Abstract
Background & Aims
Primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC) and autoimmune hepatitis (AIH) are phenotypically distinct autoimmune liver diseases that progress to cirrhosis and liver failure; however, their histological fibrosis distribution differs. We investigated the extracellular matrix (ECM) profiles of patients with PSC, PBC, and AIH to establish whether the diseases display differential patterns of ECM turnover.
Methods
Serum samples were retrospectively collected from the UK (test cohort; PSC n = 78; PBC n = 74; AIH n = 58) and Norway (validation cohort; PSC n = 138; PBC n = 28; AIH n = 27). Patients with ulcerative colitis without liver disease (n = 194) served as controls. We assessed specific serological biomarkers of ECM turnover: type III and V collagen formation (PRO-C3, PRO-C5), degradation of type III and IV collagen (C3M, C4M), biglycan (BGM) and citrullinated vimentin (VICM).
Results
Most of the ECM markers showed elevated serum levels in PBC compared with PSC or AIH (p <0.01). PRO-C3 correlated well with liver stiffness and showed the most striking differences between advanced and non-advanced liver disease; several of the other ECM markers were also associated with stage. PRO-C3 and other ECM markers were inversely associated with ursodeoxycholic acid treatment response in PBC and remission in AIH. All ECM remodelling markers were significantly elevated (p <0.05) in patients with PSC, PBC, or AIH compared with ulcerative colitis.
Conclusions
In this first study comparing ECM turnover in autoimmune liver diseases, we found increased ECM turnover in PBC compared with either PSC or AIH. The study indicates that ECM remodelling is different in PSC, PBC, and AIH, suggesting differing opportunities for therapeutic intervention.
Lay summary
The level of scarring is linked to prognosis in autoimmune liver diseases such as primary sclerosing cholangitis, primary biliary cholangitis, and autoimmune hepatitis; hence, the scarring process is a possible target for novel therapy. Investigating the scarring process using highly specific technology, we show that the scarring process is different between the 3 autoimmune liver diseases, and this may have important implications for the development of medical treatment.
Keywords: Biomarker, Fibrosis, Primary sclerosing cholangitis, Primary biliary cholangitis, PRO-C3
Abbreviations: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; APRI, AST to platelet ratio index; AUROC, area under the receiver operator characteristics curve; BGM, marker of biglycan degradation; C3M, marker of type III collagen degradation; C4M, marker of type IV collagen degradation; CI, confidence interval; ECM, extracellular matrix; ELF, enhanced liver fibrosis; GGT, gamma glutamyltransferase; HYA, hyaluronic acid; IBD, inflammatory bowel disease; INR, international normalised ratio; LSM, liver stiffness measurement; PBC, primary biliary cholangitis; PIIINP, N-terminal procollagen type III; PRO-C3, marker of type III collagen formation; PRO-C5, marker of type V collagen formation; PSC, primary sclerosing cholangitis; TE, transient elastography; TIMP-1, tissue inhibitor of metalloproteinase; UC, ulcerative colitis; VICM, marker of citrullinated vimentin degradation
Graphical abstract
Highlights
-
•
Serological biomarkers specifically targeting extracellular matrix remodelling enable evaluation of the dynamics of fibrosis evolution.
-
•
ECM turnover was increased in PBC compared with PSC and AIH.
-
•
ECM markers, particularly PRO-C3, were associated with disease stage in the autoimmune liver diseases and with clinical outcome in PSC.
Introduction
The autoimmune liver diseases primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH) share scientific and clinical challenges. In PSC, an incomplete comprehension of the pathogenesis and a lack of validated tools to evaluate effect of novel treatment, have hindered the development of improved therapy. Currently, PBC patients are treated with ursodeoxycholic acid which successfully halts disease progression in the majority whereas a substantial group of 30–40% of ursodeoxycholic acid non-responders exist and progress; AIH patients receive immune modulating therapy which induces remission in the majority but maintain a 10 times increased risk of progression to liver transplantation or death compared with the general population. Thus, a substantial number of patients with each of the 3 diseases progress to cirrhosis and liver failure and, although each is rare, collectively, they represent an important indication for liver transplantation. A better comprehension of the pathways driving each individual disease might facilitate the successful development of highly warranted effective therapies.[1], [2], [3]
Biomarkers of fibrosis have demonstrated utility in the prediction of prognosis in a spectrum of chronic liver diseases, including the autoimmune liver diseases, PBC, PSC, and AIH. Histological liver fibrosis stage was demonstrated to predict clinical outcomes but is currently not recommended in standard diagnostics in PBC and PSC.4,5 Non-invasive markers of fibrosis offer better opportunity for repeated assessments over time and have been reported as effective risk stratifiers for clinical outcomes. In PBC and PSC, independently validated biomarkers include the enhanced liver fibrosis (ELF) test, a serum marker panel, and liver stiffness measurement (LSM) using transient elastography (TE; Fibroscan, Echosens, Paris, France).[6], [7], [8], [9], [10] However, these tools, developed for chronic liver diseases in general, are usually considered as measures of fibrosis load and may not capture the diversity in fibrosis distribution and progression rate between the autoimmune liver diseases reflecting differences in pathogenesis, nor the dynamic process of fibrogenesis and fibrosis degradation and remodelling (reflecting disease activity) at any given stage of fibrosis. Serological biomarkers specifically targeting the extracellular matrix (ECM) remodelling may better assess these variations and dynamics.
We hypothesised that valuable additional information about the dynamics of fibrosis evolution in autoimmune liver disease would be provided by estimating the ECM turnover profile using serological biomarkers specifically targeting the ECM remodelling, differentiating between the formation and degradation processes related to the various compartments (the interstitial matrix and the basement membrane). New knowledge regarding the differences in pathogenesis underlying PSC and the other autoimmune liver diseases could indicate differing opportunities for therapeutic intervention. Furthermore, a dynamic evaluation of the disease activity using specific markers of ECM turnover could lead to the identification of sensitive biomarkers for disease monitoring and assessment of treatment response in therapeutic trials, responding to an unmet need for surrogates for clinical events particularly in PSC.11
We have previously shown that the marker of type III collagen formation (PRO-C3) is a strong predictor of prognosis in PSC.12 In this study, we explored several serological biomarkers specifically targeting ECM remodelling. Many of these have been demonstrated to be related to various chronic liver diseases as either diagnostic,[13], [14], [15] prognostic,16,17 or surrogate efficacy markers.18,19 These markers specifically target the end-product of tissue remodelling, that is a neo-epitope resulting from a specific protein cleaved by a specific protease, which is released into the circulation and may serve as biomarker for that pathological process. Combining both the protease and the protein may better assess the dynamic activity of a disease state, compared with other biomarkers targeting the intact protein. Using this technique, we investigated the ECM turnover profile of patients with PSC compared with PBC and AIH in 2 independent cohorts to evaluate associations with liver stiffness and disease stage for each disease and to establish whether differential patterns of ECM turnover are seen between the diseases.
