Abstract
The “Warburg effect” describes the reprogramming of glucose metabolism away from oxidative phosphorylation toward aerobic glycolysis, and it is one of the hallmarks of cancer cells. Several factors can be involved in this process, but in this review, the roles of non-coding RNAs (ncRNAs) are highlighted in several types of human cancer. ncRNAs, including microRNAs, long non-coding RNAs, and circular RNAs, can all affect metabolic enzymes and transcription factors to promote glycolysis and modulate glucose metabolism to enhance the progression of tumors. In particular, the 5′-AMP-activated protein kinase (AMPK) and the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathways are associated with alterations in ncRNAs. A better understanding of the roles of ncRNAs in the Warburg effect could ultimately lead to new therapeutic approaches for suppressing cancer.
Keywords: glycolysis, non-coding RNAs, cancer, Warburg effect
Graphical Abstract

Many ncRNAs have been implicated in the switch to glycolysis, thus increasing the overall aggressiveness of the tumor. Not only could further investigation into the mechanisms and functions of ncRNAs allow the discovery of additional prognostic biomarkers, but it could also be beneficial for the development of novel therapeutic approaches.
Main Text
Otto Heinrich Warburg first discovered that tumors tended to show a specific metabolic phenotype in the 1920s. Cancer cells, even when they have sufficient oxygen, tend to make ATP using glycolysis, rather than the oxidative phosphorylation (OXPHOS) that occurs in normal cells and generates more ATP per glucose molecule. This phenomenon of aerobic glycolysis became known as the “Warburg effect.” In glycolysis, the glucose uptake by the cancer cells is higher, and lactic acid is produced from pyruvate, thus reducing the pH of the tumor.1,2 Soon after the discovery, Warburg proposed that the mitochondrial function was fundamentally altered in tumor cells, and he even proposed that this could be a cause of cancer.3 However, he subsequently stated that mitochondrial function was not damaged in most kinds of cancer cells.4
Moreover, Warburg suggested that the increase in glucose/glutamine consumption by cancer cells could led to them becoming “glucose addicted” or “glutamine addicted,” which may induce cell death. Metabolic intermediates that are produced by cells through glucose/glutamine catabolism act as building blocks and reducing agents for the production of macromolecules and the generation of ATP.5
In addition to ATP, rapidly proliferating cancer cells have a high requirement for proteins, membrane phospholipids, nucleic acids, and fatty acids. Glycolysis can provide intermediates and substrates for producing many biological macromolecules. It also produces small molecules or precursors, such as non-essential amino acids, acetyl-coenzyme A (CoA), and ribose for the biosynthesis of nucleotides needed for the rapid replication of DNA.3,4,6 The increased metabolism of tumors tends to create high quantities of reactive oxygen species (ROS). In tumor cells, these ROS can trigger senescence and apoptosis caused by oxidative stress.7
The “reverse Warburg effect” defines a situation when cancer-associated stromal cells could metabolically support adjacent cancer cells by carrying out glycolysis. The transfer of metabolites can induce stromal cell-cancer cell coupling, which could allow cancer cells to generate more ATP in order to support proliferation.8
The switch to glycolysis away from OXPHOS therefore tends to reduce the levels of ROS generated in the mitochondria. The glycolytic intermediates provide the required sources of carbon for rapid cell proliferation, but less ATP is formed compared to OXPHOS.9 The lactate produced by glycolysis reduces the pH levels in the extracellular matrix (ECM).10 The acidic microenvironment can enhance tumor invasion and metastasis, while also increasing the resistance to treatment with ionizing radiation.11,12 Therefore, the Warburg effect can be regarded as a pathway in which tumor cells harness cellular stress to progress.13,14 In the Warburg effect, it is proposed that the cellular metabolism is adapted to increase the incorporation of molecules into the biomass, such as nucleotides, amino acids, and lipids, which are needed for production of new cells. In support of this idea, numerous signaling pathways involved in cell proliferation can also regulate the metabolic pathways that incorporate nutrients into the biomass. Moreover, specific cancer-associated mutations could enable cancer cells to take up and metabolize nutrients in a way that is conducive to proliferation rather than ATP production.3
Additionally, it has been found that glycolysis in cancer cells is regulated by N6-methyladenosine modifications in several mRNAs. Pyruvate dehydrogenase kinase 4 is involved in N6-methyladenosine-regulated glycolysis. When the 5′ untranslated region (UTR) of pyruvate dehydrogenase kinase 4 is modified with N6-methyladenosine, translation elongation is improved by binding to the YTHDF1/eEF-2 complex. Moreover, mRNA stability can also be improved by interacting with IGF2BP3.15 Although most N6-methyladenosine modifications take place on mRNAs, it has been shown that N6-methyladenosine modifications can also occur with noncoding RNAs, especially miRNAs and circular RNAs (circRNAs).16
Many proteomic and genomic pathways are involved in the initiation and progression of cancer.17, 18, 19 Genome-wide surveys of cancer cells have revealed that there are frequent changes or variations in copy number located within non-coding regions of the DNA.17,20 In the human genome, it has been reported that 95% of the total sequence do not actually code for proteins. This non-coding DNA (ncRNA) is transcribed to produce tens of thousands of functional ncRNAs. These ncRNAs include small interfering RNAs (siRNAs), microRNAs (miRNAs), long ncRNAs (lncRNAs), and antisense RNAs (asRNAs).20, 21, 22, 23 Recently, new types of ncRNAs, namely circRNAs, have been reported.24, 25, 26 A large portion of the circRNAs are produced from the exons of coding genes, but many of them do not encode any protein.24, 25, 26
Glycolysis in Cancer
As reviewed by Zhang and Yang,27 cancer cells frequently tend to display the glycolytic phenotype. Bonnet et al.28 suggested that by switching the glycolytic phenotype toward OXPHOS, this could cause the cancer cells to die. In addition, the restoration of the mitochondrial-K+ channel function was enough to enhance apoptosis because the mitochondrial-K+ channel axis of cancer cells was suppressed. This study provided two important hypotheses: (1) glycolysis facilitates the growth of tumor notwithstanding a suppressed mitochondrial-K+ channel axis, and (2) reversal of the glycolytic phenotype back to OXPHOS may enhance cancer cell death.
The lack of a sufficient blood supply, which is characteristic of rapidly growing tumors, causes areas of hypoxia in which OXPHOS is inactive, and a metabolic switch from mitochondrial respiration to glycolysis is accompanied by mitochondrial dysfunction.29,30 In functionally active mitochondria, the occurrence of aerobic glycolysis under normoxic conditions is intriguing. It is noteworthy that mutations in mitochondrial DNA (mtDNA) can affect the enzymes of OXPHOS, in particular three enzymes, that is, isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH), and fumarate dehydrogenase (FDH) (reviewed by Wallace31). Similarly, mutations in the nuclear DNA (nDNA) can also affect the bioenergetics of cancer cells.31 These changes in enzyme activity are related to many cellular pathways, and the metabolic machinery of cancer cells can be reprogrammed; for example, SDH mutations lead to the accumulation of succinate, which inhibits prolyl hydroxylase dehydrogenase (PHD) and finally causes hypoxia-inducible factor (HIF)-1α stabilization. This mechanism links HIF-1α and angiogenesis to aerobic glycolysis and lactate production.
However, the laboratory depletion of mtDNA in cancer cells using ethidium bromide (rho zero cells) led to a decreased growth rate and colony formation and inhibited tumorigenicity.32 These rho zero cells showed an increased sensitivity to cytotoxic drugs, thus refuting the argument that drug resistance was based on functional mitochondria. Warburg’s original statement that dysfunctional mitochondria were a “common cause of cancer growth” must now be seen to be more complex. Transfer of mitochondria from adjoining normal healthy cells can compensate for the function of OXPHOS.33 However, although the exact relationship between alterations of mitochondrial function and the cancer phenotype remains controversial, the energy metabolism of cancer cells, in particular glycolysis and lactate production, is considered a valid target for therapeutic intervention.
Despite the fact that glycolysis produces a lower amount of ATP compared to mitochondrial OXPHOS, tumors can still derive some benefits from the switch.34 First, the rate of turnover in the glycolytic enzymes is much higher (up to 100 times faster) compared to OXPHOS.35 The low yield of ATP but the high rate of production36 means that there are selective benefits in the case of shared energy sources, proposing an evolutionary advantage to glycolysis.37 Rapidly dividing cells such as microorganisms with a doubling time in the range of a few minutes to several hours require ATP for proliferation, while cancer cells with a relatively longer doubling time (∼24 h) require ATP more for cell maintenance, although their proliferation is still rapid compared to normal cells. Hence, for the growth of cancer, the ATP made by glycolysis is sufficient, but the rapid rate provides a selective growth benefit.38,39 In addition to ATP, cancer cells need a more abundant supply of metabolic precursors and intermediates, which are essential for the biosynthesis of macromolecules and for increasing the tumor mass.40 The accumulation of glycolytic intermediates has been found to be important for the pentose phosphate pathway (PPP), leading to the generation of ribulose-5-phosphate and NADPH. Both molecules are necessary for the biosynthesis of nucleic acids and lipids. Finally, the increased NADPH levels allows the cancer cells to maintain adequate levels of reduced glutathione (GSH), which is an important non-enzymatic antioxidant. GSH plays a fundamental role in the protection of cancer cells from their elevated levels of ROS, and from damage caused by anti-neoplastic agents and chemotherapeutic drugs.41,42 In this regard, Zhou et al.37 showed that chemoresistant cell lines had elevated levels of aerobic glycolysis, demonstrating a biochemical connection between glycolysis and chemoresistance. Additionally, aerobic glycolysis has been implicated in the resistance of cancer cells to radiotherapy.43
KRAS can act as an oncogene by altering the metabolism of cancer cells in many ways, such as increasing the uptake of glucose, and triggering glycolysis even in the presence of oxygen (the Warburg effect).44 These effects of oncogenic KRAS are due to the upregulation of the expression of glucose transporters as well as glycolytic enzymes.45 Amendola et al.46 found that hexokinase 1 (HK1) can interact with KRAS4A, which can change the activity of the kinase, and suggested that HK1 could be considered as an effector of KRAS4A. The palmitoylation-depalmitoylation cycle that KRAS4A undergoes enables co-localization with HK1 at the outer membrane of mitochondria. Therefore, KRAS4A expression in different cancer cells may drive specific metabolic alterations that could be used as therapeutic targets.
The pyruvate kinase isoform (pyruvate kinase M2 [PKM2]), an alternatively spliced product of the PKM2 gene, can alter glucose metabolism in cancer cells and increase tumorigenesis. This function is due not to its known enzyme activity, but to a direct interaction with the HIF-1 subunit HIF-1α, leading to the transactivation of HIF-1 target genes through increasing the binding of HIF-1 and recruitment of p300 to the hypoxia response elements.47
The PPP plays an important role in the biosynthesis of macromolecules, and recent studies have associated it with antioxidant protection and resistance to radiation and chemotherapy.48 The transketolase (TKTL1) enzyme is particularly involved in cell survival under starvation and stress conditions.49, 50, 51 Other reports have indicated that TKTL1 can influence the chemosensitivity of cancer cells to cetuximab52 and imatinib.53 Therefore, it is clear that aerobic glycolysis together with the pentose shunt pathway can provide multiple advantages to cancer cells, including for tumor progression and development of resistance to treatment. Therefore, tumor glycolysis could present a promising target for therapeutic intervention.
miRNA Biogenesis
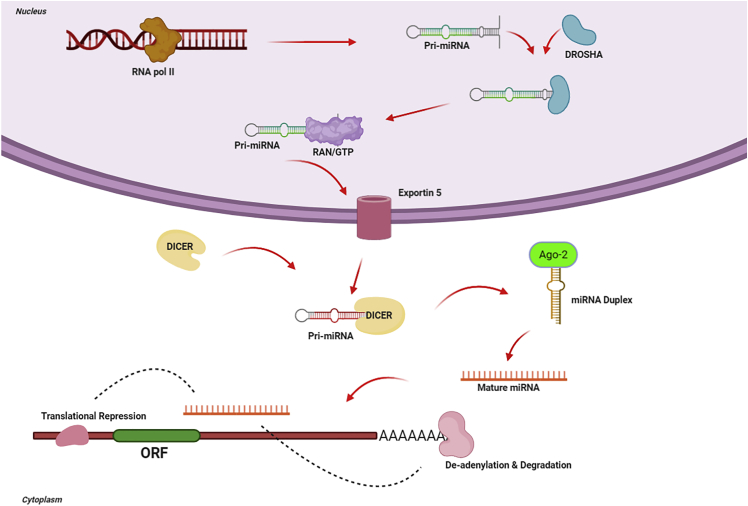
The biosynthesis mechanism of miRNA is evolutionarily conserved. The pathway involves successive endonuclease cleavage steps mediated by two ribonuclease (RNase) III enzymes called Dicer and Drosha (see Figure 1).54 The primary miRNA transcript (pri-miRNA) is produced in the nucleus by RNA polymerase II (RNA Pol II). The pri-miRNA is transformed by Drosha into a hairpin structure of ∼60–100 nt, called the precursor-miRNA (pre-miRNA) (see Figure 1).55, 56, 57, 58 The pre-miRNA is carried out of the nucleus by the interaction between Ran-GTP and exportin-5. The pre-miRNA then undergoes further processing catalyzed by Dicer (see Figure 1)59,60 to produce an ∼22-nt double-stranded RNA (dsRNA) construct composed of the passenger (miRNA∗) strand and the mature miRNA guide strand (see Figure 1). The mature miRNA strand binds to the 3′ UTR of its target genes. This binding triggers the assembly of a large protein complex called the RNA-induced silencing complex (RISC), which reduces gene expression, either by the degradation of the mRNA or by inhibiting translation.61
Figure 1.
Mechanism of miRNA Biogenesis
miRNAs are initially transcribed as a long 5′-capped and 3′-polyadenylated pri-miRNA. The Drosha complex transforms the pri-miRNA into a hairpin-shaped pre-miRNA. Exportin-5 exports the pre-miRNA into the cytoplasm, where it is further processed by Dicer. The double-stranded miRNA is dissociated, and the mature miRNA strand is combined in the RISC where it carries out gene silencing by increasing the degradation of target mRNAs or by translational inhibition.
The binding of the miRNA to the miRNA-recognition site in the 3′ UTR of the target mRNA can accommodate incomplete base pairing within the sequence. The incomplete nature of the binding between miRNA and mRNA means that any single miRNA could potentially target tens to hundreds of different mRNAs.62, 63, 64 The miRNA-RISC complex increases mRNA degradation and translational inhibition, causing silencing of the particular gene.65, 66, 67, 68, 69 These effects are heavily involved in cell differentiation, embryonic development, cell death, metabolism, and proliferation.70, 71, 72
Regulation of Glycolysis by miRNAs in Cancer
Many different oncogenes and tumor suppressor genes are regulated by miRNAs.73 The effects of miRNAs on gene regulation have been reported in the pathogenesis of cancers originating in multiple origins and composed of different cell types.74, 75, 76
Among other cellular functions, miRNA can regulate metabolic pathways, many of which are altered in cancer. The changes in tumor metabolism have been associated directly or indirectly with the downstream targets of many different miRNAs.77 Table 1 lists the glycolytic pathways involved in cancer that have been reported to be regulated by miRNAs, either directly or at the level of oncogenes.
Table 1.
