Abstract
Background
Obstructive sleep apnea (OSA) is a common form of sleep-disordered breathing with high prevalence and associated co-morbidities. It still goes largely under-reported due to events occurring in sleep and difficulty in identifying predisposing factors.
Aims
To perform questionnaire-based screening of OSA-risk in adolescents and study association of OSA-risk with craniofacial and upper airway morphology.
Material and methods
Modified STOP-BANG questionnaire was used for screening OSA-risk in adolescent orthodontic patients (10–19 years) in a government dental hospital in India. Patients were categorised into two groups: OSA-risk and non-risk, based on the questionnaire scores, and were subsequently evaluated for craniofacial and upper airway morphology, both on examination and on lateral cephalometric radiographs.
Results
Documented a high prevalence of 14% for OSA-risk in adolescent orthodontic patients. The extra-oral and intra-oral parameters found significantly associated with OSA-risk were convex profile [Odd's ratio (OR) - 3.824], steep mandibular plane angle [MPA] (OR- 79.75), Type 3/4 faucial pillars (OR- 11.227), Class II molar relationship (OR - 4.518), ovoid upper arch form (OR - 13.750). In addition, the cephalometric parameters: ANB (p– 0.025), SN-MP (p– 0.007), BA-SN (p– 0.020), PNS-AD1 (p < 0.001), PNS-AD2 (p – 0.001) also showed highly significant association to OSA-risk. The ROC curves demonstrated high sensitivity and specificity for PNS-AD1 (60%,83.3%), PNS-AD2 (73.3%, 70%) and SN-MP (60%,70%), respectively for OSA-risk.
Conclusions
The study supported applicability of modified STOP-BANG questionnaire for OSA-risk in Indian adolescents. The parameters [extra-oral, intra-oral, cephalometric and upper airway (PNS-AD1, PNS-AD2, SN-MP)] significantly associated with OSA-risk, were identified.
Keywords: Obstructive sleep apnea, Modified STOP-BANG, Craniofacial characteristics, Malocclusion
1. Introduction
Obstructive sleep apnea (OSA), a common form of sleep-disordered breathing has become a major public health challenge today, affecting all age groups. The reported prevalence of OSA is 4–9% in adults worldwide, and 2.1–4.9% in North Indian adults.1, 2, 3 In 6-15-year-old children, the prevalence is reported at 9.5%.1, 2, 3 OSA in adults is associated with severe cardiovascular, neurocognitive, and metabolic complications. Additionally, in children and adolescents, growth abnormalities, academic failure, behavioural and cognitive impairment have also been reported.4,5 Predisposing factors identified for OSA in adults include obesity, age, male sex, smoking, craniofacial, and upper airway morphological abnormalities.1 In very young children, 3-6 year-old, adenoid and tonsillar hypertrophy maybe an additional predisposing factor for OSA, with associated mouth breathing and snoring at 1.5–27.6% prevalence.5 A recent study on orthodontic patients by Abtahi S et al. (2020)6 found a high prevalence of 10.8% for paediatric OSA. The patients in this study reported with snoring and sleepiness, and associated comorbidities of obesity (18.2%), nocturnal enuresis (13.6%), and attention deficit hyperactivity disorder (31.8%).6
Despite the high prevalence, OSA remains highly under-reported due to hypopnea events occurring in sleep. Also, its gold standard for diagnosis, polysomnography (PSG), is costly and technique-sensitive and mostly unavailable in primary health centres. Hence, various questionnaires are used alternatively for screening OSA-risk (Berlin, STOP, STOP-BANG, etc.) which are more cost-effective and easily available.6 Of these, the modified STOP-BANG questionnaire in adolescents has reported high sensitivity and specificity of 64% and 82% respectively with a negative predictive value of 0.96.7 Since the majority of orthodontic patients are adolescents, this may be the preferred questionnaire in orthodontic practice.7,8
The current study aimed to assess OSA-risk using the modified STOP-BANG questionnaire in orthodontic patients and also correlate its findings with the predisposing craniofacial, malocclusion, and upper airway parameters. This study is first of its kind in Indian population and thus addresses a huge gap in the literature.
