Abstract
Aldosterone-to-renin ratio (ARR) is a screening tool for primary aldosteronism (PA), but the significance of ARR when the PA criteria are not met remains largely unknown. In this cross-sectional study we investigated the association of ARR with haemodynamic variables in 545 normotensive and never-medicated hypertensive subjects (267 men, 278 women, age range 19–72 years) without suspicion of PA. Supine haemodynamic data was recorded using whole-body impedance cardiography and radial tonometric pulse wave analysis. In sex-adjusted quartiles of ARR, determined as serum aldosterone to plasma renin activity ratio, the mean values were 282, 504, 744 and 1467 pmol/µg of angiotensin I/h, respectively. The only difference in haemodynamic variables between the ARR quartiles was higher pulse wave velocity (PWV) in the highest quartile versus other quartiles (p = 0.004), while no differences in blood pressure (BP), heart rate, wave reflections, cardiac output or systemic vascular resistance were observed between the quartiles. In linear regression analysis with stepwise elimination, ARR was an independent explanatory factor for PWV (β = 0.146, p < 0.001, R2 of the model 0.634). In conclusion, ARR was directly and independently associated with large arterial stiffness in individuals without clinical suspicion of PA. Therefore, ARR could serve as a clinical marker of cardiovascular risk.
Trial registration: ClinicalTrails.gov: NCT01742702.
Subject terms: Endocrinology, Endocrine system and metabolic diseases, Physiology, Circulation
Introduction
Primary aldosteronism (PA) is the most common form of secondary hypertension1, yet most cases remain undiagnosed2,3. The estimated prevalence of PA has conventionally ranged from 5 to 15% among hypertensive patients4–6, but according to a recent study, the prevalence might be as high as 16% to 22%7. It is known that PA is associated with significantly higher risk of cardiovascular events and target organ damage than essential hypertension at comparable levels of blood pressure5,8,9. Aldosterone excess increases the accumulation of growth factors and collagen fibres in arterial wall10,11 and it has been shown that PA patients have an increased arterial wall stiffness in comparison with patients who have essential hypertension12,13.
Screening of PA is based on the aldosterone-to-renin ratio (ARR). The Endocrine Society recommends the screening in the following cases with clinical suspicion pf PA: Blood pressure > 150/100 mmHg on three different days, drug resistant hypertension that is uncontrolled with ≥ three antihypertensive drugs, controlled hypertension with ≥ four antihypertensive drugs, hypertension with spontaneous or diuretic-induced hypokalaemia, hypertension with adrenal incidentaloma, hypertension with a family history of hypertension or cerebrovascular events at a young age (< 40 years), hypertension together with sleep apnoea, and all first-degree relatives of patients with PA1,5.
The role of ARR for screening of PA is robust, but there is a scarcity of data evaluating the significance of ARR in subjects who remain below the screening threshold for PA. Previously, ARR has been associated with the development and severity of hypertension, even in patients without excessive aldosterone levels14–18. A Japanese long-term observational analysis demonstrated that elevated ARR is associated with an increased incidence of cardiovascular disease in patients with essential hypertension19. In 60 healthy adults with a mean age 43 years, Shapiro et al. reported a direct correlation (rP = 0.298) between ARR and pulse wave velocity (PWV)20. In 2000 participants of the Framingham study, Lieb et al. found that ARR was a significant independent correlate of PWV (β = 0.20). However, the participants were not screened for the presence of PA, 33% were ingesting antihypertensive medications, and 12% had diabetes mellitus18.
In this cross-sectional study we analysed the association of ARR with several cardiovascular variables in normotensive subjects and previously undiagnosed never-medicated hypertensive patients. We tested the hypothesis whether ARR correlates with haemodynamic parameters in subjects who are not fulfilling the clinical criteria for the screening of PA.
Methods
Study subjects
The recruitment of the study subjects has previously been described in detail21–23. All subjects were examined by a physician and routine laboratory analyses for elevated blood pressure (BP) were taken24. The medical history, lifestyle behaviour and family history were documented. Alcohol use was evaluated as standard drinks (~ 12 g of absolute alcohol) per week, and smoking amount was estimated in pack-years. The exclusion criteria were history of coronary artery disease, stroke, cardiac insufficiency, valvular heart disease, chronic kidney disease25, secondary hypertension, alcohol or substance abuse26, psychiatric illnesses other than mild to moderate depression or anxiety, heart rhythm other than sinus rhythm, use of antihypertensive or uric-acid-lowering medications, and ongoing pregnancy. Altogether 606 subjects, aged 19–72 years, were eligible for the study. Data of ARR was missing in 48 subjects while 13 subjects met the screening criteria of PA (serum aldosterone > 550 pmol/l and ARR > 750 pmol/µg of angiotensin I/h, plasma potassium concentration < 3.3 mmol/l, BP > 150/100 mmHg) and were therefore excluded. The Endocrine Society screening for PA is based on BP > 150/100 mmHg on three different days1,5. In the present study the BP criteria were applied on the 5-min laboratory BP measurements (see below), and the results were confirmed by applying the criteria for the office BP measurements on a single occasion. The final study population consisted of 545 subjects.
Signed informed consent was obtained from all participants. The study complies with the declaration of Helsinki and was approved by the Ethics Committee of the Pirkanmaa Hospital District (study code R06086M).
Laboratory analyses and indexes glucose tolerance
Blood and urine samples were taken after 12 h of fasting. Plasma renin activity (GammaCoat Plasma Renin Activity 125-I RIA Kit, DiaSorin, Saluggia, Italy) and aldosterone concentration (Active Aldosterone RIA, Beckman Coulter, Fullerton, CA, USA) were determined using commercial kits. Plasma C-reactive protein (CRP), sodium, potassium, glucose, creatinine, cystatin C, uric acid, triglyceride, and total, HDL and LDL (high- and low-density lipoprotein, respectively) cholesterol concentrations were determined using Cobas Integra 800 (F. Hoffmann-LaRoche Ltd, Basel, Switzerland). Insulin and parathyroid hormone (PTH) were determined using electrochemiluminescence immunoassay (Cobas e411, Roche Diagnostics). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease – Epidemiology collaboration (CKD-EPI) cystatin C equation27. Insulin sensitivity was evaluated by the quantitative insulin sensitivity check index (QUICKI)28, and homeostatic model assessment of insulin resistance (HOMA-IR)29. In addition, the subjects were invited to participate in a standard 75-g oral glucose tolerance test (OGTT) for the determination of the Matsuda index30.