Materials and methods
Patient panels
We adopted a 2-step study design (Fig. S1). We explored characteristics of ECM turnover in a test panel consisting of PSC patients compared with PBC and AIH patients (n = 80, 76, and 57, respectively) prospectively recruited at the Royal Free Hospital, UK, then validated main findings in a previously described independent validation panel of Norwegian PSC patients (n = 138) compared with PBC and AIH patients (n = 28 and 27, respectively), all retrospectively collected from the NoPSC Biobank, Norway. Characteristics of the study population are shown in Table 1. Diagnosis of PSC was based on typical cholangiographic findings according to acknowledged criteria; the first pathological cholangiography defined the time of diagnosis of PSC.20 PBC and AIH were diagnosed based on acknowledged criteria.3,20 Duration of disease was defined as the time from the date of diagnosis to the date of serum sampling. Cases of PSC or PBC with features of AIH were included in the PSC and PBC patient panels, respectively (Table 1). Cases of secondary cholangitis or small duct PSC were excluded. Control sera from 194 patients with ulcerative colitis (UC) where PSC had been excluded (all had normal cholangiograms by magnetic resonance cholangiography and normal alkaline phosphatase [ALP]) were retrieved for comparison from the 20-year follow-up visit of a population-based Norwegian cohort.21 All patients provided informed consent in writing. The protocol was in accordance with the Declaration of Helsinki and approved by the regional committee for research ethics in southeastern Norway (ref. 2011/2572) and the UK.
Table 1.
Baseline characteristics.
| Test panel |
Validation panel |
Controls |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PSC | PBC | AIH | p value∗ | PSC | PBC | AIH | p value† | UC | |
| N | 80 | 76 | 57 | 138 | 28 | 27 | 194 | ||
| Males, n (%) | 54 (67.5) | 7 (9.2) | 9 (15.8) | <0.001 | 107 (77.5) | 4 (13.8) | 11 (40.7) | <0.001 | |
| Age, years, median (range) | 46 (20–80) | 60 (29–83) | 51 (21–76) | <0.001 | 60 (16–72) | 60 (31–72) | 43 (19–81) | ||
| Age at diagnosis, years, median (range) | 36 (16–80) | 49 (29–78) | 43 (11–70) | <0.001 | 34 (14–72) | n.a. | n.a. | – | n.a. |
| Disease duration, years, median (range) | 5 (0–28) | 7 (0–22) | 6 (0–47) | n.s. | 1.7 (–0.6–29) | n.a. | n.a. | – | n.a. |
| Features of AIH, n (%) | 7 | 3 | n.a. | <0.001 | 11 | 1 | n.a. | <0.001 | n.a. |
| IBD ever, n (%) | 56 (70.0) | 1 (1.3) | 3 (5.2) | <0.001 | 102 (74.4) | n.a. | n.a. | – | 194 |
| UC, n (% of all) | 49 (61.3) | 0 (0) | 1 (1.8) | <0.001 | 81 (59.1) | n.a. | n.a. | – | 194 (100) |
| Colorectal malignancy, n (%) | 1 | 0 | 0 | n.s. | 5 | n.a. | n.a. | – | n.a. |
| Hepatobiliary malignancy, n (%) | 1 | 1 | 0 | n.s. | 1 | n.a. | n.a. | – | n.a. |
| Liver transplantation, n (%) | 6 | 1 | 0 | 0.025 | 31 | n.a. | n.a. | – | n.a. |
| Death, n (%) | 2 | 1 | 0 | n.s. | 16 | n.a. | n.a. | – | n.a. |
| Time-follow-up, years, median (range) | 0.4 (0–1.5) | 0.6 (-0.1–1.5) | 0.7 (0–1.8) | n.s. | 0.5 (0–4.2) | n.a. | n.a. | – | n.a. |
| Disease stage measures | |||||||||
| LSM, kPa, median (range) | 10.3 (2.5–75.0) | 7.7 (3.0–37.4) | 6.9 (2.6–75.0) | n.s. | n.a. | n.a. | n.a. | –I | n.a. |
| Advanced disease, n (%) | 30 (38.0)# 41 (53.9)‡ |
27 (38.0) | 18 (33.3) | 0.05§ (<0.02)§§ | 68 (52.7)# | ||||
| ELF score, median (range) | 10.1 (7.3–14.3) | 10.1 (8.3–12.4) | 9.9 (9.4–10.3) | n.s. | 9.7 (7.1–15.7) | n.a. | n.a. | – | n.a. |
| APRI score, median (range) | 0.5 (0.1–10.4) | 0.4 (0.09–4.9) | 0.3 (0.1–25.3) | 0.030 | 0.5 (0.1–23.7) | n.a. | n.a. | – | n.a. |
| Mayo risk score, median (range) | -0.3 (-2.3 to 3.7) | n.a. | n.a. | NI | 0.1 (–2.4 to 4.1) | n.a. | n.a. | – | n.a. |
| Mayo risk score, low/intermediate-high groups, n (%) | 49/30 | n.a. | n.a. | NI | 61/68 | n.a. | n.a. | – | n.a. |
| Laboratory values | |||||||||
| ALP, U/L median (range) | 198 (21–807) | 173 (49–959) | 85 (34–218) | <0.001 | 224 (51–1459) | n.a. | n.a. | – | n.a. |
| AST, U/L median (range) | 50 (16–919) | 43 (17–318) | 27 (10–1437) | <0.001 | 68 (16–1219) | n.a. | n.a. | – | n.a. |
| ALT, U/L median (range) | 48 (9–796) | 48 (11–472) | 28 (9–519) | <0.001 | 85 (14–885) | n.a. | n.a. | – | n.a. |
| Albumin, g/L median (range) | 44 (21–807) | 44 (30–50) | 44 (23–53) | n.s. | 41 (23–50) | n.a. | n.a. | – | n.a. |
| Total bilirubin, μmol/L median (range) | 13 (3–274) | 8 (2–330) | 9 (3–124) | <0.001 | 20 (3–532) | n.a. | n.a. | – | n.a. |
| INR, median (range) | 1 (0.7–3.8) | 1 (0.8–1.4) | 1 (0.9–2.1) | <0.001 | 1 (0.8–1.8) | n.a. | n.a. | – | n.a. |
| Platelet count, 109/L median (range) | 244 (53–536) | 259 (83–658) | 225 (44–480) | 0.044 | 248 (22–903) | n.a. | n.a. | – | n.a. |
| Creatinine, median (range) | 74 (45–138) | 70 (43–166) | 68 (43–100) | 0.043 | 65 (37–111) | n.a. | n.a. | – | n.a. |
| GGT, median (range) | 186 (13–1594) | 116 (27–818) | 33 (8–324) | <0.001 | 248 (22–1620) | n.a. | n.a. | – | n.a. |
The p-value represents comparison between PSC, PBC, and AIH within the test panel.
The p-value represents comparison between PSC, PBC, and AIH within the validation panel.
Defined by LSM using the published cut-off value for F3: n = 41 (53.9%); §PSC (LSM) vs. PBC; §§PSC (LSM) vs. AIH.
Defined by Mayo score. The Mann-Whitney U test was used for comparisons between continuous non-normally distributed parameters; Student t test was used when appropriate. AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transferase; APRI, AST to platelet ratio index; AST, aspartate transferase; ELF, enhanced liver fibrosis test; GGT, gamma-glutamyl transferase; IBD, inflammatory bowel disease; LSM, liver stiffness measurements; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; UC, ulcerative colitis.
For the test panel patients and the validation panel PSC patients, the respective research databases were revised for information on clinical and laboratory data, including ascites, encephalopathy, oesophageal varices, variceal bleeding, inflammatory bowel disease (IBD) status, and colorectal or hepatobiliary malignancy at the time of serum extraction. An IBD diagnosis was based on findings at colonoscopy and histology. Diagnosis of UC and Crohn’s disease were established by accepted criteria.