Selected MicroRNAs Involved in the Regulation of Glycolysis Processes in Various Cancers
| Cancer | MicroRNAs | Expression in Cancer | Target | Model | Samples | References |
|---|---|---|---|---|---|---|
| Pancreatic cancer | miR-124 | downregulation | MCT1, PANC-1 | in vitro | 78 | |
| miR-7 | downregulation | LKB1-AMPK-mTOR | in vitro | 79 | ||
| miR-135 | upregulation | phosphofructokinase-1 | in vitro, in vivo | 80 | ||
| Glioma | miR-218 | upregulation | HK2, Bim1 | in vitro, human | 21 | 81 |
| miR-150 | upregulation | HIF-1α | in vitro | 82 | ||
| miR-451 | downregulation | GLUT1 | in vitro | 83 | ||
| miR-200b | downregulation | LDHA | in vitro | 84 | ||
| Hepatocellular carcinoma | miR-491-5p | downregulation | PKM2 | in vitro | 85 | |
| miR-142-3p | downregulation | LDHA | in vitro | 86 | ||
| miR-125b | downregulation | HK2 | in vitro, in vivo | 87 | ||
| miR-125a | downregulation | HK2 | in vitro, in vivo | 88 | ||
| miR-199a-5p | downregulation | HIF-1α | in vitro | 89 | ||
| miR-505 | downregulation | IGF-1R | in vitro, human | 60 | 90 | |
| Liver cancer | miR-34a | downregulation | LDHA | in vitro, human | 22 | 91 |
| miR-139-5p | downregulation | HK1, PFKFB3 | in vitro, in vivo | 92 | ||
| Gastric cancer | miR-21-5p | upregulation | PDHA1 | human | 46 | 93 |
| miR-34a | downregulation | LDHA | human | 73 | 94 | |
| miR-214 | upregulation | A2AR, PRDM16 | in vitro | 95 | ||
| miR-139-5p | downregulation | PRKAA1 | in vitro | 96 | ||
| miR-148b | downregulation | GLUT1 | in vitro | 97 | ||
| miR-181b | downregulation | HK2 | in vitro | 98 | ||
| Renal cell carcinoma | miR-409-3p | downregulation | PDK1 | in vitro | 99 | |
| miR-143 | downregulation | K-RAS | in vitro, in vivo, human | 100 | ||
| Osteosarcoma | miR-15b-5p | downregulation | in vitro | 101 | ||
| miR-323a-3p | downregulation | LDHA | in vitro | 102 | ||
| miR-33b | downregulation | LDHA | in vitro | 103 | ||
| miR-125b | downregulation | HK2 | in vitro | 104 | ||
| miR-186 | downregulation | PTTG1 and HIF-1 | in vitro | 105 | ||
| miR-150 | downregulation | Glut1 | in vitro | 106 | ||
| miR-185 | downregulation | hexokinase 2 | in vitro, human | 30 | 107 | |
| Testicular tumors | miR-199a-3p | downregulation | LDHA, Sp1 | in vitro | 108 | |
| Tongue squamous cell carcinoma | mitomiR-2392 | upregulation | HK2 and PKM2 | in vitro, in vivo | 109 | |
| Breast cancer | miR-27b | upregulation | PDHX | in vitro | 110 | |
| miR-31 | downregulation | DNMT3 | in vitro | 111 | ||
| miR-155 | upregulation | PIK3R1-PDK/AKT-FOXO3a-cMYC | in vitro | 112 | ||
| miR-340 | downregulation | MCU | in vitro | 113 | ||
| miR-30a-5p | downregulation | LDHA | in vitro | 114 | ||
| miR-342-3p | downregulation | MCT1 | in vitro, in vivo, human | 146 | 115 | |
| Lung cancer | miR-31-5p | upregulation | HIF-1α inhibitor | in vitro, in vivo | 116 | |
| miR-214 | upregulation | HK2, PKM2, PTEN/Akt/mTOR | in vitro | 117 | ||
| miR-449a | downregulation | LDHA | in vitro | 118 | ||
| miR-124 | downregulation | AKT-GLUT1/HK2 | in vitro | 119 | ||
| miR-155 | upregulation | HK2 | in vitro | 120 | ||
| miR-144 | downregulation | GLUT1 | in vitro | 121 | ||
| Ovarian cancer | miR-14 | upregulation | HK2, DNMT3A | in vitro | 122 | |
| miR-603 | downregulation | HK2 | human | 21 | 123 | |
| miR-21 | upregulation | HK2, PKM2, and LDHA | in vitro | 124 | ||
| miR-383 | downregulation | LDHA | in vitro | 125 | ||
| miR-203 | upregulation | PDHB | in vitro, in vivo | 126 | ||
| miR-450a | downregulation | TIMMDC1, MT-ND2, ACO2, ATP5B | in vitro, in vivo | 127 | ||
| miR-603 | upregulation | HK2 | in vitro, in vivo, human | 21 | 123 | |
| Gall bladder carcinoma | miRNA-139-5p | downregulation | PKM2 | in vitro, in vivo | 128 | |
| let-7a-5p | upregulation | PKM2, Stat3 | in vitro | 129 | ||
| miR-30d-5p | downregulation | LDHA | human | 130 | ||
| Laryngeal squamous cell carcinoma | miR-125b-5p | downregulation | HK2 | in vitro, human | 131 | |
| Prostate cancer | miR-29c | downregulation | SLC2A3 | in vitro, human | 57 | 132 |
| miR-137 | upregulation | NOX4 | in vitro | 133 | ||
| miR-101 | downregulation | NADPH, TIGAR | in vitro | 134 | ||
| miR-132 | downregulation | GLUT1 | in vitro | 135 | ||
| Bladder cancer | miR-200c | downregulation | LDHA | in vitro | 136 | |
| miR-361-5p | downregulation | Sp1/PKM2 | in vitro | 137 | ||
| miR-145 | downregulation | KLF4/PTBP1/PKMs | in vitro | 138 | ||
| miR-204-3p | downregulation | LDHA | in vitro, human | 60 | 139 | |
| Melanoma | miR-625-5p | downregulation | PKM2 | in vitro | 140 | |
| miR-137 | downregulation | GLO1 | in vitro | 141 | ||
| miR-33a-5p | downregulation | HIF-1α | in vitro, human | 20 | 142 | |
| miR-33b | downregulation | HIF-1α | in vitro | 143 | ||
| miR-370 | upregulation | PDHB | human | 41 | 144 | |
| Colorectal cancer | miR-23a∼27a∼24 cluster | upregulation | HIF-1α | in vitro | 145 | |
| miR-9-5p, miR-98-5p, and miR-199-5p | upregulation | HK2 | human | 40 | 146 | |
| miR-142-5p | upregulation | SDHB | in vitro | 147 | ||
| miR-328 | downregulation | SLC2A1/GLUT1 | human | 47 | 148 | |
| miR-1 | downregulation | HIF-1α, HK2, MCT4 | in vitro, in vivo | 149 | ||
| miR-98 | upregulation | HK2 | in vitro, human | 215 | 150 | |
| Esophageal squamous cell carcinoma | miR-143 | downregulation | HK2 | in vitro, human | 8 | 151 |
| Nasopharyngeal carcinoma | miR-34b-3, miR-449a | downregulation | LDHA | in vitro | 152 | |
| Cutaneous squamous cell carcinoma | miR-365 | upregulation | HK2, GLUT1, PDK1 | in vitro | 153 | |
| Oral squamous cell carcinoma | miR-143 | downregulation | HK2 | in vitro | 154 | |
| Thyroid cancer | miR-125a-5p | downregulation | CD147 | in vitro | 155 | |
| miR-143 | downregulation | HK2 | in vitro, in vivo | 156 |
The glucose transporter (GLUT) family of receptors (GLUT1–GLUT4) mediates transport of glucose through the plasma membrane of eukaryotic cells. GLUT3 as well as GLUT1 are mostly unmodified in many cancer types.157 However, GLUT4 activity is regulated by insulin or insulin-like growth factor (IGF) signaling, whereas the function of GLUT3 and GLUT1 is independent of insulin.158, 159, 160 Many miRNAs have been found to regulate the expression levels of GLUT3 and GLUT1, although none of these has been experimentally shown to target the mRNA levels of GLUT3 or GLUT1. However, the expression of GLUT4 was reduced by miRNA (miR)-133 and miR-223 in cardiomyocytes.161,162 Whether the expression levels of GLUT4 are involved in the metabolic phenotype in cancer remains to be investigated.
The targets of miRNAs include some glycolytic enzymes. The first step in the glycolytic pathway is the action of hexokinases (HKs) that are responsible for a transferring a phosphoryl group from ATP to the 6-hydroxyl of glucose for synthesizing glucose-6-phosphate (G6P). In this manner, glucose phosphorylation directs the glucose into the cells by maintaining the gradient essential for facilitative GLUTs. There is no evidence yet suggesting the role of miRNAs in regulating HKs.163 The miR-200 family (miR-200a, miR-200b, and miR-200c) regulates phosphoglucose isomerase that plays roles in the invasion and metastasis of cancer cells.164 Mimetics of miR-122 acting in human and rat liver cells regulate aldolase A (ALDOA).165 Phosphoglycerate kinase 1 (PGK1), enolase 1 (ENO1), ENO2, and triosephosphate isomerase 1 (TPI1) are all regulated by the miR-17-92 cluster.166 However, there are no target sites for miRNAs (miR-17-92) within these genes. The regulation of HIFs by the miR-17-92 cluster affects some glycolytic genes.166 One of the targets for miR-195 is TPI1, which was found to be downregulated in bladder cancer.167 However, a direct relationship has still to be confirmed. TPI1 and ALDOA were detected in a proteomic screen carried out in MEG-01 leukemic cells to be regulated through the miR-15a/16-1 cluster, and they were downregulated or eliminated in chronic lymphocytic leukemia (CLL) B cells.168 TPI1 was also predicted to be a direct target of the miR-15a/16-1 cluster. ALDH6A1 (aldehyde dehydrogenase 6 family member A1) was detected in a microarray screen in CLL to be regulated by the miR-15a/16-1 cluster.168 The expression levels of the target genes of phosphoglucomutase 1 (PGM1), ENO1, and PGK1 were detected using proteomic analysis in the invasive lung cancer cell line DLKP-A and found to be modulated by miR-29a.169 The mechanism involved miRNA downregulating ENO2d in the HepG2 hepatocarcinoma cell line, and this was confirmed because ENO2 levels were significantly upregulated by Drosha knockdown.170 Nevertheless, the details of ENO2-targeting miRNAs are so far unclear. Some cancer cells significantly overexpress PKM2, which is a direct target of miR-326.170 In solid tumors, the gene for PKM2 is also targeted by miR-133a and miR-133b.171
A large amount of pyruvate is converted into lactate, instead of acetyl-CoA embracing the mitochondrial Krebs cycle, within the glycolysis in cancer cells. The produced cytosol lactate leaves the cells with the help of monocarboxylate transporters (MCTs). In a study on human pancreatic cancer, the molecular mechanisms of miR-124 to regulate glycolysis were evaluated by Wu et al.,78 who found higher expression of MCT1 in PDAC tissue when comparing with normal tissue. The metabolism of lactate is influenced by the MCT1 inhibition, and the results are increased intracellular pH and decreased PANC-1 cell proliferation. The target gene of miR-124 is MCT1. The results of an in vitro study showed that the glycolytic activity of PANC-1 cells is impeded by miR-124 through targeting MCT1, which causes a decrease in the tumor phenotype by raising the intracellular pH via LDH-A and HIF-1α. The tumor growth was significantly blocked by miR-124 overexpression and MCT1 silencing within an in vivo study. In the lactate metabolic pathway, the PANC-1 progression is inhibited by miR-124 through MCT1 targeting. The results from the present study reported new findings regarding further functional studies of miR-124 that predispose potential therapeutic strategies to PDAC.78
According to Pullen et al.,172 DNA methylation at the Mct1 promoter is probably associated with cell type-specific transcriptional repression (three miRNAs, i.e., miR-29a, miR-29b, and miR-124) selectively targeting MCT1 3′ UTRs in humans and mice. The effects of the miRNAs of mutated miR-29 or miR-124 binding sites are blocked, meaning a direct function of these miRNAs on the MCT1 message. Although it is expressed in the mouse β cell line MIN6, no expression of miR-124 was evident in mature mouse islets, whereas the three miR-29 isoforms are expressed and enriched in mouse islets in large amounts. According to our results, Mct1 mRNA levels are elevated due to miR-29a inhibition in primary mouse islets, so that the isoforms of miR-29 cause β cell-specific silencing of the MCT1 transporter and influence the secretion of insulin.172
Aberrant miR-1 expression in aerobic glycolysis (the Warburg effect) was shown by Xu et al.149 in cancer cells. The aerobic glycolysis and proliferation of tumor cells were blocked by miR-1 because of Smad3 inactivation and HIF-1α targeting. Therefore, the expression of HK2 and MCT4 was decreased, indicating a new pathway that mediates aerobic glycolysis in cancer cells. The tumor glycolysis was reduced significantly following the overexpression miR-1 mimics, including the generation of lactate and the absorption of glucose, as well as cell proliferation, and ectopic Smad3 expression reversed these effects. HIF-1α is regulated by and interacts with endogenous Smad3; this activates Smad3, which is significantly suppressed by miR-1 addition. Based on their results, Smad3 has a pivotal role in the effects of miR-1 in colorectal cancer cells. This presents a mechanism for the first time so that the Warburg effect is provided easily by the miR-1/Smad3/HIF-1α axis in enhancing cancer progression, in vitro and in vivo. miR-1 is associated with tumor suppression, and thus it can act as a suppressor in molecular therapy of patients suffering from advanced colorectal cancer.149
The tumor cells frequently overexpress lactate dehydrogenase A (LDHA). Wang et al. observed higher LDHA levels in patients with colorectal cancer when comparing samples with adjacent normal tissue.173 The production of lactate and ATP and the adsorption of glucose were reduced following LDHA knockdown. A much slower growth rate was seen for the colorectal cancer cells with LDHA knockdown compared to the control cells. Their results showed that LDHA is targeted by miR-34a, miR-34c, miR-369-3p, miR-374a, and miR-4524a/b, thereby regulating glycolysis in cancer cells. In colorectal cancer tissues, an inverse correlation has been found between these miRNAs and LDHA expression. They detected a genetic loci related to the high progression of colorectal cancer, that is, rs18407893 at 11p15.4 (in the 3′ UTR of LDHA), which maps to the seed sequence identified by miR-374a. The LDHA level is lower in cancer cells overexpressing miR-374a than in miR-374a-mutant (MUT) (rs18407893 at 11p15.4). These new results can present promising therapeutic strategies to the Warburg effect and therapeutic targets of cancer energy metabolism.173
Yuan et al.136 in their studies evaluated the expression and function of LDHA in bladder cancer and reported the upregulation of LDHA in bladder cancer cells, enhancing proliferation, invasion, and glycolysis. According to their results, LDHA is targeted directly by miR-200c in bladder cancer cells. As well, LDHA-induced glycolysis, cell proliferation, and invasion were inhibited by ectopic expression of miR-200c. In fact, one of the possible therapeutic approaches can be LDHA targeting via miR-200c in bladder cancer. Mammalian cells extracted an array of cellular processes for maintaining their survival by improving glycolysis and considering low oxygen hypoxia or tension. Under these conditions, the use of genes was operated frequently by HIF-1α, which is fundamental for the helix-loop-helix transcription element of the PAS family.174 The factor of transcription act as a function of a heterodimer of HIF-1β and HIF-1α. Note that HIF-1α protein can be degraded due to existing oxygen (normoxia) by the ubiquitin-proteasomal procedure, whereas HIF-1β and HIF-1α are basically transcribed and translated.
Kelly et al.175 specified enzyme glycerol-3-phosphate dehydrogenase 1-like (GPD1L) as a new regulator of a direct target of miR-210 and HIF-1α stability. In response to the expression of miR-210, the levels of HIF-1α were stabilized by reduced levels of GPD1L, leading to enhancing HIF-1α target genes. The enhancement of HIF-1α protein levels and hypoxia-induced miR-210 suppresses GPD1L by suppression of PHD activity. Different influences of HIF-1α onto the transcription of metabolic genes were determined to be straight; nevertheless, HIF-1α additionally adjusts a number of miRNAs, including miR-210, that can regulate an excess of genes in various physiological functions. In multiple cancers, miR-210 can be overexpressed dramatically.176, 177, 178, 179 In addition, it suppresses ISCU1/2 and hence reduces the activity of prototypical iron-sulfur proteins controlling mitochondrial metabolism, consisting of complex I as well as aconitase, by taking into account hypoxic conditions.180 However, miR-210 suppresses mitochondrial respiration and can be a reason for more aerobic glycolysis in cancer, indirectly. By apparatuses independent of aerobic glycolysis, many miRNAs may overall affect the homeostasis of glucose. miR-375, known as one such miRNA, can adjust glucose homeostasis in the body with the modulation of insulin secretion. In β cells, the mentioned overexpression prevents insulin exocytosis with related mechanisms, which are independent of variations in the intracellular Ca2+ signaling and transmembrane Ca2+ fluxes.181 Many previous investigations have demonstrated that glucose homeostasis regulation using miR-375 can be mediated by taking into consideration modulation of the phosphatidylinositol 3-kinase (PI3K) signaling procedure in pancreatic β cells.182 In pancreatic adenocarcinoma cells, expression levels of miR-375 were dramatically downregulated.183,184 Hence, the accurate influence of the downregulation on the metabolism of glucose as well as the potential objectives have not been determined in the case of the tumor cells.