2. Material and Methods
This prospective, cross-sectional hospital-based study was performed after obtaining institutional ethical clearance, as part of the funded ICMR-STS project,2019 (IEC-18/3/216/JMI/ IEC /2019, dated May 21, 2019). Blinding of subjects and data entry for statistical evaluation was done by operator SB while questionnaire screening and scoring of the subjects along with an intra-oral, extra-oral examination and cephalometric evaluation of patients was done by operator LA.
The methodology outlined in Flowchart 1, had three basic components:
2.1. Screening by modified STOP-BANG questionnaire
The study was designed to screen OSA -risk in 10-19-year-old adolescent patients who reported to Orthodontic OPD of a Govt Dental Hospital for two months. Subjects, both male and female were recruited with the following inclusion criteria:
-
•
Patients 10–19 years old requiring fixed orthodontic treatment.
-
•
Healthy children with no reported growth abnormalities/syndromes.
The subjects were screened using the modified STOP-BANG questionnaire (Table 1). The components of the questionnaire were filled partly by the patients/guardians and partly by the operator. The elements of questionnaire-snoring, tiredness, academic problems, gender were answered by patient/guardian while the measurements of blood pressure (BP), height and weight for basal metabolic index (BMI), and neck circumference (NC) were done by operator LA. The values for anthropometric variables were evaluated for 95th percentile according to standardized measurements.9, 10, 11, 12
The responses to the questionnaire generated a score for each patient, which subsequently divided them into two groups:
-
•
Group 1: Non-OSA-risk (Score ≤2)
-
•
Group 2: OSA-risk (Score 3–8)
Recruitment of subjects was done till 30 subjects were included in each group.
2.2. Clinical and Cephalometric Evaluation
For both Group 1 and 2, an extra-oral and intra-oral examination were performed as per Table 2. Standardized lateral cephalograms were taken as a part of routine orthodontic records with digital CS 2200- Carestream Health® machine at 30 mA and 70 kVp with the head of the participants in natural head position (NHP) and teeth in maximum intercuspation. The radiographic films were traced using RK LED X-Ray View. The data was entered according to Table 1.
Table 2.
Comparison of demographic and clinical predictors between OSA-risk and non- OSA-risk along with statistical methods used.
| OSA | Non-OSA | Statistical test used | P-value | |
| Gender [n (%)] | ||||
| Male | 11 (36.7) | 10 (33.3) | Chi-square | 0.787 |
| Female | 19 (63.3) | 20 (66.7) | ||
| Profile | ||||
| Convex | 25 (83.3) | 17 (56.7) | Fisher exact test | 0.015* |
| Concave | 3 (10.0) | 2 (6.7) | ||
| Straight | 2 (6.7) | 11 (36.7) | ||
| Frontal examination | ||||
| Short lower face | 3 (10.0) | 5 (16.7) | Fisher exact test | 0.226 |
| Normal | 22 (73.3) | 24 (80.0) | ||
| Long lower face | 5 (16.7) | 1 (3.3) | ||
| Mandibular plane | ||||
| Flat | 0 (0) | 6 (20.0) | Fisher exact test | <0.001** |
| Normal | 8 (26.7) | 23 (76.7) | ||
| Steep | 22 (73.3) | 1 (3.3) | ||
| Faucial pillars & soft palate | ||||
| Type I | 2 (6.7) | 6 (20.0) | Fisher exact test | <0.001** |
| Type II | 2 (6.7) | 13 (43.3) | ||
| Type III | 17 (56.7) | 9 (30.0) | ||
| Type IV | 9 (30.0) | 2 (6.7) | ||
| Shape of upper arch | ||||
| Tapered | 1 (3.3) | 1 (3.3) | Fisher exact test | <0.001** |
| Ovoid | 22 (73.3) | 5 (16.7) | ||
| Square | 7 (23.3) | 24 (80.0) | ||
| Shape of lower arch | ||||
| Tapered | 9 (30.0) | 14 (46.7) | Chi-square | 0.390 |
| Ovoid | 8 (26.7) | 7 (23.3) | ||
| Square | 13 (43.3) | 9 (30.0) | ||
| Molar classification | ||||
| Class I | 4 (13.3) | 18 (60.0) | Fisher exact test | <0.001** |
| Class II div 1 | 21 (70.0) | 11 (36.7) | ||
| Class II div 2 | 3 (10.0) | 0 (0) | ||
| Class III | 2 (6.7) | 1 (3.3) | ||
| Cephalometric Analysis (Mean ± SD) | ||||
| SNA | 82.9 ± 4.5 | 82.8 ± 3.8 | Student t-test | 0.903 |
| SNB | 78.7 ± 4.9 | 79.5 ± 4.0 | Student t-test | 0.506 |
| ANB | 4.3 ± 2.9 | 2.8 ± 2.2 | Mann Whitney U test | 0.025* |
| SN-MP | 35.4 ± 5.5 | 31.2 ± 6.1 | Mann Whitney U test | 0.007* |
| PP-MP | 23.5 ± 7.5 | 22.2 ± 5.3 | Mann Whitney U test | 0.463 |
| IMPA | 100.6 ± 8.9 | 97.8 ± 6.9 | Student t-test | 0.068 |
| BaSN | 129.4 ± 6.3 | 133.2 ± 6.1 | Student t-test | 0.020 |
| PNS-AD1 | 16.5 ± 3.8 | 20.2 ± 3.4 | Mann Whitney U test | <0.001** |
| PNS-AD2 | 13.7 ± 3.6 | 17.2 ± 3.9 | Mann Whitney U test | 0.001** |
Table 1.
