Abstract
STUDY QUESTION
What is the recommended management for women and transgender men with regards to fertility preservation (FP), based on the best available evidence in the literature?
SUMMARY ANSWER
The ESHRE Guideline on Female Fertility Preservation makes 78 recommendations on organization of care, information provision and support, pre-FP assessment, FP interventions and after treatment care. Ongoing developments in FP are also discussed.
WHAT IS KNOWN ALREADY
The field of FP has grown hugely in the last two decades, driven by the increasing recognition of the importance of potential loss of fertility as a significant effect of the treatment of cancer and other serious diseases, and the development of the enabling technologies of oocyte vitrification and ovarian tissue cryopreservation (OTC) for subsequent autografting. This has led to the widespread, though uneven, provision of FP for young women.
STUDY DESIGN, SIZE, DURATION
The guideline was developed according to the structured methodology for development of ESHRE guidelines. After formulation of key questions by a group of experts, literature searches and assessments were performed. Papers published up to 1 November 2019 and written in English were included in the review.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Based on the collected evidence, recommendations were formulated and discussed until consensus was reached within the guideline group. A stakeholder review was organized after finalization of the draft. The final version was approved by the guideline group and the ESHRE Executive Committee.
MAIN RESULTS AND THE ROLE OF CHANCE
This guideline aims to help providers meet a growing demand for FP options by diverse groups of patients, including those diagnosed with cancer undergoing gonadotoxic treatments, with benign diseases undergoing gonadotoxic treatments or those with a genetic condition predisposing to premature ovarian insufficiency, transgender men (assigned female at birth), and women requesting oocyte cryopreservation for age-related fertility loss.
The guideline makes 78 recommendations on information provision and support, pre-FP assessment, FP interventions and after treatment care, including 50 evidence-based recommendations—of which 31 were formulated as strong recommendations and 19 as weak—25 good practice points and 3 research only recommendations. Of the evidence-based recommendations, 1 was supported by high-quality evidence, 3 by moderate-quality evidence, 17 by low-quality evidence and 29 by very low-quality evidence. To support future research in the field of female FP, a list of research recommendations is provided.
LIMITATIONS, REASONS FOR CAUTION
Most interventions included are not well studied in FP patients. As some interventions, e.g. oocyte and embryo cryopreservation, are well established for treatment of infertility, technical aspects, feasibility and outcomes can be extrapolated. For other interventions, such as OTC and IVM, more evidence is required, specifically pregnancy outcomes after applying these techniques for FP patients. Such future studies may require the current recommendations to be revised.
WIDER IMPLICATIONS OF THE FINDINGS
The guideline provides clinicians with clear advice on best practice in female FP, based on the best evidence currently available. In addition, a list of research recommendations is provided to stimulate further studies in FP.
STUDY FUNDING/COMPETING INTEREST(S)
The guideline was developed and funded by ESHRE, covering expenses associated with the guideline meetings, with the literature searches and with the dissemination of the guideline. The guideline group members did not receive payment. R.A.A. reports personal fees and non-financial support from Roche Diagnostics, personal fees from Ferring Pharmaceuticals, IBSA and Merck Serono, outside the submitted work; D.B. reports grants from Merck Serono and Goodlife, outside the submitted work; I.D. reports consulting fees from Roche and speaker’s fees from Novartis; M.L. reports personal fees from Roche, Novartis, Pfizer, Lilly, Takeda, and Theramex, outside the submitted work. The other authors have no conflicts of interest to declare.
DISCLAIMER
This guideline represents the views of ESHRE, which were achieved after careful consideration of the scientific evidence available at the time of preparation. In the absence of scientific evidence on certain aspects, a consensus between the relevant ESHRE stakeholders has been obtained.
Adherence to these clinical practice guidelines does not guarantee a successful or specific outcome, nor does it establish a standard of care. Clinical practice guidelines do not replace the need for application of clinical judgment to each individual presentation, nor variations based on locality and facility type.
ESHRE makes no warranty, express or implied, regarding the clinical practice guidelines and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. (Full disclaimer available at www.eshre.eu/guidelines.)
†ESHRE Pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Keywords: fertility preservation, guideline, evidence-based, oncology, transgender men, age-related fertility loss, oocyte cryopreservation, ovarian tissue cryopreservation, ovarian transposition, pregnancy, organization of care
WHAT DOES THIS MEAN FOR PATIENTS?
Fertility preservation (FP) is a term used for interventions and procedures aiming at preserving the chance of having a baby when your fertility may be damaged by your medical condition or its treatment. FP may be appropriate before undergoing treatments that can affect fertility such as in women diagnosed with cancer or other non-malignant diseases (e.g. lupus, endometriosis and Turner syndrome). FP can also be considered by transgender men and by women worried about age-related fertility loss.
The current paper summarizes the ESHRE Guideline on FP providing clinicians with evidence-based recommendations on different FP techniques and how to apply them. These techniques include egg, embryo and ovarian tissue freezing, ovarian transposition and medical treatment to protect your ovaries. In addition, the guideline also provides recommendations on how to care for, inform and support patients requiring FP. The full guideline and a patient leaflet are available on https://www.eshre.eu/FFPguideline.
Introduction
The field of fertility preservation (FP) has grown hugely in the last two decades, driven by the increasing recognition of the importance of potential loss of fertility as a very important effect of the treatment of cancer and other serious diseases, and the development of the enabling technologies of oocyte vitrification and ovarian tissue cryopreservation for subsequent autografting. This has led to the widespread, though uneven, provision of FP for many women and young girls. The very rapid development of this field in clinical practice, yet with limited data on outcomes, has led to the need for the evaluation of the underpinning evidence and the development of guidelines to assist practitioners in its safe and effective implementation.
The guideline focuses on FP options for four populations: (i) post pubertal women diagnosed with cancer undergoing gonadotoxic treatments; (ii) post pubertal women with benign diseases undergoing gonadotoxic treatments or with conditions associated with premature loss of fertility, e.g. Turner syndrome; (iii) transgender men (assigned female at birth); and (iv) women considering oocyte cryopreservation for age-related fertility loss. In all these four populations, the guideline also provides recommendations regarding patient selection to ensure safe and effective care, including during future pregnancy. While it is recognized that this does not comprehensively include all those requiring FP (notably men, prepubertal girls and boys and transgender women), it was decided to limit the scope to focus primarily on adult women.
Materials and methods
The guideline was developed according to a well-documented methodology that is universal to ESHRE guidelines (Vermeulen et al., 2017) . The guideline development group (GDG) was composed of past and present members of the coordination of the Special Interest groups (SIGs) Fertility Preservation and Quality and Safety in ART, with representation of other SIGs (SIG Psychology and counselling, and SIG Ethics and law), and addition of experts in the field, including oncologists, a scientist, and patient representatives.
In short, 21 key questions were formulated by the GDG, of which 7 were answered as narrative questions, and 14 as PICO (Patient, Intervention, Comparison, Outcome) questions. For each PICO question, databases (PUBMED/MEDLINE and the Cochrane library) were searched from inception to 1 November 2019, limited to studies written in English. From the literature searches, studies were selected based on the PICO questions, assessed for quality and summarized in evidence tables. GDG meetings were organized where the evidence and draft recommendations were presented by the assigned GDG member and discussed until consensus was reached within the group. Each recommendation was labelled as strong or weak and a grade was assigned based on the strength of the supporting evidence (High ⊕⊕⊕⊕, Moderate ⊕⊕⊕◯, Low ⊕⊕◯◯, Very low ⊕◯◯◯). Good practice points (GPPs) based on clinical expertise were added where relevant to clarify the recommendations or to provide further practical advice. ‘Research only’ recommendations were also made, and those interventions should be applied only within the context of research, with appropriate precautions and ethical approval.
Strong recommendations should be used as a recommendation to be applied for most patients, while weak recommendations require discussion and shared decision-making.
For the narrative questions, a similar literature search was conducted. Collected data were summarized in a narrative summary and conclusions were formulated.
The guideline draft and an invitation to participate in the stakeholder review were published on the ESHRE website between 6 May and 17 June 2020. All comments were processed by the GDG, either by adapting the content of the guideline and/or by replying to the reviewer. The review process was summarized in the review report which is published on the ESHRE website (www.eshre.eu/Guidelines). Overall, 71.5% of the 231 comments resulted in an adaptation or correction in the guideline text.
This guideline will be considered for update 4 years after publication, with an intermediate assessment of the need for updating 2 years after publication.
Results
Key questions and recommendations
The current document summarizes all the key questions and the recommendations from the guideline ‘Female Fertility Preservation’. Further background information and the supporting evidence for each recommendation can be found in the full version of the guideline available at https://www.eshre.eu/FFPguideline.
Organization of care
How should the care for women undergoing fertility preservation be organized?
