed to differentiate into antibody-secreting cells in vitro upon stimulation by BCR crosslinking and TLR9 engagement (Portugal et al., 2015; Sullivan et al., 2015). Similarly, DN2 cells generated in vitro by BCR crosslinking, TLR7 engagement, and cytokine (IFNγ, IL-2, IL-21, and BAFF) stimulation of naïve B cells did not continue to differentiate into antibody-secreting cells by prolonged incubation under the same conditions (Zumaquero et al., 2019). However, DN2 cells efficiently differentiated into antibody-secreting cells in the absence of BCR crosslinking, indicating that transient BCR engagement is key to optimal stimulation and differentiation of these cells (Zumaquero et al., 2019). In line with the thought that atMBCs may be preantibody-secreting cells similar to DN2 cells, Muellenbeck et al. (2013) observed secretory Ig transcripts by PCR in atMBCs but not classical MBCs. It remains to be determined whether atMBCs can be induced to secrete antibodies under the same conditions used for DN2 differentiation, but based on these recent findings it may be too early to conclude that atMBCs are dysfunctional.
4.5. Autoreactive cells
DN2 cells are predominantly expanded in SLE patients of African American descent with active disease (Jenks et al., 2018). Stimulation and subsequent differentiation of DN2 cells from these SLE patients into antibody-secreting cells resulted in the production of autoantibodies, suggesting that DN2 cells are precursors of pathogenic plasma cells (Jenks et al., 2018). Interestingly, antibodies isolated from malaria-associated atMBCs also showed increased self-and polyreactivity compared with classical MBCs (Muellenbeck et al., 2013). In addition, autoreactive T-bet+CD11c+ cells expanded in response to Plasmodium yoelii infection in mice (Rivera-Correa et al., 2017). These cells contributed to the development of Plasmodium-induced anaemia by generating autoantibodies targeting phospholipids on the erythrocyte surface, in particular phosphatidylserine (Rivera-Correa et al., 2017). Erythrocyte-reactive cells were also induced in vitro by stimulation of human PBMCs with P. falciparum lysate (Rivera-Correa et al., 2017). The abundance of atMBCs in Plasmodium-infected individuals was associated with levels of anti-phosphatidylserine IgG in plasma and the development of anaemia (Rivera-Correa et al., 2019). The presence of phosphatidylserine in the outer membrane leaflet is increased in Plasmodium-infected erythrocytes (Schwartz et al., 1987). The production of phosphatidylserine-specific autoantibodies by T-bet+CD11c+ cells may thus be beneficial during Plasmodium infections to reduce the population of infected erythrocytes, but inadvertently also target uninfected erythrocytes with this phospholipid exposed on their surface. Although future studies are necessary to determine the extent of overlap between DN2 cells and the atMBC population, the many parallels in phenotype and functionality suggest that similar drivers may underlie the formation of these cell populations in conditions of chronic inflammation.
4.6. A model for the role of atMBCs in health and disease
Based on the evidence to date, we propose a model in which atMBCs function as a normal part of the immune response. The expansion of these cells shortly after exposure to antigen, and their contraction following the clearance of infection, suggests they are activated cells that respond to antigenic stimulation. In the inflammatory conditions characteristic of chronic infections and autoimmunity, continuous antigen exposure and TLR signalling may disrupt the normal development of these cells. DN2 cells develop into ASCs only in the absence of BCR crosslinking (Zumaquero et al., 2019), suggesting continuous BCR engagement by antigen could prevent these cells from developing correctly. Similarly, TLR7/9 engagement prevents cells from repressing an inflammatory gene programme which can inhibit their development into ASCs (Stone et al., 2019). As a result, atMBCs may accumulate and become dysfunctional. During chronic or repetitive infections, it is likely that both normal and dysfunctional atMBCs are present, which could potentially explain some of the seemingly discrepant results that have been obtained.
5. Concluding remarks
AtMBCs remain an incompletely understood B cell population in the immune response to Plasmodium. There is evidence for a functional role of atMBCs in modulating the immune response, as well as for contributing to parasite antigen-specific responses and malaria-associated anaemia. As such, the precise function(s) of atMBCs remains elusive. To complicate matters, the true heterogeneity of the B cell compartment is becoming increasingly appreciated as new subpopulations of B cells with distinct phenotypes are identified. Within the atMBC population are subpopulations, defined by differences in surface marker expression, that likely also differ in function.
