Key Points
Question
In patients with coronary artery disease, is adherence to recommended fractional flow reserve (FFR) thresholds for percutaneous coronary intervention (PCI) associated with better outcomes in clinical practice?
Findings
In this retrospective cohort study that used inverse probability of treatment weighting and included 9106 patients, PCI, as compared with no PCI, was significantly associated with a lower rate of major adverse cardiac events at 5 years among patients with ischemic FFR measurements (31.5% vs 39.1%; hazard ratio [HR], 0.77) and a higher rate of major adverse cardiac events at 5 years among patients with nonischemic FFR measurements (33.3% vs 24.4%; HR, 1.37).
Meaning
Performing PCI procedures in accordance to evidenced-based FFR thresholds was associated with better outcomes.
Abstract
Importance
Fractional flow reserve (FFR) is an invasive measurement used to assess the potential of a coronary stenosis to induce myocardial ischemia and guide decisions for percutaneous coronary intervention (PCI). It is not known whether established FFR thresholds for PCI are adhered to in routine interventional practice and whether adherence to these thresholds is associated with better clinical outcomes.
Objective
To assess the adherence to evidence-based FFR thresholds for PCI and its association with clinical outcomes.
Design, Setting, and Participants
A retrospective, multicenter, population-based cohort study of adults with coronary artery disease undergoing single-vessel FFR assessment (excluding ST-segment elevation myocardial infarction) from April 1, 2013, to March 31, 2018, in Ontario, Canada, and followed up until March 31, 2019, was conducted. Two separate cohorts were created based on FFR thresholds (≤0.80 as ischemic and >0.80 as nonischemic). Inverse probability of treatment weighting was used to account for treatment selection bias.
Exposures
PCI vs no PCI.
Main Outcomes and Measures
The primary outcome was major adverse cardiac events (MACE) defined by death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Results
There were 9106 patients (mean [SD] age, 65 [10.6] years; 35.3% female) who underwent single-vessel FFR measurement. Among 2693 patients with an ischemic FFR, 75.3% received PCI and 24.7% were treated only with medical therapy. In the ischemic FFR cohort, PCI was associated with a significantly lower rate and hazard of MACE at 5 years compared with no PCI (31.5% vs 39.1%; hazard ratio, 0.77 [95% CI, 0.63-0.94]). Among 6413 patients with a nonischemic FFR, 12.6% received PCI and 87.4% were treated with medical therapy only. PCI was associated with a significantly higher rate and hazard of MACE at 5 years compared with no PCI (33.3% vs 24.4%; HR, 1.37 [95% CI, 1.14-1.65]) in this cohort.
Conclusions and Relevance
Among patients with coronary artery disease who underwent single-vessel FFR measurement in routine clinical practice, performing PCI, compared with not performing PCI, was significantly associated with a lower rate of MACE for ischemic lesions and a higher rate of MACE for nonischemic lesions. These findings support the performance of PCI procedures according to evidence-based FFR thresholds.
This cohort study assesses adherence to evidence-based fractional flow reserve (FFR) thresholds for percutaneous coronary intervention in Canada between 2013 and 2018, and associations of percutaneous coronary intervention with major adverse clinical outcomes for patients with FFR measures above and beneath ischemic thresholds.
Introduction
Fractional flow reserve (FFR) is an invasive measurement used during coronary angiography to assess the potential of a coronary stenosis to induce myocardial ischemia.1 Clinical trials have shown improved clinical outcomes when ischemic lesions (FFR ≤0.80) are treated by percutaneous coronary intervention (PCI) compared with medical therapy alone.2,3,4 Based on these data, PCI is generally recommended for lesions with ischemic FFR values. In contrast, PCI is not recommended for nonischemic FFR lesions (FFR >0.80) because it has not been demonstrated to be beneficial and may even be harmful.5,6,7,8
While studies have shown that FFR use is increasing rapidly in interventional practice,9,10,11 few have evaluated whether clinicians are adhering to recommended FFR thresholds. Data from selected registries suggest that up to 30% of patients with an ischemic FFR did not receive PCI and approximately 5% of patients received PCI even with a nonischemic FFR.12,13 Whether these observations could be extended to routine clinical practice is uncertain. Equally unknown is whether adherence to FFR thresholds is associated with improved clinical outcomes in routine clinical practice. Accordingly, the objective of this study was to use a population-based sample to construct a cohort of patients with ischemic FFR values as well as a separate cohort with nonischemic FFR values. In each cohort, utilization of PCI and its relationship with subsequent adverse cardiovascular outcomes was examined.
Methods
Data Sources
ICES (formerly known as the Institute for Clinical Evaluative Sciences) is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. The CorHealth Ontario Cardiac Registry is a prospective, provincial database that collects clinical, laboratory, and procedural characteristics on all adult patients undergoing cardiac catheterization and PCI in the province.14 The CorHealth database was linked to the following databases using unique encoded identifiers and analyzed at ICES: (1) Registered Persons Database, which contains sociodemographic and vital statistics data, and (2) the Canadian Institute for Health Information (CIHI) Discharge Abstract Database, which contains complete provincialwide capture of hospitalization events.
Study Population
Adult patients (aged >18 years) undergoing coronary angiography in Ontario, Canada, between April 1, 2013, and March 31, 2018, were identified. Exclusions included patients who received cardiac catheterization for aortic stenosis, congenital heart disease, or cardiac transplants or who underwent cardiac biopsy and donor transplant, given that FFR measurements have not been validated in these populations. Patients with ST-elevation myocardial infarction (MI), multivessel FFR, or PCI that was performed in a non-FFR artery were also excluded because of the inability to distinguish clinical outcomes related to the vessel that was assessed by FFR. Prior coronary artery bypass grafting (CABG) was also excluded because FFR measurements may be confounded by native artery patency.15 Implausible FFR values (<0.30 and ≥1.00) were also excluded.16 FFR measurements performed for left main lesions were excluded because of the lack of data on intracoronary imaging. Because elective CABG could not be ascertained after FFR assessments, patients who received CABG within 6 months after the procedure were excluded. Subsequently, 2 separate cohorts of patients, one with ischemic FFR values (FFR ≤0.80) and another with nonischemic FFR values (FFR >0.80), were created for analysis.
