Abstract
The association between use of aromatase inhibitors (AIs) and cardiovascular outcomes is controversial. While some observational studies have assessed the cardiovascular safety of AIs as upfront treatments, their cardiotoxicity as sequential treatments with tamoxifen remains unknown. Thus, we conducted a population-based cohort study using data from the United Kingdom Clinical Practice Research Datalink linked to the Hospital Episode Statistics and Office for National Statistics databases. We employed a prevalent new-user design to propensity-score match, in a 1:2 ratio, patients switching from tamoxifen to AIs with patients continuing tamoxifen between 1998 and 2016. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals for the study outcomes (myocardial infarction, ischemic stroke, heart failure, and cardiovascular mortality). Overall, 1,962 patients switching to AIs were matched to 3,874 patients continuing tamoxifen. Compared with tamoxifen, AIs were associated with an increased risk of myocardial infarction (hazard ratio (HR) = 2.08, 95% confidence interval (CI): 1.02, 4.27). The hazard ratios were elevated for ischemic stroke (HR = 1.58, 95% CI: 0.85, 2.93) and heart failure (HR = 1.69, 95% CI: 0.79, 3.62) but not cardiovascular mortality (HR = 0.87, 95% CI: 0.49, 1.54), with confidence intervals including the null value. The elevated hazard ratios observed for the cardiovascular outcomes should be corroborated in future large observational studies.
Keywords: aromatase inhibitors, breast cancer, cardiovascular disease, endocrine therapy, tamoxifen
Abbreviations
- AI
aromatase inhibitor
- ASA
acetylsalicylic acid
- BIG
Breast International Group
- CI
confidence interval
- CPRD
Clinical Practice Research Datalink
- HES
Hospital Episode Statistics
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, Tenth Revision
- IR
incidence rate
- MACE
major adverse cardiovascular events
- ONS
Office for National Statistics
- RCT
randomized controlled trial
Aromatase inhibitors (AIs) used either up front or in the sequential setting with tamoxifen have become the mainstay treatment for breast cancer in postmenopausal women (1). Indeed, compared with tamoxifen, AIs have been associated with improved efficacy in both upfront and sequential settings (2), with the latter strategy employed for up to 35% of patients (3–5). When compared with upfront tamoxifen treatment, a sequential treatment strategy with tamoxifen followed by AIs is associated with improved efficacy while reducing the incidence of the musculoskeletal symptoms typically associated with upfront AI treatment (6).
Despite their clinical benefits, there is evidence from some randomized controlled trials (RCTs) and observational studies that upfront treatment with AIs may increase the risk of cardiovascular disease outcomes when compared with tamoxifen (7–9). As a result, regulatory agencies such as the US Food and Drug Administration have identified ischemic heart disease as a potential safety concern (10). The potential biological mechanism for this association remains unclear, as some RCTs have implicated the use of AIs with hypercholesterolemia (11, 12) while other investigators have reported no association of these medications with serum cholesterol levels (13–15). Conversely, studies have shown that tamoxifen treatment is associated with a reduction in cholesterol levels (15–19).
While upfront treatment and sequential AI treatment have been shown to have similar efficacy (6), there is uncertainty among clinicians as to the choice of treatment strategy. Thus, it is imperative to fully assess the cardiovascular safety of AIs in the sequential setting when deciding on the optimal treatment strategy for patients with estrogen-receptor-positive breast cancer. To date, few RCT investigators assessing the sequential treatment strategy have reported on cardiovascular outcomes. Overall, researchers in these RCTs have reported that sequential treatment with AIs was associated with an increased risk of cardiovascular disease outcomes when compared with tamoxifen (7). However, these RCTs were designed to assess efficacy and not cardiovascular safety and used heterogeneous composite definitions for the cardiovascular outcomes. To our knowledge, no observational studies have examined the cardiotoxicity of sequential treatment with AIs as compared with tamoxifen. Thus, to address this question, we conducted a population-based cohort study to determine whether use of AIs in sequential treatment with tamoxifen is associated with an increased risk of cardiovascular outcomes when compared with upfront tamoxifen treatment among women with breast cancer.
METHODS
Data sources
We conducted a population-based matched cohort study by linking data from the United Kingdom’s Clinical Practice Research Datalink (CPRD) with information from the Hospital Episode Statistics (HES) and Office for National Statistics (ONS) databases (20). The CPRD is a primary-care-based database which captures anonymous information on medical diagnoses, procedures, lifestyle variables (such as smoking), anthropometric measurements (including body mass index), and prescriptions written by general practitioners (20). The CPRD captures data on over 4 million active patients in the United Kingdom (20) and has been shown to be representative of the United Kingdom population in regards to key characteristics such as age, ethnicity, and body mass index. Clinical diagnoses and procedures are classified according to the Read code classification system, whereas prescriptions are classified according to the United Kingdom Pricing Authority Dictionary (20). Overall, diagnoses in the CPRD have been shown to be valid (21, 22). The diagnosis of breast cancer in the CPRD has been shown to be concordant with that in the National Cancer Data Repository (96–97%) (23, 24) and medical profile reviews (98%) (23–25).
