Abstract
Corneal allograft survival is mediated by the variety of immunological reactions and wound healing process. Our aim was to explore the effects of topical administration of ripasudil, a selective Rho-associated coiled-coil protein kinase inhibitor, on corneal allograft survival. Ripasudil was administered to mice thrice a day after allogeneic corneal transplantation. Corneal graft survival, opacity, neovascularization, re-epithelization, immune cell infiltration, and mRNA levels of angiogenic and pro-inflammatory factors in the grafted cornea and draining lymph nodes (dLNs) were evaluated with slit-lamp microscopy, immunohistochemistry, flow cytometry, and polymerase chain reaction. Graft survival was significantly prolonged with lower graft opacity and neovascularization scores in 0.4% and 2.0% ripasudil-treated groups, and mRNA levels of angiogenic and pro-inflammatory factors in ripasudil-treated grafted corneas were reduced. Moreover, 0.4% and 2.0% ripasudil reduced CD45+-infiltrated leukocyte frequency, Cd11b and Cd11c mRNA levels, and the frequencies of mature dendritic cells, IFNγ-, and IL-17- producing CD4+T cells in the dLNs of recipients. Re-epithelization rate of the grafted cornea was significantly higher in the 0.4% and 2.0% ripasudil groups than in the control. Topically applied ripasudil prolonged graft survival by downregulating neovascularization and inflammation factors, while promoting corneal re-epithelization, suggesting that ripasudil may be useful for suppressing immunological rejection in corneal transplantation.
Subject terms: Eye diseases, Corneal diseases, Transplant immunology
Introduction
The cornea is the most commonly transplanted tissue worldwide1, and corneal transplantation is associated with high success rates owing to its immune privilege2. However, host factors involved in inflammation and neovascularization lead to high rejection rates even with the use of topical steroids3. These effects are observed despite treatment with high doses of non-specific immunosuppressive agents, thus often preventing long-term graft survival combined with manifestation of severe side effects, including cataracts, glaucoma, and opportunistic infections4,5.
The cornea is an avascular, transparent dome layer tissue that acts as a mechanical barrier and contributes to two-thirds of the refractive power of the eye6. Corneal neovascularization (CNV) is associated with several etiologies, including improper use of contact lens, corneal infections, chemical burns, ocular surface inflammation, trauma, limbal stem cell deficiency, and post-corneal transplantation6, and is estimated to affect 1.4 million individuals annually7. CNV decreases visual acuity with higher-order aberrations and corneal edema, contributing to a worse prognosis of corneal transplantation, accompanied by increased ocular surface inflammation and immunological responses8. Furthermore, CNV causes blindness in approximately 7 million individuals worldwide9.
CNV affects wound healing and immunological reactions after corneal transplantation by transporting immune cells into and out of the local site10. Therefore, suppression of CNV and control of immune cells are required for reducing the rates of rejection after corneal transplantation. Anti-vascular endothelial growth factor (VEGF) is widely used for preventing neovascularization, as VEGF is the primary regulator of human angiogenesis and promotes vascular endothelial cell proliferation, migration, and tube formation6.
Despite several studies reporting the beneficial effects of anti-angiogenic therapy for CNV11–13, eye drops with anti-angiogenic properties are not yet available in the clinical setting. Therefore, the development of anti-angiogenic eye drops is an unmet medical requirement, as CNV causes not only immune rejection in corneal transplantation but also vision loss due to higher-order aberrations and edema.
Rho-associated coiled-coil-containing protein kinase (ROCK), a target of the small-molecule GTP-binding protein Ras homolog family member A (Rho-A)14,15, exists as two isoforms: ROCK1 and ROCK2. The Rho-A/ROCK pathway, along with various cytokines such as VEGF, is associated with angiogenesis16,17. The ROCK inhibitor ripasudil hydrochloride hydrate (K-115) selectively inhibits both ROCK1 and ROCK2, and ripasudil (0.4%) has been approved for glaucoma treatment in Japan18. Ripasudil can be used as a potential therapeutic eye drop for retinal hypoxic neovascular diseases19,20; however, the effects of ripasudil on corneal allograft survival are not known.
Hence, in this study, we evaluated the immunotherapeutic potential of topically administered ripasudil for corneal allograft survival using a murine corneal transplantation model. The present results may provide a foundation for the clinical application of topical ripasudil in the regulation of corneal angiogenesis to improve the prognosis of corneal transplant recipients.
