Abstract
Objective:
Resuscitation after cardiac surgery needs to address multiple pathophysiological processes that are associated with significant morbidity and mortality. Functional microcirculatory derangements despite normal systemic hemodynamics have been previously described but must be tied to clinical outcomes. The authors hypothesized that microcirculatory dysfunction after cardiac surgery would include impaired capillary blood flow and impaired diffusive capacity and that subjects with the lowest quartile of perfused vessel density would have an increased postoperative lactate level and acute organ injury scores.
Design:
Prospective, observational study.
Setting:
A single, tertiary university cardiovascular surgical intensive care unit.
Participants:
25 adults undergoing elective cardiac surgery requiring cardiopulmonary bypass.
Intervention:
Sublingual microcirculation was imaged using incident dark field microscopy before and 2 to 4 hours after surgery in the intensive care unit.
Measurements and Main Results:
Compared with baseline measurements, postoperative vessel-by-vessel microvascular flow index (2.9 [2.8–2.9] v 2.5 [2.4–2.7], p < 0.0001) and perfused vessel density were significantly impaired (20.7 [19.3–22.9] v 16.3 [12.8–17.9], p < 0.0001). The lowest quartile of perfused vessel density (<12.8 mm/mm2) was associated with a significantly increased postoperative lactate level (6.0 ± 2.9 v 1.8 ± 1.2, p < 0.05), peak lactate level (7.6 ± 2.8 v 2.8 ± 1.5, p = 0.03), and sequential organ failure assessment (SOFA) score at 24 and 48 hours.
Conclusion:
In patients undergoing cardiac surgery, there was a significant decrease in postoperative microcirculatory convective blood flow and diffusive capacity during early postoperative resuscitation. Severely impaired perfused vessel density, represented by the lowest quartile of distribution, is significantly related to hyperlactatemia and early organ injury.
Keywords: microcirculation, resuscitation, cardiac surgery, critical care, shock
RESUSCITATION AFTER cardiac surgery often needs to address and reverse multiple physiological derangements that can result in significant morbidity and mortality.1,2 After cardiopulmonary bypass (CPB), postoperative hypotension is associated with microcirculatory disturbances due to ischemic-reperfusion injury, systemic inflammatory response, and microemboli formation.3 Resuscitation often targets normalized systemic hemodynamic goals to achieve adequate tissue perfusion guided by lactate and oxygen-derived flow measurements. Optimized tissue perfusion represents the final pathway for cellular oxygenation and substrate delivery. Functional microcirculatory derangements have been described after cardiac surgery, but the relationship between microcirculatory impairment and perfusion-related parameters is unclear and must be tied to clinical outcomes.4–6
During the past decade, microcirculatory imaging technology has improved.7 Incident dark field (IDF) handheld videomicroscopy has improved compared with previous generations of microcirculatory imaging and produces higher quality images.7 As a result, more detailed, functional assessments of the human microcirculation can now be obtained.
Previous studies have found significant microcirculatory impairment after cardiac surgery with cardiopulmonary bypass.3,5,8 It is unclear, however, if the severity of early alterations has an impact on meaningful clinical outcomes. Given the heterogeneity of hemodynamic disturbances that can arise after cardiac surgery, the authors hypothesized that severe alterations in microcirculatory function would be associated with the severity of postoperative lactic acidosis as well as increased postoperative organ dysfunction.
Methods
Study Design
This was a prospective study in a single, tertiary academic cardiovascular surgical intensive care unit (ICU). This study was approved by the authors’ institutional review board (IRB # 829765), and written informed consent was obtained before enrollment. All consent forms were copied in triplicate, one given to the subject, the second placed in the official medical record, and the third kept in a secured location within the principal investigator’s office. The goal was to observe the microcirculation before and after elective cardiothoracic surgery, during early resuscitation in the ICU.
Hemodynamic, laboratory, and microcirculation data were collected on each patient the day of surgery in the preoperative staging area (pre-CPB), followed by a second time point (post-CPB) during the early postoperative resuscitation period. Images were obtained within 2 to 4 hours after surgery in the ICU to identify early microcirculatory changes during the optimization phase of the clinical resuscitation, avoid interruption during patient handoff, and not interfere with initial clinical efforts. Systemic hemodynamic data along with perfusion data were collected at the time of IDF measurement. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Pennsylvania.9
Inclusion and Exclusion Criteria
Adult patients (age >18 years) undergoing elective coronary artery bypass grafting or valvular surgery requiring cardiopulmonary bypass were eligible. Informed consent and study enrollment took place immediately before the procedure, in the preoperative staging area, or medical ward before their transfer to the operating room. Patients were excluded from the study if they received postoperative intravenous nitroglycerin, nicardipine, or systemic vasodilator infusions; had active cancer; or had taken metformin within 72 hours before surgery.
