Key Points
Question
How do outcomes in the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction trial compare across worsening heart failure (HF) index admission characteristics, and is there a time-dependent risk of events according to index hospitalization subgroups?
Findings
In this secondary analysis of a randomized clinical trial, there was a risk gradient for the primary composite end point; highest in patients closest in time from index HF hospitalization and lowest in those with only outpatient worsening. This risk was reduced by vericiguat in all subgroups, without evidence of treatment heterogeneity.
Meaning
Among patients with recent worsening HF, proximity to HF hospitalization was associated with increased risk of adverse outcomes, whereas the risk reduction with vericiguat did not differ significantly across subgroups.
Abstract
Importance
The period following heart failure hospitalization (HFH) is a vulnerable time with high rates of death or recurrent HFH.
Objective
To evaluate clinical characteristics, outcomes, and treatment response to vericiguat according to prespecified index event subgroups and time from index HFH in the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction (VICTORIA) trial.
Design, Setting, and Participants
Analysis of an international, randomized, placebo-controlled trial. All VICTORIA patients had recent (<6 months) worsening HF (ejection fraction <45%). Index event subgroups were less than 3 months after HFH (n = 3378), 3 to 6 months after HFH (n = 871), and those requiring outpatient intravenous diuretic therapy only for worsening HF (without HFH) in the previous 3 months (n = 801). Data were analyzed between May 2, 2020, and May 9, 2020.
Intervention
Vericiguat titrated to 10 mg daily vs placebo.
Main Outcomes and Measures
The primary outcome was time to a composite of HFH or cardiovascular death; secondary outcomes were time to HFH, cardiovascular death, a composite of all-cause mortality or HFH, all-cause death, and total HFH.
Results
Among 5050 patients in the VICTORIA trial, mean age was 67 years, 24% were women, 64% were White, 22% were Asian, and 5% were Black. Baseline characteristics were balanced between treatment arms within each subgroup. Over a median follow-up of 10.8 months, the primary event rates were 40.9, 29.6, and 23.4 events per 100 patient-years in the HFH at less than 3 months, HFH 3 to 6 months, and outpatient worsening subgroups, respectively. Compared with the outpatient worsening subgroup, the multivariable-adjusted relative risk of the primary outcome was higher in HFH less than 3 months (adjusted hazard ratio, 1.48; 95% CI, 1.27-1.73), with a time-dependent gradient of risk demonstrating that patients closest to their index HFH had the highest risk. Vericiguat was associated with reduced risk of the primary outcome overall and in all subgroups, without evidence of treatment heterogeneity. Similar results were evident for all-cause death and HFH. Addtionally, a continuous association between time from HFH and vericiguat treatment showed a trend toward greater benefit with longer duration since HFH. Safety events (symptomatic hypotension and syncope) were infrequent in all subgroups, with no difference between treatment arms.
Conclusions and Relevance
Among patients with worsening chronic HF, those in closest proximity to their index HFH had the highest risk of cardiovascular death or HFH, irrespective of age or clinical risk factors. The benefit of vericiguat did not differ significantly across the spectrum of risk in worsening HF.
Trial Registration
ClinicalTrials.gov Identifier: NCT02861534
This secondary analysis of the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction clinical trial evaluates clinical characteristics, outcomes, and treatment response to vericiguat according to prespecified index event subgroups and time from index heart failure hospitalization.
Introduction
Despite available heart failure (HF) therapy, patients with reduced ejection fraction (HFrEF) remain at high risk of hospitalization and death. Previous chronic HFrEF trials showed that prior HF hospitalization (HFH) strongly predicted cardiovascular (CV) death and HFH, with risk declining with time from the index admission event.1,2 The Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction (VICTORIA)3 is, to our knowledge, the first positive large trial to exclusively enroll patients with worsening HFrEF in the vulnerable period (<6 months) soon after HFH, thereby offering unique opportunities to study the spectrum of patients recruited during and immediately following index HFH or an outpatient worsening HF event. Whereas the baseline characteristics and main unadjusted primary outcomes according to prespecified index event subgroups have been reported,3,4 we now provide new data informed by treatment assignment and the effect of multivariable-adjusted analyses of primary and secondary outcomes. We also assessed outcomes of patients randomized while in hospital, as well as immediately following hospitalization, to provide finer continuous detail on the association between time from index event and outcomes.
