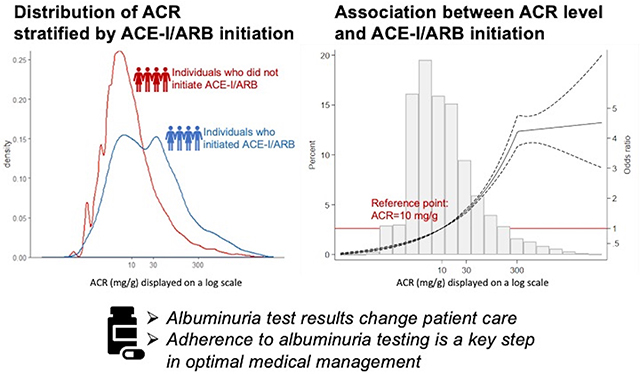
Abstract
Multiple clinical guidelines recommend an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB) in patients with elevated albuminuria, which can be measured through urine albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), or dipstick. However, how albuminuria test results relate to the prescription of ACE-I/ARB is uncertain. We identified individuals with an ACR measurement between January 1, 2004 and June 30, 2018, and no contraindications or allergy to ACE-I/ARB. We performed multivariable logistic regression analyses to evaluate the association between ACR level and prescription of ACE-I/ARB within six months after the test. We applied similar methods to investigate the association of PCR and dipstick measurement results with the prescription of ACE-I/ARB. Among 67,237 individuals with an ACR measurement, 47.7% were already taking an ACE-I or ARB at the time of first ACR measurement. Among the 35,138 individuals who were not on ACE-I/ARB, those with higher ACR levels were more likely to be prescribed ACE-I/ARB in the following 6 months, with steep increases in prescriptions until ACR 300 mg/g, after which the association plateaued. The majority (80.9%) of ACE-I/ARB prescriptions were made by family medicine and internal medicine. A similar pattern held in the cohorts tested by PCR and dipstick measurement. Our study provides evidence that albuminuria test results change patient care, suggesting that adherence to albuminuria testing is a key step in optimal medical management.
Keywords: albuminuria, urine albumin-to-creatinine ratio, urine protein-to-creatinine ratio, antihypertensive, angiotensin converting enzyme inhibitors, angiotensin receptor blockers
Graphical Abstract
Introduction
Albuminuria, defined as a urine albumin-to-creatinine ratio (ACR) ≥ 30 mg/g, is a pathologic condition which reflects kidney damage.1–3 Higher levels of albuminuria are associated with increased risks of adverse kidney events such as end-stage kidney disease, decline in eGFR, and acute kidney injury.4–6 Additionally, higher levels of albuminuria are associated with greater risks of hypertension, cardiovascular events, and all-cause mortality.4,5,7–11 Multiple clinical guidelines recommend the use of an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB) in patients with elevated albuminuria,1,12,13 as these medications provide cardio- and kidney-protective effects, particularly in the presence of albuminuria.1,12,14–19 For example, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggest use of an ACE-I or ARB as an antihypertensive agent “in diabetic adults with chronic kidney disease (CKD) and urine albumin excretion 30–300 mg/24 hours” and “in both diabetic and non-diabetic adults with CKD and urine albumin excretion > 300 mg/24 hours”.1 Similarly, the American Diabetes Association suggests that “in nonpregnant patients with diabetes and hypertension, either an ACE inhibitor or an angiotensin receptor blocker is recommended for those with modestly elevated urinary albumin-to-creatinine ratio (30–299 mg/g creatinine) and is strongly recommended for those with urinary albumin-to-creatinine ratio ≥ 300 mg/g creatinine”.20
In part due to these recommendations, testing for albuminuria is suggested for people at higher risk of CKD including those with hypertension, diabetes, or 65 years of age or older.21,22 Urine ACR is considered the preferred method of measuring albuminuria, followed by urine protein-to-creatinine ratio (PCR), and urine protein dipstick measurement.1 Urine ACR between 30 and 300 mg/g and PCR between 150 and 500 mg/g are considered “moderately increased” albuminuria; ACR above 300 mg/g and PCR above 500 mg/g are considered “severely increased” albuminuria.1 We previously reported that patients who received testing for albuminuria had a lower risk of ACE-I/ARB discontinuation.23 However, how the results of albuminuria testing relate to the prescription of ACE-I/ARB therapy is uncertain.
Using data from a large, integrated health care system, we identified individuals who received an ACR measurement and studied the association of ACR level with the utilization of ACE-I/ARB. We hypothesized that higher levels of ACR would be associated with higher odds of ACE-I/ARB initiation, supporting the notion that adherence to albuminuria testing guidelines can change management. To discern specific patterns for different albuminuria measurement methods, we also assessed the associations of PCR and dipstick measurement results with the prescription of ACE-I/ARB.
Methods
Summary data that support the findings of this study are available from the corresponding authors upon request.