Conflicting reports on atMBC functionality highlight the need for more in-depth functional studies focused on further defining the role of atMBCs in the immune response to infection. Focus on P. falciparum-specific B cells and methods to isolate these cells remain a priority as atMBCs and classical MBCs in this population may behave differently in response to infection than corresponding non-P. falciparum-specific B cells. Additionally, increased efforts to characterise the similarities and differences between human and mouse atMBCs will be invaluable in the study of these cells. Until the relationship between these populations is understood, correlating murine and human study data will be difficult.
Future studies investigating the factors that stimulate alterations in the transcriptional and surface marker profiles of atMBCs will assist in teasing out their developmental pathway during infection. In this vein, epigenetic studies probing for differences in gene expression and regulation may provide valuable insight into the evolutionary path of atMBCs relative to naïve and classical MBCs, similar to studies on the development of memory CD8+ T cells (Dogra et al., 2016; Akondy et al., 2017). Investigation of atMBC plasticity and methods to either reverse loss of function or stimulate the development of atMBCs will be valuable in vaccine development. Understanding how natural immunity to malaria is acquired will help direct efforts to design effective vaccines. As the potential positive or negative impacts of atMBCs in the development of natural immunity remain disputed, it is important to focus efforts on understanding how these cells can be harnessed to improve vaccine efficacy.
Acknowledgements
We thank Dr. Elizabeth Leadbetter for critical review and discussion. Funding: This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID), USA (R21 128466 and R21 133274). The funding source had no role in writing this review or in the decision to submit the article for publication.
References
- Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, Alexe G, Nagar S, McCausland MM, Gupta S, Tata P, Haining WN, McElrath MJ, Zhang D, Hu B, Greenleaf WJ, Goronzy JJ, Mulligan MJ, Hellerstein M, Ahmed R, 2017. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552, 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambegaonkar AA, Nagata S, Pierce SK, Sohn H, 2019. The differentiation in vitro of human Tonsil B cells with the phenotypic and functional characteristics of T-bet+ atypical memory B cells in malaria. Front. Immunol 10, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SF, Chambers MJ, Schramm CA, Plyler J, Raab JE, Kanekiyo M, Gillespie RA, Ransier A, Darko S, Hu J, Chen X, Yassine HM, Boyington JC, Crank MC, Chen GL, Coates E, Mascola JR, Douek DC, Graham BS, Ledgerwood JE, McDermott AB, 2019. Activation dynamics and immunoglobulin evolution of pre-existing and newly generated human memory B cell responses to influenza hemagglutinin. Immunity 51, 398–410.e5. [DOI] [PubMed] [Google Scholar]
- Austin JW, Buckner CM, Kardava L, Wang W, Zhang X, Melson VA, Swanson RG, Martins AJ, Zhou JQ, Hoehn KB, Fisk JN, Dimopoulos Y, Chassiakos A, O’Dell S, Smelkinson MG, Seamon CA, Kwan RW, Sneller MC, Pittaluga S, Doria-Rose NA, McDermott A, Li Y, Chun TW, Kleinstein SH, Tsang JS, Petrovas C, Moir S, 2019. Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci. Transl. Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye R, Sutton HJ, Nduati EW, Kai O, Mwacharo J, Musyoki J, Otieno E, Wambua J, Bejon P, Cockburn IA, Ndungu FM, 2020. Malaria exposure drives both cognate and bystander human B cells to adopt an atypical phenotype. Eur. J. Immunol Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayieko C, Maue AC, Jura WG, Noland GS, Ayodo G, Rochford R, John CC, 2013. Changes in B cell populations and merozoite surface protein-1-specific memory B cell responses after prolonged absence of detectable P. falciparum infection. PLoS ONE 8, e67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK, 1998. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann of Trop Med Parasitol 92 (4), 367–390. [DOI] [PubMed] [Google Scholar]
- Changrob S, McHenry AM, Nyunt MH, Sattabongkot J, Han ET, Adams JH, Chootong P, 2018. Persistence of long-lived memory B cells specific to duffy binding protein in individuals exposed to Plasmodium vivax. Sci. Rep 8, 8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Mc GI, Carrington S, 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192, 733–737. [DOI] [PubMed] [Google Scholar]