Exposures
The main exposure variable was PCI vs no PCI at the time the coronary vessel was assessed by FFR.
Outcomes
The primary outcome was a major adverse cardiac event (MACE), ascertained from the date of FFR measurement. MACE was defined using a similar definition as the Fractional Flow Reserve vs Angiography for Multivessel Evaluation 2 (FAME-2) trial.2 This was the first occurrence of (1) all-cause death, (2) hospitalization for MI, (3) hospitalization for unstable angina, or (4) urgent coronary revascularization with PCI or CABG. Death was ascertained from the Registered Persons Database, and nonfatal outcomes were from the CIHI Discharge Abstract Database.17 Urgent coronary revascularization was defined as unplanned hospitalization with PCI or CABG occurring during the same hospitalization. In secondary analyses, the association between PCI with each component of the primary outcome was determined. All outcomes were ascertained up to March 31, 2019.
Statistical Analysis
The ischemic and nonischemic cohorts were analyzed separately. In patients with an ischemic FFR, baseline characteristics were compared between patients receiving and not receiving PCI using standardized differences. A propensity score for receiving PCI was estimated using a logistic regression model. Covariates included in the model were demographics (age and sex), preexisting comorbidities (MI, prior PCI, heart failure, diabetes, cerebrovascular disease, peripheral vascular disease, hypertension, hyperlipidemia, kidney disease, dialysis, chronic obstructive pulmonary disease, smoking status, and the Charlson Comorbidity Index score), investigations (serum creatinine and left ventricular ejection fraction), symptom status (Canadian Cardiovascular Society Angina Classification at referral), and procedure-related characteristics (the number of major diseased vessels with visual stenosis ≥70% and the artery undergoing FFR assessment). Variables with missing data were assigned a category in order to be incorporated into the propensity score. Inverse probability of treatment weighting (IPTW) was used to balance differences in baseline characteristics between patients receiving and not receiving PCI.18 The PCI and no PCI groups were weighted by the stabilized inverse probability of receiving and not receiving PCI, respectively. Weighted standardized differences were used to assess the balance of baseline covariates between the PCI and no PCI groups in the weighted cohort. A standardized difference of less than 0.10 indicated good balance.19
The incidences of MACE and all-cause mortality were estimated using weighted Kaplan-Meier curves.20 The association between PCI and the hazards of MACE and all-cause mortality was estimated using weighted Cox proportional hazards models.21 For MI, unstable angina, and urgent revascularization outcomes, death served as a competing risk. Therefore, the incidence of each outcome was estimated using weighted cumulative incidence curves. Furthermore, the association between PCI with the hazards and cumulative incidence of these outcomes was modeled using cause-specific proportional hazards models as well as Fine-Gray proportional subdistributions hazards model.22,23 The proportionality assumption was verified by testing for an interaction between the exposure variable and time. The stabilized IPTWs were incorporated into the regression models without trimming.24 A robust variance estimator was used to estimate the standard error for all weighted regression models.25 Effect estimates from Cox models were reported as hazard ratios (HRs) while those from Fine-Gray models were reported as subdistribution HRs (sHRs) along with 95% CIs. Furthermore, risk differences (RDs) were estimated from the weighted cumulative incidences curves with 95% CIs derived from 2000 bootstrap resamples. The same analyses were repeated in patients with nonischemic FFR values.
