Abstract
Background
Solitary fibrous tumors (SFTs) are rare tumors, mostly derived from connective tissue mesenchymal cells that arise from the pleura. There are very few reports of primary pancreatic SFT. Preoperative diagnosis is difficult owing to the lack of distinctive radiological findings. We report a case of pancreatic SFT with particularly rare malignant findings.
Case presentation
A 60-year-old man was referred to the hospital because of a right upper quadrant mass and abnormal liver function test results. Contrast-enhanced computed tomography (CT) showed a well-defined enhanced tumor measuring approximately 8 cm in the pancreatic head. Magnetic resonance imaging (MRI) showed T1WI hypointensity, T2WI hyperintensity, and DWI hyperintensity. The main pancreatic duct and common bile duct were dilated owing to obstruction by the tumor. The following tumor markers were mildly elevated: carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), SPan-1, and DUPAN-2. The histological diagnosis obtained by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) was negative for pancreatic ductal carcinoma, malignant lymphoma and neuroendocrine tumor, suggesting the possibility of mesenchymal tumor, but the diagnosis was not confirmed. The patient was judged suitable for surgery and underwent subtotal stomach-preserving pancreatoduodenectomy with D2 lymph node dissection. On histopathological examination of the resected specimen, infiltrating spindle-shaped cells had proliferated, containing numerous mitotic figures, with necrotic findings inside the tumor. Immunostaining was positive for cluster of differentiation-34 (CD34), B cell CLL/lymphoma-2 (Bcl-2), and signal transducer and activator of transcription (STAT6). On the basis of these findings, a diagnosis of malignant pancreatic SFT was made. The patient remains free of recurrent disease after 12 months of follow-up without adjuvant therapy and he is being carefully followed up as an outpatient.
Conclusions
We experienced a case of malignant pancreatic head SFT. Immunohistochemical staining of the extracted specimens was useful for diagnosis.
Keywords: Solitary fibrous tumor, Pancreas, Malignant, Surgery
Background
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor typically located in the pleura that was first described in 1931 [1]. Several studies have since reported extra-pleural SFTs in almost every anatomic location. Most SFTs are characterized by a patternless distribution of both oval- and spindle-shaped cells in connective tissue. A correlation between either local recurrence or metastasis and histologic features such as necrosis, more than four mitoses per 10 high-power magnification fields (HPFs), increased nuclear pleomorphism, increased cellularity, tumor size larger than 10 cm, positive margins, and extra-thoracic location have been reported [2–4]. SFT of the pancreas was first reported in 1999 [5]. Because of its rarity, most reported pancreatic SFTs have demonstrated benign histopathologic features. We report a case of pancreatic SFT with malignant features confirmed by histopathology and immunohistochemical study.
Case presentation
A 60-year-old male was referred to the hospital because of a right upper quadrant mass and abnormal liver function test results. He had no significant medical history.