Clinical performa.
Part A: Modified STOP Bang questionnaire, the score of 0-2 is Non OSA risk and ≥3 (3-8) is OSA risk.
Part B: Depicts craniofacial, extra-oral and intra-oral parameters studied in OSA-risk and non-OSA-risk patients.
| Reference ID: Age/Sex: Date: |
| Part A: Questionnaire |
| Q1. Do you/your ward snore? |
| Q2. Are you/your ward tired during day? |
| A. Don't know C. Rarely E. Frequently |
| B. Never D. Occasionally F. Almost always |
| Q3. Do you/your ward wake up or stop breathing during sleep? |
| A. Don't know C. Rarely E. Frequently |
| B. Never D. Occasionally F. Almost always |
| Q4. Is your/your ward BP ≥ 95th percentile for age and height? |
| A. Yes B. No |
| Q5. Is your/your ward BMI ≥95th percentile for age? |
| A. Yes B. No |
| Q6. Are you/your ward having learning problems? |
| A. Don't know C. Rarely E. Frequently |
| B. Never D. Occasionally F. Almost always |
| Q7. Is your/your ward neck circumference ≥95th percentile for age? |
| A. Yes B. No |
| Q8. Are you/your ward male? |
| A. Yes B. No |
| Total score: …. /8 |
| Group 1: Non OSA risk (Score≤2) Group 2: OSA risk (Score 3–8) |
| Part B: Clinical Parameters |
| Group: A/B Reference ID: |
| Extra-oral Examination |
| 1. Profile |
| A. Convex B. Straight C. Concave |
| 2. Frontal examination:Lower face |
| A. Short B. Normal C. Long |
| 3. Mandibular plane angle |
| A. Flat B. Normal C. Steep |
| Intra-oral Examination |
| 4.Examination of faucial pillars and soft palate: Mallampati classification |
| A. Type I B. Type II C. Type III D. Type IV |
| 5. Shape of upper arch |
| A. Tapered B. Ovoid C. Square |
| 6. Shape of lower arch (Tapered/ovoid/square) |
| A. Tapered B. Ovoid C. Square |
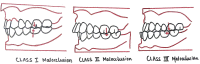
| 7. Molar relation: Angle's Classification |
| A. Class I B. Class II Div 1 C. Class II Div 2 D. Class III |
| Cephalometric parameters |
| 8. SNA (maxilla in relation to Cranial Base): … … … …degrees |
| A. High B. Average C. Low |
| 9. SNB (Mandible in relation to Cranial Base)): … … … …degrees |
| A. High B. Average C. Low |
| 10. ANB (Maxilla in relation to Mandible)): … … … …degrees |
| A. High B. Average C. Low |
| 11. SN-MP(Mandibular plane angle)- Cranium in relation to mandible): … … … …degrees |
| A. High B. Average C. Low |
| 12. PP-MP (Palatal plane in relation to Mandibular plane)): … … … …degrees |
| A. High B. Average C. Low |
| 13. IMPA (Lower incisor inclination to Mandibular plane): … … … …degrees |
| A. High B. Average C. Low |
| 14. BaSN (Basion in relation to Cranial Base)): … … … …degrees |
| A. High B. Average C. Low |
| Upper airway morphology |
| 15. PNS-AD1: … …mm |
| A. High B. Average C. Low |
| 16. PNS-AD2 (mm):: … …mm |
| A. High B. Average C. Low |
 |
 |
2.3. Statistical evaluation
All statistical analysis was performed using SPSS, version 21.0. Armonk, NY: IBM Corp. A comparison of categorical variables between the groups was done by the Chi-square test and of continuous variables between the groups by Independent t-test or Mann Whitney U test. Binary logistic regression was used to find predictors of OSA and the ROC curve was used for diagnostic accuracy of these predictors.