- Dogra P, Ghoneim HE, Abdelsamed HA, Youngblood B, 2016. Generating long-lived CD8(+) T-cell memory: Insights from epigenetic programs. EurJ Immunol, 1548–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DL, Dobano C, Baird JK, 2009. Acquired immunity to malaria. Clin. Microbiol. Rev 22, 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD, 2005. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med 202, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK, Oshansky CM, Elbein R, Thomas S, Lyon GM, Spiropoulou CF, Mehta AK, Thomas PG, Boyd SD, Ahmed R, 2016. Defining antigen-specific plasmablasts and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol 17,1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, Pereira DB, Soares IS, Martins-Filho OA, Jankovic D, Gazzinelli RT, Antonelli L, 2017. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog. 13, e1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, Tolnay M, 2013. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J. Immunol 190, 5739–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles HM, McGregor IA, 1959. Studies on the significance of high serum gamma-globulin concentrations in Gambian Africans. I. - Gamma-globulin concentrations of Gambian children in the first two years of life. Ann. Trop. Med. Parasitol 53, 492–500. [DOI] [PubMed] [Google Scholar]
- Gotz A, Tang MS, Ty MC, Arama C, Ongoiba A, Doumtabe D, Traore B, Crompton PD, Loke P, Rodriguez A, 2017. Atypical activation of dendritic cells by Plasmodium falciparum. Proc. Natl. Acad. Sci. U S A 114, E10568–E10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JT, Hollingsworth TD, Reyburn H, Drakeley CJ, Riley EM, Ghani AC, 2015. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. Biol. Sci 282, 20142657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunda R, Shamu S, Chimbari MJ, Mukaratirwa S, 2017. Economic burden of malaria on rural households in Gwanda district, Zimbabwe. Afr. J. Prim. Health Care Fam. Med 9, e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailu A, Lindtjorn B, Deressa W, Gari T, Loha E, Robberstad B, 2017. Economic burden of malaria and predictors of cost variability to rural households in south-central Ethiopia. PLoS ONE 12, e0185315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP, 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118, 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, Zhu L, Wei W, Wang C, Karnowski A, Belz GT, Ghali JR, Cook MC, Riminton DS, Veillette A, Schwartzberg PL, Mackay F, Brink R, Tangye SG, Vinuesa CG, Mackay CR, Li Z, Yu D, 2013. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 39, 770–781. [DOI] [PubMed] [Google Scholar]
- Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E, 2010. Complement receptor 2/CD21-human naïve B cells contain mostly autoreactive unresponsive clones. Blood 115, 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FE, Boss JM, Lund FE, Sanz I, 2018. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49 725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten SA, van Meijgaarden KE, Nonno FD, Baiochini A, Petrone L, Vanini V, Smits HH, Palmieri F, Goletti D, Otenhoff THM, 2016. Patients with tuberculosis have a dysfunctional circulating B-cell compartment, which normalizes following successfil treatment. PLoS Pathog. 12, e1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CC, Baccarella AM, Bayat A, Pepper M, Fontana MF, 2019. FCRL5(+) memory B cells exhibit robust recall responses. Cell Rep 27 1446–1460 e1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, Robb ML, Ostrowski MA, Deeks SG, Slifka MK, Tomaras GD, Moir S, Moody MA, Betts MR, 2017. T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, Pepper M, 2016. Somatically hypermutated Plasmodium-specific IgM(+) memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity 45, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Shinohara H, Baba Y, 2010. B cell signaling and fate decision. Annu. Rev. Immunol 28, 21–55. [DOI] [PubMed] [Google Scholar]
- Lau D, Lan LY, Andrews SF, Henry C, Rojas KT, Neu KE, Huang M, Huang Y, DeKosky B, Palm AE, Ippolito GC, Georgiou G, Wilson PC, 2017. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci. Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levack RC, Newell KL, Popescu M, Cabrera-Martinez B, Winslow GM, 2020. CD11c+ T-bet+ B cells require IL-21 and IFNγ type 1 T follicular helper cells and intrinsic Bcl-6 expression but develop normally in the absence of T-bet. J. Immunol ji2000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhou S, Qian J, Wang Y, Yu X, Dai D, Dai M, Wu L, Liao Z, Xue Z, Wang J, Hou G, Ma J, Harley JB, Tang Y, Shen N, 2017. T-bet(+)CD11c(+) B cells are critical for antichromatin immunoglobulin G production in the development of lupus. Arthritis Res. Ther 19, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly A, Liao Y, Pietrzak H, Ioannidis LJ, Sidwell T, Gloury R, Doerflinger M, Triglia T, Qin RZ, Groom JR, Belz GT, Good-Jacobson KL, Shi W, Kallies A, Hansen DS, 2019. Transcription factor T-bet in B cells modulates germinal center polarization and antibody affinity maturation in response to malaria. Cell Rep. 29 2257–2269 e2256. [DOI] [PubMed] [Google Scholar]