Study setting and study population
We identified a real-world cohort of individuals who received testing for albuminuria using data from Geisinger, an integrated health system serving 45 counties across central and northeastern Pennsylvania. The electronic health records of the system provide data on patient demographic characteristics, inpatient and outpatient encounters, problem lists, outpatient prescriptions, and laboratory test results.
We identified 83,807 individuals who received an ACR measurement between January 1, 2004 and June 30, 2018. Time of the first ACR measurement was considered the baseline date for each individual. We excluded individuals with any record of allergy to ACE-I/ARB at the time of the ACR measurement (n=3,285). Next, we excluded individuals whose systolic blood pressure was missing or below 100 mmHg based on the latest outpatient measure prior to the time of the ACR measurement (n=4,309), and those with the latest outpatient serum potassium above 5 mEq/L or no serum potassium measured at or before the time of the ACR measurement (n=6,893). Further exclusion criteria included end-stage kidney disease (n=318), pregnancy within the preceding year (n=1,294), age < 18 years (n=435), and no serum creatinine measurement by the time of the ACR measurement (n=36; Figure S1).
Exposure
The primary exposure of interest was ACR, which we log-transformed with a base of 2. To allow for a potentially non-linear relationship, we used linear spline forms of log-transformed ACR with knots corresponding to ACR values at 30 and 300 mg/g.
Outcomes
Prevalent use of ACE-I/ARB was defined as any record of ACE-I/ARB use during the three-month period prior to the ACR measurement. Among individuals who were not prevalent users of ACE-I/ARB, we investigated new prescription of ACE-I/ARB initiation within six months after the ACR measurement.
Baseline covariates
Systolic blood pressure, serum potassium, and serum creatinine at the time of the ACR testing were defined as the most recent antecedent outpatient measure. Glomerular filtration rate (GFR) was estimated using serum creatinine accounting for age, sex, and race based on the CKD Epidemiology Collaboration equation.24 We used linear spline forms of eGFR with knots at 30 and 60 mL/min/1.73 m2. We ascertained baseline comorbidities such as diabetes, congestive heart failure, myocardial infarction, and hypertension through the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes (Table S1). Data regarding statin, thiazide, calcium channel blocker, and beta blocker use at the time of the ACR testing were obtained from medication records. We also obtained demographic characteristics such as age, sex, and race/ethnicity. Calendar year at the time of the ACR testing was categorized into 2004–2008, 2009–2013, and 2014–2018.
Statistical analyses
Baseline characteristics of the study population were described using median (interquartile range) for ACR, mean (standard deviation [SD]) for other continuous variables, and count (percentage) for categorical variables. We performed multivariable logistic regression to assess the association of ACR level and other covariates with the prevalent use of ACE-I/ARB at the time of the ACR measurement.
Among individuals not on ACE-I/ARB at the time of the ACR test, we used kernel density curves to depict the distribution of log-transformed ACR levels stratified by ACE-I/ARB initiation status. We then performed multivariable logistic regression analyses to evaluate the association between ACR level and ACE-I/ARB initiation within six months of the ACR test, adjusting for baseline age, sex, race/ethnicity, calendar year of the ACR test, systolic blood pressure, serum potassium, eGFR, diabetes, congestive heart failure, myocardial infarction, hypertension, use of statin, thiazide, calcium channel blocker, and beta blocker.
Finally, among individuals who initiated ACE-I/ARB within 6 months of the ACR measurement, we assessed the proportion prescribed by providers of different specialties.
Additional analyses
We investigated associations of PCR and dipstick test result with ACE-I/ARB prescription. We applied similar inclusion criteria as described above and identified a cohort of 20,104 adults who received their first PCR measurement between January 1, 2004 and June 30, 2018, and 141,914 adults who received their first dipstick measurement during the same period. PCR was also log-transformed with a base of 2 and it was modeled using spline terms with a knot corresponding to PCR at 500 mg/g given the shape of the association as well as the clinical use of this threshold. Dipstick measurement result was treated as a categorical variable with “++ or above”, “+”, and “trace or negative” as the reference category. A similar analytic procedure was performed for each of these cohorts.
For all analyses, statistical significance was evaluated at a significance level of 0.05 based on two-sided testing. SAS software, version 9.4 (SAS Institute Inc), Stata, version 15.1 (StataCorp LLC), and R, version 3.6.0 (R Foundation for Statistical Computing) were used for statistical analyses.
This study was approved by institutional review boards at the Johns Hopkins Bloomberg School of Public Health and Geisinger Medical Center. All data were deidentified, and consent was waived.