Abnormal laboratory findings included elevated AST: 406 U/l, ALT: 397 U/l, total bilirubin: 1.01 mg/dl, direct bilirubin: 0.61 mg/dl, ALP: 4380 U/l, γ-GTP: 1548 U/l, and amylase: 509 U/l. The serum tumor markers carcinoembryonic antigen (CEA): 8.6 ng/ml, carbohydrate antigen 19-9 (CA19-9): 261 U/ml, SPan-1: 100 U/ml, and DUPAN-2: 750 U/ml were elevated, but soluble interleukin-2 receptor concentration was normal (310 U/ml).
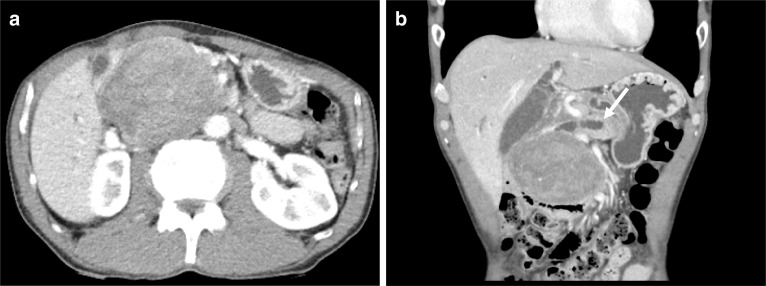
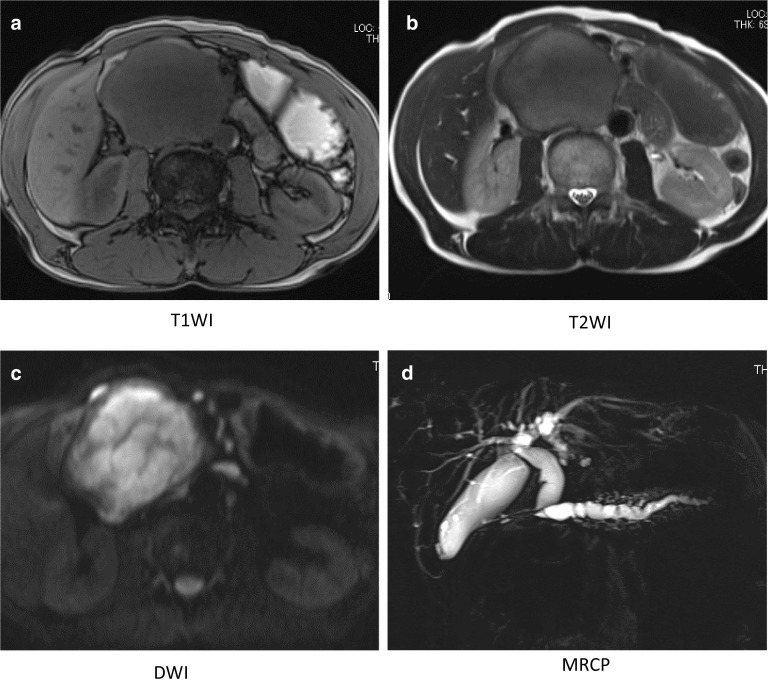
Abdominal ultrasonography revealed a well-demarked heterogeneously solid mass with a diameter of 8 cm in the head of the pancreas. Contrast-enhanced computed tomography (CT) imaging of the abdomen confirmed a 7 × 9 × 7 cm diameter exophytic mass in the head of the pancreas. The tumor was hypodense in the arterial phase, and then became weakly but uniformly hyperdense in the delayed phase. There was biliary stricture, disruption of the main pancreatic duct, and obstructive pancreatitis, but no obvious infiltration of the surrounding organs or major blood vessels or enlarged lymph nodes (Fig. 1a, b). The mass was hypointense on T1-weighted magnetic resonance imaging (MRI), hyperintense on T2-weighted images, and diffusely hyperintense on diffusion-weighted images (DWI) (Fig. 2a–c). MR cholangio-pancreatography showed dilatation of intra- and extra-hepatic bile ducts and the pancreatic duct owing to tumor obstruction (Fig. 2d).[18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET) showed heterogeneous accumulation in the pancreatic head tumor (SUV max = 7.65). Abnormal accumulation suggesting distant metastasis or lymph node metastasis was not observed (Fig. 3). These findings were atypical for a pancreatic ductal carcinoma. Trans-duodenal tumor biopsy by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) was performed. Cytological examination showed a single pattern of atypical cells with increased chromatin granularity. Histopathological examination showed proliferating short spindle-shaped cells, relatively large N/C ratio, and scattered mitotic figures. Immunostaining was positive for cluster of differentiation (CD) 34 and CD56, partially positive for cytokeratin AE1/AE3, and negative for synaptophysin, chromogranin A, somatostatin receptor type 2 (SSTR2), CD117, DOG1, S-100, smooth muscle actin (SMA), desmin, and CD31.
Fig. 1.
Abdominal CT. Enhanced CT shows a 7 × 9 × 7 cm tumor located in the pancreatic head (a). Dilatation of the distal pancreatic duct is seen (arrow) (b)
Fig. 2.
Abdominal MRI. MRI shows the tumor in the pancreatic head, with low signal intensity on T1-weighted imaging (a), low signal intensity on T2-weighted imaging (b), and high signal intensity on diffusion-weighted imaging (c). MR cholangio-pancreatography showing dilatation of intra- and extra-hepatic bile ducts and the pancreatic duct owing to tumor obstruction (d)
Fig. 3.
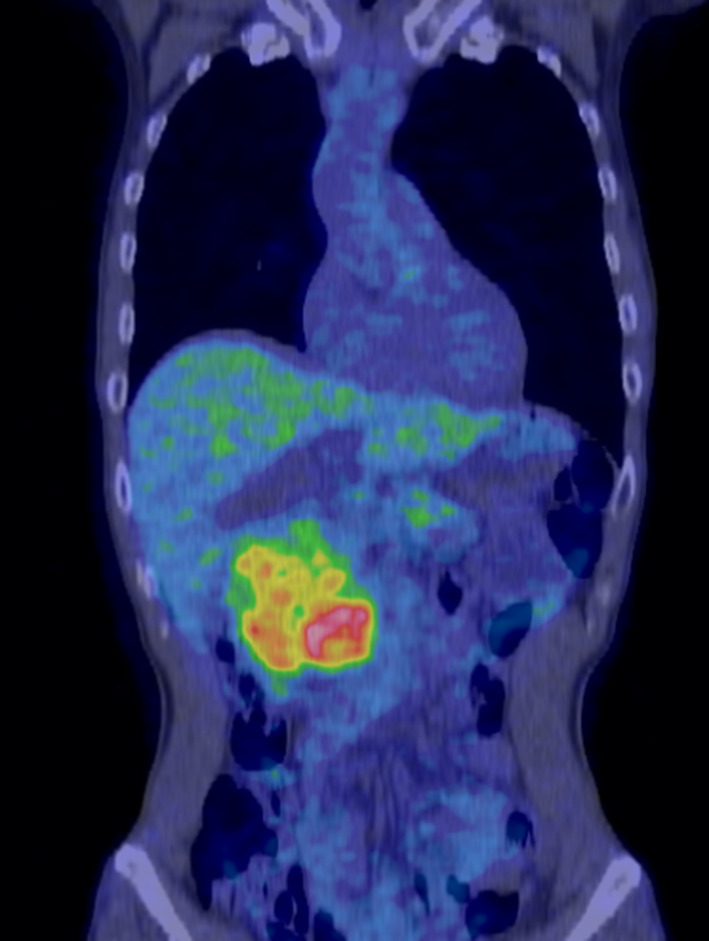
FDG-PET images. FDG-PET scan shows non-uniform increased uptake of fluorodeoxyglucose only in the pancreatic head (SUV max = 7.65)
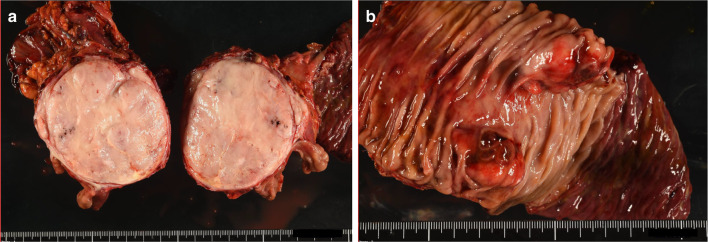