Biogenesis of lncRNAs
lncRNAs play important roles in several human diseases and also in normal development.185,186 An understanding of the biogenesis of lncRNAs has been very helpful for differentiating them from other types of ncRNAs, and also for deciphering their functional importance. The biogenesis of lncRNAs is cell stage- and cell type-dependent.187,188 Many lncRNAs have been transcribed from DNA sequences, such as promoters, intergenic regions, and enhancer regions in eukaryotic genomes.189 The first step involved in lncRNA biogenesis is cleavage by RNase P for generating mature ends. Most lncRNAs undergo 5′ capping and 3′ polyadenylation in the same way as mRNAs, but others have small nucleolar RNA (snoRNA) caps at the ends.190,191 In recent years, sub-nuclear structures called “paraspeckles” about 200–1,000 nm in size have been identified between the chromatin structures. Paraspeckles consist of several proteins wrapped around a lncRNA core.192 RNA interference (RNAi) evaluations of 40 paraspeckle proteins (PSPs) led to the identification of 4 PSPs that were essential for the formation of paraspeckles.193,194 The mechanisms for formation and regulation of many lncRNAs are not entirely understood. New techniques, such as chromatin isolation by RNA purification sequencing (ChIRP-seq), ribosome profiling, cross-linking immunoprecipitation (CLIP), phylogenetic lineage tracing, and RNA structure mapping using genetic screens and CRISPR, will provide much more information on the structure and function of lncRNAs.195, 196, 197
lncRNAs carry out regulatory functions because of their modular structure and RNA-DNA or RNA-RNA base pairing ability.20 lncRNAs regulate gene function in several ways, including (1) recruitment of the chromatin remodeling complex to alter the chromatin organizational pattern; (2) acting as “sponges” by base pairing with miRNAs to reduce their effects; (3) providing docking sites for proteins that are located within the same pathway; (4) guiding certain transcription factors (TFs) to bind to the promoters; and (5) sequestering transcription factors and keeping them away from the promoters.196
Regulation of Glycolysis by lncRNAs
The lncRNA NBR2 regulates 5′-AMP-activated protein kinase (AMPK) activity under energy stress induced by glucose starvation. Liu and Gan198 demonstrated that the knockout of NBR2 did not influence the activity of phenformin-induced AMPK, but it did decrease the expression of GLUT1, thereby decreasing glucose utilization. The lncRNA called CRNDE (colorectal neoplasia differentially expressed) promotes colorectal cancer progression and drug resistance. The intron 4 of the gene contains a highly conserved sequence (gVC-In4). Ellis et al.199 showed that in HT29 colorectal cancer cells, treatment with insulin or IGF inhibited transcription of CRNDE and gVC-In4. Knockout of gVC-In4 in vitro decreased the expression of genes involved in aerobic glycolysis and pyruvate conversion to acetyl-CoA, and it reduced the production of lactic acid. In addition, they found that the GLUT4 expression was decreased, showing that CRNDE positively regulates the GLUT4 level199 (Figure 2).
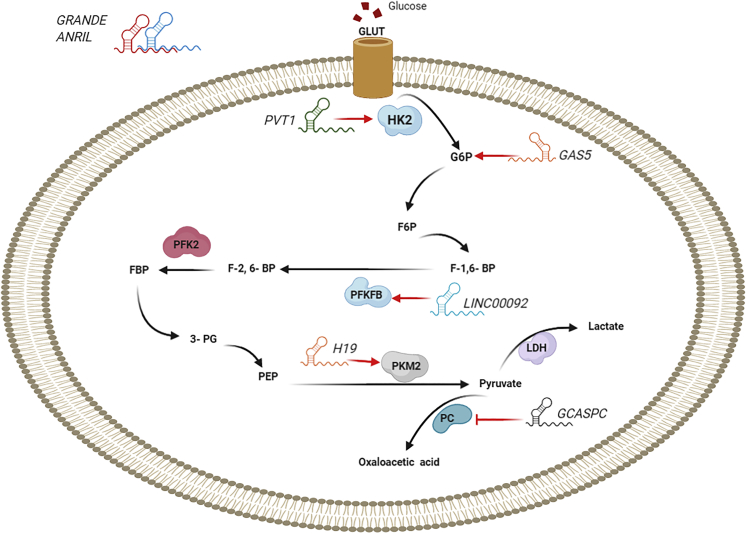
Figure 2.
lncRNAs Regulate Some Molecules Involved in Glucose Metabolism in Cancer
lncRNAs regulate glucose uptake and glycolytic flux by modulating GLUTs and glycolytic enzymes. This figure was adapted from Fan et al.200
Yiya is an lncRNA that was found to be upregulated in several cancers, and it is located on 1q41.201 The expression of Yiya is associated with cell proliferation and cell cycle regulation.201 Cyclin-dependent kinase 6 (CDK6) is considered a major regulator of the cell cycle and modulates several cell functions, although this is not completely understood.202 When CDK6 was deleted in mice, they found disorders related to hematopoiesis.203 According to some studies, CDK6 controls the PPP.204 Small molecule inhibitors of CDK6 impaired PPP activity and lactate synthesis.204 In one study, Xing et al.205 assessed the effects of Yiya expression on glycolysis in breast cancer. Yiya was associated with cytosolic CDK6 and modulated CDK6-dependent phosphorylation of the fructose bisphosphatase PFK2 (PFKFB3) enzyme, which mediates the conversion of glucose 6-phosphate to fructose-2,6-bisphosphate/fructose-1,6-bisphosphate. CDK6 silencing and CRISPR-Cas9-mediated suppression of Yiya inhibited glycolysis and tumor development. In 40% of breast cancer cases, the expression of Yiya was related to CKD6 and a poor prognosis.205
NORAD (ncRNA activated by DNA damage) is also known as LOC647979 or LINC00657.206 NORAD is known to be an oncogene and is implicated in breast cancer207 and pancreatic cancer.208 It is known that some lncRNAs can interact with miRNAs and act as RNA sponges that reduce their effect on the target mRNAs.209,210 In pancreatic cancer, NORAD functions as a sponge for miR125a3p in order to modulate the Ras homolog family member A.208 NORAD interacts with miR-202 and can promote the epithelial-mesenchymal transition, leading to metastasis and a poor prognosis in colorectal cancer.211 NORAD is also highly expressed in non-small-cell lung cancer (NSCLC) and could be related to glycolysis and increased cell viability in lung cancer as reported by Gao et al.212 These workers used quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) to investigate the expression of NORAD, miR136-5p, and markers of glycolysis and cell proliferation. The direct interaction of miR136-5p with NORAD was investigated using RNA immunoprecipitation and a luciferase reporter assay. NORAD was upregulated in NSCLC cell lines, and glycolysis and proliferation were also increased. They proposed that NORAD acted as a competing endogenous RNA for miR136-5p. Gain- and loss-of-function assays showed that miR136-5p reversed the effects of NORAD in lung cancer.212
MALAT1 (metastasis associated lung adenocarcinoma transcript 1) plays an oncogenic role in some cancers. For instance, Zhang et al.213 showed that MALAT1 participates in renal cell carcinoma by modulation of the miR-203/BIRC5 axis. Sun et al.214 also showed that MALAT1 was upregulated in ovarian cancer, and it played a role in apoptosis and proliferation by targeting miR503. Si et al.215 reported that MALAT1 could suppress apoptosis and promote autophagy by sponging miR-101 in colorectal cancer. MALAT1 is highly expressed in multiple myeloma (MM),216 and Liu et al.217 reported that downregulation of MALAT1 by RNAi activated apoptosis and suppressed proliferation.
SRY-box 13 (SOX13) is a member of the Sry-related high-mobility group box (Sox) transcription factor family, and it has been implicated in some cancers, such as glioma218 and gastric carcinoma.219 Xu et al.220 showed SOX13 was upregulated in MM.
Liu et al.221 investigated the effects of MALAT1 on glycolysis in MM by measuring the expression levels of MALAT1, SOX13, and miR-1271-5p using quantitative real-time PCR. Flow cytometry, a transwell assay, and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were used to examine apoptosis, invasion, and cell viability, respectively. The lactate production, ATP/ADP ratio, related enzyme activities, and glucose consumption were examined to quantify glycolysis, plus western blotting for the related proteins. A dual-luciferase reporter assay was used for understanding target interactions. An MM xenograft tumor model was carried out in mice. MALAT1 was overexpressed and miR-1271-5p was downregulated in MM cells. Deletion of MALAT1 inhibited invasion, glycolysis, and reduced cell viability. MALAT1 targeted miR-1271-5p, and SOX13 was shown to be a target of miR-1271-5p. MALAT1 regulated SOX13 by targeting miR-1271-5p. By downregulation of MALAT1, MM development and glycolysis were suppressed via the miR-1271-5p/SOX13 axis.221
Ftx (five prime to the X-inactivation center [XIST]) is located near XIST on the X chromosome.222 Ftx encodes a transcript of 2,300 nt with nine introns, the second and seventh of which encode two clusters of miRNAs, that is, miR-545/miR-374a and miR-421/miR-374b. The other introns encode Ftx itself, so there are no overlaps between miRNAs and Ftx. lncRNA Ftx/miR-545 participates in tumorigenesis in hepatocellular carcinoma (HCC) by affecting the PI3K/RAC-α serine/threonine-protein kinase axis via targeting the DExD/H-box helicase 58.223 The relationship between glycolysis and Ftx is not yet clear.
Peroxisome proliferator-activated receptor γ (PPARγ) combines with PPAR elements to modulate the transcription of target genes, and it heterodimerizes with the retinoid X receptor. PPARγ has a role in steatosis-associated hepatic tumorigenesis,224 and it is involved in cellular sensitivity to insulin and insulin resistance.225 It has a role in the regulation of some enzymes in carbohydrate metabolism. For instance, it promotes GLUT4 expression226 and suppresses PDK1 expression.227 PPARγ was reported to reduce the production of leptin and tumor necrosis factor (TNF)-α and improve cellular sensitivity to insulin and facilitate glucose utilization.228
Li et al.229 studied the role of Ftx in glycolysis in HCC. High expression levels of Ftx enhanced invasion, migration, and proliferation of HCC, while low expression of Ftx had the opposite effects. Ftx played a role in glycolysis, the expression of enzymes related to carbohydrate metabolism, glucose transporter expression, glucose consumption, and lactate production. PPARγ expression was related to the expression of Ftx in HCC. The inhibition of PPARγ using GW9662 in Huh7 HCC cells that overexpressed Ftx partially abrogated the increases in glucose uptake, lactate production, and relative glycolytic enzyme expression induced by Ftx. Conversely, in Bel-7402 HCC cells, activation of PPARγ rescued Ftx-mediated suppression of lactate production, glycolytic enzyme expression, and glucose uptake induced by Ftx.229
Proteins, lipids, and nucleotides derived from glycolytic metabolism are used for cell division and proliferation.38 LDHA catalyzes the last step of the glycolysis pathway. Abnormal expression levels of LDHA have been found in some cancers such as pancreatic cancer,230 HCC,231 and breast cancer.232 Suppression of LDHA decreases malignant transformation and delays tumor progression.4 Studies have investigated the mechanisms of the suppression of tumor growth after LDHA inhibition.233,234 Reduction of LDHA activity either by siRNA or by a small molecule inhibitor altered energy metabolism and caused oxidative stress and cell death in lymphoma both in vitro and in vivo.233 LDHA deletion inhibited tumorigenicity via stimulation of oxidative stress-mediated mitochondrial apoptosis in breast cancer.234 In one study, Chen et al.235 assessed the role of the lncRNA CRYBG3 on glycolysis in lung cancer. Overexpression of CRYBG3 stimulated glycolysis and enhanced glucose uptake and lactate production, while its deletion had the opposite effect.235
GLUT1 (also known as SLC2A1) is a transporter for glucose uptake, and it is expressed by cancer cells for rapid proliferation and energy production by glycolysis. SLC2A1 is highly expressed in lung cancer,236 colon cancer,237 and gastric cancer.97 Chen et al.238 showed that CREB (cyclic AMP [cAMP]-responsive element binding protein) affected glucose transport via modulating the expression of SLC2A1 and modulated the metabolism and progression of glioma. Shi et al.239 investigated the effect of LINC00174 on glycolysis in glioma. In situ hybridization and quantitative RT-PCR were used to examine the expression of LINC00174 in glioma cell lines and samples. ELISA assays, a transwell invasion assay, scratch wound healing, TUNEL (terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling), and a Cell Counting Kit-8 (CCK-8) assay were used to examine the effects on glioma cells. Western blotting, RNA pull-down, dual-luciferase reporter, and RNA immunoprecipitation assays were performed to understand the mechanism. A nude mouse xenograft model was used to understand the role of LINC00174 in glioma development. LINC00174 was overexpressed in glioma tissues and cell lines. LINC00174 deletion suppressed glycolysis, proliferation, migration, and invasion and also affected glioma tumorigenesis. Suppression of miR-152-3p or overexpression of SLC2A1 could reverse the anti-tumor effects of LINC00174 deletion on glioma. Downregulation of LINC00174 reduced the tumor volume and delayed tumor development.239
Phosphatase and tensin homolog (PTEN) is known to function as a tumor inhibitor in some cancers, such as endometrial carcinoma, kidney cancer, and melanoma.240, 241, 242 In esophageal squamous cell carcinoma (ESCC), epigenetic silencing of PTEN by promoter hypermethylation was shown to be a mechanism of PTEN reduction.243 Li et al.244 investigated the effects of LINC00184 on glycolysis in esophageal cancer. Quantitative RT-PCR was used to examine LINC00184, and it was found to be upregulated in ESCC, and at the same time PTEN was downregulated. Gain and loss of function was used to evaluate cellular processes in vivo and in vitro. Deletion of LINC00184 was found to suppress colony formation, proliferation, migration, and invasion, while glycolysis was reduced and mitochondrial OXPHOS was restored. LINC00184 promoted promoter methylation of PTEN by recruiting DNMT1. Suppression of PTEN promoter methylation increased mitochondrial OXPHOS while it inhibited glycolysis. LINC00184 was proposed to regulate glycolysis and mitochondrial OXPHOS in ESCC via Akt phosphorylation, and when Akt was inhibited by LY294002 the effects of LINC00184 on mitochondrial OXPHOS and glycolysis were reversed.244
The dysregulation of the oncogene MYC has been found to be a common event in tumorigenesis. MYC encodes a transcription factor, c-Myc, that stimulates proliferation and cell growth. Kim et al.245 showed that HIF-1 could act cooperatively with dysregulated c-Myc to promote glycolysis in the P493-6 Burkitt’s lymphoma model with an inducible MYC. The expression of the glycolysis enzyme hexokinase 2 was induced, and also pyruvate dehydrogenase kinase 1 that can inactivate pyruvate dehydrogenase and decrease mitochondrial respiration. The lncRNA PCGEM1 (prostate cancer gene expression marker 1) can affect many metabolic processes at the transcriptional level, such as the PPP, glucose metabolism, fatty acid biosynthesis, nucleic acid, and the tricarboxylic acid cycle. PCGEM1 was shown to directly interact with c-Myc, promote c-Myc chromatin recruitment, and bind to the promoters of target genes.246 The effects of c-Myc on glycolytic gene expression can increase glucose metabolism under normal oxygen conditions. The c-Myc protein level can be decreased by the lncRNA MIF (c-Myc inhibitory factor), hence suppressing glycolysis. It was proposed that lncRNA-MIF works as an endogenous competitive RNA to sequester miR-586, thereby decreasing the inhibitory effect of miR-586 against Fbxw7 (F-box/WD repeat-containing protein 7). Fbxw7 is an E3 ubiquitin ligase that can reduce the stability of c-Myc protein. Therefore, lncRNA-MIF can increase the expression of Fbxw7 and reduce the level of c-Myc protein. As shown in Figure 3, there is a feedback loop between lncRNA-MIF and c-Myc that can regulate glucose metabolism and glycolysis.247 Alternatively, the metabolic signaling pathways shown in Figure 3 do not completely reflect the current understanding of these complex networks. For example, the insulin receptor (IR) activates the PI3K/Akt signaling pathway, which is responsible for most of the metabolic activity of insulin. The IR has two splice isoforms, both of which can phosphorylate at least six known IR substrate (IRS) proteins. These IRSs are capable of interacting with eight known forms of the PI3K regulatory subunit. PI3K regulatory subunits, in turn, can associate with three forms of the PI3K catalytic subunit, and the products of PI3K enzyme activity can then activate three isoforms of Akt. The combinatorial possibilities of the IR/IRS/PI3K/Akt part of the insulin-signaling pathway alone exceed 1,000 combinations. When differential compartmentalization, stoichiometry, and the kinetics of the various downstream signaling components are taken into account, this number increases dramatically248 (Table 2).
Figure 3.
Role of PI3K/AKT/mTOR, LKB1-AMPK, and lncRNA-mediated HIF in Glucose Metabolism in Tumor Cells
The stability and synthesis of HIF-1α protein can be affected by lncRNAs, thus modulating HIF-1-mediated metabolic reprogramming. In cancer cells, the translation of HIF-1a mRNA is dependent on the activity of the mammalian target of rapamycin (mTOR) governed by the activity of upstream tumor suppressor proteins and oncoproteins. HIF-1α plays a key role in blocking mitochondrial activity and stimulating glycolic enzymes. lncRNAs can also regulate the Akt and AMPK pathways. Akt may increase oxidative phosphorylation by enhancing metabolic coupling between glycolysis and oxidative phosphorylation, through facilitating the association of mitochondrial hexokinase with VDAC in the mitochondria. Akt enhances glycolytic flux via several mechanisms. First, it increases glucose uptake and flux. Second, hyperactivated Akt activates mTORC1, which promotes HIF-1α accumulation under normoxic conditions and increases levels of GLUT1, HKII, and lactate dehydrogenase (LDH). Finally, Akt-increased cellular ATP levels help to maintain low AMPK activity, which is required for full activation of mTORC1. This figure was adapted from Fan et al.200
Table 2.