Results
Study population
There were a total of 67,237 individuals who had an ACR measurement and met the inclusion criteria (Table 1). Mean (SD) age of the cohort was 60.5 (14.9) years and 33,696 (50.1%) were female. The majority of the cohort had a clinical diagnosis of diabetes (n=36,722, 54.6%) or hypertension (n=49,530, 73.7%). Mean (SD) eGFR was 81 (24.1) mL/min/1.73 m2, and 14,189 (21.1%) individuals had an eGFR below 60 mL/min/1.73 m2 at the time of the ACR test. Median (interquartile range) ACR was 9 (4–26) mg/g. ACR ranged between 30 and 300 mg/g for 12,522 (18.6%) individuals and was above 300 mg/g for 2,927 (4.4%) individuals.
Table 1.
Characteristics of the patient cohort who received urine ACR measurement
| Patient characteristics | Overall | Baseline use of ACE-I/ARB |
|
|---|---|---|---|
| Yes | No | ||
| Total number, N | 67237 | 32099 | 35138 |
| Urine ACR, median (IQR), mg/g | 9 (4–26) | 10 (4–29) | 8 (4–23) |
| Age, mean (SD), years | 60.5 (14.9) | 62.7 (13.6) | 58.4 (15.7) |
| Systolic blood pressure, mean (SD), mmHg | 130 (16.4) | 131 (16.8) | 129 (15.9) |
| Potassium, mean (SD), mEq/L | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) |
| eGFR, mean (SD), mL/min/1.73m2 | 81 (24.1) | 78 (23.2) | 84 (24.4) |
| Female sex, N (%) | 33696 (50.1) | 15171 (47.3) | 18525 (52.7) |
| Black race, N (%) | 2526 (3.8) | 1190 (3.7) | 1336 (3.8) |
| Diabetes, N (%) | 36722 (54.6) | 17853 (55.6) | 18869 (53.7) |
| Congestive heart failure, N (%) | 4769 (7.1) | 2969 (9.3) | 1800 (5.1) |
| Hypertension, N (%) | 49530 (73.7) | 29979 (93.4) | 19551 (55.6) |
| Myocardial infarction, N (%) | 4073 (6.1) | 2604 (8.1) | 1469 (4.2) |
| Thiazide diuretics, N (%) | 17623 (26.2) | 11646 (36.3) | 5977 (17.0) |
| Calcium channel blockers, N (%) | 11504 (17.1) | 7152 (22.3) | 4352 (12.4) |
| Beta blockers, N (%) | 23270 (34.6) | 12990 (40.5) | 10280 (29.3) |
| Statin, N (%) | 31235 (46.5) | 18173 (56.6) | 13062 (37.2) |
| Calendar year, N (%) | |||
| 2004–2008 | 16719 (24.9) | 6785 (21.1) | 9934 (28.3) |
| 2009–2013 | 20898 (31.1) | 10192 (31.8) | 10706 (30.5) |
| 2014–2018 | 29620 (44.1) | 15122 (47.1) | 14498 (41.3) |
Abbreviations: ACR, albumin-to-creatinine ratio; IQR: interquartile range; SD, standard deviation; ACE-I/ARB: angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; eGFR: estimated glomerular filtration rate
Association between ACR level and prevalent use of ACE-I/ARB
A total of 32,099 (47.7%) individuals were on ACE-I/ARB therapy at the time of the initial ACR test. Individuals who were taking ACE-I/ARB therapy were more likely to have higher ACR (odds ratio (OR) per two-fold higher level: 1.11 [95% confidence interval (CI): 1.10–1.12]), serum potassium (OR per mEq/L higher level: 1.45 [95% CI: 1.38–1.52]), systolic blood pressure (OR per 10-mmHg higher level: 1.13 [95% CI: 1.12–1.14]), and eGFR (OR per 5 mL/min/1.73 m2 higher level: 1.90 [95% CI: 1.70–2.12] for eGFR below 30 mL/min/1.73 m2; 1.02 [95% CI: 1.01–1.04] for eGFR between 30 and 60 mL/min/1.73 m2; and 1.01 [95% CI: 1.00–1.02] for eGFR above 60 mL/min/1.73 m2). Additionally, ACE-I/ARB use at the time of the test was positively associated with the presence of diabetes (OR: 1.17 [95% CI: 1.13–1.21]), congestive heart failure (OR: 1.11 [95% CI: 1.04–1.19]), hypertension (OR: 3.77 [95% CI: 3.59–3.95]), statin use (OR: 1.46 [95% CI: 1.41–1.51]), thiazide use (OR: 1.61 [95% CI: 1.55–1.67]), and black race (OR: 1.23 [95% CI: 1.12–1.34]), and negatively associated with beta blocker use (OR: 0.83 [95% CI: 0.80–0.86]), older age (OR per 10-year higher age: 0.94 [95% CI: 0.92–0.95]), female sex (OR: 0.94 [95% CI: 0.91–0.98]), and more recent years (OR: 0.88 [95% CI: 0.84–0.92] and 0.86 [95% CI: 0.82–0.89] for year 2009–2013 and 2014–2018, respectively, compared with year 2004–2008).