Experimental protocol
Haemodynamic recordings were performed in a noiseless, temperature-controlled laboratory by research nurses21,22,31. Products containing caffeine, smoking or heavy meal were not allowed for ≥ 4 h, and alcohol consumption was not allowed for ≥ 24 h before the investigation. The subjects rested supine on the examination table with impedance cardiography electrodes placed on body surface, tonometric sensor for pulse wave analysis on left radial pulsation, and oscillometric brachial cuff for BP calibration to the right upper arm. The left arm with the tonometric sensor was stabilized to 90° in a support, which held the measurement probe at the heart level.
The measurement consisted of a 5-min period, during which haemodynamic data was captured continuously. For the statistical analyses, the mean values of each 1-min period of recording were calculated. The repeatability and reproducibility of the protocol have been demonstrated to be good21,22,31.
Pulse wave analysis
Radial BP and pulse wave were recorded by a tonometric sensor (Colin BP-508 T, Colin Medical Instruments Corp., USA) fixed on the radial pulse21,22. The radial BP signal was calibrated approximately every 2.5 min by brachial BP measurements. Aortic BP was derived using the SphygmoCor pulse wave monitoring system (SphygmoCor PWMx, AtCor medical, Australia)32. Aortic pulse pressure, augmentation index (AIx, augmented pressure/pulse pressure * 100), and AIx adjusted to heart rate 75/min (AIx@75) were also determined. The central forward wave amplitude was defined as the difference between waveform foot and first systolic inflection point pressure in the aortic waveform33,34.
Whole-body impedance cardiography
We used the CircMon device (JR Medical Ltd., Tallinn, Estonia) to assess changes in body electrical impedance during cardiac cycles to record heart rate, stroke volume, cardiac output, and PWV35–37. Systemic vascular resistance was calculated from radial BP and cardiac output measured by CircMon. Stroke volume, cardiac output and systemic vascular resistance were presented as indexes related to body surface area calculated using the DuBois equation38. The method and electrode configuration has been previously reported36,37.
With CircMon the recorded stroke volume and cardiac output are in good agreement with values obtained utilizing 3-dimensional echocardiography31 and the thermodilution and direct oxygen Fick methods35,36, and the PWV values show very good correlation with values measured using ultrasound or the tonometric method37,39.
Statistics
To illustrate the influence of ARR on the haemodynamic variables, the study participants were divided into quartiles of ARR. As serum aldosterone concentration was slightly higher in women than in men (see ‘Results’), the ARR quartiles 1–4 were constructed separately for women and men.
Analyses of normally distributed data were performed using analysis of variance (ANOVA) and ANOVA for repeated measurements, and non-normally distributed data using Kruskal–Wallis test with Mann–Whitney U-test in the post-hoc analyses. The Bonferroni correction was applied in all post-hoc analyses. The ANOVA for repeated measures analyses were adjusted for sex, age, BMI, and eGFR, as appropriate, by using these variables as covariates in the analyses. Normally distributed variables in the tables were presented as means and standard deviations and non-normally distributed variables as medians and 25th–75th percentiles. The figures were depicted as means and standard errors of the mean (SEM). Pearson’s correlations were calculated for normally distributed and Spearman's correlations for non-normally distributed variables. The values of the haemodynamic variables for the statistics in Table 1 and regression analysis were calculated as averages from the minutes 3–5 of the recordings when the signal was most stable. P < 0.05 was considered significant.
Table 1.
Results of the study participants in sex adjusted quartiles of aldosterone-to-renin ratio: demographic data and clinical characteristics.
| Quartile 1 (n = 135) | Quartile 2 (n = 137) | Quartile 3 (n = 137) | Quartile 4 (n = 136) | |
|---|---|---|---|---|
| Male/female (n) | 66/69 | 67/70 | 67/70 | 67/69 |
| Number of female hormone users | 16 | 12 | 21 | 21 |
| Number of oestrogen users | 11 | 10 | 18 | 16 |
| Age (years) | 41.9 (11.4) | 44.8 (11.5) | 46.0 (11.5)* | 49.5 (10.9)*† |
| Weight (kg) | 78.7 (15.8) | 79.6 (15.6) | 80.0 (14.8) | 81.8 (15.0) |
| Height (cm) | 173.2 (9.5) | 172.2 (9.6) | 173.8 (8.8) | 172.7 (9.2) |
| Body mass index (kg/m2) | 26.1 (4.2) | 26.8 (4.6) | 26.4 (4.1) | 27.3 (3.9) |
| Alcohol (standard drinks/week) | 3 [0–5] | 3 [1–6] | 3 [1–6] | 2 [0–4] |
| Current smokers (n) | 22 | 18 | 11 | 13 |
| Office measurementsa | ||||
| Systolic BP (mmHg) | 135.3 (20.0) | 137.5 (20.2) | 139.7 (18.6) | 144.1 (21.0)* |
| Diastolic BP (mmHg) | 86.2 (13.1) | 87.6 (12.2) | 88.2 (10.7) | 91.8 (11.6)*† |
| Heart rate (bpm) | 67.5 (9.6) | 67.0 (9.3) | 65.2 (9.6) | 67.8 (9.1) |
| Normotensive/hypertensive (n) | 75 / 60 | 71 / 66 | 67 / 70 | 49 / 87* |
| Haemodynamic measurementsb | ||||
| Systolic BP (mmHg) | 127.4 (18.1) | 129.0 (18.1) | 129.1 (17.8) | 132.0 (18.3) |
| Diastolic BP (mmHg) | 72.5 (11.9) | 73.4 (12.0) | 73.4 (12.0) | 76.1 (12.8) |
| Heart rate (bpm) | 63.9 (10.2) | 63.8 (10.3) | 62.1 (9.3) | 63.6 (8.1) |
| Normotensive/hypertensive (n) | 107 / 28 | 103 / 34 | 100 / 37 | 95 / 41 |
BP, blood pressure; mean (standard deviation) or median [25th–75th percentile].
