Abstract
Introduction
Early detection of dementia symptoms is critical in Down syndrome (DS) but complicated by clinical assessment barriers. The current study aimed to characterize cognitive and behavioral impairment using longitudinal trajectories comparing several measures of cognitive and behavioral functioning.
Methods
Measures included global cognitive status (Severe Impairment Battery [SIB]), motor praxis (Brief Praxis Test [BPT]), and clinical dementia informant ratings (Dementia Questionnaire for People with Learning Disabilities [DLD]). One‐year reliability was assessed using a two‐way mixed effect, consistency, single measurement intraclass correlation among non‐demented participants. Longitudinal assessment of SIB, BPT, and DLD was completed using linear mixed effect models.
Results
One‐year reliability (n = 52; 21 male) was moderate for DLD (0.69 to 0.75) and good for SIB (0.87) and BPT (0.80). Longitudinal analysis (n = 72) revealed significant age by diagnosis interactions for SIB (F(2, 115.02) = 6.06, P = .003), BPT (F(2, 85.59) = 4.56, P = .013), and DLD (F(2, 103.56) = 4.48, P = .014). SIB progression (PR) had a faster decline in performance versus no‐dementia (ND) (t(159) = −2.87; P = .013). Dementia had a faster decline in BPT performance versus ND (t(112) = −2.46; P = .041). PR showed quickly progressing scores compared to ND (t(128) = −2.86; P = .014).
Discussion
Current measures demonstrated moderate to good reliability. Longitudinal analysis revealed that SIB, BPT, and DLD changed with age depending on diagnostic progression; no change rates were dependent on baseline cognition, indicating usefulness across a variety of severity levels in DS.
Keywords: cognition, dementia, Down syndrome, longitudinal
1. INTRODUCTION
There is a clear connection between dementia, especially Alzheimer's disease (AD), and aging in people with Down syndrome (DS). Almost all individuals with DS from their mid‐30s on have neuropathology consistent with AD including amyloid plaques and neurofibrillary tangles. 1 Notably, pathological changes have been documented in individuals with DS as young as 20 years of age, or ≈50 years earlier than in neurotypical aging groups. 2 , 3 , 4 This acceleration of pathology is likely related to the location of the APP gene and other overexpressed genes on chromosome 21, which is triplicated in individuals with DS. 1 , 5 , 6 , 7 In addition to triplicated APP, individuals with DS have additional AD risk factors including oxidative damage, neuroinflammation, higher rates of apolipoprotein E ε‐4 allele (APOE ε4/ε4, ε4/ε3, ε4/ε2), propensity for sleep apnea, and premorbid intellectual disabilities. 1 , 8 , 9
Improved medical care has resulted in an increased mean life expectancy for individuals with DS to age 53, with more than 70% living past the age of 30 years and 20% living past 55 years. 5 , 10 , 11 Together with increased lifespan, the factors above contribute to greater AD risk in adults with DS than in other populations with intellectual disabilities or the general population. 12 Between the ages of 30 and 39 ≈3.4% of individuals with DS are diagnosed with dementia; however, research suggests that vulnerability to atypical aging begins around age 35, 2 , 13 and dementia prevalence increases to 40% between the ages of 50 and 59. 14 , 15 Given the exponential increase in AD‐related neuropathology and dementia diagnosis, it is essential to detect early pathological and cognitive AD changes to enhance clinical intervention and long‐term care for individuals with concurrent DS and dementia.
RESEARCH IN CONTEXT
Systematic review: Early detection of dementia is complicated by inherent clinical assessment barriers for individuals with Down syndrome (DS) including intellectual disability (ID), large intra‐ and inter‐individual variability, identification of appropriate baseline levels of functioning, selection of tests with appropriate cut scores, and patient tolerability of testing. The current study aimed to characterize the degree of cognitive and behavioral impairment using longitudinal cognitive trajectories comparing several measures of cognitive and behavioral functioning.
Interpretation: Measures of global cognitive status, motor praxis, and clinical dementia informant ratings demonstrated moderate to good 1 year reliability. Longitudinal analysis revealed that these measures changed with age depending on diagnostic progression and were not dependent on baseline level of ID, indicating their usefulness across a variety of ID levels in DS.
Future Directions: Future research should compare the current cognitive measures to biomarkers including neuroimaging, blood biomarkers, and other physiological indicators of neurodegeneration in a longitudinal fashion to further validate and enhance sensitivity to dementia‐related cognitive changes in DS.