For the test cohort, advanced liver disease was defined based on LSMs using published cut-off values for TE in PSC and PBC8,9 and LSMs or histology in AIH. PBC response was defined according to the Toronto criteria (ALP <1.67 xULN) at 24 months. AIH remission was defined according to published criteria as normalisation of IgG and alanine aminotransferase (ALT). For the PBC and AIH validation and IBD control panels, limited phenotypic information was available. Liver stiffness was not available for the validation panel.
Biochemical analyses were performed using standard routine laboratory protocols for tests including platelets, creatinine, international normalised ratio (INR), aspartate aminotransferase (AST), ALT, ALP, and gamma-glutamyltransferase (GGT). The AST to platelet index (APRI) and PSC-specific revised Mayo risk score were calculated using the published algorithms.22,23
The date of serum sample extraction was identical for the frozen sera used for analyses of biomarkers of ECM turnover and the ELF test, and routine laboratory biochemical analyses, respectively, in all cases of PSC in the validation panel and in all but 6 cases in the test panel (n = 2 for each of the 3 diseases).
Biomarkers of ECM turnover
We used validated competitive ELISAs (Nordic Bioscience, Herlev, Denmark) to assess true formation of interstitial matrix collagen PRO-C3 and type V (PRO-C5), degradation of interstitial matrix collagen type III (C3M) and basement membrane type IV collagen (C4M), degradation of the proteoglycan biglycan (BGM) and citrullinated type III intermediate filament protein vimentin (VICM), in serum samples from all of the patients in each patient panel. All biomarkers were assessed in a blinded manner according to the manufacturer[24], [25], [26], [27], [28], [29] and samples were measured within the detection range.
ELF test
We analysed frozen serum samples from the PSC patients using the commercially available ELF test (Siemens Medical Solution Diagnostics, Inc., Tarrytown, NY, USA). The assays for analysis of serum levels of tissue inhibitor of metalloproteinase-1 (TIMP-1), hyaluronic acid (HA) and intact N-terminal procollagen type III (PIIINP) were performed using the Siemens ELF test kits containing assays designed specifically for the purpose of generating the ELF test and an ADVIA Centaur XP analyser (Siemens Medical Solutions Diagnostics, Inc.).
Liver stiffness measurements
Prospectively collected LSMs using TE for the assessment of liver fibrosis were available for n = 211 patients in the test panel. LSMs were performed at median 0 month from the time of biobanking in each diagnostic group (>6 months for n = 5, 4 and 2 patients with PSC, PBC, and AIH, respectively).
Statistical analyses
We tested continuous variables for normal distribution and applied the Student t test or the Mann-Whitney U test as appropriate. The collagen III turnover was calculated as PRO-C3/C3M. We calculated the PSC-specific Mayo risk score for the PSC patients and categorised these patients into low- (≤0), medium- (>0 to ≤2) and high- (>2) risk groups using published cut-off values. Data are presented as median (range). We explored correlations between the novel ECM markers and continuous variables using Spearman's rank test. For calculation of 95% confidence intervals of rho, the Fisher r-to-z transformation was used {tanh[arctanh(r) ± 1.96/sqrt(n - 3)]}. Advanced liver disease was defined based on published cut-off values; in PSC by LSMs (≥F3; PSC: >9.6 kPa; test panel) or Mayo risk score >0 (both panels), in PBC and AIH by LSMs (≥F3; 10.7 and >10.4 kPa, respectively).8,9,30 In addition, we defined advanced disease as APRI score >1 for secondary analyses (Supplementary material). The power of ECM biomarkers and the ELF test to discriminate between patients with and without advanced disease was evaluated by the area under the receiver operating characteristics curve (AUROC) analysis; differences between AUROCs were compared with the method of DeLong.31 Optimal cut-off values to discriminate between patients were obtained from the AUROC analysis according to the Youden index. We explored associations between clinical and laboratory variables and advanced disease by univariate logistic regression analysis. ECM markers were not normally distributed and therefore normalised to tertiles before analyses; non-normally distributed standard laboratory tests (thrombocytes) were transformed by the natural logarithm. AUROCs are presented with 95% confidence interval (CI). Values of p <0.05 were considered significant. Statistical analyses were performed using MedCalc (Statistical Software version 16.8.4, MedCalc Software bvba, Ostend, Belgium) and SPSS (version 24, SPSS Inc., Chicago, IL, USA). Graphs were designed using GraphPad Prism version 8.1.2 (GraphPad Software, La Jolla, CA, USA).
Data availability
Data are available upon request and an appropriate institutional collaboration agreement.
Results
Patients
Patient characteristics are shown in Table 1. We included a test panel of 78 PSC patients, 74 PBC patients, and 58 AIH patients as well as an independent validation panel of 138 PSC patients, 28 PBC patients, and 27 AIH patients. Patients with UC with normal bile duct imaging by magnetic resonance cholangiography (n = 194) served as controls. The test and validation liver disease panels were similar for each aetiology as regards median age, gender distribution (except for a higher male proportion in the AIH validation panel), the proportion of IBD in PSC patients, and the proportion of PSC or PBC patients with features of AIH.
However, as expected, gender distribution was not equal across diagnostic groups, with a male majority amongst PSC patients and a strong female predominance for PBC patients (males: 67.5, 9.2, and 15.8% in PSC, PBC, and AIH, respectively; p <0.001). PBC patients were older compared with PSC (median age 60 vs. 45 years; p <0.001) and median age at diagnosis differed between the 3 diagnoses (Table 1). Overall, 70.0% vs. 74.4% of PSC patients had IBD in the test and validation panels, with UC affecting 61.3% and 59.1%, respectively. Comorbidity with IBD was lower in PBC and AIH patients (Table 1). In the test panel, 36 (69.2%) PBC patients were documented ursodeoxycholic acid responders and 24 AIH patients (46.2%) were in remission.
There was no significant difference in the proportion of patients with advanced disease between PSC and PBC patients within the test panel (53.9% vs. 38.0% in PSC and PBC, respectively; p = 0.053), whereas in AIH patients there was a proportion with advanced disease (33.3%) which was similar to PBC (p = 0.59) but lower compared with PSC (p <0.02). Disease duration was similar between patients with PSC, PBC, and AIH (median duration [years]: 5 [0–28], 7 [0–22] and 6 [0–47], respectively; p = 0.60). Furthermore, there were no significant differences between PSC and PBC patients regarding markers related to stage including LSMs (10.3 [2.5–75.0] vs. 7.7 [3.0–37.4], respectively; p = 0.09), APRI score (0.62 [0.15–12.65] vs. 0.53 [0.13–7.07]; p = 0.43) or, in subsets (n = 45), ELF test (10.1 [7.3–14.3] vs. 10.1 [8.3–12.4], respectively; p = 0.87) (Table 1). Information regarding disease stage was not available for PBC or AIH for the validation panel.
Comparing PSC test and validation panels, patients showed similar ELF tests (test panel: n = 45; 10.1 [7.3–14.3] and 9.7 [7.2–15.7], respectively, p = 0.66) and APRI scores (p = 0.22) indicating similar levels of fibrosis; whereas the Mayo score was higher and disease duration shorter in the validation panel compared with the test panel (Mayo score: median [range] -0.3 [-2.3–3,7] and 0.1 [-2.4–4.1], p = 0.009; duration: p <0.001).