Association between ACR level and initiation of ACE-I/ARB
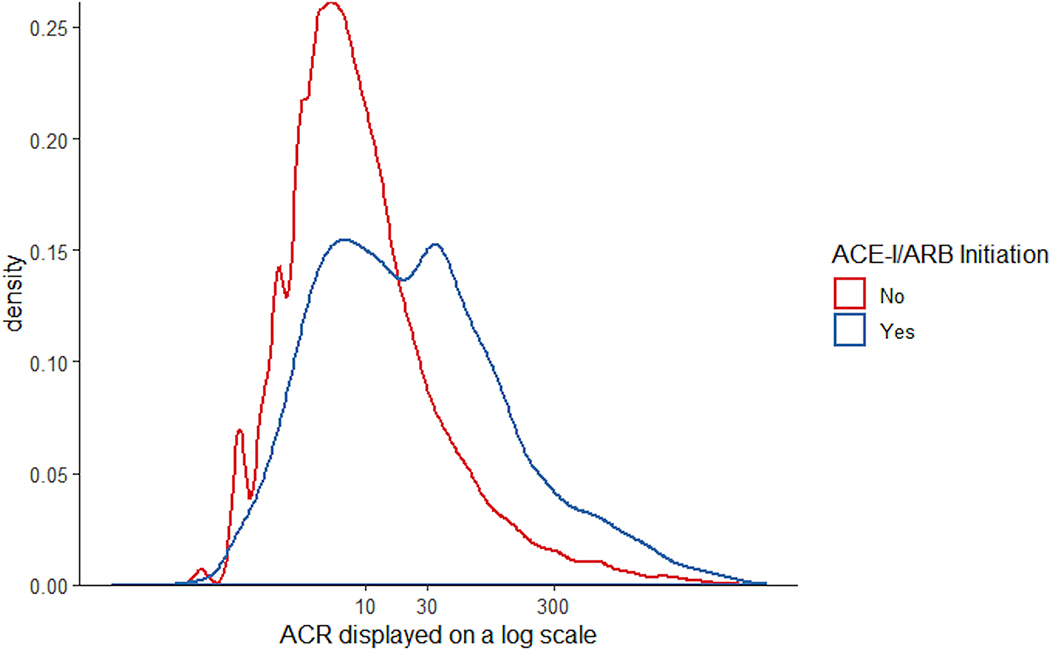
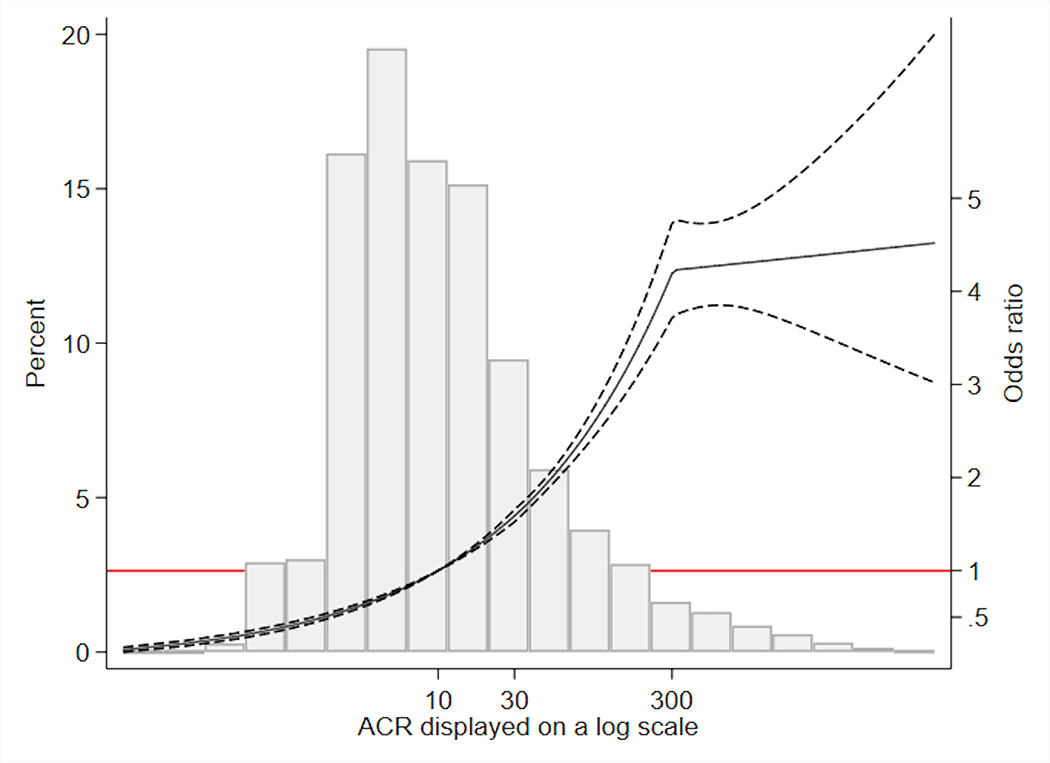
Among the 35,138 individuals who were not on ACE-I/ARB at the time of the test, 6,514 (18.5%) initiated ACE-I/ARB within 6 months. The proportion who initiated an ACE-I/ARB was 13.6%, 35.6%, and 43.1% among individuals with ACR <30, 30–300, and >300 mg/g, respectively. Among the 4,621 individuals with diabetes and ACR above 30 mg/g, 1,889 (40.9%) initiated ACE-I/ARB. Among the 426 individuals with ACR above 300 mg/g and no diabetes, 162 (38.0%) initiated ACE-I/ARB. Those who initiated ACE-I/ARB had a distribution of ACR more skewed toward higher levels than those who did not (Figure 1). Multivariable logistic regression showed significantly higher odds of initiating ACE-I/ARB with higher levels of ACR for ACR below 30 mg/g (OR per two-fold higher ACR: 1.33 [95% CI: 1.30–1.37]) and between 30 and 300 mg/g (OR: 1.34 [95% CI: 1.29–1.40]), but no significant difference associated with further increase in ACR above 300 mg/g (OR: 1.01 [95% CI: 0.93–1.10]; Figure 2). Additionally, ACE-I/ARB initiation within six months of the ACR measurement was positively associated with higher serum potassium, systolic blood pressure, eGFR, statin use, thiazide use, black race, the presence of diabetes, and hypertension, and was negatively associated with beta blocker use, calcium channel blocker use, previous diagnosis of congestive heart failure, older age, female sex, and more recent years (Table 2). Among those who initiated ACE-I/ARB, 80.9% were prescribed by family medicine and internal medicine, 1.8% by endocrinologists, 1.5% by cardiologists, 1.4% by nephrologists, 1.0% by emergency medicine, and the remaining 13.4% by providers of other specialties.
Figure 1. Distribution of ACR stratified by ACE-I/ARB initiation within six months of the ACR test among individuals not on ACE-I/ARB at the time of the test (N=35,138), displayed on a log scale.
Abbreviations: ACR, albumin-to-creatinine ratio; ACE-I/ARB: angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker
Figure 2. Association* between ACR and initiation of ACE-I/ARB among individuals not on ACE-I/ARB at the time of the ACR test (N=35,138).