3. Results and observations
A total of 213 subjects were screened in two months with modified STOP-BANG questionnaire to obtain 30 subjects in each group, OSA-risk and Non-OSA-risk. The prevalence of OSA-risk was recorded at 14%. The mean age of participants in the current study was 16±3.4 years with 65% female and 35% males. The age-wise division of the patient samples could be majorly classified into two categories:
-
•
10–14years: OSA-risk (n = 5), Non-risk (n = 9)
-
•
15–19 years: OSA-risk (n = 25), Non-risk (n = 21)
The detailed datasets used and/or analysed during the current study can be made available from the corresponding author on communication.
The results comparing the two groups demonstrated highly significant association of the following parameters with OSA-risk (Table 2):
-
•
Association of convex profile significantly higher in OSA-risk (83.3%) than non-OSA-risk (56.0%). (p ≤ 0.015)
-
•
Association of steep mandibular plane angle significantly higher in OSA-risk (73.3%) than non-OSA-risk (3.3%). (p < 0.001)
-
•
Association of Type III/IV faucial pillars significantly higher in OSA-risk (86.6%) than non-OSA-risk (46.7%). (p < 0.001)
-
•
Association of ovoid upper arch form significantly higher in OSA-risk (73.3%) than non-OSA-risk (16.7%). (p < 0.001)
-
•
Association of Class II molar relationship significantly higher in OSA-risk (80%) than non-OSA-risk (36.7%). (p < 0.001). No distinction was made in different divisions of Class II molar relationship as all Class II non-OSA-risk subjects had Class II division 1 malocclusion.
-
•
Cephalometrically, the upper airway morphology showed a significant difference between OSA-risk and non-risk. Parameters PNS-AD1 and PNS-AD2 exhibit statistically significant difference of 3.7 and 3.43 respectively (p < 0.001) between OSA-risk and Non-OSA-risk. Other cephalometric variables which exhibit higher values in OSA-risk than non-risk but are not highly significant are ANB angle (greater by 1.44°, p < 0.025) and SN-MP angle (greater by 4.2°, p < 0.007).
3.1. Receiver operator characteristic (ROC) curve
ROC was used to determine the cut-off for the predictors for OSA-risk; ANB angle ≥3.5° with the area under the curve (AUC) 0.702 given sensitivity 60% and specificity 86.7%, SN-MP angle≥35° with AUC 0.681 given sensitivity 60% and specificity 70%, PNS-AD1 ≤18 mm with AUC 0.763 given sensitivity 60% and specificity 83.3% and PND-AD2 ≤ 17 mm with AUC 0.741 given sensitivity 73.3% and specificity 70%. The AUC 0.647 for Ba-SN ≤133 was non-significant, given sensitivity 66.7% and specificity 50%.
Binary logistic regression for predictors of OSA-risk shows 7.2 times odds of convex profile compared to concave and straight profile, 79.8 times odds of steep mandibular plane compared to flat or normal plane, 11.2 times odds of Type III/IV faucial pillars compared to Type I/II faucial pillars, 13.8 times odds of ovoid upper arch compared to tapered or square arch form. In addition, change of Class I to Class II molar relationship showed 4.5 times greater odds for OSA-risk. The cephalometric angles and distances which showed greater odds for OSA-risk were: ANB angle≥ 3.5° showed 4.75 times greater odds, SN-MP angle ≥35° showed 3.5 times greater odds, Ba-SN angle ≤133° showed 2.0 times greater odds, PNS-AD1 ≤18 mm showed 5.0 times greater odds, and PNS-AD2≤17 mm showed 6.47 times greater odds. All the independent variables were significant predictors for OSA except Ba-SN, as given in the forest plot (Fig. 1).