The HES is a repository which captures all inpatient and outpatient hospital admissions. Primary and secondary diagnoses are recorded in the HES using International Classification of Diseases, Tenth Revision (ICD-10) codes, and procedures are recorded using the Office of Population Censuses and Surveys classification of interventions and procedures (fourth revision) (26). Last, the ONS database includes the electronic death certificates of all United Kingdom residents and includes primary and secondary causes of death recorded using International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes (27). Approximately 75% of general practitioner medical practices in England have been linked to HES and ONS databases since April 1, 1997, with linkage restricted to English practices that have provided consent (20). The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD and by the Research Ethics Board of the Jewish General Hospital (Montreal, Quebec, Canada).
Study population
We first identified a cohort of women at least 50 years of age who were newly diagnosed with breast cancer between April 1, 1998, and February 29, 2016 (see Web Figure 1, available at https://academic.oup.com/aje). We excluded patients with less than 1 year of medical history data, those with metastatic disease, and those with prescriptions for AIs or tamoxifen before their breast cancer diagnosis.
Prevalent new-user design
Using the cohort defined above, we employed a prevalent new-user design to match and compare patients switching from tamoxifen to AIs with patients continuing tamoxifen treatment (Web Figure 2) (28). In this approach, we divided the elapsed time since the first tamoxifen prescription into 30-day intervals. We then identified patients switching to AIs and patients receiving a prescription for tamoxifen in each of these intervals, which corresponds to the treatment decision point (29). Thus, cohort entry was determined by the date of a first AI prescription for switchers and the date of a tamoxifen prescription for patients continuing their treatment during a given interval. We then excluded patients diagnosed with metastatic disease at any time before cohort entry. This approach is illustrated in Web Figure 3.
Time-conditional propensity scores
We generated time-conditional propensity scores to estimate the predicted probability of switching to AIs versus continuing tamoxifen during each interval using conditional logistic regression (28). The propensity score model included the following variables, measured at cohort entry: age, body mass index (weight (kg)/height (m)2), Townsend Deprivation Index (30), ethnicity, cigarette smoking status, and alcohol-related disorders. The model also included the following comorbid conditions, all measured at any time before cohort entry: myocardial infarction, stroke or transient ischemic attack, heart failure, peripheral vascular disease, venous thromboembolism, chronic obstructive pulmonary disease, chronic kidney disease, and cancer (other than nonmelanoma skin cancer). The model considered non–breast cancer surgeries and the following prescription medications (all measured in the year before cohort entry): anticoagulants, antidepressants, antidiabetic drugs, antihypertensive drugs, bisphosphonates, nonsteroidal antiinflammatory drugs, opioids, acetylsalicylic acid (ASA; aspirin), non-ASA antiplatelet agents, statins, and hormone replacement therapy. Finally, breast-cancer-related variables included receipt of chemotherapy, radiation therapy, breast cancer surgery, and time between the first breast cancer diagnosis and cohort entry. Calendar time was not included in the model because it was strongly associated with the exposure and had a relatively weak association with the outcomes. This variable acted as an instrumental variable and was thus excluded from the propensity score model to minimize bias (31).
Starting with the first interval, each patient switching from tamoxifen to an AI was matched to 2 patients (to obtain the best balance of bias reduction and precision) who had received a tamoxifen prescription. Patients were matched within the same 30-day interval on duration of tamoxifen treatment and on propensity score using nearest-neighbor matching without replacement, with a caliper of 0.2 standard deviations of the logit of the propensity score (32). Tamoxifen users could contribute to the AI group, but only after the time of their switch.
Exposure ascertainment
We used an as-treated exposure definition whereby patients were followed while continuously exposed to AIs or tamoxifen. Patients were considered continuously exposed if a prescription plus a 30-day grace period overlapped with the date of the next prescription of a medication from the same class. Thus, patients were followed until reaching a study outcome (defined below, with separate follow-up for each outcome), treatment discontinuation (the end of a 30-day grace period or the date of a switch between prescriptions from different medication classes), noncardiovascular death, the end of registration with the general practice, or the end of the study period (February 29, 2016).
Cardiovascular outcomes
Separate analyses were conducted for each of the following cardiovascular outcomes: myocardial infarction, ischemic stroke, heart failure, and cardiovascular mortality (Web Table 1). Myocardial infarction, stroke, and heart failure were defined using ICD-9 and ICD-10 codes in the HES (primary or secondary diagnosis) or the ONS (underlying cause of death), and cardiovascular death was defined using ICD-9 and ICD-10 codes in the ONS. The HES has been shown to have a high (92%) positive predictive value for myocardial infarction (33), 96% specificity and negative predictive value for coronary heart disease, and perfect specificity and negative predictive value for stroke (34).
Statistical analysis
We calculated incidence rates and corresponding 95% confidence intervals based on the Poisson distribution for each exposure group. The Kaplan-Meier method was used to plot cumulative incidence curves for each exposure group. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals for each outcome, comparing use of AIs with tamoxifen. In a secondary analysis, we examined the association with the composite outcome of major adverse cardiovascular events (MACE), including nonfatal myocardial infarction, nonfatal stroke, and cardiovascular mortality. We also assessed the risk of cardiovascular outcomes by duration of use and flexibly modeled the hazard using restricted cubic splines with 3 interior knots at tertiles of follow-up time. We also assessed the hazard of MACE by previous duration of tamoxifen use.