Selected lncRNAs Involved in the Regulation of Glycolysis in Various Cancers
| Cancer | lncRNAs | Expression in Cancer | Target | Model | Samples | References |
|---|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma | PVT1 | upregulation | HIF-1α | in vitro, in vivo, human | 249 | |
| Hepatocellular carcinoma | SLC2A1-AS1 | downregulation | STAT3/FOXM1/GLUT1 pathway | in vitro, in vivo, human | 33 | 250 |
| LINC01554 | downregulation | PKM2, Akt/mTOR signaling pathway | in vitro, in vivo, human | 167 | 251 | |
| HOTAIR | upregulation | HIF-1α | in vitro, human | 38 | 252 | |
| Colorectal cancer | HNF1A-AS1 | upregulation | MYO6 | in vitro, human | 40 | 253 |
| LINRIS | upregulation | IGF2BP2 | in vitro, in vivo, human | 254 | ||
| MAFG-AS1 | upregulation | NDUFA4 | in vitro, in vivo, human | 52 | 255 | |
| GLCC1 | upregulation | LDHA | in vitro, in vivo, human | 40 | 256 | |
| Non-small-cell lung cancer | BCYRN1 | upregulation | PKM2 | in vitro, human | 20 | 257 |
| LINK-A | upregulation | HKII | in vitro, in vivo, human | 113 | 258 | |
| LINC00243 | upregulation | PDK4 | in vitro, human | 70 | 259 | |
| HOTTIP | upregulation | HMGB3 | in vitro | 260 | ||
| Multiple myeloma | MALAT1 | upregulation | SOX13 | in vitro, in vivo, human | 30 | 261 |
| Endometrial carcinoma | SNHG16 | upregulation | HK2 | in vitro, in vivo, human | 262 | |
| Glioma | LINC00174 | upregulation | SLC2A1 | in vitro, in vivo, human | 45 | 239 |
| LINC00689 | upregulation | PKM2 | in vitro, in vivo, human | 56 | 263 | |
| Esophageal cancer | LINC00184 | upregulation | PTEN | in vitro, human | 84 | 244 |
| Cervical cancer | UCA1 | upregulation | HK2 | in vitro, human | 264 | |
| Breast cancer | YIYA | upregulation | CDK6 | in vitro, human | 205 | |
| MEG3 | downregulation | PI3K/Akt pathway | in vitro, in vivo, human | 20 | 265 | |
| Ovarian cancer, CAFs | LINC00092 | upregulation | CXCL14 | in vitro, in vivo, human | 58 | 266 |
| Nasopharyngeal carcinoma | ANRIL | upregulation | mTOR signaling pathway | in vitro, human | 88 | 267 |
| Lung cancer | IGFBP4-1 | upregulation | HK2, PDK1, LDHA | in vitro, in vivo, human | 159 | 268 |
| Papillary thyroid carcinoma | ASMTL-AS1 | downregulation | FOXO1 | in vitro, in vivo, human | 95 | 269 |
| Ovarian cancer | LINC00504 | upregulation | PKM2, HK2, PDK1 | in vitro, human | 45 | 270 |
| Oral squamous cell carcinoma | p23154 | upregulation | GLUT1 | in vitro, in vivo, human | 49 | 271 |
| Pediatric acute myeloid leukemia | UCA1 | upregulation | hexokinase 2 pathway | in vitro, human | 31 | 272 |
| Osteosarcoma | PVT1 | upregulation | HK2 | in vitro, human | 46 | 273 |
CAF, cancer-associated fibroblast.
circRNA Biogenesis
In eukaryotic cells, the processing of the exons of pre-mRNAs by alternative splicing can lead to linear mRNA sequences. circRNAs are produced by a different kind or aberrant RNA splicing in contrast to linear RNAs.274 circRNAs are more resistant to degradation by RNase enzymes, and they have been found to be more stable compared to linear RNAs due to the presence of a covalent closed-loop structure.275 Vo et al.26 characterized circRNAs in more than 2,000 different cancer samples. They found a general reduction in the global abundance of circRNAs compared to the adjoining normal tissues. Many subtypes of circRNA have recently been characterized using next-generation technologies. There are now four recognized subtypes of circRNAs: (1) exonic circRNAs (ecircRNAs) derived from several or many single exons; (2) circular intronic RNAs (ciRNAs) derived only from introns; (3) exonic-intronic circRNAs (EIciRNAs), including both exons and introns; and (4) tRNA intronic circRNAs (tricRNAs) created by the splicing of pre-tRNA introns. Recently, most of the sequenced circRNAs have been determined to be ecircRNAs. The biogenesis mechanisms of circRNAs depend on the particular sub-type. ecircRNAs are produced by back splicing and lariat-driven circularization. In the lariat, the existing introns are removed, and the exons are linked together by a 5′–3′ phosphodiester bond. The downstream donor and the upstream splice acceptor sites are then closed in a circle to create a lariat, which includes the exons.276 In the downstream and upstream introns, interactions occur with RNA-binding proteins (RBPs) to form a “bridge,” followed by back splicing for the formation of the ecircRNAs.277,278 In base pairing-driven circularization of ecircRNAs, the downstream splicing donor site can be linked to the upstream splicing receptor site based on ALU complementary sequences. The introns can either be removed or maintained to produce both EIciRNAs or ecircRNAs.275 The biosynthesis of ciRNAs is mainly based on an 11-nt C-rich sequence and a 7-nt GU-rich element for directing the sites of exonucleolytic cleavage and debranching degradation.279 EIciRNAs and ciRNAs are located within the nucleus where they play role in controlling parental gene transcription, in contrast to ecircRNAs, which are localized within the cytoplasm. tricRNA is a particular intronic circRNA found in Archaea and Drosophila species.280, 281, 282 The formation of tricRNA requires the activity of tRNA splicing enzymes to divide the pre-tRNA into two different sections. One of these sections produces tRNAs, while tricRNAs are produced from the other section by formation of a 3′–5′ phosphodiester bond.281 It is accepted that the binding of RNA proteins and the presence of particular repetitive sequences surrounding the introns and the circularized exons govern the formation of circRNAs.
The mechanisms of how circRNAs regulate gene function have been investigated in several studies, leading to three main possibilities.277,283 (1) circRNAs often have miRNA binding sites that allow them to bind to miRNAs, acting as a “miRNA sponge” in order to inhibit miRNA binding to the target mRNAs. (2) circRNAs can also interact with RBPs, which leads to the depletion of RBPs and thus reduces their interaction with RNA targets. (3) It has been suggested that the circRNAs that are generated by circularization of exons might compete with the splicing of pre-mRNA into linear RNA, because they act on the same splice sites. Therefore, the mRNAs might lack the exons that were present in the circRNA, thus altering the composition of the mRNAs.284
Regulation of Glycolysis by circRNAs
As mentioned above, circRNAs have been found to be widespread and involved in many developmental steps as well as normal physiological conditions using high-throughput deep RNA sequencing as well as bioinformatics technology.275,285 Naturally occurring circRNAs play an important role in the interaction network of RNA. circRNAs are relatively stable, diverse, extremely abundant, and highly conserved.275 circRNAs are involved in processes such as controlling the expression of parental genes, acting as endogenous RNAs to sponge miRNAs, regulating RNA–protein interactions and functioning as scaffolds for the assembly of protein complexes, as well as modulating alternative splicing.286,287
It has also been discovered that circRNAs are involved in the pathogenesis of many human diseases, including cardiovascular disorders,288 nervous system disorders,289 osteoarthritis,290 diabetes,291 Alzheimer’s disease, silicosis,292 and cancer.293,294 circRNAs have important roles in the growth of cancer, stemness of the malignant cells, metastasis, and treatment resistance.295,296
There is one circRNA, named circMYC (hsa_circ_0085533), that has been found in HepG2 cancer cells, HeLa S3 cells, normal human epidermal keratinocytes, and human lung fibroblasts. It is located on chromosome 8 with a 555-base genomic length.285 circMYC is highly expressed in melanoma and is related to tumorigenesis. LDHA changes pyruvate into lactate and is one of the hallmarks of cancers.297 LDHA is considered a valid therapeutic target in many cancers, such as pancreatic cancer, lymphoma, and lung, liver, and breast cancers. Increased LDHA activity and lactate production are associated with poor prognosis and resistance to chemotherapy and radiotherapy.231,234,298,299 LDHA was found to be overexpressed in nevic melanocytes and primary and metastatic melanomas.300 In one study, Jin et al.301 assessed the effect of circMYC on glycolysis in melanoma. circMYC increased the proliferation in Mel-CV cells and human melanoma cells. Overexpression of circMYC led to inhibition of LDHA activity and lower glycolysis. circMYC targeted miR-1236 and bound to it as a molecular sponge.301
The PI3K/protein kinase B (PKB) signaling pathway is critical for cell apoptosis and survival.302 It has been proposed that some cancers are related to the incorrect activation of that pathway.303 The PI3K/PKB signaling pathway was implicated in the initiation of endometrial cancer304 and cisplatin (DDP) resistance in lung cancer.305 Understanding of the PI3K/PKB pathway could help to improve therapies for lung cancer.
Wu et al.306 studied the effects of circACACA on glycolysis in NSCLC. qPCR was used to measure the expression of miR-1183 and the circACACA Transwell assay, and CCK-8 assays were used to measure migration and proliferation. The Seahorse XFe96 analyzer was used to study mitochondrial metabolism, and western blotting was used to quantify the expression of GLUT1, matrix metalloproteinase 9, c-Myc protein, PTEN, phosphorylated (p-)PI3K, PKB, p-PKB, and PI3K. An RNA pull-down assay, RNA immunoprecipitation assay, and dual-luciferase reporter assays were used to study the correlation between circACACA and miR-1183. A xenograft tumor model was used to understand the biological role of circACACA. circACACA was highly expressed in NSCLC samples and cell lines, and the pattern was the opposite of miR-1183 expression. Deletion of circACACA suppressed glycolysis, proliferation, and migration in NSCLC. miR-1183 was targeted by circACACA, and circACACA modulated the PI3K/PKB signaling pathway by interacting with miR-1183. Reducing the expression of circACACA inhibited tumor development.306
Signal transducer and activator of transcription 3 (STAT3) has a role in ischemic cardiac hypertrophy and tumor-associated macrophage differentiation.307,308 Dong et al.309 showed that circ_0076305 regulated DDP resistance in NSCLC by positively modulating STAT3 via sponging of miR-296–5p. Xu et al.310 investigated the effects of circAKT3 on glycolysis in NSCLC. Western blotting and quantitative real-time PCR were used to examine the expression of miR-516b-5p, circAKT3, and STAT3 in NSCLC. MTT was used to measure the sensitivity to DDP. For measuring the lactate production and glycolysis in cells treated with different plasmids or 2-deoxy-glucose (2-DG), a lactate assay and glucose assay kits were used. The expression of HIF-1α in H1299 and A549 cells was detected by western blotting. Relationships between circAKT3, miR-516b-5p, and STAT3 were studied using a dual-luciferase reporter assay. circAKT3 and STAT3 were highly expressed in lung cancer while miR-516b-5p showed low expression. Deletion of circAKT3 inhibited glycolysis and increased the sensitivity to DDP. In A549 cells, the inhibition of HIF-1α-induced glycolysis abrogated the circAKT3-induced chemoresistance. miR-516b-5p was found to bind to circAKT3. Suppression of miR-516b-5p reversed the effects of circAKT3 deletion on glycolysis, as well as DDP sensitivity. Deletion of circAKT3 inhibited development of tumors in vivo by the miR-516b-5p/STAT3 axis310 (Table 3).
Table 3.
Selected Circular RNAs Involved in the Regulation of Glycolysis in Various Cancers
| Cancer | Circular RNAs | Expression in Cancer | Target | Model | Samples | Reference |
|---|---|---|---|---|---|---|
| Melanoma | circMYC | upregulation | 3′ UTR of LDHA | in vitro, human | 25 | 311 |
| Non-small-cell lung carcinoma | circACACA | upregulation | PI3K/PKB pathway | in vitro, in vivo, human | 312 | |
| Lung cancer | circAKT3 | upregulation | STAT3 | in vitro, in vivo, human | 28 | 313 |
Conclusions
Cancer is a complex disease at the cellular level, whose causative factors include environmentally induced mutations, heritable genetic conditions, and epigenetic alterations. Cancer cells are further required to modulate their metabolism in accord with the tumor microenvironment in order to survive and proliferate under the conditions imposed on them by the unrestricted growth of the tumor. The metabolic reprogramming that occurs in tumors to support cell expansion and proliferation is facilitated by the loss of tumor suppressor proteins or the activation of oncogenes. In recent times, a large number of different ncRNAs have been discovered, and these are now accepted as major mechanisms of epigenetic control of gene expression. Many ncRNAs have been implicated in the initiation, progression, and metastatic spread of different types of cancer. The dysregulated metabolism in cancer cells (called the Warburg effect) relies on switching energy production away from OXPHOS toward aerobic glycolysis. Several different sub-types of ncRNAs, including miRNAs, lncRNAs, and circRNAs, have been found to play regulatory roles in cancer metabolism, particularly in mitochondrial functions, and in the metabolism of lipids, glucose, and glutamine. Many ncRNAs have been implicated in cancer metabolic reprogramming and the switch to glycolysis, thus increasing the overall aggressiveness of the tumor. Not only could further investigations into the mechanisms and functions of ncRNAs allow the discovery of additional prognostic biomarkers, but they could also be beneficial for the development of novel therapeutic approaches.
Author Contributions
H.M. and M.R.H. contributed to the conception, design, data collection, and drafting of the manuscript. Both authors approved the final version for submission.