A series of sensitivity analyses were undertaken to test the robustness of the results. First, because clinical guidelines provide no recommendations for FFR in patients with MI, the same steps were repeated for each cohort after excluding patients presenting with acute MI. Second, the propensity score was modified to incorporate hospital-specific random effects to account for potential variation in the propensity to receive PCI at each center and the analysis was repeated using overlap weighting rather than conventional IPTW.26 Third, multiple imputation was used to impute missing values for serum creatinine and left ventricular ejection fraction in 30 data sets. The IPTW analysis was repeated in each data set and the results were pooled using Rubin rules.27 Fourth, the analysis was repeated after excluding patients who underwent CABG within 1 month and within 3 months after FFR assessment, rather than 6 months. Fifth, due to the imbalances of the number of diseased vessels prior to propensity weighting, a post hoc analysis was performed to examine outcome difference in PCI and non-PCI patients stratified by number of diseased vessels with stenosis of 70% or greater.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute). A 2-sided P < .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
Study Cohorts
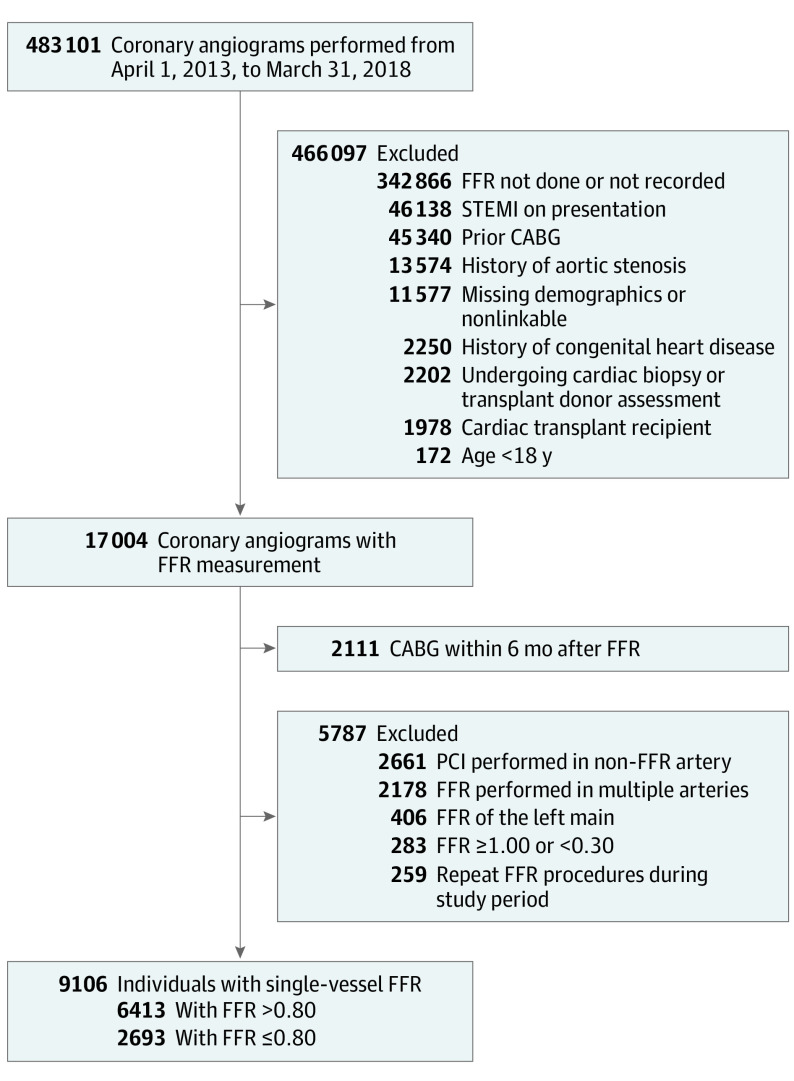
There were 9106 unique patients (29.6% with ischemic FFR and 70.4% with nonischemic FFR values) undergoing single-vessel non–left main FFR during the study period. Details of the cohort creation are shown in Figure 1. The median FFR value in these patients was 0.86 (interquartile range [IQR], 0.79-0.91), and the distribution of all FFR values is depicted in eFigure 1 in the Supplement.
Figure 1. Cohort Creation.
Selection of patients undergoing single-vessel fractional flow reserve (FFR) of non–left main lesions between April 1, 2013, and March 31, 2018, is presented. The final cohort contained 9106 unique individuals. CABG indicates coronary artery bypass grafting; PCI, percutaneous coronary intervention; and STEMI, ST-elevation myocardial infarction.
Ischemic FFR
Baseline Characteristics of Patients With Ischemic FFR
There were 2693 patients with ischemic FFR measurements. Before weighting, the mean age was 65 years and 27.0% were female (eTable 1 in the Supplement). The median FFR was 0.76 (IQR, 0.72-0.79). There were 2029 patients (75.3%) with ischemic FFRs who received PCI. A greater proportion of patients who received PCI had prior PCI procedures (36.6% vs 27.4%) or 1-vessel disease (51.3% vs 28.3%) compared with patients who did not receive PCI. In contrast, a lower proportion of patients who received PCI presented with acute MI (13.1% vs 16.4%), 2-vessel disease (18.8% vs 28.2%), and 3-vessel disease (4.6% vs 18.7%). After IPTW, all characteristics of patients who received PCI and did not receive PCI were well balanced with standardized differences less than 0.10 (Table 1; eFigure 2 in the Supplement).
Table 1. Baseline Characteristics After Propensity Score Weightinga.
| Baseline characteristics | Ischemic fractional flow reserve (≤0.80) | Nonischemic fractional flow reserve (>0.80) | ||||
|---|---|---|---|---|---|---|
| No PCI, % (n = 674) | PCI, % (n = 2022) | Standardized difference | No PCI, % (n = 5604) | PCI, % (n = 817) | Standardized difference | |
| Demographics | ||||||
| Age, mean (SD), y | 65.7 (10.4) | 64.9 (10.7) | 0.072 | 65.9 (10.7) | 66.4 (10.0) | 0.046 |
| Sex | ||||||
| Male | 74.0 | 73.2 | 0.019 | 61.2 | 65.2 | 0.084 |
| Female | 26.0 | 26.8 | 0.019 | 38.8 | 34.8 | 0.084 |
| Index presentation | ||||||
| Stable coronary artery disease | 62.1 | 63.3 | 0.025 | 63.6 | 65.3 | 0.035 |
| Unstable angina | 23.7 | 21.8 | 0.045 | 22.2 | 22.0 | 0.005 |
| Non-ST elevation myocardial infarction | 13.1 | 13.8 | 0.023 | 13.4 | 11.5 | 0.059 |
| Preexisting comorbidities | ||||||
| Hypertension | 79.4 | 79.8 | 0.009 | 78.9 | 80.1 | 0.030 |
| Hyperlipidemia | 75.5 | 76.0 | 0.011 | 74.9 | 76.3 | 0.033 |
| Myocardial infarction | 39.3 | 40.3 | 0.020 | 35.8 | 37.7 | 0.040 |
| Diabetes | 38.2 | 38.6 | 0.008 | 35.7 | 36.3 | 0.013 |
| Percutaneous coronary intervention | 34.7 | 34.7 | 0.001 | 34.4 | 37.0 | 0.054 |
| Smoking | ||||||
| Former | 31.1 | 30.1 | 0.023 | 31.2 | 32.4 | 0.026 |
| Current | 19.2 | 20.5 | 0.032 | 16.0 | 15.8 | 0.004 |