Sensitivity analyses
We conducted 4 sensitivity analyses to assess the robustness of the analyses. First, we extended the grace period between consecutive prescriptions to 60 days. Second, we conducted an analysis using inverse probability of censoring weighting with separate weights for mortality, discontinuation, and switching between treatments as competing risks. Third, we lagged the exposures by 90 days to account for a potential minimum latency period. Fourth, we adjusted for calendar time in the outcome model to account for temporal trends in the management of breast cancer and cardiovascular disease during the study period. All analyses were conducted with SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina), and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study population
In total, there were 23,525 patients with nonmetastatic breast cancer, of whom 9,783 initiated treatment with tamoxifen (Web Figures 1 and 2). These patients generated 231,988 intervals during the study period (Web Figure 2). Overall, there were 2,145 intervals in which patients switched from tamoxifen to AIs and 150,673 intervals in which patients received repeat prescription of tamoxifen. A total of 1,962 patients who switched to AIs were propensity-score-matched to 3,874 patients continuing tamoxifen (Web Figure 2). Overall, a lower proportion of the study population received AIs than continued on tamoxifen between 1998 and 2002 (8.5% vs. 17.9%), whereas a higher proportion of patients received AIs after 2002 (Web Table 2). Approximately 5% of patients were censored due to the ending of the study period, 9% due to loss to follow-up, 19% due to a treatment switch, and 62% due to treatment discontinuation.
In the unmatched population, AI users were generally similar to tamoxifen users, with the exception of venous thromboembolism, non–breast cancer malignancies, use of vitamin K antagonists, and chemotherapy, which had higher prevalences in the former group (Web Table 3). The prevalence of breast cancer surgery was higher among tamoxifen users (Web Table 3). After propensity score matching, all characteristics were well balanced between groups (Table 1). Depending on the outcome, AI users generated 3,820–3,843 person-years of follow-up, whereas tamoxifen users generated 7,120–7,134 person-years of follow-up. The median duration of follow-up for AI and tamoxifen users was 1.5 years.
Table 1.
Baseline Characteristics of Breast Cancer Patients by Type of Treatment (Aromatase Inhibitor Switchers Versus Those Continuing Tamoxifen) After Matching on Propensity Scores, United Kingdom, 1998–2016
| Characteristic | Treatment Type | Standardized Difference | |||
|---|---|---|---|---|---|
| AIs (n = 1,962) | Tamoxifen (n = 3,874) | ||||
| No. | % | No. | % | ||
| Age, yearsa | 68.2 (10.7) | 67.7 (11.1) | 0.04 | ||
| Body mass indexb | |||||
| ≤24.9 | 732 | 37.3 | 1,486 | 38.4 | 0.02 |
| 25.0–29.9 | 633 | 32.3 | 1,287 | 33.2 | 0.02 |
| ≥30.0 | 440 | 22.4 | 796 | 20.5 | 0.05 |
| Unknown | 157 | 8.0 | 305 | 7.9 | 0.00 |
| Quintile of Townsend deprivation score | |||||
| 1 | 527 | 26.9 | 1,045 | 27.0 | 0.00 |
| 2 | 536 | 27.3 | 1,066 | 27.5 | 0.00 |
| 3 | 413 | 21.1 | 799 | 20.6 | 0.01 |
| 4 | 332 | 16.9 | 669 | 17.3 | 0.01 |
| 5 | 154 | 7.8 | 295 | 7.6 | 0.01 |
| Ethnicity | |||||
| Caucasian | 1,869 | 95.3 | 3,685 | 95.1 | 0.01 |
| Other | 38 | 1.9 | 88 | 2.3 | 0.02 |
| Unknown | 55 | 2.8 | 101 | 2.6 | 0.01 |
| Cigarette smoking status | |||||
| Current smoker | 284 | 14.5 | 553 | 14.3 | 0.01 |
| Past smoker | 476 | 24.3 | 940 | 24.3 | 0.00 |
| Never smoker | 1,132 | 57.7 | 2,256 | 58.2 | 0.01 |
| Unknown | 70 | 3.6 | 125 | 3.2 | 0.02 |
| Comorbid conditions | |||||
| Alcohol-related disorder | 119 | 6.1 | 217 | 5.6 | 0.02 |
| Myocardial infarction | 41 | 2.1 | 75 | 1.9 | 0.01 |
| Stroke or transient ischemic attack | 74 | 3.8 | 136 | 3.5 | 0.01 |
| Heart failure | 67 | 3.4 | 126 | 3.3 | 0.01 |
| Peripheral vascular disease | 43 | 2.2 | 85 | 2.2 | 0.00 |
| Venous thromboembolism | 166 | 8.5 | 314 | 8.1 | 0.01 |
| COPD | 91 | 4.6 | 162 | 4.2 | 0.02 |
| Chronic kidney disease | 123 | 6.3 | 212 | 5.5 | 0.03 |
| Other cancer | 467 | 23.8 | 954 | 24.6 | 0.02 |