*Reference point at ACR 10 mg/g; adjusted for baseline age, sex, race/ethnicity, calendar year, systolic blood pressure, serum potassium, estimated glomerular filtration rate, diabetes, congestive heart failure, myocardial infarction, hypertension, use of statin, thiazide, calcium channel blocker, and beta blocker
Abbreviations: ACR, albumin-to-creatinine ratio; ACE-I/ARB: angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker
Table 2.
Associations of patient characteristics with the initiation of ACE-I/ARB among individuals not on ACE-I/ARB at the time of the ACR test (N=35,138)
| Patient characteristics | Odds ratio (95% CI) |
|---|---|
| Urine ACR, per two-fold higher | |
| ACR below 30 mg/g | 1.33 (1.30–1.37) |
| ACR between 30 and 300 mg/g | 1.34 (1.29–1.40) |
| ACR above 300 mg/g | 1.01 (0.93–1.10) |
| Age, per 10-year higher | 0.86 (0.84–0.88) |
| Female sex | 0.89 (0.83–0.94) |
| Black race | 1.48 (1.29–1.70) |
| Systolic blood pressure, per 10-mmHg higher | 1.32 (1.30–1.35) |
| Potassium, per mEq/L higher | 1.24 (1.15–1.35) |
| eGFR, per 5-mL/min/1.73 m2 higher | |
| eGFR below 30 mL/min/1.73 m2 | 2.06 (1.63–2.60) |
| eGFR between 30 and 60 mL/min/1.73 m2 | 1.09 (1.05–1.12) |
| eGFR above 60 mL/min/1.73 m2 | 1.02 (1.01–1.03) |
| Diabetes | 1.46 (1.38–1.56) |
| Congestive heart failure | 0.80 (0.69–0.93) |
| Hypertension | 1.95 (1.82–2.10) |
| Myocardial infarction | 0.92 (0.79–1.08) |
| Thiazide diuretics | 1.40 (1.30–1.52) |
| Calcium channel blockers | 0.89 (0.81–0.97) |
| Beta blockers | 0.82 (0.77–0.89) |
| Statin | 1.33 (1.25–1.42) |
| Calendar year, 2004–2008 as reference | |
| 2009–2013 | 0.78 (0.73–0.84) |
| 2014–2018 | 0.60 (0.56–0.64) |
Abbreviations: ACE-I/ARB: angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; ACR, albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate; CI, confidence interval
Results in cohorts with PCR and dipstick measurement
A total of 20,104 individuals with a PCR measurement and 141,914 individuals with a dipstick measurement met the inclusion criteria (Table S2, S3). Compared with the cohort tested with ACR, prevalence of diabetes was lower in the PCR and dipstick cohorts (33.4% and 15.3% vs. 54.6%). In contrast, the proportion with eGFR below 60 mL/min/1.73 m2 was the highest among those tested by PCR (57.2%), followed by ACR (21.1%), and dipstick (12.6%). The majority had hypertension among those tested by PCR (79.3%) and ACR (73.7%), but less than half had hypertension by the time of the initial dipstick measurement (46.9%).
