Abstract
In the present study, the growth conditions and accumulation of ectoines (ectoine and hydroxyectoine) by Paludifilum halophilum DSM 102817T under salt stress conditions have been investigated. The productivity assay of this strain for ectoines revealed that the highest cellular content was reached in the minimal glucose sea water medium (SW-15) within 15% salinity. The addition of 0.1% (w/v) aspartic acid to the medium allowed an average of four times higher biomass production, and a dry mycelial biomass of 1.76 g L−1 was obtained after 6 days of growth in shake flasks at 40 °C and 200 rpm. Among the inorganic cations supplemented to the glucose SW-15 medium, the addition of 1 mM Fe2+ yielded the highest amount of mycelial biomass (3.45 g L−1) and total ectoines content (119 mg g−1), resulting in about 410 mg L−1 of products at the end of exponential growth phase. After 1 h of incubation in an osmotic downshock solution containing 2% NaCl, 70% of this content was released by the mycelium, and recovering cells maintained a high survival, with a maximal growth rate (µmax) of about 93% of the control population exposed to 15% NaCl. During growth at optimal salinity and temperature (15% NaCl and 40 °C), P. halophilum developed a compact and circular pellets that were easy to separate by simple decantation from both fermentation media and after hypoosmotic shock. Overall, the ectoines excreting P. halophilum could be a promising resource for ectoines production in a commercially valuable culture medium and at a large-scale fermentation process.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02512-x) contains supplementary material, which is available to authorized users.
Keywords: Ectoine, Hydroxyectoine, Mycelial biomass, Paludifilum halophilum, Pelleted growth, Release of ectoines
Introduction
Hypersaline environments, whether they are natural (salt lakes, inside seas, marine stromatolites, salt marshes) or artificial (salterns, crystallizer ponds, salted foods), are extreme environments colonized by microorganisms belonging to the three domains of life (Boujelben et al. 2015; Ben abdallah et al. 2018). To cope with the high osmolarity, microorganisms use various strategies such as the reinforcement of cell walls (Zhang et al. 2016), import of K+ and Cl− (Oren 2002, 2013) or massive accumulation of various compatible solutes (Roberts 2005; Lentzen and Schwarz 2006). Compatible solutes are a chemically diverse group of highly water-soluble organic osmolytes that play a vital role during salt stress and help providing osmotic balance without interfering with the essential cellular processes and the normal metabolism of halophilic microbes (Kempf and Bremer 1998; Liu et al. 2017; Rani and Venkatesu 2018; Deng et al. 2019). Moreover, they improve protein folding and protect biomolecules such as enzymes, nucleic acids, antibodies, and even whole cells against various stress conditions (Barth et al. 2000; Ignatova and Gierasch 2006). This function-preserving property of compatible solutes has promoted considerable biotechnological interest.
One of the most abundant osmolytes present in nature is ectoine, which was originally discovered in the extremely halophilic phototrophic purple sulfur bacterium Ectothiorhodospira halochloris (Galinski et al. 1985). This discovery was followed by the detection of a hydroxylated derivative of ectoine, 5-hydroxyectoine by Inbar and Lapidot (1988) in the Gram-positive soil bacterium Streptomyces parvulus. To date, ectoines can be found within a large number of aerobic, heterotrophic bacteria, such as genus Halorhodospira, genera Halomonas, Chromohalobacter, Vibrio, and Pseudomonas of the class γ-proteobacteria (Galinski et al. 1985; Vargas et al. 2005; Tao et al. 2016; Zhao et al. 2019). However, a limited number of strains, mainly of the family of Halomonadaceae, have reached high ectoines content (150–200 mg ectoine g−1 dry cells) and therefore are considered as potential candidates for commercial production (Sauer and Galinski 1998; Guzmán et al. 2009; Wei-Chuan et al. 2017). Among them, only Halomonas elongata is currently used in industrial-scale production to deliver ectoine on the scale of tons (Kunte et al. 2014). Its excellent function-preserving characteristic has attracted considerable attention to its exploitation in the fields of cosmetic and pharmaceutical industries. It is incorporated in anti-aging cosmetic creams to stabilize the active molecules. (Graf et al. 2008; Pastor et al. 2010; Jorge et al. 2016). In addition, ectoine has been found to protect human skin cells from the effects of harmful UV radiation (Bünger and Driller 2004). Werkhäuser et al. (2014) also showed that ectoine-containing products reduced nasal and ocular symptoms in allergic rhinitis patients. Moreover, a recent study by Vyrides and stuckey (2017) confirmed that ectoine has a beneficial additive role to biological waste and wastewater treatment systems to counteract osmotic and other types of environmental stresses on microbial communities.
Beside high content, the efficiency and economic value of the manufacturing process of ectoines are determined by the cost of the fermentation medium and the downstream processing of the product. About 40–50% of the total production cost is attributed to the composition of the medium, where complex and expensive ingredients, e.g. yeast extract, are often supplemented at high concentrations in the medium to provide vitamins and other growth factors and, therefore, enhance the content of microbial cells in products (Onraedt et al. 2005; Wei-Chuan et al. 2017). Moreover, during high cell density cultivation of non-ectoine excreting strains, the dissolved oxygen limitation makes it difficult to operate the fermentor and increases the cost of production. Hence, selecting novel potential producers which are able, in cheap minimal medium, to reach high ectoines production and productivity, and easy to follow during growth and to separate from the culture medium is of special interest. In this context, filamentous bacteria belonging to the order of actinomycetale that exhibit pelleted growth morphology in submerged culture could be an interesting alternative (O’Cleirigh et al. 2005; Tesche et al. 2019). This growth mode strongly affects the overall cell performance of the process and facilitates downstream processing by simplifying solid–liquid separation procedures. To date, there are several salt-tolerant actinomycetes, mainly from the Streptomyces genus, which are capable of producing ectoines under salt stress conditions (Bursy et al. 2008; Sadeghi et al. 2014); but none of them were reported to produce important quantities.
This study proposes P. halophilum, from the Thermoactinomycetaceae family, as a new high-yield ectoines producer in a commercially valuable culture medium. This strain was isolated from the solar saltern of Sfax (Frikha-Dammak et al. 2016) in the central eastern coast of Tunisia (about 34°39′ N 10°42′ E). This ecosystem is a thalassohaline artificial environment with an area of about 1500 ha, divided into six series of ponds along 12 km of coastline with a salinity between 3.7 and 40% (w/v). In the first stage of the investigation, growth conditions for maximal mycelial biomass production and high ectoines content under salt stress conditions were investigated and a minimal medium devoid of yeast extract was defined and optimized. The second stage of the process focused on morphological growth properties of the strain under optimal fermentation conditions and its ability to release its content of ectoines following exposure to hypoosmotic shock.
Materials and methods
Bacterial strain and seed culture
The actinobacterial strain was isolated from a superficial sediment sample collected at the solar saltern of Sfax, in Tunisia, and identified as P. halophilum according to its phenotypic, chemotaxonomic and phylogenetic characters (Frikha-Dammak et al. 2016). The strain was maintained in 20% glycerol at − 80 °C. The working strain was prepared on saline Bennett’s agar plates composed of (per liter of distilled water): 1 g beef extract, 1 g yeast extract, 2 g peptone, 150 g NaCl and stored at 4 °C. For all fermentation experiments, seed cultures of P. halophilum were prepared in a 15-mL falcon tube containing 5 mL of the seawater medium (SW-15) composed of (per liter of distilled water): 4.07 g MgSO4·7H2O, 2.6 g MgCl2·6H2O, 0.4 g KCl, 47 mg NaBr, 13 mg NaHCO3, 67 mg CaCl2·2H2O, 150 g NaCl, added with 5 g L−1 glucose and yeast extract. Glucose and mineral salts were sterilized separately. The pH of the medium was adjusted to 8.4 using 2 M NaOH. After 4 days of growth at 40 °C and 200 rpm in a rotary shaker incubator (New Brunswick Scientific, NJ, USA), mycelium was collected by centrifugation (2360 g at 4 °C for 15 min) and used to inoculate in 250-mL Erlenmeyer flasks, 100 mL of the different cultures for optimization of growth and compatible solutes production.