Fig. 1.
Forest plot for predictors of OSA.
4. Discussion
The current study has identified a prevalence of 14% for OSA-risk in adolescent orthodontic patients using the modified STOP-BANG questionnaire. The reported questionnaire-based prevalence in our study on 10-19-year-old patients is higher than that reported in a questionnaire-based study by Tsukada E et al. (2018)3 on 25,211 Japanese children (mean age-10.39 years), which reported a prevalence of 9.5%. The variation can be attributed to different ethnicity of populations and a smaller sample size in the current study. The OSA-risk prevalence in orthodontic patients of our study is also greater than the orthodontic paediatric OSA patients in a recent study by Abtahi S et al. (2020),6 which reported a prevalence of 10.8%. The probable explanation of this finding can be the stage of adolescence or late adulthood of the orthodontic patients in our study, which according to the classic growth curves of Scammon, is the stage where lymphoid tissues (tonsils and adenoids) show growth and enlargement by 200% approximately, followed by involution in adulthood.13 It is known that tonsillar and adenoid hypertrophy, can further compromise the airway, cause obstruction, leading to mouth breathing and snoring and hence, OSA-risk. The age-dependent hypertrophy and involution of lymphoid tissues is also proven in an observational longitudinal study by Ishida T et al. (2018),13 on lateral cephalograms of 6-20-year-old Japanese children. They reported a decrease in size of lymphoid tissues from lower primary school (age: 8.21 ± 0.96 years), with average values 347.55 ± 12.52 mm2, 161.34 ± 9.54 mm2 to the stage of young adults (18.86 ± 1.08 years) with average values 274.48 ± 13.03 mm2, 110.97 ± 8.19 mm2 respectively.
The choice of the questionnaire for assessment of OSA-risk in the current study was based on the age of the target population (adolescents) routinely seeking orthodontic care. The results of our study show that majority of patients, both OSA-risk and non-risk fell in the age group of 15–19 years, with a mean age of 16 ± 3.4 years. The questionnaire, thus chosen for the current study, was the modified STOP-BANG questionnaire, which reported a high sensitivity of 93.8% in adolescents.8 The sensitivity of the modified SBQ was comparable to STOP- BANG questionnaire (SBQ), which according to a recent systematic review by Amra B et al.(2018), has a high sensitivity in predicting mild and severe OSA in adults (97.55% and 98.7%, respectively) as compared to other questionnaires (STOP, Berlin and Epworth Sleepiness Scale).7 The same questionnaire has also been recommended for screening OSA-risk in routine orthodontic practice for adult patients in a recent article by Behrents et al. (2019).14
The study by Behrents et al. (2019)14 also supports the predisposition of variation in craniofacial morphologies to OSA. These parameters include vertical growth, steep mandibular plane angle, long and narrow face, mandibular retrognathism, narrow and deep palate, anterior open bite and lower hyoid position.14 However, they emphasize that the strength of the relationship of these parameters with development of OSA requires further exploration and hence, justifies the objectives of our study. The results of the current study also show a significant association of OSA-risk to specific features like convex profile (p ≤ 0.015) and steep mandibular plane angle when compared to non-OSA-risk (p < 0.001). A meta-analysis (MA) by Neelapu B et al. (2017)1 evaluating craniofacial disharmony on lateral cephalograms in association with OSA also confirmed similar findings. It showed a significant increase in anterior face height along with an overall significant increase in the SN-MP angle (p = 0.00003) in OSA compared to non-OSA. Another important finding of this MA was significant reduction in PNS-Phw (in mm) (p < 0.0006) and pharyngeal space (oropharynx and pharyngeal area) in OSA patients (p < 0.0001)1 which was in direct accordance with reduced upper airway measurements (PNS- AD1 and PNS- AD2) in OSA-risk patients of our study (p < 0.001). A decrease in angular cranial base measurements (BA-SN) in OSA-risk patients of our study (p = 0.02) also found support in MA results where BA-SN difference between OSA and Non-OSA was significant (p = 0.003).1 This can be explained on the basis of reduced cranial base angle (BA-SN) correspondingly reducing cranial base flexion, further causing a decrease in sagittal airway dimensions and forward positioning of the cervical spine and posterior pharyngeal wall.15
