Abstract
Background
The relationship between gastric bare area adipose tissues invasion (GBAI) confirmed pathologically and the prognosis of gastric cancer (GC) patients is undefined. Till present, there has not been literature investigating this phenomenon. Here, we aimed at analyzing the implication of GBAI in GC.
Methods
The data of 1822 patients who underwent radical surgery between January 2000 and December 2013 at the Sun Yat-sen University Cancer Center were retrieved. Pathologically, tumor deposits (TDs) located > 5 mm from the leading edge of the primary tumor and the lymph nodes (LNs) station number 1, 2, 7, and 9 were considered GBAI. Kaplan-Meier method, log-rank test, and Cox’s proportional hazards model were employed to analyze.
Results
Two hundred and five (11.3%) patients were pathologically diagnosed with GBAI, which was more commonly found in proximal or linitis lastica than distal GC (P < 0.001). There was significant difference in 5-year survival between patients with and without GBAI for stages IIB, IIIA, IIIB, and IIIC, respectively (P < 0.009 for IIB, IIIA, and IIIB; P = 0.021 for IIIC). Among the 205 GBAI patients, 61 had detailed radiological follow-up data in which 26 (34.7%) were found to have retroperitoneal infiltration, 27 (36.0%) had peritoneal metastasis, 10 (13.3%) had hematogenous metastasis, 16 (21.3%) had lymphatic metastasis, and 16 (21.3%) had others.
Conclusions
GBAI was identified as a predictor of unfavorable prognosis for GC and was more commonly found in the proximal or linitis plastica of the stomach than in distal stomach. Retroperitoneal infiltration was one of the most commonly identified metastatic route for GC associated with GBAI after radical surgery.
Keywords: Gastric cancer, GBA, GBAI, Retroperitoneal infiltration, TDs
Introduction
The latest global cancer statistics showed that gastric cancer (GC) is now the sixth most common cancer affecting the global population but still rank third as the leading cause of cancer mortality [1]. In mainland China [2], GC remains third as the most prevalent cancer and leading cause of cancer-related death, illustrating a much higher incidence and mortality than in any other country, and most importantly, China also comprise nearly one half of the total global GC incidence [2–4].
Recurrences and metastases following radical surgery are major challenges to tackle for improving the survival outcomes of GC as these are the main factors associated with GC-related death. Currently, the most effective prognostic factors for GC are the depth of the primary tumor invasion (T), the number of metastatic regional lymph nodes (N), and status of distant metastasis (M), which are merged into known commonly used TNM staging system for GC survival prognostication. However, solely considering the TNM staging system for optimal prognostication may not be reliable as it has been showed that GC patients despite being classified within the same TNM subgroup may still have different survival outcomes, possibly related to tumor heterogeneity [5]. As such, abundant researches have tried to identify other prognostic factors such as vascular invasion, nerve invasion, adipose connective tissue invasion, ratio of lymph node metastasis, and more, but their global acceptance and application has been limited [6–9] as their clinical applicability are controversial and yet to be widely validated. Thus, more researches are still warranted.
The gastric bare area (GBA) is a special location in the stomach without any visceral peritoneum coverage and is located at a small posterosuperior area of the gastric surface, near the cardiac orifice. Specifically, it is the place where the stomach liaise with the diaphragm at the reflections of the gastrophrenic and left gastropancreatic folds, surrounded by the dorsal mesogastrium [10]. From an anatomical point of view, the GBA can be considered a bridge from abdominal organ to retroperitoneal space, saturating with areolar tissue. Till present, there has been only one radiologic study regarding the invasion of the GBA by cancer, termed as the GBA invasion, and was found as a poor prognosis factor for survival [11]. Nevertheless, this study was limited due to considering swollen lymph nodes as one kind of GBA invasion and the absence of pathological evidence. To date, there has been no research regarding the pathological confirmation of gastric bare area adipose tissues invasion (GBAI) and the relationship between GBAI and GC prognosis is yet to be elucidated.
In this present study, we aimed to identify the incidence of GBAI in a large cohort of GC and to evaluate its association with the clinicopathological characteristics and prognosis of GC.
Materials and methods
Patients
Between January 2000 and December 2013, the data of 1822 patients who underwent radical surgery at the Department of Gastric Surgery, Sun Yat-sen University Cancer Center, were retrieved. Eligibility criteria for patient inclusion comprised of (1) histologically confirmed diagnosis of gastric adenocarcinoma and R0 gastrectomy, (2) no other synchronous malignancy, (3) no preoperative chemotherapy or perioperative radiotherapy, (4) gastrectomy and lymphadenectomy based on the Japanese Gastric Cancer treatment guidelines [12], (5) more than 15 postoperative pathologically reported lymph nodes, and (6) a postoperative survival time of more than 1 month, considered the non-surgical relative death. Patients with carcinoma of the gastric stump after gastric resection for benign disease were excluded from this study. Retrieved data included the patient gender, tumor size, histological grade, status of vascular invasion, nerve invasion, adipose connective tissue invasion, GBA invasion, depth of invasion (pT), nodal status (pN), distant metastasis (pM), and the number of retrieved lymph nodes. Pathological staging was performed according to the 8th edition of the AJCC cancer staging manual.