Among the 20,104 individuals who had a PCR measurement, median (interquartile range) PCR was 110 (68–260) mg/g, and 10437 (51.9%) individuals were on ACE-I/ARB therapy at the time of the initial PCR test. Individuals who were taking ACE-I/ARB therapy were more likely to have higher PCR only for those with PCR above 500 mg/g (OR per two-fold higher PCR: 1.07 [95% CI: 1.02–1.12]), adjusting for baseline covariates. Interestingly, there was an inverse association between higher levels of PCR and prevalent use of ACE-I/ARB for PCR below 500 mg/g (OR per two-fold higher PCR: 0.94 [95% CI: 0.91–0.97]).
Of the 9,667 individuals who were not on ACE-I/ARB at the time of the PCR test, 912 (9.4%) initiated ACE-I/ARB within 6 months. Individuals who initiated ACE-I/ARB within 6 months of the PCR test had a distribution of PCR levels that was more skewed toward the higher range, compared with those did not initiate ACE-I/ARB (Figure S2). Higher PCR was independently associated with a significantly higher odds of ACE-I/ARB initiation (OR per two-fold increase in PCR: 1.47 [95% CI: 1.36–1.58] for PCR below 500 mg/g and 1.35 [95% CI: 1.23–1.48] for PCR above 500 mg/g; Figure S3). Among those who initiated ACE-I/ARB, 45.8% were prescribed by family medicine and internal medicine, 29.6% by nephrologists, 2.4% by cardiologists, 1.9% by emergency medicine, 0.9% by endocrinologists, and the remaining 19.4% by providers of other specialties.
Among the 141,914 patients who received a dipstick measurement, 39,664 (27.9%) individuals were on ACE-I/ARB therapy at the time of the initial dipstick measurement. Compared with a negative dipstick test result, having a result of “+”was negatively associated with prevalent ACE-I/ARB use (OR: 0.95 [95% CI: 0.91–0.99]); whereas a result of “++ or above” was not associated with a significant difference in prevalent ACE-I/ARB use (OR: 1.09 [95% CI: 0.99–1.21]).
Among the 102,250 individuals who were not on ACE-I/ARB at the time of the dipstick measurement, 5,234 (5.1%) initiated ACE-I/ARB within 6 months. Compared with a negative test result, both results of “+” (OR: 1.25 [95% CI: 1.16–1.36]) and “++ or above” (OR: 1.54 [95% CI: 1.29–1.84]) were independently associated with a higher odds of ACE-I/ARB initiation within 6 months of the test. Among those who initiated ACE-I/ARB, 78.5% were prescribed by family medicine and internal medicine, 3.4% by cardiologists, 2.9% by nephrologists, 1.0% by emergency medicine, 0.6% by endocrinologists, and the remaining 13.6% by providers of other specialties.
Discussion
Our study investigated the utilization of ACE-I/ARB in a large, real-world patient cohort who were tested for albuminuria. Almost half were already on ACE-I/ARB at the time of the initial ACR test. Those on ACE-I/ARB at the time of the test were more likely to have hypertension, diabetes, and congestive heart failure, which is not surprising given established benefits of ACE-I/ARB for these conditions.1,12,14,15,17 Interestingly, older individuals were less likely to be on ACE-I/ARB at the time of the test. This finding was consistent with previous studies that observed underutilization of ACE-I/ARB among older adults.25,26 Among individuals not already taking ACE-I/ARB at the time of the albuminuria test, higher ACR was positively associated with initiating ACE-I/ARB in the subsequent six months. Interestingly, the association plateaued for ACR levels above 300 mg/g; in other words, although an individual with ACR 1000 mg/g was much more likely to be prescribed an ACE-I/ARB than someone whose test showed ACR 29 mg/g, the individual was not more likely to be prescribed an ACE-I/ARB than someone with ACR 300 mg/g. In comparison, higher PCR was positively associated with ACE-I/ARB initiation for PCR levels both above and below 500 mg/g. This observation may reflect the fact that ACR above 300 mg/g is an unambiguous indication for prescribing ACE-I/ARB, with no difference in guidance with respect to ACE-I/ARB prescription once ACR passes the threshold of 300 mg/g. In comparison, the conversion of PCR to ACR is approximate, with less clear guidance on when to initiate an ACE-I/ARB, which may explain a positive association between PCR levels and ACE-I/ARB initiation across the whole range of PCR. Alternatively, it may reflect the specialty of the provider who managed the patients. Nephrologists may be more likely to both order PCR measurement to test for albuminuria and to prescribe ACE-I/ARB therapy. For example, among individuals who were prescribed ACE-I/ARB following a PCR measurement, 29.6% received prescriptions from nephrologists, compared with 1.4% among those who initiated ACE-I/ARB following an ACR measurement. Similarly, cardiologists’ prescription of ACE-I/ARB therapy was higher in patients with PCR measured compared to ACR.