1.1. Detection of incipient AD in DS
With enhanced technology and understanding of disease progression, research has increasingly focused on detection of early cognitive and behavioral changes. Notably, individuals with DS and prodromal AD demonstrate early behavioral changes that appear comparable to those of neurotypical adults with mild cognitive impairment (MCI). Specifically, behavioral changes such as delusions and violent outbursts (either physical or verbal) appear more prevalent in adults with DS early in dementia while nighttime confusion, agitation, wandering, and visual hallucinations are more prominent in later stages. 16 Such behavioral changes are generally accessible and easily studied in individuals with DS given availability of informant ratings.
Unfortunately, the onset and course of cognitive changes in individuals with DS and AD remains less clear. Early detection of cognitive changes in individuals with DS is complicated by the inherent difficulty of assessing cognition in the context of moderate to severe intellectual disability (ID). Additional assessment difficulties within this population include large intra‐ and inter‐individual variability in cognition and behavior, differential diagnostic methods, identification of an appropriate baseline, selection of a meaningful control group, and tolerability of the testing process. Despite recent reports (Firth et al. 17 ), a “gold standard” neurocognitive battery has not been established, especially as measures used for diagnosing MCI and dementia in typical aging adults are often inappropriate for use in DS populations given pre‐existing ID. 18 Moreover, few measures have established cut scores for use in DS. 19
1.2. Utility of cognitive measures
Recent efforts within the field have focused on determining the utility of cognitive measures in detecting prodromal dementia and AD in adults with DS. For instance, Walsh et al. 20 examined cognitive markers in 114 individuals with DS, 62% of whom carried dementia diagnoses, in an attempt to validate the Rapid Assessment of Developmental Disabilities (RADD) for use in dementia diagnostics. RADD scores were compared to performances on the Brief Praxis Test (BPT), Severe Impairment Battery (SIB), and Dementia Scale for People with Learning Disabilities (DLD). Results indicated strong correlations between the RADD and BPT (r = 0.842), SIB total (r = 0.921), and DLD sum of cognitive subscale scores (r = 0.889) indicating strong content concordance between measures. 20
Further research on the SIB indicates a strong association in SIB total scores between persons with AD in the general population and those with concurrent DS and dementia once adjusted for sex and functional impairment. 21 Both the SIB and DLD have longstanding established reliability and validity, 22 , 23 with such evidence supporting the appropriateness of using the SIB as a domain‐based, yet brief, assessment of cognitive change in DS.
1.3. Longitudinal assessment
Few studies have tracked longitudinal cohorts in an attempt to monitor cognitive changes and address the utility of specific cognitive measures including the SIB, BPT, and DLD. In one such study, McCarron et al. 24 followed 77 women with DS over a 14‐year period with annual visits. Notably, the DLD appeared particularly sensitive to early and progressive cognitive change over time; this was in direct contrast to the Down Syndrome Mental Status Examination (DSMSE), which appeared less sensitive and best suited for those with less severe levels of ID. 24 Despite the availability of only limited longitudinal data, researchers and clinicians alike understand the importance of early and regular cognitive screening. Clinically, longitudinal measurement has gained traction as a means of early AD detection, beginning with a baseline evaluation at age 35 and with follow‐ups annually or as needed. 25 However, to the authors’ knowledge, no studies to date have empirically examined the longitudinal trajectories and clinical utility of the BPT, SIB, and DLD in a diverse cohort of individuals with DS.
The Aging in Down Syndrome Study (ADS) at the University of Kentucky currently follows one such unique cohort. The present analyses examined longitudinal cognitive and behavioral symptoms from baseline through conversion to and progression of AD using the BPT, SIB, and DLD. Several models were tested including whether rate of change was dependent on time, age, baseline ID level, and diagnosis for each of these measures. It was hypothesized that rate of change on all measures would be greater with time accrual and increased age at baseline diagnosis. While lower ID baselines were hypothesized to be associated with initially lower baseline scores, rate of change over time was hypothesized to be independent of ID status.
2. METHODS
2.1. Participants
Research procedures were approved by the University of Kentucky Institutional Review Board. Participants completed approved protocols for informed consent or assent with guardian approval. Participants were community residing men and women with DS recruited through local DS support groups and residential facilities primarily in Kentucky, southern Indiana, and southern Ohio.
Participants were included in the test–retest reliability analysis if they had a neuropsychological evaluation and were stable with no dementia at both years. There were 52 participants who were included in the analysis. For the longitudinal analysis 95 participants had more than one visit. Participants were excluded (n = 23) from the longitudinal analysis if they had other ongoing medical conditions or environmental changes that made consensus diagnosis undeterminable. Thus, 72 participants were included in the longitudinal analysis.