Follow-up protocol
Follow-ups were performed by telephone, email, or outpatient department visits. The last follow-up date was December 2018. The postoperative follow-ups included clinical and laboratory examinations every 3 months for the first 2 years at our outpatient department, every 6 months from the third to fifth postoperative years, and then annually thereafter or until the patient died. Overall survival (OS) was defined as the time from the operation to death or the last follow-up.
Range of GBAI
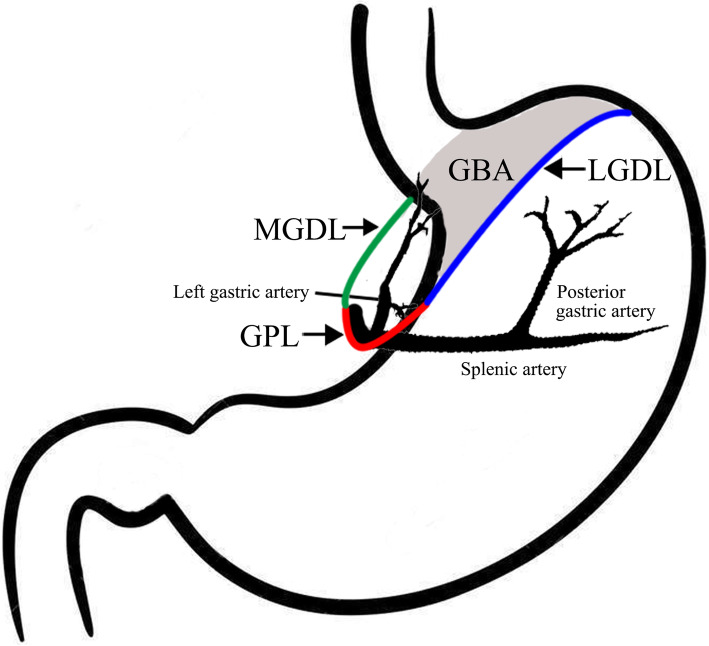
Three important ligaments compose the boundary of the GBA, namely the medial gastrodiaphragmatic ligament (MGDL), lateral gastrodiaphragmatic ligament (LGDL), and gastropancreatic ligament (GPL), as shown in Fig. 1. The hepatogastric ligament contains anterior layer and posterior layer. Rightward extension of the anterior layer becomes the peritoneum of the anterior gastric wall at the lesser curvature. The posterior layer turns posteriorly and right laterally at the lesser curvature, connecting with the right diaphragmatic crura. This peritoneum reflex from the lesser curvature to the right diaphragmatic crura is the so called MGDL which is the right boundary of the GBA. Posterior and right extension of the peritoneum of the posterior gastric wall from the fundus and the great curvature to the left diaphragmatic crura becomes the LGDL, which is the left boundary of the GBA. Inferior extensions of MGDL and LGDL become the GPL at the superior border of pancreas, which is the inferior boundary of GBA. The left gastric artery and vein pass through the GPL. Based on the 3rd English edition of Japanese classification of gastric carcinoma, the definition of the station number 1, 2, 7, and 9 LNs stations are the right paracardial LNs including those along the first branch of the ascending limb of the left gastric artery, the left paracardial LNs include those along the esophagocardiac branch of the left subphrenic artery, LNs along the trunk of the left gastric artery between its root and the origin of its ascending branch, and the LNs along the celiac artery, respectively [13]. Thence, we consider the adipose tissues invasion in the area of LNs station number 1, 2, 7, and 9 or attached to the primary tumor involving the proximal of stomach as GBAI.
Fig. 1.
Illustration of GBA, MGDL, LGDL, and GPL
Histologic evaluation of GBAI
For each patient, all postoperative pathological slides were reviewed to evaluate the presence of GBAI by one pathologist who was blinded to the clinical and survival data of patients. Existence of GBAI was examined by reviewing the adipose tissues attached to the primary tumor involving the proximal stomach, and the LNs station number 1, 2, 7, and 9. For pathologic examination of these adipose tissues, tumor deposits (TDs) located > 5 mm from the leading edge of primary tumors and LNs were considered the GBAI, irrespective of their shape, contour, and size.
In this study, adipose tissue invasion was defined as TDs residing in the adipose tissues neighboring the stomach. These TDs were identified using these three primary characteristics: (1) they were located in the adipose tissues and were separated from the primary tumor or lymph nodes; (2) there were no structures of blood or lymphatic vessel, lymphatic nodes, or nerves around them; and (3) they were enveloped by a proper fascia and could be clearly discriminated from peritoneal seeding.