Conflicts of Interest
M.R.H. declares the following potential conflicts of interest: Scientific Advisory Boards: Transdermal Cap, Cleveland, OH, USA; BeWell Global, Wan Chai, Hong Kong; Hologenix, Santa Monica, CA, USA; LumiThera, Poulsbo, WA, USA; Vielight, Toronto, ON, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics, Cambridge, MA, USA; Global Photon, Bee Cave, TX, USA; Medical Coherence, Boston MA, USA; NeuroThera, Newark, DE, USA; JOOVV, Minneapolis-St. Paul, MN, USA; AIRx Medical, Pleasanton, CA, USA; FIR Industries, Ramsey, NJ, USA; UVLRx Therapeutics, Oldsmar, FL, USA; Ultralux UV, Lansing, MI, USA; Illumiheal & Petthera, Shoreline, WA, USA; MB Lasertherapy, Houston, TX, USA; ARRC LED, San Clemente, CA, USA; Varuna Biomedical, Incline Village, NV, USA; and Niraxx Light Therapeutics, Boston, MA, USA. Consulting: Lexington International, Boca Raton, FL, USA; USHIO, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland BV, Eindhoven, the Netherlands; Johnson & Johnson, Philadelphia, PA, USA; and Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stock holdings: Global Photon, Bee Cave, TX, USA; and Mitonix, Newark, DE, USA. H.M. declares no competing interests.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. The chemical constitution of respiration ferment. Science. 1928;68:437–443. doi: 10.1126/science.68.1767.437. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantin V.R., St-Pierre J., Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen T.-L., Durán R.V. Glutamine metabolism in cancer therapy. Cancer Drug Resist. 2018;1:126–138. [Google Scholar]
- 6.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Wu Y., Wang Y., Fu A., Gong L., Li W., Li Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017;101:3015–3026. doi: 10.1007/s00253-016-8032-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilde L., Roche M., Domingo-Vidal M., Tanson K., Philp N., Curry J., Martinez-Outschoorn U. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held-Warmkessel J., Dell D.D. Lactic acidosis in patients with cancer. Clin. J. Oncol. Nurs. 2014;18:592–594. doi: 10.1188/14.CJON.592-594. [DOI] [PubMed] [Google Scholar]
- 11.Peppicelli S., Bianchini F., Calorini L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014;33:823–832. doi: 10.1007/s10555-014-9506-4. [DOI] [PubMed] [Google Scholar]
- 12.Shiraishi T., Verdone J.E., Huang J., Kahlert U.D., Hernandez J.R., Torga G., Zarif J.-C., Epstein T., Gatenby R., McCartney A. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget. 2015;6:130–143. doi: 10.18632/oncotarget.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M., Fan J., Liu Y., Yu Y., Xu J., Wen Q., Zhang J., Fu S., Wang B., Xiang L. Oncogenic role of the TP53-induced glycolysis and apoptosis regulator in nasopharyngeal carcinoma through NF-κB pathway modulation. Int. J. Oncol. 2016;48:756–764. doi: 10.3892/ijo.2015.3297. [DOI] [PubMed] [Google Scholar]
- 14.Faubert B., Vincent E.E., Griss T., Samborska B., Izreig S., Svensson R.U., Mamer O.A., Avizonis C., Shackelford D.B., Shaw R.J., Jones R.G. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc. Natl. Acad. Sci. USA. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Peng Y., Li J., Chen Z., Chen F., Tu J., Lin S., Wang H. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 2020;11:2578. doi: 10.1038/s41467-020-16306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T., Kong S., Tao M., Ju S. The potential role of RNA N6-methyladenosine in cancer progression. Mol. Cancer. 2020;19:88. doi: 10.1186/s12943-020-01204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu A.-M., Jian C., Yu A.H., Tu M.-J. RNA therapy: are we using the right molecules? Pharmacol. Ther. 2019;196:91–104. doi: 10.1016/j.pharmthera.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes J., Peruzzi P.P., Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Xu Q., Lv N., Wang L., Zhao H., Wang X., Guo J., Chen C., Li Y., Yu L. Clinical implications of genome-wide DNA methylation studies in acute myeloid leukemia. J. Hematol. Oncol. 2017;10:41. doi: 10.1186/s13045-017-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 22.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon S., Rossi J.J. Therapeutic potential of small activating RNAs (saRNAs) in human cancers. Curr. Pharm. Biotechnol. 2018;19:604–610. doi: 10.2174/1389201019666180528084059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y., He D., Peng Z., Peng W., Shi W., Wang J., Li B., Zhang C., Duan C. Circular RNAs in cancer: an emerging key player. J. Hematol. Oncol. 2017;10:2. doi: 10.1186/s13045-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Yang L., Chen L.-L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell. 2018;71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y., Wu Y.M., Dhanasekaran S.M., Engelke C.G., Cao X. The landscape of circular RNA in cancer. Cell. 2019;176:869–881.e13. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Yang J.-M. Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention. Cancer Biol. Ther. 2013;14:81–89. doi: 10.4161/cbt.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet S., Archer S.L., Allalunis-Turner J., Haromy A., Beaulieu C., Thompson R., Lee C.T., Lopaschuk G.D., Puttagunta L., Bonnet S. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y., Lu W., Chen G., Wang P., Chen Z., Zhou Y., Ogasawara M., Trachootham D., Feng L., Pelicano H. K-rasG12V transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012;22:399–412. doi: 10.1038/cr.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu W., Hu Y., Chen G., Chen Z., Zhang H., Wang F., Feng L., Pelicano H., Wang H., Keating M.J. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2012;10:e1001326. doi: 10.1371/journal.pbio.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavalli L.R., Varella-Garcia M., Liang B.C. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 1997;8:1189–1198. [PubMed] [Google Scholar]
- 33.King M.P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 34.de Souza A.C.S., Justo G.Z., de Araújo D.R., Cavagis A.D.M. Defining the molecular basis of tumor metabolism: a continuing challenge since Warburg’s discovery. Cell. Physiol. Biochem. 2011;28:771–792. doi: 10.1159/000335792. [DOI] [PubMed] [Google Scholar]
- 35.Locasale J.W., Cantley L.C. Altered metabolism in cancer. BMC Biol. 2010;8:88. doi: 10.1186/1741-7007-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y., Tozzi F., Chen J., Fan F., Xia L., Wang J., Gao G., Zhang A., Xia X., Brasher H. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 39.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 40.Deberardinis R.J., Sayed N., Ditsworth D., Thompson C.B. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Backos D.S., Franklin C.C., Reigan P. The role of glutathione in brain tumor drug resistance. Biochem. Pharmacol. 2012;83:1005–1012. doi: 10.1016/j.bcp.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Traverso N., Ricciarelli R., Nitti M., Marengo B., Furfaro A.L., Pronzato M.A., Marinari U.M., Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitroda S.P., Wakim B.T., Sood R.F., Beveridge M.G., Beckett M.A., MacDermed D.M., Weichselbaum R.R., Khodarev N.N. STAT1-dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC Med. 2009;7:68. doi: 10.1186/1741-7015-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai F.D., Lopes M.S., Zhou M., Court H., Ponce O., Fiordalisi J.J., Gierut J.J., Cox A.D., Haigis K.M., Philips M.R. K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc. Natl. Acad. Sci. USA. 2015;112:779–784. doi: 10.1073/pnas.1412811112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerr E.M., Gaude E., Turrell F.K., Frezza C., Martins C.P. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amendola C.R., Mahaffey J.P., Parker S.J., Ahearn I.M., Chen W.-C., Zhou M., Court H., Shi J., Mendoza S.L., Morten M.J. KRAS4A directly regulates hexokinase 1. Nature. 2019;576:482–486. doi: 10.1038/s41586-019-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo W., Hu H., Chang R., Zhong J., Knabel M., O’Meally R., Cole R.N., Pandey A., Semenza G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riganti C., Gazzano E., Polimeni M., Aldieri E., Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Xu X., Zur Hausen A., Coy J.F., Löchelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int. J. Cancer. 2009;124:1330–1337. doi: 10.1002/ijc.24078. [DOI] [PubMed] [Google Scholar]
- 50.Sun W., Liu Y., Glazer C.A., Shao C., Bhan S., Demokan S., Zhao M., Rudek M.A., Ha P.K., Califano J.A. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1α stabilization. Clin. Cancer Res. 2010;16:857–866. doi: 10.1158/1078-0432.CCR-09-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wanka C., Steinbach J.P., Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J. Biol. Chem. 2012;287:33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monteleone F., Rosa R., Vitale M., D’Ambrosio C., Succoio M., Formisano L., Nappi L., Romano M.F., Scaloni A., Tortora G. Increased anaerobic metabolism is a distinctive signature in a colorectal cancer cellular model of resistance to antiepidermal growth factor receptor antibody. Proteomics. 2013;13:866–877. doi: 10.1002/pmic.201200303. [DOI] [PubMed] [Google Scholar]
- 53.Zhao F., Mancuso A., Bui T.V., Tong X., Gruber J.J., Swider C.R., Sanchez P.V., Lum J.J., Sayed N., Melo J.V. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1α-induced metabolic reprograming. Oncogene. 2010;29:2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim V.N. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 55.Lee Y., Kim M., Han J., Yeom K.H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., Kim V.N. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 57.Gregory R.I., Yan K.P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 58.Denli A.M., Tops B.B., Plasterk R.H., Ketting R.F., Hannon G.J. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 59.Bohnsack M.T., Czaplinski K., Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y., Jeon K., Lee J.T., Kim S., Kim V.N. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flynt A.S., Lai E.C. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat. Rev. Genet. 2008;9:831–842. doi: 10.1038/nrg2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 63.Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baek D., Villén J., Shin C., Camargo F.D., Gygi S.P., Bartel D.P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipowicz W., Bhattacharyya S.N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 66.Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. Inhibition of translational initiation by Let-7 microRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 67.Humphreys D.T., Westman B.J., Martin D.I., Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. USA. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen C.P., Bordeleau M.-E., Pelletier J., Sharp P.A. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Nottrott S., Simard M.J., Richter J.D. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 2006;13:1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 70.Kloosterman W.P., Plasterk R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Gangaraju V.K., Lin H. MicroRNAs: key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bushati N., Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 73.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 74.Fathullahzadeh S., Mirzaei H., Honardoost M.A., Sahebkar A., Salehi M. Circulating microRNA-192 as a diagnostic biomarker in human chronic lymphocytic leukemia. Cancer Gene Ther. 2016;23:327–332. doi: 10.1038/cgt.2016.34. [DOI] [PubMed] [Google Scholar]
- 75.Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pourhanifeh M.H., Vosough M., Mahjoubin-Tehran M., Hashemipour M., Nejati M., Abbasi-Kolli M., Sahebkar A., Mirzaei H. Autophagy-related microRNAs: possible regulatory roles and therapeutic potential in and gastrointestinal cancers. Pharmacol. Res. 2020;161:105133. doi: 10.1016/j.phrs.2020.105133. [DOI] [PubMed] [Google Scholar]
- 77.Yousefpouran S., Mostafaei S., Manesh P.V., Iranifar E., Bokharaei-Salim F., Nahand J.S., Mirzaei H., Taran M., Babaei F., Sayad B., Moghoofei M. The assessment of selected miRNAs profile in HIV, HBV, HCV, HIV/HCV, HIV/HBV co-infection and elite controllers for determination of biomarker. Microb. Pathog. 2020;147:104355. doi: 10.1016/j.micpath.2020.104355. [DOI] [PubMed] [Google Scholar]
- 78.Wu D.H., Liang H., Lu S.N., Wang H., Su Z.L., Zhang L., Ma J.-Q., Guo M., Tai S., Yu S. miR-124 suppresses pancreatic ductal adenocarcinoma growth by regulating monocarboxylate transporter 1-mediated cancer lactate metabolism. Cell. Physiol. Biochem. 2018;50:924–935. doi: 10.1159/000494477. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., Tang Z.-G., Lin Y., Qu X.-G., Lv W., Wang G.-B., Li C.L. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed. Pharmacother. 2017;92:33–38. doi: 10.1016/j.biopha.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Ishak Gabra M.B., Hanse E.A., Lowman X.H., Tran T.Q., Li H., Milman N., Liu J., Reid M.A., Locasale J.W. miR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat. Commun. 2019;10:809. doi: 10.1038/s41467-019-08759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H., He J., Lanzafame R., Stadler I., Hamidi H.E., Liu H., Celli J., Hamblin M.R., Huang Y., Oakley E. Quantum dot light emitting devices for photomedical applications. J. Soc. Inf. Disp. 2017;25:177–184. doi: 10.1002/jsid.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li S.J., Liu H.L., Tang S.L., Li X.J., Wang X.Y. MicroRNA-150 regulates glycolysis by targeting von Hippel-Lindau in glioma cells. Am. J. Transl. Res. 2017;9:1058–1066. [PMC free article] [PubMed] [Google Scholar]
- 83.Guo H., Nan Y., Zhen Y., Zhang Y., Guo L., Yu K., Huang Q., Zhong Y. miRNA-451 inhibits glioma cell proliferation and invasion by downregulating glucose transporter 1. Tumour Biol. 2016;37:13751–13761. doi: 10.1007/s13277-016-5219-3. [DOI] [PubMed] [Google Scholar]
- 84.Yu F., Lu Z., Huang K., Wang X., Xu Z., Chen B., Dong P., Zheng J. MicroRNA-17-5p-activated Wnt/β-catenin pathway contributes to the progression of liver fibrosis. Oncotarget. 2016;7:81–93. doi: 10.18632/oncotarget.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Q., Dou C., Liu X., Yang L., Ni C., Wang J., Guo Y., Yang W., Tong X., Huang D. Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491-5p/PKM2 axis. Biomed. Pharmacother. 2018;107:1692–1704. doi: 10.1016/j.biopha.2018.07.071. [DOI] [PubMed] [Google Scholar]
- 86.Hua S., Liu C., Liu L., Wu D. miR-142-3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochem. Biophys. Res. Commun. 2018;496:947–954. doi: 10.1016/j.bbrc.2018.01.112. [DOI] [PubMed] [Google Scholar]
- 87.Li W., Hao J., Zhang L., Cheng Z., Deng X., Shu G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2017;65:5961–5972. doi: 10.1021/acs.jafc.7b02120. [DOI] [PubMed] [Google Scholar]
- 88.Jin F., Wang Y., Zhu Y., Li S., Liu Y., Chen C., Wang X., Zen K., Li L. The miR-125a/HK2 axis regulates cancer cell energy metabolism reprogramming in hepatocellular carcinoma. Sci. Rep. 2017;7:3089. doi: 10.1038/s41598-017-03407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kheimar A., Previdelli R.L., Wight D.J., Kaufer B.B. Telomeres and telomerase: role in Marek’s disease virus pathogenesis, integration and tumorigenesis. Viruses. 2017;9:173. doi: 10.3390/v9070173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren L., Yao Y., Wang Y., Wang S. miR-505 suppressed the growth of hepatocellular carcinoma cells via targeting IGF-1R. Biosci. Rep. 2019;39 doi: 10.1042/BSR20182442. BSR20182442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H.F., Wang Y.C., Han Y.D. MicroRNA-34a inhibits liver cancer cell growth by reprogramming glucose metabolism. Mol. Med. Rep. 2018;17:4483–4489. doi: 10.3892/mmr.2018.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hua S., Lei L., Deng L., Weng X., Liu C., Qi X., Wang S., Zhang D., Zou X., Cao C. miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene. 2018;37:1624–1636. doi: 10.1038/s41388-017-0057-3. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z., Yu M., Fei B., Fang X., Ma T., Wang D. miR-21-5p targets PDHA1 to regulate glycolysis and cancer progression in gastric cancer. Oncol. Rep. 2018;40:2955–2963. doi: 10.3892/or.2018.6695. [DOI] [PubMed] [Google Scholar]
- 94.Ping W., Senyan H., Li G., Yan C., Long L. Increased lactate in gastric cancer tumor-infiltrating lymphocytes is related to impaired T cell function due to miR-34a deregulated lactate dehydrogenase A. Cell. Physiol. Biochem. 2018;49:828–836. doi: 10.1159/000493110. [DOI] [PubMed] [Google Scholar]