Microcirculatory Measurement and Analysis
Evaluation of the patient’s sublingual microcirculation was recorded using incident dark field videomicroscopy (CytoCam, Braedius Medical BV, the Netherlands).10 At each time point, the video microscope was gently placed under the tongue until an adequate view of the microcirculation was acquired. A minimum of 3 video clips of 5 seconds’ (100 frames) duration was included at each time point per analysis, with attention to quality factors, especially the absence of pressure artefact, excess saliva, and proper location in accordance with the accepted consensus for assessing the microcirculation (Figs. 1 and 2).11,12 Each clip was deidentified and coded to be analyzed after enrollment was completed. Microcirculatory videos were exported to automated vascular analysis (AVA 3.2, Microvision Medical B.V.) format using the CCTools 2 software to be manually analyzed using a validated, web-based tool.13 Video analysis was performed at the conclusion of the data collection period by the lead investigator (J.C. G.), who was blinded to the conditions of the subject. Image quality was assessed using the 6-factor Massey quality score, which scores each video for appropriate illumination, duration, focus, content, stability, and pressure. Images were only analyzed if the Massey quality score was <10.11 Only microvessels ≤20 μm in diameter were included in the analysis. Total vessel density, proportion of perfused vessels (PPV), perfused vessel density (PVD), vessel-by-vessel microcirculatory flow index (MFIv), and microcirculatory heterogeneity index (MHI) were measured manually, according to the current best practice guidelines for reporting microcirculatory variables.12 Blood flow within each microvessel was graded as having no flow, intermittent, sluggish, or continuous flow.12 Vessels scored with intermittent or no flow were considered nonperfused, whereas continuous or sluggish scores were regarded as perfused vessels for the calculation of PPV and PVD.
Fig. 1.

Experimental setup. (A) Patient positioning during IDF measurement. (B) Anatomic sublingual triangle where measurements were obtained.
Fig. 2.

Representative still images of a patient’s (A) baseline microcirculation and (B) impaired postoperative microcirculation.
Macrocirculatory and Physiological Measurements
Cardiac output, central venous pressure, pulmonary artery pressure, and mixed venous oxygen saturation (SvO2) were measured continuously using a pulmonary artery catheter (Edwards Lifesciences LLC, Irvine, CA). Arterial blood pressure was measured using a standard invasive arterial line. Blood gas samples were drawn from an arterial line into a commercial, pre-heparinized 1-mL arterial blood sampler, then immediately analyzed with an ABL90 FLEX automatic blood gas analyzer (Radiometer America Inc., Brea, CA). Sequential organ failure assessment (SOFA) scores were calculated 24 and 48 hours after surgery.14
Perioperative Care
All patients were monitored with an invasive arterial blood pressure or noninvasive BP cuff, 5-lead electrocardiography, pulse oximetry, end-tidal capnography and gas analyzer, a pulmonary artery catheter with temperature probe, central venous pressure monitor, and transesophageal echocardiography. Induction of anesthesia was performed with intravenous fentanyl (up to 5 μg/kg), propofol (1 to 2 mg/kg), and vecuronium (0.1 mg/kg) before endotracheal intubation. Anesthesia was maintained with isoflurane in a mixture of oxygen and air. Additional doses of muscle relaxants and fentanyl were given as needed. All patients were ventilated mechanically with lung- protective ventilation. Tidal volumes were titrated to 6 to 8 mL/kg of ideal body weight and a positive end-expiratory pressure of 5 to 10 cm H2O, with the respiratory rate adjusted to achieve an end-tidal carbon dioxide measurement of 30 to 35 mmHg. Before CPB, anticoagulation with 300 IU/kg of heparin was administered to achieve an activated clotting time >450 seconds. A bypass flow rate of 2.2 to 2.4 L/min/m2 with mean arterial pressure (MAP) maintained >60 mmHg, administering intravenous phenylephrine as needed. After weaning from bypass, MAP was maintained >60 mmHg and cardiac index >2.2 L/min/m2 with the administration of fluid boluses, phenylephrine, and epinephrine infusion as needed. Postoperative cardiac function and filling status were optimized by transesophageal echocardiogram at the end of the procedure. Heparin was reversed with protamine with a dose of 1 mg for each 100 IU of heparin to restore activated clotting time to baseline values.
ICU Resuscitation and Management
All patients were warmed to achieve normothermia and resuscitated to normalized systemic hemodynamic endpoints, including an optimized intravascular filling pressure, an MAP of 65 to 80 mmHg, and a cardiac index (CI) goal of >2.0 L/min/m2. Mixed venous oxygen saturation was maintained between 60% and 70%, and lactate normalization was targeted. Phenylephrine was titrated to achieve a target MAP, and epinephrine was titrated to achieve an adequate CI after acceptable intravascular volume resuscitation. A repeated transthoracic echocardiogram was performed by the research team to evaluate left ventricular function during microcirculation assessment. A standard postoperative hemoglobin target of 8 g/dL was established for packed red blood cell transfusion.
Sample Size
This study was powered on the smallest difference between pre- and postoperative perfused vessel densities and between the highest and lowest quartile of postoperative SOFA scores using microcirculatory data from previous studies.15–17 The authors calculated that at least 18 patients would need to be enrolled to maintain 80% power to detect a significant difference in capillary flow, density, and SOFA scores. They increased this number to 25 patients to account for a variable magnitude of the effect.