Imaging assessment
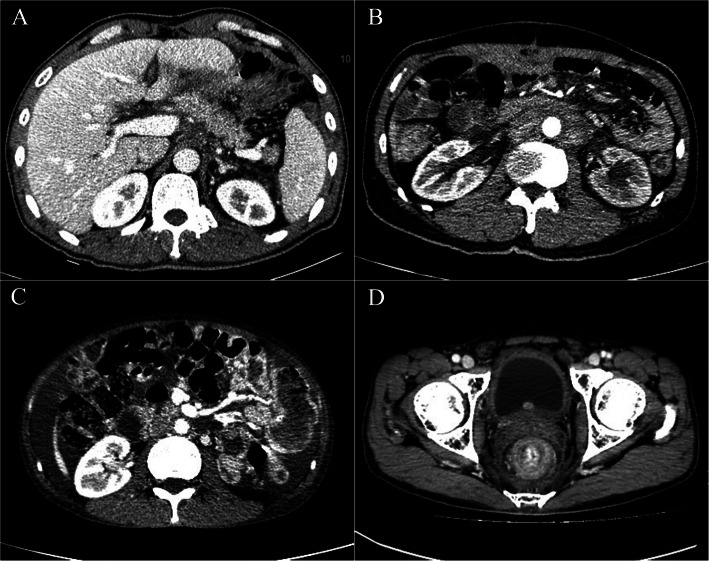
One radiologist performed all imaging reassessment. She was blinded to the clinical and survival data of patients. CT images were evaluated independently at the workstation using transverse CT (images were reconstructed with a 5-mm section thickness). Metastasis was categorized into five types, including retroperitoneal infiltration, peritoneal, hematogenous, lymphatic, and others. The radiological manifestations containing posterior-pancreas infiltration, para-aortic infiltration, mesenterium roots thickening, and thickening of the rectal lining were considered retroperitoneal infiltration, as shown in Fig. 2; peritoneal nodules/thickening, Douglas pouch nodules, and abdominal mass were considered peritoneal metastasis; liver, lung, and bone were considered hematogenous metastasis; para-aortic, left supraclavicular, mediastinum, and porta hepatis LNs were considered lymphatic metastasis; umbilical region, ovary mass, and anastomotic astium were considered others. It was difficult to classify ascites as retroperitoneal infiltration or peritoneal metastasis, so ascites was considered others.
Fig. 2.
a Posterior-pancreas infiltration. b Para-aortic infiltration. c Mesenterium roots thickening. d Thickening of the rectal lining
Statistical analysis
The 5-year overall survival rate was estimated using the Kaplan-Meier method and univariate comparisons between groups was performed using the log-rank test. In the multivariate analyses, the Cox’s proportional hazards model was carried to estimate the relative risks and to identify corresponding prognostic factors. All data analyses were performed using the SSPS software (version 22.0, Stata Corporation, TX, USA). A P value less than 0.05 (two-sided) was considered statistically significant.
Results
Clinic-pathological characteristics
Of the 1822 patients, 1220 (67.0%) were male. The mean age of the entire study cohort was 58.0 ± 12.0 years old. The mean number of lymph nodes retrieved was 27.0 ± 10.8. The median follow-up was 92 months (range 3–214 months). The 5-year OS rate for all the patients was 60.9%, and 1109 patients were alive at last follow-up.
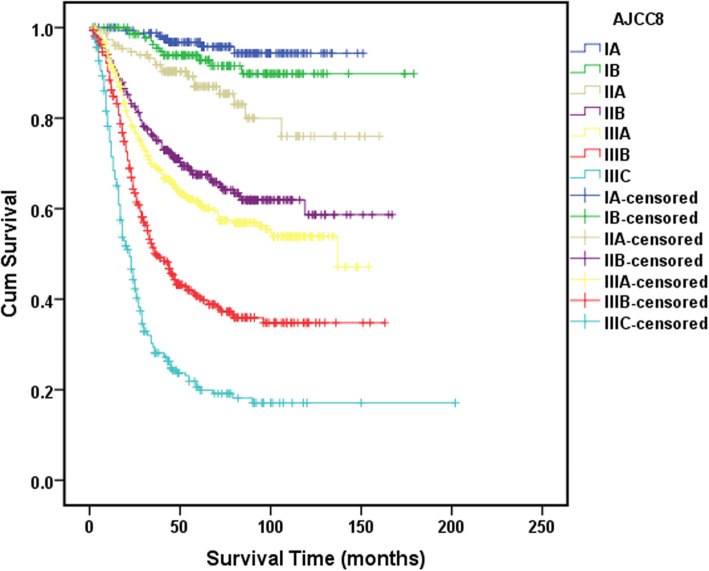
Eight parameters were significantly associated with the OS on univariate analyses, namely, age, tumor size, tumor site, vascular invasion, adipose tissues invasion, GBAI, depth of invasion (pT), and nodal status (pN) (Table 1). In the multivariate analyses for OS, age, tumor site, adipose tissues invasion, GBAI, depth of invasion (pT), and nodal status (pN) were identified as independent prognostic factors (Table 1). The 5-year survival rates for all the stages were as follows: IA, 96.0%; IB, 92.4%; IIA, 86.2%; IIB, 66.3%; IIIA, 61.1%; IIIB, 43.6%; and IIIC, 25.5% (Fig. 3).
Table 1.