The skeletal and dental malocclusion in our study revealed a significantly higher prevalence of Class II molar relationship in OSA-risk (80%) compared to non-OSA-risk (36.7%) (p < 0.001) and a cephalometric ANB angle greater by 1.44 degrees (p < 0.025) in the former compared to latter. This finding is supported by Capistrano et al. (2015)16 who correlated the facial morphologic patterns with worsened apnea-hypopnea (AHI) index, specifically in pattern II presenting with a convex profile, Class II molar relationship and increased ANB angle.16 Our study found the dental parameters of Class II molar relationship and an ovoid upper arch form resulting from transverse maxillary constriction to be significantly associated with OSA-risk (p < 0.0001). Similar results were observed in a study by Scanone A et al. (2017)17 who measured dental casts and lateral cephalograms of 60 OSA-risk individuals, average age 26 years and found Class II malocclusion to have 1.5 times greater chance of OSA-risk compared to Class I malocclusion with a statistically significant association (OR:2.5 CI 95%: 1.01–6.19; p = 0.048). However, they did not show a significant difference in transverse maxillary measurements, which was contradictory to the findings of our study. Our study has also found a significant association of Mallampati score with OSA-risk, resulting in a greater percentage of Type III and type IV faucial pillars in OSA-risk (86.6%) than non-OSA risk (46.7%) (p < 0.001) and 11.2 times odds of Type III/IV compared to Type I/II faucial pillars. The application of this score was originally used by anaesthesiologists for assessing difficulty in intubation but has also been used for predicting sleep apnea in a study on 137 suspected OSA adults by Nuckton TJ et al. (2006).18 In this study, the odds of OSA (apnea-hypopnea index ≥ 5) increased to greater than two folds with every 1-point increase in Mallampati score.
The facial phenotype in sleep apnea also seems to be influenced by upper airway anatomy due to shared embryological origins and a reciprocal effect of hard and soft tissue airway dimensions. Hence, the craniofacial parameters like mandibular retrognathism, Class II skeletal pattern, convex profile, increased SN- MP angle were found to be significantly correlated to OSA-risk in our study. A study by Sutherland et al. (2014)19 on Icelandic cohort of 822 individuals and a magnetic resonance imaging (MRI) of upper airways confirmed significant association of maxilla-mandibular relationship (r = 0.8, p < 0.0001), lower facial height (r = 0.76, p < 0.0001), mandibular length (r = 0.67, p < 0.0001) and upper airway measurements with AHI. Additionally, Lee RW et al. (2009)20 confirmed these findings on photographic analysis of extra-oral craniofacial patterns in Caucasian OSA patients versus controls. Their association of mandibular retrognathism or backwardly placed mandible with OSA, further verified the craniofacial associations of our study with OSA-risk.20
The craniofacial parameters measured in our study have been evaluated by physical examination and lateral cephalogram measurements. Previous studies have proved cephalometric analysis can be used as a complementary measure to assess OSA-risk, depicting reasonable accuracy with 93% sensitivity and 21% specificity.17 Also, since PSG, the gold standard for OSA confirmation is expensive and time-consuming, it makes questionnaires and lateral cephalograms a viable, cost effective, easily available, and more practical option for screening OSA-risk. In recent years, however, 3-dimensional cone-beam computed tomography (3-D CBCT) has been rated better in visualization and image processing of airway, but due to the higher cost and radiation dose compared to 2-D cephalograms, 3-D CBCT is still not as popular as its 2-D counterpart. A recent study by Hsu WE et al. (2019)21 compared lateral cephalogram images taken in an upright position with lateral cephalograms generated from 3D-CBCT in a supine position, for the most constricted portion of the pharynx and mandibular plane to the hyoid bone (MP-H). They found no difference in pharyngeal measurements but MP-H found a significant difference in upright and supine radiographs (p = 0.000). Hence this airway parameter must be used with caution in airway studies being conducted in 2-D lateral cephalograms.21 Lateral cephalometric studies have been conducted widely in OSA and have formulated the basis for evidence based research in the form of systematic reviews (SR) and MA, both in adults and paediatric OSA.1,22