| Non–breast cancer surgery | 480 | 24.5 | 910 | 23.5 | 0.02 |
| Medication use | |||||
| Anticoagulants | |||||
| Vitamin K antagonists | 81 | 4.1 | 141 | 3.6 | 0.03 |
| Direct oral anticoagulants | —c | — | — | — | 0.00 |
| Heparin | 17 | 0.9 | 28 | 0.7 | 0.02 |
| Antidepressants | |||||
| SSRIs | 208 | 10.6 | 371 | 9.6 | 0.03 |
| SNRIs | 48 | 2.4 | 81 | 2.1 | 0.02 |
| Tricyclic antidepressants | 217 | 11.1 | 425 | 11.0 | 0.00 |
| Other | 22 | 1.1 | 34 | 0.9 | 0.02 |
| Antidiabetic drugs | |||||
| Metformin | 90 | 4.6 | 169 | 4.4 | 0.01 |
| Sulfonylureas | 52 | 2.7 | 108 | 2.8 | 0.01 |
| Thiazolidinediones | 12 | 0.6 | 25 | 0.6 | 0.00 |
| Incretin-based drugs | — | — | — | — | 0.01 |
| Insulin | 25 | 1.3 | 37 | 1.0 | 0.03 |
| Other | — | — | — | — | 0.00 |
| Antihypertensive drugs | |||||
| Diuretics | 556 | 28.3 | 1,022 | 26.4 | 0.04 |
| β-blockers | 380 | 19.4 | 682 | 17.6 | 0.05 |
| Calcium channel blockers | 305 | 15.5 | 578 | 14.9 | 0.02 |
| ACE inhibitors | 308 | 15.7 | 575 | 14.8 | 0.02 |
| Angiotensin II receptor blockers | 138 | 7.0 | 250 | 6.5 | 0.02 |
| Other | 109 | 5.6 | 199 | 5.1 | 0.02 |
| Other drugs | |||||
| Bisphosphonates | 93 | 4.7 | 176 | 4.5 | 0.01 |
| NSAIDs | 338 | 17.2 | 667 | 17.2 | 0.00 |
| Opioids | 541 | 27.6 | 1,030 | 26.6 | 0.02 |
| ASA | 315 | 16.1 | 581 | 15.0 | 0.03 |
| Non-ASA antiplatelet agents | 30 | 1.5 | 72 | 1.9 | 0.03 |
| Statins | 350 | 17.8 | 669 | 17.3 | 0.02 |
| Hormone replacement therapy | 119 | 6.1 | 235 | 6.1 | 0.00 |
| Breast cancer-related variables | |||||
| Chemotherapy | 281 | 14.3 | 537 | 13.9 | 0.01 |
| Radiation therapy | 379 | 19.3 | 760 | 19.6 | 0.01 |
| Breast cancer surgery | 1,639 | 83.5 | 3,282 | 84.7 | 0.03 |
| Time since diagnosis, monthsa | 19.5 (13.5) | 19.2 (13.6) | 0.02 | ||
| Duration of previous tamoxifen use, monthsa | 16.4 (13.0) | 16.1 (12.8) | 0.02 | ||
Abbreviations: ACE, angiotensin-converting enzyme; AI, aromatase inhibitor; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; NSAID, nonsteroidal antiinflammatory drug; SNRI, serotonin and noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
a Values are expressed as mean (standard deviation).
b Weight (kg)/height (m2).
c Cells with fewer than 5 observations are masked, as per the confidentiality policies of the Clinical Practice Research Datalink.
Primary analysis
Switching to AIs was associated with a doubling of the risk of myocardial infarction compared with continuing tamoxifen (incidence rate (IR) = 4.7 per 1,000 person-years (95% confidence interval (CI): 2.8, 7.5) and IR = 2.0 per 1,000 person-years (95% CI: 1.1, 3.3), respectively; hazard ratio (HR) = 2.08, 95% CI: 1.02, 4.27) (Table 2). With respect to ischemic stroke, the use of AIs was associated with an elevated hazard ratio, with a confidence interval that included the null value, in comparison with continuing tamoxifen (IR = 5.0 per 1,000 person-years (95% CI: 3.0, 7.7) and IR = 3.1 per 1,000 person-years (95% CI: 1.9, 4.7), respectively; HR = 1.58, 95% CI: 0.85, 2.93) (Table 2). Overall, the use of AIs generated a higher incidence rate for heart failure than continuation of tamoxifen (IR = 3.4 per 1,000 person-years (95% CI: 1.8, 5.8) and IR = 2.0 per 1,000 person-years (95% CI: 1.1, 3.3), respectively). This generated an elevated hazard ratio, but with a confidence interval that included the null value (HR = 1.69, 95% CI: 0.79, 3.62) when AI use was compared with continuing tamoxifen (Table 2). Finally, the use of AIs was not associated with increased risk of cardiovascular mortality in comparison with continuous tamoxifen use (IR = 4.9 per 1,000 person-years (95% CI: 3.0, 7.7) and IR = 5.0 per 1,000 person-years (95% CI: 3.5, 7.0), respectively; HR = 0.87, 95% CI: 0.49, 1.54) (Table 2). The cumulative incidence curves for myocardial infarction (Figure 1A) and ischemic stroke (Figure 1B) diverged starting 3 years after switching to AIs, while the curves diverged after 2 years for heart failure (Figure 1C), albeit with fewer patients remaining at risk with long-term use (Web Table 4). For cardiovascular mortality, cumulative incidence curves overlapped during the follow-up period (Figure 1D).
