Key Points
Question
Does an association exist between cancer and subsequent Alzheimer disease (AD), and how likely is it that such a finding is associated with methodological bias rather than with a true common etiology?
Findings
In this systematic review and meta-analysis of 22 cohort and case-control studies representing 9 630 435 individuals, cancer diagnosis was associated with 11% decreased incidence of AD. Bias-adjusted metaregressions suggested that competing risks and diagnostic bias were unlikely explanations for the observed association, whereas survival bias remains to be ruled out.
Meaning
The observed inverse association between cancer and AD does not seem to be a consequence of competing risks, known confounding, or diagnostic bias.
Abstract
Importance
Observational studies consistently report inverse associations between cancer and Alzheimer disease (AD). Shared inverse etiological mechanisms might explain this phenomenon, but a systematic evaluation of methodological biases in existing studies is needed.
Objectives
To systematically review and meta-analyze evidence on the association between cancer and subsequent AD, systematically identify potential methodological biases in studies, and estimate the influence of these biases on the estimated pooled association between cancer and AD.
Data Sources
All-language publications were identified from PubMed, Embase, and PsycINFO databases through September 2, 2020.
Study Selection
Longitudinal cohort studies and case-control studies on the risk of AD in older adults with a history of any cancer type, prostate cancer, breast cancer, colorectal cancer, or nonmelanoma skin cancer, relative to those with no cancer history.
Data Extraction and Synthesis
Two reviewers independently abstracted the data and evaluated study biases related to confounding, diagnostic bias, competing risks, or survival bias. Random-effects meta-analysis was used to provide pooled estimates of the association between cancer and AD. Metaregressions were used to evaluate whether the observed pooled estimate could be attributable to each bias. The study was designed and conducted according to the Preferring Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
Incidence, hazard, or odds ratios for AD comparing older adults with vs without a previous cancer diagnosis.
Results
In total, 19 cohort studies and 3 case-control studies of the associations between any cancer type (n = 13), prostate cancer (n = 5), breast cancer (n = 1), and nonmelanoma skin cancer (n = 3) with AD were identified, representing 9 630 435 individuals. In all studies combined, cancer was associated with decreased AD incidence (cohort studies: random-effects hazard ratio, 0.89; 95% CI, 0.79-1.00; case-control studies: random-effects odds ratio, 0.75; 95% CI, 0.61-0.93). Studies with insufficient or inappropriate confounder control or greater likelihood of AD diagnostic bias had mean hazard ratios closer to the null value, indicating that these biases could not explain the observed inverse association. Competing risks bias was rare. Studies with greater likelihood of survival bias had mean hazard ratios farther from the null value.
Conclusions and Relevance
The weak inverse association between cancer and AD may reflect shared inverse etiological mechanisms or survival bias but is not likely attributable to diagnostic bias, competing risks bias, or insufficient or inappropriate control for potential confounding factors.
This systematic review and meta-analysis assesses the likelihood that methodological biases in cohort studies and case-control studies, rather than a common etiology, explain the inverse association between cancer and subsequent Alzheimer disease incidence among older adults.
Introduction
Cancer and Alzheimer disease (AD) and related dementias are consistently inversely associated in epidemiologic studies.1,2 This inverse association is counterintuitive because patients with a cancer diagnosis experience stress, treatment with cytotoxic drugs, invasive surgical procedures, and persistent pain, exposures that might decrease cognitive capacity and increase risk of developing AD relative to the cancer-free population of similar age.3,4,5,6 However, 2 meta-analyses of observational studies published up to 2014 reported an approximately 35% lower incidence of AD among older cancer survivors compared with those with no cancer history.7,8 This paradoxical inverse association has persisted in more recent studies that have implemented methodological approaches to reduce study biases that could induce this association.9,10,11
A compelling hypothesis for the inverse association between cancer and AD is a common etiology that acts in opposite directions in carcinogenesis and neurodegeneration.12 Proposed biological mechanisms involve proteins that suppress tau and amyloid-β deposition and regulate the cell cycle,13,14 common epigenetic modifications,15 and age-related dysregulation of cellular metabolism.16 If true, this potential common etiology represents a major opportunity to gain insight into the causes of both carcinogenesis and neurodegeneration.12,17,18,19
The inverse cancer-AD association could also be an artifact of methodological biases, such as bias related to handling of potential confounders, diagnostic bias, competing risks, or selective survival.17,20 The extent of these biases, or their effects on pooled risk estimates for the cancer-AD association, has not been systematically evaluated. Systematically combining evidence from studies with different biases (ie, evidence triangulation) may offer the most convincing interpretation of the literature.21,22 We thus conducted a systematic review and meta-analysis of existing literature on the association between cancer and subsequent AD risk. We evaluated the plausibility of each type of bias in each contributing study and used metaregressions to quantify the potential influence of each bias on the pooled risk estimate for the cancer-AD association.
Methods
Design and Search Strategy
The search included articles in any language published in the PubMed, Embase, and PsycINFO electronic databases through September 2, 2020. The search was conducted using the following keywords: neoplasia or cancer or malignancy and cognitive dysfunction or cognitive impairment or Alzheimer* and epidemiologic study or cohort or case-control or longitudinal study and adult or middle age or elder. This study was designed and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.23
Study Selection and Data Extraction
Inclusion criteria for the systematic review were (1) a longitudinal cohort or case-control study design that did not require mortality data to ascertain outcome; (2) the exposure variable was either a history of cancer diagnosis (yes vs no) at study baseline (prevalent cancer) or a new incident cancer diagnosis during follow-up (incident cancer), with studies categorized as including all cancer types, breast cancer, prostate cancer, colorectal cancer, or nonmelanoma skin cancer (NMSC); (3) the comparison group comprised individuals with no cancer history prior to baseline or no cancer history prior to each follow-up assessment; and (4) the outcome variable was an incident AD or dementia diagnosis. We selected breast cancer, prostate cancer, colorectal cancer, and NMSC because they account for nearly 40% of all US cancer cases and have relatively favorable survival probabilities.24
We initially screened titles and abstracts to select articles meeting our inclusion criteria. The Methods and Results sections of selected articles were reviewed for final inclusion. One reviewer (M.O.-R.) performed the literature search and screening, and 2 reviewers (M.O.-R. and L.C.K.) independently performed data extraction. Disagreements were minor and resolved through discussion between reviewers. Studies were included in the meta-analysis if a measure of association (risk ratio [RR], hazard ratio [HR], incidence rate ratio [IRR], or odds ratio [OR]) and 95% CIs were available.