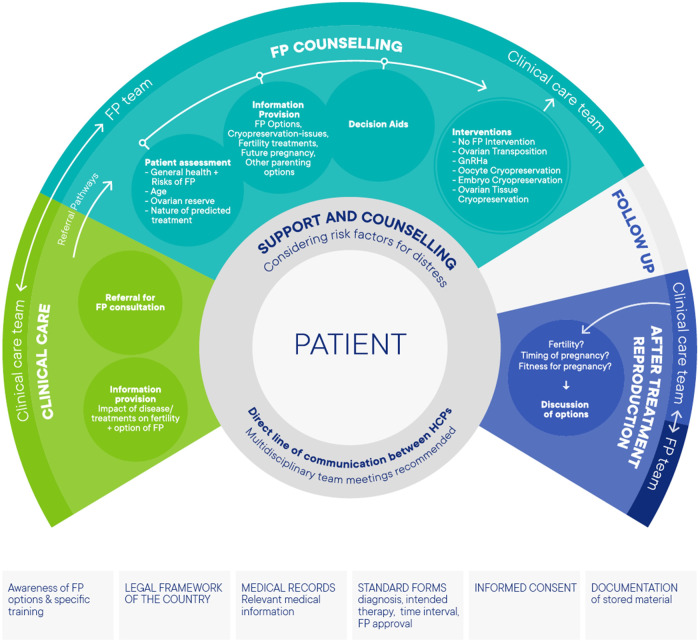
A team approach to care for women undergoing FP is advocated in the guideline. To support implementation, the following suggestions were formulated (Figure 1).
Figure 1.
Model of care for patients eligible for fertility preservation (FP).
There should be agreement within an FP service for who is responsible for all issues, including agreement on referral pathways, availability of standard forms for diagnosis, intended therapy, time intervals and a check whether FP counselling has been offered and has taken place. For FP treatment, a member of the FP team should be responsible for discussing any proposed treatment with the clinical care team before treatment initiation. Documentation and registration should be organized; all relevant medical information should be documented in the patients’ medical records, all patients undergoing FP should have been counselled about the legal and financial consequences and must have given written informed consent and accurate supporting documentation, especially about the gametes/embryos/tissue stored, is essential as storage may last for many years.
A direct link between the clinical care team and the FP team, preferably in multidisciplinary team meetings, is recommended. In addition, identification of a key individual (the ‘coordinator’) in clinical care teams is advisable to facilitate patients of reproductive age meeting with the FP team. Psychological support/counselling should be available to all patients considering FP, and specific support for particular patient groups may be required.
Expanding access to FP options is also important in the organization of FP care. The guideline group advocates improving (i) public awareness of fertility and of factors that may have negative effects on it; (ii) oncologists’ awareness of FP options; (iii) referral pathways; and (iv) availability of different FP procedures. With regard to these items, specific attention should be given to FP care for specific patient groups, such as adolescents and transgender men.
The key organizational features for establishing an FP program are summarized in Figure 2.
Figure 2.
Checklist for a high-quality fertility preservation (FP) program.
With regard to availability of FP interventions and storage of reproductive material, a survey was conducted to collect national legislative information in European countries, while recognizing that this is a constantly changing area. It was concluded that FP is available in most but not all European countries; thus, specialists should be aware of their national legislative and regulatory situation. This generally supportive legislative environment applies to patients with cancer and benign diseases, and mostly to transgender men. Provision of financial support is less widespread. This may reflect the rapidly developing nature of some FP procedures, and the ongoing change in their status from experimental towards being part of established care. With regards to the duration of storage of reproductive materials, regulations are very variable across Europe. Some countries also have different storage regulations for different materials. While a duration of storage is often applied, this may be supplemented by an upper age limit for use. Given the young age at which FP may occur, the often short allowable duration of storage (5–10 years in many countries) is inappropriate, and legislation should focus more on a maximum age of use.
Information needs and provision
What information needs to be provided to women at risk of infertility?
| Clinicians should provide information to patients regarding (i) impact of cancer, other diseases and their treatments on reproductive function; (ii) impact of cancer, other diseases and their treatment on fertility, (iii) FP options; (iv) issues related to cryopreservation storage after FP, (v) infertility and fertility treatments; (vi) pregnancy after gonadotoxic treatment or underlying condition; and (vii) other childbearing and parenting options (Peate et al., 2009; Goossens et al., 2014; Silva et al., 2018) (see Supplementary data I for more details). | STRONG ⊕⊕◯◯ |
| Information provided should be specific to the patients’ needs. | GPP |
| Age-specific information and counselling should be provided for adolescents and young adults. | GPP |
How should information on fertility preservation options be provided to patients?
| It is recommended to provide decision aids to patients who are considering FP (Peate et al., 2009; Anazodo et al., 2019; Wang et al., 2019). | STRONG ⊕⊕◯◯ |
| Healthcare professionals may consider the use of a checklist for a better provision of information to patients (Kemertzis et al., 2018). | WEAK ⊕◯◯◯ |
The full guideline includes a table of decision aids that are currently available for FP interventions. A checklist for clinicians to cover the information needs of patients undergoing FP counselling (for the four different indications) is included as Supplementary data I.
Is there a benefit of psychological support and counselling and are there particular groups that would benefit from it?
The multidisciplinary FP team counselling FP patients should be aware that maladaptive psychological processes and past psychopathology are risk factors for psychological distress during FP decision. It is recommended that patients at risk are referred for psychological support when needed.
| It is recommended that patients are offered psychological support and counselling when dealing with FP decisions, although the extent of the clinical benefit has not been studied (Chiavari et al., 2015; Greenwood et al., 2018; Logan et al., 2018; Anazodo et al., 2019). | STRONG ⊕◯◯◯ |
| Clinicians may consider referring FP patients who present risk factors for psychological distress for psychological support and counselling (Lawson et al., 2014; O'Hea et al., 2016; Shah et al., 2016; Witcomb et al., 2018; Logan et al., 2019). | WEAK ⊕◯◯◯ |
Patient selection and pre-FP assessment
Which criteria can be used to select patients for fertility preservation?
| Patients require an individual assessment of the indications and risks prior to FP interventions. | GPP |
| A multidisciplinary team is recommended to have an accurate assessment of risks. | GPP |
| For women with overt premature ovarian insufficiency (POI), FP is not recommended. | GPP |
A checklist for patient assessment and selection for FP is presented in Figure 3.
Figure 3.
Checklist for patients’ assessment and selection for fertility preservation (FP) interventions (adapted from Wallace et al. (2012)).
Which factors should be taken into account when estimating the individual risk of gonadotoxicity for a patient?
An overview of factors that increase the risk of gonadotoxicity, and of factors where evidence is not yet available in presented in Figure 4.
Figure 4.
Summary of factors to be considered when estimating the risk of gonadotoxicity.
| The risk of gonadotoxicity should be assessed in all patients undergoing gonadotoxic treatments. | GPP |
| To estimate the individual risk of gonadotoxicity, the characteristics of the proposed treatment, the patient and the disease should be considered (Tauchmanova et al., 2003; Bernhard et al., 2007; Anderson and Cameron, 2011; Abusief et al., 2012; Lawrenz et al., 2012; van der Kaaij et al., 2012; Valentini et al., 2013; Akhtar et al., 2015; Ruddy et al., 2015; Lekovich et al., 2016; Silva et al., 2016; Freour et al., 2017; Lambertini et al., 2017, 2019a,d; Dezellus et al., 2017a; Anderson et al., 2017b,c). | STRONG ⊕⊕◯◯ |
Is it relevant to do ovarian reserve testing, and for whom?
| For predicting high and low response to ovarian stimulation, use of either antral follicle count (AFC) or anti-Müllerian hormone (AMH) is recommended over other ovarian reserve tests. | STRONG ⊕⊕◯◯ |
| Assessment of pre-treatment ovarian function, in particular through AMH levels, in premenopausal women with a diagnosis of breast cancer or haematological malignancy is recommended to predict post-treatment recovery of ovarian function (Anderson et al., 2013; Dillon et al., 2013; Peigne and Decanter, 2014; Su et al., 2014; Silva et al., 2016; Dezellus et al., 2017b). | STRONG ⊕⊕◯◯ |
| Pre-treatment AMH levels should not be used as an indicator of post-treatment fertility (Hamy et al., 2016). | WEAK ⊕◯◯◯ |
| When estimating the risk of post-treatment POI, age, proposed gonadotoxic treatment type and dose, as well as pre-treatment AMH levels, should be taken into consideration (Anderson et al., 2013; Su et al., 2014; Barnabei et al., 2015). | STRONG ⊕◯◯◯ |
| Pre-treatment ovarian reserve testing could be performed in women with other malignancies, as testing is likely to be of high relevance based on indirect evidence from breast and haematological cancers (Dillon et al., 2013). | WEAK ⊕◯◯◯ |
| The relevance of ovarian reserve testing to help guide FP options or treatment decisions in systemic lupus erythematosus patients is low (Morel et al., 2013). | WEAK ⊕◯◯◯ |
| The relevance of ovarian testing to help guide FP options or treatment decisions in endometriosis patients remains inconclusive (Reinblatt et al., 2011; Benaglia et al., 2013; Ashrafi et al., 2019; Zhou et al., 2019). | WEAK ⊕◯◯◯ |
| Clinicians should be aware that in patients with endometriosis, the involvement of the ovaries and the radicality of surgery influence ovarian reserve as measured by AMH levels, but that its relevance to future fertility is unclear. | GPP |
| For women with reduced ovarian reserve (Bologna criteria, AMH <0.5 ng/ml), advice needs to be individualized and the value of FP is unclear. | GPP |
Fertility preservation interventions
Which options are available for fertility preservation in women—emergency and non-emergency?