Biomarkers of ECM remodelling in PBC, PSC, and AIH compared with UC controls
A majority of ECM markers, including PRO-C3, PRO-C5, C3M, C4M, and BGM, showed an overall difference in serum levels between PSC, PBC, and AIH in both test and validation panels (p <0.01, Fig. 1). Interestingly, the ECM turnover was overall higher in PBC compared with the other autoimmune liver diseases. PRO-C5, C3M, C4M, and BGM were significantly higher (p <0.05 to p <0.001) in PBC sera compared with PSC as well as AIH in the 2 independent panels (Fig. 1). Concerning PRO-C3, findings indicated elevated levels in PBC compared with PSC or AIH but were inconsistent between the panels (Fig. 1). None of the other markers showed any consistent significant differences between liver aetiologies. All ECM remodelling markers were significantly elevated (p <0.05) in patients with PSC compared with UC controls in both the test and validation panels (Fig. 1).
Fig. 1.
ECM markers in patients with PSC, PBC, AIH and UC.
Levels of all ECM remodelling markers were higher in all 3 autoimmune liver diseases compared with UC controls. PBC patients showed higher levels of most ECM markers compared with PSC and AIH. (A) PRO-C3 (marker of type III collagen formation), (B) PRO-C5 (marker of type V collagen formation), (C) C3M (marker of type III collagen degradation), (D) C4M (marker of type IV collagen degradation), (E) BGM (marker of biglycan degradation), and (F) VICM (marker of citrullinated vimentin degradation). Comparisons made using the Student t test; asterisks indicate statistical significances p <0.05. Differences within test and validation panels are indicated by ∗; differences between test and validation panel are indicated by ¤; differences between ulcerative colitis (UC) controls and test panel are indicated by $; differences between UC controls and validation panel are indicated by #. AIH, autoimmune hepatitis; ECM, extracellular matrix; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; UC, ulcerative colitis.
The ratio between formation and degradation of type III collagen, that is PRO-C3/C3M is shown in Fig. S2. The ratio was elevated in PSC as compared with PBC and AIH in the test panel; however, no differences between aetiologies were found in the validation panel, although there was a trend towards higher ratio in PBC as compared with PSC and AIH. In both panels, the ratio was significantly increased as compared with UC controls.
Associations of the ECM markers with liver disease stage
We explored whether the levels of the ECM markers were different between patients with non-advanced vs. advanced disease (see Materials and methods section for definitions). Several ECM markers showed elevated levels in advanced disease, with the most striking differences between advanced and non-advanced disease for PRO-C3 for all aetiologies (percent reduction in non-advanced compared with advanced disease: 68%, 56%, and 39% in PSC, PBC, and AIH, respectively) (Table 2), underscoring an important association of collagen III formation with advanced disease for all 3 autoimmune liver diseases.
Table 2.
Extracellular matrix markers in advanced compared with non-advanced autoimmune liver disease.
| PSC |
PBC |
AIH |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test panel |
Test panel |
Test panel |
|||||||||||||
| Non-advanced | Advanced | p value | Non-advanced | Advanced | p value | Non-advanced | Advanced | p value | |||||||
| N | 35 | 41 | 44 | 27 | 36 | 18 | |||||||||
| Pro-C3/C3M | 1.4 | (0.6–5.0) | 3.0 | (0.7–6.4) | <0.001 | 1.1 | (0.4–4.3) | 1.7 | (0.5–6.5) | <0.001 | 1.2 | (0.2–2.2) | 1.3 | (0.6–7.9) | 0.25 |
| Pro-C3 | 13.3 | (6.8–84.7) | 42.2 | (11.7–104.0) | <0.001 | 17.6 | (8.0–66.9) | 40.3 | (9.4–107.6) | <0.001 | 12.1 | (7.3–35.5) | 19.8 | (10.6–82.1) | 0.001 |
| Pro-C5 | 808.0 | (359.5–2,990.2) | 795.9 | (56.1–3,035.2) | 0.81 | 1,219.6 | (211.7–3,464.4) | 1,497.9 | (699.8–3,496.9) | 0.07 | 743.2 | (339.6–3,309.6) | 1,119.4 | (498.2–1,889.5) | 0.05 |
| C3M | 10.7 | (5.6–31.4) | 12.8 | (7.2–48.9) | 0.05 | 16.1 | (8.0–80.2) | 19.6 | (9.5–44.8) | 0.03 | 12.3 | (6.1–32.9) | 15.6 | (9.5–33.2) | 0.02 |
| C4M | 27.2 | (15.0–73.4) | 29.4 | (14.6–65.7) | 0.70 | 38.7 | (20.1–91.5) | 50.3 | (25.0–96.9) | 0.04 | 27.1 | (14.6–73.2) | 35.5 | (19.3–70.9) | 0.02 |
| BGM | 15.8 | (4.6–64.1) | 15.8 | (4.6–48.2) | 0.43 | 19.6 | (4.6–47.2) | 25.1 | (10.0–96.73) | 0.05 | 13.2 | (4.6–39.9) | 15.4 | (4.6–26.9) | 0.67 |
| VICM | 6.8 | (0.7–36.2) | 7.2 | (0.7–35.3) | 0.53 | 9.2 | (0.7–57.6) | 5.1 | (0.7–59.2) | 0.01 | 11.3 | (0.7–54.4) | 5.1 | (0.7–36.3) | 0.03 |
Advanced disease is defined by liver stiffness measurement using published cut-off values by transient elastography for PSC (F3; 9.6 kPa), PBC (F3; 10.7 kPa) and AIH (F3; 10.4 kPa). Median (range) values are given. The Mann-Whitney U test is used for comparisons. AIH, autoimmune hepatitis; BGM, biglycan marker; ECM, extracellular matrix; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; VICM, citrullinated vimentin.
In PSC, elevated levels of PRO-C3 and PRO-C3/C3M ratio (reflecting the balance between collagen III formation and degradation) were observed in advanced compared with non-advanced disease (Table 2), whereas no significant difference was demonstrated for the other markers. In multivariate logistic regression analyses including age, sex, disease duration, ALP, Mayo risk score, and a single ECM marker at a time, we demonstrated independent association with advanced PSC for PRO-C3 as the single variable in the final model (5.57 [2.38, 13.05], p <0.001). No independent association was found for other ECM markers. To compare findings in the test and validation panels, we further defined advanced compared with non-advanced disease using the Mayo score, confirming the associations for PRO-C3 and PRO-C3/C3M ratio with advanced PSC. Furthermore, these analyses showed significantly elevated levels in advanced disease for PRO-C5 and C3M in both panels.
In PBC and AIH, PRO-C3, C3M, and C4M (in both PBC and AIH) and PRO-C3/C3M ratio and BGM (in PBC), showed significantly elevated levels in advanced compared with non-advanced disease whereas VICM showed lower values in advanced PBC as well as AIH (Table 2). Multivariate analysis as above but substituting bilirubin and ursodeoxycholic acid response for the Mayo score showed strong and independent association with advanced PBC for PRO-C3 (12.05, 95% CI [2.89, 50.20], p = 0.001) and PRO-C3/C3M ratio (4.33, 95% CI [1.51, 12.40], p = 0.006). In AIH, multivariate analyses including sex, AIH duration, remission state and 1 ECM marker at a time showed independent association only for PRO-C3 (3.49 [1.17, 10.45], p = 0.03).
We performed AUROC analyses of the ability of the ECM markers to detect advanced disease in PSC, PBC, and AIH, respectively (Table 3). Overall, PRO-C3 performed best, showing excellent (AUC >0.8; in PSC and PBC) or good (AUC = 0.771; AIH) ability to discriminate advanced from non-advanced disease. Furthermore, in PSC, PRO-C3, and PRO-C3/C3M ratio discriminated well (AUC >0.7) between mild and advanced disease as defined by the Mayo score in both panels, whereas discriminatory ability was also demonstrated for PRO-C5, C3M, and C4M in both panels with AUC >0.6, and for BGM (AUC >0.6) in the validation panel only (Table 4). In PBC and AIH, PRO-C5, C3M, and C4M (both aetiologies) and VICM and BGM (in PBC) discriminated between advanced and non-advanced disease (Table 3).