Clinic-pathological characteristics and the univariate and multivariate survival analysis in gastric cancer patients and in patients with GBAI or not
| Variables | n (%) | 5-year survival rate (%) | P | HR (95% CI) | P | Non-GBAI (%) | GBAI | P | HR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Female | 602 (33.0) | 60.0 | 0.580 | 534 (33.0) | 68 (33.2) | 0.883 | ||||
| Male | 1220 (67.0) | 61.3 | 1083 (67.0) | 137 (66.8) | ||||||
| Age (years) | ||||||||||
| < 50 | 478 (26.2) | 66.3 | 0.004 | ref. | 432 (26.7) | 46 (22.4) | 0.176 | |||
| ≥ 50 | 1244 (73.8) | 58.9 | 1.02 (1.01–1.03) | < 0.001 | 1185 (73.3) | 159 (77.6) | ||||
| Tumor size (cm) | ||||||||||
| ≤ 5.0 | 1210 (66.4) | 66.5 | < 0.001 | ref. | 1112 (68.8) | 98 (47.8) | < 0.001 | ref. | ||
| > 5.0 | 612 (33.6) | 49.7 | 1.03 (0.89–1.20) | 0.668 | 505 (31.2) | 107 (52.2) | 1.08 (0.87–1.33) | 0.304 | ||
| Tumor site | ||||||||||
| Distal | 1006 (55.2) | 70.3 | < 0.001 | ref. | 954 (59.0) | 52 (25.4) | < 0.001 | ref. | ||
| Proximal/linitis lastica | 816 (44.8) | 49.3 | 1.43 (1.23–1.68) | < 0.001 | 663 (41.0) | 153 (74.6) | 1.63 (1.40–1.90) | < 0.001 | ||
| Histological grade | ||||||||||
| Well/moderately differentiated | 262 (14.4) | 69.5 | 0.234 | 247 (15.3) | 15 (7.3) | 0.009 | ref. | |||
| Poorly differentiated | 1197 (65.7) | 58.3 | 1054 (65.2) | 143 (69.8) | 1.18 (0.93–1.51) | 0.174 | ||||
| Undifferentiated/signet ring cell carcinoma | 363 (19.9) | 63.1 | 316 (19.5) | 47 (22.9) | 1.16 (0.87–1.54) | 0.309 | ||||
| Vascular invasion | ||||||||||
| No | 1529 (83.9) | 62.9 | < 0.001 | ref. | 1396 (86.3) | 133 (64.9) | < 0.001 | ref. | ||
| Yes | 293 (16.1) | 50.5 | 0.883 (0.72–1.08) | 0.217 | 221 (13.7) | 72 (35.1) | 1.07 (0.87–1.33) | 0.506 | ||
| Nerve invasion | ||||||||||
| No | 1465 (80.4) | 60.8 | 0.932 | 1348 (83.4) | 117 (57.1) | < 0.001 | ref. | |||
| Yes | 357 (19.6) | 61.1 | 269 (16.6) | 88 (42.9) | 0.86 (0.69–1.06) | 0.152 | ||||
| Adipose tissues invasion | ||||||||||
| No | 1437 (78.9) | 67.6 | < 0.001 | ref. | ||||||
| Yes | 385 (21.1) | 35.6 | 1.31 (1.05–1.65) | 0.019 | ||||||
| GBAI | ||||||||||
| No | 1617 (88.7) | 63.1 | < 0.001 | ref. | ||||||
| Yes | 205 (11.3) | 19.6 | 1.45 (1.12–1.89) | 0.005 | ||||||
| Depth of invasion | ||||||||||
| T1 | 235 (12.9) | 96.2 | < 0.001 | ref. | 235 (14.5) | 0 (0.0) | < 0.001 | ref. | ||
| T2 | 217 (11.9) | 83.4 | 3.35 (1.61–7.98) | 0.001 | 215 (13.3) | 2 (1.0) | 3.36 (1.61–7.00) | 0.001 | ||
| T3 | 421 (23.1) | 64.4 | 5.94 (2.99–11.80) | < 0.001 | 377 (23.3) | 44 (21.5) | 6.66 (3.35–13.25) | < 0.001 | ||
| T4a | 892 (49.0) | 45.9 | 8.46 (4.30–16.63) | < 0.001 | 744 (46.0) | 148 (72.2) | 9.33 (4.75–18.31) | < 0.001 | ||
| T4b | 57 (3.1) | 38.6 | 10.49 (4.95–22.22) | < 0.001 | 46 (2.8) | 11 (5.4) | 10.97 (5.18–23.24) | < 0.001 | ||
| Nodal status | ||||||||||
| N0 | 561 (30.8) | 80.6 | < 0.001 | ref. | 551 (34.0) | 10 (4.9) | < 0.001 | ref. | ||
| N1 | 289 (15.9) | 74.7 | 1.08 (0.80–1.46) | 0.612 | 272 (16.8) | 17 (8.3) | 1.14 (0.84–1.53) | 0.400 | ||
| N2 | 306 (16.8) | 61.1 | 1.54 (1.18–2.01) | 0.001 | 273 (16.9) | 33 (16.1) | 1.61 (1.23–2.10) | < 0.001 | ||
| N3a | 402 (22.1) | 46.8 | 2.41 (1.89–3.01) | < 0.001 | 336 (20.8) | 66 (32.2) | 2.49 (1.96–3.17) | < 0.001 | ||
| N3b | 264 (14.5) | 25.0 | 3.95 (3.04–5.14) | < 0.001 | 185 (11.4) | 79 (38.5) | 4.23 (3.27–5.47) | < 0.001 | ||
Fig. 3.
The 5-year survival analysis for all the stages according to the AJCC 8th edition staging system
Association of GBAI with GC clinicopathological characteristics
Two hundred and five (11.3%) of the 1822 investigated cases were diagnosed with GBAI on pathology, mainly observed in stage IIB, IIIA, IIIB, and IIIC (Table 1). Seven parameters were significantly associated with GBAI on univariate analysis, namely, tumor size, tumor site, histological grade, vascular invasion, nerve invasion, depth of invasion (pT), and nodal status (pN) (Table 1). In multivariate analyses, tumor site, depth of invasion (pT), and nodal status (pN) remained as independent prognostic factors for GBAI (Table 1).