Nuclear magnetic resonance (NMR) and high-performance liquid chromatography (HPLC) analyses of ectoines
To identify compatible solutes produced by P. halophilum, mycelium from a 5-mL 6-day suspension culture on the glucose SW-15 medium without yeast extract was harvested by centrifugation and extracted according to the method of Kunte et al. (1993). Briefly, 10 mg dry cell pellets was resuspended in 570 μL of a methanol/chloroform/water mixture (10/5/4, v/v/v) and mixed for 15 min at 37 °C. To precipitate proteins and extract osmolytes, 170 μL of chloroform and 170 μL of water were added. Liquid-phase separation was enhanced by gentle centrifugation, and the hydrophilic top layer containing compatible solutes was recovered, dried and reconstituted in deuterated water (D2O) for NMR experiments and in H2O for HPLC analysis. For the identification of osmolytes, NMR experiments were run on a Bruker Ascend 400 spectrometer operating at 400 MHz for proton and 100 MHz for carbon nuclei. Chemical shifts were expressed in ppm and coupling constants in Hz. Signals for the osmolytes were assigned by comparison with previously published chemical shift values from Motta et al. (2004) and confirmed by comparison with 1H-NMR spectra of commercially available pure compounds.
The identification of ectoines was also determined by HPLC–UV on the basis of their retention time in comparison with standard products (Sigma-Aldrich) in a KNAUER–NH2 analytical column, with 2 mL min−1 of flow rate and detection at λmax of 210 nm. The quantification of ectoines was also obtained by establishing the standard curve between the HPLC peak areas and the concentrations of ectoine. The standard curve was established using standard solutions from 0.2 to 0.8 mg mL−1. Linear curves and their fitting equations were established using Origin 9.0.
Effect of medium on mycelial biomass and ectoines production
Two basal minimal liquid media, SW (see composition above) and M63 composed of (per liter of distilled water): 16.3 g KH2PO4, 4.2 g KOH, 2 g (NH4)2SO4, 39.5 mg MgSO4·7H2O, 0.5 mg FeSO4·7H2O and one complex medium named Bennett’s medium (see composition above), were compared for strain growth and production of ectoines at 40 °C. All the three media were added with 5 g L−1 glucose and 150 g L−1 NaCl, and pH was adjusted to 8.4 using 2 M NaOH. In all fermentation experiments, the mycelial biomass was estimated after 6 days by measuring the dry cell weight from 2 mL of broth culture. The mycelium pellet was collected in 2-mL pre-weighted Eppendrof tubes by centrifugation at 10,000 g for 5 min and washed twice with 15% (w/v) sodium chloride solutions. The difference in weight was measured for dry cell weight after drying the tubes in 70 °C hot air oven for 24 h, where a constant and persistent weight were obtained. The amount of total intracellular ectoines was determined as described above according to the fitting equations and expressed in terms of ectoines content (mg g−1 dry mycelial biomass) and volumetric concentration (mg L−1 culture broth).
In order to improve mycelial biomass production and therefore ectoines productivity, the glucose SW-15 medium was used for the fermentation process in flasks, and to which the various components to be screened were added. In the first step and to detect an eventual auxotrophy of the strain, yeast extract with varying concentrations was added to the medium and dry mycelial biomass was determined after 6 days as described above. With the focus to keep a synthetic-medium devoid of yeast extract, different amino acids were also screened individually at 0.1% (w/v) each. Moreover, several inorganic cations (Zn2+, Mn2+, Co2+, Cu2+, Fe2+ and Ni+) which usually play an enzymological role in microbial metabolism were separately added to the basic glucose SW-15 medium at 0.5 mM, and in all experiments, biomass and ectoines production was determined as described above after 6-day culture at 40 °C and 200 rpm.
Effect of salt concentration on growth and ectoines content of P. halophilum
To investigate the effects of salt concentrations on the growth of P. halophilum, 5 mL of seed culture was inoculated in 100 mL of optimized glucose SW medium with NaCl concentration ranging from 5 to 25% (w/v). The flasks were incubated at 40 °C and 200 rpm for 6 days, and samples were withdrawn to measure mycelial biomass and ectoines content as described above. The pelleted growth characteristics of the strain at different salinities were also visually compared.
Determination of ectoines release and cell survival after osmotic downshock
P. halophilum was grown in optimized SW-15 medium at 40 °C and 200 rpm for 6 days, and then cells from 25 mL were collected from the culture broth by centrifugation at 2360 g at 4 °C for 15 min. The cells were suspended in 5 mL of distilled water containing 0, 2, 4 and 15% NaCl for ectoines release at 30 °C and under gentle shaking. After 1 h, the suspensions were centrifuged at 10,000 g for 5 min and the supernatants containing released ectoine were analyzed by HPLC. The cell pellets recovered after centrifugation were re-suspended in 25 mL of fresh SW-15 optimized medium with 15% NaCl, incubated at 40 °C under the same initial conditions, and growth was followed by samples withdrawn every day for dry cell weight as described before. The bacterial cells obtained from the culture broth subjected to 15% osmotic downshock were used as control. The survival was evaluated by the determination of maximal growth rate (µmax) in exponential growth phase of recovering cells after osmotic downshock and compared with that of control cells.
Statistical analysis
All data regarding mycelial biomass production, ectoines content and ectoines excretion were replicated three times and subjected to statistical analysis using one-way ANOVA with a Tukey test for multiple comparison procedure. Results were expressed as mean values ± standard deviation (SD). Differences were considered statistically significant if the P value was between 0.01 and 0.05, and extremely significant if the P value was less than 0.01.
Results
Identification of ectoines and effect of medium on cellular content
To investigate the nature of compatible solute accumulated in P. halophilum cells, chemical structures of extracted osmolytes were determined by NMR analysis through 1H- and 13C NMR, 1H-1H correlated spectroscopy (COSY), heteronuclear single quantum correlation (HSQC) and heteronuclear multiple bond correlation (HMBC) experiments. The 1H- and 13C NMR spectra (Fig. 1a, b) exhibited obvious signals of both ectoine and 5-hydroxyectoine demonstrating their existence as a mixture in this fraction. The singlets at 2.24 and 2.30 ppm were attributed to two methyl groups correlating each in the HMBC spectrum (Fig. S1) with an ethylenic carbon at δC = 160.5 (160.6) ppm. The correlation systems between the protons at 2.1 ppm (H-5) and both of the carbons at 55.8 ppm (C-4) and 47.9 ppm (C-6) in the HMBC spectrum as well as the connectivities between H-5 (δH = 2.1 ppm) and both of H-4 (δH = 3.34 ppm) and H-6 (δH = 3.03 ppm, t, J = 7.3 Hz) in the 1H–1H COSY spectrum proved decidedly the existence of ectoine in this fraction (Fig. S2). On the other hand, the downfield-shifted multiplet at 4.5 ppm indicated the substitution of the methylene group at the C-5 position by a hydroxyl group in 5-hydroxyectoine. This multiplet makes correlations in the COSY spectrum with both signals at 3.31 and 3.47 ppm related to H-6 and the multiplet at 4.07 ppm due to H-4. The two peaks at δC = 174.4 ppm and δC = 176.8 ppm in the 13C NMR spectrum are relate to the carboxylic acid moieties of the molecules. Relevant correlations are further presented on the COSY (Fig. S2) and HMBC (Fig. S1) spectra. The proton signals and chemical shifts of the osmolytes synthesized by P. halophilum corresponded to those for ectoine and hydroxyectoine.