*P < 0.05 versus Q1; †P < 0.05 versus Q2.
aOffice BP was available from 123–129 participants per group.
bHaemodynamic measurement systolic/diastolic BP values were 12.7–15.6/16.0–17.3 mmHg, and heart rate 2.8–4.5 beats/min, lower than in the office with no significant differences between the quartiles.
To investigate factors independently associated with PWV in the whole study population, linear regression analysis with stepwise elimination was applied. The covariates in the analysis were age, sex, BMI, categorised smoking status (never, present, previous), categorised alcohol consumption (low, moderate, high); plasma aldosterone, renin, aldosterone-to-renin ratio, HDL cholesterol, LDL cholesterol, triglycerides, CRP, uric acid, calcitriol, PTH, QUICKI, eGFR, ejection duration, heart rate, and mean aortic pressure. Coefficients (B) and standard coefficients (Beta) of regression were calculated, and assumptions of linearity were confirmed by the analysis of residuals. IBM SPSS Statistics Version 26 (IBM Corporation, Armonk, NY, USA) was used for statistics.
Results
Study population and laboratory values
The proportion of male subjects was 49%. Plasma renin activity and ARR did not significantly differ between women versus men [0.99 (0.90) vs. 0.97 (0.95) ng of angiotensin I/ml/h; 779 (527) vs. 719 (531) pmol/µg of angiotensin I/h, respectively; mean (SD)]. However, serum aldosterone concentration was higher in women than in men (561 (481) vs. 449 (208) pmol/l, respectively, p < 0.001). Therefore, the ARR results were examined in sex-adjusted quartiles (Q) (Table 1).
None of the subjects used anti-hypertensive medicines. The proportions of female hormone users did not differ between the ARR quartiles. Mean participant age was 46 years, with an age range of 19–72 years in male and 21–72 years in female subjects. Participant age was higher in Q3 versus Q1, and in Q4 versus Q1 and Q2. Mean BMI was 26.6 kg/m2 with no differences between the quartiles. Average alcohol use was moderate 4.3 standard drinks/week, and the prevalence of present and previous smokers was 12% and 31%, respectively. Alcohol intake and the prevalence of smokers were corresponding in all quartiles (Table 1).
When applying the office cut-off values of hypertension (systolic BP ≥ 140 or diastolic BP ≥ 90)24, the number of never-medicated hypertensive subjects was 283 (51.9%). Office systolic BP was higher in Q4 than in Q1, and office diastolic BP in Q4 than in Q1 and Q2. The proportion of hypertensive subjects in the office measurements was higher in Q4 than in Q1.
During the haemodynamic measurements, systolic and diastolic BP values were 13–16 and 16–17 mmHg lower, respectively, and heart rate 3–5 beats/min lower, than in the office measurements, with no significant differences between the quartiles. With the office cut-off values of hypertension, no significant differences were found in the proportion of hypertensive subjects between the quartiles during the haemodynamics measurements (Table 1).
In sex adjusted quartiles of ARR, the mean values were 282, 504, 744 and 1467 pmol/µg of angiotensin I/h, respectively (Table 2). The differences in ARR were explained by variations in plasma renin activity, as aldosterone concentrations did not differ between the quartiles. Plasma renin activity was different in all quartiles with highest values in Q1 and lowest values in Q4. In our study, age was inversely correlated both with plasma renin activity and serum aldosterone concentration (rS = − 0.369 and rS = − 0.253, respectively, p < 0.01 for both), while age and ARR were directly correlated (rS = 0.238, p < 0.01).
Table 2.
Laboratory results of the study participants in sex adjusted quartiles of aldosterone-to-renin ratio.
| Quartile 1 (n = 135) | Quartile 2 (n = 137) | Quartile 3 (n = 137) | Quartile 4 (n = 136) | |
|---|---|---|---|---|
| Aldosterone-to-renin ratio (pmol/µg of Ang I/h) | 287 [227–342] | 497 [451–560]* | 740 [674–799]*† | 1302 [1085–1614]*†‡ |
| Serum aldosterone (pmol/l) | 419 [297–552] | 448 [324–599] | 431 [336–636] | 427 [323–575] |
