Abstract
Cardiovascular diseases (CVDs) are among the leading causes of mortality and morbidity worldwide. Nitric oxide (NO) is a signalling molecule that plays a vital role in protecting and regulating cardiovascular function and homeostasis. Despite several successful outcomes of exogenous supplementation of NO for cardiovascular disease therapy, a great challenge lies in controlled and long-term delivery due to the short half-life and instability of NO. Recently, increasing attention has been paid to the in situ conversion of endogenous NO donors catalysed by functional surfaces. Based on bioinspired and biomimicking strategies, a series of surface functionalization methods for cardiovascular stents has been successfully developed. By further combining NO catalysis with other biofunctionalities, improved endothelium healing and long-term patency can be anticipated.
Highlights
-
•
Nitric oxide (NO) is a signalling molecule that plays a vital role in cardiovascular system.
-
•
Surface functionalization provides a promising strategy for the in situ generation of NO from endogenous donors.
-
•
Combining NO catalysis with other surface biofunctionalities could enhance long-term patency of the cardiovascular stents.
Cardiovascular diseases (CVDs) encompassing atherosclerosis, aortic aneurysms, restenosis, and pulmonary arterial hypertension are among the leading causes of mortality and morbidity worldwide [1]. Surgical interventions are the primary treatment for reducing mortality, including percutaneous coronary interventions (PCIs), coronary bypass and heart valve replacement. Although the development of surgical techniques and cardiovascular devices promotes the clinical curative effect, unavoidable complications are associated with thrombosis and restenosis in long-term outcomes and attributed to the interaction of blood with material surfaces and the development of neointimal hyperplasia (NIH), which limits clinical applications and is the main cause of device failure [2].
Nitric oxide (NO) is a signalling molecule that plays an important role in protecting and regulating cardiovascular functions [3]. NO is constitutively expressed in endothelial cells (ECs) through enzymatic conversion of l-arginine by nitric oxide synthase (NOS), and it plays an important role in protecting and regulating vascular functions, including suppressing vascular smooth muscle cell (VSMC) proliferation, inhibiting platelet adhesion and thrombosis, and preventing inflammation by inhibiting the adhesion and activation of leukocytes [4]. Several successful outcomes have been reported using polymeric carriers for NO delivery to treat cardiovascular diseases, but a great challenge lies in the controlled and long-term delivery due to their reactivity, short half-life (2–5 s), labile nature, and instability during storage [5]. Thus, there has been enormous effort in seeking biomaterial-based strategies to load NO donors and deliver precise amounts of NO directly to the target tissue site in a controlled manner with minimum adverse effects [[6], [7], [8]].
In addition to ‘classical’ l-arginine–NO synthase–NO signalling, accumulating evidence has revealed an alternative pathway to generate NO through the biotransformation of various endogenous NO donors (S-nitrosothiol, nitrate, nitrite, etc.) in blood and tissues that have previously been thought to be inner end products [9,10]. As a result, surface modification of bare stents by immobilizing relevant enzymes to catalyse the bioactivation of endogenous NO donors may represent a new paradigm for the future of NO-releasing stents. Recently, Yang and coworkers developed a series of surface functionalization methods based on bioinspired and biomimicking strategies to realize the long-term, stable and controllable generation of NO in cardiovascular stents.
As a current major therapeutic treatment for cardiovascular diseases, nonbiogenic metal stents are inclined to trigger a cascade of cellular and molecular events, including inflammatory responses, thrombogenic reactions, smooth muscle cell hyperproliferation accompanied by delayed arterial healing, and poor re-endothelialisation, thus leading to restenosis along with late stent thrombosis. In a recent study, Yang et al. proposed a strategy that utilized circulating RSNO molecules from blood itself for the local and sustained delivery of NO [11]. These researchers incorporated glutathione peroxidase (GPx) at the solid interface via co-immobilization of selenocystamine (SeCA) in the network of mussel-inspired polydopamine (PDA) coating. The SeCA/PDA coatings enabled the development of a catalytic surface for exceptionally long-term local NO generation over 60 days. A high SeCA/DA molar ratio resulted in an increased NO flux, and the rate could be controlled to mimic that of the native endothelium. After continuous exposure to NO donor solution for 60 days, the SeCA/PDA coatings still maintained 55% of their NO production rate, indicating long-term efficacy as a coating for blood contacting devices. Taking the biomimetic surface strategy one step further, the authors fabricated a dual-functional stent surface with NO-releasing and endothelial progenitor cell (EPC)-capturing properties by using a clickable mussel-inspired peptide mimic via mussel molecular adhesion and bioorthogonal conjugations [12]. As shown in Fig. 1, two typical vasoactive moieties, i.e., nitric oxide (NO)-generating organoselenium (SeCA) and EPC-targeting peptide (TPS), are grafted onto peptide-adhered surfaces via a bioorthogonal click reaction. The results showed that SeCA-catalysed NO generation and TPS-induced EPC capture contributed synergistically to inhibit thrombosis and SMC proliferation in vitro, finally promoting reendothelialization on the stents in vivo. Compared with traditional methods, this surface engineering strategy provides a facile approach for rational biofunctionalization of not only vascular stents but also other medical metal devices with optimal multifunction.
