Abstract
The present study was conducted to prepare a compound plant extract as a candidate animal feed additive. Firstly, Evodia rutaecarpa (ER), Schisandra sphenanthera (SS), Punica granatum (PG) and Artemisia argyi (AA) were screened out from 17 plants as materials of candidate compound plant extracts by measuring the antibacterial rate on Escherichia coli and Salmonella paratyphoid, and the scavenging capability on 2,2 diphenyl-1-picrylhydrazine radical in vitro. Secondly, proportions of the four materials were optimized with an L9 (43) orthogonal experiment. By range analysis of experimental results, two compound extracts (named as F1 and F2) with the strongest antibacterial and antioxidant functions were obtained. The ratio of ER: SS: PG: AA is 9:9:1:3 in F1 and 9:9:9:3 in F2, respectively. Finally, the effects of F1 and F2 on security and efficacy in vivo were evaluated. In healthy mice, F1 had no significant effects (p > 0.05) on all blood parameters and viscera indices, and at 1000 mg/kg bw dose significantly increased (p < 0.05) the average daily gain (ADG). F2 decreased (p < 0.05) white blood cell count at 3000 mg/kg bw and increased (p < 0.05) red blood cell count at 333 mg/kg bw. In immunosuppressed mice, both F1 and F2 improved ADG (p < 0.05) and the feed intake to gain ratio (p < 0.01), and increased the activities of hepatic superoxide dismutase (p < 0.05), catalase (p < 0.05) and total antioxygen capacity (p < 0.05), and the content of malonaldehyde (p < 0.01). In mice challenged with Escherichia coli, the antidiarrhea and reducing mortality effects of F1 were equivalent to the antibiotic. F2 failed to protect the experimental mice. These results suggested F1, a compound plant extract, show a great potential as a substitute for antibiotics in animal feed.
Keywords: Antibacterial activity, Antioxidant activity, Mice, Plant extracts
Introduction
Antibiotics have been added in feed to prevent diseases and improve growth performances in animal production for decades. However, antibiotics as feed additives have been forbidden due to enhancing public understanding about antibiotic resistance and residues in many countries including China. Consequently, studies on alternatives to antibiotics are very significant in animal feed. Phytogenic feed additives are attracting more and more interest because of no bacterial resistance or drug residues. In addition, various bioactive components such as polyphenols, alkaloids, and polysaccharides are involved in nature plants (Windisch et al. 2008), which play important roles in antibacterial, antioxidant, anti-inflammatory, antidiarrhea and immunomodulatory effects (Liu et al. 2011).
However, every plant shows its limitations in some bioactive activities, but its superiority in other ways. For example, Angelica dahurica has been widely studied for its strong antioxidant activity (Xu et al. 2011; Liang et al. 2018), but few studies could confirm its antibacterial capability. On the other hand, it is well known that bioactive components of each plant are very different. For example, the most main functional ingredient of Lycium barbarum is polysaccharides, but that of Punica granatum is polyphenols. In addition, to some extent, the extracting method and process also affect the contents of bioactive ingredients. Hypothetically, several plants which separately have strong antibacterial or antioxidant potentiality by mixed extraction together could show more extensive biological activities in vitro, whether or not the extract is also secure and effective in improving health and growth performances in vivo. This is also the most important and basic requirement of a compound plant extract that could be an alternative antibiotic feed supplement.
Therefore, in the present study 17 plants were firstly selected because they had been universally reported to have strong antibacterial and/or antioxidant activities, and among these plants some or their extracts had been demonstrated to protect animals against diarrhea and modulate immune response and antioxidant capability in vivo (Zhao et al. 2016; Zhang et al. 2017; Rajabi et al. 2017). Secondly, an aqueous extract of each plant was prepared to compare the antibacterial and antioxidant properties in vitro. Thirdly, plants which showed stronger activities were further screened out to develop compound extracts by orthogonal design. Finally, the effects of the two candidate compound extracts were evaluated in vivo.
Materials and methods
Materials
Samples of 17 plants, which were root barks of Acanthopanax gracilistylus, roots of Angelica dahurica, roots of Angelica sinensis, leaves of Artemisia argyi, roots of Astragalus membranaceus, fruits of Euodia rutaecarpa, barks of Fraxinus chinensis, roots of Glycyrrhiza uralensis, roots of Isatis indigotica, fruits of Lycium barbarum, twigs of Morus alba, seeds of Plantago major, roots of Portulaca oleracea, Pulsatilla chinensis, peels of Punica granatum, fruits of Schisandra sphenanthera and Taraxacum mongolicum, were obtained from Pharmacy of Zhejiang Chinese Medical University, Hangzhou, China. Mueller–Hinton broth was from Hangzhou Tianhe Microorganism Reagent Company Limited, Hangzhou, China. Escherichia coli and Salmonella paratyphoid were from China Institute of Veterinary Drug Control, Beijing, China. Analytical reagent-grade chemicals such as 2,2 diphenyl-1-picrylhydrazine free radical (DPPH), Folin–Ciocalteu reagent, ethanol, acetone and ether were procured from Sangon Biotech Company Limited, Shanghai, China.