Statistical Analysis
Statistical analysis was conducted using Prism v 8.0 (Graph-Pad Software, San Diego, CA). Data were assessed for normality using the D’Agostino and Pearson omnibus normality test. Global hemodynamic and microcirculation variables are reported as mean ± SD. Variables that were not normally distributed are reported as median with interquartile range (25th to 75th percentiles). Differences between variables at the initial (pre-CPB) and postoperative (post-CPB) time points were assessed using paired student t test or Wilcoxon matched-pairs signed rank test. Linear regression analysis was performed to determine whether PVD was an independent predictor of hyperlactatemia and postoperative SOFA scores. One-way ANOVA with Bonferroni post-hoc testing was performed to compare postoperative organ injury using 24- and 48-hour SOFA scores by PVD quartile.
Results
Study Population and Operative Details
The authors enrolled 25 subjects undergoing elective coronary artery bypass grafting surgery (CABG) or valvular surgery requiring cardiopulmonary bypass. Patient demographic and perioperative data are reported in Table 1. Common microvascular comorbidities included hypertension (84%), diabetes mellitus (32%), and chronic renal disease (20%). All subjects survived to hospital discharge.
Table 1.
Subject Characteristics and Demographics
| n = 25 | ||
|---|---|---|
| Age, y | 63 | ±12 |
| Sex, male | 20 | 80.0% |
| EuroSCORE II | 2.0 | |
| Performed operation | ||
| CABG | 10 | 40.0% |
| CABG + valve replacement/repair | 6 | 20.0% |
| Valvular surgery only | 9 | 40.0% |
| Comorbidities, n, % | ||
| Hypertension | 21 | 84.0% |
| Diabetes | 8 | 32.0% |
| Heart failure with reduced ejection fraction (<30%) | 2 | 8.0% |
| Chronic kidney disease | 5 | 20.0% |
| Chronic obstructive pulmonary disease | 1 | 4.0% |
| Perioperative details | ||
| Cardiopulmonary bypass time, min | 105 | ±35 |
| Cross-clamp time, min | 75 | ±27 |
| Grafts, n | 3 | (2–3) |
| Cell saver transfusion, mL | 752 | ±452 |
| Red blood cell transfusion, n | 15 | 60% |
| Fresh frozen plasma transfusion, n | 5 | 20% |
| Platelet transfusion, n | 8 | 32% |
| Crystalloid intravenous fluid, mL | 1,231 | ±850 |
| Urine output, mL | 778 | ±478 |
| CPB ultrafiltrate, mL | 816 | ±1,288 |
| SOFA score | ||
| 24 hours | 6 | (5–9) |
| 48 hours | 6 | (2–8) |
| 30-day survival | 25 | 100% |
NOTE. Data presented as mean ± standard deviation, median (quartile range), and frequency (%).
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass;
SOFA, sequential organ failure assessment.
Global Hemodynamics During Microcirculatory Measurement
Preoperative MAP was higher than postoperative MAP (94 ± 15 v 75 ± 9 mmHg; p < 0.0001). Pre- and postoperative cardiac index were similar (2.4 ± 0.7 v 2.5 ± 0.8 L/min/m2). Twenty-three patients required postoperative catecholamine support to achieve clinical macrocirculatory targets. Phenylephrine was used primarily to achieve target MAP, at a dose 0.82 ± 0.6 mcg/kg/min. Cardiac index, measured continuously by pulmonary artery catheter, was maintained with epinephrine, at a dose 0.05 ± 0.03 mcg/kg/min. Left ventricular function by point-of-care echocardiography was reassessed during microcirculation assessment and also unchanged. There was a statistically significant difference between pre- and postoperative central venous pressure, systemic vascular resistance, and lactate level despite postoperative macrocirculatory variables remaining within clinically acceptable range (Table 2).
Table 2.
Clinical Data
| Before Bypass | After Bypass | p | |
|---|---|---|---|
| Clinical and maerocirculatory data | |||
| Temperature,°C | 36.7 ± 0.3 | 36.6 ± 0.5 | 0.95 |
| Heart rate, beats/min | 73 ± 11 | 91 ± 9 | <0.0001 |
| Mean arterial pressure, mmHg | 94 ± 15 | 75 ± 9 | <0.0001 |
| Central venous pressure, mmHg | 13 ± 4 | 9 ± 3 | 0.0003 |
| Cardiac index, L/min/m2 | 2.4 ± 0.7 | 2.5 ± 0.8 | 0.56 |
| Systemic vascular resistance, dyn/s/cm−5 | 1,236 (1,128–1,617) | 1,024 (955–1,214) | 0.03 |
| Left ventricular ejection fraction,% | 50 (45–60) | 60 (50–65) | 0.006 |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.0 ± 2.1 | 12.4 ± 1.4 | 0.17 |
| Hematocrit,% | 37.1 ± 5.3 | 36.5 ± 4.3 | 0.18 |
| PaO2, mmHg | – | 134 ± 60 | – |
| Lactate, mmol/dL | 0.8 ± 0.4 | 4.3 ± 2.7 | <0.0001 |
| Peak lactate, mmol/dL | – | 5.3 ± 3.0 | – |
| Mixed venous O2 saturation, % | 69 ± 8 | – | |
| Vasopressor support | |||
| Phenylephrine, n, mcg/kg/min | – | 14; 0.6 (0.3–1.2) | – |
| Vasopressin, n, units/min | – | 4; 0.03 (0.02–0.06) | – |
| Inotropic support, %, mcg/kg/min | |||
| Epinephrine, n, meg/kg/min | – | 20; 0.04 (0.02–0.06) | – |
NOTE. Data are presented as mean ± standard deviation, median (quartile range), and frequency (%).