- 95.Yang L., Zhang W., Wang Y., Zou T., Zhang B., Xu Y., Pang T., Hu Q., Chen M., Wang L. Hypoxia-induced miR-214 expression promotes tumour cell proliferation and migration by enhancing the Warburg effect in gastric carcinoma cells. Cancer Lett. 2018;414:44–56. doi: 10.1016/j.canlet.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Sun K., Hu P., Xu F. LINC00152/miR-139-5p regulates gastric cancer cell aerobic glycolysis by targeting PRKAA1. Biomed. Pharmacother. 2018;97:1296–1302. doi: 10.1016/j.biopha.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Ding X., Liu J., Liu T., Ma Z., Wen D., Zhu J. miR-148b inhibits glycolysis in gastric cancer through targeting SLC2A1. Cancer Med. 2017;6:1301–1310. doi: 10.1002/cam4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li L.Q., Yang Y., Chen H., Zhang L., Pan D., Xie W.J. MicroRNA-181b inhibits glycolysis in gastric cancer cells via targeting hexokinase 2 gene. Cancer Biomark. 2016;17:75–81. doi: 10.3233/CBM-160619. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., He Y., Bai H., Dang Y., Gao J., Lv P. Phosphoinositide-dependent kinase 1-associated glycolysis is regulated by miR-409-3p in clear cell renal cell carcinoma. J. Cell. Biochem. 2019;120:126–134. doi: 10.1002/jcb.27152. [DOI] [PubMed] [Google Scholar]
- 100.Takai T., Tsujino T., Yoshikawa Y., Inamoto T., Sugito N., Kuranaga Y., Heishima K., Soga T., Hayashi K., Miyata K. Synthetic miR-143 exhibited an anti-cancer effect via the downregulation of K-RAS networks of renal cell cancer cells in vitro and in vivo. Mol. Ther. 2019;27:1017–1027. doi: 10.1016/j.ymthe.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weng Y., Shen Y., He Y., Pan X., Xu J., Jiang Y., Zhang Q., Wang S., Kong F., Zhao S. The miR-15b-5p/PDK4 axis regulates osteosarcoma proliferation through modulation of the Warburg effect. Biochem. Biophys. Res. Commun. 2018;503:2749–2757. doi: 10.1016/j.bbrc.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 102.Chen H., Gao S., Cheng C. miR-323a-3p suppressed the glycolysis of osteosarcoma via targeting LDHA. Hum. Cell. 2018;31:300–309. doi: 10.1007/s13577-018-0215-0. [DOI] [PubMed] [Google Scholar]
- 103.Zheng X.M., Xu C.W., Wang F. miR-33b inhibits osteosarcoma cell proliferation through suppression of glycolysis by targeting lactate dehydrogenase A (LDHA) Cell. Mol. Biol. 2018;64:31–35. [PubMed] [Google Scholar]
- 104.Wu Y., He H., Wu B., Wen J., Guo Z., Luo Y., Cao G. [miR-125b suppresses the aerobic glycolysis of osteosarcoma HOS cells by downregulating the expression of hexokinase-2] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1365–1370. [PubMed] [Google Scholar]
- 105.Xiao Q., Wei Z., Li Y., Zhou X., Chen J., Wang T., Shao G., Zhang M., Zhang Z. miR-186 functions as a tumor suppressor in osteosarcoma cells by suppressing the malignant phenotype and aerobic glycolysis. Oncol. Rep. 2018;39:2703–2710. doi: 10.3892/or.2018.6394. [DOI] [PubMed] [Google Scholar]
- 106.Yuan G., Zhao Y., Wu D., Gao Ch. miR-150 up-regulates Glut1 and increases glycolysis in osteosarcoma cells. Asian Pac. J. Cancer Prev. 2017;18:1127–1131. doi: 10.22034/APJCP.2017.18.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu C., Cai L., Li H. miR-185 regulates the growth of osteosarcoma cells via targeting hexokinase 2. Mol. Med. Rep. 2019;20:2774–2782. doi: 10.3892/mmr.2019.10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou S., Min Z., Sun K., Qu S., Zhou J., Duan H., Liu H., Liu X., Gong Z., Li D. miR-199a-3p/Sp1/LDHA axis controls aerobic glycolysis in testicular tumor cells. Int. J. Mol. Med. 2018;42:2163–2174. doi: 10.3892/ijmm.2018.3771. [DOI] [PubMed] [Google Scholar]
- 109.Fan S., Tian T., Chen W., Lv X., Lei X., Zhang H., Sun S., Cai L., Pan G., He L. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019;79:1069–1084. doi: 10.1158/0008-5472.CAN-18-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eastlack S.C., Dong S., Ivan C., Alahari S.K. Suppression of PDHX by microRNA-27b deregulates cell metabolism and promotes growth in breast cancer. Mol. Cancer. 2018;17:100. doi: 10.1186/s12943-018-0851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Y., He J., Yang L., Luo Q., Liu Z. Histone deacetylase-3 modification of microRNA-31 promotes cell proliferation and aerobic glycolysis in breast cancer and is predictive of poor prognosis. J. Breast Cancer. 2018;21:112–123. doi: 10.4048/jbc.2018.21.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao K., Li J., Chen J., Qian L., Wang A., Chen X., Xiong W., Tang J., Tang S., Chen Y. microRNA-33a-5p increases radiosensitivity by inhibiting glycolysis in melanoma. Oncotarget. 2017;8:83660–83672. doi: 10.18632/oncotarget.19014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Huang Y.Y., Wang Y., Lyu P., Hamblin M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017;7:7781. doi: 10.1038/s41598-017-07525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li L., Kang L., Zhao W., Feng Y., Liu W., Wang T., Mai H., Huang J., Chen S., Liang Y. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett. 2017;400:89–98. doi: 10.1016/j.canlet.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 115.Romero-Cordoba S.L., Rodriguez-Cuevas S., Bautista-Pina V., Maffuz-Aziz A., D’Ippolito E., Cosentino G., Baroni S., Iorio M.V., Hidalgo-Miranda A. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci. Rep. 2018;8:12252. doi: 10.1038/s41598-018-29708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu B., Cao X., Zhang W., Pan G., Yi Q., Zhong W., Yan D. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J. 2019;33:545–556. doi: 10.1096/fj.201800803R. [DOI] [PubMed] [Google Scholar]
- 117.Zhang K., Zhang M., Jiang H., Liu F., Liu H., Li Y. Down-regulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via down-regulating the expression of hexokinase 2 and pyruvate kinase isozyme M2. Biomed. Pharmacother. 2018;105:545–552. doi: 10.1016/j.biopha.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Ferguson S.W., Wang J., Lee C.J., Liu M., Neelamegham S., Canty J.M., Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci. Rep. 2018;8:1419. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao X., Lu C., Chu W., Zhang B., Zhen Q., Wang R., Zhang Y., Li Z., Lv B., Li H., Liu J. MicroRNA-124 suppresses proliferation and glycolysis in non-small cell lung cancer cells by targeting AKT-GLUT1/HKII. Tumour Biol. 2017;39 doi: 10.1177/1010428317706215. 1010428317706215. [DOI] [PubMed] [Google Scholar]
- 120.Lv X., Yao L., Zhang J., Han P., Li C. Inhibition of microRNA-155 sensitizes lung cancer cells to irradiation via suppression of HK2-modulated glucose metabolism. Mol. Med. Rep. 2016;14:1332–1338. doi: 10.3892/mmr.2016.5394. [DOI] [PubMed] [Google Scholar]
- 121.Liu M., Gao J., Huang Q., Jin Y., Wei Z. Downregulating microRNA-144 mediates a metabolic shift in lung cancer cells by regulating GLUT1 expression. Oncol. Lett. 2016;11:3772–3776. doi: 10.3892/ol.2016.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang S., Pei M., Li Z., Li H., Liu Y., Li J. Double-negative feedback interaction between DNA methyltransferase 3A and microRNA-145 in the Warburg effect of ovarian cancer cells. Cancer Sci. 2018;109:2734–2745. doi: 10.1111/cas.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu J., Wang L., Chen W., Wang Y., Zhen S., Chen H., Cheng J., Zhou Y., Li X., Zhao L. miR-603 targeted hexokinase-2 to inhibit the malignancy of ovarian cancer cells. Arch. Biochem. Biophys. 2019;661:1–9. doi: 10.1016/j.abb.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Guo N.L., Zhang J.X., Wu J.P., Xu Y.H. Isoflurane promotes glucose metabolism through up-regulation of miR-21 and suppresses mitochondrial oxidative phosphorylation in ovarian cancer cells. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170818. BSR20170818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han R.L., Wang F.P., Zhang P.A., Zhou X.Y., Li Y. miR-383 inhibits ovarian cancer cell proliferation, invasion and aerobic glycolysis by targeting LDHA. Neoplasma. 2017;64:244–252. doi: 10.4149/neo_2017_211. [DOI] [PubMed] [Google Scholar]
- 126.Xiaohong Z., Lichun F., Na X., Kejian Z., Xiaolan X., Shaosheng W. MiR-203 promotes the growth and migration of ovarian cancer cells by enhancing glycolytic pathway. Tumour Biol. 2016;37:14989–14997. doi: 10.1007/s13277-016-5415-1. [DOI] [PubMed] [Google Scholar]
- 127.Muys B.R., Sousa J.F., Placa J.R., de Araujo L.F., Sarshad A.A., Anastasakis D.G., Wang X., Li X.L., de Molfetta G.A., Ramão A. miR-450a acts as a tumor suppressor in ovarian cancer by regulating energy metabolism. Cancer Res. 2019;79:3294–3305. doi: 10.1158/0008-5472.CAN-19-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen J., Yu Y., Chen X., He Y., Hu Q., Li H., Han Q., Ren F., Li J., Li C. miR-139-5p is associated with poor prognosis and regulates glycolysis by repressing PKM2 in gallbladder carcinoma. Cell Prolif. 2018;51:e12510. doi: 10.1111/cpr.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yao A., Xiang Y., Si Y.R., Fan L.J., Li J.P., Guo W., He H.-X., Liang X.-J., Tan Y. PKM2 promotes glucose metabolism through a let-7a-5p/Stat3/hnRNP-A1 regulatory feedback loop in breast cancer cells. J. Cell. Biochem. 2019;120:6542–6554. doi: 10.1002/jcb.27947. [DOI] [PubMed] [Google Scholar]
- 130.He Y., Chen X., Yu Y., Li J., Hu Q., Xue C., Chen J., Shen S., Luo Y., Ren F. LDHA is a direct target of miR-30d-5p and contributes to aggressive progression of gallbladder carcinoma. Mol. Carcinog. 2018;57:772–783. doi: 10.1002/mc.22799. [DOI] [PubMed] [Google Scholar]
- 131.Hui L., Zhang J., Guo X. miR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. Biomed. Pharmacother. 2018;103:1194–1201. doi: 10.1016/j.biopha.2018.04.098. [DOI] [PubMed] [Google Scholar]
- 132.Ghasemi A., Rabiee N., Ahmadi S., Hashemzadeh S., Lolasi F., Bozorgomid M., Kalbasi A., Nasseri B., Shiralizadeh Dezfuli A., Aref A.R. Optical assays based on colloidal inorganic nanoparticles. Analyst (Lond.) 2018;143:3249–3283. doi: 10.1039/c8an00731d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu Q.Q., Zheng B., Weng G.B. Downregulated NOX4 underlies a novel inhibitory role of microRNA-137 in prostate cancer. J. Cell. Biochem. 2019;120:10215–10227. doi: 10.1002/jcb.28306. [DOI] [PubMed] [Google Scholar]
- 134.Huang S., Yang Z., Ma Y., Yang Y., Wang S. miR-101 enhances cisplatin-induced DNA damage through decreasing nicotinamide adenine dinucleotide phosphate levels by directly repressing Tp53-induced glycolysis and apoptosis regulator expression in prostate cancer Cells. DNA Cell Biol. 2017;36:303–310. doi: 10.1089/dna.2016.3612. [DOI] [PubMed] [Google Scholar]
- 135.Qu W., Ding S.M., Cao G., Wang S.J., Zheng X.H., Li G.H. miR-132 mediates a metabolic shift in prostate cancer cells by targeting Glut1. FEBS Open Bio. 2016;6:735–741. doi: 10.1002/2211-5463.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan D., Zheng S., Wang L., Li J., Yang J., Wang B., Chen X., Zhang X. miR-200c inhibits bladder cancer progression by targeting lactate dehydrogenase A. Oncotarget. 2017;8:67663–67669. doi: 10.18632/oncotarget.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ling Z., Liu D., Zhang G., Liang Q., Xiang P., Xu Y., Han C., Tao T. miR-361-5p modulates metabolism and autophagy via the Sp1-mediated regulation of PKM2 in prostate cancer. Oncol. Rep. 2017;38:1621–1628. doi: 10.3892/or.2017.5852. [DOI] [PubMed] [Google Scholar]
- 138.Minami K., Taniguchi K., Sugito N., Kuranaga Y., Inamoto T., Takahara K., Takai T., Yoshikawa Y., Kiyama S., Akao Y., Azuma H. miR-145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget. 2017;8:33064–33077. doi: 10.18632/oncotarget.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo J., Zhao P., Liu Z., Li Z., Yuan Y., Zhang X., Yu Z., Fang J., Xiao K. miR-204-3p inhibited the proliferation of bladder cancer cells via modulating lactate dehydrogenase-mediated glycolysis. Front. Oncol. 2019;9:1242. doi: 10.3389/fonc.2019.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang H., Feng C., Zhang M., Zeng A., Si L., Yu N., Bai M. miR-625-5p/PKM2 negatively regulates melanoma glycolysis state. J. Cell. Biochem. 2019;120:2964–2972. doi: 10.1002/jcb.26917. [DOI] [PubMed] [Google Scholar]
- 141.Lv N., Hao S., Luo C., Abukiwan A., Hao Y., Gai F., Huang W., Huang L., Xiao X., Eichmüller S.B., He D. miR-137 inhibits melanoma cell proliferation through downregulation of GLO1. Sci. China Life Sci. 2018;61:541–549. doi: 10.1007/s11427-017-9138-9. [DOI] [PubMed] [Google Scholar]
- 142.Cao K., Li J., Chen J., Qian L., Wang A., Chen X., Xiong W., Tang J., Tang S., Chen Y. MicroRNA-33a-5p increases radiosensitivity by inhibiting glycolysis in melanoma. Oncotarget. 2017;8:83660–83672. doi: 10.18632/oncotarget.19014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Y., Wu C., Li L. MicroRNA-33b inhibits cell proliferation and glycolysis by targeting hypoxia-inducible factor-1α in malignant melanoma. Exp. Ther. Med. 2017;14:1299–1306. doi: 10.3892/etm.2017.4702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Wei S., Ma W. miR-370 functions as oncogene in melanoma by direct targeting pyruvate dehydrogenase B. Biomed. Pharmacother. 2017;90:278–286. doi: 10.1016/j.biopha.2017.03.068. [DOI] [PubMed] [Google Scholar]
- 145.Jin F., Yang R., Wei Y., Wang D., Zhu Y., Wang X., Lu Y., Wang Y., Zen K., Li L. HIF-1α-induced miR-23a∼27a∼24 cluster promotes colorectal cancer progression via reprogramming metabolism. Cancer Lett. 2019;440–441:211–222. doi: 10.1016/j.canlet.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 146.Snezhkina A.V., Krasnov G.S., Zhikrivetskaya S.O., Karpova I.Y., Fedorova M.S., Nyushko K.M., Belyakov M.M., Gnuchev N.V., Sidorov D.V., Alekseev B.Y. [Overexpression of microRNAs miR-9, -98, and -199 Correlates with the Downregulation of HK2 Expression in Colorectal Cancer] Molekuliarnaia biologiia. 2018;52:220–230. doi: 10.7868/S0026898418020052. [DOI] [PubMed] [Google Scholar]
- 147.Liu S., Xiao Z., Ai F., Liu F., Chen X., Cao K., Ren W., Zhang X., Shu P., Zhang D. miR-142-5p promotes development of colorectal cancer through targeting SDHB and facilitating generation of aerobic glycolysis. Biomed. Pharmacother. 2017;92:1119–1127. doi: 10.1016/j.biopha.2017.05.134. [DOI] [PubMed] [Google Scholar]
- 148.Santasusagna S., Moreno I., Navarro A., Muñoz C., Martinez F., Hernández R., Castellano J.J., Monzo M. miR-328 mediates a metabolic shift in colon cancer cells by targeting SLC2A1/GLUT1. Clin. Transl. Oncol. 2018;20:1161–1167. doi: 10.1007/s12094-018-1836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu W., Zhang Z., Zou K., Cheng Y., Yang M., Chen H., Wang H., Zhao J., Chen P., He L. miR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death Dis. 2017;8:e2761. doi: 10.1038/cddis.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu W., Huang Y., Pan Q., Xiang P., Xie N., Yu H. MicroRNA-98 suppress Warburg effect by targeting HK2 in colon cancer cells. Dig. Dis. Sci. 2017;62:660–668. doi: 10.1007/s10620-016-4418-5. [DOI] [PubMed] [Google Scholar]
- 151.Fong L.Y., Jing R., Smalley K.J., Taccioli C., Fahrmann J., Barupal D.K., Alder H., Farber J.L., Fiehn O., Croce C.M. Integration of metabolomics, transcriptomics, and microRNA expression profiling reveals a miR-143-HK2-glucose network underlying zinc-deficiency-associated esophageal neoplasia. Oncotarget. 2017;8:81910–81925. doi: 10.18632/oncotarget.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li H., Li X., Ge X., Jia L., Zhang Z., Fang R., Yang J., Liu J., Peng S., Zhou M. miR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget. 2016;7:54838–54851. doi: 10.18632/oncotarget.10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou L., Wang Y., Zhou M., Zhang Y., Wang P., Li X., Yang J., Wang H., Ding Z. HOXA9 inhibits HIF-1α-mediated glycolysis through interacting with CRIP2 to repress cutaneous squamous cell carcinoma development. Nat. Commun. 2018;9:1480. doi: 10.1038/s41467-018-03914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun X., Zhang L. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci. Rep. 2017;37 doi: 10.1042/BSR20160404. BSR20160404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang L., El-Hussein A., Xuan W., Hamblin M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B. 2018;178:277–286. doi: 10.1016/j.jphotobiol.2017.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miao Y., Zhang L.F., Zhang M., Guo R., Liu M.F., Li B. Therapeutic delivery of miR-143 targeting tumor metabolism in poorly differentiated thyroid cancer xenografts and efficacy evaluation using 18F-FDG microPET-CT. Hum. Gene Ther. 2019;30:882–892. doi: 10.1089/hum.2018.160. [DOI] [PubMed] [Google Scholar]
- 157.Macheda M.L., Rogers S., Best J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell. Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 158.Ogura K., Sakata M., Yamaguchi M., Kurachi H., Murata Y. High concentration of glucose decreases glucose transporter-1 expression in mouse placenta in vitro and in vivo. J. Endocrinol. 1999;160:443–452. doi: 10.1677/joe.0.1600443. [DOI] [PubMed] [Google Scholar]