| Plasma renin activity (ng of Ang I/ml/h) | 1.47 [1.12–2.17] | 0.89 [0.67–1.21]* | 0.58 [0.46–0.88]*† | 0.29 [0.20–0.44]*†‡ |
| Sodium (mmol/l) | 140.4 (1.8) | 139.9 (1.8) | 140.1 (2.1) | 140.7 (1.9)† |
| 24-h urine sodium excretion (mmol)a | 153 (62) | 155 (61) | 154 (65) | 148 (57) |
| Potassium (mmol/l) | 3.8 (0.2) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) |
| 24-h urine potassium excretion (mmol)a | 85 (30) | 87 (30) | 86 (27) | 79 (22) |
| Calcium (mmol/l) | 2.30 (0.09) | 2.29 (0.10) | 2.30 (0.10) | 2.29 (0.10) |
| Parathyroid hormone (pmol/l) | 4.37 (1.59) | 4.37 (1.41) | 4.43 (1.49) | 4.71 (1.59) |
| 25OH-D3 (nmol/l) | 74.2 (33.5) | 66.1 (28.3) | 74.9 (45.4) | 70.7 (30.0) |
| 1,25(OH)2-D3 (pmol/l) | 110.2 (31.4) | 109.3 (36.3) | 107.6 (33.8) | 106.7 (34.9) |
| C-reactive protein (mg/l) | 0.7 [0.5–1.5] | 1.0 [0.5–2.2] | 0.5 [0.4–1.3]† | 1.0 [0.5–2.1]‡ |
| Creatinine (µmol/l) | 74 (13) | 72 (13) | 75 (14) | 73 (14) |
| Cystatin C | 0.81 (0.14) | 0.83 (0.14) | 0.85 (0.15) | 0.86 (0.15) |
| Estimated GFR (ml/min/1.73 m2) | 104.7 (18.1) | 100.9 (17.8) | 98.3 (18.0)* | 95.3 (17.1)* |
| Total cholesterol (mmol/l) | 5.0 (1.0) | 5.1 (1.0) | 5.0 (1.0) | 5.3 (1.0)* |
| Triglycerides (mmol/l) | 1.11 [0.67–1.38] | 1.22 [0.74–1.43] | 1.21 [0.67–1.46] | 1.27 [0.87–1.54] |
| HDL cholesterol (mmol/l) | 1.6 (0.4) | 1.6 (0.5) | 1.6 (0.4) | 1.5 (0.4) |
| LDL cholesterol (mmol/l) | 2.9 (0.9) | 3.0 (1.0) | 2.9 (0.9) | 3.2 (0.9) |
| Uric acid (µmol/l) | 302 (71) | 297 (65) | 292 (71) | 303 (86) |
| Glucose (mmol/l) | 5.3 (0.5) | 5.5 (0.5) | 5.4 (0.5) | 5.6 (0.8)* |
| Insulin (mU/l) | 6.8 [5.0–10.4] | 6.8 [5.3–9.7] | 5.9 [4.3–8.1] | 7.0 [4.7–9.4] |
| Matsuda indexb | 5.74 [3.74–8.18] | 5.94 [3.50–8.78] | 7.62 [4.44–9.69] | 5.80 [3.89–7.72] |
| HOMA-IR | 1.56 [1.14–2.61] | 1.70 [1.23–2.37] | 1.43 [1.02–2.11] | 1.69 [1.13–2.40] |
| QUICKI | 0.357 [0.331–0.375] | 0.352 [0.335–0.371] | 0.362 [0.341–0.382] | 0.353 [0.335–0.376] |
Results shown as mean (standard deviation) or median [25th–75th percentile]; GFR, estimated glomerular filtration rate from plasma cystatin C using the CKD-EPI formula27.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index.
*P < 0.05 versus Q1; †P < 0.05 versus Q2; ‡P < 0.05 versus Q3.
an = 101–118 for 24-h urine excretion results.
bn = 88–98 for Matsuda index results in each quartile.
Average measures of plasma electrolytes, PTH, CRP, uric acid, glucose metabolism and renal function were within the normal range in all quartiles (Table 2). In seven participants fasting plasma glucose ranged 7.0–10.3 mmol/l, i.e. in the diabetic range, but none of them presented with glucosuria. Altogether 53 participants had impaired fasting plasma glucose concentration ranging 6.2–6.9 mmol/l. Plasma sodium concentration was minimally higher in Q4 than in Q2. No differences were observed in 24-h excretion of sodium or potassium to the urine, plasma potassium, calcium, PTH, creatinine, uric acid, triglycerides, HDL cholesterol or LDL cholesterol. However, total cholesterol was somewhat higher in Q4 when compared to Q1. Estimated GFR was lower in Q3 and Q4 when compared to Q1. Although fasting plasma glucose concentration was higher in Q4 than in Q1, all the evaluated insulin sensitivity indices (QUICKI, HOMA-IR, Matsuda index) were invariable between the ARR quartiles (Table 2).
Haemodynamic measurements
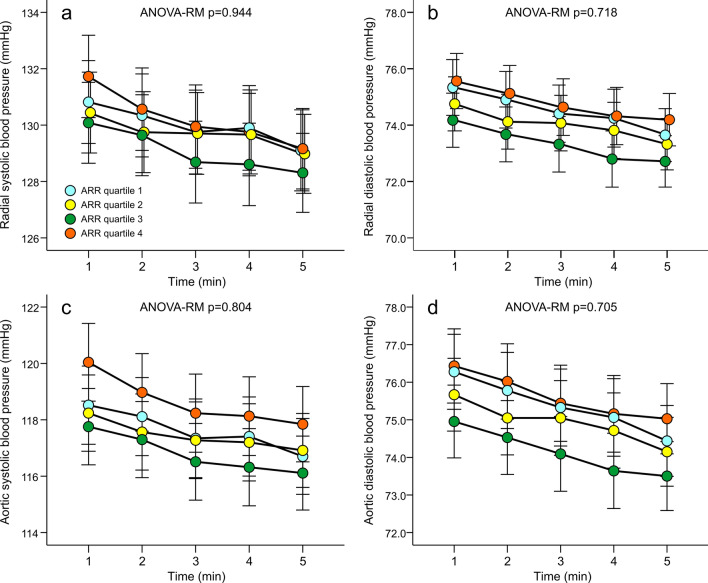
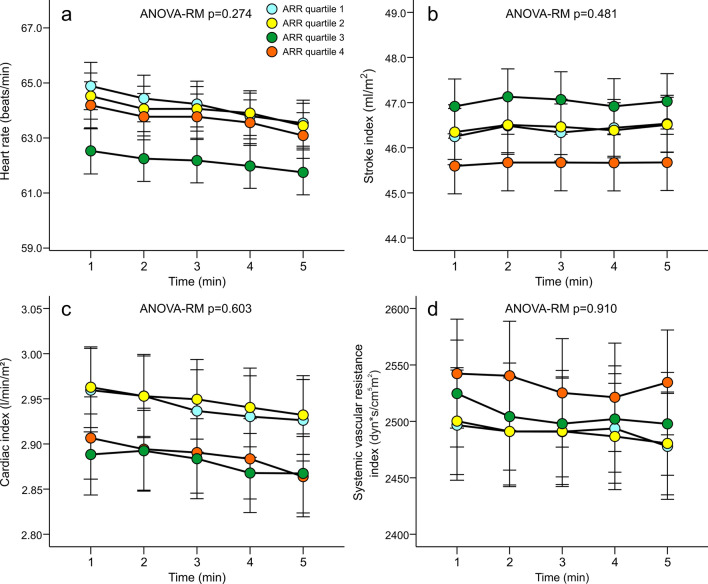
ARR and haemodynamics. The results representing the haemodynamic variables in the sex-, age-, and eGFR-adjusted quartiles of ARR are shown in Figs. 1, 2 and 3. No differences in radial or aortic BP were observed between the quartiles (Fig. 1). Heart rate, stroke index, cardiac index and systemic vascular resistance index were also similar in all quartiles (Fig. 2). No differences were found in forward wave amplitude, augmentation index or extracellular water balance between the quartiles, either. However, in the absence of differences in central BP, aortic to popliteal PWV was clearly higher in Q4 versus other quartiles (p = 0.004) (Fig. 3). Among the study subjects, the Spearman correlation between ARR and PWV was 0.219 (p < 0.01).