Results
Topical administration of ripasudil inhibited graft cornea angiogenesis and lymphangiogenesis
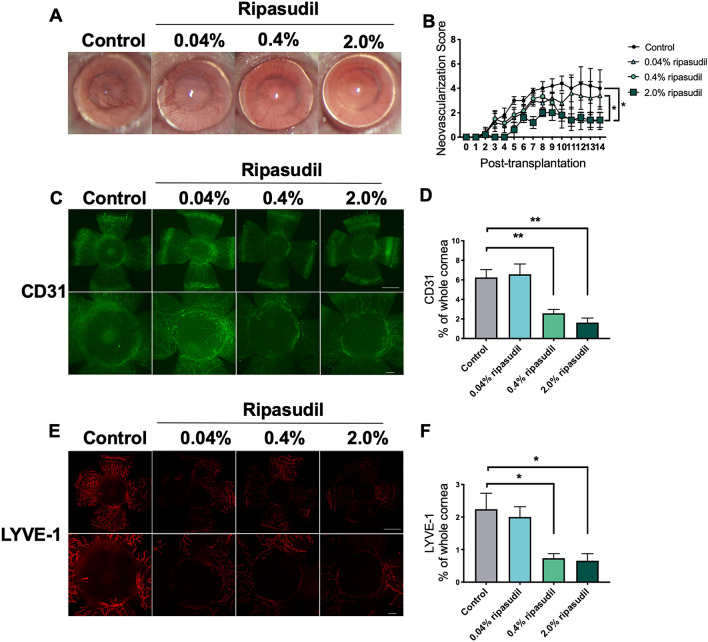
Figure 1A shows a representative image of the grafted cornea at day 14 post-transplantation. Topical administration of 0.4% and 2.0% ripasudil significantly reduced the CNV score compared to that in the control group on day 14 post-transplantation (Fig. 1B p = 0.022 and p = 0.037, respectively). Figure 1C shows the results of immunofluorescence staining for blood (CD31+) in the grafted corneas. Upper row shows the whole grafted cornea. Bottom row shows the center of the grafted cornea at higher magnification. Quantification of the vascularized area in corneal whole mounts revealed that topical administration of 0.4% and 2.0% ripasudil significantly reduced the de novo generation of CD31+ blood vessels (p = 1.000, 0.008, and 0.001 for 0.04%, 0.4%, 2.0% ripasudil versus control, respectively; n = 5, Fig. 1D). Figure 1E shows the results of immunofluorescence staining for lymphatic vessels (lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1)+) in the grafted corneas. Upper row shows the whole grafted cornea. Bottom row shows the center of the grafted cornea at higher magnification. Quantification of the vascularized area in corneal whole mounts revealed that topical administration of 0.4% and 2.0% ripasudil significantly reduced the de novo generation of LYVE-1+ lymphatic vessels (p = 1.000, 0.012, and 0.009 vs. control, respectively; n = 5, Fig. 1F).
Figure 1.
Topical administration of ripasudil suppressed neovascularization and lymphangiogenesis in murine corneal transplants. (A) Representative slit-lamp images showing grafted corneas on day 14 post-transplantation (magnification × 25). (B) Neovascularization scores of grafted corneas 14 days after transplantation (two-way ANOVA, n = 5 per group; *p < 0.01). (C) Representative immunohistochemical images of CD31 staining of grafted corneas on day 14 post-transplantation. Upper row: whole grafted cornea. Bottom row: high magnification showing center of grafted cornea. (D) Percentages of the corneal area covered with blood vessels (CD31+) in the ripasudil and control groups (one-way ANOVA, n = 5 per group; *p < 0.01). (E) Representative immunohistochemical images of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) staining of grafted corneas on day 14 post-transplantation. Upper row: whole grafted cornea. Bottom row: high magnification showing center of grafted cornea. (F) Percentages of the corneal area covered with lymphatic vessels (LYVE-1+) in the ripasudil and control groups (one-way ANOVA, n = 5 per group; *p < 0.05). The area of the whole grafted cornea covered by blood or lymphatic vessels was analyzed using ImageJ version 1.53a (National Institutes of Health, Bethesda, MD, USA; available at https://rsb.info.nih.gov/ij/index.html)57,58. Scale bar, 1000 μm. ANOVA analysis of variance.
Topical administration of ripasudil downregulated angiogenic and lymphangiogenic factors in grafted corneas
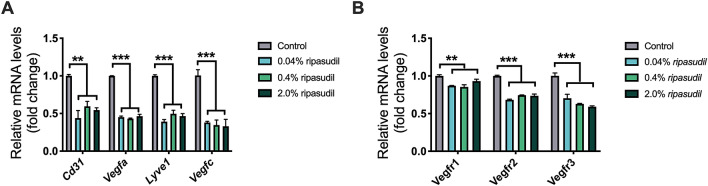
To investigate the effects of the topical administration of ripasudil on angiogenic signals in grafted corneas, we analyzed the mRNA levels of angiogenic and lymphangiogenic genes in the grafted cornea through reverse transcription-quantitative PCR (RT-qPCR) on day 14 post-transplantation (Fig. 2). Pecam1 (Cd31), Vegfa, Vegfc, Lyve1, and Vegf receptor (Vegfr)1, 2, 3 mRNA were significantly downregulated in the ripasudil groups than in the control group (Fig. 2A,B; n = 3).
Figure 2.
Expression of angiogenesis and lymphangiogenesis markers in the grafted cornea on day 14 post-transplantation. (A) mRNA levels of angiogenesis (Cd31 and Vegfa) and lymphangiogenesis (Lyve1 and Vegfc) markers in the 0.04%, 0.4%, and 2.0% ripasudil groups compared to those in the control (n = 3, one-way ANOVA; **p < 0.01, ***p < 0.001). (B) mRNA levels of Vegfr1, 2, and 3 in the 0.04%, 0.4%, and 2.0% ripasudil groups compared to those in the control (n = 3, one-way ANOVA; **p < 0.01, ***p < 0.001). Vegf vascular endothelial growth factor, Lyve1 lymphatic vessel endothelial hyaluronan receptor-1, ANOVA analysis of variance, Vegfr vascular endothelial growth factor receptor.
Topical administration of ripasudil decreased leukocyte infiltration and inflammation-related mRNA expression in grafted corneas
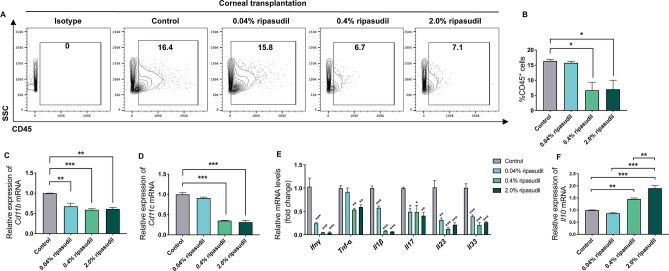
To determine the effect of ripasudil on the leukocyte infiltration of allografts, we examined CD45+ leukocytes in the grafted corneas through flow cytometry. CD45+ leukocytes in the grafted corneas were enumerated through flow cytometry on day 14 post-transplantation (Fig. 3A). The frequency of occurrence of CD45+ cells was significantly lower in the grafted corneas in the 0.4% and 2.0% ripasudil groups than in the control group (Fig. 3B, n = 3, control vs. 0.4% ripasudil, p = 0.031; control vs. 2.0% ripasudil, p = 0.039). The expression levels of Cd11b and Cd11c, surface markers of leukocytes such as neutrophils, macrophages, and dendritic cells (DCs)21–23, were significantly lower in the 0.4% and 2.0% ripasudil groups than in the control group (Fig. 3C, Fig. 3D, n = 3). The expression levels of inflammatory cytokines in the grafted corneas were assessed through RT-qPCR on day 14 post-transplantation. Interferon (Ifn)γ, Tumor necrosis factor (Tnf)-α, Interleukin (Il)1β, Il17, Il23, and Il33 mRNA were significantly downregulated in the ripasudil groups than in the control group (Fig. 3E). Furthermore, the mRNA level of the immunoregulatory cytokine Il10 was significantly higher in the grafted corneas in the 0.4% and 2.0% ripasudil groups than in the control group (Fig. 3F, n = 3, p < 0.01 and p < 0.001, respectively).