- 159.Hoffman N.J., Elmendorf J.S. Signaling, cytoskeletal and membrane mechanisms regulating GLUT4 exocytosis. Trends Endocrinol. Metab. 2011;22:110–116. doi: 10.1016/j.tem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He A., Liu X., Liu L., Chang Y., Fang F. How many signals impinge on GLUT4 activation by insulin? Cell. Signal. 2007;19:1–7. doi: 10.1016/j.cellsig.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 161.Lu H., Buchan R.J., Cook S.A. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc. Res. 2010;86:410–420. doi: 10.1093/cvr/cvq010. [DOI] [PubMed] [Google Scholar]
- 162.Horie T., Ono K., Nishi H., Iwanaga Y., Nagao K., Kinoshita M., Kuwabara Y., Takanabe R., Hasegawa K., Kita T., Kimura T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009;389:315–320. doi: 10.1016/j.bbrc.2009.08.136. [DOI] [PubMed] [Google Scholar]
- 163.Singh P.K., Mehla K., Hollingsworth M.A., Johnson K.R. Regulation of aerobic glycolysis by microRNAs in cancer. Mol. Cell. Pharmacol. 2011;3:125–134. [PMC free article] [PubMed] [Google Scholar]
- 164.Ahmad A., Aboukameel A., Kong D., Wang Z., Sethi S., Chen W., Sarkar F.H., Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–3409. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fabani M.M., Gait M.J. miR-122 targeting with LNA/2′-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA. 2008;14:336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taguchi A., Yanagisawa K., Tanaka M., Cao K., Matsuyama Y., Goto H., Takahashi T. Identification of hypoxia-inducible factor-1α as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68:5540–5545. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- 167.Ichimi T., Enokida H., Okuno Y., Kunimoto R., Chiyomaru T., Kawamoto K., Kawahara K., Toki K., Kawakami K., Nishiyama K. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int. J. Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 168.Calin G.A., Cimmino A., Fabbri M., Ferracin M., Wojcik S.E., Shimizu M., Taccioli C., Zanesi N., Garzon R., Aqeilan R.I. miR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Muniyamppa M.K., Dowling P., Henry M., Meleady P., Doolan P., Gammell P., Clynes M., Barron N. iRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur. J. Cancer. 2009;45:3104–3118. doi: 10.1016/j.ejca.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 170.Liu Q., Fu H., Sun F., Zhang H., Tie Y., Zhu J., Xing R., Sun Z., Zheng X. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong T.S., Liu X.B., Chung-Wai Ho A., Po-Wing Yuen A., Wai-Man Ng R., Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int. J. Cancer. 2008;123:251–257. doi: 10.1002/ijc.23583. [DOI] [PubMed] [Google Scholar]
- 172.Pullen T.J., da Silva Xavier G., Kelsey G., Rutter G.A. miR-29a and miR-29b contribute to pancreatic β-cell-specific silencing of monocarboxylate transporter 1 (Mct1) Mol. Cell. Biol. 2011;31:3182–3194. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang J., Wang H., Liu A., Fang C., Hao J., Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kelly T.J., Souza A.L., Clish C.B., Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1α stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol. Cell. Biol. 2011;31:2696–2706. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Greither T., Würl P., Grochola L., Bond G., Bache M., Kappler M., Lautenschläger C., Holzhausen H.J., Wach S., Eckert A.W., Taubert H. Expression of microRNA 210 associates with poor survival and age of tumor onset of soft-tissue sarcoma patients. Int. J. Cancer. 2012;130:1230–1235. doi: 10.1002/ijc.26109. [DOI] [PubMed] [Google Scholar]
- 177.Devlin C., Greco S., Martelli F., Ivan M. miR-210: more than a silent player in hypoxia. IUBMB Life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Radojicic J., Zaravinos A., Vrekoussis T., Kafousi M., Spandidos D.A., Stathopoulos E.N. MicroRNA expression analysis in triple-negative (ER, PR and Her2/neu) breast cancer. Cell Cycle. 2011;10:507–517. doi: 10.4161/cc.10.3.14754. [DOI] [PubMed] [Google Scholar]
- 179.Puisségur M.P., Mazure N.M., Bertero T., Pradelli L., Grosso S., Robbe-Sermesant K., Maurin T., Lebrigand K., Cardinaud B., Hofman V. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chan S.Y., Zhang Y.-Y., Hemann C., Mahoney C.E., Zweier J.L., Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Poy M.N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P.E., Pfeffer S., Tuschl T., Rajewsky N., Rorsman P., Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 182.El Ouaamari A., Baroukh N., Martens G.A., Lebrun P., Pipeleers D., van Obberghen E. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic β-cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bloomston M., Frankel W.L., Petrocca F., Volinia S., Alder H., Hagan J.P., Liu C.-G., Bhatt D., Taccioli C., Croce C.M. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 184.Lee E.J., Gusev Y., Jiang J., Nuovo G.J., Lerner M.R., Frankel W.L., Morgan D.L., Postier R.G., Brackett D.J., Schmittgen T.D. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schmitz S.U., Grote P., Herrmann B.G. Mechanisms of long noncoding RNA function in development and disease. Cell. Mol. Life Sci. 2016;73:2491–2509. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Delás M.J., Hannon G.J. lncRNAs in development and disease: from functions to mechanisms. Open Biol. 2017;7:170121. doi: 10.1098/rsob.170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Akerman I., Tu Z., Beucher A., Rolando D.M.Y., Sauty-Colace C., Benazra M., Nakic N., Yang J., Wang H., Pasquali L. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metab. 2017;25:400–411. doi: 10.1016/j.cmet.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morán I., Akerman I., van de Bunt M., Xie R., Benazra M., Nammo T., Arnes L., Nakić N., García-Hurtado J., Rodríguez-Seguí S. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16:435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu H., Yang L., Chen L.-L. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33:540–552. doi: 10.1016/j.tig.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 190.Vicens Q., Westhof E. Biogenesis of circular RNAs. Cell. 2014;159:13–14. doi: 10.1016/j.cell.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 191.Chen L.-L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 192.Naganuma T., Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013;10:456–461. doi: 10.4161/rna.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamazaki T., Hirose T. The building process of the functional paraspeckle with long non-coding RNAs. Front. Biosci. (Elite Ed.) 2015;7:1–41. doi: 10.2741/715. [DOI] [PubMed] [Google Scholar]
- 194.Krol J. Paraspeckles: nuclear nests helping to raise mature miRNAs. Nat. Struct. Mol. Biol. 2017;24:783–784. doi: 10.1038/nsmb.3479. [DOI] [PubMed] [Google Scholar]
- 195.Yoon J.-H., Gorospe M. Cross-linking immunoprecipitation and qPCR (CLIP-qPCR) analysis to map interactions between long noncoding RNAs and RNA-binding proteins. Methods Mol. Biol. 2016;1402:11–17. doi: 10.1007/978-1-4939-3378-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Salehi S., Taheri M.N., Azarpira N., Zare A., Behzad-Behbahani A. State of the art technologies to explore long non-coding RNAs in cancer. J. Cell. Mol. Med. 2017;21:3120–3140. doi: 10.1111/jcmm.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li J.-H., Liu S., Zheng L.-L., Wu J., Sun W.-J., Wang Z.-L., Zhou H., Qu L.-H., Yang J.-H. Discovery of protein-lncRNA interactions by integrating large-scale CLIP-seq and RNA-seq datasets. Front. Bioeng. Biotechnol. 2015;2:88. doi: 10.3389/fbioe.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu X., Gan B. lncRNA NBR2 modulates cancer cell sensitivity to phenformin through GLUT1. Cell Cycle. 2016;15:3471–3481. doi: 10.1080/15384101.2016.1249545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ellis B.C., Graham L.D., Molloy P.L. CRNDE, a long non-coding RNA responsive to insulin/IGF signaling, regulates genes involved in central metabolism. Molecular Cell Research. 2014;1843:372–386. doi: 10.1016/j.bbamcr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 200.Fan C., Tang Y., Wang J., Xiong F., Guo C., Wang Y., Zhang S., Gong Z., Wei F., Yang L. Role of long non-coding RNAs in glucose metabolism in cancer. Mol. Cancer. 2017;16:130. doi: 10.1186/s12943-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang F., Yi F., Zheng Z., Ling Z., Ding J., Guo J., Mao W., Wang X., Wang X., Ding X. Characterization of a carcinogenesis-associated long non-coding RNA. RNA Biol. 2012;9:110–116. doi: 10.4161/rna.9.1.18332. [DOI] [PubMed] [Google Scholar]
- 202.Lim S., Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 203.Scheicher R., Hoelbl-Kovacic A., Bellutti F., Tigan A.-S., Prchal-Murphy M., Heller G., Schneckenleithner C., Salazar-Roa M., Zöchbauer-Müller S., Zuber J. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125:90–101. doi: 10.1182/blood-2014-06-584417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zanuy M., Ramos-Montoya A., Villacañas O., Canela N., Miranda A., Aguilar E., Agell N., Bachs O., Rubio-Martinez J., Pujol M.D. Cyclin-dependent kinases 4 and 6 control tumor progression and direct glucose oxidation in the pentose cycle. Metabolomics. 2012;8:454–464. doi: 10.1007/s11306-011-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xing Z., Zhang Y., Liang K., Yan L., Xiang Y., Li C., Hu Q., Jin F., Putluri V., Putluri N. Expression of long noncoding RNA YIYA promotes glycolysis in breast cancer. Cancer Res. 2018;78:4524–4532. doi: 10.1158/0008-5472.CAN-17-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee S., Kopp F., Chang T.-C., Sataluri A., Chen B., Sivakumar S., Yu H., Xie Y., Mendell J.T. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu H., Li J., Koirala P., Ding X., Chen B., Wang Y., Wang Z., Wang C., Zhang X., Mo Y.Y. Long non-coding RNAs as prognostic markers in human breast cancer. Oncotarget. 2016;7:20584–20596. doi: 10.18632/oncotarget.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li H., Wang X., Wen C., Huo Z., Wang W., Zhan Q., Cheng D., Chen H., Deng X., Peng C., Shen B. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol. Cancer. 2017;16:169. doi: 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang J., Li X.-Y., Hu P., Ding Y.-S. lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncology Res. 2018;26:1411–1418. doi: 10.3727/096504018X15190844870055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gao W., Weng T., Wang L., Shi B., Meng W., Wang X., Wu Y., Jin L., Fei L. Long non-coding RNA NORAD promotes cell proliferation and glycolysis in non-small cell lung cancer by acting as a sponge for miR-136-5p. Mol. Med. Rep. 2019;19:5397–5405. doi: 10.3892/mmr.2019.10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang H., Li W., Gu W., Yan Y., Yao X., Zheng J. MALAT1 accelerates the development and progression of renal cell carcinoma by decreasing the expression of miR-203 and promoting the expression of BIRC5. Cell Prolif. 2019;52:e12640. doi: 10.1111/cpr.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sun Q., Li Q., Xie F. lncRNA-MALAT1 regulates proliferation and apoptosis of ovarian cancer cells by targeting miR-503-5p. OncoTargets Ther. 2019;12:6297–6307. doi: 10.2147/OTT.S214689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Si Y., Yang Z., Ge Q., Yu L., Yao M., Sun X., Ren Z., Ding C. Long non-coding RNA Malat1 activated autophagy, hence promoting cell proliferation and inhibiting apoptosis by sponging miR-101 in colorectal cancer. Cell. Mol. Biol. Lett. 2019;24:50. doi: 10.1186/s11658-019-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cho S.-F., Chang Y.C., Chang C.-S., Lin S.-F., Liu Y.-C., Hsiao H.-H., Chang J.G., Liu T.-C. MALAT1 long non-coding RNA is overexpressed in multiple myeloma and may serve as a marker to predict disease progression. BMC Cancer. 2014;14:809. doi: 10.1186/1471-2407-14-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu H., Wang H., Wu B., Yao K., Liao A., Miao M., Li Y., Yang W. Down-regulation of long non-coding RNA MALAT1 by RNA interference inhibits proliferation and induces apoptosis in multiple myeloma. Clin. Exp. Pharmacol. Physiol. 2017;44:1032–1041. doi: 10.1111/1440-1681.12804. [DOI] [PubMed] [Google Scholar]
- 218.He Z., Ruan X., Liu X., Zheng J., Liu Y., Liu L., Ma J., Shao L., Wang D., Shen S. FUS/circ_002136/miR-138-5p/SOX13 feedback loop regulates angiogenesis in glioma. J. Exp. Clin. Cancer Res. 2019;38:65. doi: 10.1186/s13046-019-1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bie L.-Y., Li D., Wei Y., Li N., Chen X.-B., Luo S.-X. SOX13 dependent PAX8 expression promotes the proliferation of gastric carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019;47:3180–3187. doi: 10.1080/21691401.2019.1646751. [DOI] [PubMed] [Google Scholar]
- 220.Xu H., Li J., Zhou Z.G. NEAT1 promotes cell proliferation in multiple myeloma by activating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6403–6411. doi: 10.26355/eurrev_201810_16053. [DOI] [PubMed] [Google Scholar]
- 221.Liu N., Feng S., Li H., Chen X., Bai S., Liu Y. Long non-coding RNA MALAT1 facilitates the tumorigenesis, invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13 axis. J. Cancer Res. Clin. Oncol. 2020;146:367–379. doi: 10.1007/s00432-020-03127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chureau C., Chantalat S., Romito A., Galvani A., Duret L., Avner P., Rougeulle C. Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum. Mol. Genet. 2011;20:705–718. doi: 10.1093/hmg/ddq516. [DOI] [PubMed] [Google Scholar]
- 223.Liu Z., Dou C., Yao B., Xu M., Ding L., Wang Y., Jia Y., Li Q., Zhang H., Tu K. Ftx non coding RNA-derived miR-545 promotes cell proliferation by targeting RIG-I in hepatocellular carcinoma. Oncotarget. 2016;7:25350–25365. doi: 10.18632/oncotarget.8129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Patitucci C., Couchy G., Bagattin A., Cañeque T., de Reyniès A., Scoazec J.-Y., Rodriguez R., Pontoglio M., Zucman-Rossi J., Pende M., Panasyuk G. Hepatocyte nuclear factor 1α suppresses steatosis-associated liver cancer by inhibiting PPARγ transcription. J. Clin. Invest. 2017;127:1873–1888. doi: 10.1172/JCI90327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Janani C., Ranjitha Kumari B.D. PPAR gamma gene—a review. Diabetes Metab. Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 226.Wu Z., Xie Y., Morrison R.F., Bucher N.L., Farmer S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Invest. 1998;101:22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lecarpentier Y., Claes V., Vallée A., Hébert J.-L. Thermodynamics in cancers: opposing interactions between PPAR gamma and the canonical WNT/beta-catenin pathway. Clin. Transl. Med. 2017;6:14. doi: 10.1186/s40169-017-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 229.Li X., Zhao Q., Qi J., Wang W., Zhang D., Li Z., Qin C. lncRNA Ftx promotes aerobic glycolysis and tumor progression through the PPARγ pathway in hepatocellular carcinoma. Int. J. Oncol. 2018;53:551–566. doi: 10.3892/ijo.2018.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shi M., Cui J., Du J., Wei D., Jia Z., Zhang J., Zhu Z., Gao Y., Xie K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin. Cancer Res. 2014;20:4370–4380. doi: 10.1158/1078-0432.CCR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sheng S.L., Liu J.J., Dai Y.H., Sun X.G., Xiong X.P., Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 232.Zhao Y.H., Zhou M., Liu H., Ding Y., Khong H.T., Yu D., Fodstad O., Tan M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 233.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang Z.-Y., Loo T.Y., Shen J.-G., Wang N., Wang D.-M., Yang D.-P., Mo S.-L., Guan X.-Y., Chen J.-P. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res. Treat. 2012;131:791–800. doi: 10.1007/s10549-011-1466-6. [DOI] [PubMed] [Google Scholar]
- 235.Chen H., Pei H., Hu W., Ma J., Zhang J., Mao W., Nie J., Xu C., Li B., Hei T.K. Long non-coding RNA CRYBG3 regulates glycolysis of lung cancer cells by interacting with lactate dehydrogenase A. J. Cancer. 2018;9:2580–2588. doi: 10.7150/jca.24896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim S.-J., Hwang S.-H., Kim I.J., Lee M.K., Lee C.H., Lee S.-Y., Lee E.Y. The association of 18F-deoxyglucose (FDG) uptake of PET with polymorphisms in the glucose transporter gene (SLC2A1) and hypoxia-related genes (HIF1A, VEGFA, APEX1) in non-small cell lung cancer. SLC2A1 polymorphisms and FDG-PET in NSCLC patients. J. Exp. Clin. Cancer Res. 2010;29:69. doi: 10.1186/1756-9966-29-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Goos J.A., de Cuba E.M., Coupé V.M., Diosdado B., Delis-Van Diemen P.M., Karga C., Beliën J.A., Menke-Van der Houven van Oordt C.W., Geldof A.A., Meijer G.A., DeCoDe PET Group Glucose transporter 1 (SLC2A1) and vascular endothelial growth factor A (VEGFA) predict survival after resection of colorectal cancer liver metastasis. Ann. Surg. 2016;263:138–145. doi: 10.1097/SLA.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 238.Chen J., Zhang C., Mi Y., Chen F., Du D. CREB1 regulates glucose transport of glioma cell line U87 by targeting GLUT1. Mol. Cell. Biochem. 2017;436:79–86. doi: 10.1007/s11010-017-3080-3. [DOI] [PubMed] [Google Scholar]