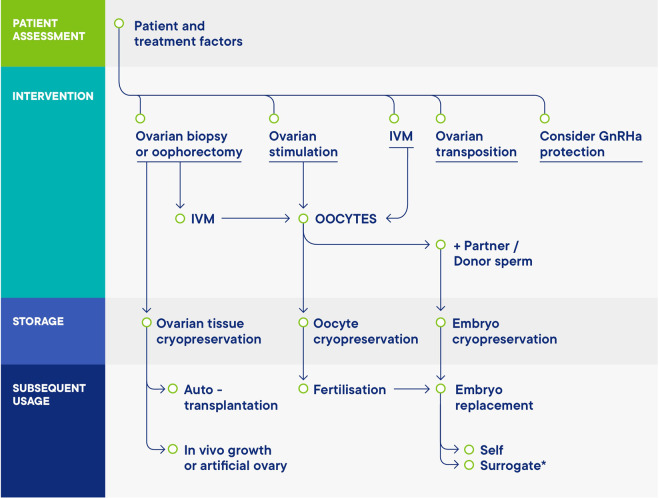
Fertility can be preserved through several procedures, including cryopreservation of oocytes, embryos or ovarian tissue, and potentially medical and surgical methods of protection (see Figure 5). Since the development of vitrification, oocyte cryopreservation is the method of choice for women undergoing treatment for age-related fertility loss, and for most women undergoing FP for medical indications. Embryo cryopreservation is even more widely available and long-established part of assisted reproduction, but the necessity for joint legal ownership with the male partner is an important consideration that may result in difficulties later on. Ovarian tissue cryopreservation (OTC) is an important option either through choice, or if there is insufficient time for ovarian stimulation. In vitro oocyte maturation (IVM) can also be considered, and in some cases, there may be a possibility of combining different approaches.
Figure 5.
Schematic overview of the options for female fertility preservation (FP). Adapted from (Anderson et al., 2015) .
Protection of the ovary against the effects of treatment would be an ideal approach. Options include GnRH agonists (mostly investigated in women with breast cancer) and ovarian transposition in women scheduled for pelvic radiotherapy.
How should ovarian stimulation be performed in cancer patients undergoing FP treatment?
| For ovarian stimulation in women seeking FP for medical reasons, the GnRH antagonist protocol is recommended for its feasibility in urgent situations, short time and safety reasons (The ESHRE Guideline Group on Ovarian Stimulation et al., 2020). | STRONG ⊕◯◯◯ |
| For patients requiring ovarian stimulation where there is a lack of urgency, the use of a long protocol may also be appropriate (The ESHRE Guideline Group on Ovarian Stimulation et al., 2020). | WEAK ⊕◯◯◯ |
| In urgent FP cycles, random-start ovarian stimulation is an option (Marklund et al., 2020; The ESHRE Guideline Group on Ovarian Stimulation et al., 2020). | WEAK ⊕⊕◯◯ |
| Double stimulation can be considered for urgent FP cycles (The ESHRE Guideline Group on Ovarian Stimulation et al., 2020; Vaiarelli et al., 2020). | WEAK ⊕⊕◯◯ |
| In ovarian stimulation for FP in oestrogen-sensitive diseases the concomitant use of anti-oestrogen therapy, such as letrozole, is probably recommended. | GPP |
| For ovarian stimulation in transgender men aiming at oocyte cryopreservation, GnRH antagonist protocols can be considered as they have been shown to be feasible and with numbers of oocytes retrieved comparable to those obtained in cisgender women when individuals have stopped previous treatment with testosterone. | WEAK ⊕◯◯◯ |
| How should ovarian stimulation be performed in transgender men undergoing FP treatment? | |
| For transgender men, the addition of letrozole to the antagonist protocol can be considered as it may enhance treatment adherence by reducing oestrogenic symptoms (Armuand et al., 2017). | GPP |
Is oocyte cryopreservation effective and safe for FP?
| Oocyte cryopreservation should be offered as an established option for FP (Rienzi et al., 2012; Cobo et al., 2013; Druckenmiller et al., 2016; Massarotti et al., 2017; Cobo et al., 2018; Rodriguez-Wallberg et al., 2019b). | STRONG ⊕⊕◯◯ |
| Women with a partner should be offered the option to cryopreserve unfertilized oocytes or to split the oocytes to attempt both embryo and oocyte cryopreservation. | GPP |
| Women should be informed of accurate, centre-specific expertise and live birth rates. They should also be informed that success rates after cryopreservation of oocytes at the time of a cancer diagnosis may be lower than in women without cancer. | GPP |
Oocyte cryopreservation for age-related fertility loss
| Women considering oocyte cryopreservation for age-related fertility loss should be fully informed regarding the success rates, risks, benefits, costs and the possible long-term consequences, both in terms of physical and psychological health (Rienzi et al., 2012; Cobo et al., 2013; Druckenmiller et al., 2016; Massarotti et al., 2017; Cobo et al., 2018; Ethics Committee of the American Society for Reproductive Medicine, 2018; Rodriguez-Wallberg et al., 2019b; Anderson et al., 2020). | STRONG ⊕◯◯◯ |
| Suitability should be determined on a case-by-case basis. | GPP |
Is embryo cryopreservation effective and safe for fertility preservation?
| Embryo cryopreservation is an established option for FP (Dolmans et al., 2005; Barcroft et al., 2013; Courbiere et al., 2013; Debrock et al., 2015; Dolmans et al., 2015; Rienzi et al., 2017; Alvarez and Ramanathan, 2018; Cobo et al., 2018; Rodriguez-Wallberg et al., 2019a). | STRONG ⊕⊕◯◯ |
| Women should be informed about the risk of losing reproductive autonomy and possible issues with ownership of stored embryos. | GPP |
| Women should be informed of accurate, centre-specific expertise and live birth rates. They should also be informed that success rates after cryopreservation of embryos at the time of a cancer diagnosis may be lower than in women without cancer. | GPP |
Should ovarian tissue cryopreservation be used for FP?
| It is recommended to offer OTC in patients undergoing moderate/high-risk gonadotoxic treatment where oocyte/embryo cryopreservation is not feasible, or at patient preference (Pacheco and Oktay, 2017; Gellert et al., 2018). | STRONG ⊕⊕◯◯ |
| OTC should probably not be offered to patients with low ovarian reserve (AMH < 0.5 ng/ml and AFC < 5) or advanced age considering the unfavourable risk/benefit. Current evidence suggest that the efficiency of OTC procedure is questionable above 36 years of age (Paradisi et al., 2016; Diaz-Garcia et al., 2018; Gellert et al., 2018). | WEAK ⊕◯◯◯ |
| The GDG considers that OTC is an innovative method for ovarian function and fertility preservation in post pubertal women. | GPP |
| Patients who have already received low gonadotoxic treatment or a previous course of chemotherapy, can be offered OTC as FP option (Poirot et al., 2019). | WEAK ⊕◯◯◯ |
| Ovarian stimulation can be performed immediately after OTC (Huober-Zeeb et al., 2011; Dittrich et al., 2013; Dolmans et al., 2014). | WEAK ⊕◯◯◯ |
| OTC at the time of oocyte pick-up after ovarian stimulation should not be performed unless in a research context. | RESEARCH ONLY |
| Ovarian transposition can be performed at the same time as OTC in patients who will receive pelvic irradiation. | GPP |
| OTC is not recommended as the primary FP procedure in transgender men but can be proposed as an experimental option when ovaries are removed during gender reassignment surgery. | GPP |
| OTC/ovarian tissue transplantation (OTT) can be considered in patients with POI-associated genetic and chromosomal disorders but requires genetic counselling and should be performed within a research protocol. | RESEARCH ONLY |
Should vitrification versus slow-freezing be used for OTC for FP?
| The slow-freezing protocol should be used for OTC as it is well-established and considered as standard (Fabbri et al., 2016; Dalman et al., 2017; Shi et al., 2017). | STRONG ⊕◯◯◯ |
| Vitrification of ovarian tissue should only be offered within a research program. | RESEARCH ONLY |
Which safety issues should be considered when replacing ovarian tissue?