2.2. Assessments
Clinical assessments included the BPT, SIB, and DLD. The BPT 26 is a measure of dyspraxia that is a modification of the original 62‐item Dyspraxia Scale for Adults with Down Syndrome. The BPT consists of 20 items selected from the original for maximum change demonstrated over 3 years. 27 The BPT requires minimal verbal demands and instead uses simple behavioral output. Low scores on the BPT indicate severe dyspraxia. The SIB 28 was developed to assess cognition in severely impaired individuals. By using one‐step commands and gestural cues, the SIB allows for non‐verbal responses and partially correct responses to assess behavioral and cognitive symptoms in individuals with severe dementia. The SIB yields a total score along with six major subscales including attention, orientation, language, memory, visuospatial ability, and construction; lower scores indicate more severe deficits. The DLD 22 is a diagnostic screening tool that measures behavioral and cognitive dysfunction as reported by caregivers or guardians. It consists of 50 items resulting in a sum of cognitive scores including: short‐term memory, long‐term memory, and spatial/temporal orientation along with a sum of social scores including speech, practical skills, mood, activity/interest, and behavioral disturbance. Higher scores on the DLD indicate more severe deterioration. DLD raters for the current study were caregivers and/or legal guardians who were responsible for daily care of the participants either in the home or an assisted living setting.
2.3. Consensus diagnosis
Dementia diagnoses were determined through an expert diagnostic consensus review consisting of one neuropsychologist, one psychologist, and three neurologists and are based on NINCDS‐ADRDA criteria. Baseline levels of ID were determined by caregiver report of prior evaluation results and by review of records when available.
2.4. Statistical analysis
A two‐way mixed effect, consistency, single measurement intraclass correlation (ICC[3,1]) was calculated for the SIB, BPT, and DLD. The objective was to quantify the reliability of primary cognitive and dementia outcome scales over a 1‐year period among non‐demented (ND) individuals with DS. Reliability was considered poor if the ICC was <0.5, moderate if ICC was between 0.5 and 0.75, good if the ICC was between 0.75 and 0.9, and excellent if ICC was >0.90. 29 All analyses were completed using R 3.6.1 30 and the “irr” package. 31 Reliable change indices were calculated using the methods described by Jacobson and Truax 32 along with Chelune et al. 33 These indices reflect the amount of change in each assessment needed to be 90% or 95% confident the change is beyond normal variability.
Longitudinal analysis of SIB, BPT, and DLD was completed using mixed effects models. The objective of this analysis was to evaluate the rate of change in the SIB, BPT, and DLD across ages for participants who remained ND versus those who progressed to dementia (PR), versus those who had dementia as baseline (DM). First, the random effects were specified using the restricted likelihood method. Random intercept versus random intercept and slope models were compared. The random intercept model was selected as there were no issues with convergence. Then main effects and interactions of the fixed effects (eg, age and disease progression) were fitted using the maximum likelihood method. Post‐hoc comparisons used the Tukey correction for multiple comparisons. Model fit was selected based on loglikelihood of nested models, Akaike information criterion (AIC), and Bayesian information criterion (BIC). All analyses were completed using R 3.6.1 30 and the “lme4” 34 and “lmerTest” 35 packages.
3. RESULTS
3.1. Test–retest reliability
Between years 1 and 2 there were 52 eligible participants who had SIB, BPT, or DLD data. Of these 49 participants, 42 to 43 participants had data for each specific test. Full participant characteristics are included in Table 1. Results of the reliability analyses are reported in Table 2. SIB and BPT test–retest reliability over the first year of assessment was good. DLD total score and subscore reliability was moderate. DLD cognitive subscore reliability had slightly better reliability than DLD social subscore (Table 2). Reliable changes indices revealed that over the first year, a decrease of 12 points on the SIB and of 10 points on the BPT were associated with 90% confidence that a real change occurred. On the DLD, increases of 11 points in the total score, 7 points in the cognitive subscore, and 6 points in the social subscore were associated with 90% confidence of real change. More conservative reliable change indices (at 95% confidence) are provided in Table 2.
TABLE 1.
Participant characteristics for test–retest reliability
| Year 1 and 2 (N = 52) | |||
|---|---|---|---|
| % (n) | Mean (SD) | Median [Q1–Q3] | |
| Sex | |||
| Male | 40.38% (21) | ||
| Female | 59.62% (31) | ||
| ID level | |||
| Borderline | 3.85% (2) | ||
| Mild | 55.77% (29) | ||
| Moderate | 38.46% (20) | ||
| Profound | 0% (0) | ||
| Severe | 0% (0) | ||
| Not documented | 1.92% (1) | ||
| Age | 38.96 (9.28) | 37.06 [32.66–44.75] | |
| Time (years) | 1.02 (0.10) | 1.02 [0.98–1.05] | |
| SIB total | 82.21 (15.92) | 87 [73.50–94] | |
| BPT total | 69.60 (8.79) | 72 [64.50–76] | |
| DLD total | 9.77 (8.38) | 7 [3–14.5] | |
| DLD cognitive | 4.44 (5.64) | 2 [0–6] | |
| DLD social | 5.33 (4.25) | 5 [2–8] | |
Abbreviations: BPT, Brief Praxis Test; DLD, Dementia Questionnaire for People with Learning Disabilities; ID, intellectual disability; SD, standard deviation; SIB, Severe Impairment Battery.