A meta-analysis by Katyal V et al. (2013)22 on lateral cephalometric parameters in paediatric OSA documented a significant association of reduced PNS- AD1 and PNS- AD2 distance with OSA. This was incidentally the most sensitive diagnostic factor of OSA-risk in our study with a sensitivity of 60% for PNS-AD1 and 73.3% for PNS-AD2, according to the ROC curves. Additionally, 3D-CBCT studies also potentiate the results of 2-D cephalometric studies. A recent CBCT study by Gahlot M (2019),23 done in 15 North Indian patients also showed an association of OSA with increased cranio-cervical angulation, gonial angle, saddle angle indicating vertical growth pattern, and greater ANB angle due to mandibular retrognathism. These findings corroborated our results on 2-D cephalogram as indicated by higher ANB and SN-MP with the ROC curve showing a true positive of 60% and false negative of 70%. The cross-sectional airway area also showed severe reduction at the retro-palatal region in 3D-CBCT, as substantiated by reduced PNS-AD1 and PNS-AD2 in the current study.23 Another recent CBCT study by Iwasaki et al. (2019)24 potentiated the role of hyoid bone placement in association with skeletal maxilla-mandibular relations. This was characterized by increased ANB angle, observing a positive correlation between the two and thus affecting the pharyngeal airway bone.24 However hyoid bone placement was not considered in the present study, which may be a limitation. Another CBCT study by Haskell JA et al. (2014)25 which measured the distance of hyoid bone to the cervical vertebral column in OSA also supports retrognathic mandible and vertical skeletal facial morphology as the cause for potential airway apnea, which was recorded in our study as increased ANB angle and SN-MP angle.25
However, our study has few limitations, the sample size was small with an unequal male to female ratio. Although the modified STOP-BANG questionnaire used in the current study has high sensitivity and specificity for OSA-risk in adolescents, no comparison with any other questionnaire has been done. No confirmation of OSA with PSG has been done after screening OSA-risk. Cost limitation and ethical issue limitation in performing 3D-CBCT for all orthodontic patients (both OSA-risk and non-OSA-risk) restricted our study and access to 2D-cephalograms. Nevertheless, the results of our study have documented significant associations of craniofacial morphology and airway with OSA-risk and have found support in both two and three-dimensional radiographic outcomes in previous literature. Hence, future research can be planned after addressing the limitations of the current study.
5. Conclusions
Extraoral and intra-oral parameters significantly associated with OSA-risk are convex profile, steep mandibular plane, type 3/4 faucial pillars, Class II molar relation, and ovoid upper arch. The ROC curves demonstrated high sensitivity and specificity for cephalometric parameters of PNS-AD1 (60%,83.3%), PNS-AD2 (73.3%, 70%) and SN-MP (60%,70%), respectively for OSA-risk.
Funding
This project is funded by the Indian Council of Medical Research (ICMR)–STS program 2019–20 (ID: 2019-05866).
References
- 1.Neelapu B.C., Kharbanda O.P., Sardana H.K. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients -a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90. doi: 10.1016/j.smrv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S.K., Kupawat S., Banga A., Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi India. Chest. 2006;130(1):149–156. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada E., Kitamura S., Enomoto M. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal A., Pakhare A.P., Bhatt G.C., Choudhary B., Patil R. Association of pediatric obstructive sleep apnea with poor academic performance: a school-based study from India. Lung India. 2018;35:132–136. doi: 10.4103/lungindia.lungindia_218_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzini M.F., Di Francesco R.C. Managing obstructive sleep apnoea in children: the role of craniofacial morphology. Clinics. 2016 Nov 1;71(11):664–666. doi: 10.6061/clinics/2016(11)08. PMID: 27982168; PMCID: PMC5108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abtahi S., Witmans M., Alsufyani N.A., Major M.P., Major P.W. Pediatric sleep-disordered breathing in the orthodontic population: prevalence of positive risk and associations. Am J Orthod Dentofacial Orthop. 2020;157(4):466–473. doi: 10.1016/j.ajodo.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Amra B., Rahmati B., Soltaninejad F., Feizi A. Screening questionnaires for obstructive sleep apnea: an updated systematic review. Oman Med J. 2018;33(3):184–192. doi: 10.5001/omj.2018.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combs D., Goodwin J.L., Quan S.F., Morgan W.J., Parthasarathy S. Modified STOP-bang tool for stratifying obstructive sleep apnea risk in adolescent children. PLoS One. 2015;18 10(11) doi: 10.1371/journal.pone.0142242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Öztürk N.A.A., Dilektaşlı A.G., Çetinoğlu E.D., Ursavaş A., Karadağ M. Diagnostic accuracy of a modified STOP-BANG questionnaire with national anthropometric obesity indexes. Turk Thorac J. 2019;20(2):103–107. doi: 10.5152/TurkThoracJ.2018.18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lande M., Varade W. Blood pressure – performing preventive services: in A bright futures handbook. https://www.yumpu.com/en/document/view/8882198/physical-examination-bright-futures-american-academy-of-
- 11.Kuczmarski R.J., Ogden C.L., Grummer-Strawn L.M. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 12.Katz S.L., Vaccani J.P., Clarke J., Hoey L., Colley R.C., Barrowman N.J. Creation of a reference dataset of neck sizes in children: standardizing a potential new tool for prediction of obesity-associated diseases? BMC Pediatr. 2014;14:159–167. doi: 10.1186/1471-2431-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida T., Manabe A., Yang S.S., Yoon H.S., Kanda E., Ono T. Patterns of adenoid and tonsil growth in Japanese children and adolescents: a longitudinal study. Sci Rep. 2018 Nov 20;8(1):17088. doi: 10.1038/s41598-018-35272-z. PMID: 30459413; PMCID: PMC6244207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrents R.G., Shelgikar A.V., Conley R.S. Obstructive sleep apnea and orthodontics: an American association of orthodontists white paper. Am J Orthod Dentofacial Orthop. 2019;156:13–28. doi: 10.1016/j.ajodo.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg B., Fraser B. The cranial base in obstructive sleep apnea. J Oral Maxillofac Surg. 1995;53:1150e4. doi: 10.1016/0278-2391(95)90621-5. [DOI] [PubMed] [Google Scholar]
- 16.Capistrano A., Cordeiro A., Capelozza Filho L. Facial morphology and obstructive sleep apnea. Dent press J Orthod. 2015;20(6):60–67. doi: 10.1590/2177-6709.20.6.060-067.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scannone A., Tosta M., Suarez A., Otero L. Cephalometric and dental measures as diagnostic tools for the obstructive sleep apnea. J Sleep Disor Treat Care. 2017;6(4) doi: 10.4172/2325-9639.1000202. [DOI] [Google Scholar]
- 18.Nuckton T.J., Glidden D.V., Browner W.S., Claman D. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29(7):903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland K., Schwab R.J., Maislin G. Facial phenotyping by quantitative photography reflects craniofacial morphology measured on magnetic resonance imaging in Icelandic sleep apnea patients. Sleep. 2014 May 1;37(5):959–968. doi: 10.5665/sleep.3670. PMID: 24790275; PMCID: PMC3985099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee R.W., Chan A.S., Grunstein R.R., Cistulli P.A. Craniofacial phenotyping in obstructive sleep apnea--a novel quantitative photographic approach. Sleep. 2009;32(1):37–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu W.E., Wu T.Y. Comparison of upper airway measurement by lateral cephalogram in upright position and CBCT in supine position. J Dent Sci. 2019;14(2):185–191. doi: 10.1016/j.jds.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katyal V., Pamula Y., Martin A.J., Daynes C.N., Kennedy J.D., Sampson W.J. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2013;143(1):20–30. doi: 10.1016/j.ajodo.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Gahlot M. A CBCT study to assess the volumetric analysis of airway, craniofacial morphology and cervical vertebral fusion anomalies in North Indian patients with obstructive sleep apnea. EC Dental Science. 2019;2:303–320. [Google Scholar]
- 24.Iwasaki T., Suga H., Yanagisawa-Minami A. Relationships among tongue volume, hyoid position, airway volume and maxillofacial form in paediatric patients with Class-I, Class-II and Class-III malocclusions. Orthod Craniofac Res. 2019;22:9–15. doi: 10.1111/ocr.12251. [DOI] [PubMed] [Google Scholar]
- 25.Haskell J.A., Haskell B.S., Spoon M.E., Feng C. The relationship of vertical skeleton-facial morphology to oropharyngeal airway shape using cone beam computed tomography: possible implications for airway restriction. Angle Orthod. 2014;84(3):548–554. doi: 10.2319/042113-309.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