These findings suggested the possible diagnosis as SFT, extra-gastrointestinal stromal tumor (GIST), and neuroendocrine carcinoma, but the diagnosis was not confirmed because of limited amount of specimen and CD56 positive tumor. The EUS-FNA results indicated that the possibility of malignant lymphoma was low. Therefore, surgical resection was the therapy of choice for this large pancreatic head tumor. The patient underwent subtotal stomach-preserving pancreatoduodenectomy with D2 lymph node dissection. Intraoperative findings did not show ascites, peritoneal dissemination, or distant metastasis. A solid tumor measuring approximately 8 cm was found in the head of the pancreas, and the body of the pancreas showed obstructive pancreatitis. The tumor was firmly adherent to the surrounding tissues, such as the gallbladder, inferior vena cava, and mesentery owing to an inflammatory reaction. The inferior vena cava, superior mesenteric artery, and common hepatic artery were safely preserved by careful separation from the tumor. However, the border between the superior mesenteric vein and the tumor was partly unclear, and partial resection was required. The resected tumor was a yellowish-white, well-circumscribed mass measuring 8 × 8 × 6 cm and was located in the pancreatic head (Fig. 4a). A prominent lesion was observed in the duodenum, which was considered to be tumor invasion (Fig. 4b).
Fig. 4.
Macroscopic images of the resected specimen seen as a solid tumor located in the pancreatic head. The cut surface of the tumor is well-demarcated, heterogeneous, and yellowish-white in color (a). Small prominent lesions are seen in the duodenal mucosa, which were considered tumor invasion (b)
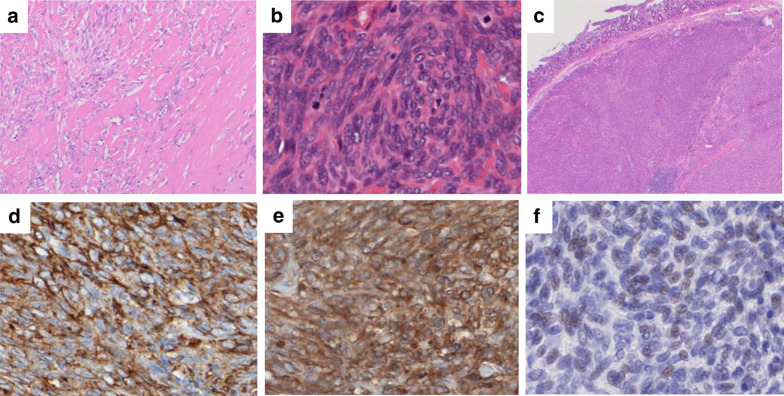
Pathological examination of the resected specimen demonstrated proliferating spindle-shaped cells involving normal pancreatic tissue, and it was considered that most of the cells were excreting and some were invasive (Fig. 5a). Fibrosis accompanied by hyalinization was observed at the margin of the tumor, and there were necrotic foci inside the tumor. Twelve mitotic figures were observed in 10 HPFs (Fig. 5b). In addition, venous infiltration and duodenal infiltration (Fig. 5c) were observed on the duodenal side in contact with the main lesion, suggesting a highly malignant tumor.
Fig. 5.
Histopathological findings of the resected specimen. Invasive growth of proliferated spindle-shaped cells in the pancreatic tumor. Hyalinized fibrosis is present at the periphery of the tumor (× 100) (a). The tumor showed high cellularity, increased mitotic figures (12/10 HPFs), and nuclear pleomorphism (increased N/C ratio) (× 200) (b). Tumor infiltration was observed on the duodenal side in contact with the main lesion (c). Immunohistochemically, the tumor cells were positive for CD34 (× 200) (d) and Bcl-2 (× 200) (e), and weakly positive for STAT6 (× 200) (f)
Immunohistochemical analysis of the resected tumor revealed that the tumor cells expressed positive results for CD34 (Fig. 5d), vimentin, and Bcl-2 (Fig. 5e), focally positive for cytokeratin AE1/AE3, and weakly positive for STAT6 (Fig. 5f). The tumor cells were negative for CD117, DOG1, SMA, desmin, S-100, synaptophysin, and chromogranin A. On the basis of the histology and immunostaining profile, the tumor was diagnosed as a malignant SFT of the pancreas.