Fig. 1.
(A) Structural formula of the clickable mussel-inspired peptide, NO-generating organoselenium, and EPC-binding peptide. (B) Surface cografting on representative vascular stents through mussel-inspired coordinative interactions and bioorthogonal click chemistry. (C) The catalytic reactions of nitric acid (NO) generation process by SeCA and the proposed mechanism of SeCA/TPS cografted stents for antiplatelet adhesion, SMC inhibition, EPC homing, EC growth, and reendothelialization.
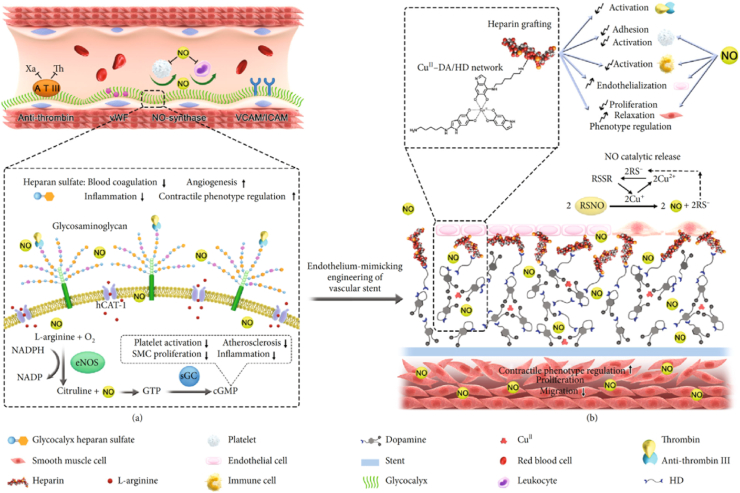
Metal–organic frameworks (MOFs) represent a new class of coordination materials created from metal bridged by organic bridging ligands. Yang et al. [13] developed a method to provide glutathione peroxidase (GPx)-like activity in the network of copper-phenolic-amine to enhance NO production capacity. The resultant NO-generating coatings, which were fabricated using a natural plant polyphenol, gallic acid, a glutathione peroxidase-like species selenocystamine, and the CuII ion, exhibited long-term, stable and controllable NO generation. Since antithrombogenicity is a crucial factor for the long-term success of vascular implants, surface heparinization was further [14] introduced through a stepwise metal (copper)-catechol-(amine) (MCA) surface chemistry strategy (Fig. 2). The heparin-grafted and NO-catalytic coating could mimic the physiological functions of native endothelium, which may address the challenges of in-stent restenosis.
Fig. 2.
Scheme of metal-catechol-amine network- (MCAN-) based coating strategy for engineering endothelium-mimicking cardiovascular stent coating. (a) Functions of healthy ECs. HS on ECs has anticoagulant function through binding with antithrombin III (ATIII) to inhibit thrombin molecules existing in a coagulation system. (b) The MCAN-based coating strategy inspired by native endothelium function. The delicate organization of metal ion-Cu2+ in the MCAN gives GPx-like activity to the modified surface while a large amount of surface primary amine groups allows sufficient covalent grafting of heparin.
Yang et al. [15] further developed mussel-inspired “built-up” surface chemistry for multifunctional surface tailoring. The REDV-functionalized pDA/CuII-DA surface perfectly combined the functions of NO and REDV, exhibiting excellent antithrombogenic properties and selectivity towards endothelial cells over smooth muscle cells. Hence, enhanced re-endothelialisation and anti-restenosis have been observed in vivo. These researchers also developed a copper-DA-HD (CuII-DA/HD) network that can catalyse the generation of therapeutic nitric oxide. The copper-catechol-(amine) networks are abundant with primary amine groups, thus facilitating the covalent immobilization of bivalirudin (BVLD) [16]. The synergistic modification of catalytic NO generation and BVLD grafting on cardiovascular stents provides a simple and effective strategy for functionalizing blood-contacting and other biomedical devices.