Data items extracted were (1) study design, country, and study start and end dates; (2) study population source, inclusion and exclusion criteria, recruitment methods, and sample size; (3) participant characteristics, including age, sex, educational level, and race/ethnicity; (4) methods of cancer and AD diagnosis ascertainment; (5) analytic strategy, including model covariates; and (6) measure of association between cancer and AD and 95% CI. When multiple estimates were reported (eg, if possible and probable AD were included as separate outcomes), we used the measure of association for the outcome corresponding to stronger certainty of AD diagnosis.
Evaluation of Methodological Study Biases with Causal Diagrams
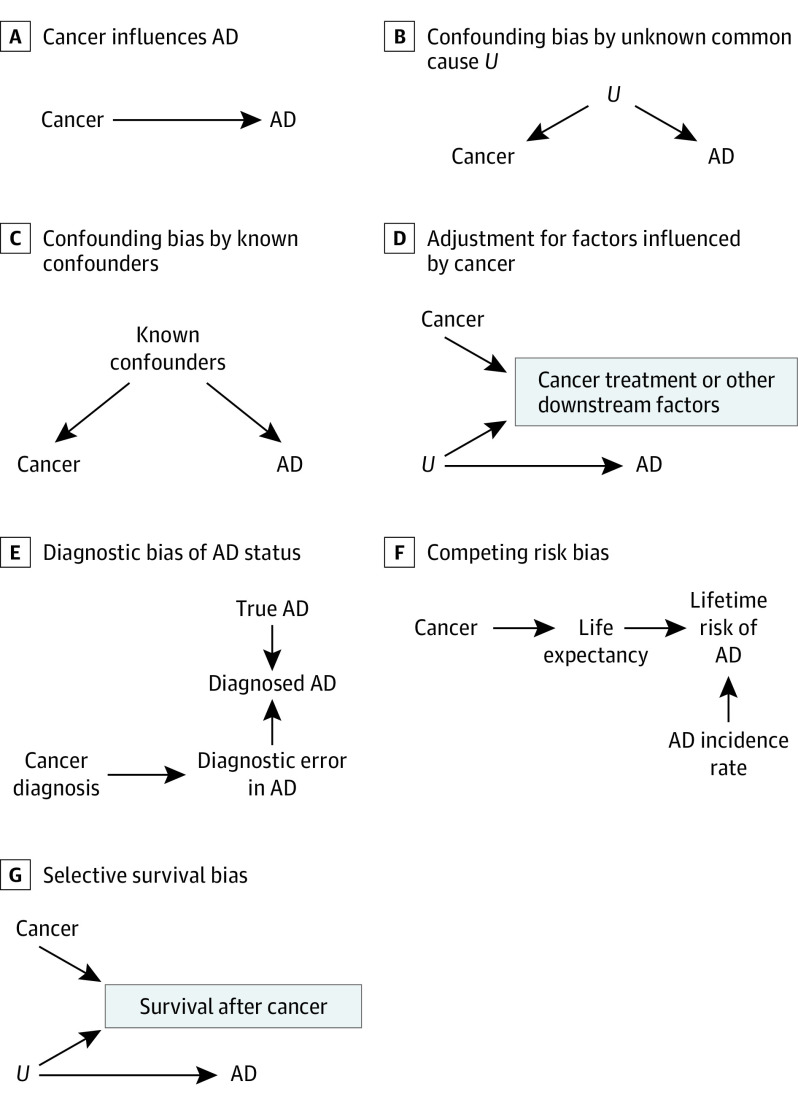
We specified several causal structures that may account for the cancer-AD association, representing each with a directed acyclic graph (DAG)25 (Figure 1). Substantive interest in the cancer-AD association arises because of the possibility that cancer influences AD (Figure 1A) or that there is an unmeasured common cause of cancer and AD (Figure 1B). We considered alternative causal structures to be “biases” (Figures 1C-G). The DAGs illustrate how each type of bias could induce a spurious association between cancer and AD, which could be in either a negative (inverse) or a positive direction. Our appraisal of potential biases in the studies included in this review was informed by the causal structures in Figure 1.
Figure 1. Directed Acyclic Graphs Depicting Alternative Explanations for the Observed Cancer–Alzheimer Disease (AD) Association.
The panel headings A through G correspond to the scenarios depicted in each panel. The directed acyclic graphs presented in panels A through G represent assumed data structures that could lead to spurious observed associations between cancer and AD. A, The direct arrow from cancer to AD indicates a causal association between cancer and subsequent AD risk. B, The direct arrows from unknown confounders U to cancer and to AD indicate that these conditions share a common cause. C-G, Alternative (noncausal) explanations for the cancer-AD association with no meaningful contribution of cancer to the etiology of neurodegeneration. C, The missing box around “Known confounders” indicates lack of statistical control for known confounders of the cancer-AD association. D, Adjustment for downstream variables, such as cancer treatment and comorbidities after cancer, is always inappropriate because it can introduce bias. E, A history of cancer diagnosis may influence the probability of receiving a diagnosis of AD. F, Cancer reduces life expectancy, and death is a competing risk to AD diagnosis. G, An unmeasured factor U promotes survival after cancer and reduces risk of AD. (The box around “Survival after cancer” indicates the restriction of the study population to those who survived cancer.)
Bias related to handling of confounders occurs when there are common causes of the exposure and outcome that are unaccounted for (Figures 1B and C). Confounders that would explain the observed inverse cancer-AD association would be those that raise risk of cancer but reduce risk of AD, ruling out many common lifestyle and social factors associated with increased risk of both conditions, such as smoking or alcohol consumption. We considered age, sex, and educational level as sociodemographic factors that should be included in a minimal adjustment set in all studies on this association. Another source of inappropriate statistical control is when models adjust for potential downstream consequences of cancer, including cancer treatment or comorbidities affected by cancer (Figure 1D). Adjustment for factors affected by the exposure of interest is a well-established source of bias.26 We considered studies that adjusted for age, sex, and educational level and those that did not adjust for downstream consequences of cancer as being less susceptible to bias owing to inappropriate handling of confounders.
Diagnostic bias of AD may occur if having a cancer diagnosis affects the probability of AD diagnosis (Figure 1E). Diagnostic bias can occur if clinicians are likely to overlook AD symptoms in patients receiving chemotherapy or other cancer treatments or if they are less likely to search for AD symptoms in patients with reduced life expectancy. Studies that ascertain AD from electronic health records have the greatest potential for diagnostic bias of AD. In Figure 1E, the arrow from cancer diagnosis to error in diagnosing AD represents this scenario. Diagnostic bias is avoided in studies in which all participants have an equal probability of AD diagnosis, independent of their cancer status, such as community-based cohorts in which cognition is routinely assessed at predetermined intervals for all participants.27 Diagnostic bias of cancer is also of interest because it could explain the inverse cancer-AD association if preexisting cognitive impairments lead to underdiagnosis or late diagnosis of cancer.28,29 We considered community-based studies and those that restricted their sample to participants who were cognitively intact at baseline as less susceptible to diagnostic bias.