Table 3.
AUROC analyses of the discriminatory ability of extracellular matrix markers for detecting advanced disease.
| Test panel |
Validation panel |
||||
|---|---|---|---|---|---|
| PSC |
PBC |
AIH |
PSC |
PSC |
|
| LSM∗ | LSM∗ | LSM∗ | Mayo | Mayo | |
| N advanced/total (%) | 37/70 (53) | 25/68 (37) | 17/52 (33) | 30/78 (38) | 68/129 (53) |
| PRO-C3/C3M | 0.830 (0.727, 0.933) p <0.001 |
0.767 (0.641, 0.892) p <0.001 |
0.585 (0.415, 0.755) p = 0.33 |
0.761 (0.656, 0.866) p <0.001 |
0.772 (0.692, 0.853) p <0.001 |
| PRO-C3 | 0.855 (0.766, 0.944) p <0.001 |
0.833 (0.722, 0.943) p <0.001 |
0.771 (0.641, 0.900) p <0.001 |
0.820 (0.727, 0.913) p <0.001 |
0.826 (0.754, 0.898) p <0.001 |
| PRO-C5 | 0.498 (0.360, 0.636) p = 0.98 |
0.643 (0.505, 0.781) p = 0.04 |
0.676 (0.529, 0.823) p = 0.02 |
0.655 (0.524, 0.786) p = 0.02 |
0.721 (0.633, 0.809) p <0.001 |
| C3M | 0.620 (0.488, 0.752) p = 0.09 |
0.673 (0.540, 0.807) p = 0.01 |
0.708 (0.566, 0.850) p = 0.004 |
0.681 (0.551, 0.810) p = 0.01 |
0.716 (0.628, 0.804) p <0.001 |
| C4M | 0.510 (0.373, 0.647) p = 0.88 |
0.668 (0.533, 0.804) p = 0.02 |
0.708 (0.561, 0.855) p = 0.01 |
0.644 (0.511, 0.776) p = 0.03 |
0.761 (0.679, 0.843) p <0.001 |
| BGM | 0.419 (0.284, 0.553) p = 0.24 |
0.644 (0.509, 0.780) p = 0.04 |
0.548 (0.377, 0.719) p = 0.58 | 0.535 (0.398, 0.673) p = 0.62 | 0.606 (0.507, 0.704) p = 0.04 |
| VICM | 0.470 (0.331, 0.609) p = 0.67 |
0.280 (0.149, 0.411) p = 0.001 |
0.339 (0.171, 0.506) p = 0.06 |
0.488 (0.357, 0.618) p = 0.85 |
0.504 (0.404, 0.605) p = 0.93 |
For analyses involving LSM: panel restricted to patients with LSM available and AST <175. Published cut-off levels for fibrosis (F3) were used (PSC >9.6 kPa, PBC >10.7 kPa; AIH >10.4 kPa). Mayo risk score cut-off 0 differentiated mild vs. moderate-high risk. Values are given as AUC (95% CI). AIH, autoimmune hepatitis; AUROC, area under the receiver operator characteristics curve; BGM, biglycan marker; ELF, enhanced liver fibrosis test; LSM, liver stiffness measurement; Mayo, PSC-specific revised Mayo risk score; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; VICM, citrullinated vimentin.
Table 4.
Correlations between extracellular matrix markers and measures of liver fibrosis or prognosis.
| Test panel |
Validation panel |
||||||
|---|---|---|---|---|---|---|---|
| LSM∗ |
Mayo |
ELF |
Mayo |
||||
| PSC | PBC | AIH | PSC | PSC | PSC | ||
| N | 70 | 67 | 52 | 79 | 138 | 129 | |
| PRO-C3/C3M | Rho (95% CI) p value |
0.533 (0.341–0.682) <0.001 |
0.449 (0.294–0.660) <0.001 |
0.246 (-0.029–0.486) 0.08 |
0.473 (0.281–0.628) <0.001 |
0.772 (0.695–0.832) <0.001 |
0.598 (0.474–0.699) <0.001 |
| PRO-C3 | Rho (95% CI) p value |
0.649 (0.489–0.767) <0.001 |
0.555 (0.363–0.702) <0.001 |
0.473 (0.230–0.661) <0.001 |
0.591 (0.425–0.718) <0.001 |
0.830 (0.770–0.876) <0.001 |
0.701 (0.601–0.779) <0.001 |
| PRO-C5 | Rho (95% CI) p value |
0.090 (-0.148–0.318) 0.44 |
0.202 (-0.040–0.422)0.09 | 0.217 (-0.059–0.462) 0.12 |
0.233 (0.013–0.432) 0.04 |
0.388 (0.181–0.478) <0.001 |
0.446 (0.296–0.575) <0.001 |
| C3M | Rho (95% CI) p value |
0.277 (0.045–0.481) 0.02 |
0.243 (0.003–0.457) 0.04 |
0.263 (-0.011–0.500) 0.06 |
0.317 (0.103–0.503) 0.004 |
0.399 (0.248–0.531) <0.001 |
0.449 (0.299–0.577) <0.001 |
| C4M | Rho (95% CI) p value |
0.160 (-0.078–0.381) 0.17 |
0.256 (0.017–0.467) 0.03 |
0.300 (0.030–0.530) 0.03 |
0.279 (0.062–0.471) 0.01 |
0.406 (0.256–0.537) <0.001 |
0.506 (0.365–0.624) <0.001 |
| BGM | Rho (95% CI) p value |
-0.089 (-0.317–0.149) 0.45 |
0.121 (-0.123–0.351) 0.31 |
-0.025 (-0.296–0.250) 0.86 |
0.084 (-0.140–0.230) 0.46 |
0.086 (-0.082–0.250) 0.32 |
0.202 (0.030–0.362) 0.02 |
| VICM | Rho (95% CI) p value |
-0.112 (-0.338–0.126) 0.34 |
-0.367 (-0.558 to -0.139) 0.002 |
-0.375 (-0.588 to -0.114) 0.006 |
-0.001 (-0.222–0.220) 0.99 |
-0.098 (-0.261–0.070) 0.25 |
-0.043 (-0.214–0.131) 0.63 |
Analysis restricted to patients with AST <175. Correlations were explored using Spearman's rank test. AIH, autoimmune hepatitis; BGM, matrix metalloproteinase mediated degradation of biglycan; C3M, degradation of type III collagen; C4M, degradation of type IV collagen; ELF, enhanced liver fibrosis test; LSM, liver stiffness measurement; Mayo, PSC-specific revised Mayo risk score; PBC, primary biliary cholangitis; PRO-C3 and PRO-C5, formation of type III and V collagen, respectively; PSC, primary sclerosing cholangitis; VICM, degradation of citrullinated vimentin.
Correlations between the ECM markers and LSMs, fibrosis scores and Mayo risk score.