| Heart failure | 13.7 | 13.2 | 0.013 | 11.4 | 12.2 | 0.025 |
| Chronic obstructive pulmonary disease | 11.6 | 11.5 | 0.003 | 11.0 | 10.1 | 0.030 |
| Cerebrovascular disease | 8.1 | 8.4 | 0.011 | 7.4 | 7.0 | 0.014 |
| Peripheral vascular disease | 6.2 | 6.0 | 0.007 | 5.5 | 5.3 | 0.007 |
| Kidney disease | 2.8 | 2.7 | 0.007 | 3.0 | 4.7 | 0.090 |
| Dialysis | 2.1 | 2.0 | 0.009 | 2.0 | 2.1 | 0.006 |
| Charlson Comorbidity Index score, mean (SD) | 1.6 (1.7) | 1.6 (1.7) | 0.001 | 1.4 (1.6) | 1.5 (1.5) | 0.043 |
| Investigations | ||||||
| Serum creatinine, mg/dLb | ||||||
| ≤1.36 | 82.3 | 82.7 | 0.012 | 84.3 | 82.6 | 0.046 |
| 1.37 to 2.04 | 7.3 | 7.0 | 0.011 | 5.7 | 7.2 | 0.062 |
| >2.04 | 2.4 | 2.4 | 0.005 | 2.2 | 2.3 | 0.006 |
| Left ventricular ejection fractionc | ||||||
| ≥50% | 49.9 | 50.5 | 0.011 | 55.6 | 55.2 | 0.007 |
| 35%-49% | 11.2 | 10.9 | 0.009 | 9.6 | 11.1 | 0.050 |
| 20%-34% | 4.0 | 4.2 | 0.009 | 3.7 | 4.9 | 0.060 |
| <20% | 1.1 | 0.9 | 0.027 | 0.7 | 1.0 | 0.030 |
| Symptom status | ||||||
| Canadian Cardiovascular Society Classd | ||||||
| 0 (least impaired) | 15.5 | 15.6 | 0.004 | 15.8 | 15.6 | 0.007 |
| 1 | 13.8 | 14.0 | 0.005 | 13.0 | 12.8 | 0.006 |
| 2 | 26.6 | 26.2 | 0.009 | 26.9 | 25.7 | 0.027 |
| 3 | 18.6 | 17.2 | 0.035 | 15.6 | 17.8 | 0.060 |
| 4 (most impaired) | 25.6 | 27.0 | 0.031 | 28.7 | 28.2 | 0.032 |
| Interventional characteristics | ||||||
| Fractional flow reserve | ||||||
| Mean (SD)e | 0.76 (0.05) | 0.74 (0.06) | 0.237 | 0.89 (0.05) | 0.87 (0.05) | 0.418 |
| Median (IQR) | 0.77 (0.74-0.80) | 0.76 (0.72-0.78) | 0.89 (0.85-0.93) | 0.86 (0.83-0.90) | ||
| Vessels with stenosis ≥70% | ||||||
| 0 | 25.6 | 25.4 | 0.005 | 65.6 | 65.7 | 0.002 |
| 1 | 46.0 | 45.7 | 0.004 | 23.0 | 22.9 | 0.002 |
| 2 | 20.7 | 21.1 | 0.010 | 8.5 | 8.7 | 0.004 |
| 3 | 7.8 | 7.8 | 0.001 | 2.8 | 2.7 | 0.009 |
| Fractional flow reserve artery | ||||||
| Left anterior descending | 80.0 | 81.4 | 0.037 | 61.5 | 60.8 | 0.014 |
| Right coronary | 12.5 | 11.9 | 0.020 | 21.0 | 21.2 | 0.006 |
| Left circumflex | 7.5 | 6.7 | 0.030 | 17.5 | 18.0 | 0.012 |
Abbreviations: IQR, interquartile range; PCI, percutaneous coronary intervention.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Sample sizes in each group are estimated after inverse probability of treatment weighting.
Serum creatinine missing in 7.9% and 7.8% of patients with ischemic and nonischemic fractional flow reserve, respectively.
Left ventricular ejection fraction missing in 33.4% and 29.0% of patients with ischemic and nonischemic fractional flow reserve, respectively.
The Canadian Cardiovascular Society classification is a score used to grade the severity of angina, with 0 indicating least impaired (no angina) and 4 indicating most impaired (angina at rest).
The distribution of fractional flow reserve measurements in patients who received and did not receive PCI are depicted in eFigure 1 in the Supplement.
Outcomes After PCI of Patients With Ischemic FFR
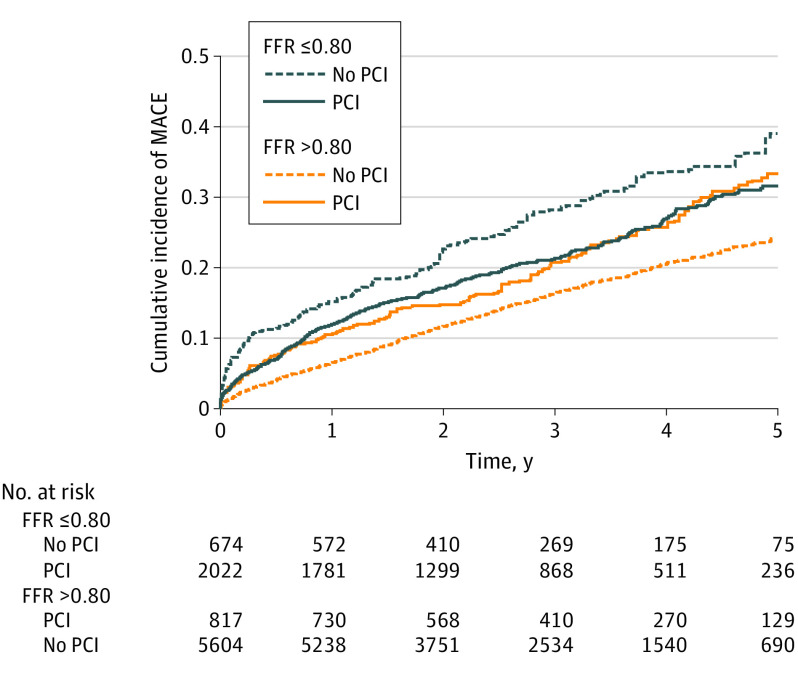
The incidence and association between PCI and outcomes are presented in Table 2. At 5 years, the incidence of MACE was 31.5% in the PCI group and 39.1% in the no PCI group (RD, −7.6% [95% CI, −15.0% to 0%]). PCI was associated with a significantly lower hazard of MACE at 5 years (HR, 0.77 [95% CI, 0.63-0.94]; Figure 2). When analyzed separately, the incidence and hazard of death (16.8% vs 20.6%; HR, 0.77 [95% CI, 0.57-1.04]), MI (8.7% vs 10.8%; HR, 0.92 [95% CI, 0.64-1.31]), unstable angina (9.9% vs 11.2%; HR, 0.81 [95% CI, 0.58-1.13]), and urgent coronary revascularization (6.2% vs 9.5%; HR, 0.71 [95% CI, 0.46-1.08]) were not statistically significantly different, although the point estimates generally favored the PCI group. PCI was also associated with a significantly lower hazard of MACE in the initial 30 days (2.8% vs 6.0%; HR, 0.47 [95% CI, 0.30-0.75]) and at 1 year (11.9% vs 15.2%; HR, 0.76 [95% CI, 0.58-0.99]) when compared with no PCI. Using the Fine-Gray model to assess the association of PCI with the incidence of nonfatal outcomes yielded similar results (eTable 2 in the Supplement).