Competing risks are events that preclude the occurrence of the primary outcome of interest.30 Here, death is a competing risk to AD, and a cancer diagnosis increases risk of death (Figure 1F). Studies that report cumulative incidence proportions of AD are subject to competing risks bias because the cumulative incidence proportion does not account for death. Cumulative incidences are usually estimated from unadjusted analyses, Kaplan-Meier curves, or regression models such as Fine and Gray.31 We considered longitudinal studies that used rate-based estimators such as HRs or IRRs30 or case-control studies that used incidence density sampling32 as having no competing risks bias.
Survival bias can occur if the study sample is enriched for individuals who have protective characteristics that promote cancer survival and reduce AD risk, which are usually unknown or unmeasured. Figure 1G illustrates this type of bias with the unknown AD protective characteristics denoted as U. Survival bias could arise even in longitudinal studies reporting HRs, because the cohort might become enriched over time for cancer survivors with unknown AD protective characteristics.33 Therefore, we considered studies on the association between NMSC and AD as less susceptible to survival bias because this type of cancer does not meaningfully raise mortality risk.34
Studies that include individuals with cancer diagnosis prior to baseline (ie, prevalent cancer cases) in their sample may be particularly vulnerable to survival bias because the sample will be limited to cancer survivors who did not receive a diagnosis of AD in the time frame from their diagnosis to the study baseline. This type of bias could be avoided by either excluding prevalent cancer cases from the analysis or providing separate estimates for prevalent and incident cancer cases. We considered studies that separated prevalent and incident cancer cases in the analysis as less susceptible to survival bias.
We also considered 2 other study characteristics that could lead to selection bias: (1) a high percentage (>5%) of missing covariate data, which decreases the analytic sample size, and, if not missing at random, may induce a bias similar to survival bias; and (2) studies with restrictive inclusion and exclusion criteria, such as including only participants with no comorbidities, which also can induce a bias similar to survival bias.
Statistical Analysis
For the systematic review, we calculated the proportion of all studies reporting a positive or negative (inverse) statistically significant association between cancer and AD (P < .05). In the meta-analyses and metaregressions, the primary outcome was an RR, OR, IRR, or HR comparing AD risk between individuals with vs without a previous cancer diagnosis. Effect estimates were transformed to the natural log (ln RR, ln OR, ln IRR, and ln HR) for analyses, then back-transformed for interpretation.
To estimate the pooled association between cancer and AD, we conducted separate meta-analyses of cohort and case-control studies, combining all cancer types. Because some studies had subgroup analyses of cancer types, we additionally conducted meta-analyses stratified by cancer type. We first estimated fixed-effect models and assessed heterogeneity using the Higgins and Thompson I2 statistic,35 followed by random-effects models. We used maximum likelihood to estimate between-study variance and the Knapp-Hartung method to estimate the variance of the summary effect estimate.36
Finally, we performed random-effects metaregressions to assess the influence of each type of methodological study bias on the pooled estimates. We developed 9 metaregression models in total, 1 model per type of study bias, adjusting for study design (case-control vs cohort) as a covariate. The metaregression coefficients represent the effects of each type of bias on the pooled cancer-AD risk estimate. Specifically, the metaregression coefficient is the difference in the pooled ln HR for cancer-AD association in studies with vs without the type of study bias being modeled. The intercepts from the metaregression models represent the pooled ln HR for cancer-AD association in studies without the type of study bias being modeled. Because we are interested in identifying types of study bias that could account for the observed inverse association between cancer and AD, metaregression coefficients that move the pooled HR away from the null value in the inverse direction indicate that the bias being modeled in that metaregression may contribute to the inverse cancer-AD association. We assessed publication bias with a funnel plot of standard error by ln HR. Analyses were conducted with R, version 3.6.3, using the Meta and Metafor packages (R Project for Statistical Computing), with a 2-sided P < .05 indicating statistical significance.
Results
Selection and Characteristics of Included Studies
Our search returned 2764 unique records; 22 studies (19 cohort studies and 3 case-control studies) met the eligibility criteria and were included (eFigure 1 in the Supplement). In total, 13 studies10,37,38,39,40,41,42,43,44,45,46,47,48 investigated all cancer types (eTable 2 in the Supplement); 5 studies49,50,51,52,53 investigated prostate cancer; 3 studies9,54,55 investigated NMSC; and 1 study56 investigated breast cancer (Table 1). Five studies that investigated all cancer types (5 of 13) additionally reported cancer type–specific subgroup analyses.
Table 1. Overview of the Studies Investigating the Association Between Cancer and AD by Cancer Type.