TABLE 2.
Summary of test–retest reliability and reliable change indices
| n | Time 1 mean (SD) | Time 2 mean (SD) | ICC (95% CI) | 90% RCI | 95% RCI | |
|---|---|---|---|---|---|---|
| SIB | 43 | 84.16 (15.00) | 86.37 (11.75) | 0.87 (0.77–0.93) | 12 | 14 |
| BPT | 42 | 70.57 (9.09) | 70.98 (8.71) | 0.80 (0.66–0.89) | 10 | 11 |
| DLD | 43 | 8.98 (8.09) | 9.19 (8.38) | 0.71 (0.53–0.83) | 11 | 13 |
| DLD – Cognitive | 43 | 3.86 (5.19) | 4.33 (6.19) | 0.75 (0.58–0.86) | 7 | 8 |
| DLD – Social | 43 | 5.12 (4.44) | 4.86 (4.09) | 0.69 (0.49–0.82) | 6 | 7 |
Note: RCI values were rounded up to next whole number.
Abbreviations: BPT, Brief Praxis Test; DLD; ICC, intraclass correlation; RCI, reliable change index; SD, standard deviation; SIB, Severe Impairment Battery.
3.2. Longitudinal analysis
There were 72 participants (n = 32 male) who were eligible for the longitudinal analysis. About 64% of participants had four or fewer follow‐up visits, the remainder having five or more, for an average number of 4.35 visits and follow‐up time of 3.53 years. Full participant characteristics are provided in Table 3.
TABLE 3.
Participant characteristics for longitudinal analysis
| % (n) | Mean (SD) | Median [Q1–Q3] | |
|---|---|---|---|
| Sex | |||
| Male | 44.44% (32) | ||
| Female | 55.56% (40) | ||
| ID level | |||
| Borderline/mild | 48.61% (36) | ||
| Moderate to profound | 47.22% (34) | ||
| Not documented | 1.39% (1) | ||
| Baseline diagnosis | |||
| No dementia | 72.22% (52) | ||
| Possible dementia | 9.72% (7) | ||
| Probable dementia | 18.06% (13) | ||
| Last diagnosis | |||
| No dementia | 56.94% (41) | ||
| Possible dementia | 9.72% (7) | ||
| Probable dementia | 33.33% (24) | ||
| Diagnosis progression | |||
| Remained non‐Demented | 53.94% (41) | ||
| Progressed | 23.61% (17) | ||
| Dementia at baseline | 19.44% (14) | ||
| Follow‐up (years) | 3.53 (2.28) | 2.91 [1.93–5.38] | |
| Age | 41.31 (10.57) | 39.50 [33–50] | |
| SIB total | 79.64 (19.06) | 87 [70–93] | |
| BPT total | 67.80 (11.71) | 71.50 [63–76] | |
| DLD total | 15.10 (13.59) | 11 [4–21] | |
| DLD cognitive | 7.52 (8.72) | 4 [1–14] | |
| DLD social | 7.58 (6.10) | 6 [3–11] |
Abbreviations: BPT, Brief Praxis Test; DLD, Dementia Questionnaire for People with Learning Disabilities; ID, intellectual disability; SD, standard deviation; SIB, Severe Impairment Battery.
3.2.1. Brief praxis test
Age (F(1,94.51) = 10.03; P = .002), ID level (F(1,60.10) = 7.35; P = .009), and diagnosis progression (F(2, 70.26) = 11.33; P < .001) were all found to be significantly associated with BPT total score. Sex was not significantly associated with BPT total score and was dropped from the model. For each year increase in age, BPT scores dropped 0.40 points (t(94.51) = −3.17; P = .002). Individuals with moderate to profound ID scored 5.79 points lower than those with borderline/mild ID (t(60.10) = −2.71; P = .009). Using a Tukey correction for multiple comparisons, individuals with DM had significantly lower BPT scores than the ND (difference = −12.36; t(93.50) = −3.32; P = .004) or PR (difference = −15.74; t(83.00) = −4.54; P < .001) cohorts. There was no significant difference between the ND and PR groups (P > .05).
Next, a model examining the interaction of age and diagnosis progression was examined. The interaction model provided better fit than the main effects model based on AIC and likelihood ratio test. BIC was not smaller in the interaction compared to the main effect model. Thus, the change in BPT scores across age depended on diagnosis progression status (F(2, 85.59) = 4.56; P = .013). Using a Tukey correction for multiple comparisons, the DM group had a significantly faster decline in BPT performance compared to the ND cohort (t(112) = −2.46; P = .041), but the PR group did not (t(112) = −2.03; P = .109). There was no significant difference between the DM and PR groups (P > .05; Figure 1A).