Microcirculatory Measurements
A total of 280 microcirculation video clips were screened for quality. After image quality assessment, 175 video assessments met the quality threshold for further analysis. The analyzed image sequences had a mean Massey quality score of 0.5 ± 0.7 (illumination 0.06 ± 0.3, duration 0.0 ± 0.1, focus 0.1 ± 0.3, content 0.0 ± 0.2, stability 0.3 ± 0.4, and pressure 0.1 ± 0.2).
Compared with baseline, postoperative microcirculatory convective flow was impaired, represented by a reduction in vessel-by-vessel MFI (pre-CPB v post-CPB MFIv: 2.9 [2.8–2.9] v 2.5 [2.4 – 2.7]; p < 0.0001) along with an increase in heterogeneity (pre-CPB v post-CPB MHI: 0.0 [0.0–0.2] v 0.5 [0.3–0.75]; p < 0.0001). Postoperative diffusive capacity was also impaired, as both proportion of perfused vessels (pre-CPB v post-CPB, PPV %: 94.3 [92.0–96.3] v 76.9 [68.5–85.0]; p < 0.0001) and perfused vessel density (pre-CPB v post-CPB, PVD: 20.7 [19.3–22.9] v 16.3 [12.8–17.9]; p < 0.0001) were reduced compared with preoperative baseline (Fig 3). Linear regression analysis did not find a correlation between postoperative MFIv and mean arterial pressure (p = 0.75) or cardiac index (p = 0.35). Postoperative perfused vessel density was not correlated with mean arterial pressure (p = 0.63) or cardiac index (p = 0.67). There was an inverse correlation between central venous pressure and microcirculatory flow index (MFIv p = 0.01, r2 = 0.25) and PVD (p = .02, r2 = 0.23). Catecholamine dose was not correlated with postoperative MFIv, MHI, total vessel density, or PVD (p = 0.47; p = 0.95; p = 0.25; p = 0.86).
Fig. 3.
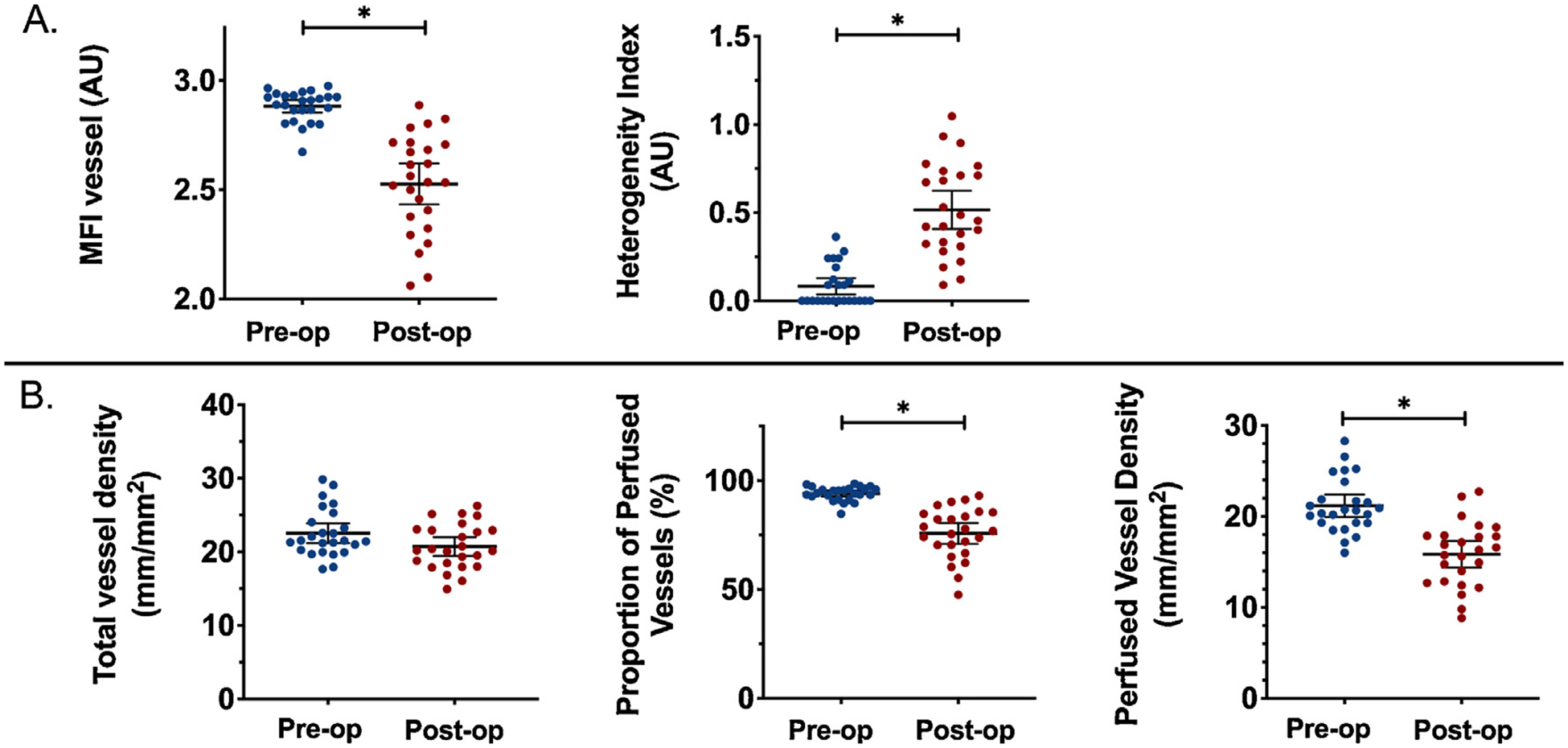
Change in microcirculatory diffusive capacity after cardiac surgery with normal macrocirculatory measurements. A significant reduction in diffusive capacity (A) and convective blood flow (B). Scatter plot represents mean, with bars representing 95% confidence interval. *p < 0.0001.