Table 2.
Risk of Adverse Cardiovascular Outcomes Among Breast Cancer Patients When Aromatase Inhibitor Switchers Are Compared With Those Continuing Tamoxifen, United Kingdom, 1998–2016
|
Outcome and Treatment Type |
No. of Events |
Person-Years of Follow-up |
Incidence | Risk Estimate | ||
|---|---|---|---|---|---|---|
| IR a | 95% CI | HR b | 95% CI | |||
| Myocardial infarction | ||||||
| Tamoxifen | 14 | 7,126 | 2.0 | 1.1, 3.3 | 1.00 | Referent |
| AIs | 18 | 3,820 | 4.7 | 2.8, 7.5 | 2.08 | 1.02, 4.27 |
| Ischemic stroke | ||||||
| Tamoxifen | 22 | 7,120 | 3.1 | 1.9, 4.7 | 1.00 | Referent |
| AIs | 19 | 3,831 | 5.0 | 3.0, 7.7 | 1.58 | 0.85, 2.93 |
| Heart failure | ||||||
| Tamoxifen | 14 | 7,128 | 2.0 | 1.1, 3.3 | 1.00 | Referent |
| AIs | 13 | 3,835 | 3.4 | 1.8, 5.8 | 1.69 | 0.79, 3.62 |
| Cardiovascular mortality | ||||||
| Tamoxifen | 36 | 7,134 | 5.0 | 3.5, 7.0 | 1.00 | Referent |
| AIs | 19 | 3,843 | 4.9 | 3.0, 7.7 | 0.87 | 0.49, 1.54 |
Abbreviations: AI, aromatase inhibitor; CI, confidence interval; HR, hazard ratio; IR, incidence rate.
a Number of cases per 1,000 person-years.
b HR obtained from the matched population.
Figure 1.
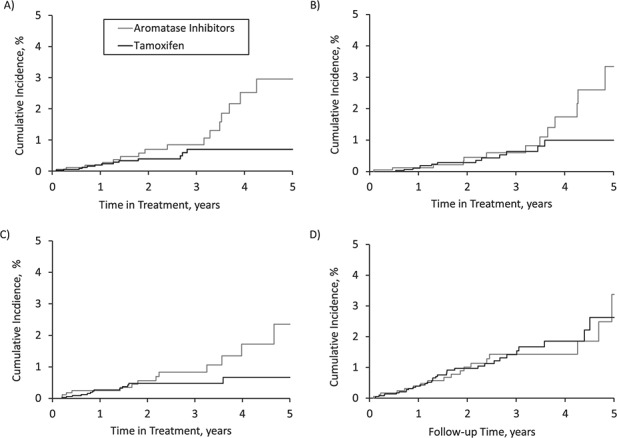
Cumulative incidence of myocardial infarction (A), ischemic stroke (B), heart failure (C), and cardiovascular mortality (D) among breast cancer patients when aromatase inhibitor switchers are compared with those continuing tamoxifen, United Kingdom, 1998–2016. Numbers of patients at risk by time since cohort entry are shown in Web Table 4.
Secondary analyses
The hazard ratio for MACE was elevated though nonsignificant with switching to AIs, as compared with continuing tamoxifen (Web Table 5; HR = 1.47, 95% CI: 0.98, 2.18). There were no systematic differences in the hazard ratio by duration of previous tamoxifen use when comparing AIs with continuing tamoxifen (Web Table 6), albeit the event rate was low in some strata. When modeling the hazard ratio with restricted cubic splines (Web Figure 4), the hazards for myocardial infarction and heart failure increased with duration of use, whereas for ischemic stroke there was an initially elevated hazard ratio that declined over time. For cardiovascular mortality, the hazard ratio remained around the null value. The hazard ratio remained elevated for MACE by time in tamoxifen treatment or duration of previous tamoxifen use (Web Figure 5).
Sensitivity analyses
Sensitivity analysis using inverse probability of censoring weighting led to results that were consistent with those of the primary analyses (Web Table 7). In contrast, extending the grace period for each prescription to 60 days (Web Table 8) led to dilution of the association towards the null value. Imposing a 90-day exposure lag period led to effect estimates that were consistent with those of the primary analysis, albeit with slightly wider confidence intervals due to a lower number of events (Web Table 9). Similarly, adjusting for calendar time in the outcome model led to results that were consistent with the primary analysis (Web Table 10).
DISCUSSION
In this population-based cohort study, treatment with AIs in the sequential setting with tamoxifen, when compared with continuing tamoxifen, was associated with a doubling of risk of myocardial infarction. The hazard ratios were also elevated, albeit nonsignificantly, for ischemic stroke and heart failure, while no association with cardiovascular mortality was seen. These results remained consistent in secondary and sensitivity analyses.
The results of this study are consistent with previous meta-analyses of RCTs, where AIs were associated with an increased the risk of ischemic events as compared with tamoxifen in the upfront setting (7, 35, 36). They also corroborate the signal for severe heart failure observed in the Breast International Group (BIG) 1-98 Trial (letrozole: 0.65%; tamoxifen: 0.33%) (13). To date, however, few RCTs have assessed the cardiovascular safety of AIs in the sequential setting with tamoxifen. In a meta-analysis of RCTs comparing AIs in sequential treatment with tamoxifen, versus upfront tamoxifen treatment, AIs were associated with a 16% increased risk of cardiovascular outcomes (relative risk = 1.16, 95% CI: 1.03, 1.32), with an elevated relative risk of ischemic cardiovascular outcomes (relative risk = 1.21, 95% CI: 0.93, 1.57) (7). In the BIG 1-98 Trial, there were imbalances in cardiovascular outcomes and ischemic heart disease in the sequential AI arm versus upfront tamoxifen (7.0% vs. 5.7% and 2.3% vs. 1.5%) after 71 months and 76 months, respectively (37). Similarly, in RCTs which randomized patients to receive AIs or continued tamoxifen after 2–3 years of tamoxifen treatment, there was a 20% increased risk of cardiovascular outcomes associated with AIs (relative risk = 1.20, 95% CI: 1.02, 1.41) (7). However, this association was not observed in RCTs that compared AIs with placebo or no treatment after 5 years of tamoxifen treatment (7). Overall, these RCTs were designed to assess efficacy and not cardiovascular safety and used a heterogeneous composite outcome definition (7, 8).
To date, 4 observational studies have compared the risk of cardiovascular outcomes between AIs and tamoxifen (9, 38–40). In one study, the use of AIs was associated with a doubling in the risk of myocardial infarction (9), while other studies did not find an association with ischemic cardiovascular outcomes (38–40). However, none of these studies specifically examined the association between AIs and cardiovascular outcomes in the sequential setting with tamoxifen. Overall, patients treated up front with AIs had more comorbidity and history of cardiovascular disease than patients treated up front with tamoxifen. However, in the present study, patients who switched to AIs were similar to patients on continuous tamoxifen treatment.
There is some evidence that an increased risk of cardiovascular events with AIs may be due to their effects on lipid levels. Indeed, in RCTs comparing AIs with tamoxifen, the use of anastrozole and letrozole was associated with an increased risk of hypercholesterolemia (41–43). However, it remains unclear whether this increased risk is due to the lipid-lowering effects of tamoxifen or to unfavorable effects of AIs. In RCTs, tamoxifen has been shown to decrease low-density lipoprotein cholesterol and total cholesterol levels between 25 mg/dL and 39 mg/dL within 3 months–1 year of initiation of tamoxifen treatment, with effects persisting for up to 5 years when on tamoxifen treatment (15, 16, 44, 45). These results are consistent with a meta-analysis of RCTs that showed that tamoxifen decreased the risks of ischemic heart disease by 34%, nonfatal myocardial infarction by 26%, and fatal myocardial infarction by 45% (7, 46). Evidence from one trial suggests that there may be a rebound effect where lipid levels return to baseline levels after discontinuation of treatment for 5 years with tamoxifen (47).
This study had several strengths. First, to our knowledge, it was the first study to specifically examine the association between AIs and cardiovascular outcomes in the sequential setting. Second, the cardiovascular outcomes in this study were defined using data from the HES and the ONS, which have been shown to have high specificity (33, 34). Third, we applied a rigorous study design whereby patients who switched to AIs were matched with patients using tamoxifen on duration of previous tamoxifen use and time-conditional propensity scores. Finally, we observed consistent results in secondary and sensitivity analyses.
This study also had some limitations. Because the CPRD records medication prescriptions issued by general practitioners, exposure misclassification is possible. However, 76% of patients in the study population initiated treatment with either tamoxifen or AIs, which is concordant with the prevalence of hormone-receptor-positive breast cancer (48–50). In addition, general practitioners in the United Kingdom are involved in routine management and treatment of patients with breast cancer (51, 52). However, some patients’ nonadherence to treatment could have led to nondifferential exposure misclassification and underestimation of the effect estimates. Second, given the observational nature of this study, residual confounding is possible. Reassuringly, the exposure groups were already similar in the unmatched population, indicating that the reason for switching was not motivated by comorbidity. In addition, we achieved near-perfect balance when matching the exposure groups on time-conditional propensity score. Finally, some secondary analyses had low statistical power because of fewer exposure events, and it was not possible to assess the risk of cardiovascular outcomes by specific AIs.
In conclusion, in this population-based study, AIs in the sequential setting were associated with a doubling of the risk of myocardial infarction, in comparison with continuous tamoxifen, among women with breast cancer. The hazard ratio was also elevated, though nonsignificantly, for ischemic stroke and heart failure, while no association with cardiovascular mortality was seen. Overall, additional large observational studies are needed to corroborate these findings in the sequential setting among patients with breast cancer.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Centre for Clinical Epidemiology, Lady Davis Institute, Jewish General Hospital, Montreal, Quebec, Canada (Farzin Khosrow-Khavar, Kristian B. Filion, Samy Suissa, Laurent Azoulay); Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Farzin Khosrow-Khavar, Kristian B. Filion, Samy Suissa, Laurent Azoulay); Gerald Bronfman Department of Oncology, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Farzin Khosrow-Khavar, Nathaniel Bouganim, Laurent Azoulay); Department of Oncology, Cedar Cancer Center, McGill University Health Center, Montreal, Quebec, Canada (Nathaniel Bouganim); and Division of Clinical Epidemiology, Department of Medicine, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Kristian B. Filion, Samy Suissa).
This study was supported by a Foundation Scheme grant from the Canadian Institutes of Health Research (grant FDN-143328). F.K.-K. is the recipient of a doctoral award from the Fonds de recherche du Québec–Santé (FRQS). K.B.F. has received a Chercheur-Boursier Junior 2 award from the FRQS and is the recipient of a William Dawson Scholar award from McGill University. S.S. holds the James McGill Chair. L.A. is the recipient of a Senior Chercheur-Boursier career award from the FRQS and a William Dawson Scholar award from McGill University.
N.B. has served as a consultant for Amgen, Inc. (Thousand Oaks, California), Novartis International AG (Basel, Switzerland), and Roche, Inc. (Basel, Switzerland). S.S. has received research funding, participated in advisory board meetings, or served as a speaker for AstraZeneca LP (London, United Kingdom), Boehringer Ingelheim GmbH (Ingelheim am Rhein, Germany), Novartis International AG (Basel, Switzerland), Pfizer, Inc. (New York, New York), and Merck & Co., Inc. (Kenilworth, New Jersey). L.A. has received consulting fees from Janssen Pharmaceuticals, Inc. (Titusville, New Jersey) for work unrelated to this study. None of the other authors have any potential conflicts to disclose.
REFERENCES
- 1. Kelly E, Lu CY, Albertini S, et al. Longitudinal trends in utilization of endocrine therapies for breast cancer: an international comparison. J Clin Pharm Ther. 2015;40(1):76–82. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 3. Bosco-Levy P, Jove J, Robinson P, et al. Persistence to 5-year hormonal breast cancer therapy: a French national population-based study. Br J Cancer. 2016;115(8):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hadji P, Ziller V, Kyvernitakis J, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat. 2013;138(1):185–191. [DOI] [PubMed] [Google Scholar]
- 5. Huiart L, Dell'Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer. 2011;104(10):1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Velde CJ, Verma S, Nes JG, et al. Switching from tamoxifen to aromatase inhibitors for adjuvant endocrine therapy in postmenopausal patients with early breast cancer. Cancer Treat Rev. 2010;36(1):54–62. [DOI] [PubMed] [Google Scholar]
- 7. Khosrow-Khavar F, Filion KB, Al-Qurashi S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in post-menopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews A, Stanway S, Farmer RE, et al. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363(8172):k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdel-Qadir H, Amir E, Fischer HD, et al. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer. 2016;68:11–21. [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration, US Department of Health and Human Services Arimidex® (Anastrozole). (Prescription package insert). Silver Spring, MD: Food and Drug Administration; 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf. Accessed May 15, 2019. [Google Scholar]
- 11. Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93(suppl 1):S23–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC Trial. Lancet Oncol. 2006;7(8):633–643. [DOI] [PubMed] [Google Scholar]
- 13. Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 Trial. J Clin Oncol. 2007;25(36):5715–5722. [DOI] [PubMed] [Google Scholar]
- 14. Hozumi Y, Suemasu K, Takei H, et al. The effect of exemestane, anastrozole, and tamoxifen on lipid profiles in Japanese postmenopausal early breast cancer patients: final results of National Surgical Adjuvant Study BC 04, the TEAM Japan Sub-Study. Ann Oncol. 2011;22(8):1777–1782. [DOI] [PubMed] [Google Scholar]
- 15. Younus M, Kissner M, Reich L, et al. Putting the cardiovascular safety of aromatase inhibitors in patients with early breast cancer into perspective: a systematic review of the literature. Drug Saf. 2011;34(12):1125–1149. [DOI] [PubMed] [Google Scholar]
- 16. Dewar JA, Horobin JM, Preece PE, et al. Long term effects of tamoxifen on blood lipid values in breast cancer. BMJ. 1992;305(6847):225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grainger DJ, Schofield PM. Tamoxifen for the prevention of myocardial infarction in humans: preclinical and early clinical evidence. Circulation. 2005;112(19):3018–3024. [DOI] [PubMed] [Google Scholar]
- 18. Guetta V, Lush RM, Figg WD, et al. Effects of the antiestrogen tamoxifen on low-density lipoprotein concentrations and oxidation in postmenopausal women. Am J Cardiol. 1995;76(14):1072–1073. [DOI] [PubMed] [Google Scholar]
- 19. Love RR, Wiebe DA, Feyzi JM, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst. 1994;86(20):1534–1539. [DOI] [PubMed] [Google Scholar]
- 20. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrett EL, Thomas SL, Smeeth L. Validity of diagnoses in the General Practice Research Database. Br J Gen Pract. 2011;61(588):438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arhi CS, Bottle A, Burns EM, et al. Comparison of cancer diagnosis recording between the Clinical Practice Research Datalink, Cancer Registry and Hospital Episodes Statistics. Cancer Epidemiol. 2018;57:148–157. [DOI] [PubMed] [Google Scholar]
- 24. Boggon R, Staa TP, Chapman M, et al. Cancer recording and mortality in the General Practice Research Database and linked cancer registries. Pharmacoepidemiol Drug Saf. 2013;22(2):168–175. [DOI] [PubMed] [Google Scholar]
- 25. Margulis AV, Fortuny J, Kaye JA, et al. Validation of cancer cases using primary care, cancer registry, and hospitalization data in the United Kingdom. Epidemiology. 2018;29(2):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. NHS Digital Hospital Episode Statistics (HES). About the HES database. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed May 1, 2019.