Fig. 1.
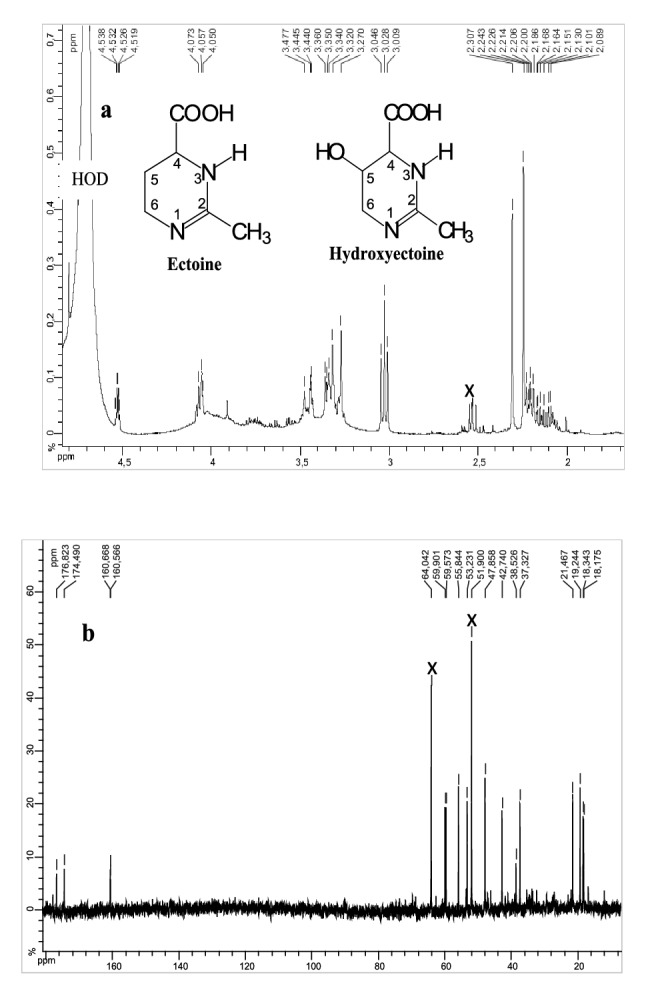
1H-NMR (a) and 13C-NMR (b) spectra of whole-cell compatible solute extracts of P. halophilum grown in minimal SW-15 medium at 15% (w/v) NaCl and with 5 g L−1 of glucose as the sole carbon source. The major solutes were ectoine (E) and hydroxyectoine (EOH)
Typical HPLC-chromatographic profiles of ectoine and hydroxyectoine standards (Fig. S3) showed that this method also offers a good resolution and an ideal symmetry of the peaks with acceptable retention time of 22.609 min and 20.027 min, respectively. In addition, good selectivity with utility in quantifying the ectoine and hydroxyectoine by the selected chromatographic parameters was obtained. Standard calibration graphs were plotted for regression analysis, and results indicated great linearity in the range of 0.2–0.8 mg mL−1 for ectoine and hydroxyectoine. The coefficient of correlation (R2) was 1, and the liner regression equation was y = 2.85e−8 x − 0.007 where y is the concentration of ectoine or hydroxyectoine (mg mL−1) and x is the peak area (Fig. S4).
In order to check the medium’s effect on the accumulation of ectoines, we monitored growth and osmolytes production in batch cultures of P. halophilum in two minimal media (SW-15 and M63-15) and one complex medium (Bennett’s medium). Table 1 shows that the highest cell dry weight of 2.1 g L−1 was reached on the Bennett’s medium, followed by M63 and SW media on which no more than 0.27 g L−1 of biomass occurred. However, maximum ectoine and hydroxyectoine concentrations of 19.6 mg L−1 and 9.08 mg L−1, respectively, were obtained on the minimal SW medium on which the intracellular content of both compatible solutes reached their highest value of 103.91 mg g−1 of P. halophilum mycelial dry weight, while the lowest content of 0.37 mg g−1 was obtained on the complex Bennett’s medium. Therefore, glucose SW-15 minimal medium appeared to be an effective medium for producing ectoines from P. halophilum and, thus, it was used as the basic medium to improve growth and accumulation of crude ectoines.
Table 1.
Effect of medium on mycelial biomass production and ectoines content of P. halophilum after 6 days of growth at 15% NaCl, 40 °C and 200 rpm
| Medium | X (g L−1) | E (mg L−1) | HE (mg L−1) | E + HE (mg L−1) | E + HE content (mg g−1) |
|---|---|---|---|---|---|
| Bennett’s | 2.1 ± 0.1 | 0.78 ± 0.15 | 0 | 0.78 ± 0.1 | 0.37 ± 0.01 |
| M63 | 0.51 ± 0.02 | 7.37 ± 1.4 | 1.01 ± 0.2 | 8.41 ± 1.6 | 16.49 ± 3.0 |
| SW | 0.27 ± 0.01 | 19.6 ± 3.9 | 9.08 ± 1.8 | 28.68 ± 5.1 | 103.91 ± 20.7 |
Each point represents the mean of three independent experiments (SD < 5% of the mean for mycelial biomass and < 20% of the mean for ectoines content)
X dry mycelial biomass, E ectoine, HE hydroxyectoine
Improvement of P. halophilum growth on glucose SW-15 medium
In order to increase the cell density of P. halophilum on glucose SW-15 medium and, hence, the volume concentration of ectoines, yeast extract which would be a source of growth factors filling an auxotrophy was added with different concentrations and batch cultures were incubated at 40 °C and 200 rpm. After 6 days, dry cell weights were determined and values are reported in Fig. 2a. An average of four times higher biomass production in the presence of 0.15% yeast extract than in its absence was observed. Moreover, the highest level of biomass produced with 0.15% yeast extract reached nearly the maximum value obtained with the complex Bennett’s medium.
Fig. 2.