To assess the clinical relevance of these results, several clinical, hemodynamic, and perfusion variables were compared by postoperative perfused vessel density quartile. Patients in the lowest quartile (<12.8 mm/mm2) exhibited higher postoperative lactate (Fig 4), peak lactate, and SOFA score at 24 hours compared with patients with a perfused vessel density in the upper 3 quartiles (Fig 5, Table 3).
Fig. 4.

Arterial lactate distribution according to PVD quartile. A significant difference between the lowest and highest PVD quartile was observed for (A) lactate at the time of microcirculation assessment and (B) patient peak lactate. *p < 0.05.
Fig. 5.

Postoperative day 1 SOFA score distribution according to PVD quartile. A significant difference between the lowest and all other PVD quartiles was observed. *p < 0.05. **p < 0.01.
Table 3.
Clinical Characteristics of Lowest Versus Upper Postoperative PVD Quartiles
| Quartile | First | Second-Fourth | p |
|---|---|---|---|
| MAP, mmHg | 76 (±10) | 74 (±8) | 0.62 |
| Cardiac index, L/min/m2 | 2.6 (±1.1) | 2.5 (±0.6) | 0.75 |
| Central venous pressure, mmHg | 11 (±2.3) | 8 (±4) | 0.15 |
| Lactate, mmol/L | 6.0 (±2.9) | 3.6 (±2.4) | 0.05* |
| Peak lactate, mmol/L | 7.6 (±2.8) | 4.6 (±2.7) | 0.02* |
| SvO2, % | 73 (±10) | 68 (±7) | 0.15 |
| SOFA 24 h | 9 (8–11) | 6 (5–7) | 0.002* |
| SOFA 48 h | 9 (6–11) | 6 (2–7) | 0.03* |
NOTE. Data presented as mean ± standard deviation, median (quartile range), and frequency (%).
MAP, mean arterial pressure; PVD, perfused vessel density; SOFA, sequential organ failure assessment.
Availability of Data and Materials
The data set supporting the conclusions of this article is available via the Zenodo research data repository.18
Discussion
Microcirculatory convective flow and diffusive capacity were impaired during early resuscitation in this cohort of patients after cardiac surgery. An acute reduction in small vessel capillary blood flow and increased heterogeneity were consistent with previous studies evaluating microcirculatory function after cardiac surgery requiring cardiopulmonary bypass.3,19 A number of potential causes have been suggested for the early loss of microcirculatory coherence with systemic hemodynamics, which include blood exposure to extracorporeal cardiopulmonary bypass circuitry, postoperative systemic inflammatory response syndrome (SIRS), ischemic-reperfusion injury, endothelial injury, impaired red blood cell rheology, or patient-specific hemodynamic endpoints that were outside generally accepted targets.5,20–23
The most notable changes in patients’ postoperative microcirculation were an increased heterogeneity, also referred to as a type 1 phenotype of microcirculatory dysfunction.12 A type 1 loss represents microcirculatory dysfunction that is heterogeneous and can cause regional tissue hypoxia due to various cellular insults, including endothelial dysfunction, hemorheological changes, or abnormal vasomotor tone due to pathologic nitric oxide release or oxidative stress. In this cohort, a reduction in the proportion of perfused vessels appeared to contribute most to the reduction in perfused vessel density. Impaired PVD has been associated with poor clinical outcomes in patients with trauma and sepsis and has been identified at the initiation of cardiopulmonary bypass and other inflammatory conditions.24–26
The authors also identified type 3 microcirculatory dysfunction, which was recognized by a reduction in postoperative vessel-by-vessel MFI and total vessel density. Type 3 dysfunction is seen as diffuse slowing of microcirculatory blood flow as a result of increased vascular resistance, and/or increased venous pressures. Postoperative central venous pressures decreased in the cohort, which made excessive postcapillary pressure a less likely cause of type 3 dysfunction. It is possible that a combined catecholamine therapy (phenylephrine and epinephrine) without concomitant vasodilator therapy could lead to excessive precapillary vasoconstriction. The authors did not include subjects who were prescribed concomitant vasodilators such as nitroglycerin, nicardipine, or phosphodiesterase inhibitors for inotropic support. Including these medications could improve postoperative microcirculatory function.6,27
Within the study cohort, each patient’s intravascular filling and cardiac function were assessed at the end of the operation by transesophageal echocardiogram to guide resuscitative interventions. Postoperative fluid resuscitation was guided by dynamic measures of volume responsiveness to avoid excessive crystalloid administration. Vasopressor and inotropic therapy were initiated if the patient needed additional hemodynamic support.28,29
The effects of vasoactive medications on the functional microcirculation are incompletely understood but theoretically will directly affect capillary blood flow via precapillary sphincter augmentation. A majority of subjects in this study received phenylephrine to reverse postoperative vasoplegia, and 4 patients received vasopressin in addition. Intraoperative phenylephrine, used to increase systemic vascular resistance after the initiation of cardiopulmonary bypass, has been found to reduce the sublingual small vessel MFI, with significant microcirculatory shunting toward vessels >25 micrometers in size.30 This effect was not observed with postoperative phenylephrine use, with which a 20% to 30% increase in MAP did not change microvascular blood flow.31 Vasopressin appeared to affect microcirculatory blood flow less than other vasoactives when used to reverse SIRS-related vasoplegia.32
Patients with chronic hypertension may require a more aggressive, individualized treatment strategy to ensure adequate end-organ perfusion after surgery.33 The postoperative MAP targets were often lower than patients’ preoperative measurements, to protect surgical anastomoses and avoid postoperative bleeding. Relatively low blood pressure targets in patients with chronic hypertension may contribute to microcirculatory impairment.