Figure 3.
Leukocyte infiltration and inflammation-related mRNA levels in corneal grafts. (A) Representative flow cytometry plots and (B) statistical analysis of flow cytometry data showing the frequency of occurrence of infiltrating CD45+ leukocytes in grafted corneas at day 14 post-transplantation (n = 3, one-way ANOVA; *p < 0.05). mRNA levels of Cd11b (C) and Cd11c (D) expressed on the surface of leukocytes, including neutrophils, macrophages, and dendritic cells, in grafted corneas on day 14 post-transplantation (Cd11b n = 3, one-way ANOVA, **p < 0.01, ***p < 0.001; Cd11c n = 3, one-way ANOVA, ***p < 0.001). (E) mRNA levels of inflammation-related markers in grafted corneas on day 14 post-transplantation (n = 3, one-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001). (F) mRNA level of Interleukin (Il)10 in the grafted cornea on day 14 post-transplantation (n = 3, one-way ANOVA; **p < 0.01, ***p < 0.001). Statistical analyses of flow cytometry data were performed using FlowJo software X 10.5.3. (FlowJo LLC, Ashland, OR, USA; purchased from https://www.flowjo.com). ANOVA analysis of variance, Ifnγ Interferonγ, Tnf-α Tumor necrosis factor-α.
Topical administration of ripasudil suppressed DC maturation, and IFNγ- and IL-17- expressing effector T cell generation in dLNs
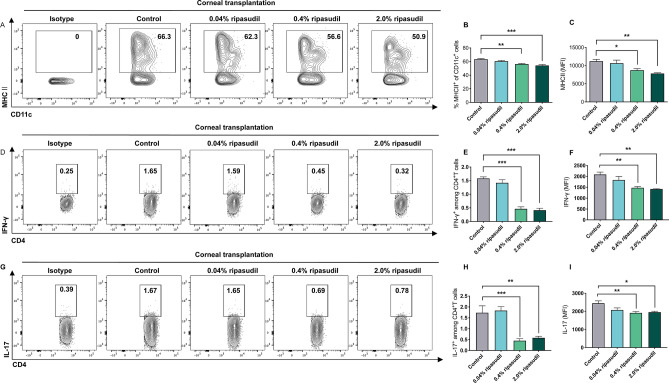
To investigate the effect of topical administration of ripasudil on dendritic cells (DCs) maturation, we determined the frequency of occurrence of CD11c+ major histocompatibility complex (MHC) II+ DCs and the expression of MHC II in the draining lymph nodes (dLNs) through flow cytometry 14 days after corneal transplantation (Fig. 4A–C). The frequency of occurrence of CD11c+ MHC II+ DCs was significantly lower in the 0.4% and 2.0% ripasudil groups than in the control group (Fig. 4B; n = 5; p = 0.002, p < 0.001, respectively). Furthermore, the mean fluorescence intensity of MHC II among dLNs was significantly lower in the 0.4% and 2.0% ripasudil groups than in the control group (Fig. 4C; n = 5; p = 0.029 and p = 0.002, respectively). The frequencies of IFNγ- and IL-17-expressing T cells (Fig. 4D,E,G,H) and mean fluorescence intensities of IFNγ and IL-17 (Fig. 4F; n = 5; p = 0.002, p = 0.001, respectively and 4I; n = 5; p = 0.007, p = 0.013, respectively) were lower in the 0.4% and 2.0% ripasudil groups than in the control.
Figure 4.
Topical administration of ripasudil suppressed dendritic cells (DCs) maturation, and Interferon (IFN)γ- and Interleukin (IL)-17-expressing effector T cell generation in draining lymph nodes (dLNs). (A) Representative flow cytometry plot showing major histocompatibility complex (MHC) II+ CD11c+ DCs in dLNs 14 days after corneal transplantation. (B) Frequencies of MHC II+ CD11c+ DCs in dLNs. (C) Mean fluorescence intensity of MHC II in CD11c+ cells from dLNs. (D) Representative flow cytometry plot showing CD4+ IFNγ+ cells. (E) Frequencies of occurrence of CD4+ IFNγ+ cells in dLNs (F) Mean fluorescence intensity of IFNγ in CD4+ cells from dLNs. (G) Representative flow cytometry plot showing CD4+ IL-17+ cells. (H) Frequencies of occurrence of CD4+ IL-17+ cells in dLNs. (I) Mean fluorescence intensity of IL-17 in CD4+ cells from dLNs. Statistical analyses of flow cytometry data were performed using FlowJo software X 10.5.3. (FlowJo LLC, Ashland, OR, USA; purchased from https://www.flowjo.com).