The patient’s postoperative course was uneventful except for gastric stasis, which was treated with conservative management. The patient was discharged 22 days after surgery, and he remains free of recurrent disease after 12 months of follow-up without adjuvant therapy.
Discussion
SFT was reported by Klemperer et al. in 1931 as a tumor of the pleura [1]. SFT is a rare tumor, and Gold et al. reported that it constituted less than 2% of all soft tissue tumors [2]. SFT is a mesenchymal tumor typically located in the thoracic cavity, but the tumor can also be found in soft tissues and organs throughout the body [6]. SFT of the pancreas is extremely rare, with a total of 29 reported cases, including the present case [5, 7–33]. A summary of these cases is shown in Tables 1 and 2. The median age at diagnosis is 53 years, and there is no gender difference (14 males and 15 females reported). The most common tumor site is the pancreatic head, with 17 cases; 9 cases were reported in the pancreatic body and 3 cases in the pancreatic tail. Pancreatic resection was performed in 28 patients, and pancreatoduodenectomy and distal pancreatectomy were often performed, as with other pancreatic malignancies. Furthermore, mass enucleation was selected in 5 cases and central pancreatic resection in 1 case.
Table 1.
Patient characteristics of pancreatic solitary fibrous tumors
| Author | Year | Age, sex | Chief complaints | Size (cm) | Location | Primary diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| Lüttges et al. [5] | 1999 | 50, F | Incidental | 5.5 | Body | NET | DP |
| Chatti et al. [7] | 2006 | 41, M | Abdominal pain | 13 | Body | NET | Enucleation |
| Gardini et al. [8] | 2007 | 62, F | Abdominal pain | 3 | Head | NET | PD |
| Miyamoto et al. [9] | 2007 | 41, F | Abdominal pain | 2 | Head–body | NET | Enucleation |
| Srinivasan et al. [10] | 2008 | 78, F | Back pain weight loss | 5 | Body | Mesenchymal tumor | DP |
| Kwon et al. [11] | 2008 | 54, M | Incidental | 4.5 | Body | NET, SPT | Median segmentectomy |
| Ishiwatari et al. [12] | 2009 | 58, F | Incidental | 3 | Head | NET | PD |
| Chetty et al. [13] | 2009 | 67, F | Incidental | 2.6 | Head | NET | PD |
| Sugawara et al. [14] | 2010 | 55, F | Incidental | 7 | Head | NA | PD |
| Santos et al. [15] | 2012 | 40, M | Incidental | 3 | Body | NA | Partial pancreatectomy |
| Tasdemir et al. [16] | 2012 | 24, F | Epigastric pain | 18.5 | Head | Mesenchymal tumor | Enucleation |
| Azadi et al. [17] | 2012 | 57, M | Incidental | 3.1 | Tail | NA | DP |
| van der Vorst et al. [18] | 2012 | 67, F | Abdominal pain | 2.8 | Head | NET | Enucleation |
| Yamanashi et al. [19] | 2012 | 50, M | Incidental | 10 | Tail | NEC | DP |
| Chen et al. [20] | 2013 | 49, F | Abdominal pain | 13 | Head | PD | |
| Hwang et al. [21] | 2014 | 53, F | Incidental | 5.2 | Head | NET, SPT | PD |
| Han et al. [22] | 2015 | 77, F | Jaundice | 1.5 | Head | SFT | Conservative |
| Estrella et al. [23] | 2015 | 52, F | Jaundice | 15 | Head | NET | PD |
| Baxter et al. [24] | 2015 | 58, F | Abdominal pain | 3.5 | Head | NET, GIST, SPT, SFT | PD |
| Paramythiotis et al. [25] | 2016 | 55, M | Abdominal pain | 3.6 | Body | NET, SPT, GIST | DP |
| Murakami et al. [26] | 2016 | 82, M | Hypokalemia hypertension, edema | 6 | Tail | NET | DP |
| Spasevska et al. [27] | 2016 | 47, M | Epigastric pain jaundice | 3.5 | Head | Cystadenocarcinoma | PD |
| Clare et al. [28] | 2017 | 39, F | Incidental | 2.2 | Head | NA | PD |
| Sheng et al. [29] | 2017 | 1, M | Jaundice | 2 | Head | NA | PD |
| D'Amico et al. [30] | 2017 | 52, M | Incidental | 2 | Body | NET | Enucleation |
| Oana et al. [31] | 2017 | 73, M | Abdominal discomfort | 7.5 | Head | NET, ACC, GIST | Partial pancreatectomy |
| Geng et al. [32] | 2020 | 48, M | Hypoglycemia | 6.5 | Body | SFT, liver metastasis |
TACE, DP left lateral liver sectionectomy |
| Qian et al. [33] | 2020 | 46, M | Hypoglycemia | 7 | Body | NEC, liver metastasis |
TACE, DP left lateral liver sectionectomy |
| Present case | 60, M | Palpable mass | 8 | Head | NEC, GIST, SFT | PD |
NET neuroendocrine tumor, DP distal pancreatectomy, PD pancreatoduodenectomy, SPT solid pseudopapillary tumor, NA not applicable, NEC neuroendocrine carcinoma, SFT solitary fibrous tumor, GIST gastrointestinal stromal tumor, ACC acinar cell carcinoma, TACE transarterial chemoembolization
Table 2.