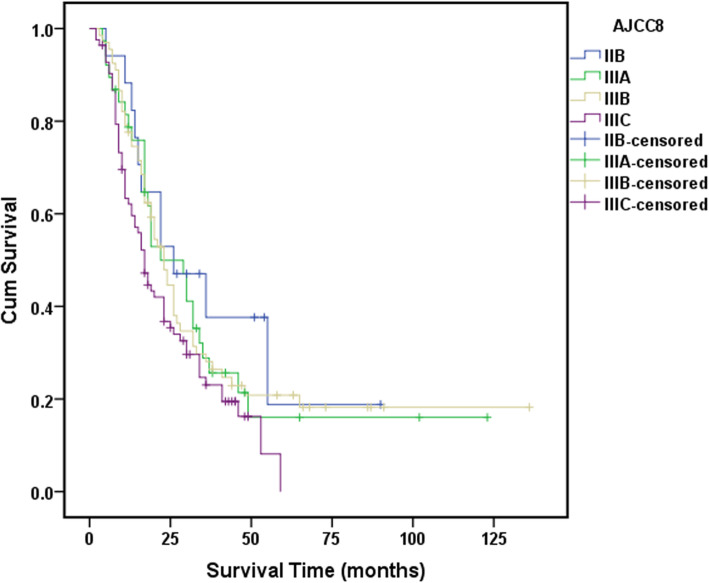
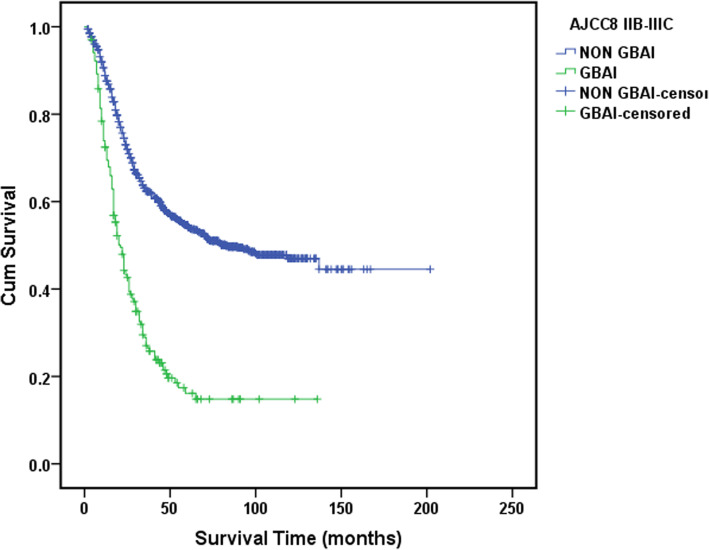
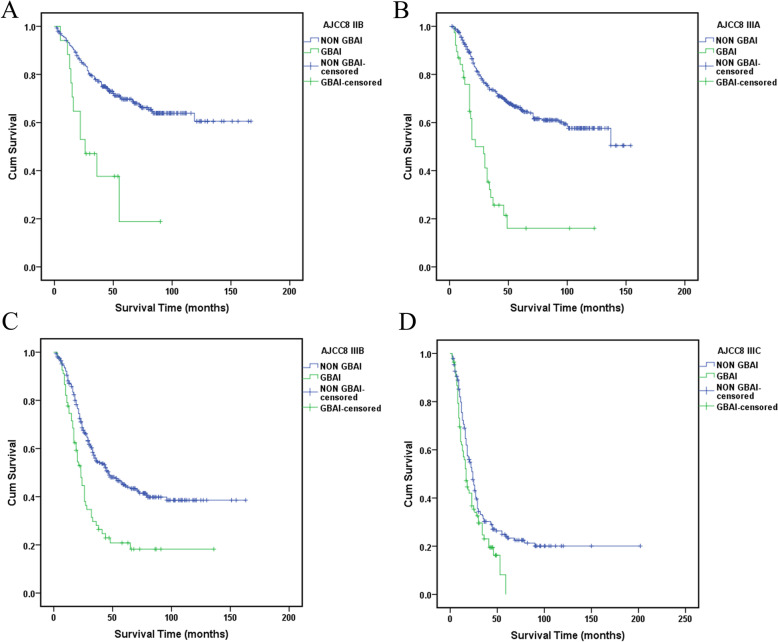
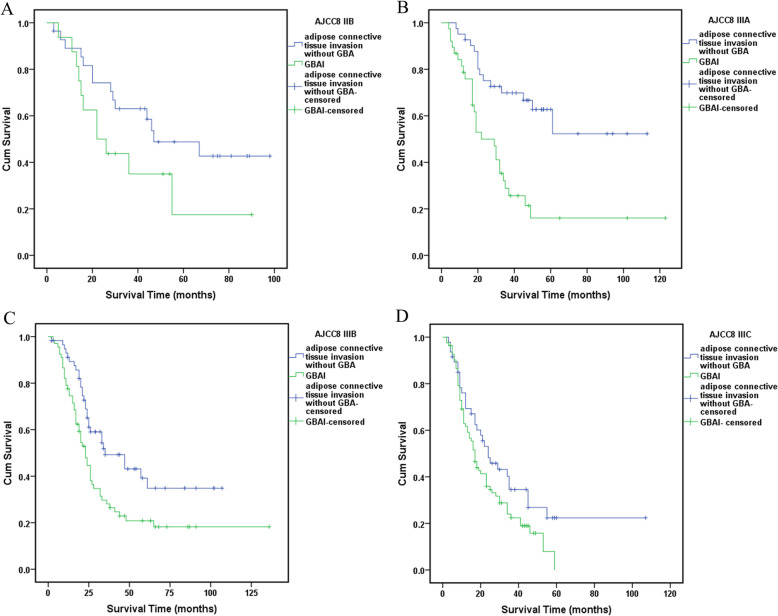
Statistically significant differences in survival were found between all the TNM stage groupings as shown in Fig. 3 (P < 0.01). However, no statistically significant difference in survival between GBAI patients in stage IIB to IIIC (P = 0.159) (Fig. 4). Interestingly, the difference in 5-year OS between all the patients with GBAI and without GBAI in stages IIB to IIIC was statistically significant (24.4% vs. 54.8%, P < 0.01) (Fig. 5). The 5-year OS for patients with GBAI were as follows: IIB, 35.3%; IIIA, 26.3%; IIIB, 23.9%; and IIIC, 21.7%. Further, statistically significant differences between patients with GBAI and without GBAI in stages IIB, IIIA, IIIB, and IIIC were also found (P < 0.01 for IIB, IIIA, and IIIB, P = 0.021 for IIIC) (Fig. 6). For the purpose of confirming the predictive value of adipose tissues invasion in GBA rather than in other place, we compared the 5-year overall survival rate between these two groups (Fig. 7). The 5-year OS for the patients with adipose tissues invasion outside GBA were as follows: IIB (n = 28), 50.0%; IIIA (n = 41), 63.4%; IIIB (n = 56), 48.3%; and IIIC (n = 47), 34.0%; The result showed statistically significant differences in 5-year OS between patients with GBAI and adipose tissues invasion outside GBA in stage IIIA, IIIB, and IIIC, respectively (P < 0.01 for IIIA and IIIB, and P = 0.050 for IIIC), apart for stage IIB (P = 0.117).
Fig. 4.
The 5-year survival analysis for GBAI cases
Fig. 5.
The 5-year survival analysis for GBAI cases and non-GBAI cases in stages IIB, IIIA, IIIB, and IIIC
Fig. 6.
The 5-year survival analysis for patients with GBAI or not in stages IIB, IIIA, IIIB, and IIIC, respectively
Fig. 7.
The 5-year survival analysis for patients with GBAI or adipose tissues invasion outside GBA in stages IIB, IIIA, IIIB, and IIIC, respectively
Metastasis status of GBAI patients