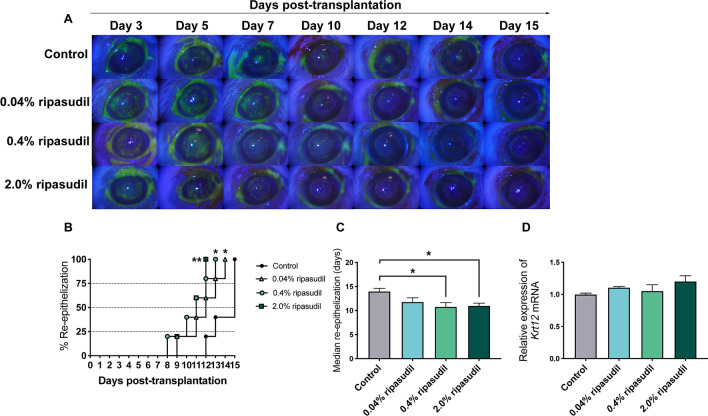
Topical administration of ripasudil promoted re-epithelization of the grafted cornea
Re-epithelization of a grafted cornea is essential not only for the potential immunological role of the corneal epithelium but also for corneal surface protection. Figure 5A shows representative images of re-epithelization of the grafted cornea obtained using a slit-lamp biomicroscope with cobalt blue light. Topical administration of ripasudil significantly promoted re-epithelization of the grafted cornea (Fig. 5B, n = 5; 0.04% ripasudil, p = 0.047; 0.4% ripasudil, p = 0.002; 2.0% ripasudil, p = 0.008). Topical administration of ripasudil improved the median re-epithelization time in the 0.4% and 2.0% ripasudil groups compared to that in the control group (Fig. 5C, p = 5; p = 0.103, p = 0.040, p = 0.024 for 0.04%, 0.4%, and 2.0% ripasudil vs. control, respectively). The expression of cytokeratin 12 (Krt12), a corneal epithelial differentiation marker24, did not differ significantly between the groups (Fig. 5D).
Figure 5.
Ripasudil promoted the re-epithelization of corneal grafts. (A) Representative images of grafted corneas with fluorescein staining after topical administration with ripasudil post-corneal transplantation (n = 5 mice per group). (B) Re-epithelization rate of corneal grafts after treatment with 0.04%, 0.4%, and 2.0% ripasudil post-corneal transplantation (*p = 0.018, *p = 0.018, **p = 0.008, respectively; log-rank test, n = 5 mice per group). (C) Median time required for re-epithelization of corneal grafts after treatment with 0.4% and 2.0% ripasudil post-corneal transplantation (Mann–Whiteney test, *p = 0.040 and *p = 0.024, respectively). (D) Relative mRNA level of cytokeratin 12 (Krt12) did not differ significantly between the groups (one-way ANOVA, n = 5 mice per group). The re-epithelization rate of corneal grafts was analyzed using ImageJ version 1.53a (National Institutes of Health, Bethesda, MD, USA; available at https://rsb.info.nih.gov/ij/index.html)57,58. ANOVA analysis of variance.
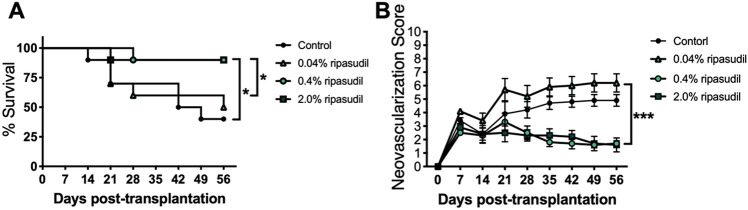
Topical administration of ripasudil prolonged graft survival
Administration of 0.4% and 2.0% ripasudil significantly prolonged graft survival (Fig. 6A, n = 7; 0.4% ripasudil, p = 0.024; 2.0% ripasudil, p = 0.025) and reduced neovascularization (Fig. 6B, n = 7; p < 0.001 for both) compared to that in the control group.
Figure 6.
Topical administration of ripasudil promoted corneal graft survival. (A) Weekly examinations of grafted corneas for 8 weeks post-transplantation treated with 0.4% and 2.0% ripasudil (log-rank test, *p = 0.026 and *p = 0.025, respectively; n = 10 mice per group). (B) Weekly examinations of neovascularization scores of grafted corneas 8 weeks post-transplantation (two-way ANOVA, ***p < 0.001, ***p < 0.001, respectively, n = 10 mice per group). (C) Weekly examinations of graft opacity scores of grafted corneas 8 weeks post-transplantation (two-way ANOVA, ***p < 0.001, ***p < 0.001, respectively, n = 10 mice per group). ANOVA analysis of variance.
Discussion
In this study, we investigated the efficacy of ripasudil, a ROCK inhibitor, in controlling the immune reactions and wound healing responses upon corneal transplantation when applied topically on a transplanted cornea. We found that topical administration of ripasudil mediates immunological and wound healing responses owing to its ability to be rapidly deactivated downstream of neovascularization and inflammation. These findings suggest that topical administration of ripasudil might be an effective treatment alternative for the management of corneal transplantation.