PRO-C3 showed the strongest correlation with established fibrosis markers compared with the other ECM markers for any of the aetiologies. In PSC, PRO-C3 displayed strong correlation with LSMs (rho >0.5, p <0.001) and excellent correlation with ELF test (validation panel; rho 0.83, p <0.001; Table 4). C3M (both panels) and C4M (validation panel only) also showed significant correlations with LSMs in PSC, linking collagen degradation to liver stiffness in PSC (Table 4). Supporting an association with fibrosis in PSC, in the validation panel, these markers as well as PRO-C5 showed significant correlations with ELF test, a well-established fibrosis marker panel associated with prognosis in PSC. No correlation with either LSMs or ELF test was seen for BGM and VICM (Table 4). Finally, in PSC, PRO-C3 correlated well with the Mayo risk score in both PSC panels (rho 0.59 and 0.70, respectively; p <0.001). PRO-C5, C3M, and C4M were also correlated with the Mayo risk score in both panels (Table 4).
In PBC and AIH, PRO-C3 showed good correlation with LSMs (rho = 0.56 and 0.48, respectively; both p <0.001). Correlations with LSMs were also demonstrated for C3M, C4M, and VICM for PBC as well as AIH (Table 4).
Associations of the ECM markers with disease activity in PBC and AIH
Several ECM markers were reduced in PBC patients who were ursodeoxycholic acid responders (n = 36; 69.2%) compared with non-responders (n = 16; 30.8%) (Fig. 2). Responders showed reduced levels for PRO-C3 (median 37.3 vs. 18.6; p = 0.002), PRO-C5 (1650.3 vs. 1096.3; 0.001), C3M (26.8 vs. 14.1; p <0.001), C4M (58.8 vs. 31.6; p <0.001), and BGM (26.7 vs. 18.8; p = 0.04). In addition, increased levels were found in responders for VICM (4.7 vs. 9.7; p <0.001). There was no difference for LSMs (median [range] 7.4 [3.0, 37.4] vs. 10.9 [4.4, 31.2]; p = 0.06).
Fig. 2.
Extracellular matrix markers are different dependent on disease activity in patients with primary biliary cholangitis.
The boxplots show significantly lower levels of extracellular matrix markers in patients with PBC who were ursodeoxycholic acid responders (n = 36) compared with non-responders (n = 16) for (A) type III collagen formation (PRO-C3; p = 0.002), (B) type V collagen formation (PRO-C5; p = 0.001), (C) C3M (p <0.001), (D) C4M (p <0.001), (E) biglycan (BGM; p <0.05), and increased level for (F) citrullinated vimentin (VICM; p <0.001). ∗ p ≤ 0.05; ∗∗ p <0.005; ∗∗∗ p ≤ 0.001. PBC, primary biliary cholangitis.
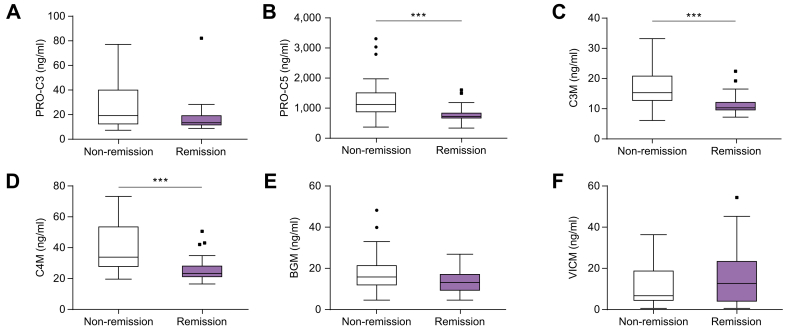
In patients with AIH with available data (n = 52), ECM markers PRO-C5, C3M, and C4M showed reduced levels in patients in remission (n = 24; 46.2%) compared with non-remission (n = 28; 53.8%) with reduced values in remission for PRO-C5, C3M, and C4M (median 1114.0 vs. 724.2; 14.6 vs. 10.3; 31.9 vs. 24.7; p = 0.001, 0.001 and 0.002, respectively) (Fig. 3) whereas no difference across disease activity state was demonstrated for BGM, VICM, or LSMs.
Fig. 3.
Extracellular matrix markers are different dependent on disease activity in patients with autoimmune hepatitis.
The boxplots show extracellular matrix markers in patients with autoimmune hepatitis in remission (n = 24) compared with non-remission for (A) type III collagen formation (PRO-C3), (B) type V collagen formation (PRO-C5), (C) degradation of type III collagen (C3M), (D) degradation of type IV collagen (C4M), (E) biglycan (BGM) and (F) citrullinated vimentin (VICM). Levels were significantly lower in patients with remission for PRO-C5, C3M and C4M (p = 0.001). ∗∗∗ p ≤ 0.001.
Outcome prediction
We have previously reported that markers of collagen formation (PRO-C3, PRO-C5) and degradation (C3M, C4M) are associated with prognosis in PSC.12 Extending analyses to BGM and VICM, AUROC analyses showed modest discriminatory ability for BGM to discriminate between PSC patients who did and did not reach liver transplantation or death during follow-up (AUC 0.63; p = 0.02), relating biglycan degradation to clinical outcome in PSC for the first time. Survival times were reduced in high-risk vs. low-risk groups (defined by optimal cut-off values as decided by Youden) for BGM (mean survival 1.80 vs. 2.87 [p = 0.009]; Table S1). BGM (hazard ratio 2.34, p = 0.012) was associated with reaching the clinical endpoint in univariate Cox-regression analysis (Table S2). Low event-rate and short follow-up in the test panel and missing outcome data for validation panel PBC and AIH patients precluded analysis for these panels.
Discussion
In this study, we have dissected the ECM remodelling in 3 autoimmune liver diseases using highly specific, targeted markers reflecting the dynamic balance between fibrogenesis and fibrosis degradation in the liver. For the first time, we demonstrate a striking difference in ECM remodelling indicating higher turnover in PBC compared with either PSC or AIH. This difference could not be explained by differences in stage, as the proportion of patients with advanced liver disease was not different between PBC and the other autoimmune liver diseases. Our results highlight the differences between the 3 diseases as regards fibrosis composition and handling, underscore the pathogenetic differences between PSC and PBC, and clearly establish PBC as a high-turnover disease compared with PSC and AIH.
The difference that we observed between PBC and PSC supports the notion that, despite the fact that PBC and PSC both give rise to biliary-type fibrosis as evidenced by histology, the pathogenesis leading to this result may be fundamentally different between the 2 diseases. Whereas the fibrogenesis in PSC is mainly driven by pro-fibrogenic factors derived from ‘reactive’ cholangiocytes, in PBC, the fibrogenesis is driven by a more defined immune-mediated inflammation and characterised by ECM degradation associated with cholangiocyte damage. Hence, portal fibrosis in PBC is more similar to a chronic wound healing reaction, which could explain the higher ECM turnover compared with PSC. In AIH, fibrogenesis is initiated in the liver lobule instead of the portal tract; hence, different pro-fibrogenic cells are likely involved. In addition, the ratio between formation and degradation of type III collagen was significantly increased in all aetiologies as compared with UC controls, further suggesting not only an increased turnover of type III collagen, but also an increased net deposition of type III collagen in all aetiologies compared with UC.
We demonstrated the association of several of the novel markers with fibrosis in autoimmune liver diseases, showing increased levels of markers of collagen formation as well as degradation, underscoring that the fibrosis in autoimmune liver diseases represents a relatively high-turnover condition, as an analogy to the balance between bone formation and resorption in osteoporosis.32 This suggests that therapeutic strategies aimed at altering the balance between fibrosis formation and degradation may benefit patients.