Epinephrine was used to augment cardiac index after adequate intravascular volume resuscitation. The macrocirculatory effects of epinephrine are well documented.34,35 Previous reports of epinephrine’s effect on the splanchnic microcirculation have been mixed.8,36 In general, there is minimal evidence to suggest that epinephrine improves regional perfusion after cardiac surgery but can contribute to increased myocardial workload, oxygen consumption, and lactate production.37,38
Other medications can also affect perioperative microcirculation, including continuous infusions of sedation and analgesics. All 25 subjects in this study received propofol as their primary sedative while intubated; however, emerging literature suggests that dexmedetomidine may decrease impairment of capillary convective blood flow and diffusive capacity.39,40
Dexmedetomidine is a selective α2-adrenergic receptor agonist that affects endothelium-dependent vascular reactivity leading to an indirect vasodilatation through the reduction of central sympathetic activity, decreased peripheral release of norepinephrine, and increased local nitric oxide production.41
The relationship between microcirculatory impairment and perfusion-related clinical changes remains controversial. Lactic acidosis after cardiac surgery has been well- described, occurring in approximately 10% to 20% of patients and is associated with increased morbidity and mortality.42 The cause for postcardiopulmonary bypass lactic acidosis remains unclear, with a number of contributing etiologies that include tissue hypoxia, hyperglycemia, exposure to catecholamines, cardiopulmonary bypass, and mitochondrial dysfunction.43–45 Elevated lactate levels have been previously associated with changes in microcirculatory function, although this cohort appeared to have lactate levels that were much higher than those in previous studies,3,8,46 Lactate levels were inversely related to PVD, with the highest lactate levels found in patients with the most significantly impaired capillary blood flow (Fig 4). While this study did not allow for a causal relationship to be made, perfusion impairments can clearly result in hypoxic hyperlactatemia. Further investigation of the relationship between microcirculatory alterations and lactate kinetics needs to be completed, to better understand the relationship between the microcirculation and postoperative lactate evolution.
Microcirculatory impairment has been associated with organ injury in patients with hemorrhagic, cardiogenic, and septic shock.47–49 The authors observed a progressive decline in PVD that was associated with an increased early SOFA score as well as postoperative lactate levels. Patients with the lowest PVD had both high lactate levels and increased SOFA scores. The authors hypothesized that the severity of early microcirculatory impairments reflects clinically relevant microvascular injury and impaired tissue oxygenation that lead to end-organ injury if left unaddressed. A resuscitation strategy that focuses primarily on catecholamines, without concomitant vasodilator therapy, may produce an unbalanced resuscitation toward excessive precapillary vasoconstriction leading to impaired tissue perfusion despite acceptable clinical end points.
Limitations
This study had several limitations. First, the observational nature of this study and smaller sample size hindered the ability to point to any direct effect of individual resuscitation efforts on clinical outcomes. This study was powered to find changes in microcirculatory function and extreme differences in SOFA score, which is a composite organ injury score. The authors acknowledge that the smaller sample size reduced the ability to differentiate between specific organ injuries within the SOFA score itself. Second, intraoperative microcirculation assessment would have helped better describe the dynamic changes that occurred after individual clinical interventions during the entire course of the patient’s critical illness. This research study focused on the presence and relationship of early microcirculatory changes after surgery with clinical outcomes, but it did not assess for persistent microvascular dysfunction, which may also contribute to postoperative organ injury.47,50,51 Third, changes in convective flow were measured using vessel-by-vessel MFI, which is a more precise estimate compared with quadrant-based MFI measurement, but it is important to note that MFIv is only a semiquantitative measurement of capillary blood flow. Lastly, comparisons of microcirculation analysis between previous studies remain challenging as advancement in handheld videomicroscopy technology has improved significantly during the past 2 decades. Current generation IDF technology has allowed more detailed visualization of capillary density and function compared with previous orthogonal polarization spectral imaging and side stream dark field imaging.