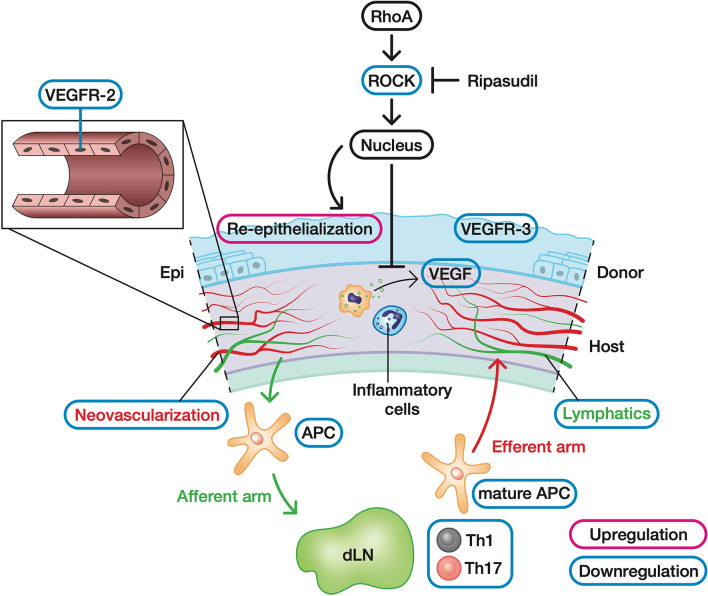
After corneal transplantation, upregulation of proangiogenic and proinflammatory factors, along with epithelial and stromal damage at the local graft site, cause corneal infiltration of immune cells and induction of neovascularization and lymphangiogenesis25,26. The circulating immune responses of afferent (lymphatic) and efferent (vascular) arms contribute to the loss of immune privilege and subsequent rejection27. These results suggest that topical administration of ripasudil locally suppressed the upregulation of proangiogenic and proinflammatory factors and promoted graft re-epithelization, resulting in the reduction of circulating immune responses (Fig. 7).
Figure 7.
Mechanism of action of topically applied ripasudil involves suppression of local angiogenesis. Topical administration of the ROCK inhibitor ripasudil suppressed the local upregulation of proangiogenic factors and promoted graft re-epithelization, resulting in the reduction in circulating immune responses. Rho-A ras homolog family member A, ROCK Rho-associated coiled-coil-containing protein kinase, VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor, APC antigen presenting cell, dLN draining lymph node.
In corneal transplantation, induced local inflammation and epithelial and stromal damage lead to infiltration of inflammatory cells, primarily neutrophils and macrophages28. The infiltrating neutrophils and macrophages secrete VEGF. VEGFR-2 along with its ligands, VEGF-A and VEGF-C, is the principal signaling receptor for vascular endothelial cells. As ripasudil selectively inhibits the ROCK pathway and VEGF secretion in these cells, VEGF-A and VEGF-C secretion may have been reduced. We found that topical administration of ripasudil downregulated VEGFR-2 because the vascular endothelial cells themselves were reduced in the grafted cornea by the immunosuppressive function of ripasudil. Previous studies have reported that ROCK inhibitors regulate the proliferation of vascular endothelial cells by reducing VEGF-induced ROCK activation20,29. To determine the direct effects of ripasudil on vascular endothelial cells, further in vitro experiments are needed.
Angiogenesis is accompanied through significant inflammation30. After corneal transplantation, inflammatory cytokines are released, and inflammatory cells are recruited to the grafted cornea25,31. Thereafter, CNV is induced in the grafted cornea, delaying the epithelialization of corneal grafts, and triggering corneal rejection32. Topical administration of ripasudil reduced the number of CD45+ infiltrating cells and inflammatory cytokine expression in the grafted cornea. TNF-α and IL-1β were significantly downregulated in the 0.4% and 2.0% ripasudil groups compared to the 0.04% ripasudil group, indicating that their downregulation contributes to corneal graft survival. In particular, of the cytokines examined in this study, only TNF-α was not suppressed upon 0.04% ripasudil administration in corneal grafts, suggesting that TNF-α is a potential key regulator of suppressing inflammation, angiogenesis, and lymphangiogenesis upon corneal transplantation33,34. A previous study reported that ROCK inhibition protected the vascular endothelium by inhibiting neutrophil adhesion35 and downregulating inflammatory cytokines including VEGF, TNF-α, matrix metalloproteinase (MMP)-2, and MMP-936–38. Furthermore, this study elucidates the efficacy of ripasudil in reducing the infiltration of inflammatory cells to sites in the grafted cornea after reducing the levels of the associated inflammatory cytokines at the site of injury.
Delayed corneal epithelial wound healing causes corneal infiltration of inflammatory cells and subsequent VEGF secretion39,40. Therefore, epithelial regeneration after corneal transplantation is important for graft survival. ROCK contributes to cell proliferation and migration14. The migration of recipient corneal epithelial cells is initiated 5 days after transplantation. Subsequently, the recipient epithelial cells disperse and are distributed in the graft cornea from the periphery to the center by approximately 10 days post-transplantation. Finally, re-epithelization of the grafted cornea is completed 15 days after transplantation41. Administration of 0.4% and 2.0% ripasudil promoted re-epithelization of the grafted cornea, concurrent with the a previous report on would healing42. As the epithelium serves as a significant barrier to graft acceptance41, rapid graft re-epithelization is critical for visual acuity, graft transparency, and protection of the stroma against infection and melting43. In particular, promotion of re-epithelization by the ROCK inhibitor may suppress the induction of inflammation, CNV, infection, and even graft rejection.
Owing to the reduction in local angiogenic factor levels and inflammation and promotion of epithelial regeneration by ripasudil administration, angiogenesis and lymphangiogenesis were suppressed, and graft survival was prolonged. DCs and Th1 and Th17 cells act as important mediators in the immune response at the early stage of corneal allograft rejection8,44–46. We found that the numbers of mature DCs and IFNγ+ Th1 and IL-17+ Th17 cells decreased in the dLNs, indicating that ripasudil administration inhibited angiogenesis and lymphangiogenesis and subsequently suppressed the reduction in antigen presentation in the dLNs and migration of the Th1 and Th17 cells to the grafted cornea. Furthermore, fasudil, a ROCK inhibitor, regulates the proportions of IFNγ+ Th1 and IL-17+ Th17 cells, indicating that the ROCK inhibitor itself may have a local immunosuppressive effect47. In the efferent arm, anti-neovascularization treatments are especially important for blocking the direct and indirect migration of donor antigen-presenting cells48. The afferent arm is characterized by migration of the allogeneic antigens via the lymphatic tissues. This study shows that topical administration of ripasudil suppressed lymphangiogenesis by downregulating VEGFR-3 along with its ligands, VEGF-C and VEGF-D. A previous study reported the interaction between angiogenesis and lymphangiogenesis49, suggesting that the anti-angiogenic property of ripasudil was responsible for suppressing angiogenesis and lymphangiogenesis in the grafted cornea.