Methods
Briefly,5 the VICTORIA trial showed that vericiguat reduced the composite primary outcome of HFH or CV death in patients with chronic HFrEF (EF <45%, New York Heart Association [NYHA] class II-IV) and a recent (<6 months) worsening HF event. Prespecified subgroups were less than 3 months or 3 to 6 months after HFH, and those with worsening HF receiving outpatient intravenous diuretics at less than 3 months (without HFH in the prior 6 months). Secondary end points were time to HFH, CV death, a composite of all-cause mortality or HFH, all-cause death, and total HFH. The trial protocol was approved by ethics committees at participating sites, and all patients provided written informed consent.
To compare outcomes among index event subgroups, we used Cox proportional hazards regression models, where multivariable adjustment variables were independent predictors for each outcome selected from a set of 66 available candidate variables (eTable 1 in Supplement 1).6 Interaction between treatment and index event subgroup was tested for each outcome. Patients with index HFH were further divided into in-hospital randomization, 30 days or less, 31 to 90 days, and greater than 90 days. Incident rates were calculated for every period and treatment group. Hazard ratios (HRs) for treatment effect were calculated for every period, along with interaction P value between treatment and finer time subgroup. Significance was determined at a 2-sided P value of less than .05. A trend of the HR of the primary end point for the treatment effect is presented over time from the date of the index hospitalization to randomization date, where time was a continuous variable.
Results
Of a total of 5050 patients in the VICTORIA trial, 3378 (67%) were randomized less than 3 months from index HFH (11% in-hospital), 871 (17%) within 3 to 6 months of HFH, and 801 (16%) within 3 months of outpatient worsening HF (median 26 days; interquartile range, 14-46 days). The baseline characteristics were well balanced between treatment arms within each index event subgroup (Table).
Table. Baseline Characteristics of the Study Population Stratified by Index Admission Subgroup and Treatment Arm.
| Characteristics | No./total No. (%) | |||||
|---|---|---|---|---|---|---|
| HF hospitalization within 3 mo | HF hospitalization 3-6 mo | Outpatient worsening within 3 mo | ||||
| Vericiguat (n = 1673) | Placebo (n = 1705) | Vericiguat (n = 454) | Placebo (n = 417) | Vericiguat (n = 399) | Placebo (n = 402) | |
| Age | ||||||
| Median (25th-75th), y | 68.00 (59.00-76.00) | 68.00 (59.00-76.00) | 69.00 (60.00-77.00) | 69.00 (60.00-77.00) | 70.00 (62.00-78.00) | 69.50 (62.00-77.00) |
| No. | 1673 | 1705 | 454 | 417 | 399 | 402 |
| Sex | ||||||
| Female | 401/1673 (24.0) | 403/1705 (23.6) | 105/454 (23.1) | 99/417 (23.7) | 99/399 (24.8) | 101/402 (25.1) |
| Male | 1272/1673 (76.0) | 1302/1705 (76.4) | 349/454 (76.9) | 318/417 (76.3) | 300/399 (75.2) | 301/402 (74.9) |
| Race/ethnicity | ||||||
| American Indian or Alaska Native | 18/1672 (1.1) | 19/1705 (1.1) | 2/454 (0.4) | 2/417 (0.5) | 4/399 (1.0) | 7/402 (1.7) |
| Asian | 397/1672 (23.7) | 391/1705 (22.9) | 86/454 (18.9) | 88/417 (21.1) | 88/399 (22.1) | 82/402 (20.4) |