Conclusion
In patients undergoing cardiac surgery, there was a significant postoperative decrease in both microcirculatory convective blood flow and diffusive capacity during early postoperative resuscitation. Severe derangements in capillary blood flow, specifically perfused vessel density, are associated with increases in postoperative lactate levels, peak lactate levels, and acute organ injury. Additional studies are required to investigate the relationship between alterations of the functional microcirculation and clinical outcomes after cardiac surgery.
Acknowledgments
We are extremely grateful to the subjects who took part in the presented study, support from the Abramson Emergency Medicine and Critical Care Research Fund, the ESICM Next Fellowship, which enabled the microcirculation research mentorship experience, and the University of Pennsylvania’s Center for Resuscitation Science for its continued research support and guidance.
Footnotes
Conflict of Interest
None. None of the authors has received any financial support or has a conflict of interest related to Cytocam or Braedius, BV.
References
- 1.Maganti MD, Rao V, Borger MA, et al. Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation 2005;112:I448–52. [DOI] [PubMed] [Google Scholar]
- 2.Cremer J, Martin M, Redl H, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996;61:1714–20. [DOI] [PubMed] [Google Scholar]
- 3.De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: Effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg 2009;88:1396–403. [DOI] [PubMed] [Google Scholar]
- 4.Veenstra G, Ince C, Barendrecht BW, et al. Differences in capillary recruitment between cardiac surgery and septic patients after fluid resuscitation. Microvasc Res 2019;123:14–8. [DOI] [PubMed] [Google Scholar]
- 5.Dekker NAM, Veerhoek D, Koning NJ, et al. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019;74:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atasever B, Boer C, Goedhart P, et al. Distinct alterations in sublingual microcirculatory blood flow and hemoglobin oxygenation in on-pump and off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011;25:784–90. [DOI] [PubMed] [Google Scholar]
- 7.Aykut G, Veenstra G, Scorcella C, et al. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp 2015;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koning NJ, Simon LE, Asfar P, et al. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2014;307:H967–75. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchings S, Watts S, Kirkman E. The Cytocam video microscope. A new method for visualising the microcirculation using Incident Dark Field technology. Clin Hemorheol Microcirc 2016;62:261–71. [DOI] [PubMed] [Google Scholar]
- 11.Massey MJ, Larochelle E, Najarro G, et al. The microcirculation image quality score: Development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care 2013;28:913–7. [DOI] [PubMed] [Google Scholar]
- 12.Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med 2018;44:281–99. [DOI] [PubMed] [Google Scholar]
- 13.Hessler M, Arnemann PH, Zamit F, et al. A new complimentary web-based tool for manual analysis of microcirculation videos: Validation of the Capillary Mapper against the current gold standard AVA 3.2. Microcirculation 2018;25:e12505. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793–800. [DOI] [PubMed] [Google Scholar]
- 15.Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009;32:35–9. [DOI] [PubMed] [Google Scholar]
- 16.Schoe A, Bakhshi-Raiez F, de Keizer N, et al. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol 2020;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domizi R, Damiani E, Scorcella C, et al. Association between sublingual microcirculation, tissue perfusion and organ failure in major trauma: A subgroup analysis of a prospective observational study. PLoS One 2019;14:e0213085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood JC, Jang DH, Hallisey SD, et al. Zenodo Data Repos. Available at: 10.5281/zenodo.3482958. [DOI]
- 19.Koning NJ, Vonk ABA, van Barneveld LJ, et al. Pulsatile flow during cardiopulmonary bypass preserves postoperative microcirculatory perfusion irrespective of systemic hemodynamics. J Appl Physiol (1985) 2012;112:1727–34. [DOI] [PubMed] [Google Scholar]
- 20.Schmid FX, Floerchinger B, Vudattu NK, et al. Direct evidence of endothelial injury during cardiopulmonary bypass by demonstration of circulating endothelial cells. Perfusion 2006;21:133–7. [DOI] [PubMed] [Google Scholar]
- 21.Ekeström S, Koul BL, Sonnenfeld T. Decreased red cell deformability following open-heart surgery. Scand J Thorac Cardiovasc Surg 1983;17:41–4. [DOI] [PubMed] [Google Scholar]
- 22.Hirai S Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg 2003;9:365–70. [PubMed] [Google Scholar]
- 23.Dekker NAM, Veerhoek D, van Leeuwen ALI, et al. Microvascular alterations during cardiac surgery using a heparin or phosphorylcholine-coated circuit. J Cardiothorac Vasc Anesth 2020;34:912–9. [DOI] [PubMed] [Google Scholar]
- 24.O’Neil MP, Fleming JC, Badhwar A, et al. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: Microcirculatory and systemic effects. Ann Thorac Surg 2012;94:2046–53. [DOI] [PubMed] [Google Scholar]
- 25.Edul VSK, Enrico C, Laviolle B, et al. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012;40:1443–8. [DOI] [PubMed] [Google Scholar]