| Source | Study design | Country | Study Period | Study participants, No. | Age at baseline, y | Educational level | White participants |
|---|---|---|---|---|---|---|---|
| All cancer types | |||||||
| Bowles et al,10 2017 | Population-based cohort study | US | 1994-2005 | 4357 (42% men); 756 prevalent cancer; 583 incident cancer | Median, 75 (IQR, 70-80) prevalent cancer; median, 73 (IQR, 69-78) cancer-free | <College degree: 49% prevalent cancer; 49% cancer-free at baseline | 92% prevalent cancer; 81% cancer-free at baseline |
| Driver et al,40 2012 | Population-based cohort study | US | 1986-2008 | 1278 (39% men); 176 prevalent cancer; 247 incident cancer | Median, 77 (range, 68-96) prevalent cancer, 76 (range, 68-96) cancer-free | Completed secondary school: 72% prevalent cancer; 67% cancer-free at baseline | Not reported |
| Frain et al,46 2017 | US veteran cohort study | US | 1996-2001 | 3 499 378 (98% men); 771 285 incident cancer | Median, 71 (IQR, 65-76) cancer group; median, 71 (IQR, 65-77) cancer-free group | Not reported | 74% cancer group; 71% cancer-free group |
| Freedman et al,41 2016 | Cohort study of Medicare population in SEER regions | US | 1992-2005 | 1 163 327 (50% men); 742 805 incident cancer | Median, 74 (range, 66-85) cancer group; median, 67 (range, 66-85) cancer-free group | Not reported | 84% cancer-free group |
| Hanson et al,45 2017 | Population-based cohort study | US | 1992-2009 | 92 425 (48% men); 2630 history of cancer and AD diagnosis | Range, 65-79 | Not reported | Not reported |
| Musicco et al,38 2013 | Population-based cohort study | Italy | 2004-2009 | 204 468 with cancer diagnosis (57% men) | Mean (SD), 72.4 (7.8) | Not reported | Not reported |
| Nudelman et al,39 2014 | Cross-sectional case-control study | US | 2003 | 1609 (56% men); 313 AD cases; 1296 AD controls | Mean (SD) age range, 77-71 (5-8); reported by categories of AD and cancer status | Mean years education, range: 15-17 (SD, 2-3); reported by categories of AD and cancer status | Range, 81%-95%; reported by categories of AD and cancer status |
| Ording et al,47 2020 | Population-based cohort study | Denmark | 1980-2013 | 949 309 cancer cases (48% men); 679 122 cases were 5:1 matched to cancer-free controls | Cancer cases: median, 83.1 (IQR, 77.9- 87.5); cancer-free controls: median, 83.5 (IQR: 78.6-87.7) | Not reported | Not reported |
| Prinelli et al,48 2018 | Population-based nested case-control study | Italy | 1991-2012 | 1515 in the original cohort; 54 AD cases (56% men); 216 AD controls (age-, sex-, and smoking-matched 1:4) | Mean (SD), 62.1 (7.2) | ≥Primary school: 61% AD cases; 67% AD controls | Not reported |
| Realmuto et al,44 2012 | Clinic-based case-control study | Italy | 2006-2010 | 378 (29% men); 126 AD cases; 256 AD controls (age- and sex-matched) | Mean (SD), 76.7 (6.8) at interview; mean (SD), 71.1 (7.5) at AD diagnosis | >8 y of education: 18% AD cases; 29% AD controls | Not reported |
| Roe et al,43 2005 | Cohort study | US | 1992-?a | 249 (35% men); 50 with cancer history at baseline | Mean (SD), 78.1 (10.2) cancer group; mean (SD), 79.5 (9.8) cancer-free group | Mean (SD), y: 15 (2.9) cancer group; 14 (23.2) cancer-free group | 100% cancer group; 94% cancer-free group |
| Roe et al,37 2010 | Population-based cohort study | US | 1992-1999 | 3020 (41% men); 522 prevalent cancer; 376 incident cancer | Mean (SD), 75.9 (5.3) prevalent cancer; mean (SD), 74.9 (5.2) cancer-free | Mean (SD), y: 13 (3.3) prevalent cancer, 13 (3.2) cancer-free group | 92% prevalent cancer; 90% cancer-free group |
| Sun et al,42 2020 | Population-based cohort study | Sweden | 1992-2015 | 2 502 258 (55% men); 732 901 incident cancer | Median birth year 1931 in cancer group and cancer-free group | ≥12 y: 18% cancer group; 17% cancer-free group | Not reported |
| PC | |||||||
| Chung et al,52 2016 | Population-based cohort study | Taiwan | 2001-2013 | 5340 men; 1335 incident PC; 4005 age-matched cancer-free men | Mean (SD), 72.2 (9.3) | Not reported | Not reported |
| Ng et al,50 2018 | Population-based cohort study | Australia | 2003-2004 | 40 304 men; 3664 incident PC with ADT; age-matched (ratio 1:10) with cancer-free men | 92% were ≥65 | Not reported | Not reported |
| Robinson et al,53 2018 | Population-based cohort study | Sweden | 2006-2014 | 146 985 men; 25 967 incident PC cases; year of birth– and county-matched (ratio 1:5) cancer-free men | Mean (SD), 76.5 (7.6) | High educational level: 18% cancer group; 18% cancer-free group | Not reported |
| Shahinian et al,51 2006 | Cohort study of Medicare population in SEER regions | US | 1992-2001 | 101 089 men; 50 613 incident PC between 1992-1997 | >66; median age, 72 in cancer-free group and cancer without ADT (75 for ADT group) | In zip code area with >12 y of education: 77% cancer group without ADT; 77% cancer-free group | In zip code area: 83% cancer group without ADT; 84% cancer-free group |
| Smith et al,49 2018 | Medicare inpatient hospital or skilled nursing facility cohort study | US | 1986-1997 | 549 525 men; 115 189 with PC | PC: mean (SD), 70.4 (2.41); cancer-free group not reported | Not reported | 100% |
| NMSC | |||||||
| Schmidt et al,9 2017 | Population-based cohort study | Denmark | 1980-2013 | 1 297 318 (49% men); 216 221 incident NMSC | Median, 68 (IQR, 58-78) | Not reported | Not reported |
| White et al,55 2013 | Population-based cohort study | US | 1993-2009 | 1102 (42% men); 109 prevalent NMSC; 32 incident NMSC | Mean (SD), 79.4 (5.1) prevalent cancer; mean (SD), 78.0 (4.8) incident cancer; mean (SD), 78.9 (5.5) cancer-free group | Mean (SD), y: 15.1 (3.3) prevalent cancer; 13.4 (4.2) incident cancer; 13.2 (3.5) cancer-free group | 97% prevalent cancer; 100% incident cancer; 67% cancer-free group |
| Wu et al,54 2011 | Cohort study | US | 2003-2009 | 241 534 (56% men); 120 767 with NMSC; age-, sex-, region-, and calendar year–matched (ratio 1:1) to cancer-free people | Mean, 76.4 | Not reported | Not reported |
| BC | |||||||
| Sun et al,56 2016 | Population-based cohort study | Taiwan | 2000-2004 | 120 985 women; 24 197 incident BC; age- and index year–matched (ratio 1:4) cancer-free women | BC: mean (SE), 49.5 (0.04); cancer-free group; mean (SE), 49.6 (0.07) | Not reported | Not reported |
Abbreviations: AD, Alzheimer disease; ADT, androgen deprivation therapy; BC, breast cancer; IQR, interquartile range; NMSC, nonmelanoma skin cancer; PC, prostate cancer; SEER, Surveillance, Epidemiology, and End Results program of the National Cancer Institute.
Final calendar year of follow-up not reported.
Studies used different methods to ascertain cancer and AD diagnostic statuses. Cancer diagnoses were identified by self-report during study interviews (6 studies), linked data from cancer registries or surveillance systems (11 studies), and claims data from hospitals, ambulatory centers, or pharmacies (7 studies) (eTable 1 in the Supplement). The AD diagnoses were ascertained using claim codes in electronic health records in more than half the studies (15 studies). Seven studies ascertained AD diagnostic status through direct clinical assessments of study participants and through histopathology (Table 2).