Methods
Preparation of plant extracts
These plant materials were pulverized and mixed with ddH2O (1: 20, m/v). After 1 h ultrasonic treatment, the extracts were heated in a microwave reaction system (CEM Corporation, Matthews, USA) at 65 °C for 20 min, and filtered under vacuum and concentrated to 0.5 g crude material/ml in an oven.
Antibacterial assay
Escherichia coli and Salmonella paratyphoid were incubated in Mueller–Hinton broth for 24 h at 37 °C, 210 rpm. Then the suspension equivalent samples were prepared according to McFarland standards with sterile saline. The bacterial suspension was added into the mixture containing 400 μl plant extract or sterile water and 4 ml medium (1:1000, v/v), and then incubated for 24 h at 37 °C, 210 rpm. Absorbance difference (ΔA) of the culture at 600 nm was determined from 0 to 24 h during incubation, through a spectrophotometer (BioTek Instruments Incorporated, Winooski, USA). All treatments were carried out in triplicate. The inhibition activity of plant extracts against the growth of bacteria was calculated as follows: Inhibition ratio = (ΔAblank − ΔAtest)/ΔAblank × 100%, where Ablank is the absorbance value of the control reaction, and Atest is the absorbance value of the test samples.
Minimum inhibitory concentration (MIC) of plant extracts was determined by the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI 2010).
DPPH radical scavenging assay
According to the method of Aluko and Monu (2003), 100 μl plant extracts of different concentrations (100, 50, 25, 12.5 and 6.25 mg/ml) were mixed with 100 μl DPPH ethanol solution (0.5 mg/ml) in a 96-well plate. Covered with preservative film, the liquids were incubated in dark at 37 °C for 30 min. The absorbance at 516 nm was measured with a spectrophotometer. ddH2O replaced the extract as the control. All treatments were carried out in triplicate. The inhibition of the DPPH radical by samples was calculated according to the following formula: Percent (%) inhibition of DPPH scavenging activity = (Ablank − Atest)/Ablank × 100%, where Ablank is the absorbance value of the control reaction, and Atest is the absorbance value of the test samples. A regression equation was fitted according to extract concentration and DPPH scavenging activity using CurveExpert 1.3 software. The concentration of every extract providing 50% inhibitions (DPPH IC50) was calculated from the inhibition percentage against extracts concentration.
Orthogonal assay
An orthogonal L9 (43) test design was constructed to develop candidate compound plant extracts. As shown in Table 2, nine experiments were conducted with four factors and three levels. Four factors were separately ER, SS, PG and AA, and the different weights of each plant material were as corresponding levels, which were 1 g (level 1), 3 g (level 2) and 9 g (level 3). The four plant materials were mixed in different proportions according to the orthogonal array to obtaine different mixtures. Then these mixtures were together extracted by the same extracting method as above and nine compound extracts obtained were used to detect bacterial inhibition ratios and DPPH IC50. Two candidate compound extracts F1 and F2 were developed using the range analysis. Then the antibacterial and antioxidant activities of the two extracts were further validated as in the above methods described.
Table 2.
Design and results of orthogonal design
| Number | ER | SS | PG | AA | Inhibition ratio (%) | DPPH IC50 (mg/ml) | |
|---|---|---|---|---|---|---|---|
| Escherichia coli | Salmonella paratyphoid | ||||||
| 1 | 3 | 2 | 1 | 3 | 93 | 100 | 24 |
| 2 | 3 | 1 | 2 | 2 | 100 | 80 | 25 |
| 3 | 2 | 1 | 3 | 3 | – | – | 15 |
| 4 | 2 | 3 | 1 | 2 | 44 | – | 36 |
| 5 | 3 | 3 | 3 | 1 | – | – | 12 |
| 6 | 1 | 2 | 3 | 2 | – | – | 10 |
| 7 | 2 | 2 | 2 | 1 | – | – | 35 |
| 8 | 1 | 3 | 2 | 3 | 87 | – | 31 |
| 9 | 1 | 1 | 1 | 1 | 26 | – | 19 |
ER Evodia rutaecarpa, SS Schisandra sphenanthera, PG Punica granatum peel, AA Artemisia argyi. –, bacterial inhibition ratio (%) was lower than 0
Determination of bioactive components in compound plant extracts
The contents of polyphenols in F1 and F2 were determined by Folin–Ciocalteu method appropriately modified (Verma et al. 2009). Five times volume of tenfold diluted Folin–Ciocalteu reagent was placed in the dark at room temperature for 10 min. The mixture was then neutralized with 10 mg/ml sodium carbonate solution and incubated at room temperature for 60 min. Finally, the absorbance of the test sample solutions obtained was measured at 750 nm using a spectrophotometer. All measurements were quantified in triplicate.
The contents of polysaccharides in F1 and F2 were detected according to the method appropriately modified (Hu et al. 2013). Three to four times volume of 95% ethanol was added to extract the solution after concentration. The mixtures were placed at room temperature for 8 h and centrifuged at 1500×g for 20 min. After precipitation, the precipitates were washed by acetone and ether, and then dissolved in ddH2O. Finally, the absorbance of the test sample solutions obtained was detected at 490 nm. All the measurements were quantified in triplicate.
Animals and housing conditions
The female BALB/c mice (20 ± 2 g) were obtained from the Zhejiang Institute for Food and Drug Control. The mice had ad libitum access to food and water and were allowed to adapt to the environment for 1 week. During the experiment, the animals were housed in plastic cages under controlled conditions at constant temperature 22 °C, with a relative humidity of 50 ± 10% and 12 h light/dark cycles. The study protocol was approved by the Institutional Committee on Animal Care based on the Chinese Specifications for the Production, Care, and Use of Laboratory Animals. Regarding the mouse survival study, we used humane end point during the experiment period. The humane end points were obvious symptoms of arching, cyanosis, hypothermia, difficulty in movement or sleepiness. Animals that reach one of these end points were euthanized by cervical dislocation.
Toxicity experiment of compound plant extracts in healthy mice
A sub-acute toxicity method was conducted to evaluate security of the candidate compound plant extracts F1 and F2 in vivo. According to the Acute Oral Toxicity Test for Food Safety National Standard (GB 15193.3-2014) approved by the National Health and Family Planning Commission of the People’s Republic of China, a total of 45 mice were orally administered with 0.2 ml ddH2O (control group) or four different doses (111, 333, 1000, and 3000 mg/kg bw) of F1 or F2 one time a day for 14 days (n = 5 per dose), with mice receiving the same treatment housed in one cage. The situation of experimental mice was monitored every day (at 9:00). Mortality, clinical signs, initial and final body weights, and feed intake were recorded. Average daily feed intake (ADFI), average daily gain (ADG) and feed intake to gain ratio (F/G) were calculated.
Sample collection
The experimental mice were etherized and killed by cervical dislocation. The samples of whole blood with ethylenediamine tetraacetic acid (EDTA) were collected. The abdominal cavity of each animal was opened. Internal organs (liver, spleen, thymus and kidneys) were removed and weighed, calculating organ indices. Viscera indices were expressed as percentage of live body weight.
Measurements of hematological traits
The samples of whole blood collected were used to analyze the counts of white blood cell (WBC), red blood cell (RBC), platelet (PLT), and the content of hemoglobin (HGB), by LH750 automatic blood cell analyzer (Beckman Coulter Incorporated, Brea, USA).
Efficacy experiment of compound plant extracts in immunosuppressed mice
According to the results of toxicity experiment, a total of 80 mice were randomly assigned into control, model, antibiotic, F1 and F2 groups (16 mice/group, 4 mice/cage). Mice in model, antibiotic, F1 and F2 groups were orally administered with 0.2 ml ddH2O, chlortetracycline (50 mg/kg bw), extract of F1 or F2 (1000 mg/kg bw) once a day for 14 days and given intraperitoneal injections of cyclophosphamide (CP) every 4 days (100 mg/kg bw for the first time and 50 mg/kg bw for the subsequent times). For the control group, ddH2O was used to replace CP, chlortetracycline or extracts.
On d 15, all these mice were weighed after 10 h fast, and half of the mice in each group received enteropathogenic Escherichia coli at 6.8 × 106 CFU/10 g bw by intraperitoneal injection. The diarrhea ratio and mortality after 48 h post-injection were recorded. The mice were observed every 12 h after ETEC injection. Mice received meloxicam via the tail vein (2 mg/kg once daily for 2 days) as an analgesic to alleviate the pain caused by the bacterial infection (Xiang et al. 2014).
Sample collection
The remaining eight mice in each group were etherized and killed by cervical dislocation. The samples of whole blood were collected. The abdominal cavity of each animal was opened. Immune organs (spleen and thymus) of the mice were removed and weighed, calculating organ indices. Viscera indices were expressed as percentage of live body weight.
The samples of serum from whole blood were prepared by centrifugation at 1500×g for 10 min at 4 °C and stored at − 80 °C.
After weighing, hepatic samples were collected and immediately frozen in liquid nitrogen, then stored at − 80 °C.
Assessment of serum and hepatic antioxidant parameters
Serum samples were used to determine the activities of superoxide dismutase (SOD), catalase (CAT) and total antioxygen capacity (T-AOC), and the content of malonaldehyde (MDA) using commercial kits (Nanjing Jiancheng Biochemical Reagent, Nanjing, China).
Hepatic samples were homogenized in nine times of saline. After 10 min centrifugation at 800×g, the supernatant of the homogenate was removed and diluted 40 times with saline to determine the activities of SOD, CAT and T-AOC, and the content of MDA.
One unit of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50%; one unit of T-AOC was defined as the amount necessary to increase the absorbance at 520 nm by 0.01 in 1 min/ml supernatant; one unit of CAT was defined as the amount of enzyme that quenched 1 μmol/s H2O2 at 405 nm.
Statistical analyses
Data from experiments in vivo were expressed as mean ± standard deviation (SD). Data obtained from toxicity experiment were analyzed by independent sample t test. Data obtained from efficacy experiment were analyzed with one-way analysis of variance following a completely random design to determine the least significant difference (LSD) at p < 0.05 using SPSS software package (IBM SPSS, New York, USA).
Results
Antibacterial and antioxidant activities of single plant extracts in vitro
The results of antibacterial activity showed that SS and ER had the higher inhibition ratio against the growth of Escherichia coli and Salmonella paratyphoid than other plant extracts (Table 1). The results of antioxidant array showed the DPPH IC50 values of all extracts were ranging from 2 to 153 mg/ml, among which PG showed the strongest scavenging DPPH radical ratio, and the second strongest was AA. Both of their DPPH IC50 values were lower than 10 mg/ml. Therefore, SS, ER, PG and AA were screened out from 17 plants as materials of candidate compound extracts.
Table 1.
Bacterial inhibition ratio and DPPH IC50 of 17 plant extracts
| Extract | Bacterial inhibition ratio (%) | DPPH IC50 (mg/ml) | |
|---|---|---|---|
| Escherichia coli coli | Salmonella paratyphoid paraparatyphoid | ||
| Acanthopanar gracilistμlus root barks | – | – | 47 |
| Angelica dahurica | – | – | 43 |
| Angelica sinensis | – | 22 | 26 |
| Artemisia argyi | – | – | 6 |
| Astragalus membranaceus roots | – | – | 90 |
| Euodia rutaecarpa | 34 | 91 | 10 |
| Fraxinus chinensis | – | 22 | 16 |
| Glycyrrhiza uralensis | – | – | 45 |
| Isatis indigotica | – | – | 153 |
| Lycium barbarum | 12 | 28 | 61 |
| Morus alba | – | – | 88 |
| Plantago major | – | – | 53 |
| Portulaca oleracea | – | – | 32 |
| Pulsatilla chinensis | – | 5 | 25 |
| Punica granatum | – | – | 2 |
| Schisandra sphenanthera fruits | 100 | 100 | 41 |
| Taraxacum mongolicum | – | – | 16 |
– Bacterial inhibition ratio (%) was lower than 0
Development of candidate compound plant extracts
The bacterial inhibition ratios and DPPH IC50 of nine compound extracts according to the orthogonal array were shown (Table 2). By range analysis, two candidate compound extracts with the best antibacterial (F1) and the strongest antioxidant (F2) activities were separately obtained. The plant material ratios of ER, SS, PG and AA were 9:9:1:3 (ER3SS3PG1AA2) in F1 and 9:9:9:3 (ER3SS3PG3AA2) in F2, respectively. The verifying results of antibacterial and antioxidant activities showed the MIC values against Escherichia coli and Salmonella paratyphoid of F1 were, respectively, 500 and 250 mg/ml, and the corresponding values of F2 were 250 and 500 mg/ml, respectively. The DPPH IC50 values of F1 and F2 were, respectively, 6.46 and 3.63 mg/ml.
The contents of polyphenols and polysaccharides of F1 were, respectively, 8.02% and 1.74%, and the values of F2 were correspondingly 10.63% and 2.16%.
Security evaluation of compound plant extracts in healthy mice
No mice were dead or had abnormal clinical symptoms during the feeding experiment. The growth performances of mice are shown in Table 3. Given F1 at 1000 mg/kg bw dose significantly increased (p < 0.05) ADG of mice than that of the control, ADFI and F/G of mice were not significantly affected (p > 0.05) by F1 and F2.
Table 3.
Effects of two compound plant extracts on growth performances in healthy mice
| Dose (mg/kg bw) | Growth performances | |||
|---|---|---|---|---|
| ADG (g) | ADFI (g) | F/G | ||
| Control | 0.39 ± 0.07 | 3.85 | 11.66 | |
| F1 | 111 | 0.41 ± 0.09 | 3.36 | 10.00 |
| 333 | 0.44 ± 0.08 | 3.33 | 9.85 | |
| 1000 | 0.47 ± 0.05* | 3.22 | 9.30 | |
| 3000 | 0.36 ± 0.30 | 3.93 | 11.89 | |
| F2 | 111 | 0.41 ± 0.09 | 3.72 | 11.17 |
| 333 | 0.42 ± 0.16 | 3.34 | 9.91 | |
| 1000 | 0.43 ± 0.08 | 3.38 | 10.03 | |
| 3000 | 0.36 ± 0.08 | 3.77 | 11.44 | |
F1 the compound extract from the raw plant material ratio of Evodia rutaecarpa: Schisandra sphenanthera:Punica granatum:Artemisia argyi is 9:9:1:3; F2 the corresponding ratio of the four plants is 9:9:9:3
ADG average daily gain, ADFI average daily feed intake, F/G ratio of feed to gain
Significant difference was *p < 0.05
The results of blood cell parameters are shown in Table 4. The values of most blood cell parameters showed no significant differences (p > 0.05) in all F1, F2 groups and the control; with the exception of WBC at 3000 mg/kg bw dose of F2 and RBC at 333 mg/kg bw dose, the former was significantly decreased (p < 0.05) and the latter was significantly increased (p < 0.05) than those of the control.
Table 4.
Effects of two compound plant extracts on blood cell parameters in healthy mice
| Dose (mg/kg bw) | Blood cell parameters | ||||
|---|---|---|---|---|---|
| WBC (109/L) | RBC (1012/L) | HGB (g/L) | PLT (109/L) | ||
| Control | 5.32 ± 1.29 | 11.5 ± 0.72 | 211 ± 12 | 11.98 ± 4.86 | |
| F1 | 111 | 5.18 ± 0.54 | 12.55 ± 1.50 | 236 ± 35 | 11.62 ± 1.87 |
| 333 | 7.66 ± 2.51 | 11.74 ± 1.40 | 204 ± 16 | 10.47 ± 0.76 | |
| 1000 | 5.08 ± 1.14 | 11.52 ± 0.90 | 218 ± 21 | 9.38 ± 1.54 | |
| 3000 | 5.75 ± 0.59 | 12.05 ± 0.86 | 211 ± 18 | 10.74 ± 1.10 | |
| F2 | 111 | 5.24 ± 0.23 | 12.22 ± 0.92 | 209 ± 14 | 9.84 ± 0.32 |
| 333 | 5.26 ± 1.94 | 12.95 ± 0.68* | 227 ± 19 | 9.30 ± 1.66 | |
| 1000 | 5.86 ± 1.20 | 11.98 ± 0.88 | 215 ± 12 | 10.40 ± 1.34 | |
| 3000 | 3.68 ± 0.45* | 11.16 ± 0.59 | 197 ± 8 | 10.81 ± 1.41 | |
F1 the compound extract from the raw plant material ratio of Evodia rutaecarpa:Schisandra sphenanthera:Punica granatum:Artemisia argyi is 9:9:1:3; F2, the corresponding ratio of the four plants is 9:9:9:3
WBC white blood cell, RBC red blood cell, HGB hemoglobin, PLT platelet
Significant differences was *P < 0.05
The results of the viscera indices are shown in Table 5. All the viscera indices including liver, spleen, thymus and kidneys of the experimental mice were not significantly affected (p > 0.05) by F1 and F2.
Table 5.
Effects of two compound plant extracts on viscera indices in healthy mice
| Dose (mg/kg bw) | Viscera indices (%) | ||||
|---|---|---|---|---|---|
| Liver | Spleen | Thymus | Kidneys | ||
| Control | 4.30 ± 0.25 | 0.32 ± 0.09 | 0.32 ± 0.06 | 1.23 ± 0.17 | |
| F1 | 111 | 4.35 ± 0.15 | 0.40 ± 0.07 | 0.40 ± 0.10 | 1.34 ± 0.06 |
| 333 | 4.26 ± 0.27 | 0.43 ± 0.13 | 0.38 ± 0.05 | 1.29 ± 0.06 | |
| 1000 | 4.37 ± 0.24 | 0.39 ± 0.07 | 0.34 ± 0.07 | 1.19 ± 0.19 | |
| 3000 | 4.73 ± 0.64 | 0.40 ± 0.05 | 0.33 ± 0.04 | 1.40 ± 0.09 | |
| F2 | 111 | 4.63 ± 0.24 | 0.34 ± 0.04 | 0.31 ± 0.08 | 1.39 ± 0.17 |
| 333 | 4.22 ± 0.30 | 0.33 ± 0.04 | 0.37 ± 0.04 | 1.34 ± 0.07 | |
| 1000 | 4.44 ± 0.17 | 0.41 ± 0.04 | 0.36 ± 0.07 | 1.45 ± 0.03 | |
| 3000 | 4.23 ± 0.19 | 0.37 ± 0.09 | 0.36 ± 0.05 | 1.33 ± 0.07 | |
F1 the compound extract from the raw plant material ratio of Evodia rutaecarpa: Schisandra sphenanthera:Punica granatum:Artemisia argyi is 9:9:1:3; F2 the corresponding ratio of the four plants is 9:9:9:3
Effects of compound plant extracts in immunosuppressed mice
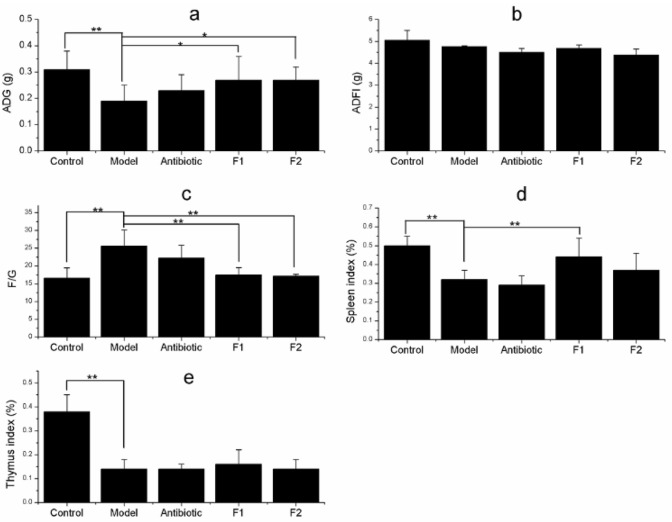
As shown in Fig. 1, ADG, the indices of spleen and thymus of mice were significantly lower (p < 0.01), and F/G was very significantly higher (p < 0.01) in the model group than the control. All the above index values had no significant difference (p > 0.05) in the antibiotic group compared with the model group. ADG of mice was significantly increased (p < 0.05), spleen index was significantly higher (p < 0.01) and F/G was significantly lower (p < 0.01) in the F1 group compared with the model, but no significant difference (p > 0.05) with the control. ADG of mice was significantly increased (p < 0.05) and F/G was significantly lowered (p < 0.01) in the F2 group than the model, but no significant difference (p > 0.05) with the control.
Fig. 1.
Growth performances and immune organ indices of immunosuppressed mice. a ADG, average daily gain; b ADFI, average daily feed intake; c F/G, feed intake to gain ratio; d spleen index; e thymus index. F1 The compound extract from the raw plant material ratio of Evodia rutaecarpa: Schisandra sphenanthera:Punica granatum:Artemisia argyi is 9:9:1:3; F2 the corresponding ratio of the four plants is 9:9:9:3. Significant differences were *P < 0.05 and **P < 0.01
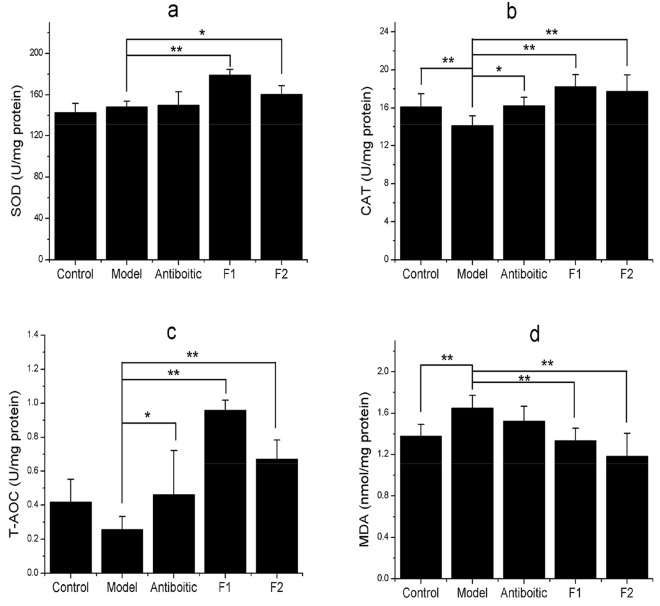
As shown in Fig. 2, the hepatic CAT activity of mice was significantly lower (p < 0.01), and MDA content was significantly increased (p < 0.01); the activities of SOD and T-AOC had no significant differences (p > 0.05) in the model group compared with the control. The hepatic CAT and T-AOC activities were significantly higher (p < 0.05), and SOD activity and MDA content had no significant differences (p > 0.05) in the antibiotic group compared with the model. The hepatic SOD activity of mice was significantly higher in both F1 (p < 0.01) and F2 (p < 0.05) than the model mice. The activities of CAT and T-AOC were significantly higher (p < 0.01), and the MDA content was significantly lower (p < 0.01) in both the F1 and F2 groups than the model.
Fig. 2.
Antioxidant activities in liver of immunosuppressed mice. a SOD superoxide dismutase (SOD) activity; b CAT, catalase (CAT) activity; c T-AOC, total antioxidant capacity; d MDA, malonaldehyde (MDA) content. F1 the compound extract from the raw plant material ratio of Evodia rutaecarpa:Schisandra sphenanthera:Punica granatum:Artemisia argyi is 9:9:1:3; F2 the corresponding ratio of the four plants is 9:9:9:3. Significant differences were *P < 0.05 and **P < 0.01
Diarrhea ratio and mortality of mice challenged with ETEC
Diarrhea ratios of the control, model, antibiotic, F1, and F2 groups were 50.0%, 66.7%, 25%, 25% and 75%, and the mortalities in these groups were correspondingly 0%, 33.3%, 0%, 0% and 37.5%, respectively.
Discussion
Antibacterial and antioxidant activities of single plant extracts
Although all plants selected in this study had been previously reported to show antibacterial or antioxidant capabilities, our results indicated most crude aqueous extracts could not inhibit the growth of Escherichia coli and Salmonella paratyphoid. In contrast, the scavenging DPPH radical property of these plant extracts was confirmed. PG has been widely studied in the past years, which showed the strongest antioxidant activity among approximately 1000 plants (Niwano et al. 2011) and significant antibacterial activity (Malviya et al. 2014). AA was also demonstrated to have antioxidant, immunomodulatory and antibacterial properties (Lan et al. 2010; Guan et al. 2019). However, in the present study antioxidant activity instead of antibacterial effect of AA was found. Similarly, our results indicated SS had strong antibacterial effect rather than antioxidant property of SS which had been demonstrated (Li et al. 2018). The inconsistent results were probably attributed to different extracting methods and tested bacteria strains. Especially, there were completely different bioactive components between aqueous extracts and organic solvent extracts.
Development of candidate compound plant extracts
For developing a compound plant extract, we originally optimized proportions of different plants using an orthogonal experiment after determining the four materials SS, ER, PG and AA. According to the theory of orthogonal design, F1 would show the strongest antibacterial activity as well as compromising antioxidant property among all possible compound plant extracts. On the other hand, F2 would have the strongest antioxidant capability and better antibacterial effect. Although in this study the subsequent verifying test of antibacterial activity in vitro could not distinguish the difference between F1 and F2 because of the limitation of the experimental method, the antioxidant effect of the two extracts in vitro had been surely confirmed. Significantly, we also offered a novel scientific method of developing compound plant extracts.
The bioactive components of compound plant extracts.
We detected two main bioactive components including polyphenols and polysaccharides which had been reported according to four plant materials of F1 and F2 (Lan et al. 2010; Zhao et al. 2015; Li et al. 2018). In contrast, the contents of both polyphenols and polysaccharides in F2 were higher than those of F1, and this was probably one of the reasons why F2 showed stronger antioxidant activity than F1 in vitro. In addition, according to previous studies (Wang et al. 2013; Liang et al. 2017; Han et al. 2017; Xia et al. 2019), the extracts F1 and F2 might also contain other components such as alkaloids, flavonoids and limonids.
Effects of compound plant extracts in mice
Our results of security evaluation showed both F1 and F2 extracts even at 3000 mg/kg bw dose orally administered had no significant negative effects on most blood cell parameters and all viscera indices in normal healthy mice, suggesting the candidate compound plant extracts would be a safe animal feed additive. Furthermore, the two extracts also improved growth performances and hepatic antioxidant function in immunosuppressed mice, even better than the antibiotic. These results indicated they would be an effective animal feed additive, especially in piglets suffering from weaning stress. In agreement with our results, PG extract improved the antibacterial activity, growth performances, antioxidant effects and immune function in some animals (Viuda-Martos et al. 2010; Rajabi et al. 2017; Jose et al. 2017). AA extract improved the antioxidant capacity and relieved the immune stress response in broilers (Zhao et al. 2016; Zhang et al. 2017). SS could also enhance immunity in immunosuppressed mice (Chen et al. 2012; Zhao et al. 2014). ER showed anti-inflammatory effects (Ko et al. 2007). Totally, the effects of F1 and F2 found in vivo in this study were mainly attributed to every material of the compound extracts.
However, there were significant differences between F1 and F2 in antidiarrhea effects and reducing mortality of mice challenged with ETEC. The effects of F1 were equivalent to the antibiotic compared with the model group. F2 failed to protect the experimental mice against diarrhea and death. Interestingly, the great differences were only attributed to different proportions of the same four plant materials between F1 and F2. More significantly, the corresponding proportions of ER and AA were higher in F1 compared with F2, and their antidiarrhea effects had been confirmed separately in mice and rabbits (Yu et al. 2000; Liu et al. 2019). Consequently, F1 was more potential to be a candidate animal feed additive, with promise as an alternative antibiotic feed supplement. Further studies of this compound plant extract in domestic animals would be very significant.
Conclusions
In summary, a compound plant extract of which materials were composed of ER, SS, PG and AA in proportion of 9:9:1:3 had been developed and demonstrated to be a safe growth-promoting, immunomodulatory, antioxidant and antidiarrhea agent in mice. It would be a promising alternative in animal feed.
Author contributions
XT and ZWX conceived of the study. YQS performed the investigation, analyzed the results and wrote the manuscript. XMM helped to revise the manuscript.
Funding
This work was supported by grants from the Zhejiang Provincial Public Welfare Technology Applied Research Project (LGN18C170004), the Science Technology Department of Zhejiang Province (2018C02035, 2018C02044 and 2016C02054), the Major Research Project from Zhejiang Province (2021-2024), and the earmarked fund for China Agriculture Research System (CARS-35). The funders had no role in study collection, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All the data used in the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Aluko RE, Monu E. Functional and bioactive properties of quinoa seed protein hydrolysates. J Food Sci. 2003;68:1254–1258. doi: 10.1111/j.1365-2621.2003.tb09635.x. [DOI] [Google Scholar]
- Chen Y, Tang J, Wang X, Sun FX, Liang SJ. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz.) Baill. Int J Biol Macromol. 2012;50:844–848. doi: 10.1016/j.ijbiomac.2011.11.015. [DOI] [PubMed] [Google Scholar]
- CLSI (2010) performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA.
- Guan X, Ge D, Li S, Huang K, Liu J, Li F (2019) Chemical composition and antimicrobial activities of Artemisia argyi lévl. et vant essential oils extracted by simultaneous distillation-extraction, subcritical extraction and hydrodistillation. Molecules 24:483. 10.3390/molecules24030483 [DOI] [PMC free article] [PubMed]
- Han B, Xin Z, Ma S, Liu W, Zhang B, Ran L, Yi L, Ren D. Comprehensive characterization and identification of antioxidants in folium Artemisiae argyi using high-resolution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1063:84–92. doi: 10.1016/j.jchromb.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Hu M, Zhang H, Feng B, Liu K, Guo S. Extraction of polysaccharides from Fomes officinalis Ames and their antitumor activity. Exp Ther Med. 2013;6(2):451–454. doi: 10.3892/etm.2013.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose T, Pattanaik AK, Jadhav SE, Dutta N, Sharma S. Nutrient digestibility, hindgut metabolites and antioxidant status of dogs supplemented with pomegranate peel extract. J Nutr Sci. 2017;6:e36. doi: 10.1017/jns.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HC, Wang YH, Liou KT, Chen CM, Chen CH, Wang WY, Chang S, Hou YC, Chen KT, Chen CF, Shen YC. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur J Pharmacol. 2007;555:211–217. doi: 10.1016/j.ejphar.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Lan MB, Zhang YH, Zheng Y, Yuan HH, Zhao HL, Gao F. Antioxidant and immunomodulatory activities of polysaccharides from moxa (Artemisia argyi) leaf. Food Sci Biotechnol. 2010;19:1463–1469. doi: 10.1007/s10068-010-0209-5. [DOI] [Google Scholar]
- Li Z, He X, Liu F, Wang J, Feng J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: properties, functions and applications. Carbohydr Polym. 2018;184:178–190. doi: 10.1016/j.carbpol.2017.12.058. [DOI] [PubMed] [Google Scholar]
- Liang X, Li B, Wu F, Li T, Wang Y, Ma Q, Liang S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement Altern Med. 2017;17:180. doi: 10.1186/s12906-017-1701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WH, Chang TW, Charng YC. Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Sci Biotechnol. 2018;27:1085–1092. doi: 10.1007/s10068-018-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Tong JM, Zhou DW. Utilization of Chinese herbal feed additives in animal production. Agric Sci China. 2011;10:1262–1272. doi: 10.1016/S1671-2927(11)60118-1. [DOI] [Google Scholar]
- Liu L, Zuo W, Li F. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning. J Anim Sci. 2019;97:1693–1700. doi: 10.1093/jas/skz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya S, Jha A, Hettiarachchy N. Antioxidant and antibacterial potential of pomegranate peel extracts. J Food Sci Technol. 2014;51:4132–4137. doi: 10.1007/s13197-013-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano Y, Saito K, Yoshizaki F, Kohno M, Ozawa T. Extensive screening for herbal extracts with potent antioxidant properties. J Clin Biochem Nutr. 2011;48:84. doi: 10.3164/jcbn.11-013FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi M, Rouzbehan Y, Rezaei J. A strategy to improve nitrogen utilization, reduce environmental impact, and increase performance and antioxidant capacity of fattening lambs using pomegranate peel extract. J Anim Sci. 2017;95:499. doi: 10.2527/jas2016.1069. [DOI] [PubMed] [Google Scholar]
- Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Fernández-López J, Pérez-Álvarez JA. Pomegranate and its many functional components as related to human health: a review. Compr Rev Food Sci F. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- Wang XX, Zan K, Shi SP, Zeng KW, Jiang Y, Guan Y, Xiao CL, Gao HY, Wu LJ, Tu PF. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia. 2013;89:1–7. doi: 10.1016/j.fifitote.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86:E140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Xia JX, Zhao BB, Zan JF, Wang P, Chen LL. Simultaneous determination of phenolic acids and flavonoids in Artemisiae argyi Folium by HPLC-MS/MS and discovery of antioxidant ingredients based on relevance analysis. J Pharm Biomed. 2019;175:1–8. doi: 10.1016/j.jpba.2019.06.031. [DOI] [PubMed] [Google Scholar]
- Xiang K, Cheng L, Luo Z, Ren J, Tian F, Tang L, Dai R. Glycyrrhizin suppresses the expressions of HMGB1 and relieves the severity of traumatic pancreatitis in rats. PLoS ONE. 2014;9:e115982. doi: 10.1371/journal.pone.0115982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SF, Ye YP, Chen FY. Chemical composition and antioxidant activities of different polysaccharides from the roots of Angelica dahurica. Chem Biodivers. 2011;8:1121–1131. doi: 10.1002/cbdv.201000233. [DOI] [PubMed] [Google Scholar]
- Yu L, Liao J, Chen C. Anti-diarrheal effect of water extract of Evodiae fructus in mice. J Ethnopharmacol. 2000;73:39–45. doi: 10.1016/S0378-8741(00)00267-1. [DOI] [PubMed] [Google Scholar]
- Zhang PF, Shi BL, Su JL, Yue YX, Cao ZX, Chu WB, Li K, Yan SM. Relieving effect of Artemisia argyi aqueous extract on immune stress in broilers. J Anim Physiol Anim Nutr. 2017;101:251–258. doi: 10.1111/jpn.12553. [DOI] [PubMed] [Google Scholar]
- Zhao T, Feng Y, Li J, Mao RW, Zou Y, Feng WW, Zheng D, Wang W, Chen Y, Yang L, Wu X. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int J Biol Macromol. 2014;65:33–40. doi: 10.1016/j.ijbiomac.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Zhao LM, Jia YL, Ma M, Duan YQ, Liu LH. Prevention effects of Schisandra polysaccharide on radiation-induced immune system dysfunction. Int J Biol Macromol. 2015;76:63–69. doi: 10.1016/j.ijbiomac.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Zhao F, Shi B, Sun D, Chen H, Tong M, Zhang P, Guo X, Yan S. Effects of dietary supplementation of Artemisia argyi aqueous extract on antioxidant indexes of small intestine in broilers. Anim Nutr. 2016;2:76–81. doi: 10.1016/j.aninu.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used in the current study are available from the corresponding author on reasonable request.