Abstract
Proteins are secreted from eukaryotic cells by several mechanisms besides the well-characterized classical secretory system. Proteins destined to enter the classical secretory system contain a signal peptide for translocation into the endoplasmic reticulum. However, many proteins lacking a signal peptide are secreted nonetheless. Contrary to conventional belief, these proteins are not just released as a result of membrane damage leading to cell leakage, but are actively packaged for secretion in alternative pathways. They are called unconventionally secreted proteins, and the best-characterized are from fungi and mammals. These proteins have extracellular functions including cell signaling, immune modulation, as well as moonlighting activities different from their well-described intracellular functions. Among the pathways for unconventional secretion are direct transfer across the plasma membrane, release within plasma membrane-derived microvesicles, use of elements of autophagy, or secretion from endosomal/multivesicular body-related components. We review the fungal and metazoan unconventional secretory pathways and their regulation, and propose experimental criteria to identify their mode of secretion.
Box 1 Terminology and abbreviations.
| Amphisome | An organelle formed by an autophagosome fusing with an MVB |
| Autophagosome | Double membrane structure that forms upon starvation |
| CUPS | Compartment for Unconventional Protein Secretion (a series of vesicles made from Golgi and endosomal membranes) |
| Endosome | A post-Golgi vesicle which also contains material derived from the plasma membrane. Sometimes called an early endosome |
| ER | Endoplasmic reticulum |
| ESCRT | The endosomal sorting complexes required for transport. Four different complexes (ESCRT 0-III) of which at least one is required to generate ILV in an MVB |
| Exosome | Small extracellular vesicles derived from the ILV of an MVB |
| Exomere | Non-vesicular nanoparticles secreted from cells, less than 50 nm in size, enriched in HSP90, (not related to ‘exomer’ coating of ER vesicles) |
| FGF | Fibroblast growth factor |
| ILV | Intraluminal vesicle, a vesicle inside of a membrane-bound organelle such as an MVB or autophagosome |
| Microvesicle | Extracellular vesicle shed from the plasma membrane, generally determined by size |
| MVB | Multivesicular body (also called the late or mature endosome). An endosome marked by intraluminal vesicles |
| PAS | Pre-autophagosomal structure. A membranous organelle that matures into an autophagosome upon binding autophagy factors. |
| PM | Plasma membrane |
| Unconventionally secreted protein | A protein lacking a classic signal sequence but nonetheless secreted from eukaryotic cells by biologically controlled processes |
| Vacuole | Yeast equivalent to a lysosome |
1. Introduction
Most proteins secreted from eukaryotic cells pass through the classical secretory pathway (Delic et al., 2013). However, a variety of proteins are secreted without passing through the organelles of the classical secretion pathway and are referred to as unconventionally (non-classically) secreted proteins. Unconventionally secreted proteins are often functional in the extracellular environment (Table 1) and their mechanisms of secretion are often linked to cell stress or disease. Therefore, it is critical that we continue to study the mechanisms of unconventional protein secretion (summarized in Fig. 1, Fig. 2, Fig. 3, Fig. 4) (Dimou and Nickel, 2018).
Table 1.
Selected Unconventionally Secreted Proteins and Likely Mechanisms of Secretion.
| Protein Name | Organism or cell type | Likely Method(s) of Unconventional Secretion | Factors involved in secretion | Extracellular function |
|---|---|---|---|---|
| a-factor | S. cerevisiae | Protein transporter | Transported by ABC transporter Ste6 (McGrath and Varshavsky, 1989) | Yeast mating pheromone (McGrath and Varshavsky, 1989) |
| Acb1 AcbA |
S. cerevisiae Dictyostelium discoideum |
CUPS CUPS |
Secretion induced during nutrient starvation, blocked by deletions to autophagy genes, ESCRT components, and plasma membrane SNARES (Duran et al., 2010). Engulfed within Snf7-stabilized CUPS membranes (Curwin et al., 2016) Acyl-CoA binding to a Golgi associated protein (GRASP) |
Cleavage by SDF-2 to stimulate pre-spore transitions into an encapsulated spore (Cabral et al., 2010) |
| Annexins AI, AII, and V | Mammals | Direct translocation across membrane | Forms oligomer that can translocate across membranes of liposomes (Popa et al., 2018) | |
| Engrailed |
Drosophila melanogaster Chicken |
Vesicular | 5% of engrailed is secreted in vesicles. Secretion relies on a sequence in its homeodomain, and is regulated by CK2 (Joliot et al., 1998) (Maizel et al., 2002) | Paracrine signaling (Maizel et al., 2002) |
| Enolase | Yeast Mammals |
Endolysosomal system | Small, non-classical exosomal EV (Jeppesen et al., 2019). Secretion mediated by SNARE TLG2 (Miura et al., 2012). Sequence specific localization based on fusion protein studies. Likely secreted in vesicles as secretion facilitated by Tlg2 (T-SNARE) fusion (Miura et al., 2012) | C. albicans enolase binds mammalian plasminogen, aiding in endothelial cell invasion (Chaffin, 2008) |
| FABP4 | Mammalian adipocytes | Secretory endosomes and lysosomes | Secreted upon treatment with lipolytic agonists such as forskolin or 3-isobutyl-1methylxanthine. Secretion is independent of Tsg101 and Hrs (ESCRT-0 and ESCRT-1) and released in a non-membrane bound form (Villeneuve et al., 2018) | Adipokine, upregulates glucose production and cardiac muscle contraction (Furuhashi et al., 2014) |
| FGF1 | Mammals | Direct translocation | Destabilizes the acidic phospholipids of the plasma membrane (Graziani et al., 2006). FGF1 folding impacts direct translocation across plasma membrane (Prudovsky et al., 2016). Upregulated in type 2 diabetes (Wang et al., 2016) | Paracrine signal. Activates FGF receptors (Ornitz and Itoh, 2015) |
| FGF2 | Mammals | Direct translocation | Interaction with ATP1A1, Tec kinase, followed by insertion into a PI(4,5)P2 PM, and emerging externally by binding heparin sulfate (La Venuta et al., 2015) (Steringer et al., 2017) | Paracrine signal. Activates FGF receptors (Ornitz and Itoh, 2015) |
| Galectin | Yeast mammals |
Not well characterized. | When expressed in S. cerevisiae, secretion relied on NCE1 and NCE2 (Cleves et al., 1996). Export mechanism requires binding to β-galactosides (Seelenmeyer et al., 2005). | Secreted from bone marrow-derived mesenchymal stem cells to Inhibit maturation of dendritic cells (Zhang et al., 2017a) |
| GAPDH | Mammals Xenopus Yeast |
Not well characterized | Found in non-vesicular fractions, and CD81, CD63, and CD9 negative exosomes of mammals (Jeppesen et al., 2019) Exosome related in Xenopus (Jella et al., 2016) Heat and starvation (Branco et al., 2014) | Iron sequestrationExternal GAPDH alters cell morphology/spreading (Yamaji et al., 2005)Exosomal GAPDH negatively regulates the sodium channel ENaC (Jella et al., 2016)Precursor for antimicrobial peptides (Branco et al., 2014) |
| HASPB | Leishmania sp. | Directly translocates across membranes, potentially associated with exosomes (Maclean et al., 2012) | Trafficked to plasma membrane after acylation in Leishmania major and during expression in CHO cells (Stegmayer et al., 2005). Reaches outer surface of plasma membrane in punctate regions (Maclean et al., 2012) | Expressed during infective parasite stage, likely aiding in infection. (Depledge et al., 2010) |
| Histones | Mammals | Amphisome | Colocalizes with autophagosome machinery (Jeppesen et al., 2019) | Extracellular function from amphisome secretion is not clear, however histones released from cell lysis act as damage-associated molecular pattern molecules (Chen et al., 2014) |
| HIV TAT | Viral/Mammal | Passage through membrane: Incorporated into extracellular vesicles | Translocation occurs in a PIP dependent manner. (Chang et al., 1997, Zeitler et al., 2015), oligomerizes to form a pore, and can also become integrated into exosomes.(Mele et al., 2018) | Contributes to cell Kaposi sarcoma lesion growth (Ensoli et al., 1993), and neurological impairments (Ajasin and Eugenin, 2020, Marino et al., 2020) |
| HMGB1 | Mammals | Endolysosomal system | Secreted in vesicles (Gardella et al., 2002). During starvation, secreted by autophagy (relies on Atg5) (Dupont et al., 2011) | Secreted from immune cells, proinflammatory cytokine which activates toll-like receptor 4 (He et al., 2018) |
| Hsp70/ Hsc70 | Yeast Mammals |
Endolysosomal system | Colocalizes with LAMP1 (Mambula and Calderwood, 2006). Associated with extracellular vesicles in tumor cells (Zhang et al., 2017b). | Proinflammatory response in cancer, targets natural killer cells and macrophages (Santos et al., 2017) C. albicans Hsp70 is involved in host invasion and infection (Sun et al., 2010) |
| Hsp90 | Mammals | Not clear, found in vesicles, but also enriched in exomere | Found in exomere, as well as CD81, CD63 and CD9 negative exosomes (Jeppesen et al., 2019). Mediates exosome release (Lauwers et al., 2018) | Wound healing (Li et al., 2012b) |
| IL1-β | Mammals | Autophagosome or Amphisome fusion with the PM | Relies on ESCRT-I protein Tsg101 (Zhang et al., 2015), ATG5 depletion in macrophages decreases IL-1β secretion (Dupont et al., 2011) and sediments with LC3 positive vesicles (Zhang et al., 2015). Sorted into autophagosomes by binding TRIM16, and secreted in a syntaxin 3 and 4 dependent manner | Cytokine during inflammatory response (Zhang et al., 2020) |
| Microvesicles & permeabilization | Inflammasome mediated permeabilization (Martin-Sanchez et al., 2016), and shed into microvesicles (Mackenzie et al., 2001) | |||
| PGK1 | Mammals Yeast |
Oncotic release | Serum removal leads to oncotic pores and the release of PGK1 in Hela cells. PGK1 release is independent of vesicular transport, and is inhibited by serum or BSA (Chirico, 2011). | Plasmin reductase, inhibiting angiogenesis and tumor growth (Lay et al., 2000) |
| Thioredoxin | Mammals | Not well characterized | Proinflammatory stimuli lead to thioredoxin-1 secretion. Not found to be vesicular (Plugis et al., 2017, Rubartellisg et al., 1992) | Cleaved extracellular TXN1 (TRX80) is a pro-inflammatory cytokine, and inhibits beta amyloid aggregation (Léveillard and Aït-ali, 2017). |
Fig. 1.