There are several limitations to this study. This study identified the inhibitory effects of ripasudil for CNV using a corneal transplantation model. However, since CNV occurs in various diseases, further studies using different CNV models are necessary. Furthermore, this study did not investigate the potential immunosuppressive effect of topical administration of ripasudil with steroids. Steroids are frequently topically administered after corneal transplantation50. Therefore, it is necessary to investigate whether adding ripasudil to the steroid after corneal transplantation will further suppress the rejection. Recent studies have reported a protective effect of ROCK inhibitors on corneal endothelial cells51,52. Although the corneal endothelial cell density in corneal transplantation was not investigated in this study, we speculate that ripasudil may be effective for the long-term survival of corneal grafts because of the protective effect of ROCK inhibitors on corneal endothelial cells.
In summary, the ROCK inhibitor ripasudil inhibited CNV and inflammation and subsequently promoted graft survival. Ripasudil administration might satisfy unmet medical needs in the treatment of corneal transplantation, which require suppression of corneal neovascularization and inflammation.
Materials and methods
Animals and anesthesia
BALB/c (H-2d) and C57BL/6 (H-2b) male mice (6–8 weeks old) were purchased from Sankyo Labo Service Corporation, Inc. (Tokyo, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Juntendo University Graduate School of Medicine (Approval No. 2020231) and were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Anesthesia was administered intraperitoneally (ketamine/xylazine solution at 120 mg/kg body weight and 20 mg/kg body weight, respectively).
Allogeneic corneal transplantation
For allogeneic corneal transplantation, the corneas of C57BL/6 mice were grafted onto BALB/c host beds as described previously53. In brief, the central cornea (2 mm in diameter) was excised from a donor C57BL/6 mouse using scissors (Vannas-Storz Instruments, San Dimas, CA, USA). The graft bed was prepared by excising a 1.5-mm site in the central cornea of a BALB/c mouse. The donor button was then placed onto the recipient bed and secured with eight interrupted 11-0 nylon sutures. After surgery, the host eyelids were closed for 3 days, and the interrupted corneal sutures were removed 7 days after surgery.
Grafted cornea assessment
The graft’s neovascularization score, opacity score, and survival rate were evaluated for 8 weeks using a slit-lamp biomicroscope. We used a standardized scoring system to assess the neovascularization score (range, 0–8) and opacity score (range, 0–5+)53. Corneas with an opacity score of 2+ for two consecutive examinations were rejected. Re-epithelization of the grafted cornea was assessed using 0.5% fluorescein staining under a slit-lamp biomicroscope with cobalt blue light.
Eye drop treatment
Ripasudil (K-115), a novel ROCK inhibitor, was obtained from Kowa Company Ltd. (Nagoya, Japan)18. To assess the pharmacological efficacy of ripasudil in the murine cornea transplantation model, 0.04%, 0.4%, and 2.0% ripasudil (n = 5–7 mice/group) was administered thrice daily in accordance with a previous study20,54,55. The vehicle of ripasudil was used as the control56, which contained sodium dihydrogen phosphate as a buffering agent, glycerin as an isotonic agent, sodium hydroxide as a pH-adjusting agent (pH range 5–7), and benzalkonium chloride as a preservative.
Corneal whole mount and immunofluorescence staining
Freshly excised corneas were washed with phosphate-buffered saline on day 14 post-transplantation. The corneal epithelium was removed after incubation with 20 mM ethylenediaminetetraacetic acid for 60 min at 37 °C, fixed in acetone for 15 min at 20–22 °C, and blocked in 2% bovine serum albumin for 60 min. The corneas were double-stained overnight for CD31 (Santa Cruz Biotechnology, Dallas, TX, USA) and LYVE-1 (AF2125, R&D Systems, MN, USA) using goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated CD31 (1:100) and purified goat anti-mouse (1:400) LYVE-1, respectively, as described previously57. Cy3-conjugated donkey anti-goat (1:2000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) antibody was then added as a secondary antibody and incubated for 2 h. Stained whole corneas were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA, USA). The stained whole mount corneas were observed under a fluorescence microscope (BZ-X710, Keyence, Osaka, Japan). The area of the whole grafted cornea covered by blood or lymphatic vessels was analyzed using ImageJ version 1.53a (National Institutes of Health, Bethesda, MD, USA; available at https://rsb.info.nih.gov/ij/index.html)57,58.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
The excised corneas were immediately submerged in RNAlater solution (Ambion, Austin, TX, USA). Total RNA was isolated from five corneas per group using a NucleoSpin RNA isolation kit (Macherey-Nagal GmbH, Duren, Germany) in accordance with the manufacturer’s instructions. The cDNA was reverse-transcribed from total RNA using random primers and the ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) in accordance with the manufacturer’s guidelines. The qPCR primers specific for mouse mRNA are enlisted in Table S1. qPCR was performed with the ABI PRISM 7300 HT sequence detection system using the FAST-SYBR Green master mix (Life Technology Japan, Tokyo, Japan). Results were analyzed using the comparative cycle threshold method, and Gapdh mRNA expression in the same cDNA was used as the internal control.