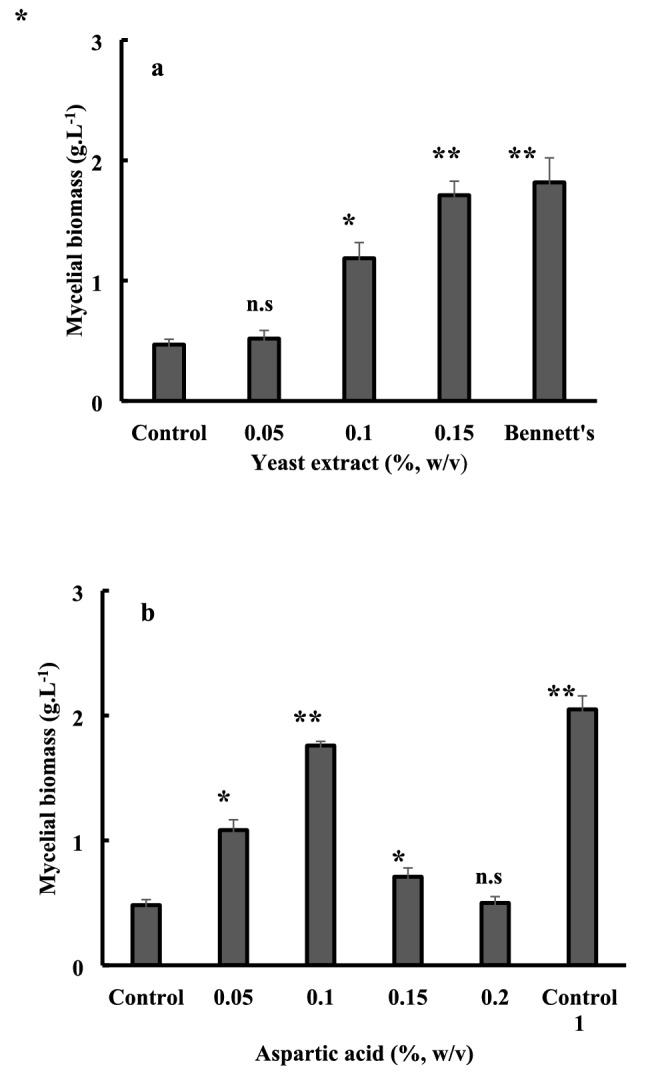
Effects of yeast extract (a) and aspartic acid (b) concentration on mycelial biomass production by P. halophilum. Cells were grown in glucose SW-15 medium at the optimal salinity (15% NaCl) for 6 days at 40 °C and 200 rpm. Mycelial biomass concentrations were determined by drying the cells at 70 °C. Control means mycelial biomass production in glucose SW-15 medium without aspartic acid or yeast extract; control 1 means mycelial biomass production in glucose SW-15 medium with 0.15% yeast extract. Each point represents the mean and error bars represent standard deviation (biological replicates, N = 3). Statistical analysis is based on one-way ANOVA with a Tukey test for multiple comparison procedures. Significant difference compared to the control (without aspartic acid or yeast extract addition): *P < 0.05, **P ≤ 0.01
Aiming to keep a synthetic-medium devoid of yeast extract for the production of high mycelial biomass and suitable for ectoines production, the potential auxotrophy of yeast extract was detected by screening several amino acids on glucose SW-15 minimum medium (Fig. S5). Results showed that only aspartic acid allowed for a significantly better growth than the control without yeast extract, and the mycelial biomass of 1.79 g L−1 obtained in the presence of 0.1% aspartic acid equalizes nearly that of the positive control with 0.15% yeast extract (Fig. 2b). The effect of inorganic cations on mycelial biomass and ectoines production was also studied. Among the different cations supplemented to SW-15, Zn2+, Mn2+ or Co2+ strongly reduced mycelial biomass and completely inhibited ectoines production by P. halophilum. As for the other metals, while Cu2+ and Ni+ did not affect growth and osmolytes accumulation, only Fe2+ showed a significant positive effect on both parameters (Fig. 3). At 1 mM Fe2+, a twofold increase in mycelial biomass and ectoines production was observed. In addition, the relative proportion of ectoine, compared to hydroxyectoine also increased from 56% in the absence of Fe2 to 70% at 1 mM Fe2+ at the end of exponential growth phase. However, the specific content of ectoines was slightly improved from about 104 to 119 mg g−1 of dry mycelial biomass.
Fig. 3.
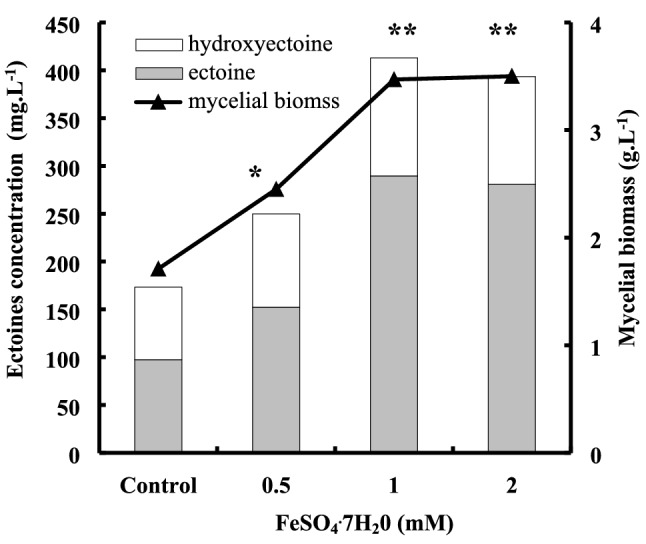
Effects of iron concentration on mycelial biomass and ectoines production by P. halophilum. Cells were cultivated in glucose SW-15 medium at the optimal salinity (15% NaCl) and in the presence of 0.5, 1 and 1.5 mM iron sulfate. After 6 days of growth at 40 °C and 200 rpm, mycelial biomass concentrations were determined by drying the cells at 70 °C, and ectoines content by HPLC. Control means mycelial biomass and ectoines production in glucose SW-15 medium no supplemented with iron. Each point represents the mean of three determinations (N = 3) and SD < 15% of the mean. Significant difference compared to the control (without iron addition): *P < 0.05, **P ≤ 0.01
Effects of increasing salinities in optimized SW medium on growth and ectoines content
To assess the effect of salinity on growth and ectoines content, cells were exposed in optimized glucose SW-15 medium with increasing concentrations of NaCl. As shown in Fig. 4a, an increase in the salinity up to 15–20% NaCl strongly stimulated growth, but further increase to 25% NaCl impaired growth. Hence, P. halophilum not only depends on a considerable salt concentration for its growth but it can also cope with a broad spectrum of salinities (from 5 to 25% NaCl). However, its growth is optimally poised for a rather narrow spectrum of salinities. The cells were also analyzed for ectoines content and the optimal salt concentration for cell growth was also favorable for ectoine accumulation. At 15% NaCl concentration, P. halophilum exhibited the highest ectoines production, reaching after 6 days of growth 119 mg g−1 (88 mg g−1 ectoine and 31 mg g−1 hydroxyectoine). At a higher salt concentration, the total ectoines dropped to only 55 mg g−1 in the presence of 25% NaCl (Fig. 4a). The mycelial cells were also examined for growth properties, and were found to form spherical pellets from the mid-exponential growth phase and were kept nearly constant during the entire period of fermentation. A comparison of the impact of growth salinities and temperatures on the morphological profiles of these pellets showed the periphery of the pellets that were obtained at salinities of 15–20% and 40 °C was rather circular and had a compact surface structure (Fig. 4b). However, at salinities lower than 10%, or temperatures higher than 40 °C, lower compact pellet surface structures were observed and pellets were relatively unstructured and irregular at the periphery (Fig. 4c).
Fig. 4.