Table 2. Outcomes of Patients With Ischemic Fractional Flow Reserve (≤0.80) After Propensity Score Weighting.
| Outcome | Follow-up time | No PCI, % [Reference] | PCI, % | Risk difference (95% CI)a | Hazard ratio (95% CI)b |
|---|---|---|---|---|---|
| Primary outcome | |||||
| MACEc | 30 d | 6.0 | 2.8 | −3.2 (−5.5 to −1.2) | 0.47 (0.30 to 0.75) |
| 1 y | 15.2 | 11.9 | −3.3 (−6.6 to 0.0) | 0.76 (0.58 to 0.99) | |
| 5 y | 39.1 | 31.5 | −7.6 (−15.0 to 0.0) | 0.77 (0.63 to 0.94) | |
| Secondary outcomes | |||||
| Death | 30 d | 1.2 | 0.6 | −0.6 (−1.6 to 0.2) | 0.45 (0.18 to 1.13) |
| 1 y | 4.7 | 4.1 | −0.6 (−2.4 to 1.2) | 0.87 (0.57 to 1.33) | |
| 5 y | 20.6 | 16.8 | −3.8 (−9.9 to 1.9) | 0.77 (0.57 to 1.04) | |
| Myocardial infarction | 30 d | 1.6 | 1.4 | −0.2 (−1.3 to 0.7) | 0.87 (0.44 to 1.72) |
| 1 y | 3.1 | 3.7 | 0.6 (−0.9 to 2.0) | 1.17 (0.73 to 1.86) | |
| 5 y | 10.8 | 8.7 | −2.1 (−7.8 to 2.4) | 0.92 (0.64 to 1.31) | |
| Unstable angina | 30 d | 2.6 | 1.0 | −1.6 (−3.2 to −0.3) | 0.36 (0.18 to 0.74) |
| 1 y | 6.6 | 4.5 | −2.1 (−4.5 to 0.2) | 0.67 (0.44 to 1.02) | |
| 5 y | 11.2 | 9.9 | −1.3 (−4.8 to 2.1) | 0.81 (0.58 to 1.13) | |
| Urgent coronary revascularizationd | 30 d | 1.8 | 0.2 | −1.6 (−3.1 to −0.5) | 0.13 (0.04 to 0.45) |
| 1 y | 4.2 | 2.5 | −1.7 (−3.9 to 0.0) | 0.57 (0.33 to 0.97) | |
| 5 y | 9.5 | 6.2 | −3.3 (−9.1 to 1.2) | 0.71 (0.46 to 1.08) | |
Abbreviations: MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
Risk differences were estimated from weighted cumulative incidence curves along with 95% CIs generated in 2000 bootstrap resamples.
Hazard ratios and 95% CIs were estimated from weighted cause-specific proportional hazards models.
MACE was defined by death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Urgent coronary revascularization was defined as unplanned hospitalization with percutaneous coronary intervention or coronary artery bypass grafting occurring during the same hospitalization.
Figure 2. Major Adverse Cardiac Events After Percutaneous Coronary Intervention (PCI).
The cumulative incidence of major adverse cardiac events in the propensity-weighted cohorts of patients is overlaid and depicted. Curves depict the incidence of events in patients with an ischemic fractional flow reserve (FFR) who had PCI (median follow-up, 3.08 years; interquartile range [IQR], 1.96-4.34) and did not have PCI (median follow-up, 2.77 years; IQR, 1.72-4.15) and with a nonischemic FFR who had PCI (median follow-up, 3.41 years; IQR, 2.18-4.73) and did not have PCI (median follow-up, 2.98 years; IQR, 1.87-4.31). The number at risk in the weighted samples is depicted for each group. Major adverse cardiac events were defined by death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Nonischemic FFR
Baseline Characteristics of Patients With Nonischemic FFR
There were 6413 patients with nonischemic FFR measurements. Before weighting, the mean age was 66 years and 38.9% were female (eTable 1 in the Supplement). The median FFR was 0.89 (IQR, 0.85-0.92). There were 810 patients (12.6%) who received PCI with nonischemic FFR measurements. A higher proportion of patients who received PCI presented with acute MI (18.6% vs 12.7%), as well as 1-vessel (43.6% vs 20.0%), 2-vessel (14.2% vs 7.7%), and 3-vessel (4.3% vs 2.6%) disease compared with patients who did not receive PCI. After IPTW, standardized differences between characteristics in patients who did and did not receive PCI were less than 0.10 (Table 1; eFigure 3 in the Supplement).