Figure 1.
Radial systolic (a) and diastolic (b) blood pressure calibrated from brachial blood pressure measurements, and aortic systolic (c) and diastolic (d) blood pressure in quartiles (n = 135–137) of aldosterone-to-renin ratio (ARR); analyses were adjusted for sex, age, and estimated glomerular filtration rate; ANOVA-RM, analysis of variance for repeated measurements, results are depicted as mean and standard error of the mean.
Figure 2.
Heart rate (a), stroke index (b), cardiac index (c) and systemic vascular resistance index (d) in quartiles (n = 135–137) of aldosterone-to-renin ratio (ARR); analyses were adjusted for sex, age, and estimated glomerular filtration rate; ANOVA-RM, analysis of variance for repeated measurements, mean and standard error of the mean.
Figure 3.
Forward wave amplitude (a), augmentation index (b), extracellular water balance (c) and aortic-to-popliteal pulse wave velocity (d) in quartiles (n = 135–137) of aldosterone-to-renin ratio (ARR); analyses were adjusted for sex, age, and estimated glomerular filtration rate; ANOVA-RM, analysis of variance for repeated measurements, mean and standard error of the mean.
Aldosterone and haemodynamics. When examined in sex-adjusted quartiles of serum aldosterone concentration, the quartiles presented with differences in age and BMI (not shown). In the sex-, age- and BMI-adjusted quartiles of serum aldosterone concentration, no differences in the haemodynamic variables were detected between the quartiles except for PWV, the value of which was lower in Q1 versus Q4 (8.12 ± 0.12 vs. 8.58 ± 0.12 m/s, p = 0.045).
Renin activity and haemodynamics. In sex-adjusted quartiles of plasma renin activity, no additional adjustments were needed (not shown). Corresponding to the results in quartiles of aldosterone concentration, all haemodynamic variables in quartiles of renin activity were similar except for PWV. However, the difference in PWV was observed between Q1 and Q2 (8.65 ± 0.13 vs. 8.08 ± 0.12 m/s, p = 0.006), while PWV in Q1, Q3 and Q4 was corresponding (ranging from 8.37 ± 0.12 to 8.65 ± 0.12 m/s, p = 0.559–1.000).
Determinants of large arterial stiffness
The results of the linear regression analyses with stepwise elimination are presented in Table 3. The independent explanatory factors for PWV were age, ejection duration, uric acid concentration, mean aortic pressure, ARR, BMI, low alcohol consumption, and heart rate (R2 = 0.634). The variables in the model included both aldosterone concentration and renin activity, yet neither of them turned out as independent predictors for PWV. QUICKI was used as an insulin sensitivity variable in the model, as the Matsuda index was not available from all participants (Table 2). In our study population the correlation between QUICKI and Matsuda index was 0.884 (p < 0.001) and between QUICKI and HOMA-IR-0.720 (p < 0.001).
Table 3.
Significant explanatory variables for aortic to popliteal pulse wave velocity in linear regression analysis with stepwise elimination.
| Pulse wave velocity (m/s) | B | Beta | P |
|---|---|---|---|
| R2 = 0.634 | |||
| (constant) | 5.046 | ||
| Age | 0.071 | 0.488 | < 0.001 |
| Ejection duration | − 0.016 | − 0.185 | < 0.001 |
| Uric acid | 0.005 | 0.199 | < 0.001 |
| Mean aortic pressure | 0.018 | 0.148 | < 0.001 |
| Aldosterone-to-renin ratio | 4.68 × 10–4 | 0.146 | < 0.001 |
| Body mass index | 0.041 | 0.103 | 0.001 |
| Heart rate | 0.017 | 0.098 | 0.010 |
| Low alcohol consumption category | − 0.220 | − 0.062 | 0.025 |
Variables included in the model correlated with pulse wave velocity with a p-value < 0.05: age, sex, BMI, categorised smoking status, categorised alcohol consumption; plasma aldosterone, renin, aldosterone-to-renin ratio, HDL cholesterol, LDL cholesterol, triglycerides, CRP, uric acid, calcitriol, PTH; QUICKI, eGFR, ejection duration, heart rate, and mean aortic pressure. HDL, high-density lipoprotein; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check index; eGFR, estimated glomerular filtration rate from plasma cystatin C using the CKD-EPI formula27.
When the office cut-off for hypertension (BP ≥ 140/90 mmHg) was applied for the laboratory measurements24, ARR was an independent explanatory factor for PWV in both the normotensive (p < 0.001, R2 = 0.580) and hypertensive (p = 0.012, R2 = 0.523) subgroups. The result did not change when applying the home BP measurement cut-off (BP ≥ 135/85 mmHg).
If the office BP measurements were applied for the exclusion of participants with potential PA, 499 subjects were eligible for the statistical analyses. Also, in this analysis ARR was an independent explanatory factor for PWV (p < 0.001), while the other explanatory factors were age, mean aortic pressure, heart rate, and plasma concentrations of HDL cholesterol, uric acid, and triglycerides (R2 = 0.572 of the model).
Discussion
In this study we investigated the association of ARR with several cardiovascular variables in normotensive subjects and never-medicated hypertensive patients without clinical suspicion of PA and without cardiovascular or renal comorbidities, and cardiovascular medications. In analyses adjusted for confounding factors, ARR was significantly associated with PWV but not with any other haemodynamic variable. The linear regression analyses confirmed that ARR was an independent explanatory factor for PWV, an acknowledged marker of large arterial stiffness40,41. As increased arterial stiffness is a strong independent predictor of cardiovascular events41, while higher ARR is associated with an increased incidence of cardiovascular disease19 and predicts future stroke in hypertensive patients42, a higher ARR may predispose to the future development of cardiovascular diseases.
Age is the most significant explanatory factor for large arterial stiffness43,44. In the present study, subjects in the highest ARR quartile were older than in the other quartiles. Consequently, the Figs. 1, 2 and 3 were adjusted for differences in age in addition to sex and estimated GFR. Moreover, despite the presence of age in the regression model, ARR was an independent explanatory factor for PWV. The inverse correlation between age and plasma renin is known from previous studies45. High sodium intake would also lower renin1,5, but this was not the cause for lower renin in the ARR quartiles 2–4 versus quartile 1, as 24-h sodium excretion to the urine was similar in all quartiles.
The screening positive cases with putative PA were excluded from our study population that was predominantly normotensive during the haemodynamic measurements. We found that ARR was directly and independently associated with large arterial stiffness, while the linear regression model displayed no direct association between serum aldosterone concentration or plasma renin activity and PWV. This finding may reflect higher renin-independent aldosterone release in the participants with high ARR. Indeed, a continuum of renin-independent aldosteronism in normotensive subjects has been previously identified46,47. High aldosterone levels related to prevailing plasma renin activity may predispose to the chronic adverse effects of aldosterone in the vascular system already prior to the clinical diagnosis of PA or hypertension46. The molecular basis for this continuum of aldosterone secretion may be explained by the aldosterone-producing cell clusters that have been discovered in the zona fasciculata of morphologically normal non-neoplastic adrenal glands: these clusters may be found in > 50% of normal adrenal glands, leading to mild autonomous aldosterone secretion and increased ARR with increasing age far more commonly than previously perceived48,49.
Some former studies have suggested a direct association between ARR and arterial stiffness. Shapiro et al. reported a significant association of ARR with PWV in 60 normotensive subjects, but in contrast to our study, their approach did not provide evidence of ARR as an independent explanatory factor of PWV20. In the Framingham study, Lieb et al. found that ARR was directly associated with PWV and four other measures of vascular pathophysiology18. As a major difference to our study, medicated hypertensive subjects and patients with known diabetes were not excluded from their analyses. Also, there was a 3-year interval between the laboratory examinations and haemodynamic measurements, whereas in our study these procedures were performed within a median period of 8 days from one another (25th to 75th percentile 2–17 days). A small study in 24 patients with essential hypertension by Mahmud and Feely found no correlation between ARR and PWV, but showed that aldosterone antagonist-induced decrease in systolic BP correlated with pre-treatment ARR50. However, in 102 patients with confirmed PA, no correlation was found between ARR and baseline PWV, or between ARR and the reduction in PWV following adrenalectomy51. Yet, the subsequent increase in plasma renin activity after adrenalectomy was an explanatory factor for the reduction in PWV during 6 months of follow-up51. Altogether, the effects of aldosterone excess on large arterial stiffness may be manifested early in the course of the disease, and the beneficial changes in the vasculature following surgical treatment do directly correlate with the plasma concentrations of aldosterone or ARR.
Noteworthy, in the present study the systolic and diastolic BP values were 13–16 and 16–17 mmHg lower, respectively, during the haemodynamic laboratory measurements than in the office measurements. This resembles the white-coat effect in hypertension, which has been related to large arterial stiffness in both untreated52 and treated hypertensive patients53. Out-of-office BP monitoring provides better assessment of overall BP and response to treatment in patients with white-coat hypertension54. The average difference between the office and laboratory BP in our study was rather large, whereby haemodynamic measurements under standard conditions can also result in clearly lower BP when compared with the office measurements. The correlations between office and laboratory measurements were 0.70 and 0.65 for systolic and diastolic BP, respectively. We used the algorithm recommended by The Endocrine Society for the screening of PA patients1,5, in order to exclude potential PA patients from the study population. Regardless of whether office BP > 150/100 mmHg measured on a single occasion, or average BP > 150/100 mmHg during haemodynamic measurements, was applied for the exclusion of participants, ARR was an independent explanatory factor for PWV. Of note, the results were corresponding when studied in normotensive and hypertensive subjects with two different cut-off values for hypertension (140/90 or 135/85 mmHg).
Seven of the present participants had fasting plasma glucose in the diabetic range, 53 participants had impaired fasting plasma glucose, while 89% of the participants presented with glucose values within the normal range. PA has been linked to abnormalities in glucose metabolism, especially to insulin resistance and impaired glucose tolerance55–57. Even in the absence of PA, plasma aldosterone concentration was related to insulin resistance in 251 male African American subjects58. Higher ARR was also associated with insulin resistance in 483 young adult African Americans without cardiovascular or renal disease59. In our study, no differences in insulin sensitivity between the ARR quartiles were observed as the indices QUICKI, HOMA-IR and Matsuda did not deviate. Therefore, the differences in PWV between the ARR quartiles could not be explained by variations in insulin sensitivity. Also the plasma concentrations of uric acid, the levels of which are usually elevated in insulin resistance and metabolic syndrome23, were similar in quartiles of ARR.
Changes in calcium metabolism may influence BP60, and induce alterations in the components of the renin-angiotensin system in the vasculature61. Moreover, vitamin D receptor activation downregulates the synthesis of renin in the juxtaglomerular cells and influences the expression of other components of the renin-angiotensin system in the kidney62,63. In the present study, the plasma concentrations of calcium and vitamin D metabolites were similar in the ARR quartiles. Therefore, putative changes in the metabolism of calcium and vitamin D were not the explanations for the differences in arterial stiffness between the ARR quartiles.