| Black or African American | 85/1672 (5.1) | 87/1705 (5.1) | 32/454 (7.0) | 31/417 (7.4) | 6/399 (1.5) | 8/402 (2.0) |
| Multiple | 134/1672 (8.0) | 129/1705 (7.6) | 24/454 (5.3) | 28/417 (6.7) | 25/399 (6.3) | 23/402 (5.7) |
| Native Hawaiian or other Pacific Islander | 1/1672 (0.1) | 5/1705 (0.3) | 2/454 (0.4) | 6/417 (1.4) | 0/399 | 0/402 |
| White | 1037/1672 (62.0) | 1074/1705 (63.0) | 308/454 (67.8) | 262/417 (62.8) | 276/399 (69.2) | 282/402 (70.1) |
| Region | ||||||
| Asia Pacific | 409/1673 (24.4) | 417/1705 (24.5) | 104/454 (22.9) | 98/417 (23.5) | 79/399 (19.8) | 76/402 (18.9) |
| Eastern Europe | 568/1673 (34.0) | 589/1705 (34.5) | 134/454 (29.5) | 132/417 (31.7) | 146/399 (36.6) | 125/402 (31.1) |
| Latin and South America | 234/1673 (14.0) | 226/1705 (13.3) | 54/454 (11.9) | 55/417 (13.2) | 74/399 (18.5) | 81/402 (20.1) |
| North America | 170/1673 (10.2) | 170/1705 (10.0) | 69/454 (15.2) | 63/417 (15.1) | 42/399 (10.5) | 46/402 (11.4) |
| Western Europe | 292/1673 (17.5) | 303/1705 (17.8) | 93/454 (20.5) | 69/417 (16.5) | 58/399 (14.5) | 74/402 (18.4) |
| BMI | ||||||
| Median (25th-75th) | 26.78 (23.66-30.72) | 26.75 (23.76-31.06) | 26.90 (23.84-30.52) | 26.53 (23.65-30.36) | 27.00 (23.48-30.82) | 27.68 (24.01-32.24) |
| No. | 1665 | 1688 | 446 | 408 | 398 | 399 |
| Medical history | ||||||
| Ejection fraction, % | ||||||
| Median (25th-75th) | 29.00 (22.00-35.00) | 28.00 (22.00-35.00) | 29.00 (23.00-35.00) | 28.00 (21.00-35.00) | 31.00 (25.00-38.00) | 33.00 (25.00-38.00) |
| No. | 1665 | 1702 | 453 | 416 | 398 | 402 |
| ≤40% | 1557/1665 (93.5) | 1579/1702 (92.8) | 419/453 (92.5) | 392/416 (94.2) | 360/398 (90.5) | 360/402 (89.6) |
| NYHA class | ||||||
| I | 0/1671 | 1/1705 (0.1) | 0/453 | 0/416 | 0/399 | 1/402 (0.2) |
| II | 942/1671 (56.4) | 998/1705 (58.5) | 293/453 (64.7) | 254/416 (61.1) | 243/399 (60.9) | 245/402 (60.9) |
| III | 697/1671 (41.7) | 684/1705 (40.1) | 158/453 (34.9) | 157/416 (37.7) | 155/399 (38.8) | 152/402 (37.8) |
| IV | 32/1671 (1.9) | 22/1705 (1.3) | 2/453 (0.4) | 5/416 (1.2) | 1/399 (0.3) | 4/402 (1.0) |
| Atrial fibrillation | 734/1672 (43.9) | 806/1705 (47.3) | 209/454 (46.0) | 197/416 (47.4) | 155/399 (38.8) | 167/402 (41.5) |
| Atrial fibrillation or flutter | 760/1672 (45.5) | 843/1705 (49.4) | 214/454 (47.1) | 207/416 (49.8) | 160/399 (40.1) | 174/402 (43.3) |
| Diabetes | 837/1672 (50.1) | 776/1705 (45.5) | 209/454 (46.0) | 192/416 (46.2) | 180/399 (45.1) | 175/402 (43.5) |
| Hypertension | 1323/1672 (79.1) | 1349/1705 (79.1) | 373/454 (82.2) | 330/416 (79.3) | 306/399 (76.7) | 314/402 (78.1) |
| Coronary artery disease | 938/1672 (56.1) | 922/1705 (54.1) | 277/454 (61.0) | 242/416 (58.2) | 247/399 (61.9) | 236/402 (58.7) |
| Stroke | 193/1672 (11.5) | 196/1705 (11.5) | 56/454 (12.3) | 55/416 (13.2) | 33/399 (8.3) | 45/402 (11.2) |
| Vital signs | ||||||
| Systolic blood pressure, mm Hg | ||||||
| Median (25th-75th) | 118.00 (108.00-130.00) | 118.00 (108.00-131.00) | 121.00 (110.00-135.00) | 119.00 (109.50-131.00) | 120.00 (109.00-131.00) | 121.50 (112.00-133.00) |
| No. | 1669 | 1701 | 450 | 412 | 399 | 402 |
| Diastolic blood pressure, mm Hg | ||||||
| Median (25th-75th) | 72.00 (65.00-80.00) | 73.00 (65.00-80.00) | 71.50 (64.00-81.00) | 72.00 (65.00-79.00) | 72.00 (64.00-80.00) | 73.00 (65.00-81.00) |
| No. | 1669 | 1701 | 450 | 412 | 399 | 402 |
| Concomitant medications | ||||||
| ACE-I or ARB | 1222/1671 (73.1) | 1247/1705 (73.1) | 326/451 (72.3) | 300/413 (72.6) | 299/399 (74.9) | 306/401 (76.3) |
| β-Blocker | 1550/1671 (92.8) | 1579/1705 (92.6) | 426/451 (94.5) | 387/413 (93.7) | 373/399 (93.5) | 376/401 (93.8) |
| MRA | 1192/1671 (71.3) | 1248/1705 (73.2) | 297/451 (65.9) | 288/413 (69.7) | 258/399 (64.7) | 262/401 (65.3) |
| Sacubitril-valsartan | 228/1671 (13.6) | 258/1705 (15.1) | 78/451 (17.3) | 61/413 (14.8) | 54/399 (13.5) | 52/401 (13.0) |
| Triple therapy | 1010/1671 (60.4) | 1063/1705 (62.3) | 254/451 (56.3) | 241/413 (58.4) | 216/399 (54.1) | 225/401 (56.1) |
| SOC devices | ||||||
| ICD | 437/1671 (26.2) | 460/1705 (27.0) | 142/451 (31.5) | 131/413 (31.7) | 117/399 (29.3) | 112/401 (27.9) |
| Biventricular pacemaker | 225/1671 (13.5) | 236/1705 (13.8) | 84/451 (18.6) | 76/413 (18.4) | 61/399 (15.3) | 57/401 (14.2) |
| Laboratory results | ||||||
| Creatinine, mg/dL | ||||||
| Median (25th-75th) | 1.20 (0.90-1.60) | 1.20 (0.90-1.60) | 1.20 (0.90-1.60) | 1.20 (0.95-1.50) | 1.20 (0.90-1.50) | 1.10 (0.90-1.50) |
| No. | 1638 | 1678 | 442 | 404 | 394 | 400 |
| eGFR, mL/min/1.73 m2 | ||||||
| Median (25th-75th) | 58.40 (40.04-76.40) | 57.53 (39.93-77.77) | 58.40 (41.69-76.48) | 58.69 (43.56-76.22) | 58.70 (42.70-80.30) | 60.32 (45.12-77.58) |
| No. | 1638 | 1680 | 442 | 405 | 394 | 400 |
| Sodium, mEq/L | ||||||
| Median (25th-75th) | 140 (138-142) | 140 (138-142) | 141 (139-142) | 140 (139-142) | 141 (138-142) | 140 (138-142) |
| No. | 1640 | 1677 | 443 | 404 | 393 | 400 |
| Core laboratory NT-proBNP value at randomization, pg/mL | ||||||
| Median (25th-75th) | 3111.0 (1608.0-5852.0) | 3029.0 (1636.0-5634.0) | 2574.0 (1675.0-5121.0) | 2619.5 (1544.0-4816.0) | 2244.0 (1316.0-4395.0) | 2299.5 (1258.0-4126.0) |
| No. | 1598 | 1617 | 431 | 390 | 385 | 384 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IV, intravenous; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B type natriuretic peptide; NYHA, New York Heart Association; SOC, standard of care.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 76.25; sodium to millimoles per liter, multiply by 1.
We observed a gradient of risk across the index event subgroups (eFigure 1 and eTable 2 in Supplement 1), with highest risk of the primary composite end point in HFH less than 3 months (40.9 events per 100 patient-years), followed by HFH 3 to 6 months (29.6 events per 100 patient-years), and lowest risk in the outpatient worsening subgroup (23.4 events per 100 patient-years). Compared with the outpatient worsening subgroup, the adjusted risk of CV death or HFH was higher in the HFH less than 3 months subgroup (adjusted HR, 1.48; 95% CI, 1.27-1.73), but no longer significantly different in the HFH 3 to 6 months subgroup (adjusted P = .25). Similar trends were observed for the secondary outcomes (eTable 2 in Supplement 1). There was no statistical difference in vericiguat’s effect on primary or secondary outcomes across index event subgroups (eTable 3 in Supplement 1; Figure 1).