FIGURE 1.
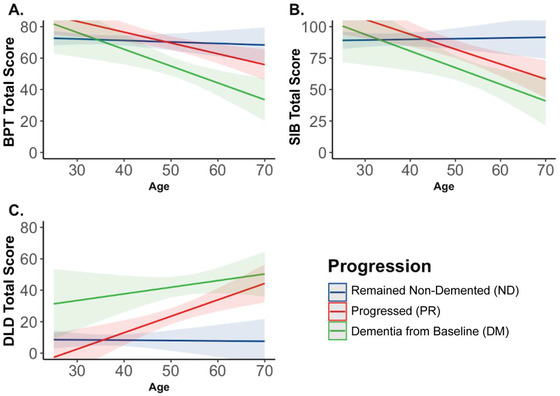
Predicted changes in primary outcome measures by age and progression status. A) BPT total score declined with increasing age at a greater rate among those who had dementia from baseline (DM) compared to those who remained non‐demented (ND); B) SIB total score declined with increasing age at a greater rate among those who progressed (PR) compared to those who remained non‐demented (ND); C) There were no significant differences in rates of DLD change between groups, but there was a main effect of group whereby the DM group had significantly higher DLD total scores than the ND and PR groups
3.2.2. Severe impairment battery
Age (F(1,127.77) = 6.77; P = .010), ID level (F(1,67.52) = 11.22; P = .001), and diagnosis progression (F(2, 78.83) = 6.69; P = .002) were all found to be significantly associated with SIB total score. Sex was not significantly associated with SIB total score and was dropped from the model. For each year increase in age, SIB scores dropped 0.48 points (t(127.77) = −2.60; P = .010). Individuals with moderate to profound ID scored 11.30 points lower than those with borderline/mild ID (t(67.53) = −3.35; P = .001). Using a Tukey correction for multiple comparisons, individuals with DM had significantly lower SIB scores than the ND (difference = −18.06; t(97.50) = −3.19; P = .005) or PR (difference = −16.75; t(80.50) = −3.17; P = .006) cohorts. There was no significant difference between the ND and PR groups (P > .05).
Next, a model examining the interaction of age and diagnosis progression was examined. The interaction model provided better fit than the main effects model based on AIC, BIC, and likelihood ratio test. Thus, the change in SIB scores across age depended on diagnosis progression status (F(2, 115.02) = 6.06; P = .003). Using a Tukey correction for multiple comparisons, the PR group had a significantly faster decline in SIB performance compared to the ND cohort (t(159) = −2.87; P = .013), but the DM group did not (t(118) = −2.35; P = .052). There was no significant difference between the DM and PR groups (P > .05; Figure 1B).
3.2.3. Dementia questionnaire for people with learning disabilities
Age (F(1,110.03) = 4.56; P = .035) and diagnosis progression (F(2, 76.26) = 23.52; P < .001) were found to be significantly associated with SIB total score. Sex and ID level were not significantly associated with DLD total score and were dropped from the model. For each year increase in age, DLD scores increased 0.33 points (t(110.03) = 2.14; P = .035). Using a Tukey correction for multiple comparisons, individuals with PR (difference = 11.00; t(82.00) = 2.87; P = .014) or DM (difference = 29.60; t(90.30) = 6.63; P < .001) had significantly higher DLD scores than ND. Furthermore, those with DM had elevated DLD scores compared to the PR group (difference = 18.50; t(76.00) = 4.53; P < .001).
Next, a model examining the interaction of age and diagnosis progression was examined. The interaction model provided better fit than the main effects model based on AIC and likelihood ratio test. BIC was not smaller in the interaction compared to the main effect model. Thus, the change in DLD scores across age depended on diagnosis progression status (F(2, 103.56) = 4.48; P = .014). Using a Tukey correction for multiple comparisons, the PR group had a significantly faster increase in DLD score compared to the ND cohort (t(128) = −2.86; P = .014). There was no significant difference between the DM and PR or ND groups (both P values > 0.05; Figure 1C). However, there was a main effect of diagnosis progression whereby the DM group had significantly higher DLD total scores than ND (difference = 31.08; t(103.00) = 5.59; P < .001) and PR (difference = 22.41; t(101.20) = 3.69; P = .001) groups (Figure 1C).
4. DISCUSSION
This study sought to characterize and compare over time the cognitive trajectories of individuals with DS who remain non‐demented, those who progress to dementia, and those monitored after a dementia diagnosis. The approach was two‐fold: (1) to estimate test–retest reliability of a short battery using the subsample of individuals who remained non‐demented over the course of the 1‐year study period and (2) to estimate and compare rates of change over time attributable to changes in clinical consensus diagnosis.