Table 2. Study Methods of AD Diagnosis Ascertainment.
| Method and criteria | Source |
|---|---|
| Direct within-study assessments of participants | |
| The National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria | Bowles et al,10 2017; Driver et al,40 2012; Nudelman et al,39 2014; Realmuto et al,44 2012; Roe et al,43 2005; Roe et al,37 2010; White et al,55 2013 |
| Cognitive testing to evaluate multiple domains of cognitive function | Bowles et al,10 2017; Driver et al,40 2012; Nudelman et al,39 2014 |
| Histopathology | Roe et al,43 2005 |
| Electronic health records | |
| Medical claims using ICD-9 code 331.0 (AD diagnosis) | Chung et al,52 2016; Frain et al,46 2017; Freedman et al,41 2016; Hanson et al,45 2017; Prinelli et al,48 2018; Musicco et al,38 2013; Shahinian et al,51 2006; Smith et al,49 2018; Sun et al,56 2016; Wu et al,54 2011 |
| Medical claims using ICD-9 code 290.0 (dementia, senile), 290.21 (with depressive features), 290.3 (acute confusional state) | Frain et al,46 2017; Shahinian et al,51 2006; Smith et al,49 2018; Sun et al,56 2016; Sun et al,42 2020 |
| Medical claims using ICD-9 code 294 (other organic chronic psychotic conditions), 297 (delusional disorders), 310 (specific nonpsychotic mental disorders due to organic brain damage) | Smith et al,49 2018; Sun et al,56 2016 |
| Medical claims with ICD-10 codes (G30-G30.9-AD diagnosis) | Ording et al,47 2020; Prinelli et al,48 2018; Robinson et al,53 2018; Schmidt et al,9 2017; Sun et al,42 2020 |
| Medical claims with ICD-8 codes | Ording et al,47 2020; Schmidt et al,9 2017 |
| Pharmacy claims for donepezil, rivastigmine, galantamine, memantine | Chung et al,52 2016; Musicco et al,38 2013; Ng et al,50 2018; Prinelli et al,48 2018; Robinson et al,53 2018 |
| Mortality registry, exemption code for AD | Musicco et al,38 2013; Prinelli et al,48 2018 |
Abbreviations: AD, Alzheimer disease; ICD-8, International Classification of Diseases, Eighth Revision; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
Overview of Study Results and Meta-analysis
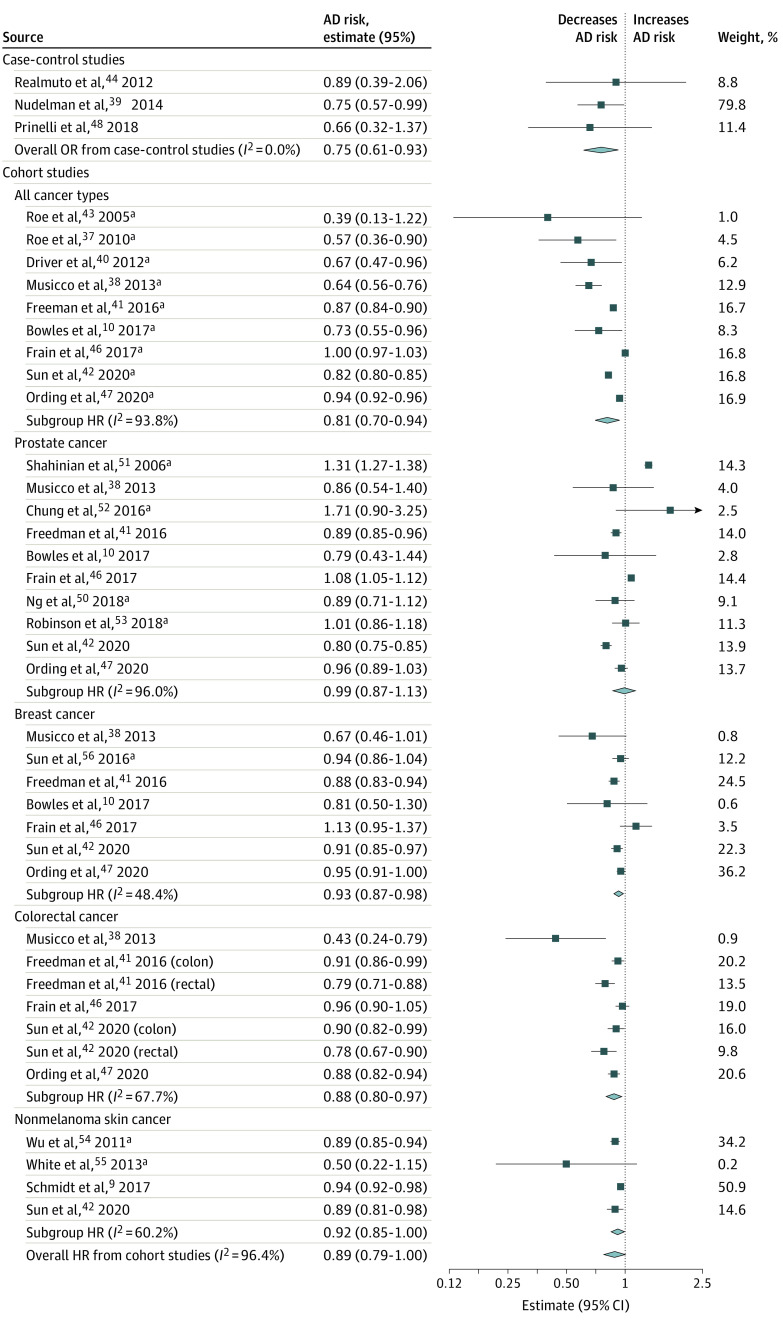
Overall, 11 of 22 included studies9,10,37,38,39,40,41,42,47,49,54 (50%) observed a statistically significant inverse association between cancer and AD, and 7 of 22 studies43,44,45,50,55,56,57 (32%) observed an inverse association that was nonstatistically significant. Two studies51,52 observed positive associations between cancer and AD, and 2 other studies46,53 found effectively null results. In the meta-analysis of all cohort studies (16 studies), the pooled fixed-effect HR for AD in cancer survivors compared with people with no cancer history was 0.94 (95% CI, 0.93-0.95). The I2 statistic was 96.4%, indicating high heterogeneity. The corresponding random-effects model provided a summary HR of 0.89 (95% CI, 0.79-1.00) (Figure 2). The random-effects OR for case-control studies was 0.75 (95% CI, 0.61-0.93) (Figure 2).
Figure 2. Forest Plot of Random-Effects Models for the Pooled Cancer–Alzheimer Disease (AD) Risk Estimatesa.
Random-effects meta-analyses were stratified by cancer type and study design. HR indicates hazard ratio; OR, odds ratio. Solid squares represent individual study estimates. The diamonds represent pooled estimates from the random-effects models.
aThe random-effects meta-analysis for cohort studies (16 studies) includes only the main study results to avoid double counting study participants when cancer type-specific subgroup analyses were performed.