| For OTT, a one-step laparoscopy procedure should be performed as it is considered safe without causing additional surgical risk (Schmidt et al., 2011; Beckmann et al., 2017, 2018). | STRONG ⊕⊕◯◯ |
| OTT at the orthotopic site is recommended to restore fertility (Beckmann et al., 2017; Gellert et al., 2018). | STRONG ⊕⊕◯◯ |
| The decision to perform OTT in oncological patients requires a multidisciplinary approach. | GPP |
| It is recommended to evaluate the presence of residual neoplastic cells in the ovarian cortex (and in the residual medulla when available) using appropriate techniques in all cancer survivors before OTT and patients should be informed about this risk (Abir et al., 2010; Bittinger et al., 2011; Fabbri et al., 2012; Greve et al., 2012; Bastings et al., 2013; Dolmans et al., 2013, 2016; Jahnukainen et al., 2013; Luyckx et al., 2013; Bockstaele et al., 2015; Rodriguez-Iglesias et al., 2015; Kristensen et al., 2017; Anderson et al., 2017d; Andersen et al., 2018; Gellert et al., 2018; Masciangelo et al., 2018; Shapira et al., 2018). | STRONG ⊕◯◯◯ |
| OTT is not recommended in cases where the ovary is involved in the malignancy (Kristensen et al., 2017; Masciangelo et al., 2018). | STRONG ⊕◯◯◯ |
| OTT and pregnancy can be considered in hormone-sensitive tumours such as endometrial cancer treated by fertility-sparing strategy or breast cancer, after complete remission of the disease (Lambertini et al., 2018a). | STRONG ⊕⊕◯◯ |
| There appears to be no increased risk of congenital abnormalities for children born after OTT (Pacheco and Oktay, 2017; Gellert et al., 2018). | WEAK ⊕◯◯◯ |
| Long-term risks in human are considered to be low but a long-term follow-up of patients after OTT is recommended. | GPP |
| OTT can be offered in BRCA patients, as an alternative when egg or embryo freezing is not feasible, but the ovarian tissue must be completely removed after subsequent pregnancy (Lambertini et al., 2018a, 2019b). | WEAK ⊕◯◯◯ |
Should IVM be used for FP?
| IVM should be regarded as an innovative FP procedure (Moria et al., 2011; Creux et al., 2018; Grynberg et al., 2019). | STRONG ⊕◯◯◯ |
| IVM requires specific expertise and should only be performed when oocyte cryopreservation is required but ovarian stimulation not feasible. | GPP |
| IVM after ex vivo extraction can be offered as an experimental procedure. | WEAK ⊕◯◯◯ |
Should GnRH agonists be used for ovarian protection in patients undergoing gonadotoxic treatment?
| GnRH agonists during chemotherapy should be offered as an option for ovarian function protection in premenopausal breast cancer patients receiving chemotherapy; however, limited evidence exists on their protective effect on the ovarian reserve and the potential for future pregnancies (Lambertini et al., 2015, 2018c). | STRONG ⊕⊕⊕⊕ |
| In women with breast cancer, GnRH agonists during chemotherapy should not be considered an option for FP instead of cryopreservation techniques (Lambertini et al., 2015, 2018c). | STRONG ⊕⊕⊕◯ |
| In malignancies other than breast cancer, GnRH agonists should not be routinely offered as an option for ovarian function protection and FP without discussion of the uncertainty about its benefit (Gilani et al., 2007; Senra et al., 2018). | STRONG ⊕◯◯◯ |
| GnRH agonists during chemotherapy may be considered as an option for ovarian function protection in premenopausal patients with autoimmune diseases receiving cyclophosphamide. However, it should be acknowledged that limited data are available in this setting (Ben-Aharon et al., 2010; Marder et al., 2012; Brunner et al., 2015). | WEAK ⊕⊕◯◯ |
| GnRH agonists should not be considered an equivalent or alternative option for FP but can be offered after cryopreservation techniques or when they are not possible. | GPP |
Should transposition of ovaries be used for ovarian protection?
| Where pelvic radiotherapy without chemotherapy is planned, women may be offered ovarian transposition with the aim to prevent POI (Gubbala et al., 2014; Hoekman et al., 2019). | WEAK ⊕⊕◯◯ |
| Women with reduced ovarian reserve and women at risk of having ovarian metastases are inappropriate candidates for ovarian transposition. | GPP |
After treatment care
How should patients be re-assessed before use of stored material?
| Before the use of stored material, fitness for pregnancy should be thoroughly assessed, taking into account treatment late effects, the age of the patient and the interval since treatment. | STRONG ⊕◯◯◯ |
| The need for psychological counselling, pre-conception counselling and fertility treatment counselling should be considered for all patients. Local guidelines for counselling should be followed. | GPP |
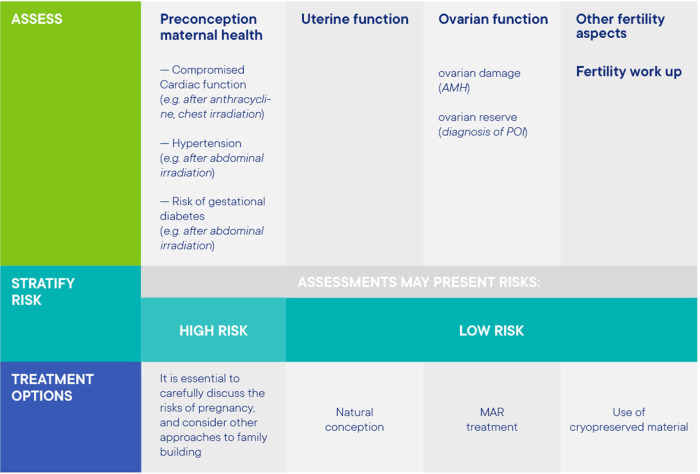
Information on patient re-assessment before attempting pregnancy (with or without the use of stored material) is summarized in Figure 6. With regards to pre-conception counselling, a checklist was prepared outlining the reproductive options after FP for cancer patients and for transgender men (Supplementary data II).
Figure 6.
Patient re-assessment before attempting pregnancy (with or without the use of stored material).
What is the effect of previous gonadotoxic treatments and underlying conditions on obstetric outcomes?
With regards to obstetric outcomes after oocyte cryopreservation for age-related fertility loss, it has been shown that there are risks of due to older age at pregnancy, which increase after the age of 45 (Aoyama et al., 2019). More research is needed on the number of women who return to use their frozen oocytes, pregnancy complications, and live birth rates in these women.
| Preconception counselling and appropriate obstetric monitoring is recommended in women intending to become pregnant after gonadotoxic treatments (Fossa et al., 2005; Madanat-Harjuoja et al., 2013; Ji et al., 2016; Anderson et al., 2017a; van der Kooi et al., 2018). | STRONG ⊕⊕⊕◯ |
| An interval of at least 1 year following chemotherapy completion is suggested before attempting a pregnancy in order to reduce the risk of pregnancy complications (Hartnett et al., 2018). | STRONG ⊕◯◯◯ |
| Radiotherapy to a field that included the uterus increases the risk of pregnancy complications; this risk is age (higher at prepubertal ages) and dose dependent. These pregnancies should be treated as high risk and managed in a centre with advanced maternity services (Sanders et al., 1996; Critchley and Wallace, 2005; Signorello et al., 2010; Teh et al., 2014; Tarin et al., 2016; van de Loo et al., 2019). | STRONG ⊕◯◯◯ |
| After completion of the recommended treatment, pregnancy is safe in women who have survived breast cancer. This is independent of oestrogen receptor status of the tumour (Hartman and Eslick, 2016; Sun et al., 2018; Lambertini et al., 2018b; Lee et al., 2019; Schuurman et al., 2019). | STRONG ⊕⊕◯◯ |
| Pregnancy after treatment for breast cancer should be closely monitored, as there is an increased risk of preterm birth and low birth weight. Patients should be informed about these risks (Hartman and Eslick, 2016; Sun et al., 2018; Lee et al., 2019; Schuurman et al., 2019; Lambertini et al., 2019c). | STRONG ⊕⊕⊕◯ |
| Reliable non-hormonal contraception is mandatory during tamoxifen treatment. It is recommended to stop tamoxifen for at least 3 months before attempting pregnancy. | GPP |
| Women with endometrial cancer should be followed up for high-risk pregnancy and monitored by an oncologist due to the risk of relapse (Chao et al., 2011; Park et al., 2013). | STRONG ⊕◯◯◯ |
| The risk of preterm birth is increased after treatment for early cervical cancer and these pregnancies should be treated as high risk and managed in a centre with advanced maternity services (Bentivegna et al., 2016; Kyrgiou et al., 2017; Zhang et al., 2017). | STRONG ⊕⊕◯◯ |
| Women previously treated for cancer require individual assessment of their obstetric risks and potential additional obstetric surveillance (Longhi et al., 2000; do Rosario et al., 2006; Haggar et al., 2013; Marklund et al., 2018). | STRONG ⊕◯◯◯ |
| Healthcare professionals should have a high level of awareness of the risk of depression and increased dysphoria during and after pregnancy care for transgender men (Light et al., 2014; Obedin-Maliver and Makadon, 2016; Brandt et al., 2019). | WEAK ⊕◯◯◯ |
Future FP interventions
What are ongoing developments with regards to FP?