Figure 1. Kaplan-Meier Graphs by Index Event Subgroup Showing Time to First Primary Composite End Point by Treatment Arm.
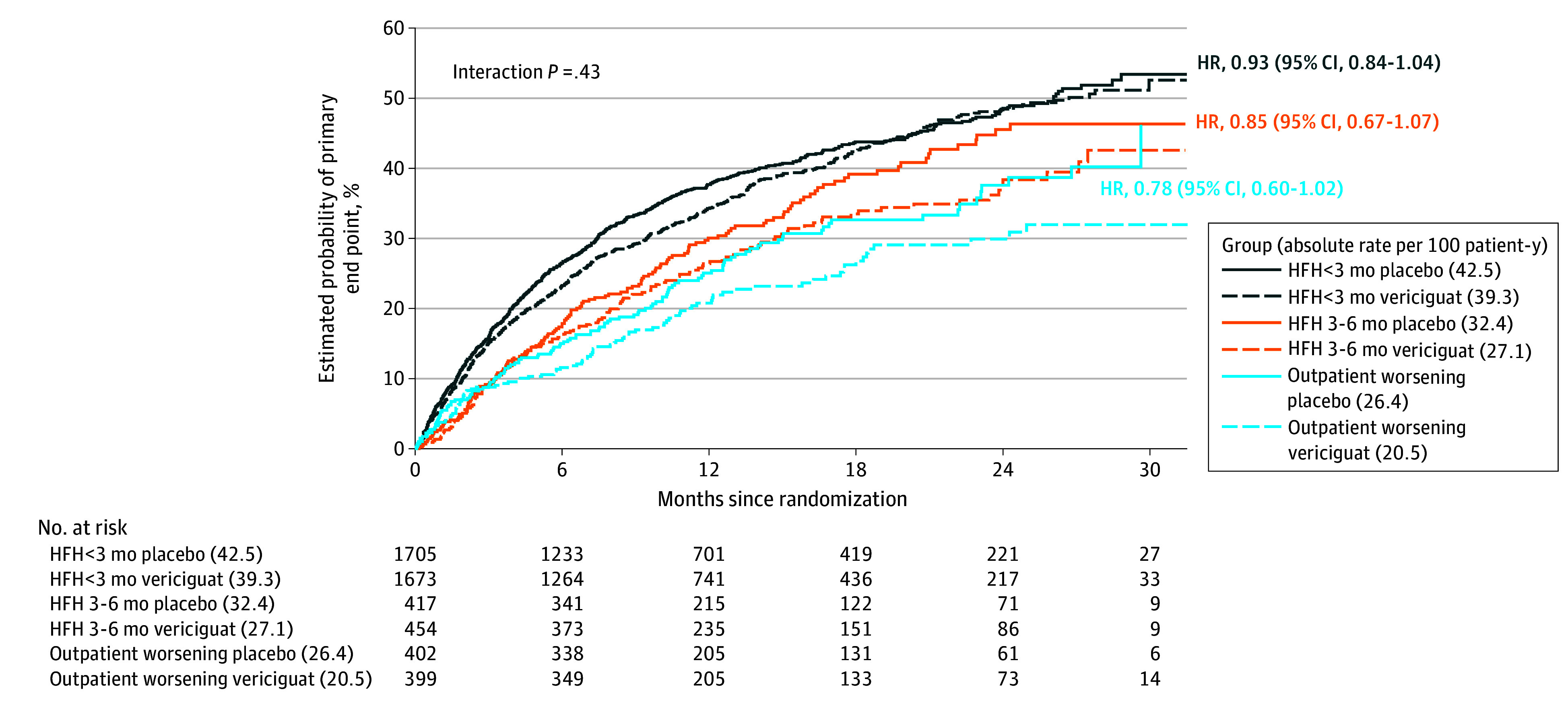
Black lines represent patients randomized within 3 months of HF hospitalization, orange lines those randomized within 3 to 6 months of HF hospitalization, and blue lines those randomized with 3 months of intravenous (IV) diuretic use for heart failure. Reported hazard ratios are populated from unadjusted models. HRH indicates heart failure hospitalization.