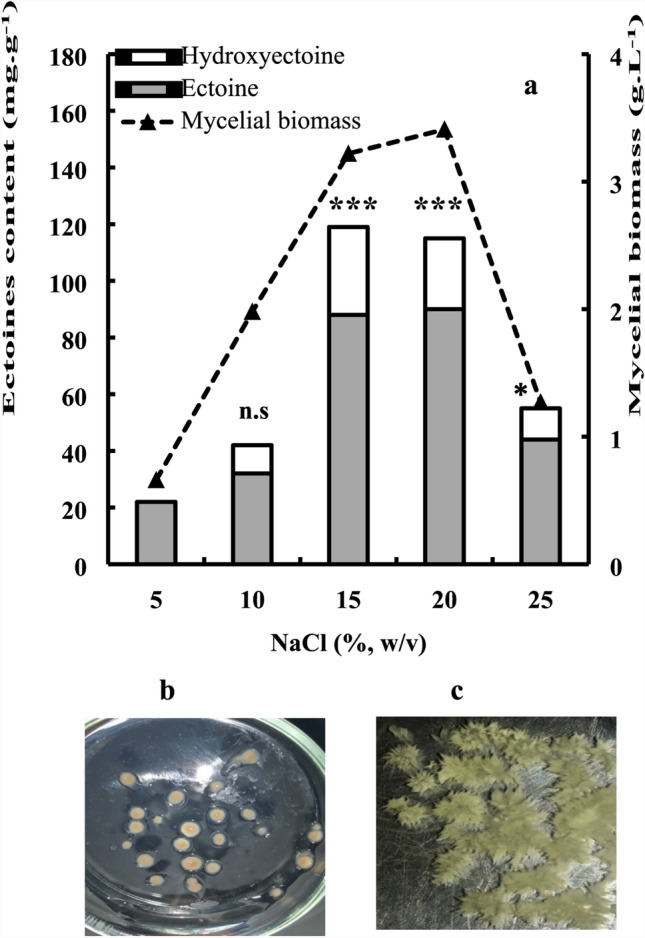
a Effects of increasing salinities on mycelial biomass production and ectoines content of P. halophilum. Cells were grown in optimized glucose SW-15 medium supplemented with various NaCl concentrations ranging from 5 to 25% (w/v). After 6 days of growth at 40 °C and 200 rpm, the concentrations of mycelial biomass were determined by drying the cells at 70 °C, and ectoines content by HPLC. Values are the mean of three independent experiments (N = 3) and SD < 10% of the mean. Significant difference compared to the control (with 5% NaCl addition): *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. b Compact and circular pellets morphology of P. halophilum obtained after 6 days of mycelial growth at optimal growth conditions (NaCl: 15%; T: 40 °C); c Unstructured and irregular pellets morphology of P. halophilum obtained after 6 days of mycelial growth at non-optimal growth conditions (NaCl:10%; T: 44 °C)
Growth phase effect on ectoines production in optimized SW medium
To test whether ectoines biosynthesis in P. halophilum is growth phase dependent, cells were grown in the glucose SW-optimized medium in the presence of 15% NaCl, at 40 °C and 200 rpm. As shown in Fig. 5, after a lag phase of one day, P. halophilum entered the exponential phase of growth, and the stationary phase was observed from the 7th day of incubation. The intracellular content of ectoine initially increased from 56.2 mg g−1 dry weight in the early exponential growth phase to 88 mg g−1 dry weight in the late exponential phase and then decreased to 29.1 mg g−1 dry weight in the late stationary phase. The hydroxyectoine content increased from the late exponential growth phase and then was maintained nearly constant in the stationary phase. Additionally, the ectoine/hydroxyectoine ration decreased from 4.6 in the early exponential phase to 2.8 in the late growth phase and to about 0.5 in the stationary phase. However, total ectoines concentration reached its maximum value of 410 mg L−1 on the sixth day of growth. The final optimized SW medium could be defined as follows: 5 g glucose, 4.07 g MgSO4·7H2O, 2.6 g MgCl2·6H2O, 0.4 g KCl, 47 mg NaBr, 13 mg NaHCO3, 67 mg CaCl2·2H2O, 278 mg FeSO4·7H2O, 1 g aspartic acid, 150 g NaCl in 1 L of distilled water adjusted to pH 8.4 and incubated at 40 °C and 200 rpm. Under these conditions, the dry mycelial biomass and ectoines concentration after 6 days reached their highest values of 3.45 g L−1 and 410 mg L−1, respectively.
Fig. 5.

Effect of growth phase on mycelial biomass production and ectoines content of P. halophilum. Cells were grown in optimized glucose SW-15 medium (15% salinity, 40 °C and 200 rpm), and samples were withdrawn every day for dry cell weight by drying the cells at 70 °C and ectoines content by HPLC. Each point represents the mean of three independent experiments (N = 3) and SD < 10% of the mean
Effect of osmotic downshock on ectoines release and survival of recovering cells
As shown in Fig. 6, 87 and 70% intracellular ectoines were released after 1 h when the mycelial cells were exposed, respectively, to 0 and 2% NaCl in distilled water. The amount of released ectoines diminished to 34% when the NaCl concentration in the osmotic downshock solution was increased to 4%. However, at 15% salinity mycelial cells released only 5% of the intracellular ectoines after 1 h of exposition. A comparison of the growth curves of recovered cells revealed that the 2% osmotic downshock recovering cells followed the same profile than the control exposed to 15% NaCl (data not shown). As depicted in Fig. 6, the µmax (per day) for the 2% treated and control cells were very similar at 0.56 and 0.6 day−1, respectively.
Fig. 6.
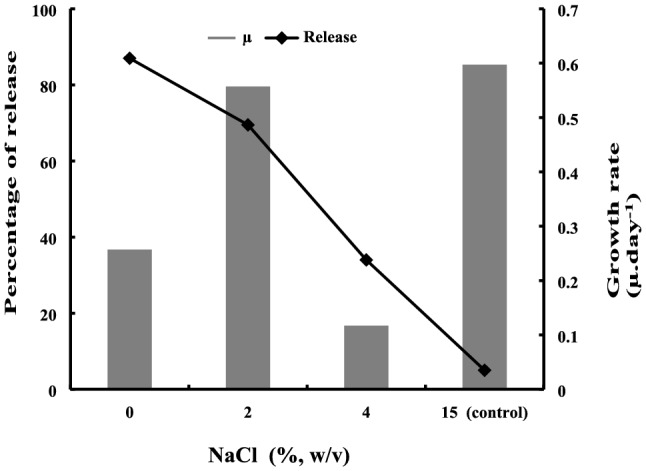
Effect of NaCl concentration in the downshock solution on ectoine release rates and growth rates of recovering cells. Cells of P. halophilum from 25 mL were harvested after 6 days of growth in optimized SW-15 medium (15% salinity, 40 °C and 200 rpm), and cells were collected and suspended in 5 mL of distilled water containing 0, 2, 4 and 15% NaCl. After 1 h of incubation at 30 °C and under gentle shaking, the suspensions were centrifuged, the supernatants containing released ectoine were analyzed by HPLC, and maximum growth rates of recovering cells in exponential growth phase were determined in fresh SW-15 optimized medium with 15% NaCl. Each point represents the mean of three independent experiments (N = 3) and SD < 10% of the mean
Discussion
The data presented in this study address the growth of P. halophilum under salt stress conditions and demonstrate that this saltern-living thermoactinomycete belongs to the group of microorganisms that synthesize ectoine and hydroxyectoine compatible solutes to counteract the negative effects of high external osmolarity of the medium. There are several reports in the literature which prove that actinomycete strains, mainly from the Streptomyces genus such as S. clavuligerus, S. griseus, S. parvulus, S. peucetius, and S. antibioticus are also capable of synthesizing ectoine and 5-hydroxyectoine under salt stress (Malin and Lapidot 1996). Furthermore, ectoine and 5-hydroxyectoine biosynthetic genes have been identified in several Streptomyces (Ikeda et al. 2003; Prabhu et al. 2004; Sadeghi et al. 2014), and our database searches revealed that they are also present in the genome sequences of P. halophilum (https://www.ncbi.nlm.nih.gov/nuccore/NOWF00000000). Taken together, these findings suggest that both ectoines are rather common compatible solutes synthesized by members of the halophilic actinomycetes as a physiological stress response to increased salinity.
Interestingly, the composition of the medium was found to be an important factor affecting ectoines accumulation in P. halophilum. At the same temperature and NaCl concentration, the basal SW medium stimulate higher ectoines production than M63 and Bennett’s. P. halophilum produced only 0.37 and 16.49 mg g−1 ectoines after 6 days of cultivation in Bennett’s and M63 media containing 15% NaCl, while the ectoines content increased to 103.9 mg g−1, after 6 days of cultivation in glucose SW-15 containing the same concentration of NaCl (Table 1). In general, halophilic and halotolerant organisms use a group of solutes (cocktails of solutes), and not just a single solute, for osmotic balance (Da Costa et al. 1998; Leon et al. 2018). The SW and M63 are minimal media, while Bennett’s is a complex medium containing precursors for various solutes. Thus, with the availability of these precursors in the culture medium, the bacterial cells tend to synthesize several molecules that together contribute to osmotic balance. Besides the medium, the presence of NaCl is a key factor that significantly influences cell growth and ectoines accumulation of P. halophilum. Previous studies have established that the amount of ectoines in bacterial cells is controlled by the concentration of NaCl in the culture medium (Detkova and Boltyanskaya 2007; Van-Thuoc et al. 2010). In the present study, ectoines content produced by P. halophilum increased and reached the maximum value when the NaCl concentration in the culture medium was increased to 15% (Fig. 4). With a concentration between 10 and 15%, a small amount of hydroxyectoine was also detected (Van-Thuoc et al. 2010; Öztürk et al. 2015). In congruity with earlier studies (Borges et al. 2002; Van-Thuoc et al. 2013), ectoines seem to be the better stress protectors for various biological functions of the strain, but up to a certain NaCl concentration. For instance, they can protect the functionality of proteins against osmotic stress (Kolp et al. 2006; Richter et al. 2019) by using the preferential exclusion model, which involves the exclusion of osmolytes from the protein surface to hydrate the protein, slow the diffusion of water molecules and preserve the native state of the protein (Kunte et al. 2014; Eiberweiser et al. 2015). They can also stabilize lipid bilayers (Harishchandra et al. 2010), protect DNA from damage by ionizing radiation (Schröter et al. 2017), and provide hydroxyl radical scavenging activity (Brands et al. 2019). Together, these results imply that NaCl stress is a key regulator for synthesis and accumulation of ectoine and hydroxyectoine. The ectABCD genes, related to ectoines synthesis in many halophilic strains, as H. elongata or Nicardiopsis gilva, present different degrees of transcriptional regulation in the range from 5 to 15% NaCl concentration, particularly at 15% (He et al. 2015; Han et al. 2018).