In the 205 GBAI patients, only 61 had medical records with detail data of radiological examinations during follow-up (Table 2). Of them, 26 (34.7%) had retroperitoneal infiltration, 27 (36.0%) had peritoneal metastasis, 10 (13.3%) had hematogenous metastasis, 16 (21.3%) had lymphatic metastasis, and 16 (21.3%) had other types of metastasis.
Table 2.
The detail of metastasis status of GBAI patients
| Metastasis paths (n) | Radiological manifestations | n |
|---|---|---|
| Retroperitoneal infiltration (26) | Mesenterium roots thickening | 17 |
| Para-aortic infiltration | 4 | |
| Posterior-pancreas infiltration | 6 | |
| Rectum circled thickening | 7 | |
| Peritoneal metastasis (27) | Peritoneal nodules/thickening | 18 |
| Douglas pouch nodules | 12 | |
| Abdominal mass | 5 | |
| Hematogenous metastasis (10) | Liver | 7 |
| Lung | 4 | |
| Bone | 1 | |
| Lymphatic metastasis (16) | Para-aortic | 14 |
| Left supraclavicular | 3 | |
| Mediastinum | 3 | |
| Porta hepatis | 2 | |
| Others (16) | Umbilical region | 3 |
| Ovary mass | 6 | |
| Anastomotic astium | 3 | |
| Ascites | 4 |
Discussion
Adipose tissues invasion, also known as TDs, is defined as the satellite peritumoral lesion in peritumoral adipose tissue of a primary carcinoma without any histological evidence of any residual lymph node or vessels structure. It was firstly recognized in rectal cancer by Gabriel in 1935 [14]. Quantity of researches confirmed that TDs was not only limited to colorectal cancer but also common to different tumor types, including gastric, biliary duct, and pancreatic cancers [15–18]. The impact of TDs on GC outcomes had been investigated by many researchers and was associated with poor prognosis. In the 8th edition of AJCC gastric cancer staging manual [19], TDs are considered regional LN metastases for the purposes of gastric cancer staging. However, some research suggested that TDs in GC should be incorporated into the T category and treated as a form of serosal invasion [18, 20]. Nevertheless, the prognostic impact of TDs is undeniable, and the algorithm of staging with TDs is controversial.