- McGregor IA, Gilles HM, 1960. Studies on the signifcance of high serum gamma-globulin concentrations in Gambian Africans. II. - Gamma-globulin concentrations of Gambian children in the fourth, fifth, and sixth years of life. Ann. Trop. Med. Parasitol 54, 257–280. [DOI] [PubMed] [Google Scholar]
- Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS, 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med 205, 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H, 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, Esen M, Theisen M, Mordmuller B, Wardemann H, 2013. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J. Exp. Med 210, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, Cancro MP, 2016. Cutting edge: IL-4, IL-21, and IFN-gamma interact to govern T-bet and CD11c Expression in TLR-activated B cells. J. Immunol 197, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng-Adjei N, Portugal S, Holla P, Li S, Sohn H, Ambegaonkar A, Skinner J, Bowyer G, Doumbo OK, Traore B, Pierce SK, Crompton PD, 2017. Malaria-induced interferon-gamma drives the expansion of Tbethi atypical memory B cells. PLoS Pathog. 13, e1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD, 2015. Circulating Th1-Cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 13, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero B, Mantovani S, Ludovisi S, Varchetta S, Mele D, Paolucci S, Baldanti F, Mondelli MU, 2015. Skewed B cells in chronic hepatitis C virus infection maintain their ability to respond to virus-induced activation. J. Viral Hepat 22, 391–398. [DOI] [PubMed] [Google Scholar]
- Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K, 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun 76, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Gardner PJ, Schweighoffer E, McLaughlin S, Hosking C, Tumwine I, Davis RS, Potocnik AJ, Tybulewicz VL, Langhorne J, 2018. Plasmodium-specific atypical memory B cells are short-lived activated B cells. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper K, Grimbacher B, Eibel H, 2013. B-cell biology and development. J. Allergy Clin. Immunol 131, 959–971. [DOI] [PubMed] [Google Scholar]
- Portugal S, Doumtabe D, Traore B, Miller LH, Troye-Blomberg M, Doumbo OK, Dolo A, Pierce SK, Crompton PD, 2012. B cell analysis of ethnic groups in Mali with differential susceptibility to malaria. Malar. J 11, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, Skinner J, Virtaneva K, Sturdevant DE, Porcella SF, Doumbo OK, Doumbo S, Kayentao K, Ongoiba A, Traore B, Sanz I, Pierce SK, Crompton PD, 2015. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM, 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293, 1461–1470. [DOI] [PubMed] [Google Scholar]
- Riley EM, Olerup O, Bennett S, Rowe P, Allen SJ, Blackman MJ, Troye-Blomberg M, Holder AA, Greenwood BM, 1992. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciparum. Int. Immunol 4, 1055–1063. [DOI] [PubMed] [Google Scholar]
- Rivera-Correa J, Guthmiller JJ, Vijay R, Fernandez-Arias C, Pardo-Ruge MA, Gonzalez S, Butler NS, Rodriguez A, 2017. Plasmodium DNA-mediated TLR9 activation of T-bet(+) B cells contributes to autoimmune anaemia during malaria. Nat. Commun 8, 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Correa J, Mackroth MS, Jacobs T, Schulze Zur Wiesch J, Rolling T, Rodriguez A, 2019. Atypical memory B-cells are associated with Plasmodium falciparum anemia through anti-phosphatidylserine antibodies. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Feltrer A, Carneiro I, Smith L, Schellenberg JR, Greenwood B, Schellenberg D, 2010. The age patterns of severe malaria syndromes in sub-Saharan Africa across a range of transmission intensities and seasonality settings. Malar. J 9, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, Boyle MJ, Tappero J, Muhindo M, Kamya MR, Dorsey G, Drakeley C, Ssewanyana I, Smith DL, Greenhouse B, 2016. Quantifying heterogeneous malaria exposure and clinical protection in a cohort of ugandan children. J. Infect. Dis 214, 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, Kamya MR, Rosenthal PJ, Rek J, Dorsey G, Nankabirwa J, Staedke SG, Kilama M, Drakeley C, Ssewanyana I, Smith DL, Greenhouse B, 2018. Quantification of anti-parasite and anti-disease immunity to malaria as a function of age and exposure. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P, 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 118, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P, 2013. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc. Natl. Acad. Sci. U S A 110, E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P, 2017. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Invest 127, 1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, Hom J, Lee FE, 2019. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol 10, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharer CD, Blalock EL, Mi T, Barwick BG, Jenks SA, Deguchi T, Cashman KS, Neary BE, Patterson DG, Hicks SL, Khosroshahi A, Eun-Hyung Lee F, Wei C, Sanz I, Boss JM, 2019. Epigenetic programming underpins B cell dysfunction in human SLE. Nat. Immunol 20, 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen A, Teirlinck AC, Bijker EM, Roestenberg M, Hermsen CC, Hoffman SL, Sauerwein RW, 2014. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J. Immunol 192, 3719–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RS, Olson JA, Raventos-Suarez C, Yee M, Heath RH, Lubin B, Nagel RL, 1987. Altered plasma membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Blood 69, 401–407. [PubMed] [Google Scholar]
- Shretta R, Avancena A, Hatefi A, 2016. The economics of malaria control and elimination: a systematic review. Malar J 15 (1). 10.1186/s12936-016-1635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Peel JN, Scharer CD, Risley CA, Chisolm DA, Schultz MD, Yu B, Ballesteros-Tato A, Wojciechowski W, Mousseau B, Misra RS, Hanidu A, Jiang H, Qi Z, Boss JM, Randall TD, Brodeur SR, Goldrath AW, Weinmann AS, Rosenberg AF, Lund FE, 2019. T-bet transcription factor promotes antibody-secreting cell differentiation by limiting the inflammatory effects of IFN-gamma on B cells. Immunity 50 1172–1187 e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RT, Kim CC, Fontana MF, Feeney ME, Jagannathan P, Boyle MJ, Drakeley CJ, Ssewanyana I, Nankya F, Mayanja-Kizza H, Dorsey G, Greenhouse, 2019. FCRL5 delineates functionally impaired memory B cells associated with Plasmodium falciparum exposure. PLoS Pathog. 11, e1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundling C, Ronnberg C, Yman V, Asghar M, Jahnmatz P, Lakshmikanth T, Chen Y, Mikes J, Forsell MN, Sonden K, Achour A, Brodin P, Persson KE, Farnert A, 2019. B cell profiling in malaria reveals expansion and remodelling of CD11c+ B cell subsets. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Feng D, Wang R, Ghose B, Hu T, Ji L, Wu T, Fu H, Huang Y, Feng Z, 2017. Economic burden of malaria inpatients during National Malaria Elimination Programme: estimation of hospitalization cost and its interprovince variation. Malar. J 16, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, Liu H, Autoimmunity Molecular Medicine T, Manna Z, Goldbach-Mansky R, Hasni S, Siegel R, Sanjuan M, Streicher K, Cancro MP, Kolbeck R, Ettinger R, 2018. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet (+) B cells in SLE. Nat. Commun 9, 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz K, Wehr C, Drager R, Schmidt S, Eibel H, Schlesier M, Peter HH, 2002. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology 206, 502–513. [DOI] [PubMed] [Google Scholar]
- Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, Warnatz K, 2004. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin. Immunol 113, 161–171. [DOI] [PubMed] [Google Scholar]
- Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I, 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol 178, 6624–6633. [DOI] [PubMed] [Google Scholar]
- Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, Hernandez JN, Doumbo OK, Pierce SK, Branch OH, Crompton PD, 2011. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS ONE 6, e15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK, 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol 183, 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD, 2010. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 6, e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2019. World Malaria Report. World Health Organization, Geneva: http://www.who.int/publications/i/item/world-malaria-report-2019. [Google Scholar]
- Wilson TJ, Fuchs A, Colonna M, 2012. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J. Immunol 188, 4741–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap XZ, Hustin LSP, Sauerwein RW, 2019. TH1-polarized TFH cells delay naturally-acquired immunity to malaria. Front. Immunol 10,1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Goldschmidt T, Salter H, 2012. Possible allelic structure of IgG2a and IgG2c in mice. Mol. Immunol 50, 169–171. [DOI] [PubMed] [Google Scholar]
- Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, Rosal-Vela A, Botta D, Bradley JE, Wojciechowski W, Ptacek T, Danila MI, Edberg JC, Bridges SL Jr., Kimberly RP, Chatham WW, Schoeb TR, Rosenberg AF, Boss JM, Sanz I, Lund FE, 2019. IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