This study suggests that providers do follow clinical guidelines recommending ACE-I/ARB use in individuals with elevated albuminuria. However, in this cohort of individuals without recorded contraindications to ACE-I/ARB such as hyperkalemia, hypotension, or allergy to ACE-I/ARB, only 43.1% of those with ACR > 300 mg/g and only 40.9% of individuals with diabetes and ACR >30 mg/g initiated ACE-I/ARB within 6 months of the test. Underutilization of ACE-I/ARB was also observed in a previous study reporting that 54% of the diabetic patients with albuminuria received ACE-I/ARB.27 Of note, these utilization rates were obtained among individuals who were tested for albuminuria. Underutilization would likely be higher among individuals who do not undergo albuminuria testing.
While individuals with previous diagnosis of congestive heart failure were more likely to be on ACE-I/ARB at the time of the ACR measurement, those who were not on ACE-I/ARB at the time of the ACR measurement were less likely to initiate ACE-I/ARB after the ACR measurement. This may reflect selection bias: those who were not on ACE-I/ARB already may have intolerance or a contraindication to therapy. However, previous studies suggest underutilization of ACE-I/ARB among individuals with congestive heart failure may be due to fear of adverse events such as kidney function deterioration and hypotension, and insufficient appreciation of the benefits of these medications or the prognostic implications of heart failure.28–30 Our findings similarly highlight underutilization of ACE-I/ARB among individuals with elevated albuminuria. Given that the majority of the population received prescriptions from family medicine and internal medicine providers, educational efforts focused on these providers may help overcome barriers of ACE-I/ARB utilization among individuals with elevated albuminuria.
There are several limitations of this study. First, we used medication prescription records to ascertain ACE-I/ARB use. Therefore, our study results reflect the pattern in ACE-I/ARB prescription rather than the actual dispense or intake of these medications. Second, data were not captured for the drug classes of sodium-glucose cotransporter 2 inhibitors or glucagon-like peptide 1 receptor agonists, since randomized controlled trials supporting their indications in individuals with albuminuria were first published near the end of the study period. Third, the study cohort was mainly white and our findings may thus have limited generalizability to other racial and ethnicity groups. Fourth, albuminuria level was based on only one measurement. Fifth, we did not take dosage into consideration. Future studies are needed to evaluate dosage of ACE-I/ARB in real-world clinical practice to examine potential opportunities to maximize therapeutic potential. Sixth, our study was from a primarily rural setting, which may have limited access to specialty care. Therefore, the observed ACE-I/ARB utilization patterns may not generalize to urban settings. Finally, we were unable to account for socioeconomic status.
Our study assessed the association between albuminuria test results and the prescription of ACE-I/ARB in a large, integrated health system. We examined the pattern for patients with ACR, PCR, and dipstick measurement individually. Instead of categorizing ACR and PCR values into “normal to mildly increased”, “moderately increased” and “severely increased” albuminuria, we evaluated a continuous form of these variables with splines in the model to provide insights on the effect of increase in the measurement within each category while comprehensively addressing indications and contraindications for the initiation of ACE-I/ARB. Finally, we excluded people with recorded allergies to ACE-I/ARB.
Perspectives
Using data from a large, integrated health system, we found that higher levels of ACR were associated with a higher likelihood of initiating ACE-I/ARB. A similar trend was found for other methods of albuminuria testing including PCR and dipstick measurement. These results provide evidence that results from albuminuria testing change patient care, suggesting that adherence to albuminuria testing is a key step in optimal medical management.
Supplementary Material
Novelty and Significance.
What is new
Our study investigates how results of albuminuria testing relate to the prescription of renin-angiotensin system blockade.
We examined renin-angiotensin system blockade prescription patterns after different albuminuria testing methods including urine albumin-to-creatinine ratio, protein-to-creatinine ratio, and dipstick measurement.
What is relevant
Renin-angiotensin system blockade is a first-line antihypertensive and provides cardio- and kidney-protective effects in the presence of albuminuria.
Albuminuria testing is low, and some hypothesize that under-testing stems from uncertainty as to whether the results of albuminuria tests change management.
Summary
Higher levels of albuminuria were associated with a higher likelihood of renin-angiotensin system blockade prescription. This study provides evidence that results from albuminuria testing change patient care, suggesting that adherence to albuminuria testing is a key step in optimal medical management.