Our research showed that 205 (11.3%) of 1822 patients were pathologically diagnoses as GBAI and were commonly found in stage IIB, IIIA, IIIB, and IIIC. GBAI status was related to T staging, N staging, and tumor site in multivariate analysis, signifying that deeper gastric wall infiltration and larger number of LNs metastasis could relate to greater risk of identifying GBAI. There are similar survival curves among stage IIB, IIIA, IIIB, and IIIC with GBAI (P = 0.159, Fig. 4), and Kaplan-Meier plot showed some overlapping survival curves among each other, implying there is no significant difference in prognosis for patients with GBAI. Upon further analysis, the distance between 5-year survival curves of the patients staged IIB with GBAI and without GBAI, staged IIIA with GBAI and without GBAI, staged IIIB with GBAI and without GBAI, and staged IIIC with GBAI and without GBAI become widely, respectively, reflecting higher mortality risks of the patients with GBAI than without GBAI in the same stage (P < 0.009 for IIB, IIIA, and IIIB, and P = 0.021 for IIIC, Fig. 6). Significant differences in the 5-year survival rates were also observed between patients with GBAI and with TDs outside GBA in patients staged as IIIA, IIIB, and IIIC (P < 0.009 for IIIA and IIIB, and P = 0.050 for IIIC, Fig. 7). Although, P value of stage IIB is 0.117, with no statistically significant differences, we noted that the distance between two curves spread increasingly wide interval, possibly caused due to the small sample size of stage IIB (n = 28 vs. n = 16).
The 5-year survival rates for the patients with GBAI were as follows: IIB, 35.3%; IIIA, 26.3%; IIIB, 23.9%; and IIIC, 21.7%. Also, the 5-year survival rates for all patients staged as IIIB and IIIC were 43.6% and 25.5%. The 5-year survival rate of the patients staged as IIB with GBAI was similar to all patients staged as IIIB, and the 5-year survival rates of patients staged as IIIA, IIIB, and IIIC were similar to all patients staged as IIIC. These results demonstrated that GBAI was associated with poorer prognosis than others in the same stage.
From the anatomic point of view, once tumor invade GBA which is a bridge from abdominal to retroperitoneal space, it will spread to the retroperitoneal space subsequently [21]. A mass of researches regarding severe acute pancreatitis [22–30] suggested that there are contiguous spaces in the retroperitoneal spaces which are directly or indirectly related with each other, for instance the peripancreatic region, perirenal and posterior pararenal space, and the root of the small bowel mesentery, as well as pelvic retroperitoneal space. These contiguous spaces are the anatomy basic for retroperitoneal infiltration.
Researches showed that adipocytes, which were found in the close proximity to tumors, along tumor margins, or within the tumor body, exhibited both short- and long-range interactions with cancer cells [31–33]. These adipocytes, the so called cancer-associated adipocytes (CAAs), also referred to as tumor-infiltrating adipocytes, influence tumor biology in a number of ways, including by promoting angiogenesis and inflammation. The CAAs are the molecular biological basic for retroperitoneal infiltration. Hence, cancer cells of patients with GBAI will go through GBA, like a bridge, diffusing to retroperitoneal space under the interactions with CAAs, causing retroperitoneal infiltration which was different from peritoneal metastasis, as shown in Fig. 2. This can be the possible reason of the poor prognosis when cancer invades the GBA. Our follow-up data showed there were 26 (42.6%) cases with retroperitoneal infiltration, which were in accordance with this perspective.
In our research, we found that GBAI in the proximal or linitis plastica GC was more common than distal GC. The GBA is located at the posterior of proximal gastric wall, when the proximal primal tumor penetrates serosa or lymph node metastasis at this area, the cancer cells will invade adipose tissues in GBA easier, being one of the causes for the poor prognosis of proximal GC as compared to of distal GC of the same TNM subgroup. This perspective resonates with Wu et al. [21].
Despite the important findings described, there are still some limitations worth acknowledging in this present study. First, our population cohort was from a single institution, based on relatively limited retrospective data on GBAI, of which only 61 patients had medical records detailing the radiological finding on metastatic route. Second, the basis of the scientific hypothesis of the present study was from clinical observation of retroperitoneal infiltration which is believed to be one of the most commonly identified metastatic route for GC. Nevertheless, future multicenter prospective studies are necessary to validate our findings.
Conclusions
GBAI was identified as a predictor of unfavorable prognosis for GC and was more commonly found in the proximal or linitis plastica of the stomach than in distal stomach. Retroperitoneal infiltration was one of the most commonly identified metastatic route for GC associated with GBAI after radical surgery.
Acknowledgements
Not applicable.
Abbreviations
- GC
Gastric cancer
- AJCC
American Joint Committee on Cancer
- TNM
Tumor node metastasis
- TDs
Tumor deposits
- GBA
Gastric bare area
- GBAI
Gastric bare area tissues adipose invasion
- MGDL
Medial gastrodiaphragmatic ligament
- LGDL
Lateral gastrodiaphragmatic ligament
- GPL
Gastropancreatic ligament
- CAAs
Cancer-associated adipocytes
Authors’ contributions
All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn).
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee of Sun Yat-Sen University Cancer Center, number B2020-005-62.
Consent for publication
We obtained the patients’ or legal representative’s consent for publication of this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yongming Chen and Shuhang Xu contributed equally to this work.
Contributor Information
Yuanfang Li, Email: liyuanf@sysucc.org.cn.