Our study has limitations. The cross-sectional design cannot substantiate causality. A potential selection bias caused by the recruitment of voluntary subjects and the exclusion protocol must be acknowledged. The haemodynamic recordings lasted for five minutes and the values of the last three minutes were used for the analyses, which gives a rather short window of observation. Still, the analyses were based on average on 190 cardiac cycles in each subject (mean HR in the study population was 63.3 beats/min). In addition, we applied indirect non-invasive methods requiring mathematical processing to derive PWV, stroke volume and cardiac output from the bioimpedance signal36, and central aortic BP waveform from the applanation tonometry signal32. Therefore, the results must be interpreted with caution, albeit the methods have been validated against direct or invasive measurements31,35,37. However, the approach to examine central haemodynamics instead of plainly focusing on brachial artery pressure, may be better related with the level of cardiovascular risk64,65.
In conclusion, a direct association between ARR and PWV was observed in 545 normotensive and never-treated hypertensive subjects when the screening criteria of PA were not met. Therefore, our results indicate that ARR is related to arterial stiffness in individuals without clinical suspicion of PA according to the prevailing guidelines. Altogether, increased ARR in the absence of stage II and more severe hypertension should be recognized as an indicator of increased cardiovascular risk.
Acknowledgements
The authors are deeply grateful to Paula Erkkilä, RN and Reeta Kulmala, RN for invaluable contribution to the haemodynamic measurements. The authors wish to acknowledge CSC – IT Center for Science, Finland, for computational resources. The study was supported by the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital, Finnish Foundation for Cardiovascular Research, Päivikki and Sakari Sohlberg Foundation, Pirkanmaa Regional Fund of the Finnish Cultural Foundation, Emil Aaltonen Foundation, and Ida Montin Foundation.
Author contributions
E.K. and I.P. reviewed the literature and wrote the original version of the manuscript. I.P., E.K. and H.H. performed the statistical analyses. P.N., J.K., A.T. and I.P. carried out the clinical examinations of patients. E.K., P.N., M.K.C., J.K., A.T., J.M., M.V., N.M. and I.P. participated in the design of the technical details and methodology of the study. O.N. was responsible for hormonal laboratory analyses. All authors contributed to the discussion and editing the manuscript. I.P. was responsible for designing and conducting the study. All authors take the responsibility for the contents of the manuscript.
Data availability
Analyses and generated datasets during the current study are not available publicly as our clinical database contains several indirect identifiers and the informed consent obtained does not allow publication of individual patient data. The datasets are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young WF. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J. Intern. Med. 2019;285:126–148. doi: 10.1111/joim.12831. [DOI] [PubMed] [Google Scholar]
- 2.Mulatero P, et al. Guidelines for primary aldosteronism: uptake by primary care physicians in europe. J. Hypertens. 2016;34:2253–2257. doi: 10.1097/HJH.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 3.Rossi E, Perazzoli F, Negro A, Magnani A. Diagnostic rate of primary aldosteronism in emilia-romagna, Northern Italy, during 16 years (2000–2015) J. Hypertens. 2017;35:1691–1697. doi: 10.1097/HJH.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 4.Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies—a review of the current literature. Horm. Metab. Res. 2012;44:157–162. doi: 10.1055/s-0031-1295438. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 6.Monticone S, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J. Am. Coll. Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann. Intern. Med. 2020;173:10–20. doi: 10.7326/M20-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milliez P, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Monticone S, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4. [DOI] [PubMed] [Google Scholar]
- 10.Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin. Sci. 2007;113:267–278. doi: 10.1042/CS20070123. [DOI] [PubMed] [Google Scholar]
- 11.Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension. 2005;46:1227–1235. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- 12.Strauch B, et al. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am. J. Hypertens. 2006;19:909–914. doi: 10.1016/j.amjhyper.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary MK, et al. Primary aldosteronism: higher volume load, cardiac output and arterial stiffness than in essential hypertension. J. Intern. Med. 2020 doi: 10.1111/joim.13115. [DOI] [PubMed] [Google Scholar]
- 14.Tomaschitz A, et al. Aldosterone/renin ratio determines peripheral and central blood pressure values over a broad range. J. Am. Coll. Cardiol. 2010;55:2171–2180. doi: 10.1016/j.jacc.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Newton-Cheh C, et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49:846–856. doi: 10.1161/01.HYP.0000258554.87444.91. [DOI] [PubMed] [Google Scholar]
- 16.Gaddam KK. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch. Intern. Med. 2008;168:1159. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meneton P, et al. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged caucasian populations. J. Hum. Hypertens. 2008;22:550–558. doi: 10.1038/jhh.2008.27. [DOI] [PubMed] [Google Scholar]
- 18.Lieb W, et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 2009;119:37–43. doi: 10.1161/CIRCULATIONAHA.108.816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisaka T, et al. Association of elevated plasma aldosterone-to-renin ratio with future cardiovascular events in patients with essential hypertension. J. Hypertens. 2012;30:2322–2330. doi: 10.1097/HJH.0b013e328359862d. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro Y, Boaz M, Matas Z, Fux A, Shargorodsky M. The association between the renin–angiotensin–aldosterone system and arterial stiffness in young healthy subjects. Clin. Endocrinol. (Oxf.). 2008;68:510–512. doi: 10.1111/j.1365-2265.2008.03176.x. [DOI] [PubMed] [Google Scholar]
- 21.Tahvanainen A, et al. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand. J. Clin. Lab. Invest. 2009;69:128–137. doi: 10.1080/00365510802439098. [DOI] [PubMed] [Google Scholar]
- 22.Tikkakoski AJ, et al. Hemodynamic alterations in hypertensive patients at rest and during passive head-up tilt. J. Hypertens. 2013;31:906–915. doi: 10.1097/HJH.0b013e32835ed605. [DOI] [PubMed] [Google Scholar]
- 23.Kangas P, et al. Changes in hemodynamics associated with metabolic syndrome are more pronounced in women than in men. Sci. Rep. 2019;9:18377. doi: 10.1038/s41598-019-54926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams B, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 25.KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [PubMed] [Google Scholar]
- 26.Treatment of alcohol abuse. Current Care Guideline by the Finnish Medical Society Duodecim and the Finnish Society of Addiction Medicine. (2015). https://www.kaypahoito.fi/web/kh/suositukset/suositus?id=hoi50028#K1. Accessed 10 August 2020.