Flow cytometry analysis
Corneas and ipsilateral dLNs were harvested, and single-cell suspensions were prepared as described previously53,59. To avoid non-specific staining, cells were blocked with an anti-FcR blocking antibody (eBioscience, San Diego, CA, USA). The isolated cells were stained with the respective antibodies. Mature DCs were stained with anti-CD11c Alexa488 (N418, BioLegend, CA, USA), anti-CD45 PE (30-F11, eBioscience), and anti-I-A/I-E PeCy7 (M5/114.15.2, BioLegend). For intracellular IFNγ and IL-17 staining, the cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate, and 500 ng/mL ionomycin (Sigma-Aldrich, St. Louis, MO, USA) for 6 h at 37 °C in a 5% CO2 incubator in the presence of GolgiStop (0.7 μL per 100 μL cell culture; BD Biosciences, San Jose, CA, USA) to inhibit cytokine secretion. The cells were then stained with anti-CD4 FITC, anti-IFNγ APC (XMG1.2), and anti-IL-17 PECy7 (TC11-18H10.1) (BioLegend) antibodies. All antibodies and their matched isotype controls, and the fixation and permeabilization buffers were purchased from eBioscience. The stained cells were examined using LSR Fortessa (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo software X 10.5.3. (FlowJo LLC, Ashland, OR, USA; purchased from https://www.flowjo.com).
Statistical analysis
Experiments with more than two groups were analyzed using one-way or two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison post-hoc test. The Mann–Whitney test was performed to compare medians between the groups. Kaplan–Meier analysis was performed to construct corneal graft re-epithelization curves and to evaluate graft survival post-corneal transplantation, and the log-rank test was performed to compare corneal re-epithelization of the grafted corneas and graft survival post-corneal transplantation. Data are presented as mean ± standard error of mean values and were considered statistically significant at p < 0.05. All statistical analyses were performed using Prism version 8.0 software (GraphPad, La Jolla, CA, USA).
Supplementary information
Acknowledgements
The staff of the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine provided technical assistance. A joint research agreement was concluded between Juntendo University and Kowa Company, Ltd., and this joint research project was funded in part by Kowa Company, Ltd.
Author contributions
T.I. conceived of the study. T.I. initiated the study design and K.F., Y.O., J.Z., K.F., H.S., M.M., M.O., and N.N. helped with implementation. T.I. and J.S. wrote the paper. T.F., and A.M. provided critical advice to the project and edited the paper. All authors contributed to refinement of the study protocol and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
Drs. Inomata, Funaki, and Murakami have a patent REJECTION REACTION SUPPRESSANT of K-115 issued. All other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76882-w.
References
- 1.Gain P, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 2.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Sanfilippo F, MacQueen JM, Vaughn WK, Foulks GN. Reduced graft rejection with good HLA-A and B matching in high-risk corneal transplantation. N. Engl. J. Med. 1986;315:29–35. doi: 10.1056/NEJM198607033150105. [DOI] [PubMed] [Google Scholar]
- 4.Streilein JW, Yamada J, Dana MR, Ksander BR. Anterior chamber-associated immune deviation, ocular immune privilege, and orthotopic corneal allografts. Transplant. Proc. 1999;31:1472–1475. doi: 10.1016/S0041-1345(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 5.Qazi Y, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J. Clin. Cell Immunol. 2013 doi: 10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JH, et al. Corneal neovascularization: an anti-VEGF therapy review. Surv. Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005;19:1133–1135. doi: 10.1038/sj.eye.6701973. [DOI] [PubMed] [Google Scholar]
- 8.Inomata T, Hua J, Di Zazzo A, Dana R. Impaired function of peripherally induced regulatory T cells in hosts at high risk of graft rejection. Sci. Rep. 2016;6:39924. doi: 10.1038/srep39924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann BO, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch. Ophthalmol. 2008;126:71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Azimzade Y, Hong J, Mashaghi A. Immunophysical analysis of corneal neovascularization: mechanistic insights and implications for pharmacotherapy. Sci. Rep. 2017;7:12220. doi: 10.1038/s41598-017-12533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock F, Konig Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 12.Cursiefen C, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–1637. doi: 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari G, et al. Topical ranibizumab as a treatment of corneal neovascularization. Cornea. 2013;32:992–997. doi: 10.1097/ICO.0b013e3182775f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr. Biol. 1999;9:136–145. doi: 10.1016/S0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- 16.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 2003;23:211–217. doi: 10.1161/01.ATV.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- 17.Hata Y, et al. Antiangiogenic properties of fasudil, a potent Rho-Kinase inhibitor. Jpn. J. Ophthalmol. 2008;52:16–23. doi: 10.1007/s10384-007-0487-5. [DOI] [PubMed] [Google Scholar]
- 18.Garnock-Jones KP. Ripasudil: first global approval. Drugs. 2014;74:2211–2215. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 19.Hida Y, et al. Effects of ripasudil, a ROCK inhibitor, on retinal edema and nonperfusion area in a retinal vein occlusion murine model. J. Pharmacol. Sci. 2018;137:129–136. doi: 10.1016/j.jphs.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M, et al. Vascular normalization by ROCK inhibitor: therapeutic potential of ripasudil (K-115) eye drop in retinal angiogenesis and hypoxia. Invest. Ophthalmol. Vis. Sci. 2016;57:2264–2276. doi: 10.1167/iovs.15-17411. [DOI] [PubMed] [Google Scholar]
- 21.Sahu SK, et al. Mast cells initiate the recruitment of neutrophils following ocular surface injury. Invest. Ophthalmol. Vis. Sci. 2018;59:1732–1740. doi: 10.1167/iovs.17-23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005;115:2363–2372. doi: 10.1172/jci23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inomata T, et al. Perlecan-deficient mutation impairs corneal epithelial structure. Invest. Ophthalmol. Vis. Sci. 2012;53:1277–1284. doi: 10.1167/iovs.11-8742. [DOI] [PubMed] [Google Scholar]