Histological features and outcomes of pancreatic solitary fibrous tumors
| Author | Positive immunohistochemistry | Malignant features | Diagnosis of malignant SFT | Recurrence | Outcome | Follow-up |
|---|---|---|---|---|---|---|
| Lüttges et al. [5] | CD34, CD99, Bcl-2, vimentin | No | No | No | Alive | 20 mo |
| Chatti et al. [7] | CD34, CD99, Bcl-2, vimentin | No | No | No | Died postoperative complications | 3 d |
| Gardini et al. [8] | CD34, CD99, Bcl-2, vimentin, SMA (focal) | NA | No | No | Alive | 16 mo |
| Miyamoto et al. [9] | CD34, Bcl-2 | No | No | No | Alive | 7 mo |
| Srinivasan et al. [10] | CD34, Bcl-2 | No | No | No | Alive | 7 mo |
| Kwon et al. [11] | CD34, CD99, vimentin | No | No | No | NA | NA |
| Ishiwatari et al. [12] | CD34, Bcl-2 | Necrosis | No | No | Alive | 42 mo |
| Chetty et al. [13] | CD34, CD99, Bcl-2 | No | No | No | Alive | 6 mo |
| Sugawara et al. [14] | CD34 | No | No | No | NA | NA |
| Santos et al. [15] | CD34, beta-catenin | No | No | No | NA | NA |
| Tasdemir et al. [16] | CD34, Bcl-2, beta-catenin, vimentin, Ki67 < 2% | No | No | No | Alive | 3 mo |
| Azadi et al. [17] | CD34, Bcl-2, Ki67 < 5% | No | No | No | NA | NA |
| van der Vorst et al. [18] | CD34, CD99, Bcl-2 | No | No | No | NA | NA |
| Yamanashi et al. [19] | CD34, vimentin, Bcl-2 | Intra-pancreatic metastasis, necrosis, > 2 mitoses/HPFs, hypercellularity | Yes | Intra-pancreatic | Alive | 32 mo |
| Chen et al. [20] | CD34, Bcl-2, vimentin, CD68, muscle-specific actin | Necrosis | No | No | Alive | 30 mo |
| Hwang et al. [21] | CD34, Bcl-2, muscle-specific actin, CD10, ER, PR | No | No | No | Alive | 30 mo |
| Han et al. [22] | CD34, CD99 | No | No | – | No progression | 10 mo |
| Estrella et al. [23] | CD34, Bcl-2, keratin (rare), p16, p53 | Nuclear atypia, necrosis 17 mitoses/10 HPFs, | Yes | No | Alive | 40 mo |
| Baxter et al. [24] | CD34, Bcl-2 | NA | No | No | NA | NA |
| Paramythiotis et al. [25] | CD34, CD99, Bcl-2, vimentin, S-100 (focal) | No | No | No | Alive | 40 mo |
| Murakami et al. [26] | STAT6, CD34, Bcl-2, ACTH (focal), POMC (focal), NSE (focal) | No | No | No | Died sepsis | 4 mo |
| Spasevska et al. [27] | CD34, vimentin, CD99, Bcl-2 (focal), nuclear beta-catenin (focal) | No | No | No | Died postoperative complications | 1 wk |
| Clare et al. [28] | STAT6, CD34, Bcl-2, CD56, cytokeratin CAM5.2, AE1/AE3 | 6/10 HPFs | Yes | No | Alive | 40 mo |
| Sheng et al. [29] | CD34, vimentin, SMA (focal), Ki67 < 3% | Mild–moderate nuclear pleomorphism 2–5/10 HPFs hypercellularity | No | No | Alive | 12 mo |
| D'Amico et al. [30] | STAT6, CD34 | No | No | No | Alive | 24 mo |
| Oana et al. [31] | CD34, Bcl-2 | No | No | No | Alive | 36 mo |
| Geng et al. [32] | STAT6, CD34, Bcl-2, CD31, PHH-3, D2-40, Ki67 > 10% | 4–5/10 HPFs necrosis | Yes | Residual liver tumor ( +) | Alive | 6 mo |
| Qian et al. [33] | STAT6, CD34, Bcl-2, Ki67 10% | Heterotypic cell 4–5/10 HPFs local infarction | Yes | NA multiple recurrence | Alive | 10 mo |
| Present case | STAT6, CD34, Bcl-2, vimentin, cytokeratin AE1/AE3(focal) | Hypercellularity 12/10 HPFs necrosis invasive growth | Yes | No | Alive | 12 mo |
SFT solitary fibrous tumor, HPFs high-power fields, CD cluster of differentiation, Bcl-2 B cell CLL/lymphoma-2, STAT6 signal transducer and activator of transcription 6, ER estrogen receptor, PR progesterone receptor, SMA smooth muscle actin, NA not applicable
Pancreatic SFT shows a well-defined mass with an internal heterogeneous contrast effect with CT, and exhibits hypointensity on T1WI and hyperintensity on T2WI with MRI, in most cases. These features are atypical, which makes it difficult to distinguish SFT from other soft tissue tumors [17, 32]. In our case, the tumor was visualized as a well-defined tumor with a weak contrast effect, and exhibited hypointensity on T1WI and hyperintensity on T2WI, with diffuse strong hyperintensity on DWI. On the basis of the above findings, we considered that the differential diagnoses of the tumor should include malignant lymphoma, neuroendocrine tumor, acinar cell carcinoma, extra-GIST, and SFT, but it was not possible to make a diagnosis, preoperatively. Histopathological examination, including immunohistochemical staining, was considered important, and a tumor biopsy was performed. However, biopsy also could not confirm the diagnosis, but malignant lymphoma could be ruled out, so we selected surgery as treatment.
Histologically, SFT shows two features: a patternless appearance in which elliptical- to spindle-shaped tumor cells grow randomly, and a hemangiopericytic growth pattern owing to vascular proliferation and perivascular sclerosis. Because SFT is a mesenchymal tumor, immunohistochemical staining is positive for CD34 and vimentin, and negative for mesothelial cell-derived cytokeratin and epithelial membrane antigen. Staining is also negative for S-100, which is positive for neurogenic tumors, and negative for c-kit, which is positive for GIST. These features are useful for distinguishing SFT from other mesenchymal tumors [6, 19]. Recently, it was revealed that NAB2–STAT6 fusion was the driver mutation in SFT, and the transcriptional repressor of the cell division pathway is converted to the transcriptional activator [34]. Therefore, STAT6 has been proven to be more sensitive (98%) and specific (85%) for SFT [32]. The present case was finally diagnosed as a SFT of the pancreas because the tumor was positive for STAT6.
Most SFTs are benign, but some are known to recur or metastasize. Previous reports have shown the histopathologic features of malignant SFT as (1) high cellularity; (2) more than 4 mitotic figures per 10 HPFs; (3) nuclear pleomorphism; (4) hemorrhage and necrosis; (5) tumor diameter ≥ 10 cm, and (6) positive margins [2–4]. Demicco et al. also reported that age > 55 years is a poor prognostic factor [35]. In the present case, the tumor was positive for STAT6, also showed high cellularity, increased mitotic figures (12/10 HPFs), nuclear pleomorphism (increased N/C ratio), necrosis inside the tumor, and invasive proliferative findings. The patient was 60 years old, so all findings fulfilled the malignant features except for tumor size. On the basis of the histology and immunohistochemical staining profile, we made a diagnosis of malignant SFT.
Unlike patients in other reports, our case was characterized by partially positive expression of cytokeratin AE1/AE3. SFT is classically negative for cytokeratin, but Cavazza et al. reported a malignant pleural SFT in which the majority of the neoplastic cells strongly expressed cytokeratin AE1/AE3. The authors reported that cytokeratin AE1/AE3-positive cells were lightly scattered in the pleural SFT primary lesion, and that 70% of the tumor cells were positive in the intrathoracic disseminated lesion 4 years after resection [36]. According to previous reports of pancreatic SFT, two cases were positive for cytokeratin and keratin that also had histopathologically malignant findings with numerous mitotic figures [23, 28]. Including our case, in pancreatic SFT, like pleural SFT, cytokeratin positivity may indicate high malignant potential.
To our knowledge, only five cases of pancreatic SFT had malignant findings, and two had distant metastases at the time of diagnosis. Twenty-two patients who underwent surgical treatment and had no malignant findings were free from recurrence; their prognosis was considered favorable. In our case, no recurrence was observed 12 months postoperatively. Because a case of recurrence and metastasized pancreatic malignant SFT has been reported, periodic follow-up with image examination is recommended. To date, there is no established postoperative adjuvant therapy or treatment for recurrence. We await the future accumulation of cases.
Conclusion
We experienced a case of pancreatic head SFT. Immunohistochemical staining of the excised specimen was useful for diagnosis. Careful follow-up is demanding because of several malignant features.