To increase the spectrum of FP options, innovative technologies and novel in vitro avenues are continually being developed. This includes technologies involving transplantation into the patient, such as transplantation of the whole ovary after cryopreservation and procedures to optimize the use of transplanted ovarian cortex tissue, such as in vitro activation, processes to reduce ischaemia by promoting revascularization, techniques to eliminate residual malignant cells, methods for transplantation of follicles isolated from ovarian cortex tissue as bioprosthetic ovaries, and methods for transplantation of isolated cells into the remaining (gonadotoxic-exposed) ovary. Another line of research focuses on technologies that do not involve transplantation, such as in vitro matured oocytes from cultured ovarian cortex tissue, in vitro matured oocytes from primordial follicles isolated from ovarian cortex tissue and in vitro matured oocytes from cells isolated from the ovary. In addition, research is conducted in the use of in vitro matured oocytes from induced pluripotent stem cells (in vitro gametogenesis) or from mesenchymal stromal cells. Treatments to prevent gonadotoxic-induced POI have been recently reviewed (Spears et al., 2019).
Regarding future FP interventions, the GDG formulated the following conclusion: It is important to stress that emerging technologies, however promising, need to be followed by rigorous clinical trials, ensuring internationally accepted standards, to demonstrate efficacy and safety before they can be offered as medical treatment. Moreover, a scientific-medical consensus is required regarding safety and functional criteria that needs to be achieved before considering clinical use of in vitro-derived human oocytes. In this regard, a societal debate on the ethical issues and what emerging technologies may be considered acceptable for human reproductive purposes is recommended.
Discussion
The current paper summarizes the 78 recommendations on information provision and support, pre-FP assessment, FP interventions and after treatment care from the ESHRE Guideline on ‘Female Fertility Preservation’. This Guideline covers all aspects of FP on four different patient populations, specifically cancer patients, patients with benign diseases, transgender men and women requesting FP for age-related fertility loss, and was written by a multidisciplinary group with gynaecologists and fertility specialists, oncologists, a psychologist, a bioethicist, an embryologist, a scientist, and patient representatives.
According to the World Health Organization, individuals and couples have the right to decide the number, timing and spacing of their children (https://www.who.int/news-room/fact-sheets/detail/infertility). FP can be considered one option in preventing infertility and an important part of realizing the right to have a family. The importance of FP has also been demonstrated at the start of the COVID-19 pandemic, when ESHRE recommended not starting fertility treatments with the exception of treatments for FP. It has been shown that although most fertility treatments were suspended, FP interventions were continued in most centres throughout Europe (Vermeulen et al., 2020).
Notwithstanding the importance and relevance of FP, research data on many aspects are scarce. As a basis for the current guideline, a broad and formal literature review was conducted. Few relevant RCTs have been performed, with evidence for most interventions deriving from case series. Research gaps were detected in several areas, and these are documented in a list of recommendations for further research (Supplementary data III). Although the literature searches focused on the four patient populations separately, most studies included in the guideline reported on women with cancer, mostly breast cancer, with very few studies on FP in benign diseases, and even fewer on FP in transgender men and in women requesting FP for age-related fertility loss. For the latter patient groups, most reports focus on feasibility, acceptability and ethical considerations, rather than efficacy, efficiency and safety. To advance the field of female FP, more research is needed on the efficacy and safety of both established and newer techniques with a focus on achievement of live birth, but also on patient preferences and indications for FP. Indeed, one key aspect of the provision of FP is the need to widen access, while offering it to appropriately selected patients with a relevant indication. The variable provision of FP interventions deprives some women from having a family. However, applying FP to all patients undergoing gonadotoxic treatment, even with limited gonadotoxicity, will result in large amounts of stored but unused reproductive cells and tissue, creating unnecessary burden and costs for some patients involved and for health services. The risk of gonadotoxicity can be estimated or quantified in those patients receiving specific treatments that have been studied in sufficient detail, albeit generally with ovarian reserve testing which has very limited value for predicting future fertility. For many patients, the risk and benefits of FP interventions require multidisciplinary discussion and decision-making with regards to FP. New targeted treatments in oncology including immunotherapy largely have unknown gonadotoxicity (Lambertini et al., 2020). Providing appropriate information to patients and supporting their decision-making is crucial, both on whether to proceed with FP, and when it has become time to attempt pregnancy. Patient information leaflets summarizing the guideline’s most relevant information for patients are available and can be used as a template, and links to decision aids are provided.
Despite the limitations of guidelines in general, and the limitations in the evidence supporting the current guideline, the guideline group is confident that this document will help best practice in female FP.
Supplementary Material
Acknowledgements
The Guideline Development Group would like to acknowledge the help of many clinicians and professional organizations who refereed the content of the Guideline and submitted helpful comments to the draft version.
Authors’ roles
R.A.A. chaired the GDG and hence fulfilled a leading role in collecting the evidence, writing the manuscript and dealing with reviewer comments. N.V., as methodological expert, performed all literature searches for the guideline, provided methodological support and coordinated the guideline development. S.D. and C.M. represented the patient perspective in the guideline group. All other authors, listed in alphabetical order, as GDG members, contributed equally to the manuscript, by drafting key questions, synthesizing evidence, writing the different parts of the guideline and discussing recommendations until consensus within the group was reached.
Funding
The study has no external funding; all costs for meetings were covered by ESHRE.
Conflict of interest
R.A.A. reports personal fees and non-financial support from Roche Diagnostics, personal fees from Ferring Pharmaceuticals, IBSA and Merck Serono, outside the submitted work; D.B. reports grants from Merck Serono and Goodlife, outside the submitted work; I.D. reports consulting fees from Roche and speaker’s fees from Novartis; M.L. reports personal fees from Roche, Novartis, Pfizer, Lilly, Takeda and Theramex, outside the submitted work. The other authors have no conflicts of interest to declare.
Footnotes
ESHRE Pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
References
- Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, Meirow D, Felz C, Avigad S. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod 2010;25:1708–1712. [DOI] [PubMed] [Google Scholar]
- Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. Relationship between reproductive history, anthropometrics, lifestyle factors, and the likelihood of persistent chemotherapy-related amenorrhea in women with premenopausal breast cancer. Fertil Steril 2012;97:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S, Youssef I, Soudy H, Elhassan TA, Rauf SM, Maghfoor I. Prevalence of menstrual cycles and outcome of 50 pregnancies after high-dose chemotherapy and auto-SCT in non-Hodgkin and Hodgkin lymphoma patients younger than 40 years. Bone Marrow Transplant 2015;50:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod 2018;33:2051–2059. [DOI] [PubMed] [Google Scholar]
- Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, Bradford N, Cohn R, Birdsall M, Barr R. et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update 2019;25:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CY, Bollerup AC, Kristensen SG. Defining quality assurance and quality control measures in connection with ovarian tissue cryopreservation and transplantation: a call to action. Hum Reprod 2018;33:1201–1204. [DOI] [PubMed] [Google Scholar]