- 239.Shi J., Zhang Y., Qin B., Wang Y., Zhu X. Long non-coding RNA LINC00174 promotes glycolysis and tumor progression by regulating miR-152-3p/SLC2A1 axis in glioma. J. Exp. Clin. Cancer Res. 2019;38:395. doi: 10.1186/s13046-019-1390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Que W.C., Qiu H.Q., Cheng Y., Liu M.B., Wu C.Y. PTEN in kidney cancer: a review and meta-analysis. Clin. Chim. Acta. 2018;480:92–98. doi: 10.1016/j.cca.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 241.Dong Y., Richards J.-A., Gupta R., Aung P.P., Emley A., Kluger Y., Dogra S.K., Mahalingam M., Wajapeyee N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene. 2014;33:4632–4642. doi: 10.1038/onc.2013.409. [DOI] [PubMed] [Google Scholar]
- 242.Feng Z.-Z., Chen J.-W., Yang Z.-R., Lu G.-Z., Cai Z.-G. Expression of PTTG1 and PTEN in endometrial carcinoma: correlation with tumorigenesis and progression. Med. Oncol. 2012;29:304–310. doi: 10.1007/s12032-010-9775-x. [DOI] [PubMed] [Google Scholar]
- 243.Sun Z., Ji N., Bi M., Wang S., Liu X., Wang Z. PTEN gene is infrequently hypermethylated in human esophageal squamous cell carcinoma. Tumour Biol. 2015;36:5849–5857. doi: 10.1007/s13277-015-3256-y. [DOI] [PubMed] [Google Scholar]
- 244.Li W., Huang K., Wen F., Cui G., Guo H., He Z., Zhao S. LINC00184 silencing inhibits glycolysis and restores mitochondrial oxidative phosphorylation in esophageal cancer through demethylation of PTEN. EBioMedicine. 2019;44:298–310. doi: 10.1016/j.ebiom.2019.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 245.Kim J.W., Gao P., Liu Y.-C., Semenza G.L., Dang C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell. Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hung C.-L., Wang L.-Y., Yu Y.-L., Chen H.-W., Srivastava S., Petrovics G., Kung H.J. A long noncoding RNA connects c-Myc to tumor metabolism. Proc. Natl. Acad. Sci. USA. 2014;111:18697–18702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang P., Cao L., Fan P., Mei Y., Wu M. lncRNA-MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting Fbxw7-mediated c-Myc degradation. EMBO Rep. 2016;17:1204–1220. doi: 10.15252/embr.201642067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Taniguchi C.M., Emanuelli B., Kahn C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 249.Sun J., Zhang P., Yin T., Zhang F., Wang W. Upregulation of lncRNA PVT1 facilitates pancreatic ductal adenocarcinoma cell progression and glycolysis by regulating miR-519d-3p and HIF-1A. J. Cancer. 2020;11:2572–2579. doi: 10.7150/jca.37959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shang R., Wang M., Dai B., Du J., Wang J., Liu Z., Qu S., Yang X., Liu J., Xia C. Long noncoding RNA SLC2A1-AS1 regulates aerobic glycolysis and progression in hepatocellular carcinoma via inhibiting the STAT3/FOXM1/GLUT1 pathway. Mol. Oncol. 2020;14:1381–1396. doi: 10.1002/1878-0261.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zheng Y.-L., Li L., Jia Y.-X., Zhang B.-Z., Li J.-C., Zhu Y.-H., Li M.Q., He J.Z., Zeng T.T., Ban X.J. LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 2019;9:796–810. doi: 10.7150/thno.28992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hu M., Fu Q., Jing C., Zhang X., Qin T., Pan Y. lncRNA HOTAIR knockdown inhibits glycolysis by regulating miR-130a-3p/HIF1A in hepatocellular carcinoma under hypoxia. Biomed. Pharmacother. 2020;125:109703. doi: 10.1016/j.biopha.2019.109703. [DOI] [PubMed] [Google Scholar]
- 253.Guo X., Zhang Y., Liu L., Yang W., Zhang Q. HNF1A-AS1 regulates cell migration, invasion and glycolysis via modulating miR-124/MYO6 in colorectal cancer cells. Onco. Targets Ther. 2020;13:1507–1518. doi: 10.2147/OTT.S231249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang Y., Lu J.-H., Wu Q.-N., Jin Y., Wang D.-S., Chen Y.-X., Liu J., Luo X.J., Meng Q., Pu H.Y. lncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer. 2019;18:174. doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cui S., Yang X., Zhang L., Zhao Y., Yan W. lncRNA MAFG-AS1 promotes the progression of colorectal cancer by sponging miR-147b and activation of NDUFA4. Biochem. Biophys. Res. Commun. 2018;506:251–258. doi: 10.1016/j.bbrc.2018.10.112. [DOI] [PubMed] [Google Scholar]
- 256.Tang J., Yan T., Bao Y., Shen C., Yu C., Zhu X., Tian X., Guo F., Liang Q., Liu Q. lncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat. Commun. 2019;10:3499. doi: 10.1038/s41467-019-11447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lang N., Wang C., Zhao J., Shi F., Wu T., Cao H. Long non-coding RNA BCYRN1 promotes glycolysis and tumor progression by regulating the miR-149/PKM2 axis in non-small-cell lung cancer. Mol. Med. Rep. 2020;21:1509–1516. doi: 10.3892/mmr.2020.10944. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 258.Zhao W., Li W., Dai W., Huang N., Qiu J. LINK-A promotes cell proliferation through the regulation of aerobic glycolysis in non-small-cell lung cancer. OncoTargets Ther. 2018;11:6071–6080. doi: 10.2147/OTT.S171216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Feng X., Yang S. Long non-coding RNA LINC00243 promotes proliferation and glycolysis in non-small cell lung cancer cells by positively regulating PDK4 through sponging miR-507. Mol. Cell. Biochem. 2020;463:127–136. doi: 10.1007/s11010-019-03635-3. [DOI] [PubMed] [Google Scholar]
- 260.Shi J., Wang H., Feng W., Huang S., An J., Qiu Y., Wu K. Long non-coding RNA HOTTIP promotes hypoxia-induced glycolysis through targeting miR-615-3p/HMGB3 axis in non-small cell lung cancer cells. Eur. J. Pharmacol. 2019;862:172615. doi: 10.1016/j.ejphar.2019.172615. [DOI] [PubMed] [Google Scholar]
- 261.Liu N., Feng S., Li H., Chen X., Bai S., Liu Y. Long non-coding RNA MALAT1 facilitates the tumorigenesis, invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13 axis. J. Cancer Res. Clin. Oncol. 2020;146:367–379. doi: 10.1007/s00432-020-03127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang G., Ma A., Jin Y., Pan G., Wang C. lncRNA SNHG16 induced by TFAP2A modulates glycolysis and proliferation of endometrial carcinoma through miR-490-3p/HK2 axis. Am. J. Transl. Res. 2019;11:7137–7145. [PMC free article] [PubMed] [Google Scholar]
- 263.Liu X., Zhu Q., Guo Y., Xiao Z., Hu L., Xu Q. lncRNA LINC00689 promotes the growth, metastasis and glycolysis of glioma cells by targeting miR-338-3p/PKM2 axis. Biomed. Pharmacother. 2019;117:109069. doi: 10.1016/j.biopha.2019.109069. [DOI] [PubMed] [Google Scholar]
- 264.Wu F., Zhou D., Cui Y., Shen G., Li Y., Wei F. Long non-coding RNA UCA1 modulates the glycolysis of cervical cancer cells by miR-493-5p/HK2. Int. J. Clin. Exp. Pathol. 2018;11:3943–3951. [PMC free article] [PubMed] [Google Scholar]
- 265.Zhu M., Wang X., Gu Y., Wang F., Li L., Qiu X. MEG3 overexpression inhibits the tumorigenesis of breast cancer by downregulating miR-21 through the PI3K/Akt pathway. Arch. Biochem. Biophys. 2019;661:22–30. doi: 10.1016/j.abb.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 266.Zhao L., Ji G., Le X., Wang C., Xu L., Feng M., Zhang Y., Yang H., Xuan Y., Yang Y. Long noncoding RNA LINC00092 acts in cancer-associated fibroblasts to drive glycolysis and progression of ovarian cancer. Cancer Res. 2017;77:1369–1382. doi: 10.1158/0008-5472.CAN-16-1615. [DOI] [PubMed] [Google Scholar]
- 267.Zou Z.W., Ma C., Medoro L., Chen L., Wang B., Gupta R., Liu T., Yang X.Z., Chen T.T., Wang R.Z. lncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yang B., Zhang L., Cao Y., Chen S., Cao J., Wu D., Chen J., Xiong H., Pan Z., Qiu F. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol. Cancer. 2017;16:154. doi: 10.1186/s12943-017-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Feng Z., Chen R., Huang N., Luo C. Long non-coding RNA ASMTL-AS1 inhibits tumor growth and glycolysis by regulating the miR-93-3p/miR-660/FOXO1 axis in papillary thyroid carcinoma. Life Sci. 2020;244:117298. doi: 10.1016/j.lfs.2020.117298. [DOI] [PubMed] [Google Scholar]
- 270.Liu Y., He X., Chen Y., Cao D. Long non-coding RNA LINC00504 regulates the Warburg effect in ovarian cancer through inhibition of miR-1244. Mol. Cell. Biochem. 2020;464:39–50. doi: 10.1007/s11010-019-03647-z. [DOI] [PubMed] [Google Scholar]
- 271.Wang Y., Zhang X., Wang Z., Hu Q., Wu J., Li Y., Ren X., Wu T., Tao X., Chen X. lncRNA-p23154 promotes the invasion-metastasis potential of oral squamous cell carcinoma by regulating Glut1-mediated glycolysis. Cancer Lett. 2018;434:172–183. doi: 10.1016/j.canlet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 272.Zhang Y., Liu Y., Xu X. Knockdown of lncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J. Cell. Biochem. 2018;119:6296–6308. doi: 10.1002/jcb.26899. [DOI] [PubMed] [Google Scholar]
- 273.Song J., Wu X., Liu F., Li M., Sun Y., Wang Y., Wang C., Zhu K., Jia X., Wang B., Ma X. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem. Biophys. Res. Commun. 2017;490:217–224. doi: 10.1016/j.bbrc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 274.Cocquerelle C., Mascrez B., Hétuin D., Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 275.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zaphiropoulos P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc. Natl. Acad. Sci. USA. 1996;93:6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 278.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 279.Zhang Y., Zhang X.-O., Chen T., Xiang J.-F., Yin Q.-F., Xing Y.-H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 280.Abelson J., Trotta C.R., Li H. tRNA splicing. J. Biol. Chem. 1998;273:12685–12688. doi: 10.1074/jbc.273.21.12685. [DOI] [PubMed] [Google Scholar]
- 281.Salgia S.R., Singh S.K., Gurha P., Gupta R. Two reactions of Haloferax volcanii RNA splicing enzymes: joining of exons and circularization of introns. RNA. 2003;9:319–330. doi: 10.1261/rna.2118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lu Z., Filonov G.S., Noto J.J., Schmidt C.A., Hatkevich T.L., Wen Y., Jaffrey S.R., Matera A.G. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA. 2015;21:1554–1565. doi: 10.1261/rna.052944.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Du W.W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Huang S., Yang B., Chen B.J., Bliim N., Ueberham U., Arendt T., Janitz M. The emerging role of circular RNAs in transcriptome regulation. Genomics. 2017;109:401–407. doi: 10.1016/j.ygeno.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 285.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang Y., Liang W., Zhang P., Chen J., Qian H., Zhang X., Xu W. Circular RNAs: emerging cancer biomarkers and targets. J. Exp. Clin. Cancer Res. 2017;36:152. doi: 10.1186/s13046-017-0624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang M., Xin Y. Circular RNAs: a new frontier for cancer diagnosis and therapy. J. Hematol. Oncol. 2018;11:21. doi: 10.1186/s13045-018-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., Kohlmaier A., Herbst A., Northoff B.H., Nicolaou A. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bai Y., Zhang Y., Han B., Yang L., Chen X., Huang R., Wu F., Chao J., Liu P., Hu G. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood–brain barrier integrity. J. Neurosci. 2018;38:32–50. doi: 10.1523/JNEUROSCI.1348-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Liu Q., Zhang X., Hu X., Dai L., Fu X., Zhang J., Ao Y. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a miR-136 “sponge” in human cartilage degradation. Sci. Rep. 2016;6:22572. doi: 10.1038/srep22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhao Z., Li X., Jian D., Hao P., Rao L., Li M. hsa_circ_0054633 in peripheral blood can be used as a diagnostic biomarker of pre-diabetes and type 2 diabetes mellitus. Acta Diabetol. 2017;54:237–245. doi: 10.1007/s00592-016-0943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhou Z., Jiang R., Yang X., Guo H., Fang S., Zhang Y., Cheng Y., Wang J., Yao H., Chao J. circRNA mediates silica-induced macrophage activation via HECTD1/ZC3H12A-dependent ubiquitination. Theranostics. 2018;8:575–592. doi: 10.7150/thno.21648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Geng Y., Jiang J., Wu C. Function and clinical significance of circRNAs in solid tumors. J. Hematol. Oncol. 2018;11:98. doi: 10.1186/s13045-018-0643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chen L., Zhang S., Wu J., Cui J., Zhong L., Zeng L., Ge S. Retracted. Oncogene. 2019;38:5750. doi: 10.1038/s41388-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Guarnerio J., Bezzi M., Jeong J.C., Paffenholz S.V., Berry K., Naldini M.M., Lo-Coco F., Tay Y., Beck A.H., Pandolfi P.P. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 296.Liang H.-F., Zhang X.-Z., Liu B.-G., Jia G.-T., Li W.-L. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am. J. Cancer Res. 2017;7:1566–1576. [PMC free article] [PubMed] [Google Scholar]
- 297.Ždralević M., Brand A., Di Ianni L., Dettmer K., Reinders J., Singer K., Peter K., Schnell A., Bruss C., Decking S.M. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J. Biol. Chem. 2018;293:15947–15961. doi: 10.1074/jbc.RA118.004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Augoff K., Hryniewicz-Jankowska A., Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. 2015;358:1–7. doi: 10.1016/j.canlet.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 299.Xie H., Hanai J., Ren J.-G., Kats L., Burgess K., Bhargava P., Signoretti S., Billiard J., Duffy K.J., Grant A. Targeting lactate dehydrogenase—a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ho J., de Moura M.B., Lin Y., Vincent G., Thorne S., Duncan L.M., Hui-Min L., Kirkwood J.M., Becker D., Van Houten B., Moschos S.J. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol. Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jin C., Dong D., Yang Z., Xia R., Tao S., Piao M. circMYC regulates glycolysis and cell proliferation in melanoma. Cell Biochem. Biophys. 2020;78:77–88. doi: 10.1007/s12013-019-00895-0. [DOI] [PubMed] [Google Scholar]
- 302.Nicholson K.M., Anderson N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 303.Mahajan K., Mahajan N.P. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J. Cell. Physiol. 2012;227:3178–3184. doi: 10.1002/jcp.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dong P., Konno Y., Watari H., Hosaka M., Noguchi M., Sakuragi N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 2014;12:231. doi: 10.1186/s12967-014-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen K., Abuduwufuer A., Zhang H., Luo L., Suotesiyali M., Zou Y. SNHG7 mediates cisplatin-resistance in non-small cell lung cancer by activating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6935–6943. doi: 10.26355/eurrev_201908_18733. [DOI] [PubMed] [Google Scholar]
- 306.Wu W., Xi W., Li H., Yang M., Yao X. Circular RNA circ-ACACA regulates proliferation, migration and glycolysis in non-small-cell lung carcinoma via miR-1183 and PI3K/PKB pathway. Int. J. Mol. Med. 2020;45:1814–1824. doi: 10.3892/ijmm.2020.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yuan L., Qiu L., Ye Y., Wu J., Wang S., Wang X., Zhou N., Zou Y. Heat-shock transcription factor 1 is critically involved in the ischaemia-induced cardiac hypertrophy via JAK2/STAT3 pathway. J. Cell. Mol. Med. 2018;22:4292–4303. doi: 10.1111/jcmm.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kumar V., Cheng P., Condamine T., Mony S., Languino L.R., McCaffrey J.C., Hockstein N., Guarino M., Masters G., Penman E. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dong Y., Xu T., Zhong S., Wang B., Zhang H., Wang X., Wang P., Li G., Yang S. circ_0076305 regulates cisplatin resistance of non-small cell lung cancer via positively modulating STAT3 by sponging miR-296-5p. Life Sci. 2019;239:116984. doi: 10.1016/j.lfs.2019.116984. [DOI] [PubMed] [Google Scholar]
- 310.Xu Y., Jiang T., Wu C., Zhang Y. circAKT3 inhibits glycolysis balance in lung cancer cells by regulating miR-516b-5p/STAT3 to inhibit cisplatin sensitivity. Biotechnol. Lett. 2020;42:1123–1135. doi: 10.1007/s10529-020-02846-9. [DOI] [PubMed] [Google Scholar]
- 311.Jin C., Dong D., Yang Z., Xia R., Tao S., Piao M. circMYC regulates glycolysis and cell proliferation in melanoma. Cell Biochem. Biophys. 2020;78:77–88. doi: 10.1007/s12013-019-00895-0. [DOI] [PubMed] [Google Scholar]
- 312.Wu W., Xi W., Li H., Yang M., Yao X. Circular RNA circACACA regulates proliferation, migration and glycolysis in nonsmallcell lung carcinoma via miR1183 and PI3K/PKB pathway. Int. J. Mol. Med. 2020;45:1814–1824. doi: 10.3892/ijmm.2020.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xu Y., Jiang T., Wu C., Zhang Y. circAKT3 inhibits glycolysis balance in lung cancer cells by regulating miR-516b-5p/STAT3 to inhibit cisplatin sensitivity. Biotechnol. Lett. 2020;42:1123–1135. doi: 10.1007/s10529-020-02846-9. [DOI] [PubMed] [Google Scholar]