Outcomes After PCI of Patients With Nonischemic FFR
The incidence and association between PCI and outcomes for the nonischemic cohort are presented in Table 3. The 5-year incidence of MACE was 33.3% in patients who received PCI and 24.4% in patients who did not (RD, 8.9% [95% CI, 3.1%-14.7%]). PCI of a nonischemic lesion was associated with a significantly higher hazard of MACE at 5 years (HR, 1.37 [95% CI, 1.14-1.65]; Figure 2). When analyzed separately, there was no statistically significant association between PCI and death at 5 years (15.0% vs 13.9%; HR, 0.94 [95% CI, 0.68-1.29]). However, PCI was associated with a significantly higher incidence and hazard of MI (8.5% vs 5.0%; HR, 1.69 [95% CI, 1.20-2.37]), unstable angina (13.4% vs 7.8%; HR, 1.78 [95% CI, 1.35-2.35]), and urgent coronary revascularization (7.3% vs 3.9%; HR, 1.76 [95% CI, 1.21-2.57]). Similarly, PCI was associated with a significantly higher hazard of MACE at 30 days (3.1% vs 1.5%; HR, 2.11 [95% CI, 1.26-3.54]) and 1 year (10.6% vs 6.5%; HR, 1.67 [95% CI, 1.27-2.21]) when compared with no PCI. The association of PCI with the incidence of nonfatal outcomes was consistent when using the Fine-Gray model (eTable 2 in the Supplement).
Table 3. Outcomes of Patients With Nonischemic Fractional Flow Reserve (>0.80) After Propensity Score Weighting.
| Outcome | Follow-up time | No PCI, % [Reference] | PCI, % | Risk difference (95% CI)a | Hazard ratio (95% CI)b |
|---|---|---|---|---|---|
| Primary outcome | |||||
| MACEc | 30 d | 1.5 | 3.1 | 1.6 (0.3 to 3.1) | 2.11 (1.26 to 3.54) |
| 1 y | 6.5 | 10.6 | 4.1 (1.7 to 6.7) | 1.67 (1.27 to 2.21) | |
| 5 y | 24.4 | 33.3 | 8.9 (3.1 to 14.7) | 1.37 (1.14 to 1.65) | |
| Secondary outcomes | |||||
| Death | 30 d | 0.4 | 0.2 | −0.2 (−0.5 to 0.0) | 0.41 (0.12 to 1.42) |
| 1 y | 2.5 | 2.1 | −0.4 (−1.6 to 1.1) | 0.84 (0.44 to 1.61) | |
| 5 y | 13.9 | 15.0 | 1.1 (−3.3 to 5.9) | 0.94 (0.68 to 1.29) | |
| Myocardial infarction | 30 d | 0.6 | 2.0 | 1.4 (0.3 to 2.7) | 3.56 (1.70 to 7.46) |
| 1 y | 1.8 | 3.5 | 1.7 (0.4 to 3.4) | 2.05 (1.26 to 3.32) | |
| 5 y | 5.0 | 8.5 | 3.5 (0.8 to 6.3) | 1.69 (1.20 to 2.37) | |
| Unstable angina | 30 d | 0.5 | 1.4 | 0.9 (0.0 to 2.1) | 3.02 (1.26 to 7.24) |
| 1 y | 2.4 | 5.7 | 3.3 (1.4 to 5.4) | 2.40 (1.63 to 3.52) | |
| 5 y | 7.8 | 13.4 | 5.6 (2.1 to 9.7) | 1.78 (1.35 to 2.35) | |
| Urgent coronary revascularizationd | 30 d | 0.2 | 0.2 | 0 (−0.3 to 0.3) | 0.97 (0.27 to 3.45) |
| 1 y | 1.1 | 2.4 | 1.3 (0.2 to 2.5) | 2.20 (1.29 to 3.74) | |
| 5 y | 3.9 | 7.3 | 3.4 (0.6 to 6.2) | 1.76 (1.21 to 2.57) | |
Abbreviations: MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
Risk differences were estimated from weighted cumulative incidence curves along with 95% CIs generated in 2000 bootstrap resamples.
Hazard ratios and 95% CIs were estimated from weighted cause-specific proportional hazards models.
MACE was defined by death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Urgent coronary revascularization was defined as unplanned hospitalization with percutaneous coronary intervention or coronary artery bypass grafting occurring during the same hospitalization.
Sensitivity Analysis
After excluding 1385 patients who presented with acute MI, there were 2261 patients with ischemic FFR measurements and 5460 with nonischemic FFR measurements (eTable 3 in the Supplement). PCI was associated with a significantly lower hazard of MACE at 5 years (29.5% vs 34.3%; HR, 0.78 [95% CI, 0.62-0.99]) in comparison with no PCI in the ischemic cohort, but a significantly higher hazard of MACE at 5 years in the nonischemic cohort (30.2% vs 23.6%; HR, 1.30 [95% CI, 1.05-1.62]; eTable 4 in the Supplement). Results also remained similar when using overlap weights and incorporating a hospital-specific random effects term (eTable 5 in the Supplement), when the 7.8% of missing values for serum creatinine and 27.8% for left ventricular ejection fraction were imputed (eTable 6 in the Supplement), when the timing of patients undergoing CABG who were excluded was altered to 1 month and 3 months after FFR assessment (eTable 7 in the Supplement), and when patients were stratified by the number of diseased vessels (eTable 8 in the Supplement).
Discussion
In this population-based study of patients with coronary artery disease undergoing FFR measurement for single-vessel, non–left main disease in routine clinical practice, some patients did not receive PCI in lesions that had ischemic FFR values, while others received PCI in lesions that were considered as nonischemic. PCI, compared with no PCI, was significantly associated with better clinical outcomes in ischemic lesions and worse outcomes in nonischemic lesions. These findings support the performance of PCI procedures according to evidence-based FFR thresholds.