Acknowledgements
We thank Jane Charbonneau, DVM, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Abbreviations
- Bcl-2
B cell CLL/lymphoma-2
- CA19-9
Carbohydrate antigen 19-9
- CD
Cluster of differentiation
- CEA
Carcinoembryonic antigen
- CT
Computed tomography
- DWI
Diffusion-weighted image
- EUS-FNA
Endoscopic ultrasound-guided fine needle aspiration
- FDG-PET
[18F]-fluorodeoxyglucose positron emission tomography-computed tomography
- GIST
Gastrointestinal stromal tumor
- HPFs
High-power magnification fields
- MRI
Magnetic resonance imaging
- SFT
Solitary fibrous tumor
- SMA
Smooth muscle actin
- SSTR2
Somatostatin receptor type 2
- STAT6
Signal transducer and activator of transcription
Authors’ contributions
YT and TH drafted and revised the article. TH, HT, MO, and YN performed the surgery. WM, TT, TH, and SM contributed to perioperative care. TH and TK contributed to the study design. HM and TO are responsible for the histopathological diagnosis. YN supervised the writing of the manuscript. All authors approved the submitted version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was not funded by any educational or commercial organization.
Availability of data and materials
The authors declare that all the data in this article are available within the article.
Ethics approval and consent to participate
This study was carried out in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuka Taguchi, Email: novilnio.5@gmail.com.
Takanobu Hara, Email: harataka66@gmail.com.
Hiroaki Tamura, Email: tamh@hmedc.or.jp.
Masahito Ogiku, Email: mohgiku@yahoo.co.jp.
Mana Watahiki, Email: ipeanlaboon3.m@gmail.com.
Toru Takagi, Email: kyokui100031@gmail.com.
Takashi Harada, Email: gaku0122@mac.com.
Shinichiro Miyazaki, Email: miya19770529@gmail.com.
Tadataka Hayashi, Email: hayashi@hmedc.or.jp.
Toshikazu Kanai, Email: kanai@hmedc.or.jp.
Hiroki Mori, Email: mori_h@hmedc.or.jp.
Takachika Ozawa, Email: t.ozawa@hmedc.or.jp.
Yoshiro Nishiwaki, Email: y.nishiwk@hmedc.or.jp.
References
- 1.Klemperer P, Rabin CB. Primary neoplasms of the pleura. A report of five cases. Arch Pathol. 1931;11:385–412. [Google Scholar]
- 2.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. doi: 10.1002/cncr.10328. [DOI] [PubMed] [Google Scholar]
- 3.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 5.Luttges J, Mentzel T, Hubner G, Kloppel G. Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Arch. 1999;435:37–42. doi: 10.1007/s004280050428. [DOI] [PubMed] [Google Scholar]
- 6.Davanzo B, Emerson RE, Lisy M, Koniaris LG, Kays JK. Solitary fibrous tumor. Transl Gastroenterol Hepatol. 2018;3:94. doi: 10.21037/tgh.2018.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatti K, Nouira K, Ben Reguigua M, Bedioui H, Oueslati S, Laabidi B, et al. Solitary fibrous tumor of the pancreas. A case report. Gastroenterol Clin Biol. 2006;30:317–319. doi: 10.1016/S0399-8320(06)73174-8. [DOI] [PubMed] [Google Scholar]
- 8.Gardini A, Dubini A, Saragoni L, Padovani F, Garcea D. Benign solitary fibrous tumor of the pancreas: a rare location of extra-pleural fibrous tumor. Single case report and review of the literature. Pathologica. 2007;99:15–18. [PubMed] [Google Scholar]
- 9.Miyamoto H, Molena DA, Schoeniger LO, Haodong X. Solitary fibrous tumor of the pancreas: a case report. Int J Surg Pathol. 2007;15:311–314. doi: 10.1177/1066896907302419. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan VD, Wayne JD, Rao MS, Zynger DL. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. JOP. 2008;9:526–530. [PubMed] [Google Scholar]
- 11.Kwon HJ, Byun JH, Kang J, Park SH, Lee MG. Solitary fibrous tumor of the pancreas: imaging findings. Korean J Radiol. 2008;9(Suppl):S48–51. doi: 10.3348/kjr.2008.9.s.s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishiwatari H, Hayashi T, Yoshida M, Kuroiwa G, Sato Y, Kobune M, et al. A case of solitary fibrous tumor of the pancreas. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1078–1085. [PubMed] [Google Scholar]
- 13.Chetty R, Jain R, Serra S. Solitary fibrous tumor of the pancreas. Ann Diagn Pathol. 2009;13:339–343. doi: 10.1016/j.anndiagpath.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara Y, Sakai S, Aono S, Takahashi T, Inoue T, Ohta K, et al. Solitary fibrous tumor of the pancreas. Jpn J Radiol. 2010;28:479–482. doi: 10.1007/s11604-010-0453-x. [DOI] [PubMed] [Google Scholar]
- 15.Santos LA, Santos VM, Oliveira OC, De Marco M. Solitary fibrous tumour of the pancreas: a case report. An Sist Sanit Navar. 2012;35:133–136. doi: 10.4321/S1137-66272012000100013. [DOI] [PubMed] [Google Scholar]
- 16.Tasdemir A, Soyuer I, Yurci A, Karahanli I, Akyildiz H. A huge solitary fibrous tumor localized in the pancreas: a young women. JOP. 2012;13:304–307. [PubMed] [Google Scholar]
- 17.Azadi J, Subhawong A, Durand DJ. F-18 FDG PET/CT and Tc-99m sulfur colloid SPECT imaging in the diagnosis and treatment of a case of dual solitary fibrous tumors of the retroperitoneum and pancreas. J Radiol Case Rep. 2012;6:32–37. doi: 10.3941/jrcr.v6i3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Vorst JR, Vahrmeijer AL, Hutteman M, Bosse T, Smit VT, van de Velde CJ, et al. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J Gastrointest Surg. 2012;4:180–184. doi: 10.4240/wjgs.v4.i7.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanashi T, Toriumi F, Yahagi M, Shiratori F, Igarashi K, Nishi T, et al. A case of primary malignant solitary fibrous tumor of the pancreas. Jpn J Gastroenterol Surg. 2012;45:961–969. doi: 10.5833/jjgs.45.961. [DOI] [Google Scholar]
- 20.Chen JW, Lu T, Liu HB, Tong SX, Ai ZL, Suo T, et al. A solitary fibrous tumor in the pancreas. Chin Med J (Engl) 2013;126:1388–1389. [PubMed] [Google Scholar]
- 21.Hwang JD, Kim JW, Chang JC. Imaging findings of a solitary fibrous tumor in pancreas: a case report. J Korean Soc Radiol. 2014;70:53–57. doi: 10.3348/jksr.2014.70.1.53. [DOI] [Google Scholar]
- 22.Han SH, Baek YH, Han S-y, Lee SW, Jeong JS, Cho JH, et al. Solitary fibrous tumor of the pancreas: a case report and review of the literature. Korean J Med. 2015;88:293–298. doi: 10.3904/kjm.2015.88.3.293. [DOI] [Google Scholar]
- 23.Estrella JS, Wang H, Bhosale PR, Evans HL, Abraham SC. Malignant solitary fibrous tumor of the pancreas. Pancreas. 2015;44:988–994. doi: 10.1097/MPA.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 24.Baxter AR, Newman E, Hajdu CH. Solitary fibrous tumor of the pancreas. J Surg Case Rep. 2015;12:1–4. doi: 10.1016/j.ijscr.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paramythiotis D, Kofina K, Bangeas P, Tsiompanou F, Karayannopoulou G, Basdanis G. Solitary fibrous tumor of the pancreas: case report and review of the literature. World J Gastrointest Surg. 2016;8:461–466. doi: 10.4240/wjgs.v8.i6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K, Nakamura Y, Felizola SJ, Morimoto R, Satoh F, Takanami K, et al. Pancreatic solitary fibrous tumor causing ectopic adrenocorticotropic hormone syndrome. Mol Cell Endocrinol. 2016;436:268–273. doi: 10.1016/j.mce.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Spasevska L, Janevska V, Janevski V, Noveska B, Zhivadinovik J. Solitary fibrous tumor of the pancreas: a case report and review of the literature. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2016;37:115–120. doi: 10.1515/prilozi-2016-0024. [DOI] [PubMed] [Google Scholar]
- 28.Clare F, Russell D, Rocha FG. Malignant solitary fibrous tumor of the pancreas. J Pancreas. 2017;18:159–162. [Google Scholar]
- 29.Sheng Q, Xu W, Liu J, Shen B, Deng X, Wu Y, et al. Pancreatic solitary fibrous tumor in a toddler managed by pancreaticoduodenectomy: a case report and review of the literature. OncoTargets Ther. 2017;10:1853–1858. doi: 10.2147/OTT.S133650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Amico FE, Ruffolo C, Romano M, Di Domenico M, Sbaraglia M, DeiTos AP, et al. Rare neoplasm mimicking neuroendocrine pancreatic tumor: a case report of solitary fibrous tumor with review of the literature. Anticancer Res. 2017;37:3093–3097. doi: 10.21873/anticanres.11665. [DOI] [PubMed] [Google Scholar]
- 31.Oana S, Matsuda N, Sibata S, Ishida K, Sugai T, Matsumoto T. A case of a "wandering" mobile solitary fibrous tumor occurring in the pancreas. Clin J Gastroenterol. 2017;10:535–540. doi: 10.1007/s12328-017-0774-8. [DOI] [PubMed] [Google Scholar]
- 32.Geng H, Ye Y, Jin Y, Li BZ, Yu YQ, Feng YY, et al. Malignant solitary fibrous tumor of the pancreas with systemic metastasis: a case report and review of the literature. World J Clin Cases. 2020;8:343–352. doi: 10.12998/wjcc.v8.i2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian X, Zhou D, Gao B, Wang W. Metastatic solitary fibrous tumor of the pancreas in a patient with Doege-Potter syndrome. Hepatobiliary Surg Nutr. 2020;9:112–115. doi: 10.21037/hbsn.2019.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 36.Cavazza A, Rossi G, Agostini L, Roncella S, Ferro P, Fedeli F. Cytokeratin-positive malignant solitary fibrous tumour of the pleura: an unusual pitfall in the diagnosis of pleural spindle cell neoplasms. Histopathology. 2003;43:606–608. doi: 10.1111/j.1365-2559.2003.01703.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all the data in this article are available within the article.