- Anderson R A Mitchell R T Kelsey T W Spears N Telfer E E Wallace W H B.. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. The Lancet Diabetes & Endocrinology 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Anderson C, Engel SM, Mersereau JE, Black KZ, Wood WA, Anders CK, Nichols HB. Birth outcomes among adolescent and young adult cancer survivors. JAMA Oncol 2017. a;3:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Engel SM, Weaver MA, Zevallos JP, Nichols HB. Birth rates after radioactive iodine treatment for differentiated thyroid cancer. Int J Cancer 2017. b;141:2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 2011;96:1336–1343. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Davies MC, Lavery SA; Royal College of Obstetricians and Gynaecologists. Elective egg freezing for non-medical reasons: scientific impact paper no. 63. BJOG 2020;127:e113–e121. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mansi J, Coleman RE, Adamson DJA, Leonard RCF. The utility of anti-Mullerian hormone in the diagnosis and prediction of loss of ovarian function following chemotherapy for early breast cancer. Eur J Cancer 2017. c;87:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer 2013;49:3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Wallace WHB, Telfer EE. Ovarian tissue cryopreservation for fertility preservation: clinical and research perspectives. Hum Reprod Open 2017. d;2017:hox001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Pinto R, Ray JG, Hill AD, Scales DC, Lapinsky SE, Hladunewich MA, Seaward GR, Fowler RA. Association of maternal age with severe maternal morbidity and mortality in Canada. JAMA Netw Open 2019;2:e199875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armuand G Dhejne C Olofsson J I Rodriguez-Wallberg K A.. Transgender men's experiences of fertility preservation: a qualitative study. Hum Reprod 2017;32:383–390. [DOI] [PubMed] [Google Scholar]
- Ashrafi M, Arabipoor A, Hemat M, Salman-Yazdi R. The impact of the localisation of endometriosis lesions on ovarian reserve and assisted reproduction techniques outcomes. J Obstet Gynaecol 2019;39:91–97. [DOI] [PubMed] [Google Scholar]
- Barcroft J, Dayoub N, Thong KJ. Fifteen year follow-up of embryos cryopreserved in cancer patients for fertility preservation. J Assist Reprod Genet 2013;30:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabei A, Strigari L, Marchetti P, Sini V, De Vecchis L, Corsello SM, Torino F. Predicting ovarian activity in women affected by early breast cancer: a meta-analysis-based nomogram. Oncologist 2015;20:1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update 2013;19:483–506. [DOI] [PubMed] [Google Scholar]
- Beckmann MW, Dittrich R, Lotz L, Oppelt PG, Findeklee S, Hildebrandt T, Heusinger K, Cupisti S, Muller A. Operative techniques and complications of extraction and transplantation of ovarian tissue: the Erlangen experience. Arch Gynecol Obstet 2017;295:1033–1039. [DOI] [PubMed] [Google Scholar]
- Beckmann MW, Dittrich R, Lotz L, van der Ven K, van der Ven HH, Liebenthron J, Korell M, Frambach T, Sutterlin M, Schwab R. et al. Fertility protection: complications of surgery and results of removal and transplantation of ovarian tissue. Reprod Biomed Online 2018;36:188–196. [DOI] [PubMed] [Google Scholar]
- Benaglia L, Bermejo A, Somigliana E, Faulisi S, Ragni G, Fedele L, Garcia-Velasco JA. In vitro fertilization outcome in women with unoperated bilateral endometriomas. Fertil Steril 2013;99:1714–1719. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Pharmacological interventions for fertility preservation during chemotherapy: a systematic review and meta-analysis. Breast Cancer Res Treat 2010;122:803–811. [DOI] [PubMed] [Google Scholar]
- Bentivegna E, Maulard A, Pautier P, Chargari C, Gouy S, Morice P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil Steril 2016;106:1195–1211. [DOI] [PubMed] [Google Scholar]
- Bernhard J, Zahrieh D, Castiglione-Gertsch M, Hurny C, Gelber RD, Forbes JF, Murray E, Collins J, Aebi S, Thurlimann B. et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients–the International Breast Cancer Study Group Trial VIII. JCO 2007;25:263–270. [DOI] [PubMed] [Google Scholar]
- Bittinger SE, Nazaretian SP, Gook DA, Parmar C, Harrup RA, Stern CJ. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril 2011;95:803.e803–803.e6. [DOI] [PubMed] [Google Scholar]
- Bockstaele L, Boulenouar S, Van Den Steen G, Dechene J, Tsepelidis S, Craciun L, Noel JC, Demeestere I. Evaluation of quantitative polymerase chain reaction markers for the detection of breast cancer cells in ovarian tissue stored for fertility preservation. Fertil Steril 2015;104:410–417. [DOI] [PubMed] [Google Scholar]
- Brandt JS, Patel AJ, Marshall I, Bachmann GA. Transgender men, pregnancy, and the “new” advanced paternal age: a review of the literature. Maturitas 2019;128:17–21. [DOI] [PubMed] [Google Scholar]
- Brunner HI, Silva CA, Reiff A, Higgins GC, Imundo L, Williams CB, Wallace CA, Aikawa NE, Nelson S, Klein-Gitelman MS. et al. Randomized, double-blind, dose-escalation trial of triptorelin for ovary protection in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol 2015;67:1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AS, Chao A, Wang CJ, Lai CH, Wang HS. Obstetric outcomes of pregnancy after conservative treatment of endometrial cancer: case series and literature review. Taiwan J Obstet Gynecol 2011;50:62–66. [DOI] [PubMed] [Google Scholar]
- Chiavari L, Gandini S, Feroce I, Guerrieri-Gonzaga A, Russell-Edu W, Bonanni B, Peccatori FA. Difficult choices for young patients with cancer: the supportive role of decisional counseling. Support Care Cancer 2015;23:3555–3562. [DOI] [PubMed] [Google Scholar]
- Cobo A, Garcia-Velasco J, Domingo J, Pellicer A, Remohi J. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018;33:2222–2231. [DOI] [PubMed] [Google Scholar]
- Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril 2013;99:1485–1495. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Decanter C, Bringer-Deutsch S, Rives N, Mirallie S, Pech JC, De Ziegler D, Carre-Pigeon F, May-Panloup P, Sifer C. et al. Emergency IVF for embryo freezing to preserve female fertility: a French multicentre cohort study. Hum Reprod 2013;28:2381–2388. [DOI] [PubMed] [Google Scholar]
- Creux H, Monnier P, Son WY, Buckett W. Thirteen years' experience in fertility preservation for cancer patients after in vitro fertilization and in vitro maturation treatments. J Assist Reprod Genet 2018;35:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HOD, Wallace WHB. Impact of cancer treatment on uterine function. JNCI Monographs 2005;2005:64–68. [DOI] [PubMed] [Google Scholar]
- Dalman A, Deheshkar Gooneh Farahani NS, Totonchi M, Pirjani R, Ebrahimi B, Rezazadeh Valojerdi M. Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: an evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. Cryobiology 2017;79:29–36. [DOI] [PubMed] [Google Scholar]
- Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D'Hooghe TM. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT. Hum Reprod 2015;30:1820–1830. [DOI] [PubMed] [Google Scholar]
- Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, Delozier T, Levy C, Bendavid C, Debled M. et al. Prospective evaluation of serum anti-Mullerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer 2017. a;79:72–80. [DOI] [PubMed] [Google Scholar]
- Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, Delozier T, Levy C, Bendavid C, Debled M. et al. Prospective evaluation of serum anti-Mullerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer 2017. b;79:72–80. [DOI] [PubMed] [Google Scholar]
- Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, Cobo A, Remohi J, Pellicer A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril 2018;109:478–485.e2. [DOI] [PubMed] [Google Scholar]
- Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril 2013;99:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich R, Lotz L, Mueller A, Hoffmann I, Wachter DL, Amann KU, Beckmann MW, Hildebrandt T. Oncofertility: combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod Biol Endocrinol 2013;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Rosario PW, Barroso AL, Rezende LL, Padrao EL, Borges MA, Purisch S. Malformations in the offspring of women with thyroid cancer treated with radioiodine for the ablation of thyroid remnants. Arq Bras Endocrinol Metabol 2006;50:930–933. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril 2005;83:897–901. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Hollanders de Ouderaen S, Demylle D, Pirard C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J Assist Reprod Genet 2015;32:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans MM, Iwahara Y, Donnez J, Soares M, Vaerman JL, Amorim CA, Poirel H. Evaluation of minimal disseminated disease in cryopreserved ovarian tissue from bone and soft tissue sarcoma patients. Hum Reprod 2016;31:2292–2302. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril 2013;99:1514–1522. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Marotta ML, Pirard C, Donnez J, Donnez O. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol 2016;127:474–480. [DOI] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an Ethics Committee opinion. Fertil Steril 2018;110:1022–1028. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Vicenti R, Macciocca M, Martino NA, Dell'Aquila ME, Pasquinelli G, Morselli-Labate AM, Seracchioli R, Paradisi R. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod 2016;31:1838–1849. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Vicenti R, Macciocca M, Pasquinelli G, Lima M, Parazza I, Magnani V, Venturoli S. Cryopreservation of ovarian tissue in pediatric patients. Obstet Gynecol Int 2012;2012:910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossa SD, Magelssen H, Melve K, Jacobsen AB, Langmark F, Skjaerven R. Parenthood in survivors after adulthood cancer and perinatal health in their offspring: a preliminary report. J Natl Cancer Inst Monogr 2005;2005:77–82. [DOI] [PubMed] [Google Scholar]
- Freour T, Barriere P, Masson D. Anti-mullerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. Eur J Cancer 2017;74:1–8. [DOI] [PubMed] [Google Scholar]
- Gellert SE, Pors SE, Kristensen SG, Bay-Bjorn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet 2018;35:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani MM, Hasanzadeh M, Ghaemmaghami F, Ramazanzadeh F. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia-Pacific J Clin Oncol 2007;3:79–83. [Google Scholar]
- Goossens J, Delbaere I, Van Lancker A, Beeckman D, Verhaeghe S, Van Hecke A. Cancer patients' and professional caregivers' needs, preferences and factors associated with receiving and providing fertility-related information: a mixed-methods systematic review. Int J Nurs Stud 2014;51:300–319. [DOI] [PubMed] [Google Scholar]
- Greenwood EA, Pasch LA, Hastie J, Cedars MI, Huddleston HG. To freeze or not to freeze: decision regret and satisfaction following elective oocyte cryopreservation. Fertil Steril 2018;109:1097–1104. [DOI] [PubMed] [Google Scholar]
- Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, Ralfkiær E, Andersen CY. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood 2012;120:4311–4316. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Dagher Hayeck B, Papanikolaou EG, Sifer C, Sermondade N, Sonigo C. BRCA1/2 gene mutations do not affect the capacity of oocytes from breast cancer candidates for fertility preservation to mature in vitro. Hum Reprod 2019;34:374–379. [DOI] [PubMed] [Google Scholar]
- Gubbala K, Laios A, Gallos I, Pathiraja P, Haldar K, Ind T. Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. J Ovarian Res 2014;7:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggar F, Pereira G, Preen D, Woods J, Martel G, Boushey R, Mamazza J, Einarsdottir K. Maternal and neonatal outcomes in pregnancies following colorectal cancer. Surg Endosc 2013;27:2327–2336. [DOI] [PubMed] [Google Scholar]
- Hamy AS, Porcher R, Eskenazi S, Cuvier C, Giacchetti S, Coussy F, Hocini H, Tournant B, Perret F, Bonfils S. et al. Anti-Mullerian hormone in breast cancer patients treated with chemotherapy: a retrospective evaluation of subsequent pregnancies. Reprod Biomed Online 2016;32:299–307. [DOI] [PubMed] [Google Scholar]
- Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat 2016;160:347–360. [DOI] [PubMed] [Google Scholar]
- Hartnett KP, Mertens AC, Kramer MR, Lash TL, Spencer JB, Ward KC, Howards PP. Pregnancy after cancer: does timing of conception affect infant health? Cancer 2018;124:4401–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekman EJ, Broeders E, Louwe LA, Nout RA, Jansen FW, de Kroon CD. Ovarian function after ovarian transposition and additional pelvic radiotherapy: a systematic review. Eur J Surg Oncol 2019;45:1328–1340. [DOI] [PubMed] [Google Scholar]
- Huober-Zeeb C, Lawrenz B, Popovici RM, Strowitzki T, Germeyer A, Stute P, von Wolff M. Improving fertility preservation in cancer: ovarian tissue cryobanking followed by ovarian stimulation can be efficiently combined. Fertil Steril 2011;95:342–344. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Tinkanen H, Wikstrom A, Dunkel L, Saarinen-Pihkala UM, Makinen S, Asadi Azarbaijani B, Oskam IC, Vettenranta K, Laine T. et al. Bone marrow remission status predicts leukemia contamination in ovarian biopsies collected for fertility preservation. Leukemia 2013;27:1183–1185. [DOI] [PubMed] [Google Scholar]
- Ji J, Sundquist J, Sundquist K. Stillbirth and neonatal death among female cancer survivors: a national cohort study. Int J Cancer 2016;139:1046–1052. [DOI] [PubMed] [Google Scholar]
- Kemertzis MA, Ranjithakumaran H, Hand M, Peate M, Gillam L, McCarthy M, Super L, McQuillan S, Drew S, Jayasinghe Y. et al. Fertility preservation toolkit: a clinician resource to assist clinical discussion and decision making in pediatric and adolescent oncology. J Pediatr Hematol Oncol 2018;40:e133–e139. [DOI] [PubMed] [Google Scholar]
- Kristensen SG, Giorgione V, Humaidan P, Alsbjerg B, Bjorn AB, Ernst E, Andersen CY. Fertility preservation and refreezing of transplanted ovarian tissue-a potential new way of managing patients with low risk of malignant cell recurrence. Fertil Steril 2017;107:1206–1213. [DOI] [PubMed] [Google Scholar]
- Kyrgiou M, Athanasiou A, Kalliala IEJ, Paraskevaidi M, Mitra A, Martin-Hirsch PP, Arbyn M, Bennett P, Paraskevaidis E. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev 2017;11:CD012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Campbell C, Bines J, Korde LA, Izquierdo M, Fumagalli D, Del Mastro L, Ignatiadis M, Pritchard K, Wolff AC. et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst 2019. a;111:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Ceppi M, Cognetti F, Cavazzini G, De Laurentiis M, De Placido S, Michelotti A, Bisagni G, Durando A, Valle E. et al. Dose-dense adjuvant chemotherapy in premenopausal breast cancer patients: a pooled analysis of the MIG1 and GIM2 phase III studies. Eur J Cancer 2017;71:34–42. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA, Jr, Ugolini D, Pronzato P, Loibl S, Moore HC, Partridge AH. et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol 2015;26:2408–2419. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Di Maio M, Poggio F, Pagani O, Curigliano G, Mastro LD, Paluch-Shimon S, Loibl S, Partridge AH, Azim HA., Jr et al. Knowledge, attitudes and practice of physicians towards fertility and pregnancy-related issues in young BRCA-mutated breast cancer patients. Reprod Biomed Online 2019. b;38:835–844. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim Jr HA, Desir J, Delbaere A, t’Kint de Roodenbeke M-D, de Azambuja E, Ignatiadis M. et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol 2018. a;29:237–243. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, Jensen MB, Gelber S, Del Grande M, Ignatiadis M. et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst 2018. b;110:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Martel S, Campbell C, Guillaume S, Hilbers FS, Schuehly U, Korde L, Azim HA, Jr, Di Cosimo S, Tenglin RC. et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer 2019. c;125:307–316. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, Boni L, Unger JM, Anderson RA, Mehta K. et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. JCO 2018. c;36:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Olympios N, Lequesne J, Calbrix C, Fontanilles M, Loeb A, Leheurteur M, Demeestere I, Di Fiore F, Perdrix A. et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-mullerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol 2019. d;9:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, Paluch-Shimon S, Halaska MJ, Uzan C, Meissner J. et al. ; ESMO Guidelines Committee. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2020; 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, Henes M. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma–evaluation by using antimullerian hormone and retrieved oocytes. Fertil Steril 2012;98:141–144. [DOI] [PubMed] [Google Scholar]
- Lawson AK, Klock SC, Pavone ME, Hirshfeld-Cytron J, Smith KN, Kazer RR. Prospective study of depression and anxiety in female fertility preservation and infertility patients. Fertil Steril 2014;102:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Kim BW, Park S, Park S, Lee JE, Choi YJ, Kim SY, Woo SU, Youn HJ, Lee I. Childbirth in young Korean women with previously treated breast cancer: the SMARTSHIP study. Breast Cancer Res Treat 2019;176:419–427. [DOI] [PubMed] [Google Scholar]
- Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet 2016;33:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol 2014;124:1120–1127. [DOI] [PubMed] [Google Scholar]
- Logan S, Perz J, Ussher JM, Peate M, Anazodo A. A systematic review of patient oncofertility support needs in reproductive cancer patients aged 14 to 45 years of age. Psychooncology 2018;27:401–409. [DOI] [PubMed] [Google Scholar]
- Logan S, Perz J, Ussher JM, Peate M, Anazodo A. Systematic review of fertility-related psychological distress in cancer patients: informing on an improved model of care. Psychooncology 2019;28:22–30. [DOI] [PubMed] [Google Scholar]
- Longhi A, Porcu E, Petracchi S, Versari M, Conticini L, Bacci G. Reproductive functions in female patients treated with adjuvant and neoadjuvant chemotherapy for localized osteosarcoma of the extremity. Cancer 2000;89:1961–1965. [DOI] [PubMed] [Google Scholar]
- Luyckx V, Durant JF, Camboni A, Gilliaux S, Amorim CA, Van Langendonckt A, Irenge LM, Gala JL, Donnez J, Dolmans MM. Is transplantation of cryopreserved ovarian tissue from patients with advanced-stage breast cancer safe? A pilot study. J Assist Reprod Genet 2013;30:1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madanat-Harjuoja LM, Lahteenmaki PM, Dyba T, Gissler M, Boice JD, Jr, Malila N. Stillbirth, early death and neonatal morbidity among offspring of female cancer survivors. Acta Oncol 2013;52:1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder W, McCune WJ, Wang L, Wing JJ, Fisseha S, McConnell DS, Christman GM, Somers EC. Adjunctive GnRH-a treatment attenuates depletion of ovarian reserve associated with cyclophosphamide therapy in premenopausal SLE patients. Gynecol Endocrinol 2012;28:624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund A, Eloranta S, Wikander I, Kitlinski ML, Lood M, Nedstrand E, Thurin-Kjellberg A, Zhang P, Bergh J, Rodriguez-Wallberg KA. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer-a prospective nationwide Swedish multicenter study. Hum Reprod 2020;35:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund A, Nasiell J, Berger AS, Fagerberg A, Rodriguez-Wallberg KA. Pregnancy achieved using donor eggs in cancer survivors with treatment-induced ovarian failure: obstetric and perinatal outcome. J Women's Health (2002) 2018;27:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciangelo R, Bosisio C, Donnez J, Amorim CA, Dolmans MM. Safety of ovarian tissue transplantation in patients with borderline ovarian tumors. Hum Reprod 2018;33:212–219. [DOI] [PubMed] [Google Scholar]