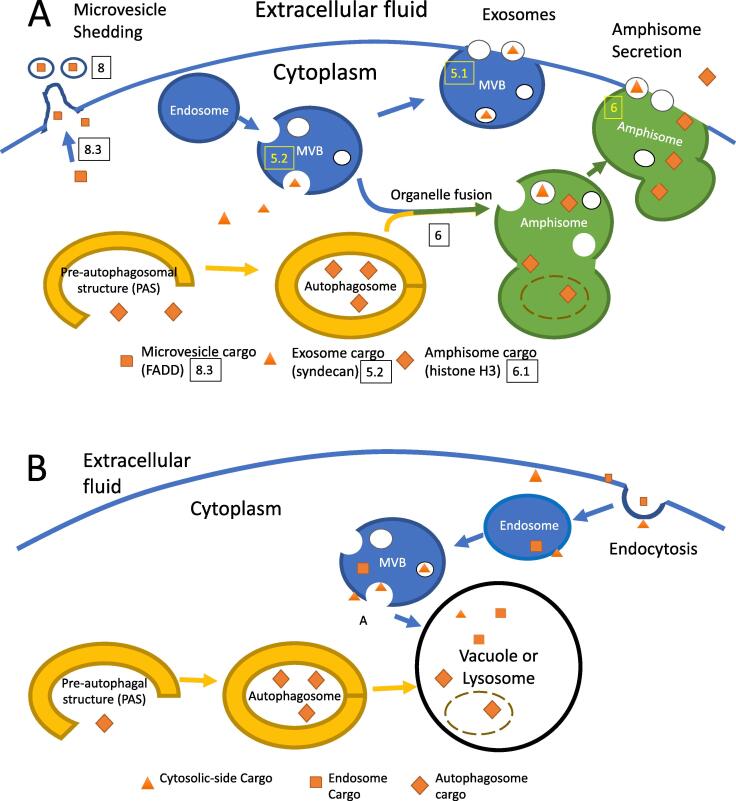
Cargo uptake and secretion vs. degradation in the endolysosomal system. Endosome-derived vesicles are shown in blue, and autophagosome-derived structures are ochre. Amphisomes form by fusion of endosome-derived and autophagosome-derived vesicles, and are colored green. The boxed numbers refer to the relevant section of the text. Proteins in the cytosol can be taken up into intraluminal vesicles of an MVB or incorporated into an autophagosome when a PAS (pre-autophagosomal structure) matures into an autophagosome during starvation. Squares represent cargo taken up into microvesicles; triangles, cargo taken up into exosomes; and diamonds, cargo taken up into double-membrane bounded autophagosomes. Examples of proteins in each category are listed at the bottom of each panel. A) Unconventional secretion by incorporation into vesicles. The endosomal-MVB pathway of secretion is shown, as is the amphisome pathway that results from fusion of the autophagosomes (ochre) with the MVBs (blue) to yield amphisomes (green) with the autophagosomal inner membrane dissolved (dotted line). B) Pathways to the lysosome/vacuole: endocytosis and autophagy. External membrane proteins (orange squares) are enveloped into the lumen of MVBs by ESCRT- dependent or independent pathways, targeted to the vacuole, and digested after fusion. Cytosolic proteins (diamonds and triangles) can be incorporated into ILVs in the MVB, or into the lumen of an autophagosome. Fusion of either body with the lysosome/vacuole leads to dissolution of ILV and autophagosomal inner membranes and subsequent degradation of protein cargo. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
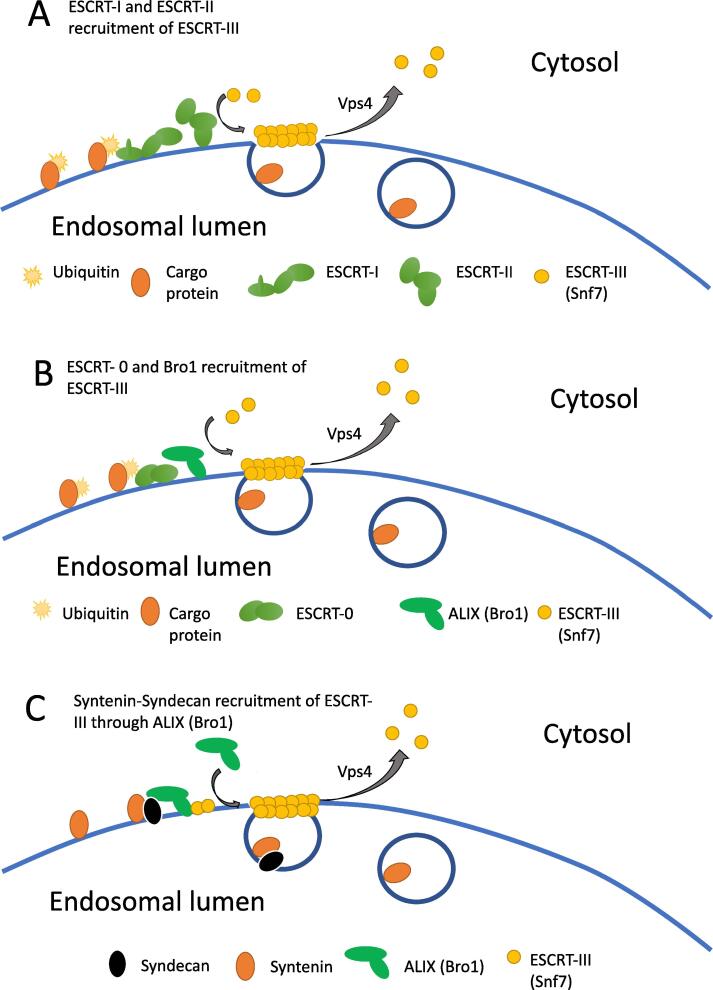
Mechanisms of intraluminal vesicle formation. In each case, ESCRT-II or ALIX is required for assembly of the ESCRT-III complex, and Vps4 facilitates ESCRT-III disassembly and membrane scission to form an ILV. A) Cargo at the MVB surface is ubiquitinated, recruiting ESCRT-I and II to recruit ESCRT-III (Snf7) pinching and scission. B) ESCRT-0 recruits ALIX/Bro1 to recruit ESCRT-III. C) Syntenin-syndecan-ALIX recruitment of ESCRT-III (ubiquitin-independent).
Fig. 3.
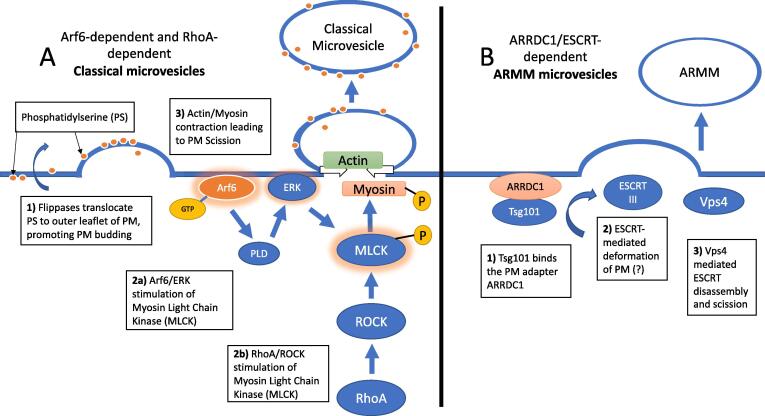
Two mechanisms of microvesicle shedding. A) Classical microvesicles formed by 1) translocation of phosphatidylserine to the outer leaflet and deformation of the PM, 2) stimulation of MLCK by either Arf6/ERK (2a) or RhoA/Rock (2b) (Li et al., 2012a), and 3) actin-myosin mediated vesicle scission. B) ARMM microvesicle formation by 1) Tsg101, 2) ESCRT-III, and 3) Vps4-mediated disassembly.
Fig. 4.
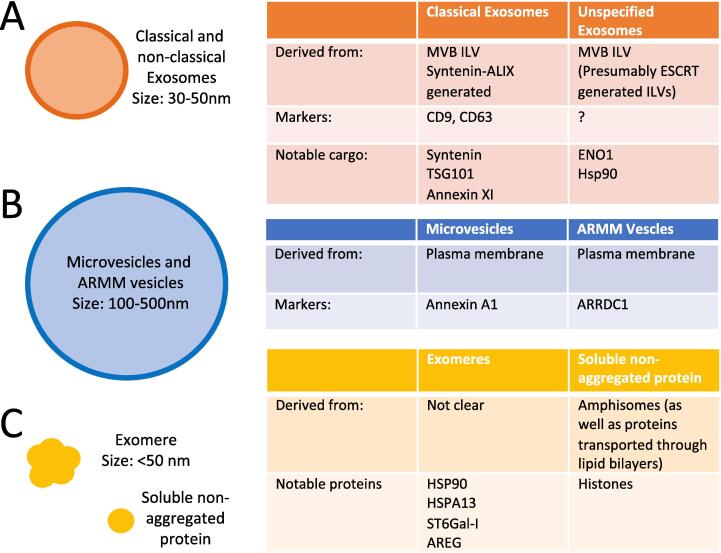
Summary of extracellular vesicles, nanoparticles, and proteins released from membrane bound organelles of mammalian cells. Biogenesis summaries and marker proteins are given where known. A) Classical and non-classical exosomes (vesicles derived from the MVB), discussed in Section 5. B) Microvesicles and ARMM vesicles (derived from pinching off from the PM), section 8. C) Non vesicular nanoparticles and proteins (section 9).
Unconventional protein secretion mechanisms are diverse. For example, In yeast, the ABC transporter Ste6 secretes a-factor across the plasma membrane (McGrath and Varshavsky, 1989). In mammals, some proteins are secreted by forming pore structures and moving directly across plasma membrane. Finally, a wide range of proteins in both yeast and mammals are taken up into intraluminal vesicles of multivesicular bodies or autophagosomes and released upon fusion with the plasma membrane. This review will summarize key criteria for demonstrating that a protein is unconventionally secreted, categorize unconventional secretory pathways, list characteristics associated with the various mechanisms, and suggest directions for future research.
1.1. An overview of classical secretion
Classically secreted proteins are translocated into the endoplasmic reticulum (ER), either co-translationally or post-translationally. The key steps and components of the co- and post-translational translocation pathways were identified by a combination of biochemical and genetic approaches (Barlowe and Millera, 2013, Delic et al., 2013, Rapoport et al., 2017). Material in these reviews is summarized here. In the co-translation translocation pathway, signal recognition particle (SRP) binds to the signal sequence located on the N-terminus of a secretory protein as it emerges from the ribosome. SRP pauses translation and guides the nascent chain-ribosome-mRNA complex to the ER by binding to SRP receptor (encoded by SRP101, and SRP102) located on the cytosolic surface of the ER. Upon hydrolysis of GTP, SRP returns to the cytosol and translation resumes, thereby translocating the nascent chain through the Sec61 translocon. The signal sequence is removed during translocation, and the mature protein folds in the lumen of the ER with the assistance of molecular chaperones such as BiP. In the post-translational translocation pathway, presecretory protein synthesis is completed in the cytosol and then the proteins are translocated into the ER with the assistance of the Sec62-Sec63 complex. In addition to Sec62 and Sec63, the complex contains Sec61, Sec71, Sec72, Sbh1, and Sss1 in the yeast model system. Due to the conserved nature of signal sequences, bioinformatics algorithms can distinguish between proteins that enter the secretory system and cytosolic proteins (Zhao et al., 2019).
Molecular chaperones play an important role in post-translational translocation (Chirico et al., 1988, Deshaies et al., 1988). In the cytosol, Hsp70 (Ssa1 in yeast) maintains the presecretory protein in a translocation-competent conformation and guides it to the Sec complex, where Sec62-Sec63 binds Sec61 and primes the channel to accept a protein substrate (Wu et al., 2019). Here Sec63 positions Ssa1 to deliver substrates to the Sec61 pore (Itskanov and Park, 2019). In the lumen of the ER, Kar2 (yeast ortholog of BiP) and Lhs1 bind incoming presecretory proteins and aid their translocation by trapping or ratcheting (Brodsky et al., 1995). Post-translational translocation of proteins has been described in yeast using several model proteins including prepro-α-factor. However, very few examples of post-translational translocation have been described in higher eukaryotes, even though orthologs to the yeast proteins are present (Johnson et al., 2013).
In the ER, newly co- or post-translationally translocated proteins are processed similarly. If they were glycosylated during translocation, their carbohydrate chains can be modified in the ER before they are transported in COPII vesicles to the Golgi apparatus. The proteins may be further glycosylated in the Golgi before they are packaged into secretory or transport vesicles. Vesicles may proceed to the plasma membrane without delay (constitutive secretory pathway) or remain in the cytosol until fusion is signaled (regulated secretory pathway). Upon fusion with the plasma membrane, the secretory proteins are released into the extracellular milieu (Novick et al., 1981).
After the classical secretory pathway was described, exceptions which bypassed it began to emerge. For example, some secreted proteins lack a signal sequence and never enter the ER or Golgi. They are called unconventionally secreted proteins (Dimou and Nickel, 2018). The idea of unconventional protein secretion is sometimes met with skepticism, and the mechanism of secretion can be difficult to elucidate. A summary of suggested experimental criteria to show a protein is unconventionally secreted is presented in Box 1, along with suggested criteria to guide a researcher in characterizing the mechanism of secretion.
Box 2.
Experimental analysis of unconventional secretion. There are several pathways known for unconventional secretion. This box serves as an experimentally-based guide to those pathways, which are discussed in this article with examples of specific proteins for each pathway.
-
•A protein is unconventionally secreted if it fulfills the following criteria:
-
•Lack a classical signal sequence.
-
•Secretion should not be affected by blocking the classical secretion pathway, by deleting or mutating classical secretory components, or by using chemical inhibitors (e.g. brefeldin A).
-
•If unconventional secretion can be blocked, classical secretion should not be affected. (In other words, classically secreted proteins, such as invertase, or GPI-anchored adhesins should continue to be secreted).
-
•
-
•To show a protein is unconventionally secreted by a specific transporter (section 3)
-
•The specific transporter should be identified and the secreted protein should physically interact with it. Genetic knockout or knockdown of a transporter should inhibit secretion. For example, yeast with a ste6 loss of function mutation are sterile due to lack a-factor secretion.
-
•
-
•To show a protein is unconventionally secreted by translocating across the plasma membrane, it should (section 4)
-
•Bind to the plasma membrane, which often requires cofactors or other proteins, and insert into the plasma membrane, which is often linked to a membrane remodeling.
-
•Have the ability to enter or leave liposomes, or other model systems in vitro (Prudovsky et al., 2013).
-
•
-
•To show a protein is unconventionally secreted by entering the lumen of an MVB, autophagosome, or amphisome (sections 5–7)
-
•The protein should colocalize with endosome or autophagosome markers
-
•Deleting or downregulating genes required for autophagy or endosome function should alter secretion
-
•Exosomal proteins of interest should be found in extracellular vesicles positive for MVB markers such as CD9, CD63, or CD81 in mammalian cells. Currently there are no comparable markers in fungal extracellular vesicles. However, the ESCRT proteins Hse1 and Vps27 (ESCRT-0) are found in fungal extracellular vesicles of C. albicans (Bielska and May, 2019).
-
•
-
•To show a protein is unconventionally secreted by microvesicles (section 8)
-
•Proteins of interest should be released in a vesicle shedding from the plasma membrane. Classical microvesicles will contain annexin A1, whereas ARMM microvesicles will contain ARRDC1 (Jeppesen et al., 2019). Microvesicle shedding can be imaged with transmission electron microscopy, and microvesicles can be sorted from other extracellular vesicles using high resolution centrifugation.