In summary, although NO-releasing coatings have been employed in the development of drug-eluting stents, long-term NO delivery proved to be a main challenge. Utilization of a biofunctional surface for in situ catalysis of NO enables the specific spatial and temporal release of NO at the site of stenting, thereby regulating the exposure and effects of NO on vascular cell activity and thus maximizing beneficial effects and minimizing side effects. Metal–organic frameworks represent a versatile and promising candidate for the future development of functional surfaces. By combining catalytic NO generation with other biofunctional molecules (such as heparin, REDV peptide, etc.), it will ultimately drive progress in full endothelialisation on the stent surfaces and is expected to facilitate a new category of cardiovascular stents for clinical applications.
CRediT authorship contribution statement
Meng Qian: Writing - original draft. Qi Liu: Writing - original draft. Yan Wei: Review & editing. Zhikun Guo: Review & editing. Qiang Zhao: Supervision, Writing - review & editing.
Conflict of interest
None.
Declaration of competing interest
The authors declare that they have no competing interest.
Acknowledgement
This study was supported by grants from the National Key R&D Program of China (2018YFE0200503), National Natural Science Foundation of China (81925021 and 81871500), and Natural Science Foundation of Tianjin (18JCJQJC46900).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zhikun Guo, Email: gzk@xxmu.edu.cn.
Qiang Zhao, Email: qiangzhao@nankai.edu.cn.
References
- 1.Rabinovich-Nikitin I., Lieberman B., Martino T.A. Circadian-regulated cell death in cardiovascular diseases. Circulation. 2019;139:965–980. doi: 10.1161/CIRCULATIONAHA.118.036550. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi N., De Mel A., Alavijeh O.S. Nitric oxide donors for cardiovascular implant applications. Small. 2013;9:22–35. doi: 10.1002/smll.201200458. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X., Zhang J., Feng G. Nitric oxide-releasing biomaterials for biomedical applications. Curr. Med. Chem. 2016 doi: 10.2174/0929867323666160729104647. [DOI] [PubMed] [Google Scholar]
- 4.Vong L.B., Bui T.Q., Tomita T. Novel angiogenesis therapeutics by redox injectable hydrogel - regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials. 2018;167:143–152. doi: 10.1016/j.biomaterials.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Midgley A.C., Wei Y., Li Z. Nitric‐oxide‐releasing biomaterial regulation of the stem cell microenvironment in regenerative medicine. Adv. Mater. 2020;32:1805818. doi: 10.1002/adma.201805818. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q., Zhang J., Song L. Biomaterials; 2013. Polysaccharide-based Biomaterials with On-Demand Nitric Oxide Releasing Property Regulated by Enzyme Catalysis. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Lu Y., Qin K. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Contr. Release. 2015;28:179–188. doi: 10.1016/j.jconrel.2015.05.283. [DOI] [PubMed] [Google Scholar]
- 8.Hou J., Pan Y., Zhu D. Targeted delivery of nitric oxide via a 'bump-and-hole'-based enzyme-prodrug pair. Nat. Chem. Biol. 2019;15:151–160. doi: 10.1038/s41589-018-0190-5. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg J.O., Gladwin M.T., Ahluwalia A. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466-c1. [DOI] [PubMed] [Google Scholar]
- 11.Zhilu Y., Ying Y., Li Z. Mussel-inspired catalytic selenocystamine-dopamine coatings for long-term generation of therapeutic gas on cardiovascular stents. Biomaterials. 2018;178:1–10. doi: 10.1016/j.biomaterials.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z., Zhao X., Hao R. Bioclickable and mussel adhesive peptide mimics for engineering vascular stent surfaces. Proc. Natl. Acad. Sci. Unit. States Am. 2020 doi: 10.1073/pnas.2003732117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhilu Y., Ying Y., Kaiqin X. Metal-phenolic surfaces for generating therapeutic nitric oxide gas. Chem. Mater. 2018:8b01876. doi: 10.1021/acs.chemmater.8b01876. [DOI] [Google Scholar]
- 14.Yang Y., Gao P., Wang J. Endothelium-mimicking multifunctional coating modified cardiovascular stents via a stepwise metal-catechol-(amine) surface engineering strategy. Research. 2020:1–20. doi: 10.34133/2020/9203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Maitz M.F., Yang Z. Biomaterials; 2020. Mussel-inspired "Built-Up" Surface Chemistry for Combining Nitric Oxide Catalytic and Vascular Cell Selective Properties; p. 241. [DOI] [PubMed] [Google Scholar]
- 16.Gao P., Qiu H., Xiong K. Biomaterials; 2020. Metal-catechol-(amine) Networks for Surface Synergistic Catalytic Modification: Therapeutic Gas Generation and Biomolecule Grafting; p. 119981. [DOI] [PubMed] [Google Scholar]