- Massarotti C, Scaruffi P, Lambertini M, Remorgida V, Del Mastro L, Anserini P. State of the art on oocyte cryopreservation in female cancer patients: a critical review of the literature. Cancer Treat Rev 2017;57:50–57. [DOI] [PubMed] [Google Scholar]
- Morel N, Bachelot A, Chakhtoura Z, Ghillani-Dalbin P, Amoura Z, Galicier L, Aumaitre O, Piette J-C, Pourrat J, Boutin D. et al. ; on behalf of the PLUS group. Study of anti-Müllerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab 2013;98:3785–3792. [DOI] [PubMed] [Google Scholar]
- Moria A, Das M, Shehata F, Holzer H, Son WY, Tulandi T. Ovarian reserve and oocyte maturity in women with malignancy undergoing in vitro maturation treatment. Fertil Steril 2011;95:1621–1623. [DOI] [PubMed] [Google Scholar]
- O’Hea EL, Monahan BR, Cutillo A, Person SD, Grissom G, Boudreaux ED. Predictors of psychological distress and interest in mental health services in individuals with cancer. J Health Psychol 2016;21:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obedin-Maliver J, Makadon HJ. Transgender men and pregnancy. Obstet Med 2016;9:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci 2017;24:1111–1120. [DOI] [PubMed] [Google Scholar]
- Paradisi R, Macciocca M, Vicenti R, Rossi S, Morselli-Labate AM, Mastroroberto M, Seracchioli R, Fabbri R. New insights in the selection and management of cancer patients applicants for ovarian tissue cryopreservation. Gynecol Endocrinol 2016;32:881–885. [DOI] [PubMed] [Google Scholar]
- Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, Nam JH. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol 2013;121:136–142. [DOI] [PubMed] [Google Scholar]
- Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat 2009;116:215–223. [DOI] [PubMed] [Google Scholar]
- Peigne M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reprod Biol Endocrinol 2014;12:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot C, Fortin A, Lacorte JM, Akakpo JP, Genestie C, Vernant JP, Brice P, Morice P, Leblanc T, Gabarre J. et al. ; for the CAROLéLISA Cooperative Group. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum Reprod 2019;34:1083–1094. [DOI] [PubMed] [Google Scholar]
- Reinblatt SL, Ishai L, Shehata F, Son WY, Tulandi T, Almog B. Effects of ovarian endometrioma on embryo quality. Fertil Steril 2011;95:2700–2702. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, Remohi J, Ragni G, Ubaldi FM. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod 2012;27:1606–1612. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Iglesias B, Novella-Maestre E, Herraiz S, Diaz-Garcia C, Pellicer N, Pellicer A. New methods to improve the safety assessment of cryopreserved ovarian tissue for fertility preservation in breast cancer patients. Fertil Steril 2015;104:1493–1502. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Marklund A, Lundberg F, Wikander I, Milenkovic M, Anastacio A, Sergouniotis F, Wanggren K, Ekengren J, Lind T. et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden-Trends in patients' choices and benefit of the chosen methods after long-term follow up. Acta Obstet Gynecol Scand 2019. a;98:604–615. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Waterstone M, Anastácio A. Ice age: cryopreservation in assisted reproduction—an update. Reprod Biol 2019. b;19:119–126. [DOI] [PubMed] [Google Scholar]
- Ruddy KJ, Guo H, Barry W, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis MJ. et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res Treat 2015;151:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R. et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996;87:3045–3052. [PubMed] [Google Scholar]
- Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, Ottosen C, Andersen CY. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril 2011;95:695–701. [DOI] [PubMed] [Google Scholar]
- Schuurman TN, Witteveen PO, van der Wall E, Passier JLM, Huitema ADR, Amant F, Lok CAR. Tamoxifen and pregnancy: an absolute contraindication? Breast Cancer Res Treat 2019;175:17–25. [DOI] [PubMed] [Google Scholar]
- Senra JC, Roque M, Talim MCT, Reis FM, Tavares RLC. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:77–86. [DOI] [PubMed] [Google Scholar]
- Shah MS, Letourneau JM, Niemasik EE, Bleil M, McCulloch CE, Rosen MP. The role of in-depth reproductive health counseling in addressing reproductive health concerns in female survivors of nongynecologic cancers. J Psychosoc Oncol 2016;34:305–317. [DOI] [PubMed] [Google Scholar]
- Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, Schiff E, Orvieto R, Meirow D. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril 2018;109:48–53. [DOI] [PubMed] [Google Scholar]
- Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep 2017;7:8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, Mertens AC, Whitton JA, Robison LL, Boice JD., Jr Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet 2010;376:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Almeida-Santos AT, Melo C, Ribeiro Rama AC. Antineoplastic agents and (in)fertility: informing patients to improve decisions. J Adolesc Young Adult Oncol 2018;7:306–314. [DOI] [PubMed] [Google Scholar]
- Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod 2016;31:2737–2749. [DOI] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Haunschild C, Chung K, Komrokian S, Boles S, Sammel MD, DeMichele A. Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer 2014;120:3691–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Ding X, Wu Y, Yang L. Meta-analysis of associations between maternal breast cancer and the risk of adverse delivery outcomes. Int J Gynecol Obstet 2018;140:146–152. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Garcia-Perez MA, Cano A. Obstetric and offspring risks of women's morbid conditions linked to prior anticancer treatments. Reprod Biol Endocrinol 2016;14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchmanova L, Selleri C, De Rosa G, Esposito M, Orio F, Jr, Palomba S, Bifulco G, Nappi C, Lombardi G, Rotoli B. et al. Gonadal status in reproductive age women after haematopoietic stem cell transplantation for haematological malignancies. Hum Reprod 2003;18:1410–1416. [DOI] [PubMed] [Google Scholar]
- Teh WT, Stern C, Chander S, Hickey M. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int 2014;2014:482968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ESHRE Guideline Group on Ovarian Stimulation, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G. et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open 2020;2020:hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiarelli A, Cimadomo D, Conforti A, Schimberni M, Giuliani M, D’Alessandro P, Colamaria S, Alviggi C, Rienzi L, Ubaldi FM. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the Bologna criteria: a case series. Fertil Steril 2020;113:121–130. [DOI] [PubMed] [Google Scholar]
- Valentini A, Finch A, Lubiński J, Byrski T, Ghadirian P, Kim-Sing C, Lynch HT, Ainsworth PJ, Neuhausen SL, Greenblatt E. et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol 2013;31:3914–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo L, van den Berg MH, Overbeek A, van Dijk M, Damen L, Lambalk CB, Ronckers CM, van den Heuvel-Eibrink MM, Kremer LCM, van der Pal HJ. et al. Uterine function, pregnancy complications, and pregnancy outcomes among female childhood cancer survivors. Fertil Steril 2019;111:372–380. [DOI] [PubMed] [Google Scholar]
- van der Kaaij MA, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser EC, Allgeier A, Meulemans B, Simons AH, Lugtenburg PJ. et al. Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol 2012;30:291–299. [DOI] [PubMed] [Google Scholar]
- van der Kooi ALF, Brewster DH, Wood R, Nowell S, Fischbacher C, van den Heuvel-Eibrink MM, Laven JSE, Wallace WHB, Anderson RA. Perinatal risks in female cancer survivors: a population-based analysis. PLoS One 2018;13:e0202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen N Le Clef N D'Angelo A Tilleman K Veleva Z Nelen W, Manual for ESHRE guideline development. , ESHRE, 2017.https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guideline-development-process (date last accessed, 5 November 2020). [Google Scholar]
- Vermeulen N, Ata B, Gianaroli L, Lundin K, Mocanu E, Rautakallio-Hokkanen S, Tapanainen JS, Veiga A. A picture of medically assisted reproduction activities during the COVID-19 pandemic in Europe. Hum Reprod Open 2020;2020:hoaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WH, Critchley HO, Anderson RA. Optimizing reproductive outcome in children and young people with cancer. J Clin Oncol 2012;30:3–5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Anazodo A, Logan S. Systematic review of fertility preservation patient decision aids for cancer patients. Psychooncology 2019;28:459–467. [DOI] [PubMed] [Google Scholar]
- Witcomb GL, Bouman WP, Claes L, Brewin N, Crawford JR, Arcelus J. Levels of depression in transgender people and its predictors: results of a large matched control study with transgender people accessing clinical services. J Affect Disord 2018;235:308–315. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li W, Kanis MJ, Qi G, Li M, Yang X, Kong B. Oncologic and obstetrical outcomes with fertility-sparing treatment of cervical cancer: a systematic review and meta-analysis. Oncotarget 2017;8:46580–46592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chen C, Hu C, Wang Y, Zhang X, Wu R. Predictive value of the serum anti-Mullerian level for spontaneous pregnancy in women after endometriosis surgery. J Int Med Res 2019;47:5643–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.