-
•
-
•To show a protein is unconventionally secreted in exomeres or as a non-vesicular free protein: (section 9)
-
•Exomeres should be isolated by size using a technique such as AF4 (asymmetric flow field-flow fractionation) (Zhang et al., 2018), density gradient fractionation (Jeppesen et al., 2019), or pelleting at high speed (Zhang et al., 2019). Isolated nanoparticles should lack a plasma membrane.
-
•Free proteins should not be associated with membrane bound organelles upon release.
-
•
2. Unconventionally secreted proteins are functional
Many unconventionally secreted proteins have extracellular functions, consistent with the notion that their secretion is biologically controlled. For example, enolase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are key components of glycolysis and have alternative extracellular roles (see Table 1). In S. cerevisiae, external GAPDH can be cleaved into antimicrobial peptides (Branco et al., 2014). In mammals, it can be used for iron sequestration (Sheokand et al., 2013). Hsp70 is an abundant cytosolic protein that when released from Cryptococcus neoformans plays a role in host-cell interactions and infections (Silveira et al., 2013). In mammalian cells, externalized Hsp70 can interact with LOX-1, a cell surface receptor (Calderwood et al., 2016). When released in vesicles, Hsp70 can act as a proinflammatory signal in response to bacteria (Anand et al., 2010, Maio, 2011). Hsp90-alpha (a truncated form of Hsp90) promotes tissue repair in mammals by inducing migration through extracellular signaling (Li et al., 2012b). Thus, unconventionally secreted proteins have important extracellular functions.
3. Direct transport across the plasma membrane by ABC transporters
A few proteins translocate through the plasma membrane with the help of ABC-type transport proteins.
3.1. Yeast a-factor
One of the best-known examples of an unconventional protein secretory system is that used by yeast a-factor, a mating pheromone. Yeast a-factor is coded by MFA1, and is translated as a 36 amino acid polypeptide precursor lacking a classical N-terminal signal sequence. In the cytosol, a cysteine residue near the C-terminus of the precursor protein is prenylated, the three C-terminal amino acids are cleaved off, and the cysteine residue is carboxymethylated. Next, its N-terminus is truncated in two sequential steps yielding the mature 12-amino acid a-factor (reviewed in Michaelis and Barrowman, 2012). a-factor exits the cell by passing through Ste6, an ABC transporter located in the plasma membrane (McGrath and Varshavsky, 1989). ABC transporters utilize energy from ATP to pump cargo, typically small, amphipathic molecules (Wilkens, 2015).
3.2. Hsp70
ABC transporters have also been implicated in unconventional secretion of Hsp70 in mammalian cells. It was proposed that Hsp70 exits mammalian cells via endolysosomal vesicles having first entered them in an ABC-transporter dependent manner after a heat shock (Mambula and Calderwood, 2006). This idea was based on evidence that the ABC-transporter inhibitor glibenclamide interferes with Hsp70 secretion. Mambula and Calderwood (2006) suggested Hsp70 is a substrate of ABC A1. However, glibenclamide is a sulfonylurea agent that interferes with membrane polarization in pancreatic β-cells (Aittoniemi et al., 2009, Fuhlendorff et al., 1998). At high doses, glibenclamide can target ABC A1, but ABC A1 is only known as a transporter of small molecules, such as lipids and free cholesterol (Phillips, 2018, Terao et al., 2011). A second drug, DIDS (4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid) targeting ABC transporters had a similar effect on Hsp70 secretion (Mambula and Calderwood, 2006). DIDS globally targets membrane depolarization through inhibition of anion transport (Akerboom et al., 1991). We propose that glibenclamide and DIDS inhibit Hsp70 secretion indirectly by targeting membrane depolarization.
4. Transporter-independent unconventional protein secretion
Some proteins, such as FGF1, FGF2, HIV-TAT, and annexins bypass organelles and vesicles and are directly translocated across the plasma membrane without the assistance of a transporter. Generally, these proteins are translated in the cytosol, bind lipids after they are post-translationally modified, undergo a conformational change facilitated by other proteins, and then transport across the plasma membrane (Popa et al., 2018, Prudovsky et al., 2002, Steringer et al., 2012, Stewart et al., 2018).
4.1. FGF1
In mammalian cells, FGF1 (fibroblast growth factor 1) is unconventionally secreted. Externally, FGF1 acts as an autocrine signal and activates four different variants of the tyrosine kinase FGF receptor (Ornitz and Itoh, 2015). FGF1 secretion is linked to Notch signaling (Kacer et al., 2011) and is induced by stress, such as heat shock (Jackson et al., 1992) or starvation (Shin et al., 1996). Upon heat shock, FGF1 dimerizes, associates with S100A13, Syt1, SphK1 and Cu2+, and then the resulting complex interacts with the plasma membrane. The complex destabilizes the plasma membrane and then translocates across the lipid bilayer, while simultaneously externalizing acidic phospholipids (Prudovsky et al., 2013). During translocation, FGF1 exists in a partially unfolded state, which may aid in this process (Prudovsky et al., 2013).
4.2. FGF2
FGF2 also directly crosses the plasma membrane but in a manner distinct from that of FGF1. The membrane-bound protein ATP1 A1 recruits FGF2 monomers to the plasma membrane, where it subsequently binds PIP2 (phosphatidylinositol 4,5-bisphosphate). PIP-induced oligomerization of FGF2 is dependent on an exposed Cys residue (La Venuta et al., 2015). Tec kinase phosphorylates FGF2 at tyrosine 82, which has been shown to stabilize FGF2 oligomerization in vitro (Steringer et al., 2012). FGF2 oligomers insert into the plasma membrane and form a pore (La Venuta et al., 2015, Steringer et al., 2012). Upon emergence from the plasma membrane, FGF2 oligomers are released into the extracellular space and bind to external heparan sulfate proteoglycans creating a reservoir of FGF2 (reviewed in La Venuta et al., 2015).
In addition to its intercellular signaling function following unconventional secretion, it has a robust intracrine pathway. FGF2 contains nuclear and nucleolar targeting signals (Sheng et al., 2004). In the nucleus, FGF2 can bind the upstream binding factor (UBF) and stimulate rRNA transcription (Sheng et al., 2005).
4.3. HIV-Tat
Unconventional secretion by direct translocation across the plasma membrane also plays an important role in viral infection. The HIV transcription factor Tat is a virulence factor that is unconventionally secreted. Once released from HIV infected cells, it can be taken up by uninfected cells (Chang et al., 1997). HIV-Tat is released in a PIP2-dependent manner and, like FGF2, oligomerizes to form a pore in the membrane, and it subsequently binds to heparan sulfate (Chang et al., 1997; (Mele et al., 2018, Zeitler et al., 2015). In addition to the pore structure, monomers can destabilize the plasma membrane to spontaneously pass through, and HIV-Tat can also be incorporated into exosomes (as in Fig. 1A) (Mele et al., 2018). Thus, unconventional secretion has a role in infectious diseases.
4.4. Annexins
Annexins are a family of proteins (12 in vertebrates) that bind phospholipids in a calcium-dependent manner and have a variety of extracellular functions, such as activation of G protein-coupled receptors, anti-coagulation, protection of the placental surface, and coating of microvesicles (Schloer et al., 2018). Annexin translocation across the plasma membrane is initiated by calcium-dependent membrane binding (Schloer et al., 2018). Membrane remodeling is likely involved in annexin transport (Stewart et al., 2018). HeLa cells treated with cinnamycin to induce phospholipid flipping have increased levels of annexin A2 and A5 on their surface. Deleting the scramblase TMEM16F, which translocates phospholipids across monolayers of a phospholipid bilayer, prevented annexins A2 and A5 from translocating to the cell surface (Stewart et al., 2018). Annexin A5 can also translocate across membranes of liposomes (Popa et al., 2018, Stewart et al., 2018). Annexin VI can form voltage-gated ion channels in artificial membranes at acidic pH after undergoing a conformational change (Golczak et al., 2001). Together these results suggest that unconventional secretion of annexins involves a conformational change, coupled with binding to and remodeling the plasma membrane while forming a pore structure. Thus, many annexins, Tat, and FGF2 unconventional secretion may involve similar mechanisms.
4.5. Phosphoglycerate kinase 1
Phosphoglycerate kinase 1 (PGK1) is a glycolytic enzyme that is unconventionally secreted. It has been found in the cell wall of C. albicans (Perumal et al., 2012) and S. cerevisiae (Miura, 2018). Additionally, it is secreted from mammalian cells where it can function as a disulfide reductase of plasmin. Extracellular PGK1 also activates autocatalytic production of angiostatin and a subsequent decrease in the rate of tumor growth (Lay et al., 2000). PGK1 and other proteins may also be released from cells by gentle, nonlethal manipulations of cell cultures. For example, washing cells such as HeLa cells with serum-free media - a very mild stress - resulted in the rapid release of PGK1 in non-vesicular form through a process termed oncotic release (Chirico, 2011).
5. Unconventional secretion through membrane-bound structures: endosomes, autophagosomes, amphisomes, CUPS, and microvesicles
There are three known mechanisms by which cytosolic proteins can be taken up into membrane-bound organelles or structures for secretion: 1) entering the endolysosomal system through incorporation into multivesicular bodies or lysosomes (Fig. 1A, blue), 2) engulfment by autophagosomes or autophagy derived structures such as amphisomes (Fig. 1A, ochre and green), and 3) secretion by microvesicles pinching off from the plasma membrane (Fig. 1A, top left). The mechanisms of secretion through these pathways are discussed below.
5.1. The endolysosomal system, incorporation of cytoplasmic proteins into multivesicular bodies (MVB), and release as exosomes
Multivesicular bodies (late endosomes) are formed by the maturation of early endosomes (Fig. 1A and 1B). Together they are key components of the endolysosomal system, which directs endocytosis, recycling, and degradation of extracellular material and cell membrane components. During maturation of MVBs, the membrane invaginates and captures cytosolic proteins into vesicles that pinch off into the lumen of the MVB (Fig. 1A). Also, details of cytoplasmic entry into MVBs are discussed in section 5.2 and in Fig. 2; and microvesicle formation is described in section 8 and in Fig. 3.
The intraluminal vesicles have two fates. They can be unconventionally secreted as exosomes upon fusion of the MVB with the cell membrane (Choi et al., 2013, Hessvik and Llorente, 2018). Alternatively, proteins in ILVs can be digested upon fusion of the MVB with the vacuole or lysosomes (Fig. 1B). The signals that determine these alternate fates of MVB and autophagosomal cargo proteins are currently unknown.
The tetraspanins CD63, CD81, and CD9 have traditionally been considered exosome markers (Hessvik et al., 2016, Jakobsen et al., 2015, Raposo and Stoorvogel, 2013, Willms et al., 2016), and association with these markers has been used an indicator of exosomes cargo. Although several proteins have been identified as exosomal cargo (Baietti et al., 2012, Clayton et al., 2005, Willms et al., 2016), many such identifications are now in question (Jeppesen et al., 2019).
Jeppesen and coworkers (2019) used very gentle, high resolution ultracentrifugation and direct immunoaffinity capture to isolate exosomes and define their contents. Their analysis distinguished classical exosomes from other extracellular vesicles. They showed that the membrane of classical exosomes contains CD63, CD81, CD9, flotillin -1 and -2, EGFR, integrin beta1 and alpha2, and Na/K ATPase. Proteins associated with the biogenesis of exosomes include syntenin-1, ALIX, TSG101, and ARRDC1 (Jeppesen et al., 2019). Importantly, classical exosomes lack metabolic enzymes, such as GAPDH, enolase 1, and pyruvate kinase M; these were previously identified as exosomal cargo. The exosomes also lack annexin A1, A2, and V, but annexin VII and X1 are weakly associated with them. Rab7 is absent from classical exosomes, but Rab11 and Rab27A are weakly associated with them. In addition, they lack certain components of nucleosomes, autophagy, miRNA machinery, RNA-binding proteins, and the cytoskeleton. In summary, the limited number of components suggests that cargo is specifically selected during classical exosome biogenesis.
Ubiquitin-dependent (Anand et al., 2019) and ubiquitin-independent (Baietti et al., 2012, Gauvreau et al., 2009) pathways can generate and sort proteins into exosomes (Fig. 2). Both mechanisms of ILV formation are discussed below.
5.2. Intraluminal vesicle (ILV) generation at the MVB
An ensemble of ESCRT proteins are found in four ESCRT complexes that control the formation of intraluminal vesicles (see Table 2). Critically, there are several ways that these complexes trigger the assembly of ESCRT-III, whose polymerization promotes membrane constriction and eventual vesicle fission with the assistance of the AAA ATPase vacuolar protein-associating sorting protein 4 (Vps4) (Fig. 2).
Table 2.
ESCRT complex components in yeast and mammals.
| Complex name | Yeast names | Mammalian Names | Function in ILV biogenesis |
|---|---|---|---|
| ESCRT-0 | Hse1 Vps27 |
Stam1/2 HRS |
Binds phosphatidylinositol-3-phosphate of the multivesicular body outer leaflet (Slagsvold et al., 2006) and ubiquitin (Mosesso et al., 2019). Recruits Bro1 (ALIX), leading to localized assembly of the ESCRT-III in yeast (Tang et al., 2016). |
| ESCRT-I | Vps23 Vps28 Vps37 Mvb12 |
TSG101 hVps28 Vps37 MVB12 |
Binds ubiquitinated proteins on the surface of the MVB and deform membranes for other proteins to bind, but do not form buds (Schöneberg et al., 2016). |
| ESCRT-II | Vps36 Vps22 Vps25 |
EAP45 EAP30 EAP20 |
Binds ubiquitinated proteins on the surface of the MVB, ESCRT-I (Schöneberg et al., 2016) and PI3P (Feyder et al., 2015), Vps25 activates the Vps20 subunit of ESCRT-III (Teis et al., 2010) |
| ESCRT-III | Vps20 Snf7 Vps24 Vps2 |
CHMP6 CHMP4 CHMP3 CHMP2 |
ESCRT-III monomers such as Snf7 are recruited and activated by upstream effectors, and polymerize to deform membranes. Snf7 exists in two conformations- a closed conformation associated with the cytosol, and an open, active conformation which binds membranes and polymerizes (Schöneberg et al., 2016). Snf7 can be activated by Vps20 through Vps25 (ESCRT-II) (Teis et al., 2010) or directly by Bro1 (Tang et al., 2016, Wemmer et al., 2011) |
| VPS4 | Vps4 Vps60 Vta1 |
SKD1 CHMP5 LIP5 |
ESCRT-III is disassembled and recycled to the cytosol by the Vps4 complex consisting of the hexameric, pore forming AAA + ATPase (Schöneberg et al., 2016). |
| ALIX/Bro1 | Bro1 | ALIX | Auxiliary component of the ESCRT system. Recruits ESCRT-III for budding and scission (Tang et al., 2016, Wemmer et al., 2011). Utilized to generate “classical exosomes” (Jeppesen et al., 2019) (discussed below) by binding syndecan-syntenins in mammals (Baietti et al., 2012, Friand et al., 2015) |
In the ubiquitin-dependent ILV formation pathways (Fig. 2A and B), the cytosolic domains of membrane-bound endosomal proteins are ubiquitinated (Ahmed et al., 2019). Ubiquitination serves as a binding signal for ESCRT protein assembly in fungi and metazoans (Mosesso et al., 2019). Deubiquitinating enzymes, such as Doa4, remove ubiquitin before sorting is completed (Kimura et al., 2014), and the VPS4 complex leads to ESCRT-III disassembly and vesicle closure (Schöneberg et al., 2016).
Several models suggest that ubiquitin-dependent ESCRT assembly occurs in at least two parallel pathways (Tang et al., 2016) (Fig. 2A and 2B). In the first pathway (Fig. 2A), ESCRT-III is recruited through ESCRT-I and II bound to ubiquitinated proteins (Tang et al., 2016). Here, ESCRT-II binds PI3P and ESCRT-II’s two Vps25 subunits activate ESCRT-III oligomerization (Feyder et al., 2015, Teis et al., 2010). This process is coupled with enrichment of ceramide in microdomains of endosomal membranes (April et al., 2008) and phosphatidic acid (Friand et al., 2015, Ghossoub et al., 2014) to promote negative membrane curvature. There is evidence that ESCRT-0 can assemble the ESCRT I/ESCRT II complex prior to ESCRT-III polymerization (Henne et al., 2011).
In the second pathway (Fig. 2B), ESCRT-0 binds ubiquitinated cargo, as well as endosome-specific phosphatidylinositol 3-phosphate (PI3P) (Henne et al., 2011). In yeast ESCRT-0 then recruits Bro1 (mammalian ALIX), which can directly activate the ESCRT-III protein Snf7 to polymerize, deform endosomal membranes, and lead to ILV formation (Tang et al., 2016). Both of these pathways are ubiquitin dependent.
In addition to the two ubiquitin-dependent pathways, there is a ubiquitin-independent pathway (Fig. 2C). In this pathway, membrane cargo (e.g. syndecan) can be selectively recruited into ILVs by syntenin, an adaptor for recruiting ALIX (Baietti et al., 2012). Membrane cargo marked with ALIX leads to assembly of ESCRT-III, and membrane constriction and vesicle fission proceeds as described above (Baietti et al., 2012). Syntenin can also bind tetraspanins such as CD63, but this is not required for exosome biogenesis (Friand et al., 2015).
Some intraluminal vesicles of MVBs are released as exosomes after MVBs are transported along microtubules to the cell membrane. With the assistance of Rab proteins, actin, and SNAREs, MVBs fuse with the cell membrane and the exosomes are released into the extracellular milieu (Fig. 1A) (Hessvik and Llorente, 2018).
5.3. Alternative pathways to an ILV, vacuole, or lysosome
MVBs fusing with each other or with vacuoles can also generate ILVs at points of docking and fusion, and selectively sequester proteins into these ILVs. This process is called the Intraluminal Fragment Pathway (Mattie et al., 2017, Mcnally and Brett, 2018). Notably, this process is ESCRT-independent (Mcnally and Brett, 2018). Vacuolar membrane can invaginate to capture cytosolic proteins into in intravacuolar membrane vesicles during microautophagy (Reggiori and Klionsky, 2013). However, details of vesicle formation are unknown, and the secretion of proteins that had been sequestered into vacuolar ILVs during microautophagy or passage through the Intraluminal Fragment Pathway has not been demonstrated.
5.4. Lysosomal secretion
Metazoan lysosomes (or endolysosomes) can fuse with the plasma membrane (Li and Kane, 2009). For example, osteoclasts - cells that resorb bone - use lysosomal vesicles to export bone-degrading enzymes, such as cathepsin K. The vesicles fuse with the plasma membrane and release their contents into the bone resorption cavity created by the osteoclasts. The low pH of the cavity facilitates hydroxyapatite dissolution and provides optimal conditions for the activity of released lysosomal enzymes. The acidic pH is generated by a membrane-bound, ATP-dependent proton pump (Lacombe et al., 2013).
5.4.1. FABP4 secretion
One example of a protein that is secreted through a lysosome is FABP4. FABP4 is a fatty acid binding protein, a type of intracellular lipid chaperone which binds hydrophobic ligands. Elevated levels of intracellular and secreted FABP4 are linked to type 2 diabetes and several cardiovascular diseases (Furuhashi et al., 2014). FABP4 lacks a signal sequence and its secretion is stimulated by lipolysis-mediated signaling (Mita et al., 2015).
Mammalian FABP4 enters endosome and lysosome compartments, and is secreted as lysosomes fuse with the plasma membrane (Villeneuve et al., 2018). FABP4 secretion is independent of Tsg101 (ESCRT-0) and Hrs (ESCRT-I), suggesting it does not enter the lysosome through canonical ILV formation at the MVB surface (Villeneuve et al., 2018). FABP4 secretion is blocked in the absence of autophagy-related protein Beclin-1 (Atg6) and Sirtuin-1 signaling (Josephrajan et al., 2019). However, canonical autophagosome formation is also dispensable for FABP4 secretion (Villeneuve et al., 2018) and Atg6 plays a role in endosomal trafficking (Cao and Klionsky, 2007). Taken together, FABP4 enters a lysosome in an autophagy- and ESCRT-independent pathway.
FABP4 is released in a non-vesicular form, an observation that suggests FABP4 is translocated directly into the lumen of the endosome or lysosome rather than being sequestered into an ILV. The mechanism of its translocation has not been specifically characterized (Villeneuve et al., 2018). A possibility is chaperone-mediated autophagy, where HSC70 unfolds proteins, facilitating their translocation into the lysosome through an interaction with LAMP2A (Tang et al., 2017). Chaperone-mediated autophagy substrates have a KFERQ-like motif, and the FABP4 protein sequence contains an in silico predicted KFERQ-like motif (Kirchner et al., 2019).
6. Secretion through autophagosomes and amphisomes
Autophagosomes are double-membrane organelles that capture other organelles and large areas of cytoplasm and deliver them to vacuoles (lysosomes) (Fig. 1). Autophagosomes form during a starvation-induced complex process for breaking down cellular components. In addition to their role in autophagy, molecular components of autophagosomes are critical for unconventional secretion of some proteins (Bruns et al., 2011, Cruz-Garcia et al., 2014, Duran et al., 2010), and some unconventionally secreted cargo, such as IL-1β, are localized in autophagosomes (Zhang et al., 2015). Protein components of autophagy (Atg proteins) assemble at a vesicular structure called a phagophore, sometimes called a pre-autophagosomal structure, or phagophore assembly site (PAS; Fig. 1) (Fader and Colombo, 2006, Tanida et al., 2004). The PAS expands to form an autophagosome upon starvation (Reggiori and Klionsky, 2013) where cytosolic contents are taken up. A canonical autophagosome then fuses with the vacuole, where its contents are recycled (Fig. 1B).
Autophagosomes sometimes fuse with multivesicular bodies to form structures called amphisomes (Fig. 1A) (Klionsky et al., 2014). Amphisomes can fuse with the plasma membrane and deliver cargo to the external environment as a mechanism of unconventional protein secretion, or fuse with the lysosome, where their contents are degraded. Proteins such as Park7 (also known as DJ-1, a paralog of Hsp31-34 in yeast) are unconventionally secreted under the same conditions that cause amphisomes to form, leading to speculation that they are taken up into amphisomes and released when the amphisomes fuse with the plasma membrane (Urano et al., 2018). However there are still many unknowns, including a specific molecular signal that causes amphisomes to fuse with the plasma membrane rather than the lysosome. One of the best characterized mechanisms of unconventional protein secretion through amphisomes, using Histone H3 as a model, is discussed below.
6.1. Histone H3
Histones H3 is unconventionally secreted through amphisomes. It can be taken up into autophagosomes and is found within the same structure as the autophagy marker LC3 (Dou et al., 2015, Jeppesen et al., 2019). Many of the same LC3 structures also contained the MVB marker CD63, and, therefore, were amphisomes. H3 was found in the lumen of amphisomes but not within its ILVs. The LC3-CD63 positive amphisomes fused with the plasma membrane and released H3 in a nonvesicular form. This result can be explained by the degradation of the inner autophagosomal membrane in a mature autophagosome or amphisome, while ILVs, such as exosomes, remain intact (Fig. 1A) (Jeppesen et al., 2019, Tsuboama et al., 2016, Zhao and Zhang, 2019). Therefore, histone H3 secretion proceeds as follows: Histone H3 is taken up into an LC3-positive autophagosome, the autophagosome matures and its inner membrane degrades, the autophagosome fuses with CD63 positive endosomes to form an amphisome, and the amphisome fuses with the plasma membrane and releases a non-vesicular form of H3 (Fig. 1A) (Jeppesen et al., 2019).
6.2. IL-1β
IL-1β is a cytokine that is released by either pyroptosis (cell leakage) or through an autophagy-mediated unconventional protein secretion mechanism. Upon inflammation, IL-1β is processed from pro-IL-1β to a mature state by caspase-1, and the mature fragment is subsequently released from cells. In macrophages, a multi-protein complex called the inflammasome promotes the externalization of IL-1β by plasma membrane permeabilization (Martin-Sanchez et al., 2016). However, IL-1β can also be released without compromising the plasma membrane as described below.
Human embryonic kidney cells (HEK293T) co-expressing pro-IL-1β and pro-caspase1 (pre-interleukin 1β convertase) secrete mature IL-1β (Zhang et al., 2015) without the inflammasome-mediated pyroptosis, demonstrating that IL-1β can be secreted without compromising the plasma membrane. IL-1β secretion was linked specifically to autophagy in macrophages and later in other cell lines by using nutrient starvation and pharmacological inhibition of autophagy. Starvation recruits IL-1β to an LC3-positive structure (Dupont et al., 2011), and pharmacological inhibition of autophagy blocks IL-1β secretion (Zhang et al., 2015). Therefore, IL-1β can be secreted through an autophagy-dependent, inflammasome-independent pathway. Unlike pyroptosis, the autophagy-dependent, inflammasome-independent secretion of IL1-β is controlled (Sitia and Rubartelli, 2020).
Evidence also supports IL-1β sorting into autophagosomes and then sorting to the plasma membrane instead of the lysosome (Fig. 1). An activated inflammasome leads to caspase-1 maturation and processing of pro-IL-1β into a mature form. Mature IL-1β binds membranes containing Sec22b through TRIM, a specialized cargo receptor. This complex is recruited to LC3-II positive membranes, which subsequently mature into autophagosomes. Finally, autophagosomes fuse with the plasma membrane, with the assistance of syntaxin 3 & 4, and IL-1β is released (Kimura et al., 2017).