Acknowledgments
Sources of funding
Research reported in this publication was supported by R01 DK115534 (PI: Dr. Grams and Inker) and R01 DK100446 (PI: Dr. Grams and Coresh) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). The funding sources had no role in the design and conduct of the study, analysis, or interpretation of the data; and preparation or final approval of the manuscript prior to publication.
Footnotes
Disclosures
None
Reference
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322(13):1294–1304. doi: 10.1001/jama.2019.14745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 4.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–2360. doi: 10.1001/jama.2012.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wada T, Haneda M, Furuichi K, et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18(4):613–620. doi: 10.1007/s10157-013-0879-4 [DOI] [PubMed] [Google Scholar]
- 6.James MT, Grams ME, Woodward M, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirayama A, Konta T, Hozawa A, et al. Slight increase in urinary albumin excretion within the normal range predicts incident hypertension in a community-based Japanese population: the Takahata study. Hypertens Res. 2015;38(1):56–60. doi: 10.1038/hr.2014.117 [DOI] [PubMed] [Google Scholar]
- 8.Astor BC, Hallan SI, Miller ER, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226–1234. doi: 10.1093/aje/kwn033 [DOI] [PubMed] [Google Scholar]
- 9.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. doi: 10.1001/archinte.167.22.2490 [DOI] [PubMed] [Google Scholar]
- 10.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal N, Zelnick L, Bhat Z, et al. Burden and Outcomes of Heart Failure Hospitalizations in Adults With Chronic Kidney Disease. J Am Coll Cardiol. 2019;73(21):2691–2700. doi: 10.1016/j.jacc.2019.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Microvascular Complications and Foot Care. Diabetes Care. 2015;38(Supplement 1):S58–S66. doi: 10.2337/dc15-S012 [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol (Engl Ed). 2016;69(12):1167. doi: 10.1016/j.rec.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Ferrari R, Guardigli G, Ceconi C. Secondary Prevention of CAD with ACE Inhibitors: A Struggle Between Life and Death of the Endothelium. Cardiovasc Drugs Ther. 2010;24(4):331–339. doi: 10.1007/s10557-010-6244-x [DOI] [PubMed] [Google Scholar]
- 16.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 18.Marin R, Ruilope LM, Aljama P, et al. A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19(10):1871–1876. [DOI] [PubMed] [Google Scholar]
- 19.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 20.Association AD. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes−2020. Diabetes Care. 2020;43(Supplement 1):S135–S151. doi: 10.2337/dc20-S011 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. Albuminuria. National Kidney Foundation; https://www.kidney.org/atoz/content/albuminuria Published December 24, 2015. Accessed May 22, 2020. [Google Scholar]
- 22.American Society for Clinical Pathology. Don’t request just a serum creatinine to test adult patients with diabetes and/or hypertension for CKD; use the Kidney Profile (serum Creatinine with eGFR and urinary albumin-creatinine ratio). https://www.choosingwisely.org/clinician-lists/ascp-serum-creatinine-to-test-for-ckd/. Accessed June 30, 2020.
- 23.Qiao Y, Shin J-I, Sang Y, et al. Discontinuation of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Chronic Kidney Disease. Mayo Clin Proc. October 2019. doi: 10.1016/j.mayocp.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkelmayer WC, Fischer MA, Schneeweiss S, Wang PS, Levin R, Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46(6):1080–1087. doi: 10.1053/j.ajkd.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 26.Pappoe LS, Winkelmayer WC. ACE inhibitor and angiotensin II type 1 receptor antagonist therapies in elderly patients with diabetes mellitus: are they underutilized? Drugs Aging. 2010;27(2):87–94. doi: 10.2165/11316430-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 27.Rosen AB, Karter AJ, Liu JY, Selby JV, Schneider EC. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high-risk clinical and ethnic groups with diabetes. J Gen Intern Med. 2004;19(6):669–675. doi: 10.1111/j.1525-1497.2004.30264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed A Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine? J Am Geriatr Soc 2002;50(7):1297–1300. doi: 10.1046/j.1532-5415.2002.50321.x [DOI] [PubMed] [Google Scholar]
- 29.Houghton AR, Cowley AJ. Why are angiotensin converting enzyme inhibitors underutilised in the treatment of heart failure by general practitioners? International Journal of Cardiology. 1997;59(1):7–10. doi: 10.1016/S0167-5273(96)02904-X [DOI] [PubMed] [Google Scholar]
- 30.Bart BA, Gattis WA, Diem SJ, O’Connor CM. Reasons for Underuse of Angiotensin-Converting Enzyme Inhibitors in Patients With Heart Failure and Left Ventricular Dysfunction. The American Journal of Cardiology. 1997;79(8):1118–1120. doi: 10.1016/S0002-9149(97)00060-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.