As intracellular compounds, ectoines production in P. halophilum is also linked to the amount of mycelial biomass obtained on the basal SW-15 medium; hence improving osmolytes accumulation is also linked to the cellular production. In order to improve the mycelial biomass and with the focus on keeping a synthetic medium, 0.1% (w/v) aspartic acid, allowed reaching about four times higher of biomass than that obtained on the SW-15 control medium (Fig. 2b). The role of this particular amino acid in the growth of P. halophilum as a ready precursor for protein synthesis is evident, but its contribution to more and faster intracellular compatible solutes synthesis, and therefore higher ability to support the constraints imposed by high osmolarity on cellular hydration, physiology, and growth cannot been discarded. Indeed, previous researchers have shown that the routes for ectoines biosynthesis starts by l-aspartate conversion to ß-aspartylphosphate and then l-asparte-ß-semialdehyde (Ono et al. 1999; Reshetnikov et al. 2011). The improvement of mycelial biomass and ectoines production was also observed when 1 mM Fe2+ was supplemented to the glucose SW-15 medium and reached about twice the values of the control without iron (Fig. 3). The critical role of iron, compared to Cu2+ and Ni+, which did not affect growth and ectoines accumulation could be related to its structural role, or most likely to its critical role for enzyme catalysis as is the case of ectoine synthase (ectC), showing previously an iron-dependent enzyme (Reuter et al. 2010; Widderich et al. 2016). In addition, in studying the interplay between iron homeostasis and the salt stress response of Chromohalobacter salexigens, Argandona et al. (2010) found that the amount and relative proportion of ectoine and 5-hydroxyectoine were affected by iron concentration in the growth medium. These authors ascribed an activator function of the Fur regulatory protein for the transcription of the ectoines biosynthetic genes.
In the optimized SW-15 medium, the total ectoines content produced by P. halophilum increased with growth and reached the maximum value of about 119 mg g−1 at the end of the exponential growth phase. This content generated by the strain, still slightly lower to that of other efficient producers such as H. organivorans (165 mg g−1) (Van Thuoc et al. 2019) or the commercial ectoine producers H. elongata (155 mg g−1) (Sauer and Galinski 1998), could be further improved in controlled bioreactor fermentations. During the exponential growth phase of P. halophilum, a mixture of ectoine and 5-hydroxyectoine, with ectoine dominating the ectoine/5-hydroxyectoine solute pool was observed (Fig. 5). However, in stationary phase culture, a shift in relative proportions in favor of hydroxyectoine was detected. The substantial increase in 5-hydroxyectoine content when cells enter stationary phase was also shown in the moderate halophile Salibacillus salexigens (Bursy et al. 2007), Streptomyces coelicolor A3 (Bursy et al. 2008) and Virgibacillus halodenitrificans (Tao et al. 2016). This implies that the hydroxylated derivative of ectoine possesses stress-relieving properties that allow the cell to better cope with the multitude of challenges imposed by the stationary phase (Klauck et al. 2007). Interestingly, 70% of the intracellular ectoines content were released after 1 h of hypoosmotic shock at 2% NaCl without significant loss in viability of the recovered mycelial cells (Fig. 6). Indeed, the recovered population after the hypoosmotic shock kept 93% of its growth rate when compared to the control cells exposed in the same conditions to 15% NaCl. These results are better than those obtained with the commercial strain H. elongata, where the ectoine release and bacterial survival rates have been reported at 64% and 86%, respectively, in the presence of 3% NaCl (Sauer and Galinski 1998).
Being filamentous bacteria, salt-stressed cells of P. halophilum were also found to form, from the mid-exponential phase, a compact pelleted growth aspect (Fig. 5b). This growth mode has the great advantage of maintaining a very low viscosity of the culture medium and thus minimizing the limitations of mixing and mass transfer phenomena which represent the main obstacles to a possible large-scale culture of the strain (Sinha et al. 2001; Wucherpfennig et al. 2010). This pellet growth mode of filamentous bacteria also facilitates the downstream step of the biotechnological process by simplifying the procedure of separation of the cells from the liquid culture medium by simple decantation. In addition, where bacterial contamination was encountered, the culture fluid rapidly developed turbidity rather than the typical hyphal pellets. However, the major disadvantages of pelleted growth forms are mass transfer limitations leading to significant concentration gradients of oxygen and other nutrients from the culture fluid to the inner pellet core (Hille et al. 2005; Van Dissel et al. 2014). These limitations, if they are not well controlled, can finally result in autolytic processes within the inner part of big bacterial pellets. Thus, a correlation between the change of the size and the specific content in ectoines of the formed pellets should be established in a controlled bioreactor.
Conclusions
Our data firstly demonstrated that ectoines biosynthesis is key features for the physiological adjustment process of P. halophilm to high osmotic stress conditions. The strain displayed the highest mycelial biomass production and cellular content of these compatible solutes in mineral glucose SW medium added with 15% NaCl, 0.1% aspartic acid and 1 mM Fe2+. In addition, our results on the release of ectoines, following 1 h of exposure to hypoosmotic shock without significant loss of cellular viability, indicated the potential use of this strain for commercial ectoines production. Currently, investigation on growth of P. halophilum in controlled fermentor to reach high cell density and higher intracellular content of ectoines is in progress in our laboratory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the General Direction of Scientific Research (DGRS) of Tunisia for financial support through the project PRF No PRF2019-D1P1.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflict of interest.
References
- Argandona M, Nieto JJ, Iglesias-Guerra F, Calderon MI, Garcia-Estepa R, Vargas C. Interplay between iron homeostasis and the osmotic stress response in the halophilic bacterium Chromohalobacter salexigens. Appl Environ Microbiol. 2010;76:3575–3589. doi: 10.1128/AEM.03136-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Huhn M, Matthey B, Klimka A, Galinski EA, Engert A. Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl Environ Microbiol. 2000;66:1572–1579. doi: 10.1128/aem.66.4.1572-1579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah M, Karray F, Kallel N, Armougom F, Mhiri N, Quéméneur M, Cayol JL, Erauso G, Sayadi S. Abundance and diversity of prokaryotes in ephemeral hypersaline lake Chott El Jerid using Illumina Miseq sequencing, DGGE and qPCR assays. Extremophiles. 2018;22:811–823. doi: 10.1007/s00792-018-1040-9. [DOI] [PubMed] [Google Scholar]
- Borges N, Ramos A, Raven NDH, Sharp RJ. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles. 2002;6:209–216. doi: 10.1007/s007920100236. [DOI] [PubMed] [Google Scholar]
- Boujelben I, Martínez-García M, van Pelt J, Maalej S. Diversity of cultivable halophilic archaea and bacteria from superficial hypersaline sediments of Tunisian solar salterns. Antony van Leeuwenhoeck. 2015;106:675–692. doi: 10.1007/s10482-014-0238-9. [DOI] [PubMed] [Google Scholar]
- Brands S, Schein P, Castro-Ochoa KF, Galinski EA. Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch Biochem Biophys. 2019;674:108097. doi: 10.1016/j.abb.2019.108097. [DOI] [PubMed] [Google Scholar]
- Bünger J, Driller H. Ectoine: an effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol Physiol. 2004;17:232–237. doi: 10.1159/000080216. [DOI] [PubMed] [Google Scholar]
- Bursy J, Pierik AJ, Pica N, Bremer E. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J Biol Chem. 2007;282:31147–31155. doi: 10.1074/jbc.M704023200. [DOI] [PubMed] [Google Scholar]
- Bursy J, Kuhlmann AU, Pittelkow M, Hartmann H, Jebbar M, Pierik AJ, Bremer E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl Environ Bacteriol. 2008;74:7286–7296. doi: 10.1128/AEM.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa MS, Santos H, Galinski EA. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Eng Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- Deng Y, Huang L, Zhang C, Xie P, Cheng J, Wang X. Physicochemical and functional properties of water soluble gum from Chaenomeles speciosa seeds. J Bioresour Bioprod. 2019;4:222–230. [Google Scholar]
- Detkova EN, Boltyanskaya YV. Osmoadaptation of haloalkaliphilic bacteria: role of osmoregulators and their possible practical application. Microbiology. 2007;76:511–522. [PubMed] [Google Scholar]
- Eiberweiser A, Nazet A, Kruchinin SE, Fedotova MV, Buchner R. Hydration and ion binding of the osmolyte ectoine. J Phys Chem B. 2015;119:15203–15211. doi: 10.1021/acs.jpcb.5b09276. [DOI] [PubMed] [Google Scholar]
- Frikha-Dammak D, Fardeau ML, Cayol JL, Ben Fguira-Fourati L, Najeh S, Ollivier B, Maalej S. Paludifilum halophilum gen. nov. sp. nov. a thermoactinomycete isolated from superficial sediment of a solar saltern. Int J Syst Evol Microbiol. 2016;66:1–8. doi: 10.1099/ijsem.0.001523. [DOI] [PubMed] [Google Scholar]
- Galinski EA, Pfeiffer HP, Trüper HG. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem. 1985;149:135–139. doi: 10.1111/j.1432-1033.1985.tb08903.x. [DOI] [PubMed] [Google Scholar]
- Graf R, Anzali S, Buenger J, Pfluecker F, Driller H. The multifunctional role of ectoine as a natural cell protectant. Clin Dermatol. 2008;26:326–333. doi: 10.1016/j.clindermatol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Guzmán H, Van-Thuoc D, Martín J, Hatti-Kaul R, Quillaguamán J. A process for the production of ectoine and poly(3-hydroxybutyrate) by Halomonas boliviensis. Appl Microbiol Biotechnol. 2009;84:1069–1077. doi: 10.1007/s00253-009-2036-2. [DOI] [PubMed] [Google Scholar]
- Han J, Gao Q-X, Zhang Y-G, Li L, Mohamad OAA, Narsing Rao MP, Xiao M, Hozzein WN, Alkhalifah DHM, Tao Y, Li W-J. Transcriptomic and ectoine analysis of halotolerant Nocardiopsis gilva YIM 90087T under salt stress. Front Microbiol. 2018;9:618. doi: 10.3389/fmicb.2018.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harishchandra RK, Wulff S, Lentzen G, Neuhaus T, Galla HJ. The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys Chem. 2010;150:37–46. doi: 10.1016/j.bpc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- He YZ, Gong J, Yu HY, Tao Y, Zhang S, Dong ZY. High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb Cell Fact. 2015;14:55. doi: 10.1186/s12934-015-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A, Neu TR, Hempel DC, Horn H. Oxygen profiles and biomass distribution in biopellets of Aspergillus niger. Biotechnol Bioeng. 2005;92:614–623. doi: 10.1002/bit.20628. [DOI] [PubMed] [Google Scholar]
- Ignatova Z, Gierasch LM. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc Natl Acad Sci USA. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- Inbar L, Lapidot A. The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus. Use of 13C- and 15N-labeled L-glutamate and 13C and 15N NMR spectroscopy. J Biol Chem. 1988;263:16014–16022. [PubMed] [Google Scholar]
- Jorge CD, Borges N, Bagyan I, Bilstein A, Santos H. Potential applications of stress solutes from extremophiles in protein folding diseases and healthcare. Extremophiles. 2016;20:251–259. doi: 10.1007/s00792-016-0828-8. [DOI] [PubMed] [Google Scholar]
- Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- Klauck E, Typas A, Hengge R. The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli. Sci Prog. 2007;90:103–127. doi: 10.3184/003685007X215922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolp S, Pietsch M, Galinski EA, Gutschow M. Compatible solutes as protectants for zymogens against proteolysis. Biochim Biophys Acta. 2006;1764:1234–1242. doi: 10.1016/j.bbapap.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Kunte HJ, Galinski EA, Trüper HG. A modified FMOC-method for the detection of aminoacid type osmolytes and tetrahydropyrimidines (ectoines) J Microbiol Methods. 1993;17:129–136. [Google Scholar]
- Kunte HJ, Lentzen G, Galinski E. Industrial production of the cell protectant ectoine: protection, mechanisms, processes, and products. Curr Biotechnol. 2014;3:10–25. [Google Scholar]
- Lentzen G, Schwarz T. Extremolytes: natural compounds from extremophiles for versatile applications. Appl Microbiol Biotechnol. 2006;72:623–634. doi: 10.1007/s00253-006-0553-9. [DOI] [PubMed] [Google Scholar]
- León MJ, Hoffmann T, Sánchez-Porro C, Heider J, Ventosa A, Bremer E. Compatible solute synthesis and import by the moderate halophile Spiribacter salinus: physiology and genomics. Front Microbiol. 2018;9:108. doi: 10.3389/fmicb.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Ding XW, Narsing Rao MP, Zhang B, Zhang YG, Liu FH. Morphological and transcriptomic analysis reveals the osmoadaptive response of endophytic fungus Aspergillus montevidensis ZYD4 to high salt stress. Front Microbiol. 2017;8:1789. doi: 10.3389/fmicb.2017.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin G, Lapidot A. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J Bacteriol. 1996;178:385–395. doi: 10.1128/jb.178.2.385-395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta A, Romano I, Gambacorta A. Rapid and sensitive NMR method for osmolyte determination. J Microbiol Methods. 2004;58:289–294. doi: 10.1016/j.mimet.2004.04.012. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Casey JT, Walsh PK, O’Shea DG. Morphological engineering of Streptomyces hygroscopicus var.geldanus: regulation of pellet morphology through manipulation of broth viscosity. Appl Environ Microbiol. 2005;68:305–310. doi: 10.1007/s00253-004-1883-0. [DOI] [PubMed] [Google Scholar]
- Ono H, Sawada K, Khunajakr N, Tao T, Yamamoto M, Hiramoto M, Shinmyo A, Takano M, Murooka Y. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J Bacteriol. 1999;181:91–99. doi: 10.1128/jb.181.1.91-99.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onraedt AE, Walcarius BA, Soetaert WK, Vandamme EJ. Optimization of ectoine synthesis through fed-batch fermentation of Brevibacterium epidermis. Biotechnol Prog. 2005;21:1206–1212. doi: 10.1021/bp0500967. [DOI] [PubMed] [Google Scholar]
- Oren A. Intracellular salt concentration and ion metabolism in halophilic microorganisms. In: Seckbach J, editor. Halophilic microorganisms and their environments. 5. Dordrecht: Kluwer Academic Publishers; 2002. pp. 207–231. [Google Scholar]
- Oren A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front Microbiol. 2013;4:315. doi: 10.3389/fmicb.2013.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk HU, Sariyar Akbulut B, Ayan B, Poli A. Moderately halophilic bacterium Halomonas sp. AAD12: a promising candidate as a hydroxyectoine producer. J Microb Biochem Technol. 2015;7:262–268. [Google Scholar]
- Pastor JM, Salvador M, Argandona M, Bernal V, Reina-Bueno M, Csonka LN, Iborra JL, Vargas C, Nieto JJ, Canovas M. Ectoines in cell stress protection: uses and biotechnological production. Biotechnol Adv. 2010;28:782–801. doi: 10.1016/j.biotechadv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Prabhu J, Schauwecker F, Grammel N, Keller U, Bernhard M. Functional expression of the ectoine hydroxylase gene (thpD) from Streptomyces chrysomallus in Halomonas elongata. Appl Environ Microbiol. 2004;70:3130–3132. doi: 10.1128/AEM.70.5.3130-3132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani A, Venkatesu P. Changing relations between proteins and osmolytes: a choice of nature. Phys Chem Chem Phys. 2018;20:20315–20333. doi: 10.1039/c8cp02949k. [DOI] [PubMed] [Google Scholar]
- Reshetnikov AS, Khmelenina VN, Mustakhimov II, Trotsenko YA. Genes and enzymes of ectoine biosynthesis in halotolerant methanotrophs. Methods Enzymol. 2011;495:15–30. doi: 10.1016/B978-0-12-386905-0.00002-4. [DOI] [PubMed] [Google Scholar]
- Reuter K, Pittelkow M, Bursy J, Heine A, Craan T, Bremer E. Synthesis of 5-hydroxyectoine from ectoine: crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD. PLoS ONE. 2010;5(5):e10647. doi: 10.1371/journal.pone.0010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AA, Mais C-N, Czech L, Geyer K, Hoeppner A, Smits SHJ, Erb TJ, Bange G, Bremer E. Biosynthesis of the stress-protectant and chemical chaperon ectoine: biochemistry of the transaminase EctB. Front Microbiol. 2019;10:2811. doi: 10.3389/fmicb.2019.02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MF. Organic compatible solutes of halotolerant and halophilic microorganism. Saline Syst. 2005;1:5. doi: 10.1186/1746-1448-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A, Soltani BM, Nekouei MK, Jouzani GS, Mirzaei HH, Sadeghizadeh M. Diversity of the ectoines biosynthesis genes in the salt tolerant Streptomyces and evidence for inductive effect of ectoines on their accumulation. Microbiol Res. 2014;169:699–708. doi: 10.1016/j.micres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Sauer T, Galinski EA. Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng. 1998;57:306–313. [PubMed] [Google Scholar]
- Schröter MA, Meyer S, Hahn MB, Solomun T, Sturm H, Kunte HJ. Ectoine protects DNA from damage by ionizing radiation. Sci Rep. 2017;7:15272. doi: 10.1038/s41598-017-15512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha J, Bae JT, Park JP, Kim KH, Song CH, Yun JW. Changes in morphology of Paecilomyces japonika and their effect on broth reology during production of exo-biopolymers. Appl Microbiol Biotechnol. 2001;56:88–92. doi: 10.1007/s002530100606. [DOI] [PubMed] [Google Scholar]
- Tao P, Li H, Yu Y, Gu J, Liu Y. Ectoine and 5-hydroxyectoine accumulation in the halophile Virgibacillus halodenitrificans PDB-F2 in response to salt stress. Appl Microbiol Biotechnol. 2016;100:6779–6789. doi: 10.1007/s00253-016-7549-x. [DOI] [PubMed] [Google Scholar]
- Tesche S, Rösemeier-Scheumann R, Lohr J, Hanke R, Büchs J, Krull R. Salt-enhanced cultivation as a morphology engineering tool for filamentous actinomycetes: increased production of labyrinthopeptin A1 in Actinomadura namibiensis. Eng Life Sci. 2019;19:781–794. doi: 10.1002/elsc.201900036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dissel D, Claessen D, van Wezel GP. Morphogenesis of Streptomyces in submerged cultures. Adv Appl Microbiol. 2014;89:1–45. doi: 10.1016/B978-0-12-800259-9.00001-9. [DOI] [PubMed] [Google Scholar]
- Van-Thuoc D, Guzmán H, Quillaguamán J, Hatti-Kaul R. High productivity of ectoines by Halomonas boliviensis using a combined twostep fed-batch culture and milking process. J Biotechnol. 2010;147:46–51. doi: 10.1016/j.jbiotec.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Van-Thuoc D, Hashim SO, Hatti-Kaul R, Mamo G. Ectoine mediated protection of enzyme from the effect of pH and temperature stress: a study using Bacillus halodurans xylanase as a model. Appl Microbiol Biotechnol. 2013;97:6271–6278. doi: 10.1007/s00253-012-4528-8. [DOI] [PubMed] [Google Scholar]
- Van-Thuoc D, Thi Hien T, Sudesh K. Identification and characterization of ectoine-producing bacteria isolated from Can Gio mangrove soil in Vietnam. Ann Microbiol. 2019;69:819–828. [Google Scholar]
- Vargas C, Jebbar M, Carrasco R, Blanco C, Calderon M, Iglesias-guerra F, Nieto JJ. Ectoines as compatible solutes and carbon and energy sources for the halophilic bacterium Chromohalobacter salexigens. J Appl Microbiol. 2005;100:98–107. doi: 10.1111/j.1365-2672.2005.02757.x. [DOI] [PubMed] [Google Scholar]
- Vyrides I, Stuckey DC. Compatible solute addition to biological systems treating waste/wastewater to counteract osmotic and other environmental stresses: a review. Crit Rev Biotechnol. 2017;37:865–879. doi: 10.1080/07388551.2016.1266460. [DOI] [PubMed] [Google Scholar]
- Wei-Chuan C, Ching-Cha H, John Chi-Wei L, Yu-Kaung C, Li-Fen W, Yu-Hong W. Production and characterization of ectoine using a moderately halophilic strain Halomonas salina BCRC17875. J Biosci Bioeng. 2017;125:1–7. doi: 10.1016/j.jbiosc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Werkhäuser N, Bilstein A, Sonnemann U. Treatment of allergic rhinitis with ectoine containing nasal spray and eye drops in comparison with azelastine containing nasal spray and eye drops or with cromoglycic acid containing nasal spray. J Allergy. 2014 doi: 10.1155/2014/176597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widderich N, Kobus S, Höppner A, Riclea R, Seubert A, Dickschat JS, Heider J, Smits SHJ, Bremer E. Biochemistry and crystal structure of ectoine synthase: a metal-containing member of the cupin superfamily. PLoS ONE. 2016 doi: 10.1371/journal.pone.0151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Kiep KA, Driouch H, Wittmann C, Krull R. Morphology and rheology in filamentous cultivations. Adv Appl Micobiol. 2010;72:89–136. doi: 10.1016/S0065-2164(10)72004-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Zhang Y, Wang Z, Zhao M, Su N, et al. Quantitative proteomics reveals membrane protein-mediated hypersaline sensitivity and adaptation in halophilic Nocardiopsis xinjiangensis. J Proteome Res. 2016;15:68–85. doi: 10.1021/acs.jproteome.5b00526. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shannan L, Peiwen L, Simian S, Cuiqing M, Ping X, Haijun S, Chunyu Y. High ectoine production by an engineered Halomonas hydrothermalis Y2 in a reduced salinity medium. Microb Cell Fact. 2019;18:184. doi: 10.1186/s12934-019-1230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