Zhiwei Zhou, Email: zhouzhw@sysucc.org.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res= Chung-kuo yen cheng yen chiu. 2015;27(1):2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019;39(1):10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeruttun SR, Xu L, Wang F, Yi X, Fang C, Liu Z, et al. A homogenized approach to classify advanced gastric cancer patients with limited and adequate number of pathologically examined lymph nodes. Cancer Commun. 2019;39(1):32. doi: 10.1186/s40880-019-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishibeppu K, Komatsu S, Ichikawa D, Imamura T, Kosuga T, Okamoto K, et al. Venous invasion as a risk factor for recurrence after gastrectomy followed by chemotherapy for stage III gastric cancer. BMC Cancer. 2018;18(1):108. doi: 10.1186/s12885-018-4052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YX, Shao QS, Yang Q, Wang YY, Yang J, Zhao ZK, et al. Clinicopathological characteristics and prognosis of early gastric cancer after gastrectomy. Chin Med J (Engl) 2012;125(5):770–774. [PubMed] [Google Scholar]
- 8.Graham Martinez C, Knijn N, Verheij M, Nagtegaal ID, van der Post RS. Tumour deposits are a significant prognostic factor in gastric cancer - a systematic review and meta-analysis. Histopathology. 2019;74(6):809–816. doi: 10.1111/his.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Squires MH, Poultsides GA, Fields RC, Weber SM, Votanopoulos KI, et al. Impact of lymph node ratio in selecting patients with resected gastric cancer for adjuvant therapy. Surgery. 2017;162(2):285–294. doi: 10.1016/j.surg.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannister LHBM, Collins P. Gray's anatomy. 38. London: Longman Group; 1995. pp. 1754–1755. [Google Scholar]
- 11.Sun RJ, Tang L, Li XT, Li ZY, Sun YS. CT findings in diagnosis of gastric bare area invasion: potential prognostic factors for proximal gastric carcinoma. Jpn J Radiol. 2019;37(7):518–525. doi: 10.1007/s11604-019-00837-z. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer A Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14(2):113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel WBDC, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;23:395–413. doi: 10.1002/bjs.1800239017. [DOI] [Google Scholar]
- 15.Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS, et al. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162(2):177–183. doi: 10.1016/j.jss.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Gresta LT, Rodrigues-Junior IA, de Castro LP, Cassali GD, Cabral MM. Assessment of vascular invasion in gastric cancer: a comparative study. World J Gastroenterol. 2013;19(24):3761–3769. doi: 10.3748/wjg.v19.i24.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puppa G, Ueno H, Kayahara M, Capelli P, Canzonieri V, Colombari R, et al. Tumor deposits are encountered in advanced colorectal cancer and other adenocarcinomas: an expanded classification with implications for colorectal cancer staging system including a unifying concept of in-transit metastases. Modern Pathol. 2009;22(3):410–415. doi: 10.1038/modpathol.2008.198. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z, Wang ZN, Xu YY, Zhu GL, Huang BJ, Xu Y, et al. Prognostic significance of tumor deposits in gastric cancer patients who underwent radical surgery. Surgery. 2012;151(6):871–881. doi: 10.1016/j.surg.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Brierley JDGM, Wittekind C, Amin MB. TNM classification of maligant tumours. 8. Oxford: Wiley Black-well; 2017. [Google Scholar]
- 20.Anup S, Lu J, Zheng CH, Li P, Xie JW, Wang JB, et al. Prognostic significance of perigastric tumor deposits in patients with primary gastric cancer. BMC Surg. 2017;17(1):84. doi: 10.1186/s12893-017-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu B, Min PQ, Yang K. Utility of multidetector CT in the diagnosis of gastric bare area invasion by proximal gastric carcinoma. Abdom Imaging. 2007;32(3):284–289. doi: 10.1007/s00261-006-9058-3. [DOI] [PubMed] [Google Scholar]
- 22.Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36(3):253–260. doi: 10.1097/00004836-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Garcea G, Jackson B, Pattenden CJ, Sutton CD, Neal CP, Dennison AR, et al. Early warning scores predict outcome in acute pancreatitis. J Gastrointestinal Surg. 2006;10(7):1008–1015. doi: 10.1016/j.gassur.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Mortele KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52(1):67–72. doi: 10.1016/j.ejrad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa K, Idoguchi K, Tanaka H, Tohma Y, Ukai I, Watanabe H, et al. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol. 2006;60(3):445–452. doi: 10.1016/j.ejrad.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 26.De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas. 2007;34(2):185–190. doi: 10.1097/mpa.0b013e31802d4136. [DOI] [PubMed] [Google Scholar]
- 27.Korobkin M, Silverman PM, Quint LE, Francis IR. CT of the extraperitoneal space: normal anatomy and fluid collections. AJR Am J Roentgenol. 1992;159(5):933–942. doi: 10.2214/ajr.159.5.1414803. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell AM, Duddy L, Lee C, Lee MJ. CT of pelvic extraperitoneal spaces: an anatomical study in cadavers. Clin Radiol. 2007;62(5):432–438. doi: 10.1016/j.crad.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Mindell HJ, Mastromatteo JF, Dickey KW, Sturtevant NV, Shuman WP, Oliver CL, et al. Anatomic communications between the three retroperitoneal spaces: determination by CT-guided injections of contrast material in cadavers. AJR Am J Roentgenol. 1995;164(5):1173–1178. doi: 10.2214/ajr.164.5.7717227. [DOI] [PubMed] [Google Scholar]
- 30.Thornton FJ, Kandiah SS, Monkhouse WS, Lee MJ. Helical CT evaluation of the perirenal space and its boundaries: a cadaveric study. Radiology. 2001;218(3):659–663. doi: 10.1148/radiology.218.3.r01fe17659. [DOI] [PubMed] [Google Scholar]
- 31.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner M, Dudley AC. A three-party alliance in solid tumors: adipocytes, macrophages and vascular endothelial cells. Adipocyte. 2013;2(2):67–73. doi: 10.4161/adip.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324(2):142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn).