Abstract
We examined associations of individual- and neighborhood-level life-course (LC) socioeconomic status (SES) with incident dementia in the Atherosclerosis Risk in Communities cohort. Individual- and neighborhood-level SES were assessed at 3 life epochs (childhood, young adulthood, midlife) via questionnaire (2001–2002) and summarized into LC-SES scores. Dementia was ascertained through 2013 using cognitive exams, telephone interviews, and hospital and death certificate codes. Cox regression was used to estimate hazard ratios of dementia by LC-SES scores in race-specific models. The analyses included data from 12,599 participants (25% Black) in the United States, with a mean age of 54 years and median follow-up of 24 years. Each standard-deviation greater individual LC-SES score was associated with a 14% (hazard ratio (HR) = 0.86, 95% confidence interval (CI): 0.81, 0.92) lower risk of dementia in White and 21% (HR = 0.79, 95% CI: 0.71, 0.87) lower risk in Black participants. Education was removed from the individual LC-SES score and adjusted for separately to assess economic factors of LC-SES. A standard-deviation greater individual LC-SES score, without education, was associated with a 10% (HR = 0.90, 95% CI: 0.84, 0.97) lower dementia risk in White and 15% (HR = 0.85, 95% CI: 0.76, 0.96) lower risk in Black participants. Neighborhood LC-SES was not associated with dementia. We found that individual LC-SES is a risk factor for dementia, whereas neighborhood LC-SES was not associated.
Keywords: dementia, disparities, life course, socioeconomic status
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- APOE
apolipoprotein E
- CI
confidence interval
- HR
hazard ratio
- LC
life-course
- SES
socioeconomic status
Chronic diseases in older adults are caused by a complex accumulation and interaction of lifetime exposures, particularly socioeconomic status (SES) (1). SES reflects the “social and economic factors that influence which positions individuals or groups will hold within the structure of a society.” (2) SES collected across the life course can be used to quantify the accumulation of risk factors over the progression of life epochs (3, 4). Life epochs generally include childhood, young adulthood, midlife, and older adulthood, and they can be measured at the individual and neighborhood levels. Life-course (LC) SES models hypothesize that life epochs do not occur independently of one another, but events occurring during these periods can accumulate and interact leading to increased risk of chronic disease over a lifetime (3, 4).
SES is an especially crucial component in the development of dementia due to the importance of cognitive reserve (5). The concept of cognitive reserve reflects the observation that cognitive function does not always correspond to observable brain pathology (5). While there is no standard measure of cognitive reserve, measures of SES, particularly education, are widely used proxies, because they signify beneficial environmental exposures (5). However, it is unclear whether benefits of economic success (such as high income and wealth) are associated with reduced risk of dementia independent of educational attainment. Further, Alzheimer’s disease and related dementias have biological and behavioral risk factors whose associations might be confounded or modified by SES (6). For instance, confounding of associations between midlife vascular risk factors and incident dementia by SES might not be eliminated by adjustment for mid- or late life SES alone, necessitating the use of LC-SES measures (7–9). We are interested in characterizing the association between LC-SES and dementia, assessing separately the potential associations of economic versus educational dimensions of SES.
A number of studies have found a significant inverse association between individual-level SES and cognitive decline and dementia (10–24). However, the methods used to measure SES have varied widely, and many relied on SES measured only during mid- or late life. A life-course approach to understanding dementia is important in order to better classify risk factors that might have a cumulative impact on disease risk but are (partially or fully) masked by examining only one life epoch (4, 7). Among studies that have assessed LC-SES and cognitive function, very few have measured neighborhood-level SES. Neighborhood SES adds context to individual SES and might independently influence dementia risk through physical and social characteristics of neighborhoods that contribute to disparities and influence individual behaviors and stress levels (25).
Using the Atherosclerosis Risk in Communities (ARIC) Study cohort, we hypothesized that higher cumulative LC-SES was inversely associated with risk of incident dementia, with both higher individual- and neighborhood-level LC-SES independently contributing to lower dementia risk. We also hypothesized individual-level economic measures of LC-SES would be associated with lower risk of dementia independent of individual educational attainment.
METHODS
ARIC is a prospective cohort study that enrolled 15,792 mostly White and Black participants who were aged 45–64 years at visit 1 (1987–1989) from Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. After institutional review board approval and informed consent, participants completed 6 clinic visits during 1987–2017 as well as cognitive assessments integrated into these clinic visits as part of the ARIC Neurocognitive Study. For our analysis, incident dementia was ascertained from visit 1 (1987–1989) through visit 5 (2011–2013). Participants were excluded if they were not White or Black (or were Black participants from Maryland or Minnesota (n = 93), due to small numbers), they did not participate in the LC-SES ancillary study (2001–2002) (n = 2,626), they developed dementia before the LC-SES questionnaire was administered (n = 141), or they were missing visit-1 (1987–1989) covariates (n = 323). After exclusions, 12,599 participants were included in the analytical sample.
Individual- and neighborhood-level LC-SES data were obtained retrospectively using telephone questionnaires administered in 2001–2002. Questions evaluated SES factors including education, occupation, occupational role, home ownership, family income, and family wealth over 3 life epochs: childhood (approximately age 10 years—SES pertained to parental SES), young adulthood (approximately age 30 years), and middle/older adulthood (aged 45–64 years when participants entered the ARIC study) (Table 1). Individual-level LC-SES scores were created by summarizing SES variables related to the 3 epochs following an approach developed by Carson et al. (26). For individual LC-SES, response variables related to each epoch had a range of possible values between 0 (lowest SES) and 5 (highest SES) (26). These epoch scores were summed for an individual LC-SES score ranging between 0 and 15 (26). We also assessed individual LC-SES without education by removing individual-level education from the score (keeping parental education in the score) for a possible LC-SES score without education ranging from 0 to 13. We then adjusted for individual education separately in the models to determine whether economic factors of SES were independently associated with dementia.
Table 1.
Individual and Neighborhood Life-Course Socioeconomic Factors and Scoringa, Atherosclerosis Risk in Communities Study, United States, 1987–1989
| Individual LC-SES | Neighborhood LC-SES | ||
|---|---|---|---|
| Variable | Score | Variable | z Score b |
| Childhood (Age 10 Years) | |||
| Parental education | <8th grade = 0 8th grade = 1 >8th grade = 2 |
Adult education | Proportion with high-school or college degree |
| Parental occupation | Manual = 0 Nonmanual = 1 |
Adult occupational role | Proportion with managerial roles |
| Parental occupational role | Nonmanagerial = 0 Managerial = 1 |
Dwellings occupied by owner | Proportion of homes occupied by owner |
| Parental home ownership | Rent or other = 0 Own home = 1 |
Log median home value | Median value of homes |
| Young Adulthood (Age 30 Years) | |||
| Education | Less than high school = 0 High school = 1 Beyond high school = 2 |
Adult education | Proportion with high-school or college degree |
| Occupation | Manual = 0 Nonmanual = 1 |
Adult occupational role | Proportion with managerial roles |
| Occupational role | Nonmanagerial = 0 Managerial = 1 |
Log median income | Median family income |
| Home ownership | Rent or other = 0 Own home = 1 |
Dwellings occupied by owner Log median home value |
Proportion of homes occupied by owner Median value of homes |
| Middle/Older Adulthood (Ages 45–64 Years) | |||
| Income, $ | <25,000 = 0 25,000–34,999 = 1 >35,000 = 2 |
Adult education | Proportion with high-school or college degree |
| Occupation | Manual = 0 Non-manual = 1 |
Adult occupational role | Proportion with managerial roles |
| Occupational role | Non-managerial = 0 Managerial = 1 |
Log median income | Median family income |
| Home ownership | Rent or other = 0 Own home = 1 |
Dwellings occupied by owner Median home value Households with passive income |
Proportion of homes occupied by owner Median value of homes Proportion with income besides wages/salary |
Abbreviation: LC-SES, life-course socioeconomic status.
a Adapted from Carson et al. (26).
b Values for z scores derived from census-tract data representing the location a participant reported living during each epoch.
Participants were questioned about previous addresses, and responses were processed with corrections for spelling errors and confirmation of city and county names. Fewer than 15% of childhood address were flagged for errors. Of the flagged addresses, over 70% were corrected for misspellings or incorrect county names. Data from subsequent epochs had fewer errors flagged. Addresses were then geocoded using a commercial geocoder and assigned census tracts. Neighborhood-level LC-SES variables were identified in a factor analysis from available census data over the 3 life epochs representing several decades (26); z scores were calculated by subtracting individual neighborhood SES variable values (derived from census tract data at each epoch) from the group mean and dividing by the standard deviation. Because of the potential impact of segregation on SES and different racial distributions across the 4 ARIC field centers, race-specific z scores were obtained for each census variable and summed to develop a summary z score for neighborhood LC-SES where a higher z score indicated higher SES (26). We created race-specific, distribution-based tertiles of the neighborhood-level LC-SES score for analysis.
Covariate information was collected at visit 1 (1987–1989) and included age, sex, apolipoprotein E (APOE)  4 allele status, body mass index, tobacco-smoking status, hypertension, diabetes, alcohol-drinking status, high-density lipoprotein cholesterol, and total cholesterol. Body mass index was calculated as measured weight (kg) divided by height (m) squared. Hypertension was defined as having a systolic blood pressure of >140 mm Hg, diastolic blood pressure of >90 mm Hg, or self-report of antihypertensive medication use. Diabetes was defined as nonfasting serum glucose of ≥200 mg/dL, fasting glucose of ≥126 mg/dL, self-report of diabetes diagnosis from a physician, or report of taking medication for diabetes or high blood sugar.
4 allele status, body mass index, tobacco-smoking status, hypertension, diabetes, alcohol-drinking status, high-density lipoprotein cholesterol, and total cholesterol. Body mass index was calculated as measured weight (kg) divided by height (m) squared. Hypertension was defined as having a systolic blood pressure of >140 mm Hg, diastolic blood pressure of >90 mm Hg, or self-report of antihypertensive medication use. Diabetes was defined as nonfasting serum glucose of ≥200 mg/dL, fasting glucose of ≥126 mg/dL, self-report of diabetes diagnosis from a physician, or report of taking medication for diabetes or high blood sugar.
Dementia cases were identified from clinic examinations conducted at visit 5 (2011–2013), surveillance of hospitalization and death certificate codes, and cognitive screening during follow-up calls (27). A neuropsychological battery was administered at visits 2 (1990–1992), 4 (1996–1998), and 5 (2011–2013). Cognitive tests were administered using standardized protocols, and scores were converted to z scores in order to assess change over time. We identified cognitively impaired participants as those with significant cognitive decline from visits 2–5, failure in at least 1 cognitive domain, or with a Mini-Mental State Examination score of <21 for Whites and <19 for Blacks (27). These participants, as well as a random sample of unimpaired participants, were given additional physical and neurological exams, including brain magnetic resonance imaging, and their informants were interviewed using the Clinical Dementia Rating Scale and the Functional Activities Questionnaire (27). Information on suspected cases was reviewed by a committee of clinicians, and participants were classified as cognitively normal, having adjudicated mild cognitive impairment, or having adjudicated dementia (27). For participants who did not attend visit 5, additional dementia cases from visits 1–5 were identified via surveillance of hospital discharge International Classification of Diseases codes and death certificate codes related to dementia. In addition, starting in 2011, screening was conducted with telephone-based cognitive assessments during annual and semiannual follow-up calls as well as informant interviews for deceased participants suspected to have had dementia.
Individual- and neighborhood-level LC-SES measures were ascertained retrospectively causing several variables to have missing data. The amount of missing data for individual LC-SES variables was approximately 14%. For neighborhood LC-SES, the amount of missing data was approximately 25% and due primarily to changes in census questions over several decades. To address this issue, we used multiple imputation by chained equations (28) to impute individual- and neighborhood-level LC-SES scores. Using a fully conditional method algorithm and imputation models with 4 variables pertinent to each life epoch (sex, race, age, and APOE  4 allele status), we created 10 sets of imputations in SAS, version 9.4 (SAS Inc., Cary, North Carolina) (28).
4 allele status), we created 10 sets of imputations in SAS, version 9.4 (SAS Inc., Cary, North Carolina) (28).
We described prevalences and means of baseline covariates and individual LC-SES. Incidence rates of dementia between visits 1 (1987–89) and 5 (2011–13) stratified by life epoch (childhood, young adulthood, and middle/older adulthood) and race-specific, distribution-based individual and neighborhood LC-SES tertiles were estimated using Poisson regression. Cox regression was used to assess the association between LC-SES and risk of dementia. We modeled LC-SES in several ways: 1) individual-level LC-SES score (ranging from 0–15), 2) individual-level LC-SES score after removing education and adjusting for education separately in the model (ranging from 0–13), 3) neighborhood LC-SES score with adjustment for individual LC-SES score separately in the model, 4) neighborhood LC-SES alone, and 5) a neighborhood × individual LC-SES interaction term. To account for clustering of individual-level SES within neighborhood, we also performed a Cox regression with a random effect for neighborhood-level LC-SES. This design consequence was of small magnitude and did not visibly affect individual LC-SES estimates, so it is not presented.
Two models were tested for each of the Cox analyses. Model 1 adjusted for age, sex, and APOE  4 allele status. Model 2 adjusted for model 1 covariates plus visit-1 body mass index, hypertension, diabetes, high-density lipoprotein cholesterol, total cholesterol, alcohol-drinking status, and tobacco-smoking status. We used a restricted cubic spline model to investigate the continuous nonlinear relationship between individual-level LC-SES and hazard of dementia with knots specified at the 5th, 50th, and 95th percentiles. To test the proportional hazards assumption, we included an interaction term between each LC-SES measure and log follow-up time, and the assumption was met. The analysis was repeated without applying multiple imputation by chained equations procedures to impute missing LC-SES data and results were similar. All statistical analysis was conducted using SAS, version 9.4 (SAS Inc.).
4 allele status. Model 2 adjusted for model 1 covariates plus visit-1 body mass index, hypertension, diabetes, high-density lipoprotein cholesterol, total cholesterol, alcohol-drinking status, and tobacco-smoking status. We used a restricted cubic spline model to investigate the continuous nonlinear relationship between individual-level LC-SES and hazard of dementia with knots specified at the 5th, 50th, and 95th percentiles. To test the proportional hazards assumption, we included an interaction term between each LC-SES measure and log follow-up time, and the assumption was met. The analysis was repeated without applying multiple imputation by chained equations procedures to impute missing LC-SES data and results were similar. All statistical analysis was conducted using SAS, version 9.4 (SAS Inc.).
RESULTS
Among the 12,599 participants included in the analysis, 9,675 (75%) were White and 3,248 (25%) were Black, and they had a mean age of 54 (standard deviation, 5.7) years at visit 1 (1987–89). At baseline, Blacks were more likely than Whites to carry the APOE  4 allele, have not completed high school, have a family income of less than $25,000, smoke tobacco, be nondrinkers, have a higher body mass index, have higher high-density lipoprotein cholesterol, have more prevalent hypertension, and have more prevalent diabetes (Table 2). Blacks also had a lower individual-level LC-SES score than did Whites.
4 allele, have not completed high school, have a family income of less than $25,000, smoke tobacco, be nondrinkers, have a higher body mass index, have higher high-density lipoprotein cholesterol, have more prevalent hypertension, and have more prevalent diabetes (Table 2). Blacks also had a lower individual-level LC-SES score than did Whites.
Table 2.
Baseline Characteristics Stratified by Race, Atherosclerosis Risk in Communities Study, United States, 1987–1989
| White (n = 9,570) | Black (n = 3,029) | |||
|---|---|---|---|---|
| Risk Factor | Mean (SD) | % | Mean (SD) | % |
| Age, years | 53.9 (5.6) | 52.9 (5.7) | ||
| Male sex | 45.6 | 35.7 | ||
APOE  4 allele carriers 4 allele carriers |
26.3 | 39.0 | ||
| Below high-school educationa | 15.4 | 38.0 | ||
| Family income under $25,000b | 14.4 | 53.5 | ||
| Current tobacco smoker | 21.8 | 26.2 | ||
| Current alcohol drinker | 65.7 | 31.2 | ||
| Body mass indexc | 26.9 (4.8) | 29.8 (6.1) | ||
| Hypertensiond | 25.3 | 52.6 | ||
| Diabetese | 7.5 | 15.6 | ||
| Total cholesterol, mg/dL | 214.2 (40.3) | 214.8 (44.7) | ||
| HDL cholesterol, mg/dL | 51.1 (16.8) | 55.4 (17.1) | ||
| Individual LC-SES scoref | 10.2 (2.5) | 7.5 (2.7) | ||
Abbreviations: APOE, apolipoprotein E; HDL, high-density lipoprotein; LC, life course; SD, standard deviation; SES, socioeconomic status.
a Based on self-report of some high-school education or less at visit 1.
b Based on self-report of income at visit 1.
c Weight (kg)/height (m)2.
d Defined as diastolic blood pressure of >90 mm Hg, systolic blood pressure of >140 mm Hg, or use of hypertensive medication.
e Defined as nonfasting blood glucose of ≥200 mg/dL, fasting blood glucose of ≥126 mg/dL, self-report of diabetes, or reporting taking medication for diabetes or high blood sugar.
f SES score based on sum of scores from 3 life epochs, childhood (age 10 years), young adulthood age 30 years), and middle/older adulthood (study baseline age 45–64 years).
Three life epochs
A total 1,707 cases of incident dementia occurred (1,170 cases in Whites and 537 cases in Blacks) over a median follow-up of 24 years. SES at each life epoch was examined using race-specific distribution-based tertiles (Web Table 1, available at https://academic.oup.com/aje). In both Blacks and Whites—after adjustment for age, sex, and APOE  4 allele status—being in the lowest race-specific tertile of individual LC-SES at each life epoch (childhood, young adulthood, and middle/older adulthood) was associated with the highest incidence of dementia, followed by the middle SES tertile, and then the highest SES tertile (Figure 1). In both races, these differences in the incidence rates of dementia by individual SES tertile were statistically significant for young and middle/older adulthood but not for childhood. Among Whites, low SES in young adulthood was associated with a 36% (relative risk = 1.36, 95% confidence interval (CI): 1.18, 1.56) greater dementia risk compared with high young-adulthood SES. Low SES in middle/older adulthood was associated with a 49% (relative risk = 1.49, 95% CI: 1.25, 1.76) greater dementia risk compared with high SES. Among Blacks, low young-adulthood SES was associated with a 41% (relative risk = 1.41, 95% CI: 1.16, 1.71) greater risk and low middle/older-adulthood SES was associated with a 53% (relative risk = 1.53, 95% CI: 1.23, 1.90) greater risk of dementia compared with their respective high-SES tertiles. There was also a statistically significant interaction between SES tertile and race for each life epoch, indicating a stronger association between low SES and dementia in Blacks compared with Whites.
4 allele status—being in the lowest race-specific tertile of individual LC-SES at each life epoch (childhood, young adulthood, and middle/older adulthood) was associated with the highest incidence of dementia, followed by the middle SES tertile, and then the highest SES tertile (Figure 1). In both races, these differences in the incidence rates of dementia by individual SES tertile were statistically significant for young and middle/older adulthood but not for childhood. Among Whites, low SES in young adulthood was associated with a 36% (relative risk = 1.36, 95% confidence interval (CI): 1.18, 1.56) greater dementia risk compared with high young-adulthood SES. Low SES in middle/older adulthood was associated with a 49% (relative risk = 1.49, 95% CI: 1.25, 1.76) greater dementia risk compared with high SES. Among Blacks, low young-adulthood SES was associated with a 41% (relative risk = 1.41, 95% CI: 1.16, 1.71) greater risk and low middle/older-adulthood SES was associated with a 53% (relative risk = 1.53, 95% CI: 1.23, 1.90) greater risk of dementia compared with their respective high-SES tertiles. There was also a statistically significant interaction between SES tertile and race for each life epoch, indicating a stronger association between low SES and dementia in Blacks compared with Whites.
Figure 1.

Incidence rates (per 1,000 person years) of dementia adjusted for age, sex, and apolipoprotein E  4 allele status and stratified by life epoch and race-specific individual socioeconomic (SES) tertiles, Atherosclerosis Risk in Communities Study, United States, 1987–2013. Childhood: age 10 years; young adulthood: age 30 years; middle/older adulthood: ages 45–64 years. Statistically significant (P < 0.05) difference across life epoch SES tertiles was seen for Whites and Blacks for young adulthood and middle/older adulthood.
4 allele status and stratified by life epoch and race-specific individual socioeconomic (SES) tertiles, Atherosclerosis Risk in Communities Study, United States, 1987–2013. Childhood: age 10 years; young adulthood: age 30 years; middle/older adulthood: ages 45–64 years. Statistically significant (P < 0.05) difference across life epoch SES tertiles was seen for Whites and Blacks for young adulthood and middle/older adulthood.
When examining neighborhood SES, there were no statistically significant differences in dementia risk at any SES level (low, middle, high) for any of the 3 epochs, nor was there a statistically significant race interaction.
Individual LC-SES
We assessed the race-specific associations between dementia and individual LC-SES score as a continuous variable calculating the hazard ratios per increment of the pooled standard deviation (Table 3). Among Whites, after full adjustment, a standard-deviation greater individual LC-SES score was associated with a 14% lower risk of dementia (hazard ratio (HR) = 0.86, 95% CI: 0.81, 0.92). Among Blacks, a standard-deviation greater individual LC-SES score was associated with a 21% lower risk of dementia (HR = 0.79, 95% CI: 0.71, 0.87) after full adjustment.
Table 3.
Hazard Ratios for Dementia per Pooled Standard-Deviationa Increment of Individual Life-Course Socioeconomic Status, Atherosclerosis Risk in Communities Study, United States, 1987–2013
|
White (n = 9,570; 1,171 Events) |
Black (n = 3,029; 537 Events) |
|||
|---|---|---|---|---|
| Model | HR | 95% CI | HR | 95% CI |
| Model 1b | 0.83 | 0.77, 0.88 | 0.77 | 0.70, 0.85 |
| Model 2c | 0.86 | 0.81, 0.92 | 0.79 | 0.71, 0.87 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Pooled standard deviation = 2.80.
b Model 1: adjusted for age, sex, and apolipoprotein E 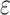 4 allele status.
4 allele status.
c Model 2: model 1 with the addition of body mass index, hypertension, diabetes, high-density lipoprotein cholesterol, total cholesterol, alcohol-drinking status, and tobacco-smoking status.
We then assessed the association between individual LC-SES independent of individual educational attainment. Education was removed from the individual LC-SES score calculation (keeping in parental education) and adjusted for separately in the models (Table 4). For Whites, a standard-deviation greater individual LC-SES without education was associated with a 10% lower risk of dementia (HR = 0.90, 95% CI: 0.84, 0.97) after model adjustments. In Blacks, a standard-deviation increment of individual LC-SES without education was associated with a 15% lower risk of dementia (HR = 0.85, 95% CI: 0.76, 0.96) after model 2 adjustments.
Table 4.
Hazard Ratios for Dementia per Pooled Standard-Deviationa Increment of Individual Life-Course Socioeconomic Status, With Separate Adjustment for Education, Atherosclerosis Risk in Communities Study, United States, 1987–2013
|
White (n = 9,674; 1,180 Events) |
Black (n = 3,248; 572 Events) |
|||
|---|---|---|---|---|
| Model and Educational Level | HR | 95% CI | HR | 95% CI |
| Model 1b | ||||
| LC-SES | 0.88 | 0.82, 0.95 | 0.86 | 0.77, 0.98 |
| Up to some high school | 1.00 | Referent | 1.00 | Referent |
| High-school graduate | 0.75 | 0.64, 0.87 | 0.74 | 0.59, 0.92 |
| Some college or more | 0.74 | 0.62, 0.88 | 0.68 | 0.52, 0.87 |
| Model 2c | ||||
| LC-SES | 0.90 | 0.84, 0.97 | 0.85 | 0.76, 0.96 |
| Up to some high school | 1.00 | Referent | 1.00 | Referent |
| High-school graduate | 0.80 | 0.68, 0.93 | 0.76 | 0.61, 0.95 |
| Some college or more | 0.81 | 0.68, 0.96 | 0.74 | 0.57, 0.96 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Pooled standard deviation = 2.80.
b Model 1: adjusted for age, sex, and apolipoprotein E  4 allele status.
4 allele status.
c Model 2: model 1 with the addition of body mass index, hypertension, diabetes, high-density lipoprotein cholesterol, total cholesterol, alcohol-drinking status, and tobacco-smoking status.
Using restricted cubic splines, we found that among both Whites and Blacks, the association between LC-SES (with and without education) and risk of dementia was linear (Web Figure 1).
Neighborhood LC-SES
We assessed the association between neighborhood LC-SES at each life epoch and risk of incident dementia and results were null for all 3 of the epochs explored. We also found no association between neighborhood-level LC-SES and incident dementia (Table 5). After adjustments, including adjustment for individual-level LC-SES, there were no statistically significant, independent associations between neighborhood-level LC-SES and dementia among Whites (model 2, for Whites, HR = 1.00, 95% CI: 0.96, 1.04; and for Blacks, HR = 1.06, 95% CI: 1.00, 1.13). We fitted a model without adjustment for individual-level LC-SES, and we fitted a separate model with individual LC-SES included and testing an individual × neighborhood LC-SES interaction term. Both of these models were nonsignificant.
Table 5.
Hazard Ratios for Dementia per Standard-Deviationa Increment of Neighborhood Life-Course Socioeconomic Status, Atherosclerosis Risk in Communities Study, United States, 1987–2013
| White | Black | |||
|---|---|---|---|---|
| (n = 9,570; 1,170 Events) | (n = 3,029; 537 Events) | |||
| Model | HR | 95% CI | HR | 95% CI |
| Model 1b | 0.99 | 0.95, 1.03 | 1.05 | 0.99, 1.12 |
| Model 2c | 1.00 | 0.96, 1.04 | 1.06 | 1.00, 1.13 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Standard deviation = 1 for White and Black participants.
b Model 1: adjusted for age, sex, and apolipoprotein E 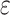 4 allele status.
4 allele status.
c Model 2: model 1 with the addition of body mass index, hypertension, diabetes, high-density lipoprotein cholesterol, total cholesterol, alcohol-drinking status, and tobacco-smoking status.
DISCUSSION
This prospective cohort study of community-dwelling Black and White adults followed for 24 years had 4 main findings. Higher individual LC-SES was associated among both Whites and Blacks with moderately lower incidence of dementia; examining life epochs, these associations were statistically significant for young adulthood and middle/older adulthood SES, and the pattern was similar for childhood SES although not statistically significant. After removing education from the individual-level LC-SES score and adjusting for educational attainment separately, a higher individual LC-SES score was associated with lower risk of dementia, suggesting that measures of economic status (income, home ownership, and wealth) might be associated with incident dementia independent of education. Finally, among both Whites and Blacks, there was no association between neighborhood-level LC-SES and incident dementia independent of individual-level LC-SES.
The results of this analysis suggest that low individual-level LC-SES as well as low SES at a given life epoch are risk factors for dementia. These findings corroborate previous studies of LC-SES in relation to cognitive decline or dementia, which found that, across the life course, markers of high SES were associated with lower risk of cognitive impairment in older adulthood (13, 15, 19, 20, 22, 24, 29). We also found the association between SES and incident dementia to be stronger among Blacks compared with Whites. This might be because of the systematic disadvantages that Blacks face due to racism and prejudice that compound the influences of SES on dementia risk. Relatedly, there are known regional and racial differences in social mobility across the United States that might be reflected in ARIC given the differences in race distribution across study sites (30).
We also found that individual-level LC-SES was inversely associated with dementia independent of individual-level education. While education is an important indicator of SES and likely a proxy for cognitive reserve, our findings suggest that other (primarily economic) SES factors also contribute to the association between LC-SES and dementia. These results mirror what other studies of LC-SES have found: a statistically significant association, albeit weaker than for education, between economic factors and cognitive impairment (13, 19, 22). In studies that used economic SES measures from middle or older adulthood only, results have been more mixed, with some studies finding an association (16, 17, 31) but most finding no association (11, 12, 14, 18). Efforts to reduce risk of dementia at the population level must address economic inequalities that are foundational to proximal causes of differences in dementia risk, such as education (32).
Finally, the lack of association between neighborhood-level LC-SES and dementia indicates that individual level factors might be more important than neighborhood factors in the causation of dementia. These findings corroborate Canadian and British cohort studies that found no association between neighborhood-level SES and risk of dementia (16, 31) but differ from a Korean study that found that higher neighborhood SES was associated with higher cognitive test scores (23). However, all 3 of these studies assessed neighborhood-level factors in mid- or late life. To our knowledge, ours is the only study of neighborhood-level LC-SES and dementia, making this a novel finding. Further examination of the relationship between neighborhood SES and dementia is needed, particularly neighborhood-level LC-SES. There is evidence that neighborhood-level environmental factors related to SES, such as lead exposure and air pollution, increase dementia risk (33–35).
Our study has several strengths, including a large sample size, large number of dementia cases, long follow-up period, multifaceted assessment of dementia, and the ability to incorporate SES over the entire life course. By not having to rely on mid- or late-life SES measures, we could account for SES over the entire latency period of dementia, which is believed to span multiple decades (36). The LC-SES approach allowed for exploration of the cumulative association of SES, without making assumptions about relative importance of individual epochs. ARIC participants were asked about childhood and young adulthood SES in mid/late life, and issues of recall bias were a concern. The cumulative approach is a more conservative way to incorporate life-course SES data in light of these limitations; however, associations with separate epochs were displayed in Figure 1. Recall bias might be related to the lack of association between childhood SES and dementia despite a stepwise association by SES level (low to high) similar to patterns seen in mid- and late life. In addition, to our knowledge, no studies have previously examined the relationship between neighborhood level LC-SES and dementia or whether neighborhood and individual LC-SES interact.
Despite our study’s strengths, there were limitations to our analyses. In the ascertainment of dementia cases, selection bias related to censoring and death over follow-up might have occurred and distorted hazard ratio estimates. To reduce selection bias, the ARIC study used a variety of strategies to completely identify dementia cases among participants that did not attend every ARIC visit, including annual follow-up calls with telephone interviews for cognitive status and surveillance of hospitals and death certificate codes. However, for a subset of cases identified by hospitalization and death certificate codes, SES might influence the likelihood of diagnosis. Second, cognitive tests used to identify cognitive decline and dementia might lack convergent validity between race groups due to cultural biases and socioeconomic differences (37, 38). Race-specific analyses were conducted to minimize race-related differences in validity of cognitive testing that might be related to SES and cultural background. Further, by using race-specific analyses, we avoided issues of comparison related to differences in attainable LC-SES over the lifetimes of Whites and Blacks due to segregation and discrimination. However, there might still be issues of generalizability and exchangeability due to lack of geographic variability in ARIC.
A third limitation was that individual LC-SES variables relied on participants’ knowledge and ability to remember, at midlife, the conditions they experienced during childhood and early adulthood. Recall bias might underlie the lack of statistically significant association between childhood SES and dementia despite a stepwise association by SES level (low to high) similar to the patterns seen in mid- and late life. However, while memory might not be precise, in measuring SES, the significance is in identifying where in the hierarchy of social position an individual fell relative to others like them. This means that precise measurement was not as important as relative knowledge of one’s circumstances, which were not likely forgotten. We also found a high level of concordance between individual and neighborhood SES at each epoch, indicating that memory of SES and census estimates based on historical addresses were congruent. A fourth limitation was that missing data, particularly within neighborhood-level LC-SES variables, required multiple imputation by chained equations methods, but a sensitivity analysis without the imputed values yielded similar results.
Our analysis indicates that incident dementia is inversely associated with individual-level LC-SES, whereas neighborhood-level LC-SES is not associated. Additional research is needed to identify critical periods over the life course where SES factors have the greatest impact on dementia risk and might warrant targeted intervention that aims to enable social and economic opportunities. In addition, further examination of neighborhood-level SES using a LC-SES model is needed.
Supplementary Material
ACKNOWLEDGMENTS
Author Affiliations: Department of Public Health Sciences, University of California Davis Medical Center, Davis, California (Kristen M. George); Division of Epidemiology and Community Health, University of Minnesota School of Public Health, Minneapolis, Minnesota (Kristen M. George, Pamela L. Lutsey, Theresa Osypuk, Aaron R. Folsom); Department of Epidemiology, University of Kentucky College of Public Health, Lexington, Kentucky (Anna Kucharska-Newton); Division of General Medicine, Columbia University Medical Center, New York, New York (Priya Palta); and Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Gerardo Heiss).
K.M.G. received support from the National Heart, Lung, and Blood Institute (training grant T32HL007779). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I). Neurocognitive data were collected with support from the National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders (grants U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917), and with previous brain magnetic resonance imaging examinations funded by the National Heart, Lung, and Blood Institute (grant R01-HL70825).
Conflict of interest: none declared.
REFERENCES
- 1. Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. [DOI] [PubMed] [Google Scholar]
- 2. Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. [DOI] [PubMed] [Google Scholar]
- 3. Galobardes B, Shaw M, Lawlor DA, et al. Methods in Social Epidemiology. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- 4. Kuh D, Ben-Shlomo Y, Lynch J, et al. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 6. Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5(1):87–96. [DOI] [PubMed] [Google Scholar]
- 7. Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knopman DS, Gottesman RF, Sharrett AR, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: the Atherosclerosis Risk in Communities study. Alzheimers Dement. 2018;14(11):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu YT, Prina AM, Brayne C. The association between community environment and cognitive function: a systematic review. Soc Psychiatry Psychiatr Epidemiol. 2015;50(3):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusmaully J, Dugravot A, Moatti JP, et al. Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: a cohort study. PLoS Med. 2017;14(6):e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staff RT, Chapko D, Hogan MJ, et al. Life course socioeconomic status and the decline in information processing speed in late life. Soc Sci Med. 2016;151:130–138. [DOI] [PubMed] [Google Scholar]
- 13. Dekhtyar S, Wang HX, Scott K, et al. A life-course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatry. 2015;23(9):885–896. [DOI] [PubMed] [Google Scholar]
- 14. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glymour MM, Kawachi I, Jencks CS, et al. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. J Epidemiol Community Health. 2008;62(6):532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer C, Yeung E, Hansen T, et al. Impact of socioeconomic status on the prevalence of dementia in an inner city memory disorders clinic. Int Psychogeriatr. 2009;21(6):1096–1104. [DOI] [PubMed] [Google Scholar]
- 17. Marengoni A, Fratiglioni L, Bandinelli S, et al. Socioeconomic status during lifetime and cognitive impairment no-dementia in late life: the population-based aging in the Chianti area (InCHIANTI) study. J Alzheimers Dis. 2011;24(3):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russ TC, Stamatakis E, Hamer M, et al. Socioeconomic status as a risk factor for dementia death: individual participant meta-analysis of 86,508 men and women from the UK. Br J Psychiatry. 2013;203(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marden JR, Tchetgen Tchetgen EJ, Kawachi I, et al. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melrose RJ, Brewster P, Marquine MJ, et al. Early life development in a multiethnic sample and the relation to late life cognition. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Everson-Rose SA, Mendes de Leon CF, Bienias JL, et al. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158(11):1083–1089. [DOI] [PubMed] [Google Scholar]
- 22. Turrell G, Lynch JW, Kaplan GA, et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):S43–S51. [DOI] [PubMed] [Google Scholar]
- 23. Kim GH, Lee HA, Park H, et al. Effect of individual and district-level socioeconomic disparities on cognitive decline in community-dwelling elderly in Seoul. J Korean Med Sci. 2017;32(9):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haan MN, Zeki Al-Hazzouri A, Aiello AE. Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: the Sacramento Area Latino Study on Aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i102–i110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 26. Carson AP, Rose KM, Catellier DJ, et al. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Ann Epidemiol. 2007;17(4):296–303. [DOI] [PubMed] [Google Scholar]
- 27. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karlamangla AS, Miller-Martinez D, Aneshensel CS, et al. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170(3):331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oishi S, Koo M, Buttrick NR. The socioecological psychology of upward social mobility. Am Psychol. 2019;74(7):751–763. [DOI] [PubMed] [Google Scholar]
- 31. Cadar D, Lassale C, Davies H, et al. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English Longitudinal Study of Ageing. JAMA Psychiat. 2018;75(7):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galea S, Vaughan R. Socioeconomic status, principles, and pragmatism: a public health of consequence, June 2019. Am J Public Health. 2019;109(6):842–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reuben A. Childhood Lead exposure and adult neurodegenerative disease. J Alzheimers Dis. 2018;64(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuller-Thomson E, Jopling SA. Exposure to lead in petrol and increased incidence of dementia. Lancet. 2017;389(10087):2371. [DOI] [PubMed] [Google Scholar]
- 35. Kilian J, Kitazawa M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer's disease—evidence from epidemiological and animal studies. Biom J. 2018;41(3):141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masters CL, Bateman R, Blennow K, et al. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 37. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Froehlich TE, Bogardus ST Jr, Inouye SK. Dementia and race: are there differences between African Americans and caucasians? J Am Geriatr Soc. 2001;49(4):477–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