Test‐retest reliability estimates were acceptable for all three measures in the battery, with particularly good reliability for the SIB and BPT. DLD test‐retest reliability was moderate overall but was stronger for the cognitive subscale than the social subscale. The more objective, task‐oriented approach of SIB and BPT versus the more subjective, caregiver‐rating approach of the DLD may explain these reliability differences across measures, whereas DLD subscale differences in reliability potentially stem from the additional subjectivity involved in rating social behavior versus cognitive ability within the DLD. Overall, these estimates concord with prior studies and provide replication in an independent sample. 22 , 23 , 36
Moreover, the 90% confidence reliable change indexes computed for use as a clinical tool to identify meaningful changes are nearly identical to previously suggested cutoff scores for the DLD and its subscales, supporting those estimates with a larger sample size and a more extensive consensus process for group formation. 22 Specifically, a 1‐year change in DLD Total Score of 11 points or more, in DLD Cognitive score of 6 points or more, or in DLD Social score of 7 points or more represented meaningful change with 90% confidence. Furthermore, the current study adds to the DS literature by determining that a 1‐year change in SIB score of 12 points or more, or in BPT score of 11 points or more, reflect a meaningful change with 90% confidence. These replicated and novel change indices help provide context for what is and is not a meaningful change.
Longitudinal changes in global mental status, praxis, and Clinical Dementia Rating were generally in accordance with previous literature associating increased cognitive and functional impairment by various measures, including the DLD, with passage of time, age at baseline, and baseline level of ID. 24 In the present study, BPT and SIB scores were sensitive to baseline ID level, whereas DLD score was not. This may be as expected for the DLD, which was designed explicitly to account for developmental ID status, and for the SIB, which was not. More surprising is that the BPT was sensitive to baseline ID level despite its development for use with individuals with DS. More crucially, the rate of change on all three of our clinical measures was independent of baseline ID status, demonstrating that longitudinal monitoring does ameliorate the barrier that baseline ID presents to measurement of cognition in the context of assessment for dementia. This time‐dependent effect demonstrates that the DLD, SIB, and BPT can detect changes in DS individuals across a variety of ID levels and therefore could be useful in a clinic setting.
Individuals with dementia at study onset had, in general, a faster decline than individuals who remained non‐demented throughout. However, trajectories for individuals who progressed from non‐demented to dementia during the study were not different from the ND or DM groups despite trends to that effect (potentially due to the small sample size of this group). These findings suggest a limited utility of global mental status and praxis for early detection or prodromal changes in contrast to much larger and complex cognitive assessments (eg, Firth et al. 17 and Startin et al. 37 ). Ratings of clinical dementia symptoms using the DLD showed equivalent, significant decline for both the progression and dementia at baseline groups compared to individuals who remained non‐demented throughout the observation period. This effect held for both the cognitive and the social domains of the DLD. These longitudinal findings accord with and extend prior reports of early social/behavioral changes prior to clinical diagnosis of dementia. 38 Moreover, results from the current study indicate that perceived cognitive and social/behavioral changes may appear on rating instruments such as the DLD before mental status and praxis measures such as the SIB and BPT. This result supports a previous longitudinal study of cognitive decline in DS, indicating that informant ratings may better detect executive and cognitive function declines. 39
Mental status and praxis measures showed the expected performance reductions across diagnostic categories, as well as the expected decline over time in individuals with DS and comorbid dementia. They were not, however, as sensitive to early changes of incipient dementia as informant ratings. Relative sensitivity of the DLD to early changes of incipient dementia compared to other measures may be due to a number factors, including differences in instrument content, involvement of caregivers in the assessment process, or difficulty motivating and engaging individuals with DS and possible dementia in the formal assessment process, as well as floor or ceiling effects on the objective measures used.
The present study has several limitations. Wide inter‐individual variability in performance on clinical assessments, even within diagnostic status and ID level, limits detection of reliable effects and likely makes the clinical recommendations for identifying reliable change in individual scores overly conservative. Considerable intra‐individual variability in performance on clinical assessments and in diagnostic status over time poses similar problems and urges further caution regarding generalizability of the present findings. Furthermore, due to the recruitment limitations and high rate of loss to follow‐up inherent in studying this special population, sample sizes within diagnostic groups are relatively small, and most participants have been enrolled for a relatively short time. Finally, data from the clinical battery were included in the overall consensus adjudication of diagnoses, presenting the threat of criterion contamination endemic to clinical dementia research. The present cohort enrolls continuously, and future sample growth and follow‐up will mitigate the majority of these limitations. Ultimately, the effect of criterion contamination will be addressed with additional validation against neuropathology found at autopsy.