- 27. United Kingdom Office for National Statistics Statistics we produce. https://www.ons.gov.uk/aboutus/whatwedo/statistics/statisticsweproduce. Accessed May 1, 2019.
- 28. Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459–468. [DOI] [PubMed] [Google Scholar]
- 29. Brookhart MA. Counterpoint: the treatment decision design. Am J Epidemiol. 2015;182(10):840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Centre for Research Methods Geographical Referencing Learning Resources. Townsend Deprivation Index. http://www.restore.ac.uk/geo-refer/36229dtuks00y19810000.php. Accessed May 15, 2019.
- 31. Wyss R, Girman CJ, LoCasale RJ, et al. Variable selection for propensity score models when estimating treatment effects on multiple outcomes: a simulation study. Pharmacoepidemiol Drug Saf. 2013;22(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346(7909):f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kivimaki M, Batty GD, Singh-Manoux A, et al. Validity of cardiovascular disease event ascertainment using linkage to UK hospital records. Epidemiology. 2017;28(5):735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. [DOI] [PubMed] [Google Scholar]
- 36. Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260–267. [DOI] [PubMed] [Google Scholar]
- 37. BIG 1-98 Collaborative Group Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361(8):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haque R, Shi J, Schottinger JE, et al. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2(12):1590–1597. [DOI] [PubMed] [Google Scholar]
- 39. Kamaraju S, Shi Y, Smith E, et al. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin Cardiol. 2019;42(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ligibel JA, James OMA, Fisher M, et al. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat. 2012;131(2):589–597. [DOI] [PubMed] [Google Scholar]
- 41. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) Trial efficacy and safety update analyses. Cancer. 2003;98(9):1802–1810. [DOI] [PubMed] [Google Scholar]
- 42. Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. [DOI] [PubMed] [Google Scholar]
- 43. Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian Tamoxifen Anastrozole (ITA) Trial. Ann Oncol. 2006;17(suppl 7):vii10–vii14. [DOI] [PubMed] [Google Scholar]
- 44. Love RR, Newcomb PA, Wiebe DA, et al. Effects of tamoxifen therapy on lipid and lipoprotein levels in postmenopausal patients with node-negative breast cancer. J Natl Cancer Inst. 1990;82(16):1327–1332. [DOI] [PubMed] [Google Scholar]
- 45. Sawada S, Sato K, Kusuhara M, et al. Effect of anastrozole and tamoxifen on lipid metabolism in Japanese postmenopausal women with early breast cancer. Acta Oncol. 2005;44(2):134–141. [DOI] [PubMed] [Google Scholar]
- 46. Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18(11):937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Markopoulos C, Chrissochou M, Antonopoulou Z, et al. Duration of tamoxifen effect on lipidemic profile of postmenopausal breast cancer patients following deprivation of treatment. Oncology. 2006;70(4):301–305. [DOI] [PubMed] [Google Scholar]
- 48. Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lumachi F, Santeufemia DA, Basso SM. Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem. 2015;6(3):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khosrow-Khavar F, Yin H, Barkun A, et al. Aromatase inhibitors and the risk of colorectal cancer in postmenopausal women with breast cancer. Ann Oncol. 2018;29(3):744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. National Institute for Clinical Excellence Improving Outcomes in Breast Cancer. London, United Kingdom: National Institute for Clinical Excellence; 2002. www.nice.org.uk/guidance/csg1/resources/improving-outcomes-in-breast-cancer-update-pdf-773371117. Accessed May 5, 2019. [Google Scholar]
- 52. National Institute for Health and Care Excellence Early and Locally Advanced Breast Cancer: Diagnosis and Management. (NICE guideline NG101). London, United Kingdom: National Institute for Health and Care Excellence; 2018. https://www.nice.org.uk/guidance/ng101/chapter/Recommendations#endocrine-therapy. Accessed May 15, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