PRO-C3 showed the strongest association with fibrosis of the ECM markers, as underscored by the tight (in PSC and PBC) or moderate (in AIH) correlation of PRO-C3 with LSMs and serum-based fibrosis scores such as the ELF test and APRI score. We demonstrated the presence of higher levels in advanced disease for PRO-C3 for all aetiologies. Overall, PRO-C3 showed the most consistent difference between stages in autoimmune liver disease. In line with this, PRO-C3 performed best for stage discrimination, with good to excellent ability to discriminate advanced from non-advanced disease in PSC as well as PBC and AIH.
Of the other markers, collagen degradation markers C3M and C4M showed most consistent association with other measures of fibrosis, including correlations to LSMs and ELF test, presence of higher levels in advanced compared with non-advanced disease (in PBC and AIH) and good performance for stage discrimination in PBC and AIH but not in PSC. Interestingly, this points to a possible role for destruction of the basement membrane, which type IV collagen is the main component of, in the pathogenesis of the autoimmune liver diseases. Moreover, the associations between these diseases and a BGM are of interest because biglycan is an important TGF-beta binding protein that releases TGF-beta upon degradation, putatively driving fibrogenesis;26 hence our observations should lead to further exploration of biglycan as a therapeutic target.
Thus, our study confirms increased ECM remodelling in advanced compared with non-advanced disease as previously reported for other liver diseases.16,18,25 Stage definition and evaluation of the level of fibrosis is not straightforward in PSC and PBC as liver biopsy is not routinely performed and because the patchy distribution of fibrosis may affect the reliability of histology. Hence, we defined advanced disease based on disease-specific cut-off values for moderate fibrosis (F3) for LSMs by TE, for which the association with histological stage and clinical outcome has been validated in independent studies in PBC as well as PSC.8,9 Furthermore, to compare test and validation panels in PSC, we defined disease severity groups using the PSC-specific Mayo risk score, which is the most commonly used predictive tool in PSC although not validated at the individual level.22 We believe that the evaluation of the ECM markers against several measures of disease stage or severity, including LSMs, strengthens our analysis; however, independent validation of the associations between the ECM markers and LSMs in larger disease-specific patient panels is warranted. Conceivably, ECM marker analysis may allow improved stage distinction in PSC as well as PBC compared with LSMs, given that serum markers better reflect the status of the whole organ compared with the limited assessment by LSMs which may not be representative in diseases with patchy distribution such as PSC and PBC; however, the present study does not allow any conclusions in this regards.
Our demonstration of excellent correlation of PRO-C3 with LSMs and serum scores of fibrosis and the strong ability to discriminate between advanced and non-advanced disease, support the association of PRO-C3 with stage as a marker of prognosis and hence our previous report showing strong predictive ability of PRO-C3 in PSC.12 PRO-C3 is a marker of formation of collagen III, one of the most abundant collagens in liver cirrhosis. In contrast, PIIINP which is used as a marker of liver fibrosis alone or as part of the ELF test, reflects the total pool of collagen III, including formation, degradation, and stable versions of the collagen. Potentially, this might yield improved reflection of the dynamics of fibrogenesis to PRO-C3 as compared with PIIINP or the ELF test.
We report higher levels of PRO-C3 and other ECM markers in ursodeoxycholic acid non-responders compared with responders for PBC and in patients with active disease compared with remission for AIH, indicating an association of ECM markers with disease activity in autoimmune liver diseases. Our findings are in line with previous publications demonstrating that PRO-C3 is associated with disease activity in HIV patients under treatment for C3M and C4M;18 moreover, reduction in PRO-C3 levels as well as the ELF test was reported following treatment in PSC with NGM282, an FGF19 analogue, as a further indication that PRO-C3 levels may reflect disease activity.33 Interestingly, the serum levels of the ECM markers observed in this study were much higher compared with that previously found in other liver diseases such as NAFLD.34,35 We speculate that this might be caused by pro-fibrogenic effects of bile acids via activation of portal fibroblasts. In support of this, findings from NGM Bio show that the change in bile acid concentration is positively correlated to the change in PRO-C3 in patients with non-alcoholic steatohepatitis.36,37 Further studies in larger, disease-specific patient panels are warranted to establish whether PRO-C3 is a good candidate surrogate marker of disease activity in PBC and other autoimmune liver diseases.
We have previously demonstrated excellent ability for PRO-C3 to detect patients reaching a clinical endpoint during follow-up in PSC, whereas PRO-C5 and C3M showed significant but weaker outcome discriminatory abilities.12 The present extension of the analysis to novel ECM markers relates biglycan degradation to clinical outcome in PSC for the first time and indicates a role for BGM for risk stratification in PSC, although further studies are warranted.
The analysis of a broad panel of novel ECM markers in a parallel design of patients with 3 different autoimmune liver diseases and the validation in independent panels for each aetiology represent strengths of the study. The number of patients differed between aetiologies (PSC > PBC or AIH) with relatively small validation groups for PBC and AIH, and this represents a limitation which may have affected power and hence results. Stage definition was complicated by the lack of liver biopsy; however, liver biopsy is not routinely indicated in either PSC or PBC, furthermore we employed LSMs to define stage in the majority of test panel patients. LSMs was not available in Norway at the time of inclusion of patients in the validation panel, but we were able to define stage in PSC validation patients using the Mayo risk score. The levels of several of the ECM markers were different between the test and validation panels with in general higher levels in the validation panel for part or all of the aetiologies for a majority of the markers, although all of the samples were analysed by the same protocol, in the same batch and the same laboratory. We can only speculate that this might be related to differences in ethnicity of the patients, with a purely Caucasian validation panel and a mixed-ethnicity test panel. The short follow-up time and relatively few events in the test panel precluded independent validation of analyses regarding the predictive abilities of the ECM markers.
In this first comparison of ECM markers in autoimmune liver diseases we found an overall increased ECM turnover in PBC compared with either PSC or AIH, indicating that the ECM remodelling is different in PSC, PBC, and AIH. This might suggest differing opportunities for therapeutic intervention for patients with PSC, PBC, and AIH; hence, further investigation of ECM remodelling markers is warranted. Furthermore, we have demonstrated for the first time the close and consistent association of the ECM remodelling marker PRO-C3 with liver stiffness and stage in PSC, PBC, and AIH, respectively.
Authors' contributions
Guarantor of the article and supervisor of the project: DT.
Study conception and design: DT, MV, MAK, THK, MP.
Collection of biological samples and clinical data: JRH, FS, KMB, BM.
Contributed to the designing of the laboratory analyses: DJL, MAK.
Performed the laboratory analyses: MJN, TMJ.
Contributed to the ELF Test analyses: DT, MP, FS.
Designed and performed the statistical analyses: MV.
Contributed to the interpretation of the data: DT, JRH, KMB, MP, MAK, THK, MJN, MV.
Drafted the manuscript: MV, MJN, DT.
Reviewed the manuscript for critical content and approved the final version of the manuscript: all authors.
Funding
The study was sponsored by the Danish Science Foundation.
Conflicts of interest
MJN, DJL, TM-J, and MAK are full-time employees at Nordic Bioscience. MAK and DJL hold stocks in Nordic Bioscience. MJN, DJL and MAK are among the original inventors and patent holders for PRO-C3, PRO-C5, C3M, and C4M. MV has received fees as an advisory board member for Intercept. DT has received fees for an advisory board with Intercept and speakers fees from Intercept and Falk. JRH has served on advisory boards for Orkla Health and Novartis, and received research support from Biogen, all unrelated to the present study.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors thank Liv Wenche Thorbjørnsen for assistance in the collection of serum samples. M. Pinzani and D. Thorburn gratefully receive funding from UCL NIHR BRC (funding 2017–2022) and PSC partners (funding 2017–2019).