A recent paper used IL-1β secretion to identify an unconventional transport system into the ER-Golgi complex (ERGIC) (Zhang et al., 2020, Zhang et al., 2015). Based on knowledge that IL-1β secretion requires unfolding of cytoplasmic pro-IL1β, the authors blocked unfolding by fusing DHFR to IL1β, then preventing unfolding of the DHFR with its ligand aminopterin. The fusion protein then became associated and was cross-linked with the ERGIC protein TMED10, among 10 other transmembrane proteins. shRNA knockdowns and deletions show that TMED10 was required for robust secretion. The IL-1β-TMED10 complex assembles into a transmembrane complex that acts as a translocation pore for the IL-1β. The ERGIC IL-1β cargo is then secreted through diverse MVB-associated and autophagosomal-associated pathways. Knocking down TMED-10 also reduces secretion of galectins, the chaperone HSPB5, Tau, anexinA1, other forms of IL-1 and some other interleukins, but not some other unconventionally secreted proteins. The authors propose that TMED10-dependent secretion be called the TMED10-channeled UPS (THU) pathway.
The THU pathway would explain the observation that knockdown of the ESCRT-1 component Tsg101 only partially reduces secretion (Bänfer et al., 2018). In addition, common secretion through THU would explain results of Tapia et al., (2019) that IL-1β shares some secretion elements, but not all, with IL-1α and IL-18. Similarly, inhibition of autocatalyzed plasma membrane translocation of Tau is only partial (Merezhko et al., 2018), consistent with secretion through the THU pathway as well. Thus, the THU pathway is one of several routes for unconventional secretion of some cytoplasmic proteins.
6.3. Enterovirus export through secretory autophagy
Enteroviruses, such as poliovirus, are released through autophagy (Mutsafi and Altan-bonnet, 2018). Poliovirus capsids colocalize to LC3-II in mammalian cells and are released in phosphatidylserine-rich vesicles, while host cells remain intact (Chen et al., 2015). Interestingly, autophagosomes containing viruses are sorted to the plasma membrane rather than the lysosome likely because they lack syntaxin17, a SNARE for fusing with the lysosome (Chen et al., 2015). Therefore, secretory autophagy is a process that is clinically relevant as a mechanism of viral infection and further investigations may shed light on how unconventionally secreted proteins are sorted to the plasma membrane rather than to the lysosome.
7. CUPS
In yeast, two model proteins (discussed below) are unconventionally secreted through a distinct compartment called CUPS (compartment for unconventional protein secretion). Nutrient starvation, autophagy genes and MVB components are required for unconventional protein secretion through CUPS (Duran et al., 2010). However, CUPS is not a canonical autophagosome or MVB (Cruz-Garcia et al., 2018, Cruz-Garcia et al., 2014, Curwin et al., 2016). Rather, CUPS is derived from ER, Golgi and endosomal membranes which are remodeled in the absence of glucose (Cruz-Garcia et al., 2014).
CUPS formation can be traced by tracking the Golgi protein Grh1 (a homologue of the mammalian Golgi protein GRASP55 and GRASP65) after starvation. Within 30 min, GRH1 migrates from ER exit sites and the early Golgi to several distinct foci, which are recognized as CUPS (Bruns et al., 2011, Cruz-Garcia et al., 2014). Transmission electron microscopy shows CUPS is initially comprised of tubules and small vesicles, and is later stabilized by engulfment of ESCRT-III-coated saccules (Curwin et al., 2016).
7.1. Acb1
Acb1 (Acyl-CoA-binding protein) is unconventionally secreted from S. cerevisiae via CUPS upon nitrogen and glucose starvation (Curwin et al., 2016, Duran et al., 2010). Acb1 is a homologue of the Dictyostelium discoideum protein AcbA, which is unconventionally secreted and processed into a signal to initiate prospore encapsulation. Acb1 secreted from S. cerevisiae can activate D. discoideum encapsulation, enabling a functional screen of Acb1 secretion.
Acb1 secretion requires several autophagy proteins (Atg5, Atg7, Atg8, and Atg12), Grh1 (a Golgi protein), as well as the MVB proteins Vps23 (ESCRT-I) and Snf7 (ESCRT-III) (Curwin et al., 2016, Duran et al., 2010). In starvation media, Acb1 is recruited to CUPS, becomes engulfed within saccules, and the mature encapsulated structure is stabilized by Snf7 (Curwin et al., 2016). Acb1 secretion also requires Sso1, a plasma membrane t-SNARE (Duran et al., 2010), suggesting the stabilized form of CUPS or a derivative vesicle directly fuses with the plasma membrane.
7.2. SOD1
Human SOD1 (superoxide dismutase 1) is a factor in amyotrophic lateral sclerosis (ALS). SOD1 lacks a signal sequence and is associated with exosomes and secretion through CUPS (Basso et al., 2013, Garcia et al., 2016). Overexpressing SOD1 in mouse astrocytes stimulates exosome release, and overexpressing a familial mutant (SOD1G93A) increases exosome release, including exosomes containing SOD1G93A (Basso et al., 2013, Silverman et al., 2019). S. cerevisiae also expresses a form of Sod1 that lacks a signal sequence. Nutrient starved S. cerevisiae unconventionally secretes Sod1 to the cell wall, and mutations targeting a diacidic sequence that is conserved between S. cerevisiae and humans decreases Sod1 secretion, suggesting Sod1 is selectively secreted in both species (Garcia et al., 2016). Yeast Sod1 follows a similar secretion pathway to Acb1 and requires the Golgi/CUPS associated protein Grh1, ESCRT-I Vps23, and ESCRT-III Snf7 (Garcia et al., 2016).
8. Microvesicles
Microvesicles are vesicles which are shed directly from the plasma membrane and contain unconventionally secreted cargo. Cytosolic proteins can be recruited into microvesicles as they form (Fig. 1A). Microvesicle formation requires the plasma membrane to pinch outwards in a process similar to viral budding.
8.1. Characteristics of microvesicles
Microvesicles are traditionally differentiated from exosomes by their size (Oliveira et al., 2010, Bello-morales et al., 2018, Choi et al., 2013, Niel et al., 2018). Microvesicles are typically 50–1000 nm in diameter (Niel et al., 2018) while exosomes are between 30 and 150 nm (Fig. 4A and B) (Niel et al., 2018, Meldolesi, 2018).
Microvesicles are heterogenous and several different molecular markers have been used to characterize them. When large microvesicles were isolated by size only, they contained the ER protein GP96 (also known as GRP94, a mammalian form of Hsp90 found in the ER). Exosomes are smaller and do not contain GP96, so GP96 is a microvesicle marker (Kowal et al., 2016). When microvesicles were immune-isolated, distinct subpopulations were found to contain either ARRDC1 (arrestin domain containing protein 1) and TSG101; or annexin A1, annexin A2, and ARF6. Notably, TSG101 was found in small extracellular vesicles (40–100 nm) (Jeppesen et al., 2019), which had previously been identified as microvesicles based on the vesicle diameter (Kowal et al., 2016). The number of classes of microvesicles suggests they are formed by multiple pathways.
There are several factors which can stimulate the release of microvesicles from the plasma membrane. Mammalian cells treated with the viral proteins HIV-1 R5, X4 gp120, or Tat increase the number of extracellular vesicles, which can induce inflammation, oxidative stress and senescence in other cells (Hijmans et al., 2019). However, the origin of these microvesicles is not clear because they were not sorted by size, or more importantly, markers (Jeppesen et al., 2019).
8.2. Models of microvesicle formation
Several models of microvesicle formation have been proposed based on studies using mammalian cells, such as erythrocytes and cancer cells. In general, they involve the plasma membrane deformation and scission.
One mechanism of microvesicle formation is similar to blebbing, during which the cytoskeleton adjacent to the site of shedding on plasma membrane is disassembled and phosphatidylserine is translocated to the outer leaflet causing the plasma membrane to bulge (Fig. 3A) (Kalra et al., 2016). This process is linked directly to Ca2+ signaling (Pollet et al., 2018). Finally, signaling cascades localized at the microvesicle budding area lead to membrane scission through activation of myosin light chain kinase (MLCK) and subsequent actin-myosin contraction (Fig. 3A). Two distinct signaling pathways are known to activate MLCK and release microvesicles: 1) Arf6 activation of phospholipase D, ERK, and MLCK (Kalra et al., 2016, Muralidharan-Chari et al., 2009, Pollet et al., 2018) and 2) RhoA-ROCK mediated signaling of MLCK (in an Arf6 independent manner) (Li et al., 2012a). Microvesicles released in this manner are called microvesicles or “classical microvesicles” (Jeppesen et al., 2019).
A second population called ARMM microvesicles, or ARMMs (arrestin domain-containing protein 1 mediated microvesicles) (Jeppesen et al., 2019, Nabhan et al., 2012) are shed by an ESCRT-mediated mechanism (Fig. 3B). In short, the ubiquitin ligase adapter protein ARRDC1 recruits the ESCRT-I TSG101 (Vps23 in S. cerevisiae) to the plasma membrane (Pollet et al., 2018). TSG101 recruits ESCRT-III leading to the outward pinching of the plasma membrane to generate a microvesicle. Vps4 is recruited to the plasma membrane. It disassembles ESCRT-III and mediates membrane scission (Pollet et al., 2018). Deleting the ESCRT Snf7 in S. cerevisiae increases the size of both microvesicles and exosomes (Oliveira et al., 2010), thereby implicating ESCRTs in formation of at least some microvesicles.
8.3. Microvesicle cargo
Some microvesicles contain clinically important cargo. For example, during inflammation, induction of an NLRP3 inflammasome leads to sorting FADD (Fas-associated protein with death domain) into microvesicles (Mouasni et al., 2019). In addition, oncogenic fibroblasts release microvesicles containing FAK (focal adhesion kinase), and these vesicles can promote anchorage-independent growth in non-oncogenic fibroblasts (Kreger et al., 2016).
Microvesicles are also a vector for viral export. Human oligodendrocytes infected with Herpes-1 virus shed microvesicles containing infectious virions (Bello-morales et al., 2018). Viruses also hijack host-cell machinery to generate microvesicles. For example, HIV Gag and Ebola EvVp40 contain amino acid sequences that mimic ARRDC1′s binding site for TSG101. They recruit ESCRT proteins at the plasma membrane and generate viral buds (Martin-Serrano et al., 2003, Martin-Serrano et al., 2001). Thus, microvesicles can play an important role in virulence.
9. Secreted proteins which are not associated with vesicles
As the secretome of cells was analyzed, it became apparent that many cytosolic proteins are unconventionally secreted in a form that is not enclosed in lipid bilayer vesicles (Jeppesen et al., 2019, Zhang et al., 2018). These can be placed into two categories: Proteins released in a soluble (or non-aggregated) form, including proteins discussed above such as IL-1β, a-factor, histones, and Acb1; and proteins secreted as distinct clusters of newly characterized nanoparticles called exomeres (Zhang et al., 2018). How proteins can be secreted in one form and not the other is not clear at this time.
9.1. Protein secretion into non-vesicular exomeres
Exomeres are nonvesicular nanoparticles less than 50 nm in diameter (Fig. 4C) (Zhang et al., 2018). Abundant markers for exomeres are Hsp90 (Zhang et al., 2018) and HSPA13 (Jeppesen et al., 2019). Exomeres have a distinct protein composition. They are enriched in extracellular matrix proteins, components of the proteasome (Zhang et al., 2018), metabolic proteins (e.g. hexokinase, glucose-6-phosphate isomerase, GAPDH, pyruvate kinase, and enolase) (Jeppesen et al., 2019), and nucleotide binding proteins (e.g. Argonaut and APP) (Zhang et al., 2019). Although exomeres lack a lipid bilayer, they contain trace amounts of lipids common to microvesicles including sterols enriched for esterified cholesterols (Zhang et al., 2019).
Many proteins associated with exomeres have extracellular moonlighting functions as demonstrated in mammals (Takaoka et al., 2014, Sheokand et al., 2013) and yeast (Branco et al., 2014, Gozalbo et al., 1998). Exomeres also contain functional proteins and can modify external proteins. For instance, exomeric St6gal (β-galactoside α2,6-sialyltransferase 1) is functional, and modifies N-glycosylated proteins of recipient cells in culture to activate prolonged EGFR signaling in recipient cells (Zhang et al., 2019). Currently, the origin of exomeres is unknown.
A summary of these various secretion mechanisms is shown in Fig. 4, and characteristics of individual unconventionally secreted proteins are listed in Table 1.
10. Is unconventional secretion frequently induced by stress?
Many proteins are unconventionally secreted upon cell stress (Table 3) (Sitia and Rubartelli, 2020). For example, IL-1β secretion and Acb1 secretion rely on induction of autophagy. FGF1 is secreted following heat shock or starvation, and histone H3 is secreted through amphisomes after induction of autophagy. Washing cells, such as HeLa cells, with serum-free media can also result in the rapid unconventional secretion of proteins through a process termed oncotic release (Chirico, 2011). Additionally, many heat shock proteins, such as Hsp90 are released in exosomes upon heat shock (Clayton et al., 2005). While unconventional secretion of some proteins occurs under normal conditions, (Delgado et al., 2001, López-villar et al., 2006, Perumal et al., 2012, Zhang et al., 2018) different types of stress can activate or stimulate their release. As Sitia and Rubartelli (2020) note, the controlled secretion of some unconventionally secreted proteins under stress conditions can limit an inflammatory response, and is distinct from a damage associated molecular pattern which is unregulated.
Table 3.
Proteins Unconventionally Secreted After Stress
| Protein Name | Organism | Inducer | References |
|---|---|---|---|
| ACB1 | Yeast | Nutrient starvation/Autophagy | (Duran et al., 2010) (Malhotra, 2013) |
| FGF1 | Mammals | Heat shock Type 2 diabetes | (Jackson et al., 1992) (Wang et al., 2016) |
| FADD (Fas Associated Death Domain) | Mammals | Inflammasome | (Mouasni et al., 2019) |
| IL-1β | Mammals | Autophagy/mTOR | (Zhang et al., 2015) |
| Histones | Mammals | Autophagy | (Jeppesen et al., 2019) |
| HMGB1 | Mammals | LPS induction | (Gardella et al., 2002) |
| Hsp90 | Mammals | Heat Shock | (Clayton et al., 2005) |
| PGK1 | Mammals | Change in colloidal osmotic pressure (oncotic release) | (Chirico, 2011) |
11. Summary and conclusion
Although most proteins secreted from eukaryotic cells pass through the classical secretory pathway, some are secreted through unconventional pathways. For example, yeast a-factor is translocated across the plasma membrane by an ABC transporter. Other proteins, such as IL-1β, FGF1, and FGF2, interact with the plasma membrane first and then are directly translocated across it.
Additionally, many cytosolic proteins are sorted into vesicles for unconventional protein secretion. They can be taken up into double membrane structures such as MVBs and autophagosomes which then fuse with the plasma membrane and be released into the extracellular milieu. They can also be sorted into microvesicles that are shed from the plasma membrane.
However, not all unconventionally secreted proteins are released in a vesicular form, even if a key step in their secretion involves incorporation into a membrane-bound structure. Proteins, such as histones and FABP4, are sorted from the cytosol in membrane-bounded organelles such as MVBs or amphisomes, and are then secreted as free proteins.
There are many examples of unconventionally secreted proteins in a variety of eukaryotes. Unconventionally secreted proteins are biologically functional outside of the cell and their presence is not simply due to cell leakage. Many unconventionally proteins are released due to stress, and their mechanisms of secretion are important biological processes to further characterize. Characterizing these proteins will tell us more about these processes and will also increase the sizes of datasets used to predict which proteins are unconventionally secreted, as well as the pathways involved (Zhao et al., 2019).
Many unconventional secretory proteins are clinically important. Proteins such as IL-1β and FGF2 are cytokines and their secretion results in signaling. FABP4 secretion is linked to diabetes and cardiovascular disease (Furuhashi et al., 2014) and studying its mechanism of secretion can help develop treatments. Extracellular vesicles contribute to a variety of pathologies (Cesselli et al., 2018). Microvesicle contents from cancer cells can be oncogenic, and controlling either microvesicle generation or cargo can limit tumor growth (Kreger et al., 2016, Li et al., 2012a). During classical exosome biogenesis, ESCRT-III is recruited to the MVB by the same protein motifs that viral proteins use at the PM to generate viral buds (Baietti et al., 2012, Dussupt et al., 2009, Hurley and Odorizzi, 2012). Studying the mechanisms of unconventional protein secretion is critical to developing treatments for diseases.
Although much has been learned about unconventional protein secretion in recent years, further investigation is warranted because of the biological and clinical importance of the unconventionally secreted proteins. Attention should be applied to identifying the signal sequences or motifs that direct proteins to specific unconventional secretory pathways. The recent identification of the diacidic signal (Cruz-Garcia et al., 2018) and the motif-1 of IL-1β (Zhang et al. 2020) paved the way for identifying cognate transport machinery. In addition, such signals can be used to identify all members of that class of unconventionally secreted proteins. Once a candidate signal has been identified, it should be verified through the use of a reporter protein. Some unconventionally secreted proteins may contain more than one targeting sequence that can direct them to different unconventional secretory pathways. Indeed, IL-1β contains both motif-1 and a KFERQ-like sequence that can direct it to either ERGIC's TMED-10 or the lysosome’s LAMP2A, respectively. How the target sequence and, thus, the pathway is chosen is ripe for investigation. The study of unconventional protein secretion would benefit from the greater use of in vivo models to complement work using cell cultures and cell-free systems. Some cell culture systems rely on the overexpression of unconventional secretory proteins because they are often found in trace amounts. Overexpression may have unforeseen consequences. Thus, it is important to demonstrate that the components and steps of unconventional secretion identified in vitro are also used in vivo. The identification of the THU unconventional secretory pathway may serve as a paradigm in the field (Zhang et al., 2020). The use of in vitro and in vivo approaches yielded a wealth of information about the pathway's signals, cargo, translocation machinery, and subcellular location at the ERGIC. The various unconventional secretory proteins congregating at the ERGIC subcompartment is reminiscent of old friends having one last shot of unconventional whiskey before leaving through the back door.
Author contributions
M.J.C prepared writing and figures about unconventional protein secretion, W.J.C. contributed text and background information related to classical secretion, as well as ESCRT assembly and classical exosome content. PNL organized, edited, and revised the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Katie Holmes for her close reading and insightful comments on the manuscript.
References
- Ahmed I., Akram Z., Iqbal M.N., Munn A.L. The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview. Int. J. Biol. Macromol. 2019;127:1–11. doi: 10.1016/j.ijbiomac.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Aittoniemi J., Fotinou C., Craig T.J., De Wet H., Proks P., Ashcroft F.M. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajasin D., Eugenin E.A. HIV-1 tat: role in bystander toxicity. Front. Cell. Infect. Microbiol. 2020;10:1–15. doi: 10.3389/fcimb.2020.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom, T., Narayanaswamit, V., Sies, H., 1991. Transport in Canalicular Plasma Membrane Vesicles from Rat Liver, 13147–13152. [PubMed]
- Anand P.K., Anand E., Bleck C.K.E., Anes E., Griffiths G. Exosomal hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S., Samuel M., Kumar S., Mathivanan S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Biophys. Acta – Proteins Proteomics. 2019;1867 doi: 10.1016/j.bbapap.2019.02.005. [DOI] [PubMed] [Google Scholar]
- April, C., Page, S.E.E.L., Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., Schwille, P., Brügger, B., Simons, M., 2008. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes 319. [DOI] [PubMed]
- Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., Zimmermann P., David G. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Bänfer S., Schneider D., Dewes J., Strauss M.T., Freibert S.A., Heimerl T., Maier U.G., Elsässer H.P., Jungmann R., Jacob R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E4396–E4405. doi: 10.1073/pnas.1718921115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C.K., Millera E. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193:383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso M., Pozzi S., Tortarolo M., Fiordaliso F., Bisighini C., Pasetto L., Spaltro G., Lidonnici D., Gensano F., Battaglia E., Bendotti C., Bonetto V. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: Implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J. Biol. Chem. 2013;288:15699–15711. doi: 10.1074/jbc.M112.425066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-morales R., Praena B., Nuez D., Rejas T., Guerra M. crossm Role of microvesicles in the spread of herpes simplex virus 1. J. Virol. 2018;92:1–19. doi: 10.1128/JVI.00088-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielska E., May R.C. Extracellular vesicles of human pathogenic fungi. Curr. Opin. Microbiol. 2019;52:90–99. doi: 10.1016/j.mib.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Branco P., Francisco D., Chambon C., Hébraud M., Arneborg N., Almeida M.G., Caldeira J., Albergaria H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014;98:843–853. doi: 10.1007/s00253-013-5411-y. [DOI] [PubMed] [Google Scholar]
- Brodsky J.L., Goeckeler J., Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns C., Mccaffery J.M., Curwin A.J., Duran J.M., Malhotra V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 2011;195:979–992. doi: 10.1083/jcb.201106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral M., Anjard C., Malhotra V., Loomis W.F., Kuspa A. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot. Cell. 2010;9:1009–1017. doi: 10.1128/EC.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S.K., Gong J., Murshid A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Klionsky D.J. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Cesselli D., Parisse P., Aleksova A., Veneziano C., Cervellin C., Zanello A., Beltrami A.P. Extracellular vesicles: how drug and pathology interfere with their biogenesis and function. Front. Physiol. 2018;9:1–15. doi: 10.3389/fphys.2018.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C., Samaniego F., Nair B.C., Buonaguro L., Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. Aids. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Chen R., Kang R., Fan X.G., Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:1–9. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Du W., Hagemeijer M.C., Takvorian P.M., Pau C., Cali A., Brantner C.A. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W.J. Protein release through nonlethal oncotic pores as an alternative nonclassical secretory pathway. BMC Cell Biol. 2011;12:1–12. doi: 10.1186/1471-2121-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W.J., Waters M.G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Choi D.S., Kim D.K., Kim Y.K., Gho Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- Clayton A., Turkes A., Navabi H., Mason M.D., Tabi Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- Cleves A.E., Cooper D.N., Barondes S.H., Kelly R.B., Francisco S. A new pathway for protein export in Saccharomyces cerevisiae. J. Cell Biol. 1996;133:1017–1026. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Garcia D., Curwin A.J., Popoff J.F., Bruns C., Duran J.M., Malhotra V. Remodeling of secretory compartments creates CUPS during nutrient starvation. J. Cell Biol. 2014;207:695–703. doi: 10.1083/jcb.201407119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Garcia D., Malhotra V., Curwin A.J. Unconventional protein secretion triggered by nutrient starvation. Semin. Cell Dev. Biol. 2018;83:22–28. doi: 10.1016/j.semcdb.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Curwin A., Brouwers N., Adell M.A.Y., Teis D., Turacchio G., Parashuraman S., Ronchi P., Malhotra V. ESCRT-III drives the final stages of CUPS maturation for unconventional protein secretion. Elife. 2016;1–25 doi: 10.7554/eLife.16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.L., Connor E.O., Azorın I., Renau-piqueras J., Gil M.L., Gozalbo D. The glyceraldehyde-3-phosphate dehydrogenase polypeptides encoded by the Saccharomyces cerevisiae TDH1, TDH2 and TDH3 genes are also cell wall proteins. Microbiology. 2001;147:411–417. doi: 10.1099/00221287-147-2-411. [DOI] [PubMed] [Google Scholar]
- Delic M., Valli M., Graf A.B., Pfeffer M., Mattanovich D., Gasser B. The secretory pathway: exploring yeast diversity. FEMS Microbiol. Rev. 2013;37:872–914. doi: 10.1111/1574-6976.12020. [DOI] [PubMed] [Google Scholar]
- Depledge D.P., MacLean L.M., Hodgkinson M.R., Smith B.A., Jackson A.P., Ma S., Uliana S.R.B., Smith D.F. Leishmania-specific surface antigens show sub-genus sequence variation and immune recognition. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies, R.J., Koch, B.D., Werner-washburnet, M., Craigt, E.A., Schekman, R., 1988. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides 800–805. [DOI] [PubMed]
- Dimou E., Nickel W. Unconventional mechanisms of eukaryotic protein secretion. Curr. Biol. 2018;28:R406–R410. doi: 10.1016/j.cub.2017.11.074. [DOI] [PubMed] [Google Scholar]
- Dou Z., Xu C., Donahue G., Shimi T., Pan J.A., Zhu J., Ivanov A., Capell B.C., Drake A.M., Shah P.P., Catanzaro J.M., Ricketts M.D., Lamark T., Adam S.A., Marmorstein R., Zong W.X., Johansen T., Goldman R.D., Adams P.D., Berger S.L. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–109. doi: 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N., Jiang S., Pilli M., Ornatowski W., Bhattacharya D., Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1?? EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran J.M., Anjard C., Stefan C., Loomis W.F., Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010;188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussupt V., Javid M.P., Abou-Jaoudé G., Jadwin J.A., De La Cruz J., Nagashima K., Bouamr F. The nucleocapsid region of HIV-1 gag cooperates with the PTAP and LYPX nL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R.A., Wingfield P., Gallo R.C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader C.M., Colombo M.I. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy. 2006;2:122–125. doi: 10.4161/auto.2.2.2350. [DOI] [PubMed] [Google Scholar]