In this study, approximately 25% of patients with ischemic FFR did not undergo PCI, in keeping with a prior study demonstrating that revascularization is underutilized in about 30% of patients with appropriate indications.28 While this study did not evaluate reasons explaining the underuse of PCI, a prospective registry found that an absence of symptoms, negative noninvasive studies, or diffuse disease without focal stenosis are potential factors.12 Other reasons may include patient characteristics such as old age, multiple competing comorbidities, patient preferences, or even physician factors.
In the recently published International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial,29 patients with stable coronary artery disease and moderate to severe ischemia were randomized to a conservative or an invasive strategy, and no significant difference in adverse cardiovascular events was demonstrated between the 2 treatments. While this study suggesting benefits associated with PCI may appear at odds with the results of ISCHEMIA, several differences between the studies should be noted. First, in the ISCHEMIA trial, 76% of patients had multivessel disease, 25% of those randomized to the invasive strategy underwent CABG, and only 20% had revascularization guided by FFR. In contrast, patients underwent PCI exclusively in the present study and all patients had revascularization guided by FFR of a single vessel. Second, the early hazards associated with revascularization in ISCHEMIA was driven by periprocedural MI.29 In contrast, this study captured clinical events and only included repeat hospitalization for MI after the index procedure.
The frequency of PCI for nonischemic lesions observed in this study was higher than other registries.12,13 For instance, 6% of PCI procedures were performed in nonischemic lesions in pooled registries from Europe13 and 4% of patients in a South Korean registry.12 Why clinicians may opt to perform PCI for nonischemic lesions is not clear. First, because FFR is a continuous measurement with a continuous risk spectrum,30 clinicians may feel inclined to perform PCI at borderline FFR values (0.81-0.85). Second, it is also possible that clinicians have a higher propensity to perform PCI among patients who have positive noninvasive stress tests or those with anginal symptoms despite a negative FFR. Regardless of the reason, this study suggests that deviating from accepted FFR thresholds is more common in routine clinical practice than previously reported from registries.
The results of this study are in line with registries that have reported on the risks of performing PCI for nonischemic FFR values.12,13 However, in these registries, the association between PCI and components of MACE, particularly MI, were not reported13 and few patients had FFR values greater than 0.85.12 They are also in keeping with the concerns for harm raised by extended follow-up at 15 years of a clinical trial that a higher risk of late MI was detected after PCI for nonischemic lesions.31,32 These results, therefore, support current class III recommendations against revascularization in the absence of ischemia and suggest clinicians avoid performing PCI for nonischemic lesions.7,8,33
Limitations
This study has several limitations. First, observational studies evaluating clinical effectiveness of PCI are potentially subjected to selection bias and, therefore, should not be used to replace findings from well-conducted randomized studies.12 Even though balance was achieved in each cohort by IPTW, and sensitivity analyses were performed to ensure the results were robust, it is still possible that patients selected for PCI differed in terms of risk compared with patients not receiving PCI. Yet, the observation of worse outcomes among patients with nonischemic FFR who received PCI was unlikely due to selection of healthier patients for treatment. Furthermore, better outcomes observed among patients with ischemic FFR who received PCI was similar to the FAME studies.2,32
Second, FFR measurements were not reviewed in a core laboratory. Interpretation was left to the discretion of the operator, which is reflective of real-world clinical practice.
Third, this study excluded patients who had CABG within 6 months after the index FFR because of the intention to evaluate whether adherence to FFR thresholds is associated with better outcomes with PCI. In sensitivity analysis, results remained consistent after excluding CABG within 1 month and 3 months after FFR.
Fourth, periprocedural MI defined by troponin elevations early after PCI could not be ascertained in this cohort. However, outcome definitions included new hospitalization events, which remain clinically relevant.
Fifth, anatomic data on nonobstructive lesions (ie, stenosis 20%-69%) were not available. Instead, IPTW was performed using obstructive coronary artery disease (stenosis ≥70%), which has been shown to have the greatest prognostic association.34
Sixth, information on whether patients had a staged PCI or PCI due to clinical deterioration after the index FFR procedure was not available. Thus, a more conservative analytical approach was used by including patients who did not receive PCI during the index FFR in the no PCI group, which would tend to bias toward the null for effective treatment.
Seventh, patients with ST-elevation MI as well as patients undergoing multivessel PCIs, left main FFR, and CABG were excluded analogous to randomized studies of FFR. Accordingly, the findings should not be generalized to patients not evaluated in this study.
Conclusions
Among patients with coronary artery disease who underwent single-vessel FFR measurement in routine clinical practice, performing PCI, compared with not performing PCI, was significantly associated with a lower rate of MACE for ischemic lesions and a higher rate of MACE for nonischemic lesions. These findings support the performance of PCI procedures according to evidence-based FFR thresholds.