When patients with an index HFH were divided into those in hospital (n = 571) and in 30-day intervals (≤30 [n = 1461]; >30-60 [n = 827]; >60-90 [n = 516]; >90-120 [n = 371]; >120-150 [n = 234]; and >150 days [n = 253]) following index hospitalization, a wider gradient of risk was observed that was highest in hospitalized patients (event rate 52.0 per 100 patient-years) (Figure 2A). The primary composite event rate decreased from 40.5 events per 100 patient-years in those randomized less than 30 days, to 41.0, 33.0, 30.9, 29.0, and 27.2 events per 100 patient-years in those randomized 31 to 60, 61 to 90, 91 to 120, 121 to 150, and more than 150 days following index hospitalization, respectively. The relative risk reduction with vericiguat vs placebo was not significantly different among these more finely divided subgroups (interaction P = .35) (Figure 2A). The continuous association between time since HFH and treatment effect of vericiguat showed a trend (interaction P = .09) toward greater benefit with longer duration since HFH (Figure 2B). A trend of the HR of the primary endpoint for the treatment effect is presented over time from the date of the index hospitalization to randomization date, where time was a continuous variable. When the continuous association between time since intravenous diuretic for outpatient worsening and the treatment effect of vericiguat was explored over the 60-day window of observation, there was no association to treatment benefit (eFigure 2 in Supplement 1).
Figure 2. Risk of the Primary Composite End Point, and Treatment Effect of Vericiguat Compared With Placebo, Analyzed by Finer Cuts of Time From Index Heart Failure (HF) Event (A) as Well as Using Time From Index Hospitalization as a Continuous Variable (B).
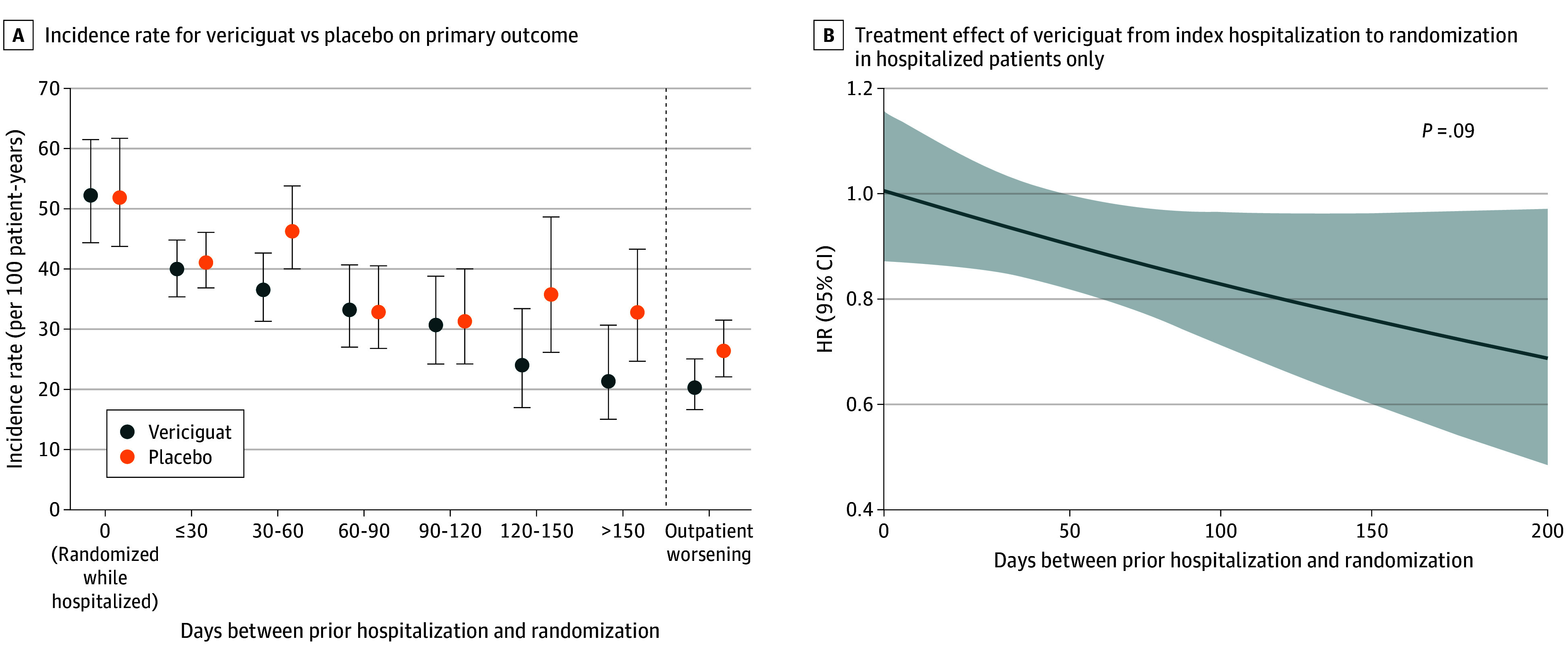
A, Incidence rates (and their 95% confidence intervals) of the primary composite end point are shown according to finer cuts of time from index event. Data on days to index event were missing in 16 patients. B, The treatment effect of vericiguat (hazard ratio [HR] with 95% confidence interval) is shown as a function of time as a continuous variable from index event to randomization among hospitalized patients. Data are presented for 4219 patients randomized within 200 days.
Safety events were infrequent in all subgroups (eTable 4 in Supplement 1), without difference between treatment arms (symptomatic hypotension range 6.2%-10.3% and syncope range 2.9%-5.8% for subgroups). Specifically, for patients randomized in-hospital, there were 24 (8.1%) cases of symptomatic hypotension with vericiguat vs 17 (6.2%) for placebo, and 17 (5.8%) syncopal episodes with vericiguat arm vs 11 (4.0%) with placebo. These adverse event rates were similar or lower than observed in the respective treatment arms of the outpatient worsening subgroup.
Discussion
Among patients with worsening chronic HF in the VICTORIA trial, those randomized within a 3-month window of HFH experienced an approximately 50% higher rate of CV death or HFH than those with outpatient worsening without HFH, even after adjusting for prognostically relevant clinical covariates, background therapy, and laboratory parameters (including N-terminal pro hormone B-type natriuretic peptide). This risk was further accentuated in those within 1 month of HFH (>40 events per 100 patient-years) and again amplified among patients randomized within their index hospitalization (>50 events per 100 patient-years). Notwithstanding this spectrum of risk, there was no statistical evidence for heterogeneity of treatment benefit with vericiguat across the range of times from index event to 6 months.
Our results are aligned with prior chronic HFrEF trials reporting increasing risk with proximity to a prior HFH event,1,2 but extend those observations to a higher risk population studied both closer to a prior HFH or outpatient worsening without HFH. The primary event rate in the HFH less than 3 months group in VICTORIA (40.9 events per 100 patient-years) was approximately 2 times higher than the corresponding rate in the highest risk group of Candesartan in Heart Failure: Assessment of Mortality and Morbidity (CHARM; those randomized 0-2 months from HFH) and Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM; also randomized <3 months from HFH). Furthermore, the primary event rate in the VICTORIA outpatient worsening subgroup (23.4 events per 100 patient-years) was more than 2 times higher than the corresponding rates in the lowest risk group of CHARM (those without prior HFH), and more similar to the event rates in the highest-risk CHARM group (those randomized 0-2 months following HFH).1 The additional risk in the VICTORIA subgroups likely reflect the additive effects of the higher natriuretic peptide entry criteria and baseline levels as compared with prior trials.7,8 These results emphasize that patients with outpatient worsening HF (especially with elevated natriuretic peptides) are at significant risk of HFH and CV death comparable with that of HFH9,10 and should be strongly considered for additional disease-modifying therapies. The benefit of vericiguat, added to standard guidelines-directed medical therapy, is particularly noteworthy in this setting, where a numerically greatest relative risk reduction with vericiguat was evident.
We observed a trend toward greater treatment benefit with increasing time from HFH. Whether this would extend beyond the vulnerable 6 months after HFH to more “stable” HFrEF outpatients warrants further study. Whereas vericiguat treatment was not associated with more adverse events than placebo among in-hospital patients their very high risk of recurrent HFH and CV death may not be modifiable as suggested in prior trials that enrolled in-hospital patients with worsening HF.11,12
Strengths and Limitations
Our study has both strengths and limitations. Strengths include the unique VICTORIA patient population, enriched for risk by the dual requirements of proximity to the HF index event as well as high natriuretic peptides, while also exploring a risk spectrum from in-hospital randomization to outpatient worsening HF. Nonetheless, VICTORIA was not powered for these exploratory post hoc analyses in smaller subgroups. Underrepresentation of women and minority ethnic groups was also evident.
Conclusions
Our results have implications for future HF trial design, suggesting that high-entry natriuretic peptides and proximity to a worsening HF event select for patients particularly prone to recurrent HFH out of proportion to CV death, compared with HF trials in lower risk HF populations. Furthermore, clinical events accrue very quickly over time in these patients, shortening the follow-up duration in an event-driven trial powered for the composite outcome. Finally, our findings highlight the residual risk and need for novel therapies in patients with recent worsening HF.
eTable 1. Candidate variable list
eTable 2. Hazard ratios for primary and secondary endpoints by index admission subgroups
eTable 3. Treatment effect by index admission event subgroup
eTable 4. Safety events of interest and serious adverse events by index event group
eFigure 1. Kaplan-Meier analysis
eFigure 2. Treatment effect of vericiguat compared with placebo analyzed using time from intravenous diuretic administration for outpatient worsening as a continuous variable
Nonauthor Collaborators. The VICTORIA Study Group.
References
- 1.Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7(4):590-595. doi: 10.1161/CIRCHEARTFAILURE.113.001281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon SD, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM-HF trial. JACC Heart Fail. 2016;4(10):816-822. doi: 10.1016/j.jchf.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong PW, Pieske B, Anstrom KJ, et al. ; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883-1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 4.Pieske B, Patel MJ, Westerhout CM, et al. ; VICTORIA Study Group . Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail. 2019;21(12):1596-1604. doi: 10.1002/ejhf.1664 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong PW, Roessig L, Patel MJ, et al. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 2018;6(2):96-104. doi: 10.1016/j.jchf.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 6.Ezekowitz JA, O'Connor CM, Troughton RW, et al. N-terminal pro-B-type natriuretic peptide and clinical outcomes in the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA). JACC Heart Fail. 2020;8(11):931-939. doi:10.1016/j.jchf.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Butler J, Anstrom KJ, Armstrong PW. Comparing the benefit of novel therapies across clinical trials: insights from the VICTORIA trial. Circulation. 2020;142(8):717-719. doi: 10.1161/CIRCULATIONAHA.120.047086 [DOI] [PubMed] [Google Scholar]
- 8.Dewan P, Jhund PS, McMurray JJV. VICTORIA in context. Eur J Heart Fail. Published online May 3, 2020 doi: 10.1002/ejhf.1833 [DOI] [PubMed] [Google Scholar]
- 9.Okumura N, Jhund PS, Gong J, et al. ; PARADIGM-HF Investigators and Committees . Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254-2262. doi: 10.1161/CIRCULATIONAHA.115.020729 [DOI] [PubMed] [Google Scholar]
- 10.Skali H, Dwyer EM, Goldstein R, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014;16(5):560-565. doi: 10.1002/ejhf.71 [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Konstam MA, Burnett JC Jr, et al. ; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332-1343. doi: 10.1001/jama.297.12.1332 [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Böhm M, Greene SJ, et al. ; ASTRONAUT Investigators and Coordinators . Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309(11):1125-1135. doi: 10.1001/jama.2013.1954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Candidate variable list
eTable 2. Hazard ratios for primary and secondary endpoints by index admission subgroups
eTable 3. Treatment effect by index admission event subgroup
eTable 4. Safety events of interest and serious adverse events by index event group
eFigure 1. Kaplan-Meier analysis
eFigure 2. Treatment effect of vericiguat compared with placebo analyzed using time from intravenous diuretic administration for outpatient worsening as a continuous variable
Nonauthor Collaborators. The VICTORIA Study Group.