- Feyder S., De Craene J.-O., Bär S., Bertazzi D., Friant S. Membrane trafficking in the yeast saccharomyces cerevisiae model. Int. J. Mol. Sci. 2015;0067:1509–1525. doi: 10.3390/ijms16011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friand V., David G., Zimmermann P. Syntenin and syndecan in the biogenesis of exosomes. Biol. Cell. 2015;107:331–341. doi: 10.1111/boc.201500010. [DOI] [PubMed] [Google Scholar]
- Fuhlendorff J., Rorsman P., Kofod H., Brand C.L., Rolin B., MacKay P., Shymko R., Carr R.D. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47:345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- Furuhashi M., Saitoh S., Shimamoto K., Miura T. Fatty acid-binding protein 4 (FABP4): Pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin. Med. Insights Cardiol. 2014;2014:23–33. doi: 10.4137/CMC.S17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, D.C., Brouwers, N., Duran, J.M., Mora, G., Curwin, A.J., 2016. A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1, pp. 1–10. [DOI] [PMC free article] [PubMed]
- Gardella S., Andrei C., Ferrera D., Lotti L.V., Torrisi M.R., Bianchi M.E., Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau M.-E., Cote M.-H., Bourgeois-Daigneault M.-C., Rivard L., Xiu F., Shaw A., Steimle V., Thibodeau J. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic. 2009;2009:1518–1527. doi: 10.1111/j.1600-0854.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- Ghossoub R., Lembo F., Rubio A., Gaillard C.B., Bouchet J., Vitale N., Slavík J., Machala M., Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- Golczak M., Kicinska A., Bandorowicz-pikula J., Buchet R. Acidic pH-induced folding of annexin VI is a prerequisite for its insertion into lipid bilayers and formation of ion channels by the protein molecules. FASEB J. 2001;15 doi: 10.1096/fj.00-0523fje. [DOI] [PubMed] [Google Scholar]
- Gozalbo D., Gil-Navarro I., Azorín I., Renau-Piqueras J., Martínez J.P., Gil M.L. The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is also a fibronectin and laminin binding protein. Infect. Immun. 1998;66:2052–2059. doi: 10.1128/iai.66.5.2052-2059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani I., Bagala C., Duarte M., Soldi R., Kolev V., Tarantini F., Krishnaswamy T., Kumar S., Doyle A., Neivandt D., Yu C., Maciag T., Prudovsky I. Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. BBRC. 2006;349:192–199. doi: 10.1016/j.bbrc.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Bianchi M.E., Coleman T.R., Tracey K.J., Al-Abed Y. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol. Med. 2018;24:1–9. doi: 10.1186/s10020-018-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., Emr S.D. The ESCRT Pathway. Dev. Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik N.P., Øverbye A., Brech A., Torgersen M.L., Jakobsen I.S., Sandvig K., Llorente A. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell. Mol. Life Sci. 2016;73:4717–4737. doi: 10.1007/s00018-016-2309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans, J.G., Stockelman, K., Levy, M., Brewster, L.M., Bammert, T.D., Greiner, J.J., Connick, Elizabeth, Desouza, C.A., JJ, G., Connick, E, Ca, D., 2019. Effects of HIV-1 gp120 and TAT-derived microvesicles on endothelial cell function, 1242–1249. https://doi.org/10.1152/japplphysiol.01048.2018. [DOI] [PMC free article] [PubMed]
- Hurley J.H., Odorizzi G. Get on the exosome bus with ALIX. Nat. Cell Biol. 2012;14:654–655. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- Itskanov S., Park E. Structure of the posttranslational Sec protein-translocation channel complex from yeast. Science (80-) 2019;363:84–87. doi: 10.1126/science.aav6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A., Friedman S., Zhan X., Engleka K.A., Forough R., Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen K.R., Paulsen B.S., Bæk R., Varming K., Sorensen B.S., Jørgensen M.M. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles. 2015;4:1–10. doi: 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jella K.K., Yu L., Yue Q., Friedman D., Duke B.J., Alli A.A. Exosomal GAPDH from proximal tubule cells regulate ENaC activity. PLoS ONE. 2016;11:1–20. doi: 10.1371/journal.pone.0165763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen D.K., Fenix A.M., Franklin J.L., Rome L.H., Burnette D.T., Coffey R.J., Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., Liebler D.C., Ping J., Liu Q., Evans R., Fissell W.H., Patton J.G., Rome L.H., Burnette D.T., Coffey R.J. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N., Powis K., High S. Post-translational translocation into the endoplasmic reticulum. Biochim. Biophys. Acta – Mol. Cell Res. 2013;1833:2403–2409. doi: 10.1016/j.bbamcr.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Joliot, A., Maizel, A., Rosenberg, D., Trembleau, A., Dupas, S., Volovitch, M., Prochiantz, A., 1998. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein 8, 856–863. [DOI] [PubMed]
- Josephrajan A., Hertzel A.V., Bohm E.K., McBurney M.W., Imai S.I., Mashek D.G., Kim D.H., Bernlohr D.A. Unconventional secretion of adipocyte fatty acid binding protein 4 is mediated by autophagic proteins in a sirtuin-1-dependent manner. Diabetes. 2019;68:1767–1777. doi: 10.2337/db18-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacer D., Mcintire C., Kirov A., Kany E., Roth J., Liaw L., Small D., Friesel R., Basilico C., Tarantini F., Verdi J., Prudovsky I. Regulation of non-classical FGF1 release and FGF-dependent cell transformation by CBF1-mediated notch signaling. J. Cell. Physiol. 2011;226:3064–3075. doi: 10.1002/jcp.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H., Drummen G.P.C., Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T., Jia, J., Kumar, S., Choi, S.W., Gu, Y., Mudd, M., Dupont, N., Jiang, S., Peters, R., Farzam, F., Jain, A., Lidke, K.A., Adams, C.M., Johansen, T., Deretic, V., 2017. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy 36, 42–60. https://doi.org/10.15252/embj.201695081. [DOI] [PMC free article] [PubMed]
- Kimura Y., Kawawaki J., Kakiyama Y., Shimoda A., Tanaka K. The ESCRT-III adaptor protein Bro1 controls functions of regulator for free ubiquitin chains 1 (Rfu1) in ubiquitin homeostasis. J. Biol. Chem. 2014;289:21760–21769. doi: 10.1074/jbc.M114.550871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner P., Bourdenx M., Madrigal-Matute J., Tiano S., Diaz A., Bartholdy B.A., Will B., Cuervo A.M. Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Eskelinen E., Deretic V. Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes… Wait, I’m confused. Autophagy. 2014;10:549–551. doi: 10.4161/auto.28448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal, J., Arras, G., Colombo, M., Jouve, M., Paul, J., Primdal-bengtson, B., 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. https://doi.org/10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed]
- Kreger B.T., Dougherty A.L., Greene K.S., Cerione R.A., Antonyak M.A. Microvesicle cargo and function changes upon induction of cellular transformation. J. Biol. Chem. 2016;291:19774–19785. doi: 10.1074/jbc.M116.725705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Venuta G., Zeitler M., Steringer J.P., Müller H.M., Nickel W. The startling properties of fibroblast growth factor 2: How to exit mammalian cells without a signal peptide at hand. J. Biol. Chem. 2015;290:27015–27020. doi: 10.1074/jbc.R115.689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe J., Karsenty G., Ferron M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle. 2013;12:2744–2752. doi: 10.4161/cc.25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers E., Wang Y., Gallardo R., Rousseau F., Lauwers E., Wang Y., Gallardo R., Van Der Kant R., Michiels E., Swerts J. Hsp90 mediates membrane deformation and exosome release. Mol. Cell. 2018:689–702. doi: 10.1016/j.molcel.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Lay A.J., Jiang X.M., Kisker O., Flynn E., Underwood A., Condron R., Hogg P.J. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- Léveillard, T., Aït-ali, N., 2017. Review Article Cell Signaling with Extracellular Thioredoxin and Thioredoxin- Like Proteins : Insight into Their Mechanisms of Action 2017. [DOI] [PMC free article] [PubMed]
- Li B., Antonyak M.A., Zhang J., Cerione R.A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740–4749. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.C., Kane P.M. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta – Mol. Cell Res. 2009;1793:650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Sahu D., Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. BBA - Mol. Cell Res. 2012;1823:730–741. doi: 10.1016/j.bbamcr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-villar E., Monteoliva L., Larsen M.R., Sachon E. Genetic and proteomic evidences support the localization of yeast enolase in the cell surface. Proteomics. 2006:107–118. doi: 10.1002/pmic.200500479. [DOI] [PubMed] [Google Scholar]
- Mackenzie A., Wilson H.L., Kiss-toth E., Dower S.K., North R.A., Surprenant A., Sheffield S. Rapid Secretion of Interleukin-1 NL by Microvesicle Shedding. Immunity. 2001;8:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Maclean L.M., Toole P.J.O., Stark M., Marrison J., Seelenmeyer C., Nickel W., Smith D.F. Trafficking and release of Leishmania metacyclic HASPB on macrophage invasion. Cell Microbiol. 2012;14:740–761. doi: 10.1111/j.1462-5822.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio A. De. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. Cell Stress Chaperones. 2011:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A., Tassetto M., Filhol O., Cochet C., Prochiantz A., Joliot A. Engrailed homeoprotein secretion is a regulated process. Development. 2002;3553:3545–3553. doi: 10.1242/dev.129.15.3545. [DOI] [PubMed] [Google Scholar]
- Malhotra V. Unconventional protein secretion: an evolving mechanism. EMBO J. 2013;32:1660–1664. doi: 10.1038/emboj.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula S.S., Calderwood S.K. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Marino J., Wigdahl B., Nonnemacher M.R. Extracellular HIV-1 tat mediates increased glutamate in the CNS leading to onset of senescence and progression of HAND. Front. Aging Neurosci. 2020;12:1–8. doi: 10.3389/fnagi.2020.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez, F., Diamond, C., Zeitler, M., Gomez, A.I., Bagnall, J., Spiller, D., White, M., Daniels, M.J.D., 2016. Inflammasome-dependent IL-1 β release depends upon membrane permeabilisation 1219–1231. https://doi.org/10.1038/cdd.2015.176. [DOI] [PMC free article] [PubMed]
- Martin-Serrano J., Yaravoy A., Perez-Caballero D., Bieniasz P.D. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J., Zang T., Bieniasz P.D. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- Mattie, S., Mcnally, E.K., Karim, M.A., Vali, H., 2017. How and why intralumenal membrane fragments form during vacuolar lysosome fusion 28, 309–321. https://doi.org/10.1091/mbc.E15-11-0759. [DOI] [PMC free article] [PubMed]
- McGrath J.P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989;340:400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Mcnally E.K., Brett C.L. The intralumenal fragment pathway mediates ESCRT-independent surface transporter down-regulation. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-07734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J. Review exosomes and ectosomes in intercellular communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- Mele A.R., Marino J., Chen K., Pirrone V., Janetopoulos C., Wigdahl B., Klase Z., Nonnemacher M.R. Defining the molecular mechanisms of HIV-1 Tat secretion: PtdIns(4,5)P 2 at the epicenter. Traffic. 2018;19:655–665. doi: 10.1111/tra.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merezhko M., Brunello C.A., Yan X., Vihinen H., Jokitalo E., Uronen R.L., Huttunen H.J. Secretion of tau via an unconventional non-vesicular mechanism. Cell Rep. 2018;25:2027–2035.e4. doi: 10.1016/j.celrep.2018.10.078. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Barrowman J. Biogenesis of the Saccharomyces cerevisiae pheromone a-factor, from yeast mating to human disease. Microbiol. Mol. Biol. Rev. 2012;76:626–651. doi: 10.1128/MMBR.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita T., Furuhashi M., Hiramitsu S., Ishii J., Hoshina K., Ishimura S., Fuseya T., Watanabe Y., Tanaka M., Ohno K., Akasaka H., Ohnishi H., Yoshida H., Saitoh S., Shimamoto K., Miura T. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity. 2015;23:359–367. doi: 10.1002/oby.20954. [DOI] [PubMed] [Google Scholar]
- Miura N. Evaluation of unconventional protein secretion by saccharomyces cerevisiae and other fungi. Cells. 2018;7 doi: 10.3390/cells7090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N., Kirino A., Endo S., Morisaka H., Kuroda K., Takagi M., Ueda M. Tracing putative trafficking of the glycolytic enzyme enolase via SNARE-driven unconventional secretion. Eukaryot. Cell. 2012;11:1075–1082. doi: 10.1128/EC.00075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesso N., Nagel M.-K., Isono E. Ubiquitin recognition in endocytic trafficking – with or without ESCRT-0. J. Cell Sci. 2019;132:jcs232868. doi: 10.1242/jcs.232868. [DOI] [PubMed] [Google Scholar]
- Mouasni S., Gonzalez V., Schmitt A., Bennana E., Guillonneau F., Mistou S., Avouac J., Ea H.K., Devauchelle V., Gottenberg J.E., Chiocchia G., Tourneur L. The classical NLRP3 inflammasome controls FADD unconventional secretion through microvesicle shedding. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-1412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsafi, Y., Altan-bonnet, N., 2018. Enterovirus Transmission by Secretory Autophagy 1–8. https://doi.org/10.3390/v10030139. [DOI] [PMC free article] [PubMed]
- Nabhan J.F., Hu R., Oh R.S., Cohen S.N., Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niel G. Van, Angelo G.D., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Publ. Gr. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Novick, P., Ferro, S., Schekman, R., 1981. Order of Events in the Yeast Secretory Pathway 25, 461–469. [DOI] [PubMed]
- Oliveira D.L., Nakayasu E.S., Joffe L.S., Guimares A.J., Sobreira T.J.P., Nosanchuk J.D., Cordero R.J.B., Frases S., Casadevall A., Almeida I.C., Nimrichter L., Rodrigues M.L. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. WIREs Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal, P., Gutierrez, D., Xim, P., 2012. Cell surface shaving of Candida albicans biofilms , hyphae , and yeast form cells 2331–2339. https://doi.org/10.1002/pmic.201100588. [DOI] [PubMed]
- Phillips M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018;59:749–763. doi: 10.1194/jlr.R082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plugis N.M., Palanski B.A., Weng C.H., Albertelli M., Khosla C. Thioredoxin-1 selectively activates transglutaminase 2 in the extracellular matrix of the small intestine: implications for celiac disease. J. Biol. Chem. 2017;292:2000–2008. doi: 10.1074/jbc.M116.767988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet H., Conrard L., Cloos A.S., Tyteca D. Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules. 2018 doi: 10.3390/biom8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa, S.J., Stewart, S.E., Moreau, K., 2018. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol., https://doi.org/10.1016/j.semcdb.2018.02.022. [DOI] [PMC free article] [PubMed]
- Prudovsky I., Bagala C., Tarantini F., Mandinova A., Soldi R., Bellum S., Maciag T. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J. Cell Biol. 2002;158:201–208. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky, I., Kacer, D., Davis, J., Shah, V., Jayanthi, S., Huber, I., Dakshinamurthy, R., Ganter, O., Neivandt, D., Guvench, O., Krishnaswamy, T., Kumar, S., 2016. Folding of fibroblast growth factor 1 is critical for its nonclassical release. https://doi.org/10.1021/acs.biochem.5b01341. [DOI] [PMC free article] [PubMed]
- Prudovsky, I., Krishnaswamy, T., Kumar, S., Sterling, S., Neivandt, D., 2013. Protein-Phospholipid interactions in nonclassical protein secretion: problem and methods of study 3734–3772. https://doi.org/10.3390/ijms14023734. [DOI] [PMC free article] [PubMed]
- Rapoport T.A., Li L., Park E. Structural and mechanistic insights into protein translocation. Annu. Rev. Cell Dev. Biol. 2017;33:369–390. doi: 10.1146/annurev-cellbio-100616-060439. [DOI] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Klionsky D.J. Autophagic processes in yeast: mechanism, machinery and regulation. Genetics. 2013;194:341–361. doi: 10.1534/genetics.112.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli, A., Bajetto, A., Allavena, G., Wollmanq, E., Sitia, R., 1992. Secretion of Thioredoxin by Normal and Neoplastic Cells through a Leaderless Secretory 24161–24164. [PubMed]
- Santos, T.G., Martins, V.R., Glaucia Noeli, M.H., 2017. Unconventional Secretion of Heat Shock Proteins in Cancer 1–17. https://doi.org/10.3390/ijms18050946. [DOI] [PMC free article] [PubMed]
- Schloer S., Pajonczyk D., Rescher U. Annexins in translational research: Hidden treasures to be found. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg J., Lee I.H., Iwasa J.H., Hurley J.H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 2016;18:5–17. doi: 10.1038/nrm.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelenmeyer, C., Wegehingel, S., Tews, I., Künzler, M., Aebi, M., Nickel, W., 2005. Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1 373–381. https://doi.org/10.1083/jcb.200506026. [DOI] [PMC free article] [PubMed]
- Sheng Z., Lewis J.A., Chirico W.J. Nuclear and nucleolar localization of 18-kDa fibroblast growth factor-2 is controlled by C-terminal signals. J. Biol. Chem. 2004;279:40153–40160. doi: 10.1074/jbc.M400123200. [DOI] [PubMed] [Google Scholar]
- Sheng Z., Liang Y., Lin C.-Y., Comai L., Chirico W.J. Direct regulation of rRNA transcription by fibroblast growth factor 2. Mol. Cell. Biol. 2005;25:9419–9426. doi: 10.1128/mcb.25.21.9419-9426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheokand N., Kumar S., Malhotra H., Tillu V., Raje C.I., Raje M. Secreted glyceraldehye-3-phosphate dehydrogenase is a multifunctional autocrine transferrin receptor for cellular iron acquisition. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3816–3827. doi: 10.1016/j.bbagen.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Shin J.T., Opalenik S.R., Wehby J.N., Mahesh V.K., Jackson A., Tarantini F., Maciag T., Thompson J.A. Serum-starvation induces the extracellular appearance of FGF-1. Biochim. Biophys. Acta - Mol. Cell Res. 1996;1312:27–38. doi: 10.1016/0167-4889(96)00013-4. [DOI] [PubMed] [Google Scholar]
- Silveira C.P., Piffer A.C., Kmetzsch L., Fonseca F.L., Soaresa D., Staats C.C., Rodrigues M.L., Schrank A., Vainstein M.H. The heat shock protein (Hsp) 70 of Cryptococcus neoformans is associated with the fungal cell surface and influences the interaction between yeast and host cells. Fungal Genet. Biol. 2013;60:53–63. doi: 10.1016/j.fgb.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Silverman J.M., Christy D., Shyu C.C., Moon K.M., Fernando S., Gidden Z., Cowan C.M., Ban Y., Greg Stacey R., Grad L.I., McAlary L., Mackenzie I.R., Foster L.J., Cashman N.R. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)G93A ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J. Biol. Chem. 2019;294:3744–3759. doi: 10.1074/jbc.RA118.004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A. Evolution, role in inflammation, and redox control of leaderless secretory proteins. J. Biol. Chem. 2020;295:7799–7811. doi: 10.1074/jbc.REV119.008907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagsvold T., Pattni K., Malerød L., Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Stegmayer, C., Kehlenbach, A., Tournaviti, S., Wegehingel, S., Zehe, C., Denny, P., Smith, D.F., Schwappach, B., Nickel, W., 2005. Direct transport across the plasma membrane of mammalian cells of Leishmania HASPB as revealed by a CHO export mutant. https://doi.org/10.1242/jcs.01645. [DOI] [PubMed]
- Steringer J.P., Bleicken S., Andreas H., Zacherl S., Laussmann M., Temmerman K., Contreras F.X., Bharat T.A.M., Lechner J., Müller H.M., Briggs J.A.G., García-Sáez A.J., Nickel W. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J. Biol. Chem. 2012;287:27659–27669. doi: 10.1074/jbc.M112.381939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steringer J.P., Lange S., Čujová S., Šachl R., Poojari C., Lolicato F., Beutel O., Müller H.M., Unger S., Coskun Ü., Honigmann A., Vattulainen I., Hof M., Freund C., Nickel W. Key steps in unconventional secretion of fibroblast growth factor 2 reconstituted with purified components. Elife. 2017;6:1–36. doi: 10.7554/eLife.28985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.E., Ashkenazi A., Williamson A., Rubinsztein D.C., Moreau K. Transbilayer phospholipid movement facilitates the translocation of annexin across membranes. J. Cell Sci. 2018;131 doi: 10.1242/jcs.217034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.N., Solis N.V., Phan Q.T., Bajwa J.S., Kashleva H., Thompson A., Liu Y., Dongari-Bagtzoglou A., Edgerton M., Filler S.G. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka Y., Goto S., Nakano T., Tseng H.P., Yang S.M., Kawamoto S., Ono K., Chen C.L. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep05204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Buchkovich N.J., Mike Henne W., Banjade S., Kim Y.J., Emr S.D. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. Elife. 2016;5:1–12. doi: 10.7554/eLife.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Wang X.W., Liu Z.H., Sun Y.M., Tang Y.X., Zhou D.H. Chaperone-mediated autophagy substrate proteins in cancer. Oncotarget. 2017;8:51970–51985. doi: 10.18632/oncotarget.17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I., Ueno T., Kominami E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia V.S., Daniels M.J.D., Palazón-Riquelme P., Dewhurst M., Luheshi N.M., Rivers-Auty J., Green J., Redondo-Castro E., Kaldis P., Lopez-Castejon G., Brough D. The three cytokines IL-1β, IL-18, and IL-1α share related but distinct secretory routes. J. Biol. Chem. 2019;294:8325–8335. doi: 10.1074/jbc.RA119.008009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D., Saksena S., Judson B.L., Emr S.D. filaments for cargo sorting and multivesicular body vesicle formation. EMBO J. 2010;29:871–883. doi: 10.1038/emboj.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y., Ayaori M., Ogura M., Yakushiji E., Uto-Kondo H., Hisada T., Ozasa H., Takiguchi S., Nakaya K., Sasaki M., Komatsu T., Iizuka M., Horii S., Mochizuki S., Yoshimura M., Ikewaki K. Effect of sulfonylurea agents on reverse cholesterol transport in vitro and vivo. J. Atheroscler. Thromb. 2011;18:513–530. doi: 10.5551/jat.7641. [DOI] [PubMed] [Google Scholar]
- Tsuboama, K., Koyama-honda, I., Sakamaki, Y., Koike, M., Morishita, H., Mizushima, N., 2016. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane 354, 5485–5491. [DOI] [PubMed]
- Urano Y., Mori C., Fuji A., Konno K., Yashirogi S., Ando M., Saito Y., Noguchi N. 6-Hydroxydopamine induces secretion of PARK7 / DJ-1 via autophagy-based unconventional secretory pathway. Autophagy. 2018;00:1–16. doi: 10.1080/15548627.2018.1493043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve J., Bassaganyas L., Lepreux S., Chiritoiu M., Costet P., Ripoche J., Malhotra V., Schekman R. Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J. Cell Biol. 2018;217:649–665. doi: 10.1083/jcb.201705047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang Q., Yu S., Pan R., Jiang D., Liu Y., Hu H., Sun W., Hong X., Xue H., Qian W., Wang D., Zhou L., Mao C., Yuan G. Fibroblast growth factor 1 levels are elevated in newly diagnosed type 2 diabetes compared to normal glucose tolerance controls. Endocr. J. 2016;63:359–365. doi: 10.1507/endocrj.EJ15-0627. [DOI] [PubMed] [Google Scholar]
- Wemmer M., Azmi I., West M., Davies B., Katzmann D., Odorizzi G. Bro1 binding to Snf7 regulates ESCRT-III membrane scission activity in yeast. J. Cell Biol. 2011;192:295–306. doi: 10.1083/jcb.201007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens, S., 2015. Structure and mechanism of ABC transporters 9, 1–9. https://doi.org/10.12703/P7-14. [DOI] [PMC free article] [PubMed]
- Willms E., Johansson H.J., Mäger I., Lee Y., Blomberg K.E.M., Sadik M., Alaarg A., Smith C.I.E., Lehtiö J., Andaloussi S.E.L., Wood M.J.A., Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Nat. Publ. Gr. 2016;1–12 doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Cabanos C., Rapoport T.A. Letter. Nature. 2019 doi: 10.1038/s41586-018-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji R., Chatani E., Harada N., Sugimoto K., Inui H., Nakano Y. Glyceraldehyde-3-phosphate dehydrogenase in the extracellular space inhibits cell spreading. Biochim. Biophys. Acta - Gen. Subj. 2005;1726:261–271. doi: 10.1016/j.bbagen.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Zeitler M., Steringer J.P., Möller H.M., Mayer M.P., Nickel W. HIV-Tat protein forms phosphoinositide-dependent membrane pores implicated in unconventional protein secretion. J. Biol. Chem. 2015;290:21976–21984. doi: 10.1074/jbc.M115.667097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Ge, X., Guo, X., Guan, S., Li, X., Gu, W., Xu, W., 2017. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1 2017. https://doi.org/10.1155/2017/3248605. [DOI] [PMC free article] [PubMed]
- Zhang G., Liu Z., Ding H., Zhou Y., Doan H.A., Sin K.W.T., Zhu Z.J., Flores R., Wen Y., Gong X., Liu Q., Li Y.P. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., Mark M.T., Molina H., Martin A.B., Bojmar L., Fang J., Rampersaud S., Hoshino A., Matei I., Kenific C.M., Nakajima M., Mutvei A.P., Sansone P., Buehring W., Wang H., Jimenez J.P., Cohen-gould L., Paknejad N., Brendel M., Manova-todorova K., Cubillos-ruiz J.R., Galletti G., Giannakakou P., Cuervo A.M. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018;20 doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Kenny, S.J., Ge, L., Xu, K., Schekman, R., 2015. Translocation of interleukin-1 b into a vesicle intermediate in autophagy- mediated secretion 1–23. https://doi.org/10.7554/eLife.11205. [DOI] [PMC free article] [PubMed]
- Zhang M., Liu L., Lin X., Wang Y., Li Y., Guo Q., Li S., Sun Y., Tao X., Zhang D., Lv X., Zheng L., Ge L. A translocation pathway for vesicle-mediated unconventional protein secretion. Cell. 2020;181:637–652.e15. doi: 10.1016/j.cell.2020.03.031. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Higginbotham J.N., Jeppesen D.K., Franklin J.L., Bellis S.L., Coffey R.J., Zhang Q., Higginbotham J.N., Jeppesen D.K., Yang Y., Li W., Mckinley E.T., Allen R.M., Vickers K.C., Liu Q., Franklin J.L., Bellis S.L., Coffey R.J. Transfer of functional cargo in exomeres article transfer of functional cargo in exomeres. Cell Reports. 2019;27:940–954.e6. doi: 10.1016/j.celrep.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Poschmann G., Waldera-Lupa D., Rafiee N., Kollmann M., Stühler K. OutCyte: a novel tool for predicting unconventional protein secretion. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-55351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.G., Zhang H. Autophagosome maturation: an epic journey from the ER to lysosomes. J. Cell Biol. 2019;218:757–770. doi: 10.1083/jcb.201810099. [DOI] [PMC free article] [PubMed] [Google Scholar]