The current study revealed moderate to good reliability of assessments for dementia in DS. Future prospective studies should evaluate the predictive utility of reliable change indices. Furthermore, longitudinal results revealed that the SIB, BPT, and DLD changed with age depending on diagnosis progression across all levels of ID in DS. While the SIB and BPT demonstrated that individuals who progressed to dementia or were demented from baseline had faster rates of decline, the DLD demonstrated the greatest separation in non‐demented, progression, and demented cohorts.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors would like to thank Stacey Brothers, Katie McCarty, Roberta Davis, and Amelia Anderson‐Mooney, PhD for their assistance with data collection. We also want to thank our team of neurologists—Drs. Gregory Jicha, Donita Lightner, and William Robertson—for their clinical expertise.
Koehl L, Harp J, Van Pelt KL, Head E, Schmitt FA. Longitudinal assessment of dementia measures in Down syndrome. Alzheimer's Dement. 2020;12:e12075 10.1002/dad2.12075
Funding information
National Institute of Child Health and Human Development (HDR01064993); National Institute on Aging (P30 AG028383 & 1T32AG057461).
REFERENCES
- 1. Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev 2007;13(3):237‐246. 10.1002/mrdd.20163 [DOI] [PubMed] [Google Scholar]
- 2. Malamud N. Neuropathology of organic brain syndromes associated with aging In Aging and the Brain. Springer; 1972:63‐87. [Google Scholar]
- 3. Noetzel M. Dementia in Down Syndrome In Morris JC, ed. Handbook of Dementing Illnesses. New York: Marcel Dekker, Ink; 1994:243‐264. [Google Scholar]
- 4. Wisniewski K, Wisniewski H, Wen G. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278‐282. [DOI] [PubMed] [Google Scholar]
- 5. Bush A, Beail N. Risk factors for dementia in people with down syndrome: issues in assessment and diagnosis. Am J Ment Retard 2004;109(2):83‐97. [DOI] [PubMed] [Google Scholar]
- 6. Fuentes JJ, Genesca L, Kingsbury TJ, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin‐mediated signaling pathways. Hum Mol Genet 2000;9(11):1681‐1690. 10.1093/hmg/9.11.1681 [DOI] [PubMed] [Google Scholar]
- 7. Pucharcos C, Fuentes JJ, Casas C, et al. Alu‐splice cloning of human Intersectin (ITSN), a putative multivalent binding protein expressed in proliferating and differentiating neurons and overexpressed in Down syndrome. Eur J Hum Genet 1999;7(6):704‐712. 10.1038/sj.ejhg.5200356 [DOI] [PubMed] [Google Scholar]
- 8. Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep 2016;39(3):699‐704. 10.5665/sleep.5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zigman WB. Atypical aging in Down syndrome. Dev Disabil Res Rev 2013;18(1):51‐67. 10.1002/ddrr.1128 [DOI] [PubMed] [Google Scholar]
- 10. Baird PA, Sadovnick AD. Life expectancy in Down syndrome. J Pediatr 1987;110(6):849‐854. 10.1016/s0022-3476(87)80395-5 [DOI] [PubMed] [Google Scholar]
- 11. Presson AP, Partyka G, Jensen KM, et al. Current estimate of Down syndrome population prevalence in the United States. J Pediatr 2013;163(4):1163‐1168. 10.1016/j.jpeds.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zigman WB, Schupf N, Devenny DA, et al. Incidence and prevalence of dementia in elderly adults with mental retardation without down syndrome. Am J Ment Retard 2004;109(2):126‐141. [DOI] [PubMed] [Google Scholar]
- 13. Zigman WB, Silverman W, Wisniewski HM. Aging and Alzheimer's disease in Down syndrome: clinical and pathological changes. Dev Disabil Res Rev 1996;2(2):73‐79. [Google Scholar]
- 14. Holland AJ, Hon J, Huppert FA, Stevens F, Watson P. Population‐based study of the prevalence and presentation of dementia in adults with Down's syndrome. Br J Psychiatry 1998;172, 493‐498. 10.1192/bjp.172.6.493 [DOI] [PubMed] [Google Scholar]
- 15. Strydom A, Shooshtari S, Lee L, et al. Dementia in older adults with intellectual disabilities‐epidemiology, presentation, and diagnosis. J Policy Pract Intellect Disabil 2010;7(2):96‐110. [Google Scholar]
- 16. Urv TK, Zigman WB, Silverman W. Psychiatric symptoms in adults with Down syndrome and Alzheimer's disease. Am J Intellect Dev Disabil 2010;115(4):265‐276. 10.1352/1944-7558-115.4.265 [DOI] [PubMed] [Google Scholar]
- 17. Firth NC, Startin CM, Hithersay R, et al. Aging related cognitive changes associated with Alzheimer's disease in Down syndrome. Ann Clin Transl Neurol 2018;5(6):741‐751. 10.1002/acn3.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krinsky‐McHale SJ, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev 2013;18(1):31‐42. 10.1002/ddrr.1126 [DOI] [PubMed] [Google Scholar]
- 19. Nieuwenhuis‐Mark RE. Diagnosing Alzheimer's dementia in Down syndrome: problems and possible solutions. Res Dev Disabil 2009;30(5):827‐838. 10.1016/j.ridd.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 20. Walsh DM, Doran E, Silverman W, Tournay A, Movsesyan N, Lott IT. Rapid assessment of cognitive function in down syndrome across intellectual level and dementia status. J Intellect Disabil Res 2015;59(11):1071‐1079. 10.1111/jir.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dick MB, Doran E, Phelan M, Lott IT. Cognitive Profiles on the severe impairment battery are similar in Alzheimer disease and Down syndrome with dementia. Alzheimer Dis Assoc Disord 2016;30(3):251‐257. 10.1097/WAD.0000000000000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evenhuis HM. Further evaluation of the Dementia Questionnaire for Persons with Mental Retardation (DMR). J Intellect Disabil Res 1996;40 Pt 4):369‐373. [DOI] [PubMed] [Google Scholar]
- 23. Witts P, Elders S. The ‘Severe Impairment Battery’: assessing cognitive ability in adults with Down syndrome. Br J Clin Psychol 1998;37(Pt 2):213‐216. [DOI] [PubMed] [Google Scholar]
- 24. McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res 2014;58(1):61‐70. 10.1111/jir.12074 [DOI] [PubMed] [Google Scholar]
- 25. Burt DB, Aylward EH. Test battery for the diagnosis of dementia in individuals with intellectual disability. Working Group for the Establishment of Criteria for the Diagnosis of Dementia in Individuals with Intellectual Disability. J Intellect Disabil Res 2000;44(Pt 2):175‐180. 10.1046/j.1365-2788.2000.00264.x [DOI] [PubMed] [Google Scholar]
- 26. Dalton AJ. The Dyspraxia Scale for adults with Down syndrome In Prasher V, ed. Neuropsychological Assessments of Dementia in Down Syndrome and Intellectual Disabilities. London: Springer; 2008:67‐89. [Google Scholar]
- 27. Dalton AJ. Onset of dyspraxia in aging persons with Down syndrome: longitudinal studies. J Intellect Dev Disabil 1998;23(1):13‐24. 10.1080/13668259800033551 [DOI] [Google Scholar]
- 28. Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol 1994;51(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 29. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15(2):155‐163. doi:papers3://publication/doi/10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 31. Gamer M, Lemon J, Fellows I, Singh P. irr: Various Coefficients of Interrater Reliability and Agreement (Version 0.84.1). 2019. Available at https://CRAN.R-project.org/package=irr
- 32. Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59(1):12 10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- 33. Chelune GJ, Naugle RI, Lüders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: Practice effects and base‐rate information. Neuropsychology 1993;7(1):41 10.1037/0894-4105.7.1.41 [DOI] [Google Scholar]
- 34. Bates D, Machler M, Bolker B, Walker S. Fitting linear mixed‐effects models using {lme4}. J Stat Softw 2015;67(1):1‐48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 35. Kuznetsova A, Brockhoff PB, Christensen RHB. {lmerTest} Package: tests in linear mixed effects models. J Stat Softw 2017;82(13):1‐26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 36. Sano M, Aisen PS, Dalton AJ, Anderews HF, Tsai W, The International Down Syndrome and Alzheimer's Disease Consortium . Assessment of Aging Individuals with Down syndrome in clinical trials: results of baseline measures. J Policy Pract Intellect Disabil 2005;2(2):126‐138. [Google Scholar]
- 37. Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer's disease in Down syndrome. Alzheimers Dement 2019;15(2):245‐257. 10.1016/j.jalz.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Urv TK, Zigman WB, Silverman W. Maladaptive behaviors related to dementia status in adults with Down syndrome. Am J Ment Retard 2008;113(2):73‐86. 10.1352/0895-8017(2008)113[73:MBRTDS]2.0.CO: ;2 [DOI] [PubMed] [Google Scholar]
- 39. Startin CM, Lowe B, Hamburg S, Hithersay R, Strydom A, LonDown S, Consortium . Validating the Cognitive Scale for Down Syndrome (CS‐DS) to detect longitudinal cognitive decline in adults with Down syndrome. Front Psychiatry 2019;10:158 10.3389/fpsyt.2019.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]