Heterogeneity was observed in stratified meta-analyses of cohort studies using all types of cancer (I2 = 93.8%) and prostate cancer (I2 = 96.0%), with less heterogeneity in analyses of breast cancer (I2 = 48.4%), colorectal cancer (I2 = 67.7%), and NMSC (I2 = 60.2%). The meta-analysis of case-control studies using all types of cancer did not exhibit heterogeneity (I2 = 0.0%). Because heterogeneity was substantial in most meta-analyses, we interpret the random-effects models as the primary findings (Figure 2).
Most studies had at least 1 type of bias, but the estimated pooled HR remained in the inverse direction when accounting for each of these biases in the metaregressions (Table 3). Details on specific biases by study are shown in eTable 3 in the Supplement). The most common biases were survival bias (cancer type that raises subsequent mortality risk; 20 studies), diagnostic bias (cancer status might influence AD ascertainment; 15 studies), and confounding bias (missing adjustment for at least 1 key sociodemographic factor; 120 studies). In studies subject to AD diagnostic bias, the cancer-AD HR was 0.94 (95% CI, 0.58-1.52), which was closer to the null value than in studies not susceptible to AD diagnostic bias (HR, 0.73; 95% CI, 0.58-0.90) (Table 3). The AD diagnostic bias accounted for 16.7% of the between-study variance in cancer-AD risk estimates. The effects of survival and confounding biases were small and accounted for less than 10% of between-study variance in cancer-AD risk estimates (Table 3).
Table 3. Overview of Methodological Study Biases.
| Estimate | Types of methodological study biases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bias from handling of potential confounders | Diagnostic bias | Competing risks, estimated cumulative risks | Survival bias and related biases | ||||||
| Missing adjustment for age, sex, or educational level | Adjusted for factors influenced by cancer | Cognitively impaired individuals not excluded at baseline | Cancer status might influence AD diagnosis | Prevalent cancers not separated from incident cancers | Cancer type that raises subsequent mortality risk | High % of missing data | Restrictive inclusion and exclusion criteria | ||
| Studies with bias, No. | 12 | 4 | 5 | 15 | 6 | 6 | 20 | 3 | 1 |
| Metaregression estimatesa | |||||||||
| Pooled ln HR (95% CI) in studies without the bias | −0.15 (−0.34 to 0.04) | −0.15 (−0.28 to −0.02) | −0.09 (−0.22 to 0.03) | −0.32 (−0.54 to −0.10) | −0.13 (−0.26 to 0.00) | −0.09 (−0.20 to 0.02) | −0.19 (−0.57 to 0.20) | −0.10 (−0.22 to 0.01) | −0.12 (−0.24 to 0.00) |
| Difference in ln HR (95% CI) for studies with the bias | 0.04 (−0.20 to 0.29) | 0.13 (−0.13 to 0.39) | −0.14 (−0.45 to 0.16) | 0.26 (0.01 to 0.52) | 0.09 (−0.32 to 0.50) | −0.34 (−0.71 to 0.03) | 0.07 (−0.33 to 0.48) | −0.46 (−1.13 to 0.22) | −0.01 (−0.92 to 0.90) |
| R2, % | 1.6 | 32.4 | 22.1 | 16.7 | 6.7 | 21.1 | 5.5 | 16.3 | 6.2 |
Abbreviations: AD, Alzheimer disease; ln HR, natural log hazard ratio.
Metaregressions adjusted for study design (case-control vs cohort) as a covariate.
Less common types of bias included inappropriate adjustment for downstream consequences of cancer, which were most commonly cancer treatments46,51 or comorbidities after cancer37 (4 studies); prevalent cancers not separated from incident cancers (4 studies); and individuals with cognitive impairment not being excluded at baseline (3 studies). Studies with inappropriate adjustment for downstream consequences of cancer had a mean HR of 0.98 (95% CI, 0.66-1.48), closer to the null value than studies without this bias (HR, 0.86; 95% CI, 0.76-0.99). Studies that combined prevalent and incident cancers had a mean HR farther from the null value (HR, 0.65; 95% CI, 0.40-1.05) than studies that did not combine prevalent and incident cancers (HR, 0.91; 95% CI, 0.82-1.02) (Table 3). Studies that did not exclude individuals with cognitive impairment at baseline had estimates farther from the null value than studies that excluded these individuals (Table 3). We observed an association between study sample size and the magnitude of the cancer-AD risk estimate, suggestive of publication bias (eFigure 2 in the Supplement).
Discussion
In this systematic review and meta-analysis, we observed that individuals with history of cancer had a mean 11% lower risk of AD than those with no cancer history.7,8 We conducted metaregressions to quantify the directions and magnitudes of the effects of study biases on the pooled cancer-AD risk estimates. We found that biases due to inappropriate handling of potential confounders, diagnostic bias, and competing risks bias were unlikely to explain the inverse cancer-AD association. However, survival bias remains a possible explanation for the inverse cancer-AD association.
Our results align with earlier meta-analyses, which reported approximately 35% lower AD risk in cancer survivors than in those with no cancer history.7,8 Our evaluation of study biases sheds light on whether current evidence is sufficient to rule out methodological study biases as explanations for the cancer-AD association. Surprisingly, the studies most susceptible to diagnostic bias of AD status influenced the pooled cancer-AD risk estimate toward the null value, which suggests that diagnostic bias is an unlikely explanation for the observed inverse association.20 People with cancer history may be more likely to receive an AD diagnosis than cancer-free individuals owing to increased surveillance and detection through increased interaction with the health care system.6 Our results indicate that survival bias may contribute to the observed inverse cancer-AD association.
The best evidence against survival bias is from 4 studies of NMSC, a cancer with high survival rates. Another strategy to address survival bias is to report HRs stratified by time since cancer diagnosis because bias from survival should increase with more time elapsed since cancer diagnosis. Three studies41,42,46 included in this review presented time-varying HRs, but evidence from these reports is inconclusive.
Strengths and Limitations
This review adhered to PRISMA guidelines, and we systematically quantified methodological biases that may spuriously explain the association under study. We integrated evidence from observational studies using different methodological approaches to understand the cancer-AD association. We did not consider all study designs that might be relevant to understanding this association, such as mendelian randomization,58,59 negative control studies, and studies with proxy measurements of AD (such as longitudinal decline of cognitive function,11 neuroimaging,39 or other functional assessments of the brain).60 Furthermore, many studies in this review were subject to multiple methodological biases; however, the number of studies was too small to allow evaluation of the impact of multiple biases simultaneously.
Conclusions
This study found a weak inverse association between cancer and AD that does not appear to be explained by bias related to handling of confounders, diagnostic bias, or competing risks. Integrating results from different methodological approaches to this research question increases confidence that cancer and AD may share a common causal factor, potentially offering a novel path to understanding the shared etiologies of carcinogenesis and neurodegeneration. Survival bias cannot yet be ruled out as an explanation. Further studies designed to minimize survival bias are necessary to help determine whether survival or a true common etiology between cancer and AD explains the observed association.
eFigure 1. PRISMA Flow Diagram of Screening and Inclusion Process
eTable 1. Study Methods of Cancer Diagnosis Ascertainment
eTable 2. Cancer Types Reported in Studies in the Category “All Cancer Types”
eTable 3. Overview of Methodological Study Biases
eFigure 2. Funnel Plot of Study Standard Error (a Function of Sample Size) by lnHR for Longitudinal Cohort Studies Estimating HRs for AD Risk (k = 16)
References
- 1.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80-93. doi: 10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nolen SC, Evans MA, Fischer A, Corrada MM, Kawas CH, Bota DA. Cancer—incidence, prevalence and mortality in the oldest-old: a comprehensive review. Mech Ageing Dev. 2017;164:113-126. doi: 10.1016/j.mad.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med. 2017;177(8):1146-1153. doi: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboalela N, Lyon D, Elswick RK Jr, et al. Perceived stress levels, chemotherapy, radiation treatment and tumor characteristics are associated with a persistent increased frequency of somatic chromosomal instability in women diagnosed with breast cancer: a one year longitudinal study. PLoS One. 2015;10(7):e0133380. doi: 10.1371/journal.pone.0133380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermelink K, Voigt V, Kaste J, et al. Elucidating pretreatment cognitive impairment in breast cancer patients: the impact of cancer-related post-traumatic stress. J Natl Cancer Inst. 2015;107(7):djv099. doi: 10.1093/jnci/djv099 [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Hurria A. New challenges in psycho-oncology research IV: cognition and cancer: conceptual and methodological issues and future directions. Psychooncology. 2018;27(1):3-9. doi: 10.1002/pon.4564 [DOI] [PubMed] [Google Scholar]
- 7.Ma LL, Yu JT, Wang HF, et al. Association between cancer and Alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis. 2014;42(2):565-573. doi: 10.3233/JAD-140168 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Guo S, Zhang X, et al. Inverse relationship between cancer and Alzheimer’s disease: a systemic review meta-analysis. Neurol Sci. 2015;36(11):1987-1994. doi: 10.1007/s10072-015-2282-2 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt SAJ, Ording AG, Horváth-Puhó E, Sørensen HT, Henderson VW. Non-melanoma skin cancer and risk of Alzheimer’s disease and all-cause dementia. PLoS One. 2017;12(2):e0171527. doi: 10.1371/journal.pone.0171527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowles EJA, Walker RL, Anderson ML, Dublin S, Crane PK, Larson EB. Risk of Alzheimer’s disease or dementia following a cancer diagnosis. PLoS One. 2017;12(6):e0179857. doi: 10.1371/journal.pone.0179857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ospina-Romero M, Abdiwahab E, Kobayashi L, et al. Rate of memory change before and after cancer diagnosis. JAMA Netw Open. 2019;2(6):e196160-e196160. doi: 10.1001/jamanetworkopen.2019.6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driver JA. Understanding the link between cancer and neurodegeneration. J Geriatr Oncol. 2012;3(1):58-67. doi: 10.1016/j.jgo.2011.11.00724071493 [DOI] [Google Scholar]
- 13.Li Q, Dong Z, Lin Y, et al. The rs2233678 polymorphism in PIN1 promoter region reduced cancer risk: a meta-analysis. PLoS One. 2013;8(7):e68148. doi: 10.1371/journal.pone.0068148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma SL, Tang NLS, Tam CWC, et al. A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer’s disease. Neurobiol Aging. 2012;33(4):804-813. doi: 10.1016/j.neurobiolaging.2010.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremolizzo L, Rodriguez-Menendez V, Brighina L, Ferrarese C. Is the inverse association between Alzheimer’s disease and cancer the result of a different propensity to methylate DNA? Med Hypotheses. 2006;66(6):1251-1252. doi: 10.1016/j.mehy.2005.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology. 2014;15(6):547-557. doi: 10.1007/s10522-014-9523-2 [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M. Cancer and dementia: it’s complicated. Alzheimer Dis Assoc Disord. 2015;29(2):177-182. doi: 10.1097/WAD.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabarés-Seisdedos R, Rubenstein JL. Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat Rev Neurosci. 2013;14(4):293-304. doi: 10.1038/nrn3464 [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Leurgans S. Is there a link between cancer and Alzheimer disease? Neurology. 2010;74(2):100-101. doi: 10.1212/WNL.0b013e3181cbb89a [DOI] [PubMed] [Google Scholar]
- 20.van der Willik KD, Schagen SB, Ikram MA. Cancer and dementia: two sides of the same coin? Eur J Clin Invest. 2018;48(11):e13019. doi: 10.1111/eci.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866-1886. doi: 10.1093/ije/dyw314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlsson H, Kendler KS. Applying causal inference methods in psychiatric epidemiology: a review. JAMA Psychiatry. 2020;77(6):637-644. doi: 10.1001/jamapsychiatry.2019.3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Cancer Society Cancer Facts & Figures 2019. American Cancer Society; 2019. [Google Scholar]
- 25.Glymour MM, Greenland S. Chapter 12: causal diagrams In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed Lippincott Williams & Wilkins; 2008:184-194. [Google Scholar]
- 26.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511-1519. doi: 10.1093/ije/dyt127 [DOI] [PubMed] [Google Scholar]
- 27.Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology. 2019;30(2):291-302. doi: 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756. doi: 10.1001/jama.285.21.2750 [DOI] [PubMed] [Google Scholar]
- 29.Bayles KA, Tomoeda CK, Cruz RF, Mahendra N. Communication abilities of individuals with late-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(3):176-181. doi: 10.1097/00002093-200007000-00009 [DOI] [PubMed] [Google Scholar]
- 30.Wolkewitz M, Cooper BS, Bonten MJM, Barnett AG, Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ. 2014;349:g5060. doi: 10.1136/bmj.g5060 [DOI] [PubMed] [Google Scholar]
- 31.Bakoyannis G, Touloumi G. Practical methods for competing risks data: a review. Stat Methods Med Res. 2012;21(3):257-272. doi: 10.1177/0962280210394479 [DOI] [PubMed] [Google Scholar]
- 32.Cheung YB, Ma X, Lam KF, Li J, Milligan P. Bias control in the analysis of case-control studies with incidence density sampling. Int J Epidemiol. 2019;48(6):1981-1991. doi: 10.1093/ije/dyz116 [DOI] [PubMed] [Google Scholar]
- 33.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13-15. doi: 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubió-Casadevall J, Hernandez-Pujol AM, Ferreira-Santos MC, et al. Trends in incidence and survival analysis in non-melanoma skin cancer from 1994 to 2012 in Girona, Spain: a population-based study. Cancer Epidemiol. 2016;45:6-10. doi: 10.1016/j.canep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 36.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106-112. doi: 10.1212/WNL.0b013e3181c91873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musicco M, Adorni F, Di Santo S, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013;81(4):322-328. doi: 10.1212/WNL.0b013e31829c5ec1 [DOI] [PubMed] [Google Scholar]
- 39.Nudelman KN, Risacher SL, West JD, McDonald BC, Gao S, Saykin AJ; Alzheimer’s Disease Neuroimaging Initiative . Association of cancer history with Alzheimer’s disease onset and structural brain changes. Front Physiol. 2014;5:423. doi: 10.3389/fphys.2014.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman DM, Wu J, Chen H, et al. Associations between cancer and Alzheimer’s disease in a U.S. Medicare population. Cancer Med. 2016;5(10):2965-2976. doi: 10.1002/cam4.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun M, Wang Y, Sundquist J, Sundquist K, Ji J. The association between cancer and dementia: a national cohort study in Sweden. Front Oncol. 2020;10:73. doi: 10.3389/fonc.2020.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64(5):895-898. doi: 10.1212/01.WNL.0000152889.94785.51 [DOI] [PubMed] [Google Scholar]
- 44.Realmuto S, Cinturino A, Arnao V, et al. Tumor diagnosis preceding Alzheimer’s disease onset: is there a link between cancer and Alzheimer’s disease? J Alzheimers Dis. 2012;31(1):177-182. doi: 10.3233/JAD-2012-120184 [DOI] [PubMed] [Google Scholar]
- 45.Hanson HA, Horn KP, Rasmussen KM, Hoffman JM, Smith KR. Is cancer protective for subsequent Alzheimer’s disease risk? evidence from the Utah population database. J Gerontol B Psychol Sci Soc Sci. 2017;72(6):1032-1043. doi: 10.1093/geronb/gbw040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frain L, Swanson D, Cho K, et al. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimers Dement. 2017;13(12):1364-1370. doi: 10.1016/j.jalz.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ording AG, Horváth-Puhó E, Veres K, et al. Cancer and risk of Alzheimer’s disease: small association in a nationwide cohort study. Alzheimers Dement. 2020;16(7):953-964. doi: 10.1002/alz.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prinelli F, Adorni F, Leite MLC, et al. Different exposures to risk factors do not explain the inverse relationship of occurrence between cancer and neurodegenerative diseases: an Italian nested case-control study. Alzheimer Dis Assoc Disord. 2018;32(1):76-82. doi: 10.1097/WAD.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 49.Smith MA, Bowen RL, Nguyen RQ, Perry G, Atwood CS, Rimm AA. Putative gonadotropin-releasing hormone agonist therapy and dementia: an application of Medicare hospitalization claims data. J Alzheimers Dis. 2018;63(4):1269-1277. doi: 10.3233/JAD-170847 [DOI] [PubMed] [Google Scholar]
- 50.Ng HS, Koczwara B, Roder D, Vitry A. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: an Australian population-based cohort study. Prostate Cancer Prostatic Dis. 2018;21(3):403-410. doi: 10.1038/s41391-018-0036-y [DOI] [PubMed] [Google Scholar]
- 51.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465-471. doi: 10.1001/archinte.166.4.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung SD, Lin HC, Tsai MC, Kao LT, Huang CY, Chen KC. Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology. 2016;4(3):481-485. doi: 10.1111/andr.12187 [DOI] [PubMed] [Google Scholar]
- 53.Robinson D, Garmo H, Van Hemelrijck M, et al. Androgen deprivation therapy for prostate cancer and risk of dementia. Eur Urol Suppl. 2018;17(2):e1075. doi: 10.1016/S1569-9056(18)31582-3 [DOI] [Google Scholar]
- 54.Wu JH, Guo Z, Steinerman JR, Kumar S, Berman R. Incidence of Alzheimer’s disease in the elderly with non-melanoma skin cancer. Pharmacoepidemiol Drug Saf. 2011;20:S304. [Google Scholar]
- 55.White RS, Lipton RB, Hall CB, Steinerman JR. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology. 2013;80(21):1966-1972. doi: 10.1212/WNL.0b013e3182941990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun LM, Chen HJ, Liang JA, Kao CH. Long-term use of tamoxifen reduces the risk of dementia: a nationwide population-based cohort study. QJM. 2016;109(2):103-109. doi: 10.1093/qjmed/hcv072 [DOI] [PubMed] [Google Scholar]
- 57.Prinelli F, Musicco M, Adorni F, et al. Occurrence of cancer and Alzheimer’s disease are inversely associated in elderly persons: a population-based incidence study. Funct Neurol. 2013;28:39-40. [DOI] [PubMed] [Google Scholar]
- 58.Feng YA, Cho K, Lindstrom S, et al. ; IGAP Consortium, Colorectal Transdisciplinary Study (CORECT); Discovery, Biology, and Risk of Inherited Variants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Transdisciplinary Research in Cancer of the Lung (TRICL) . Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Hum Genet. 2017;136(10):1341-1351. doi: 10.1007/s00439-017-1831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pathak GA, Zhou Z, Silzer TK, Barber RC, Phillips NR; Alzheimer’s Disease Neuroimaging Initiative, Breast and Prostate Cancer Cohort Consortium, and Alzheimer’s Disease Genetics Consortium . Two-stage bayesian GWAS of 9576 individuals identifies SNP regions that are targeted by miRNAs inversely expressed in Alzheimer’s and cancer. Alzheimers Dement. 2020;16(1):162-177. doi: 10.1002/alz.12003 [DOI] [PubMed] [Google Scholar]
- 60.Yarchoan M, James BD, Shah RC, et al. Association of cancer history with Alzheimer’s disease dementia and neuropathology. J Alzheimers Dis. 2017;56(2):699-706. doi: 10.3233/JAD-160977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram of Screening and Inclusion Process
eTable 1. Study Methods of Cancer Diagnosis Ascertainment
eTable 2. Cancer Types Reported in Studies in the Category “All Cancer Types”
eTable 3. Overview of Methodological Study Biases
eFigure 2. Funnel Plot of Study Standard Error (a Function of Sample Size) by lnHR for Longitudinal Cohort Studies Estimating HRs for AD Risk (k = 16)