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found at https://doi.org/10.1016/j.jhepr.2020.100178.
Supplementary data
References
- 1.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Corpechot C., Poupon R., Chazouilleres O. New treatments/targets for primary biliary cholangitis. JHEP Rep. 2019;1:203–213. doi: 10.1016/j.jhepr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the L EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 4.de Vries E.M., Verheij J., Hubscher S.G., Leeflang M.M., Boonstra K., Beuers U. Applicability and prognostic value of histologic scoring systems in primary sclerosing cholangitis. J Hepatol. 2015;63:1212–1219. doi: 10.1016/j.jhep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Vesterhus M., Hov J.R., Holm A., Schrumpf E., Nygard S., Godang K. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology. 2015;62:188–197. doi: 10.1002/hep.27825. [DOI] [PubMed] [Google Scholar]
- 7.de Vries E.M.G., Farkkila M., Milkiewicz P., Hov J.R., Eksteen B., Thorburn D. Enhanced liver fibrosis test predicts transplant-free survival in primary sclerosing cholangitis, a multi-centre study. Liver Int. 2017;37:1554–1561. doi: 10.1111/liv.13402. [DOI] [PubMed] [Google Scholar]
- 8.Corpechot C., Carrat F., Poujol-Robert A., Gaouar F., Wendum D., Chazouilleres O. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198–208. doi: 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- 9.Corpechot C., Gaouar F., El Naggar A., Kemgang A., Wendum D., Poupon R. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146:970–979. doi: 10.1053/j.gastro.2013.12.030. quiz e915-76. [DOI] [PubMed] [Google Scholar]
- 10.Ehlken H., Wroblewski R., Corpechot C., Arrive L., Rieger T., Hartl J. Validation of transient elastography and comparison with spleen length measurement for staging of fibrosis and clinical prognosis in primary sclerosing cholangitis. PLoS One. 2016;11:e0164224. doi: 10.1371/journal.pone.0164224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponsioen C.Y., Chapman R.W., Chazouilleres O., Hirschfield G.M., Karlsen T.H., Lohse A.W. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–1367. doi: 10.1002/hep.28256. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen M.J., Thorburn D., Leeming D.J., Hov J.R., Nygard S., Moum B. Serological markers of extracellular matrix remodeling predict transplant-free survival in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2018;48:179–189. doi: 10.1111/apt.14806. [DOI] [PubMed] [Google Scholar]
- 13.Leeming D.J., Karsdal M.A., Byrjalsen I., Bendtsen F., Trebicka J., Nielsen M.J. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38:1086–1096. doi: 10.1111/apt.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen M.J., Kazankov K., Leeming D.J., Karsdal M.A., Krag A., Barrera F. Markers of collagen remodeling detect clinically significant fibrosis in chronic hepatitis C patients. PLoS One. 2015;10:e0137302. doi: 10.1371/journal.pone.0137302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen M.J., Karsdal M.A., Kazankov K., Gronbaek H., Krag A., Leeming D.J. Fibrosis is not just fibrosis – basement membrane modelling and collagen metabolism differs between hepatitis B- and C-induced injury. Aliment Pharmacol Ther. 2016;44:1242–1252. doi: 10.1111/apt.13819. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen M.J., Veidal S.S., Karsdal M.A., Orsnes-Leeming D.J., Vainer B., Gardner S.D. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–437. doi: 10.1111/liv.12700. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen M.J., Lehmann J., Leeming D.J., Schierwagen R., Klein S., Jansen C. Circulating elastin fragments are not affected by hepatic, renal and hemodynamic changes, but reflect survival in cirrhosis with TIPS. Dig Dis Sci. 2015;60:3456–3464. doi: 10.1007/s10620-015-3783-9. [DOI] [PubMed] [Google Scholar]
- 18.Leeming D.J., Anadol E., Schierwagen R., Karsdal M.A., Byrjalsen I., Nielsen M.J. Combined antiretroviral therapy attenuates hepatic extracellular matrix remodeling in HIV patients assessed by novel protein fingerprint markers. AIDS. 2014;28:2081–2090. doi: 10.1097/QAD.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 19.Karsdal M.A., Hjuler S.T., Luo Y., Rasmussen D.G.K., Nielsen M.J., Holm Nielsen S. Assessment of liver fibrosis progression and regression by a serological collagen turnover profile. Am J Physiol Gastrointest Liver Physiol. 2019;316:G25–31. doi: 10.1152/ajpgi.00158.2018. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Lunder A.K., Hov J.R., Borthne A., Gleditsch J., Johannesen G., Tveit K. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology. 2016;151:660–9 e.664. doi: 10.1053/j.gastro.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Kim W.R., Therneau T.M., Wiesner R.H., Poterucha J.J., Benson J.T., Malinchoc M. A revised natural history model for primary sclerosing cholangitis. Mayo Clinic Proc. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 23.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen M.J., Nedergaard A.F., Sun S., Veidal S.S., Larsen L., Zheng Q. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 25.Leeming D.J., Veidal S.S., Karsdal M.A., Nielsen M.J., Trebicka J., Busk T. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015;50:584–592. doi: 10.3109/00365521.2014.996590. [DOI] [PubMed] [Google Scholar]
- 26.Genovese F., Barascuk N., Larsen L., Larsen M.R., Nawrocki A., Li Y. Biglycan fragmentation in pathologies associated with extracellular matrix remodeling by matrix metalloproteinases. Fibrogenesis Tissue Repair. 2013;6:9. doi: 10.1186/1755-1536-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sand J.M., Larsen L., Hogaboam C., Martinez F., Han M., Rossel Larsen M. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis – validation of two novel biomarker assays. PLoS One. 2013;8:e84934. doi: 10.1371/journal.pone.0084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassiliadis E., Oliveira C.P., Alvares-da-Silva M.R., Zhang C., Carrilho F.J., Stefano J.T. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]
- 29.Barascuk N., Veidal S.S., Larsen L., Larsen D.V., Larsen M.R., Wang J. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Hartl J., Denzer U., Ehlken H., Zenouzi R., Peiseler M., Sebode M. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769–775. doi: 10.1016/j.jhep.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 31.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 32.Henriksen K., Christiansen C., Karsdal M.A. Role of biochemical markers in the management of osteoporosis. Climacteric. 2015;18(Suppl 2):10–18. doi: 10.3109/13697137.2015.1101256. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfield G.M., Chazouilleres O., Drenth J.P., Thorburn D., Harrison S.A., Landis C.S. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Daniels S.J., Leeming D.J., Eslam M., Hashem A.M., Nielsen M.J., Krag A. ADAPT: an algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced gibrosis. Hepatology. 2019;69:1075–1086. doi: 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y., Oseini A., Gagnon R., Charles E.D., Sidik K., Vincent R. An evaluation of the collagen fragments related to fibrogenesis and fibrolysis in nonalcoholic steatohepatitis. Sci Rep. 2018;8:12414. doi: 10.1038/s41598-018-30457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison S.A., Rossi S.J., Paredes A.H., Trotter J.F., Bashir M.R., Guy C.D. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanyal A., Ling L., Jaros M.J., Baxter B., Kowdley K.V., DePaoli A.M. Changes in serum bile acids correlate with 7alpha-hydroxy-4-cholesten-3-one and fibrogenesis biomarker PRO-C3 with NGM282 therapy in patients with nonalcoholic steatohepatitis. EASL. 2019 LBP-30. 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request and an appropriate institutional collaboration agreement.