- 27.Inker LA, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz A, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 29.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiol.-Endocrinol. Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 31.Koskela JK, et al. Association of resting heart rate with cardiovascular function: a cross-sectional study in 522 Finnish subjects. BMC Cardiovasc. Disord. 2013;13:102. doi: 10.1186/1471-2261-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CH, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.CIR.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 33.Kaess BM, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell GF, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 35.Kööbi T, Kaukinen S, Ahola T, Turjanmaa VM. Non-invasive measurement of cardiac output: whole-body impedance cardiography in simultaneous comparison with thermodilution and direct oxygen fick methods. Intensive Care Med. 1997;23:1132–1137. doi: 10.1007/s001340050469. [DOI] [PubMed] [Google Scholar]
- 36.Kööbi T, Kaukinen S, Turjanmaa VM, Uusitalo AJ. Whole-body impedance cardiography in the measurement of cardiac output. Crit. Care Med. 1997;25:779–785. doi: 10.1097/00003246-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Kööbi T, Kähönen M, Iivainen T, Turjanmaa V. Simultaneous non-invasive assessment of arterial stiffness and haemodynamics—a validation study. Clin. Physiol. Funct. Imaging. 2003;23:31–36. doi: 10.1046/j.1475-097X.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 38.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 1916;17:863–871. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 39.Wilenius M, et al. Central wave reflection is associated with peripheral arterial resistance in addition to arterial stiffness in subjects without antihypertensive medication. BMC Cardiovasc. Disord. 2016;16:131. doi: 10.1186/s12872-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurent S, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 41.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 42.Satoh M, et al. Aldosterone-to-renin ratio as a predictor of stroke under conditions of high sodium intake: the Ohasama study. Am. J. Hypertens. 2012;25:777–783. doi: 10.1038/ajh.2012.33. [DOI] [PubMed] [Google Scholar]
- 43.Safar ME, London GM. Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. J. Hypertens. 2000;18:1227–1535. doi: 10.1097/00004872-200018110-00001. [DOI] [PubMed] [Google Scholar]
- 44.McEniery CM, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J. Am. Coll. Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Drayer JI, Weber MA, Laragh JH, Sealey JE. Renin subgroups in essential hypertension. Clin. Exp. Hypertens. A. 1982;4:1817–1834. doi: 10.3109/10641968209061643. [DOI] [PubMed] [Google Scholar]
- 46.Markou A, et al. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J. Clin. Endocrinol. Metab. 2013;98:1409–1416. doi: 10.1210/jc.2012-3353. [DOI] [PubMed] [Google Scholar]
- 47.Rene B, et al. Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69:950–956. doi: 10.1161/HYPERTENSIONAHA.116.08952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nanba K, et al. Age-related autonomous aldosteronism. Circulation. 2017;136:347–355. doi: 10.1161/CIRCULATIONAHA.117.028201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimoto K, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc. Natl. Acad. Sci. USA. 2015;112:E4591–E4599. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am. J. Hypertens. 2005;18:50–55. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Liao C-W, et al. Time course and factors predicting arterial stiffness reversal in patients with aldosterone-producing adenoma after adrenalectomy: prospective study of 102 patients. Sci. Rep. 2016;6:20862. doi: 10.1038/srep20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Simone G, et al. Estimate of white-coat effect and arterial stiffness. J. Hypertens. 2007;25:827–831. doi: 10.1097/HJH.0b013e32801d1f62. [DOI] [PubMed] [Google Scholar]
- 53.Barochiner J, et al. Arterial stiffness in treated hypertensive patients with white-coat hypertension. J. Clin. Hypertens. 2017;19:6–10. doi: 10.1111/jch.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal R, Weir MR. Treated hypertension and the white coat phenomenon: office readings are inadequate measures of efficacy. J. Am. Soc. Hypertens. 2013;7:236–243. doi: 10.1016/j.jash.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Widimský J, et al. Impaired insulin action in primary hyperaldosteronism. Physiol. Res. 2000;49:241–244. [PubMed] [Google Scholar]
- 56.Sindelka, G. et al. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp. Clin. Endocrinol. Diabetes. 108, 21-25 (2000). [DOI] [PubMed]
- 57.Catena C, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J. Clin. Endocrinol. Metab. 2006;91:3457–3463. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 58.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Hypertension, insulin resistance, and aldosterone: sex-specific relationships. J. Clin. Hypertens. 2009;11:130–137. doi: 10.1111/j.1751-7176.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huan Y, Deloach S, Keith SW, Goodfriend TL, Falkner B. Aldosterone and aldosterone: renin ratio associations with insulin resistance and blood pressure in African Americans. J. Am. Soc. Hypertens. 2012;6:56–65. doi: 10.1016/j.jash.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Oinonen L, et al. Plasma total calcium concentration is associated with blood pressure and systemic vascular resistance in normotensive and never-treated hypertensive subjects. Blood Press. 2020;29:137–148. doi: 10.1080/08037051.2019.1696180. [DOI] [PubMed] [Google Scholar]
- 61.Porsti I, et al. High calcium diet down-regulates kidney angiotensin-converting enzyme in experimental renal failure. Kidney Int. 2004;66:2155–2166. doi: 10.1111/j.1523-1755.2004.66006.x. [DOI] [PubMed] [Google Scholar]
- 62.Freundlich M, et al. Suppression of renin–angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 63.Pörsti IH. Expanding targets of vitamin D receptor activation: downregulation of several ras components in the kidney. Kidney Int. 2008;74:1371–1373. doi: 10.1038/ki.2008.424. [DOI] [PubMed] [Google Scholar]
- 64.Roman MJ, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 65.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension. 2016;67:183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analyses and generated datasets during the current study are not available publicly as our clinical database contains several indirect identifiers and the informed consent obtained does not allow publication of individual patient data. The datasets are available from the corresponding author on reasonable request.