- 26.Trzeciak S, McCoy JV, Phillip Dellinger R, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 2008;34:2210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai Y, Chu Y, Wu H, et al. High-dose nitroglycerin administered during rewarming preserves erythrocyte deformability in cardiac surgery with cardiopulmonary bypass [e-pub ahead of print]. Microcirculation 2020:e12608 10.1111/micc.12608. Accessed May 10, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Aya HD, Cecconi M, Hamilton M, et al. Goal-directed therapy in cardiac surgery: A systematic review and meta-analysis. Br J Anaesth 2013;110:510–7. [DOI] [PubMed] [Google Scholar]
- 29.Johnston LE, Thiele RH, Hawkins RB, et al. Goal-directed resuscitation following cardiac surgery reduces acute kidney injury: A quality initiative pre-post analysis. J Thorac Cardiovasc Surg 2020;159:1868–77. [DOI] [PubMed] [Google Scholar]
- 30.Maier S, Hasibeder WR, Hengl C, et al. Effects of phenylephrine on the sublingual microcirculation during cardiopulmonary bypass. Br J Anaesth 2009;102:485–91. [DOI] [PubMed] [Google Scholar]
- 31.Nygren A, Thorén A, Ricksten SE. Vasopressors and intestinal mucosal perfusion after cardiac surgery: Norepinephrine vs phenylephrine. Crit Care Med 2006;34:722–9. [DOI] [PubMed] [Google Scholar]
- 32.van Loon LM, Stolk RF, van der Hoeven JG, et al. Effect of vasopressors on the macro- and microcirculation during systemic inflammation in humans in vivo. Shock 2020;53:171–4. [DOI] [PubMed] [Google Scholar]
- 33.Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA 2017;318:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linton NWF, Linton RAF. Haemodynamic response to a small intravenous bolus injection of epinephrine in cardiac surgical patients. Eur J Anaesthesiol 2005;20:298–304. [DOI] [PubMed] [Google Scholar]
- 35.Royster RL, Butterworth JF, Prielipp RC, et al. A randomized, blinded, placebo-controlled evaluation of calcium chloride and epinephrine for inotropic support after emergence from cardiopulmonary bypass. Anesth Analg 1992;74:3–13. [DOI] [PubMed] [Google Scholar]
- 36.Sakka SG, Hofmann D, Thuemer O, et al. Increasing cardiac output by epinephrine after cardiac surgery: Effects on indocyanine green plasma disappearance rate and splanchnic microcirculation. J Cardiothorac Vasc Anesth 2007;21:351–6. [DOI] [PubMed] [Google Scholar]
- 37.Günnicker M, Brinkmann M, Donovan T, et al. The efficacy of amrinone or adrenaline on low cardiac output following cardiopulmonary bypass in patients with coronary artery disease undergoing preoperative -blockade. Thorac Cardiovasc Surg 1995;43:153–60. [DOI] [PubMed] [Google Scholar]
- 38.Totaro RJ, Raper RF. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med 1997;25:1693–9. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Zhang K, Wang W, et al. Dexmedetomidine versus propofol sedation improves sublingual microcirculation after cardiac surgery: A randomized controlled trial. J Cardiothorac Vasc Anesth 2016;30:1509–15. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed H, Hosny H, Tawadros Md P, et al. Effect of dexmedetomidine infusion on sublingual microcirculation in patients undergoing on-pump coronary artery bypass graft surgery: A prospective randomized trial. J Cardiothorac Vasc Anesth 2019;33:334–40. [DOI] [PubMed] [Google Scholar]
- 41.Miranda ML, Balarini MM, Bouskela E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Surv Anesthesiol 2015;59:258–9. [DOI] [PubMed] [Google Scholar]
- 42.Ranucci M, De Toffol B, Isgrò G, et al. Hyperlactatemia during cardiopulmonary bypass: Determinants and impact on postoperative outcome. Crit Care 2006;10:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raper RF, Cameron G, Walker D, et al. Type B lactic acidosis following cardiopulmonary bypass. Crit Care Med 1997;25:46–51. [DOI] [PubMed] [Google Scholar]
- 44.Sundin M, Almeida J, Osawa E, et al. Early lactate-guided therapy in cardiac surgery patients: A randomized controlled trial. Crit Care 2014;18: P170. [Google Scholar]
- 45.Maillet JM, Le Besnerais P, Cantoni M, et al. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest 2003;123:1361–6. [DOI] [PubMed] [Google Scholar]
- 46.Yeh YC, Wang MJ, Chao A, et al. Correlation between early sublingual small vessel density and late blood lactate level in critically ill surgical patients. J Surg Res 2013;180:317–21. [DOI] [PubMed] [Google Scholar]
- 47.Hutchings SD, Naumann DN, Hopkins P, et al. Microcirculatory impairment is associated with multiple organ dysfunction following traumatic hemorrhagic shock: The MICROSHOCK Study. Crit Care Med 2018;46:e889–96. [DOI] [PubMed] [Google Scholar]
- 48.Wijntjens GW, Fengler K, Fuernau G, et al. Prognostic implications of microcirculatory perfusion vs macrocirculatory perfusion in cardiogenic shock: A CULPRIT-SHOCK substudy. Eur Heart J Acute Cardiovasc Care 2020;9:108–19. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez G, Boerma EC, Dubin A, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care 2013;28:538–614. [DOI] [PubMed] [Google Scholar]
- 50.Fiorese Coimbra KT, de Freitas FGR, Bafi AT, et al. Effect of increasing blood pressure with noradrenaline on the microcirculation of patients with septic shock and previous arterial hypertension. Crit Care Med 2019;47:1033–40. [DOI] [PubMed] [Google Scholar]
- 51.Spronk PE, Ince C, Gardien MJ, et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002;360:1395–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the conclusions of this article is available via the Zenodo research data repository.18