- 25.Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J. Immunol. 2016;196:3983–3991. doi: 10.4049/jimmunol.1600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong W, et al. Angiogenesis and lymphangiogenesis in corneal transplantation: a review. Surv. Ophthalmol. 2018;63:453–479. doi: 10.1016/j.survophthal.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan SK, Dohlman TH, Dana R. Corneal lymphatics: role in ocular inflammation as inducer and responder of adaptive immunity. J. Clin. Cell Immunol. 2014 doi: 10.4172/2155-9899.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cursiefen C, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, et al. Rho-associated coiled-coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics. 2018;8:6053–6069. doi: 10.7150/thno.30305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clements JL, Dana R. Inflammatory corneal neovascularization: etiopathogenesis. Semin. Ophthalmol. 2011;26:235–245. doi: 10.3109/08820538.2011.588652. [DOI] [PubMed] [Google Scholar]
- 31.Di Zazzo A, et al. Proangiogenic function of T cells in corneal transplantation. Transplantation. 2017;101:778–785. doi: 10.1097/TP.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017;62:816–827. doi: 10.1016/j.survophthal.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 34.Dana R. Comparison of topical interleukin-1 versus tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 35.Arita R, et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celik F, et al. Comparison of the effect of intravitreal bevacizumab and intravitreal fasudil on retinal VEGF, TNFα, and caspase 3 levels in an experimental diabetes model. Int. J.. Ophthalmol. 2014;7:57–61. doi: 10.3980/j.issn.2222-3959.2014.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma DW, et al. The effect of fasudil via Rho/ROCK signaling pathway on the inflammation and fibrosis in human mesangial cells in high glucose medium. Zhonghua Nei Ke Za Zhi. 2011;50:580–584. [PubMed] [Google Scholar]
- 38.Yang X, Zhang Y, Wang S, Shi W. Effect of fasudil on growth, adhesion, invasion, and migration of 95D lung carcinoma cells in vitro. Can. J. Physiol. Pharmacol. 2010;88:874–879. doi: 10.1139/y10-047. [DOI] [PubMed] [Google Scholar]
- 39.Wilson SE, et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 40.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 41.Hori J, Streilein JW. Dynamics of donor cell persistence and recipient cell replacement in orthotopic corneal allografts in mice. Invest. Ophthalmol. Vis. Sci. 2001;42:1820–1828. [PubMed] [Google Scholar]
- 42.Zeng P, et al. Fasudil hydrochloride, a potent ROCK inhibitor, inhibits corneal neovascularization after alkali burns in mice. Mol. Vis. 2015;21:688–698. [PMC free article] [PubMed] [Google Scholar]
- 43.Dellaert MM, et al. Influence of topical human epidermal growth factor on postkeratoplasty re-epithelialisation. Br. J. Ophthalmol. 1997;81:391–395. doi: 10.1136/bjo.81.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahvildari M, et al. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation. 2016;100:525–532. doi: 10.1097/TP.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, et al. A pathogenic role of IL-17 at the early stage of corneal allograft rejection. Transpl. Immunol. 2009;21:155–161. doi: 10.1016/j.trim.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Inomata T, et al. Corneal tissue from dry eye donors leads to enhanced graft rejection. Cornea. 2018;37:95–101. doi: 10.1097/ICO.0000000000001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song J, et al. Inhibition of ROCK activity regulates the balance of Th1, Th17 and Treg cells in myasthenia gravis. Clin. Immunol. 2019;203:142–153. doi: 10.1016/j.clim.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen P, Yiu SC. Strategies for local gene therapy of corneal allograft rejection. Middle East Afr. J. Ophthalmol. 2013;20:11–25. doi: 10.4103/0974-9233.106382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osaki T, Serrano JC, Kamm RD. Cooperative effects of vascular angiogenesis and lymphangiogenesis. Regen. Eng. Transl. Med. 2018;4:120–132. doi: 10.1007/s40883-018-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudde T, Minassian DC, Larkin DF. Randomised controlled trial of corticosteroid regimens in endothelial corneal allograft rejection. Br. J. Ophthalmol. 1999;83:1348–1352. doi: 10.1136/bjo.83.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okumura N, Kinoshita S, Koizumi N. The role of Rho kinase inhibitors in corneal endothelial dysfunction. Curr. Pharm. Des. 2017;23:660–666. doi: 10.2174/1381612822666161205110027. [DOI] [PubMed] [Google Scholar]
- 52.Okumura N, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012;181:268–277. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Inomata T, Mashaghi A, Di Zazzo A, Dana R. Ocular surgical models for immune and angiogenic responses. J. Biol. Methods. 2015;2(3):e27. doi: 10.14440/jbm.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okumura N, et al. Effect of the Rho-associated kinase inhibitor eye drop (ripasudil) on corneal endothelial wound healing. Invest. Ophthalmol. Vis. Sci. 2016;57:1284–1292. doi: 10.1167/iovs.15-18586. [DOI] [PubMed] [Google Scholar]
- 55.Alkharashi M, AlAbbasi O, Magliyah M. Perioperative use of Rho-kinase inhibitors has beneficial effect on corneal endothelium after phacoemulsification. Middle East Afr. J. Ophthalmol. 2019;26:246–249. doi: 10.4103/meajo.MEAJO_27_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isobe T, Kasai T, Kawai H. Ocular penetration and pharmacokinetics of ripasudil following topical administration to rabbits. J. Ocul Pharmacol. Ther. 2016;32:405–414. doi: 10.1089/jop.2016.0028. [DOI] [PubMed] [Google Scholar]
- 57.Inomata T, et al. Kinetics of angiogenic responses in corneal transplantation. Cornea. 2017;36:491–496. doi: 10.1097/ICO.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inomata T. A new immunotherapy using regulatory T-cells for high-risk corneal transplantation. Juntendo Med. J. 2017;63:2–7. doi: 10.14789/jmj.63.2. [DOI] [Google Scholar]
- 59.Ogawa M, Inomata T, Shiang T, Tsubota K, Murakami A. Method for selective quantification of immune and inflammatory cells in the cornea using flow cytometry. J. Biol. Methods. 2018;5:e102. doi: 10.14440/jbm.2018.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).