eTable 1. Baseline Characteristics Before Propensity Score Weighting
eTable 2. Association Between Percutaneous Coronary Intervention and the Incidence of Non-fatal Outcomes After Propensity Score Weighting
eTable 3. Outcomes of Patients Presenting With Ischemic Fractional Flow Reserve (≤ 0.80) and Without Myocardial Infarction After Propensity Score Weighting
eTable 4. Outcomes of Patients Presenting With Non-ischemic Fractional Flow Reserve (> 0.80) and Without Myocardial Infarction After Propensity Score Weighting
eTable 5. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve With a Multi-level Propensity Score Accounting for Hospital Site
eTable 6. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve After Multiple Imputation for Missing Serum Creatinine and Left Ventricular Ejection Fraction
eTable 7. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve After Excluding Coronary Artery Bypass Grafting After Index Procedure
eTable 8. Outcomes in Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve Stratified by the Number of Diseased Coronary Artery Disease Vessels
eFigure 1. Distribution of Fractional Flow Reserve Measurements
eFigure 2. Standardized Differences Before and After Propensity Weighting in Patients With Ischemic FFR
eFigure 3. Standardized Differences Before and After Propensity Weighting in Patients With Non-ischemic FFR
References
- 1.Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703-1708. doi: 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 2.De Bruyne B, Fearon WF, Pijls NH, et al. ; FAME 2 Trial Investigators . Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208-1217. doi: 10.1056/NEJMoa1408758 [DOI] [PubMed] [Google Scholar]
- 3.Smits PC, Abdel-Wahab M, Neumann FJ, et al. ; Compare-Acute Investigators . Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234-1244. doi: 10.1056/NEJMoa1701067 [DOI] [PubMed] [Google Scholar]
- 4.Engstrøm T, Kelbæk H, Helqvist S, et al. ; DANAMI-3—PRIMULTI Investigators . Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671. doi: 10.1016/S0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Pursnani S, Kumar S, Bagos PG. Percutaneous coronary intervention versus optimal medical therapy for prevention of spontaneous myocardial infarction in subjects with stable ischemic heart disease. Circulation. 2013;127(7):769-781. doi: 10.1161/CIRCULATIONAHA.112.131961 [DOI] [PubMed] [Google Scholar]
- 6.Bech GJ, De Bruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928-2934. doi: 10.1161/01.CIR.103.24.2928 [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(17):2212-2241. doi: 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi: 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Desai NR, Bradley SM, Parzynski CS, et al. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA. 2015;314(19):2045-2053. doi: 10.1001/jama.2015.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh RV, Liu G, Plomondon ME, et al. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. 2020;75(4):409-419. doi: 10.1016/j.jacc.2019.10.060 [DOI] [PubMed] [Google Scholar]
- 11.Elbaz-Greener G, Masih S, Fang J, Roifman I, Wijeysundera HC. Temporal trends in fractional flow reserve use in patients undergoing coronary angiography: a population-based study. CJC Open. 2019;1(1):10-18. doi: 10.1016/j.cjco.2018.11.004 [DOI] [Google Scholar]
- 12.Ahn JM, Park DW, Shin ES, et al. ; IRIS-FFR Investigators . Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017;135(23):2241-2251. doi: 10.1161/CIRCULATIONAHA.116.024433 [DOI] [PubMed] [Google Scholar]
- 13.Van Belle E, Rioufol G, Pouillot C, et al. ; Investigators of the Registre Français de la FFR–R3F . Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation. 2014;129(2):173-185. doi: 10.1161/CIRCULATIONAHA.113.006646 [DOI] [PubMed] [Google Scholar]
- 14.Madan M, Bagai A, Overgaard CB, et al. Same-day discharge after elective percutaneous coronary interventions in Ontario, Canada. J Am Heart Assoc. 2019;8(13):e012131. doi: 10.1161/JAHA.119.012131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pijls NH, Botman KJ. Functional assessment of bypass grafts by fractional flow reserve. Eur J Cardiothorac Surg. 2007;31(3):381-382. doi: 10.1016/j.ejcts.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve: a useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183-3193. doi: 10.1161/01.CIR.92.11.3183 [DOI] [PubMed] [Google Scholar]
- 17.Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8(2):204-212. doi: 10.1161/CIRCOUTCOMES.114.001416 [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45-49. doi: 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089-3110. doi: 10.1002/sim.2174 [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34(2):187-220. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837-2849. doi: 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417-2418. doi: 10.1001/jama.2020.7819 [DOI] [PubMed] [Google Scholar]
- 27.Rubin D. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 2004. [Google Scholar]
- 28.Ko DT, Guo H, Wijeysundera HC, et al. ; Cardiac Care Network (CCN) of Ontario Variations in Revascularization Practice in Ontario (VRPO) Working Group . Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol. 2012;60(19):1876-1884. doi: 10.1016/j.jacc.2012.06.056 [DOI] [PubMed] [Google Scholar]
- 29.Maron DJ, Hochman JS, Reynolds HR, et al. ; ISCHEMIA Research Group . Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbato E, Toth GG, Johnson NP, et al. A prospective natural history study of coronary atherosclerosis using fractional flow reserve. J Am Coll Cardiol. 2016;68(21):2247-2255. doi: 10.1016/j.jacc.2016.08.055 [DOI] [PubMed] [Google Scholar]
- 31.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105-2111. doi: 10.1016/j.jacc.2007.01.087 [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182-3188. doi: 10.1093/eurheartj/ehv452 [DOI] [PubMed] [Google Scholar]
- 33.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation/American Heart Association Task Force . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354-e471. [DOI] [PubMed] [Google Scholar]
- 34.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754-1763. doi: 10.1001/jama.2014.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics Before Propensity Score Weighting
eTable 2. Association Between Percutaneous Coronary Intervention and the Incidence of Non-fatal Outcomes After Propensity Score Weighting
eTable 3. Outcomes of Patients Presenting With Ischemic Fractional Flow Reserve (≤ 0.80) and Without Myocardial Infarction After Propensity Score Weighting
eTable 4. Outcomes of Patients Presenting With Non-ischemic Fractional Flow Reserve (> 0.80) and Without Myocardial Infarction After Propensity Score Weighting
eTable 5. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve With a Multi-level Propensity Score Accounting for Hospital Site
eTable 6. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve After Multiple Imputation for Missing Serum Creatinine and Left Ventricular Ejection Fraction
eTable 7. Outcomes of Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve After Excluding Coronary Artery Bypass Grafting After Index Procedure
eTable 8. Outcomes in Patients Presenting With Ischemic and Non-ischemic Fractional Flow Reserve Stratified by the Number of Diseased Coronary Artery Disease Vessels
eFigure 1. Distribution of Fractional Flow Reserve Measurements
eFigure 2. Standardized Differences Before and After Propensity Weighting in Patients With Ischemic FFR
eFigure 3. Standardized Differences Before and After Propensity Weighting in Patients With Non-ischemic FFR